Membrane-Coated Biomimetic Nanoparticles: A State-of-the-Art Multifunctional Weapon for Tumor Immunotherapy
Abstract
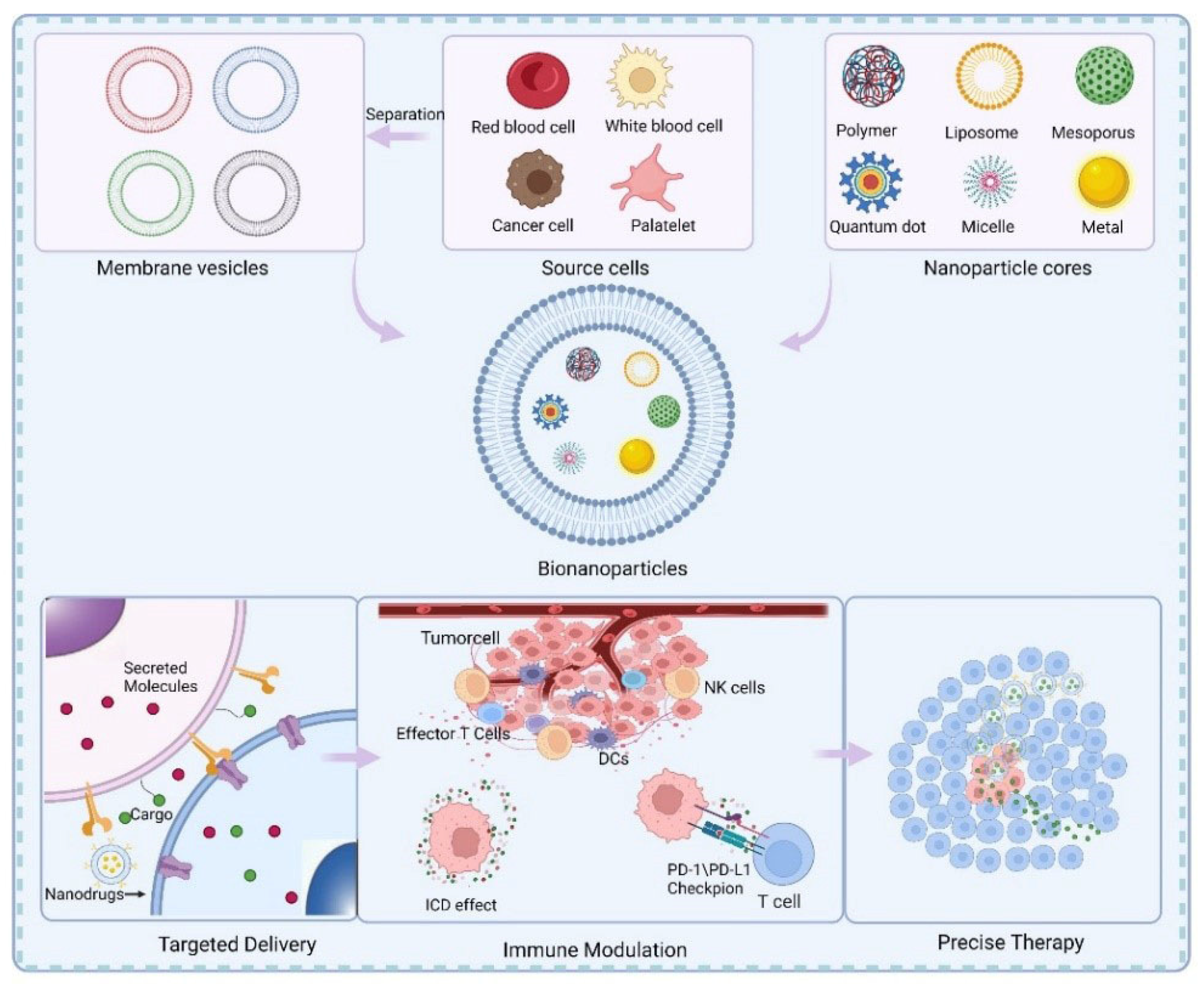
:1. Introduction
2. Background of Membrane Coating Technology for Tumor Immunotherapy
2.1. Introduction of Cell Membranes
2.2. Membrane Coating Technology
3. Cell Membrane-Coated Nanoparticles for Immunotherapy
3.1. Red Blood Cell Membrane-Coated Nanoparticles
3.2. Leukocyte Membrane-Coated Nanoparticles
3.3. Platelet Membrane-Coated Nanoparticles
3.4. Tumor Cell Membrane-Coated Nanoparticles
3.5. Hybrid Membrane-Coated Nanoparticles
4. Conclusions and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Ma, Y.; Zhang, Y.; Li, X.; Zhao, Y.; Li, M.; Jiang, W.; Tang, X.; Dou, J.; Lu, L.; Wang, F.; et al. Near-Infrared II Phototherapy Induces Deep Tissue Immunogenic Cell Death and Potentiates Cancer Immunotherapy. ACS Nano 2019, 13, 11967–11980. [Google Scholar] [CrossRef] [PubMed]
- Abbott, M.; Ustoyev, Y. Cancer and the Immune System: The History and Background of Immunotherapy. Semin. Oncol. Nurs. 2019, 35, 150923. [Google Scholar] [CrossRef] [PubMed]
- Helmy, K.Y.; Patel, S.A.; Nahas, G.R.; Rameshwar, P. Cancer immunotherapy: Accomplishments to date and future promise. Ther. Deliv. 2013, 4, 1307–1320. [Google Scholar] [CrossRef] [PubMed]
- Ilyas, F.Z.; Beane, J.D.; Pawlik, T.M. The State of Immunotherapy in Hepatobiliary Cancers. Cells 2021, 10, 2096. [Google Scholar] [CrossRef] [PubMed]
- Liu, D. CAR-T “the living drugs”, immune checkpoint inhibitors, and precision medicine: A new era of cancer therapy. J. Hematol. Oncol. 2019, 12, 113. [Google Scholar] [CrossRef] [PubMed]
- Kaymak, I.; Williams, K.S.; Cantor, J.R.; Jones, R.G. Immunometabolic Interplay in the Tumor Microenvironment. Cancer Cell 2021, 39, 28–37. [Google Scholar] [CrossRef]
- Bader, J.E.; Voss, K.; Rathmell, J.C. Targeting Metabolism to Improve the Tumor Microenvironment for Cancer Immunotherapy. Mol. Cell 2020, 78, 1019–1033. [Google Scholar] [CrossRef]
- Anderson, N.M.; Simon, M.C. The tumor microenvironment. Curr. Biol. 2020, 30, R921–R925. [Google Scholar] [CrossRef]
- Binnewies, M.; Roberts, E.W.; Kersten, K.; Chan, V.; Fearon, D.F.; Merad, M.; Coussens, L.M.; Gabrilovich, D.I.; Ostrand-Rosenberg, S.; Hedrick, C.C.; et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat. Med. 2018, 24, 541–550. [Google Scholar] [CrossRef]
- Tuccitto, A.; Shahaj, E.; Vergani, E.; Ferro, S.; Huber, V.; Rodolfo, M.; Castelli, C.; Rivoltini, L.; Vallacchi, V. Immunosuppressive circuits in tumor microenvironment and their influence on cancer treatment efficacy. Virchows. Arch. 2019, 474, 407–420. [Google Scholar] [CrossRef] [PubMed]
- Sau, S.; Alsaab, H.O.; Bhise, K.; Alzhrani, R.; Nabil, G.; Iyer, A.K. Multifunctional nanoparticles for cancer immunotherapy: A groundbreaking approach for reprogramming malfunctioned tumor environment. J. Control Release 2018, 274, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Ha, D.; Yang, N.; Nadithe, V. Exosomes as therapeutic drug carriers and delivery vehicles across biological membranes: Current perspectives and future challenges. Acta Pharm. Sin. B 2016, 6, 287–296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, Z.; Ott, P.A.; Wu, C.J. Towards personalized, tumour-specific, therapeutic vaccines for cancer. Nat. Rev. Immunol. 2018, 18, 168–182. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xie, F.; Yin, Y.; Zhang, Q.; Jin, H.; Wu, Y.; Pang, L.; Li, J.; Gao, J. Immunotherapy of Tumor RNA-Loaded Lipid Nanoparticles Against Hepatocellular Carcinoma. Int. J. Nanomed. 2021, 16, 1553–1564. [Google Scholar] [CrossRef]
- Sadeghi, M.; Koushki, K.; Mashayekhi, K.; Ayati, S.H.; Keshavarz Shahbaz, S.; Moghadam, M.; Sankian, M. DC-targeted gold nanoparticles as an efficient and biocompatible carrier for modulating allergic responses in sublingual immunotherapy. Int. Immunopharmacol. 2020, 86, 106690. [Google Scholar] [CrossRef] [PubMed]
- Dobrovolskaia, M.A.; Aggarwal, P.; Hall, J.B.; McNeil, S.E. Preclinical studies to understand nanoparticle interaction with the immune system and its potential effects on nanoparticle biodistribution. Mol. Pharm. 2008, 5, 487–495. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Guo, Q.; Zhao, H.; Zhang, L.; Li, J.; Gao, J.; Qian, W.; Li, B.; Chen, H.; Wang, H.; et al. Novel dual-control poly(N-isopropylacrylamide-co-chlorophyllin) nanogels for improving drug release. Nanomedicine 2012, 7, 383–392. [Google Scholar] [CrossRef]
- Parayath, N.N.; Amiji, M.M. Therapeutic targeting strategies using endogenous cells and proteins. J. Control. Release 2017, 258, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Jokerst, J.V.; Lobovkina, T.; Zare, R.N.; Gambhir, S.S. Nanoparticle PEGylation for imaging and therapy. Nanomedicine 2011, 6, 715–728. [Google Scholar] [CrossRef] [Green Version]
- Fang, R.H.; Jiang, Y.; Fang, J.C.; Zhang, L. Cell membrane-derived nanomaterials for biomedical applications. Biomaterials 2017, 128, 69–83. [Google Scholar] [CrossRef] [Green Version]
- Burgess, B.L.; Cavigiolio, G.; Fannucchi, M.V.; Illek, B.; Forte, T.M.; Oda, M.N. A phospholipid-apolipoprotein A-I nanoparticle containing amphotericin B as a drug delivery platform with cell membrane protective properties. Int. J. Pharm. 2010, 399, 148–155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, R.H.; Kroll, A.V.; Gao, W.; Zhang, L. Cell Membrane Coating Nanotechnology. Adv. Mater. 2018, 30, e1706759. [Google Scholar] [CrossRef] [PubMed]
- Dergunov, S.A.; Kim, M.D.; Shmakov, S.N.; Pinkhassik, E. Building Functional Nanodevices with Vesicle-Templated Porous Polymer Nanocapsules. Acc. Chem. Res. 2019, 52, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zou, M.-Z.; Qin, S.-Y.; Cheng, Y.-J.; Ma, Y.; Sun, Y.-X.; Zhang, X.-Z. Recent Advances of Cell Membrane-Coated Nanomaterials for Biomedical Applications. Adv. Funct. Mater. 2020, 39, 2003559. [Google Scholar] [CrossRef]
- Wibroe, P.P.; Anselmo, A.C.; Nilsson, P.H.; Sarode, A.; Gupta, V.; Urbanics, R.; Szebeni, J.; Hunter, A.C.; Mitragotri, S.; Mollnes, T.E.; et al. Bypassing adverse injection reactions to nanoparticles through shape modification and attachment to erythrocytes. Nat. Nanotechnol. 2017, 12, 589–594. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Ruan, J.; Lv, G.; Shan, Q.; Fan, Z.; Wang, H.; Du, Y.; Ling, L. Cell membrane-based biomimetic nanoparticless for advanced drug delivery in cancer therapy: A comprehensive review. Colloids Surf. B Biointerfaces 2022, 215, 112503. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.-M.J.; Zhang, L.; Aryal, S.; Cheung, C.; Fang, R.H.; Zhang, L. Erythrocyte membrane-camouflaged polymeric nanoparticles as a biomimetic delivery platform. Proc. Natl. Acad. Sci. USA 2011, 108, 10980–10985. [Google Scholar] [CrossRef] [Green Version]
- Copp, J.A.; Fang, R.H.; Luk, B.T.; Hu, C.M.; Gao, W.; Zhang, K.; Zhang, L. Clearance of pathological antibodies using biomimetic nanoparticles. Proc. Natl. Acad. Sci. USA 2014, 111, 13481–13486. [Google Scholar] [CrossRef] [Green Version]
- Rao, L.; Cai, B.; Bu, L.L.; Liao, Q.Q.; Guo, S.S.; Zhao, X.Z.; Dong, W.F.; Liu, W. Microfluidic Electroporation-Facilitated Synthesis of Erythrocyte Membrane-Coated Magnetic Nanoparticles for Enhanced Imaging-Guided Cancer Therapy. ACS Nano 2017, 11, 3496–3505. [Google Scholar] [CrossRef]
- Xu, E.; Wu, X.; Zhang, X.; Zul, K.; Raza, F.; Su, J.; Qiu, M. Study on the protection of dextran on erythrocytes during drug loading. Colloids Surf. B Biointerfaces 2020, 189, 110882. [Google Scholar] [CrossRef]
- Desilets, J.; Lejeune, A.; Mercer, J.; Gicquaud, C. Nanoerythrosomes, a new derivative of erythrocyte ghost: IV. Fate of reinjected nanoerythrosomes. Anticancer Res. 2001, 21, 1741–1747. [Google Scholar]
- Cheng, J.; Teply, B.A.; Sherifi, I.; Sung, J.; Luther, G.; Gu, F.X.; Levy-Nissenbaum, E.; Radovic-Moreno, A.F.; Langer, R.; Farokhzad, O.C. Formulation of functionalized PLGA-PEG nanoparticles for in vivo targeted drug delivery. Biomaterials 2007, 28, 869–876. [Google Scholar] [CrossRef] [Green Version]
- Fan, Y.; Cui, Y.; Hao, W.; Chen, M.; Liu, Q.; Wang, Y.; Yang, M.; Li, Z.; Gong, W.; Song, S.; et al. Carrier-free highly drug-loaded biomimetic nanosuspensions encapsulated by cancer cell membrane based on homology and active targeting for the treatment of glioma. Bioact. Mater. 2021, 6, 4402–4414. [Google Scholar] [CrossRef] [PubMed]
- Xia, Q.; Zhang, Y.; Li, Z.; Hou, X.; Feng, N. Red blood cell membrane-camouflaged nanoparticles: A novel drug delivery system for antitumor application. Acta. Pharm. Sin. B 2019, 9, 675–689. [Google Scholar] [CrossRef] [PubMed]
- Kang, T.; Zhu, Q.; Wei, D.; Feng, J.; Yao, J.; Jiang, T.; Song, Q.; Wei, X.; Chen, H.; Gao, X.; et al. Nanoparticles Coated with Neutrophil Membranes Can Effectively Treat Cancer Metastasis. ACS Nano 2017, 11, 1397–1411. [Google Scholar] [CrossRef] [PubMed]
- Ye, H.; Wang, K.; Wang, M.; Liu, R.; Song, H.; Li, N.; Lu, Q.; Zhang, W.; Du, Y.; Yang, W.; et al. Bioinspired nanoplatelets for chemo-photothermal therapy of breast cancer metastasis inhibition. Biomaterials 2019, 206, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Miao, Y.; Ni, W.; Xiao, H.; Zhang, J. Cancer cell membrane coated silica nanoparticles loaded with ICG for tumour specific photothermal therapy of osteosarcoma. Artif. Cells Nanomed. Biotechnol. 2019, 47, 2298–2305. [Google Scholar] [CrossRef] [Green Version]
- Nie, D.; Dai, Z.; Li, J.; Yang, Y.; Xi, Z.; Wang, J.; Zhang, W.; Qian, K.; Guo, S.; Zhu, C.; et al. Cancer-Cell-Membrane-Coated Nanoparticles with a Yolk-Shell Structure Augment Cancer Chemotherapy. Nano Lett. 2020, 20, 936–946. [Google Scholar] [CrossRef]
- Dehaini, D.; Wei, X.; Fang, R.H.; Masson, S.; Angsantikul, P.; Luk, B.T.; Zhang, Y.; Ying, M.; Jiang, Y.; Kroll, A.V.; et al. Erythrocyte-Platelet Hybrid Membrane Coating for Enhanced Nanoparticle Functionalization. Adv. Mater. 2017, 29, 1606209. [Google Scholar] [CrossRef] [Green Version]
- Yu, W.; He, X.; Yang, Z.; Yang, X.; Xiao, W.; Liu, R.; Xie, R.; Qin, L.; Gao, H. Sequentially responsive biomimetic nanoparticles with optimal size in combination with checkpoint blockade for cascade synergetic treatment of breast cancer and lung metastasis. Biomaterials 2019, 217, 119309. [Google Scholar] [CrossRef]
- Feng, Q.; Li, Y.; Wang, N.; Hao, Y.; Chang, J.; Wang, Z.; Zhang, X.; Zhang, Z.; Wang, L. A Biomimetic Nanogenerator of Reactive Nitrogen Species Based on Battlefield Transfer Strategy for Enhanced Immunotherapy. Small 2020, 16, e2002138. [Google Scholar] [CrossRef]
- Liang, X.; Ye, X.; Wang, C.; Xing, C.; Miao, Q.; Xie, Z.; Chen, X.; Zhang, X.; Zhang, H.; Mei, L. Photothermal cancer immunotherapy by erythrocyte membrane-coated black phosphorus formulation. J. Control. Release 2019, 296, 150–161. [Google Scholar] [CrossRef] [PubMed]
- Parodi, A.; Quattrocchi, N.; van de Ven, A.L.; Chiappini, C.; Evangelopoulos, M.; Martinez, J.O.; Brown, B.S.; Khaled, S.Z.; Yazdi, I.K.; Enzo, M.V.; et al. Synthetic nanoparticles functionalized with biomimetic leukocyte membranes possess cell-like functions. Nat. Nanotechnol. 2013, 8, 61–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, G.; Sun, Z.; Li, S.; Peng, X.; Li, W.; Zhou, L.; Ma, Y.; Gong, P.; Cai, L. Cell-Membrane Immunotherapy Based on Natural Killer Cell Membrane Coated Nanoparticles for the Effective Inhibition of Primary and Abscopal Tumor Growth. ACS Nano 2018, 12, 12096–12108. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Xu, C.; Jin, Y.; Li, Y.; Zhong, C.; Ma, J.; Yang, J.; Zhang, N.; Li, Y.; Wang, C.; et al. Artificial Mini Dendritic Cells Boost T Cell-Based Immunotherapy for Ovarian Cancer. Adv. Sci. 2020, 7, 1903301. [Google Scholar] [CrossRef]
- Bahmani, B.; Gong, H.; Luk, B.T.; Haushalter, K.J.; DeTeresa, E.; Previti, M.; Zhou, J.; Gao, W.; Bui, J.D.; Zhang, L.; et al. Intratumoral immunotherapy using platelet-cloaked nanoparticles enhances antitumor immunity in solid tumors. Nat. Commun. 2021, 12, 1999. [Google Scholar] [CrossRef]
- Chen, M.; Qiao, Y.; Cao, J.; Ta, L.; Ci, T.; Ke, X. Biomimetic doxorubicin/ginsenoside co-loading nanoparticles for chemoimmunotherapy of acute myeloid leukemia. J. Nanobiotechnol. 2022, 20, 273. [Google Scholar] [CrossRef]
- Luo, J.; Wang, X.; Shi, Z.; Zeng, Y.; He, L.; Cao, J.; Sun, Y.; Zhang, T.; Huang, P. Enhancement of antitumor immunotherapy using mitochondria-targeted cancer cell membrane-biomimetic MOF-mediated sonodynamic therapy and checkpoint blockade immunotherapy. J. Nanobiotechnol. 2022, 20, 228. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, X.; Li, B.; Wei, X.; Chen, Y.; Zhang, Y.; Wang, Y.; Zhang, W.; Liu, S.; Liu, Z.; et al. Intelligent Biomimetic Nanoplatform for Systemic Treatment of Metastatic Triple-Negative Breast Cancer via Enhanced EGFR-Targeted Therapy and Immunotherapy. ACS Appl. Mater. Interfaces 2022, 14, 23152–23163. [Google Scholar] [CrossRef]
- Hou, L.; Gong, X.; Yang, J.; Zhang, H.; Yang, W.; Chen, X. Hybrid-Membrane-Decorated Prussian Blue for Effective Cancer Immunotherapy via Tumor-Associated Macrophages Polarization and Hypoxia Relief. Adv. Mater. 2022, 34, e2200389. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Xu, Y.; Ji, W.; Li, L.; Qiu, L.; Zhou, S.; Qian, Z.; Zhang, H. Hybrid Membrane Nanovaccines Combined with Immune Checkpoint Blockade to Enhance Cancer Immunotherapy. Int. J. Nanomed. 2022, 17, 73–89. [Google Scholar] [CrossRef] [PubMed]
- Zang, S.; Huang, K.; Li, J.; Ren, K.; Li, T.; He, X.; Tao, Y.; He, J.; Dong, Z.; Li, M.; et al. Metabolic reprogramming by dual-targeting biomimetic nanoparticles for enhanced tumor chemo-immunotherapy. Acta Biomater. 2022, 148, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Wu, M.; Chen, J.; Liu, Y.; Chen, Y.; Fan, G.; Liu, Y.; Cheng, J.; Wang, Z.; Wang, S.; et al. Cancer-Erythrocyte Hybrid Membrane-Camouflaged Magnetic Nanoparticles with Enhanced Photothermal-Immunotherapy for Ovarian Cancer. ACS Nano 2021, 15, 19756–19770. [Google Scholar] [CrossRef]
- Li, P.; Luo, Z.; Liu, P.; Gao, N.; Zhang, Y.; Pan, H.; Liu, L.; Wang, C.; Cai, L.; Ma, Y. Bioreducible alginate-poly(ethylenimine) nanogels as an antigen-delivery system robustly enhance vaccine-elicited humoral and cellular immune responses. J. Control Release 2013, 168, 271–279. [Google Scholar] [CrossRef]
- Oldenborg, P.A.; Zheleznyak, A.; Fang, Y.F.; Lagenaur, C.F.; Gresham, H.D.; Lindberg, F.P. Role of CD47 as a marker of self on red blood cells. Science 2000, 288, 2051–2054. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, D.; Song, Q.; Wu, T.; Zhuang, X.; Bao, Y.; Kong, M.; Qi, Y.; Tan, S.; Zhang, Z. Erythrocyte Membrane-Enveloped Polymeric Nanoparticles as Nanovaccine for Induction of Antitumor Immunity against Melanoma. ACS Nano 2015, 9, 6918–6933. [Google Scholar] [CrossRef] [PubMed]
- Banz, A.; Cremel, M.; Rembert, A.; Godfrin, Y. In situ targeting of dendritic cells by antigen-loaded red blood cells: A novel approach to cancer immunotherapy. Vaccine 2010, 28, 2965–2972. [Google Scholar] [CrossRef]
- Cremel, M.; Guerin, N.; Horand, F.; Banz, A.; Godfrin, Y. Red blood cells as innovative antigen carrier to induce specific immune tolerance. Int. J. Pharm 2013, 443, 39–49. [Google Scholar] [CrossRef]
- Godfrin, Y.; Horand, F.; Cremel, M. Can red blood cells prove to be a useful tool in tumor immunotherapy? Immunotherapy 2012, 4, 871–873. [Google Scholar] [CrossRef]
- Babatunde, K.A.; Wang, X.; Hopke, A.; Lannes, N.; Mantel, P.Y.; Irimia, D. Chemotaxis and swarming in differentiated HL-60 neutrophil-like cells. Sci. Rep. 2021, 11, 778. [Google Scholar] [CrossRef]
- Harjunpaa, H.; Llort Asens, M.; Guenther, C.; Fagerholm, S.C. Cell Adhesion Molecules and Their Roles and Regulation in the Immune and Tumor Microenvironment. Front. Immunol. 2019, 10, 1078. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Myers, J.A.; Miller, J.S. Exploring the NK cell platform for cancer immunotherapy. Nat. Rev. Clin. Oncol. 2021, 18, 85–100. [Google Scholar] [CrossRef] [PubMed]
- Sivori, S.; Falco, M.; Della Chiesa, M.; Carlomagno, S.; Vitale, M.; Moretta, L.; Moretta, A. CpG and double-stranded RNA trigger human NK cells by Toll-like receptors: Induction of cytokine release and cytotoxicity against tumors and dendritic cells. Proc. Natl. Acad. Sci. USA 2004, 101, 10116–10121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shaw, D.M.; Merien, F.; Braakhuis, A.; Dulson, D. T-cells and their cytokine production: The anti-inflammatory and immunosuppressive effects of strenuous exercise. Cytokine 2018, 104, 136–142. [Google Scholar] [CrossRef]
- Li, X.; Zhang, W.; Lin, J.; Wu, H.; Yao, Y.; Zhang, J.; Yang, C. T cell membrane cloaking tumor microenvironment-responsive nanoparticles with a smart “membrane escape mechanism” for enhanced immune-chemotherapy of melanoma. Biomater. Sci. 2021, 9, 3453–3464. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Hu, C.; Tong, F.; Liu, R.; Zhou, Y.; Qin, L.; Ouyang, L.; Gao, H. Tumor Microenvironment-Responsive Dual Drug Dimer-Loaded PEGylated Bilirubin Nanoparticles for Improved Drug Delivery and Enhanced Immune-Chemotherapy of Breast Cancer. Adv. Funct. Mater. 2019, 29, 1901896. [Google Scholar] [CrossRef]
- Zhai, Y.; Wang, J.; Lang, T.; Kong, Y.; Rong, R.; Cai, Y.; Ran, W.; Xiong, F.; Zheng, C.; Wang, Y.; et al. T lymphocyte membrane-decorated epigenetic nanoinducer of interferons for cancer immunotherapy. Nat. Nanotechnol. 2021, 16, 1271–1280. [Google Scholar] [CrossRef]
- Kang, M.; Hong, J.; Jung, M.; Kwon, S.P.; Song, S.Y.; Kim, H.Y.; Lee, J.R.; Kang, S.; Han, J.; Koo, J.H.; et al. T-Cell-Mimicking Nanoparticles for Cancer Immunotherapy. Adv. Mater. 2020, 32, e2003368. [Google Scholar] [CrossRef] [PubMed]
- Tuettenberg, A.; Schmitt, E.; Knop, J.; Jonuleit, H. Dendritic cell-based immunotherapy of malignant melanoma: Success and limitations. J. Dtsch. Dermatol. Ges. 2007, 5, 190–196. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, X.; Xu, M.; Zhao, X.; Yang, C.; Li, K.; Zhao, F.; Hu, H.; Qiao, M.; Chen, D.; et al. A Self-amplifying ROS-sensitive prodrug-based nanodecoy for circumventing immune resistance in chemotherapy-sensitized immunotherapy. Acta Biomater. 2022, in press. [CrossRef]
- Wang, S.; Song, Y.; Cao, K.; Zhang, L.; Fang, X.; Chen, F.; Feng, S.; Yan, F. Photothermal therapy mediated by gold nanocages composed of anti-PDL1 and galunisertib for improved synergistic immunotherapy in colorectal cancer. Acta Biomater. 2021, 134, 621–632. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Bartolo, R.; Li, J.; Shahbazi, M.A.; Santos, H.A. Biomimetic platelet membrane-coated nanoparticles for targeted therapy. Eur. J. Pharm Biopharm. 2022, 172, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.; Park, W.; Park, S.B.; Rhim, W.K.; Han, D.K. Recent trends in cell membrane-cloaked nanoparticles for therapeutic applications. Methods 2020, 177, 2–14. [Google Scholar] [CrossRef]
- Ballerini, P.; Contursi, A.; Bruno, A.; Mucci, M.; Tacconelli, S.; Patrignani, P. Inflammation and Cancer: From the Development of Personalized Indicators to Novel Therapeutic Strategies. Front. Pharmacol. 2022, 13, 838079. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.-L.; Shen, B.-X.; Lin, M.-T.; Tong, M.-Q.; Zheng, Y.-W.; Jiang, X.; Yang, W.-G.; Yuan, J.-D.; Yao, Q.; Zhao, Y.-Z. Homing of ICG-loaded liposome inlaid with tumor cellular membrane to the homologous xenografts glioma eradicates the primary focus and prevents lung metastases through phototherapy. Biomater. Sci. 2018, 6, 2410–2425. [Google Scholar] [CrossRef] [PubMed]
- Pereira-Silva, M.; Chauhan, G.; Shin, M.D.; Hoskins, C.; Madou, M.J.; Martinez-Chapa, S.O.; Steinmetz, N.F.; Veiga, F.; Paiva-Santos, A.C. Unleashing the potential of cell membrane-based nanoparticles for COVID-19 treatment and vaccination. Expert Opin. Drug Deliv. 2021, 18, 1395–1414. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-Y.; Cheng, H.; Xie, B.-R.; Qiu, W.-X.; Zeng, J.-Y.; Li, C.-X.; Wan, S.-S.; Zhang, L.; Liu, W.-L.; Zhang, X.-Z. Cancer Cell Membrane Camouflaged Cascade Bioreactor for Cancer Targeted Starvation and Photodynamic Therapy. ACS Nano 2017, 11, 7006–7018. [Google Scholar] [CrossRef]
- Yang, R.; Xu, J.; Xu, L.; Sun, X.; Chen, Q.; Zhao, Y.; Peng, R.; Liu, Z. Cancer Cell Membrane-Coated Adjuvant Nanoparticles with Mannose Modification for Effective Anticancer Vaccination. ACS Nano 2018, 12, 5121–5129. [Google Scholar] [CrossRef]
- Meng, X.; Wang, J.; Zhou, J.; Tian, Q.; Qie, B.; Zhou, G.; Duan, W.; Zhu, Y. Tumor cell membrane-based peptide delivery system targeting the tumor microenvironment for cancer immunotherapy and diagnosis. Acta Biomater. 2021, 127, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Li, A.; Jiang, L.; Gu, Y.; Liu, J. Hybrid Membrane-Coated Biomimetic Nanoparticles (HM@BNPs): A Multifunctional Nanomaterial for Biomedical Applications. Biomacromolecules 2021, 22, 3149–3167. [Google Scholar] [CrossRef]
- Jiang, Q.; Liu, Y.; Guo, R.; Yao, X.; Sung, S.; Pang, Z.; Yang, W. Erythrocyte-cancer hybrid membrane-camouflaged melanin nanoparticles for enhancing photothermal therapy efficacy in tumors. Biomaterials 2019, 192, 292–308. [Google Scholar] [CrossRef]
- Chen, H.Y.; Deng, J.; Wang, Y.; Wu, C.Q.; Li, X.; Dai, H.W. Hybrid cell membrane-coated nanoparticles: A multifunctional biomimetic platform for cancer diagnosis and therapy. Acta Biomater. 2020, 112, 1–13. [Google Scholar] [CrossRef]



| Types of Cell Membrane | Core Nanoparticles | Applications | References |
|---|---|---|---|
| RBCM | Hyaluronidase-responsive nanoparticles | Metastasis, PDT, cancer immunotherapy | [40] |
| Hollow mesoporous TiO2 | cancer immunotherapy | [41] | |
| Black phosphorus quantum dot | PTT, tumor immunotherapy | [42] | |
| WBCM | Silicon nanoparticle | PDT, cancer immunotherapy | [43] |
| Hyaluronidase responsive nanoparticles | Cancer immune chemotherapy combination | [44] | |
| PLGA nanoparticle | Cancer immunotherapy | [45] | |
| Platelet membrane | Polylactic acid (PLA) nanoparticle cores | Cancer immunotherapy | [46] |
| DOX/Rg3 liposomes | Cancer immunotherapy | [47] | |
| Cancer cell membrane | Triphenylphosphine (TPP) metal zirconium framework | Cancer immunotherapy, tumor metastasis | [48] |
| (PLGA)-based intelligent bionic nanoplatform | Cancer immunotherapy | [49] | |
| Hybrid membrane | A hollow mesoporous Prussian blue (HMPB) nanoparticles | Cancer immunotherapy | [50] |
| Mesoporous silica nanoparticle | cancer immunotherapy | [51] | |
| Solid lipid nanoparticles | cancer immunotherapy | [52] | |
| (ICG)-loaded magnetic nanoparticles | Cancer immunotherapy | [53] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Zhang, X.; Li, H.; Liu, J.; Wei, W.; Gao, J. Membrane-Coated Biomimetic Nanoparticles: A State-of-the-Art Multifunctional Weapon for Tumor Immunotherapy. Membranes 2022, 12, 738. https://doi.org/10.3390/membranes12080738
Zhang Y, Zhang X, Li H, Liu J, Wei W, Gao J. Membrane-Coated Biomimetic Nanoparticles: A State-of-the-Art Multifunctional Weapon for Tumor Immunotherapy. Membranes. 2022; 12(8):738. https://doi.org/10.3390/membranes12080738
Chicago/Turabian StyleZhang, Yuanyuan, Xinyi Zhang, Haitao Li, Jianyong Liu, Wei Wei, and Jie Gao. 2022. "Membrane-Coated Biomimetic Nanoparticles: A State-of-the-Art Multifunctional Weapon for Tumor Immunotherapy" Membranes 12, no. 8: 738. https://doi.org/10.3390/membranes12080738
APA StyleZhang, Y., Zhang, X., Li, H., Liu, J., Wei, W., & Gao, J. (2022). Membrane-Coated Biomimetic Nanoparticles: A State-of-the-Art Multifunctional Weapon for Tumor Immunotherapy. Membranes, 12(8), 738. https://doi.org/10.3390/membranes12080738







