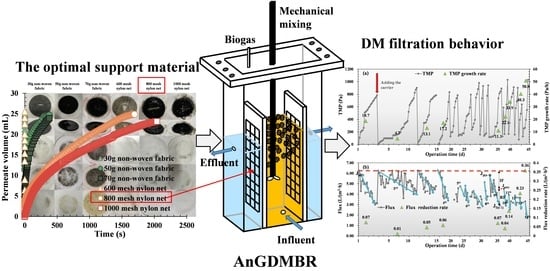
A Novel Anaerobic Gravity-Driven Dynamic Membrane Bioreactor (AnGDMBR): Performance and Fouling Characterization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Dead-End Filtration Tests
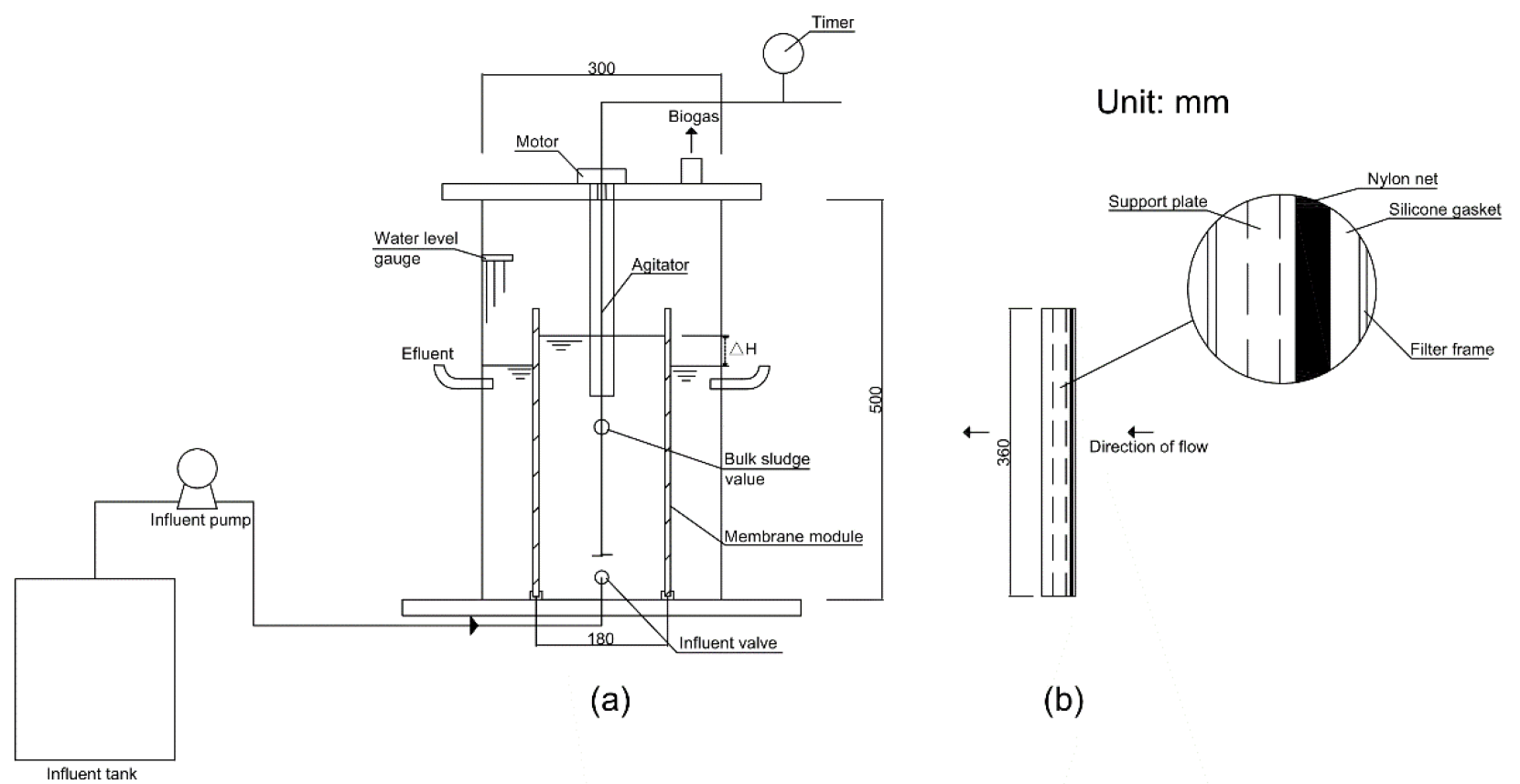
2.2. Bioreactor Setup, Operation, and In-Situ Mechanical Cleaning
2.3. Characterization of the DM Layer
2.3.1. The Retention Capacity of the DM Layer
2.3.2. Fouling Calculation of the DM Layer
2.3.3. Fouling Resistance Measurement and Scanning Electron Microscopy (SEM) Characterization of DM Layers
2.4. Microbial Community Analysis
2.5. Other Physico-Chemical Analysis
3. Results and Discussion
3.1. Optimal Support Material for DM Formation and Filtration
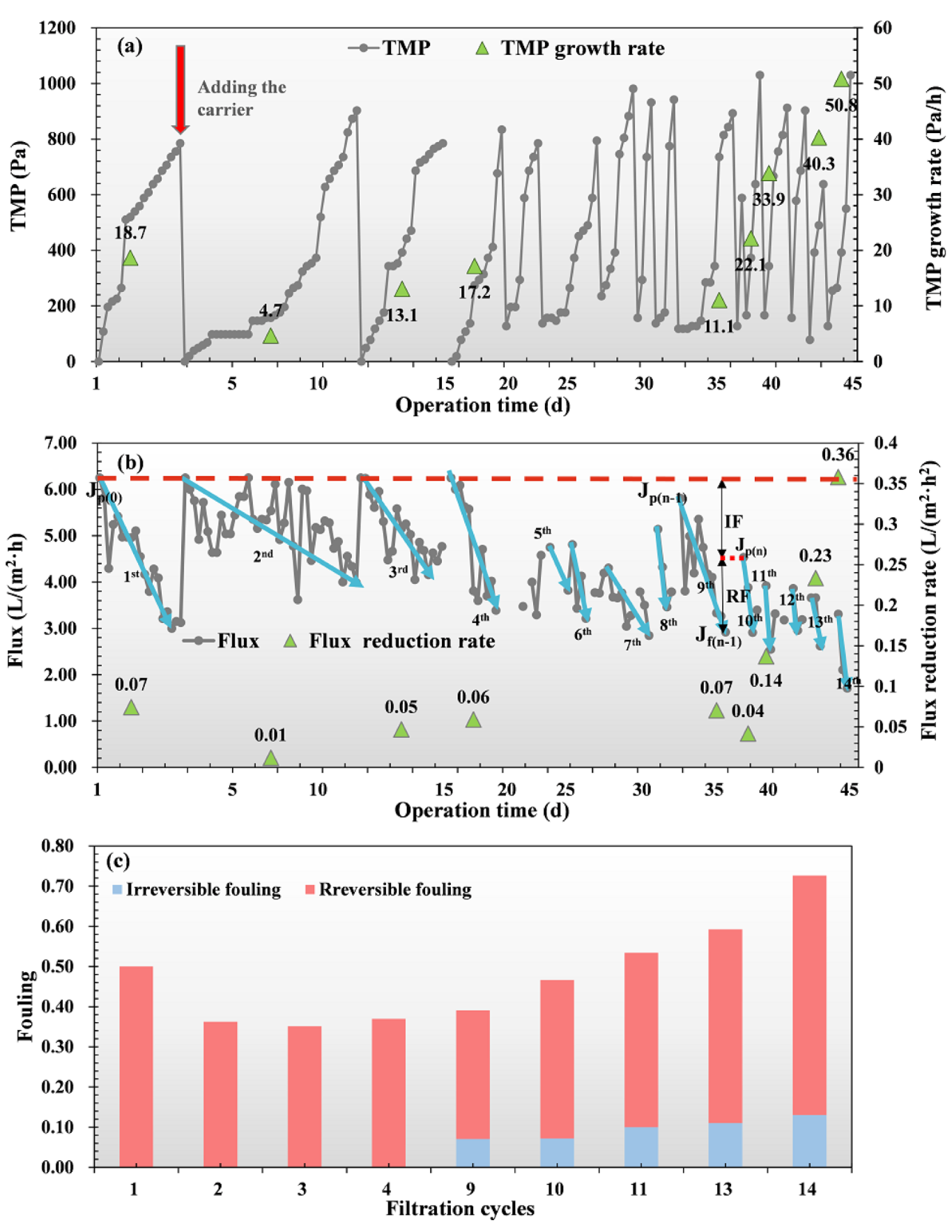
3.2. Performance of AnGDMBR
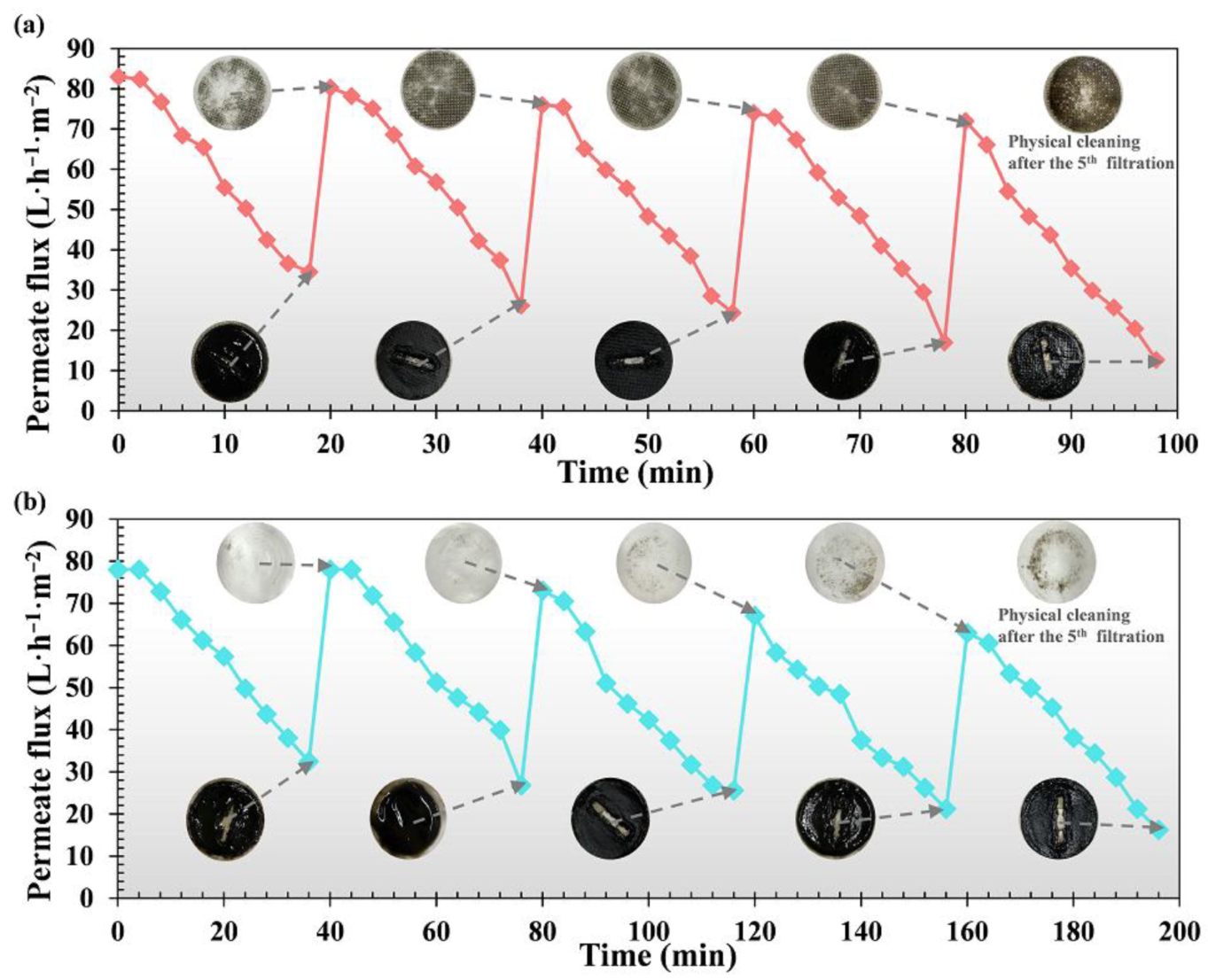
3.3. DM Formation and Filtration Behavior
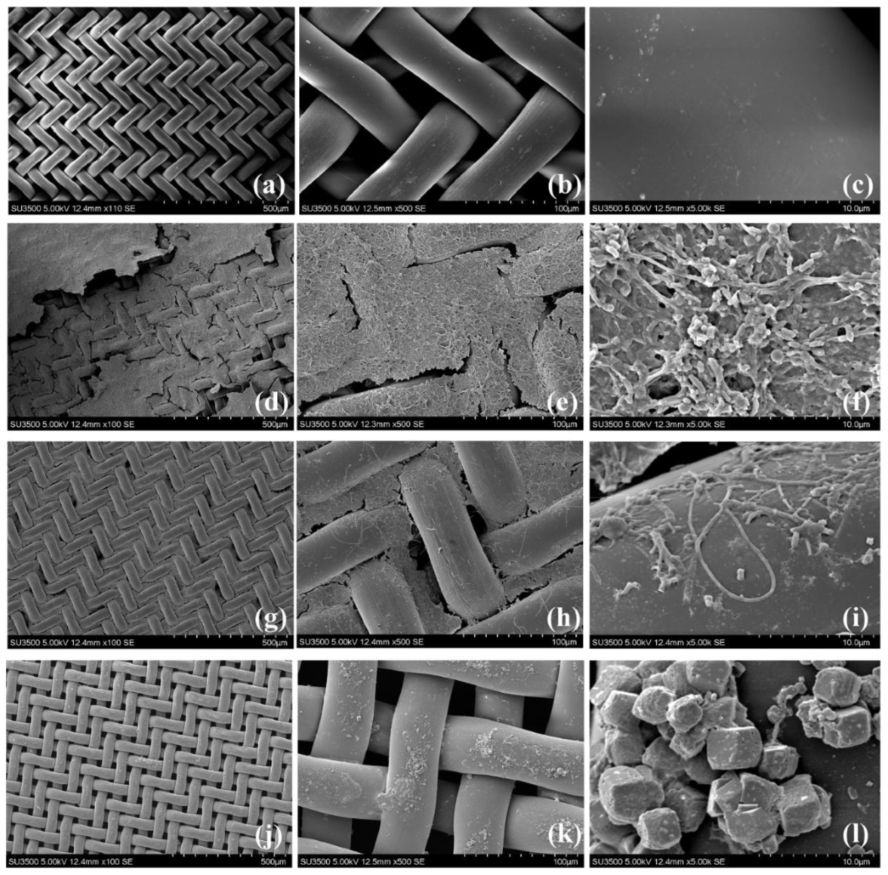
3.4. Morphological Structures of DM Layers Characterized by SEM Analysis
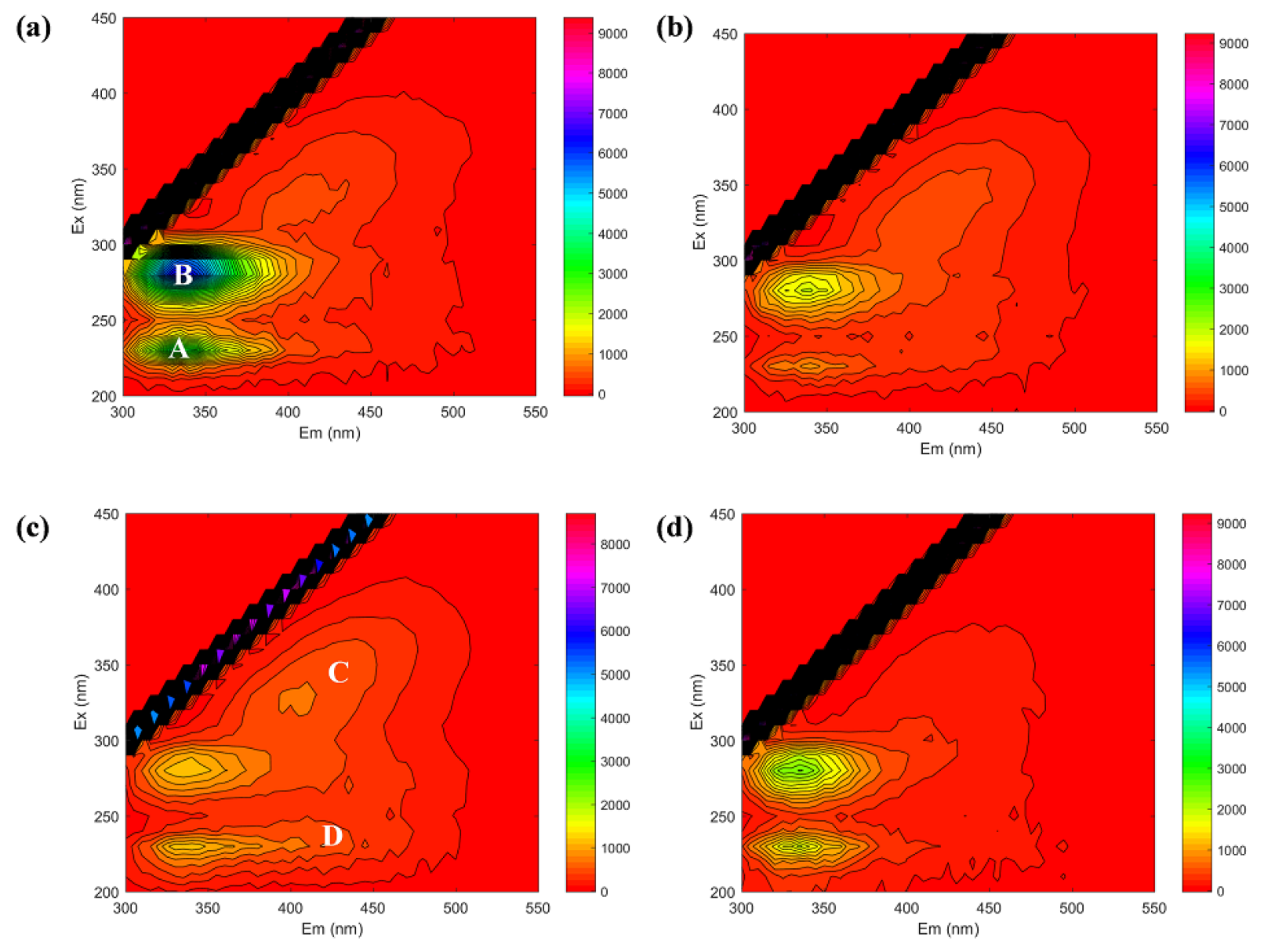
3.5. Microbial Community Analysis of Bulk Sludge and Cake Layer
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Skouteris, G.; Hermosilla, D.; López, P.; Negro, C.; Blanco, Á. Anaerobic membrane bioreactors for wastewater treatment: A review. Chem. Eng. J. 2012, 198–199, 138–148. [Google Scholar] [CrossRef]
- Smith, A.L.; Stadler, L.B.; Cao, L.; Love, N.G.; Raskin, L.; Skerlos, S.J. Navigating Wastewater Energy Recovery Strategies: A Life Cycle Comparison of Anaerobic Membrane Bioreactor and Conventional Treatment Systems with Anaerobic Digestion. Environ. Sci. Technol. 2014, 48, 5972–5981. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Peng, W.; Zhang, M.; Chen, J.; Hong, H.; Zhang, Y. A review on anaerobic membrane bioreactors: Applications, membrane fouling and future perspectives. Desalination 2013, 314, 169–188. [Google Scholar] [CrossRef]
- Jeison, D.; van Lier, J.B. Cake formation and consolidation: Main factors governing the applicable flux in anaerobic submerged membrane bioreactors (AnSMBR) treating acidified wastewaters. Sep. Purif. Technol. 2007, 56, 71–78. [Google Scholar] [CrossRef]
- Meng, F.; Zhang, H.; Yang, F.; Liu, L. Characterization of Cake Layer in Submerged Membrane Bioreactor. Environ. Sci. Technol. 2007, 41, 4065–4070. [Google Scholar] [CrossRef]
- An, Y.; Wang, Z.; Wu, Z.; Yang, D.; Zhou, Q. Characterization of membrane foulants in an anaerobic non-woven fabric membrane bioreactor for municipal wastewater treatment. Chem. Eng. J. 2009, 155, 709–715. [Google Scholar] [CrossRef]
- Hu, Y.S.; Wang, X.C.C.; Ngo, H.H.; Sun, Q.Y.; Yang, Y. Anaerobic dynamic membrane bioreactor (AnDMBR) for wastewater treatment: A review. Bioresour. Technol. 2018, 247, 1107–1118. [Google Scholar] [CrossRef]
- Yang, Y.; Zang, Y.; Hu, Y.; Wang, X.C.; Ngo, H.H. Upflow anaerobic dynamic membrane bioreactor (AnDMBR) for wastewater treatment at room temperature and short HRTs: Process characteristics and practical applicability. Chem. Eng. J. 2020, 383, 123186. [Google Scholar] [CrossRef]
- Hu, Y.; Yang, Y.; Wang, X.C.; Ngo, H.H.; Sun, Q.; Li, S.; Tang, J.; Yu, Z. Effects of powdered activated carbon addition on filtration performance and dynamic membrane layer properties in a hybrid DMBR process. Chem. Eng. J. 2017, 327, 39–50. [Google Scholar] [CrossRef]
- Ersahin, M.E.; Ozgun, H.; Dereli, R.K.; Ozturk, I.; Roest, K.; van Lier, J.B. A review on dynamic membrane filtration: Materials, applications and future perspectives. Bioresour. Technol. 2012, 122, 196–206. [Google Scholar] [CrossRef]
- Ersahin, M.E.; Tao, Y.; Ozgun, H.; Gimenez, J.B.; Spanjers, H.; van Lier, J.B. Impact of anaerobic dynamic membrane bioreactor configuration on treatment and filterability performance. J. Membr. Sci. 2017, 526, 387–394. [Google Scholar] [CrossRef]
- Yurtsever, A.; Basaran, E.; Ucar, D.; Sahinkaya, E. Self-forming dynamic membrane bioreactor for textile industry wastewater treatment. Sci. Total Environ. 2021, 751, 141572. [Google Scholar] [CrossRef]
- Pan, W.; Ouyang, H.; Tan, X.; Deng, R.; Gu, L.; He, Q. Anaerobic dynamic membrane bioreactors for synthetic blackwater treatment under room temperature and mesophilic conditions. Bioresour. Technol. 2022, 355, 127295. [Google Scholar] [CrossRef]
- Chung, C.M.; Yamamoto, K.; Cho, K. A submerged membrane bioreactor under unprecedentedly short hydraulic retention time enabled by non-woven fabric pre-filtration and electrochemical membrane cleaning. J. Membr. Sci. 2019, 592, 117355. [Google Scholar] [CrossRef]
- Anantharaman, A.; Chun, Y.; Hua, T.; Chew, J.W.; Wang, R. Pre-deposited dynamic membrane filtration—A review. Water Res. 2020, 173, 115558. [Google Scholar] [CrossRef] [PubMed]
- Saleem, M.; Masut, E.; Spagni, A.; Lavagnolo, M.C. Exploring dynamic membrane as an alternative for conventional membrane for the treatment of old landfill leachate. J. Environ. Manag. 2019, 246, 658–667. [Google Scholar] [CrossRef] [PubMed]
- Paçal, M.; Semerci, N.; Çallı, B. Treatment of synthetic wastewater and cheese whey by the anaerobic dynamic membrane bioreactor. Environ. Sci. Pollut. Res. 2019, 26, 32942–32956. [Google Scholar] [CrossRef]
- Gupta, K.; Chellam, S. Revealing the mechanisms of irreversible fouling during microfiltration—The role of feedwater composition. J. Environ. Chem. Eng. 2022, 10, 107362. [Google Scholar] [CrossRef]
- Huang, J.; Wu, X.; Liang, Z.; Yu, Y.; Liu, G. Water flushing irremovable biofilms on support material in dynamic membrane bioreactor: Formation, composition, and microbial community. Chemosphere 2021, 271, 129813. [Google Scholar] [CrossRef]
- Ersahin, M.E.; Ozgun, H.; van Lier, J.B. Effect of Support Material Properties on Dynamic Membrane Filtration Performance. Sep. Sci. Technol. 2013, 48, 2263–2269. [Google Scholar] [CrossRef]
- Yang, Y.; Deng, W.; Hu, Y.; Chen, R.; Wang, X.C. Gravity-driven high flux filtration behavior and microbial community of an integrated granular activated carbon and dynamic membrane bioreactor for domestic wastewater treatment. Sci. Total Environ. 2022, 825, 153930. [Google Scholar] [CrossRef] [PubMed]
- Martin-Garcia, I.; Monsalvo, V.; Pidou, M.; Le-Clech, P.; Judd, S.J.; McAdam, E.J.; Jefferson, B. Impact of membrane configuration on fouling in anaerobic membrane bioreactors. J. Membr. Sci. 2011, 382, 41–49. [Google Scholar] [CrossRef]
- Tang, J.; Wang, X.C.; Hu, Y.; Ngo, H.H.; Li, Y. Dynamic membrane-assisted fermentation of food wastes for enhancing lactic acid production. Bioresour. Technol. 2017, 234, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Pronk, W.; Ding, A.; Morgenroth, E.; Derlon, N.; Desmond, P.; Burkhardt, M.; Wu, B.; Fane, A.G. Gravity-driven membrane filtration for water and wastewater treatment: A review. Water Res. 2019, 149, 553–565. [Google Scholar] [CrossRef] [PubMed]
- Ding, A.; Liang, H.; Li, G.; Szivak, I.; Traber, J.; Pronk, W. A low energy gravity-driven membrane bioreactor system for grey water treatment: Permeability and removal performance of organics. J. Membr. Sci. 2017, 542, 408–417. [Google Scholar] [CrossRef] [Green Version]
- Krishnan, S.; Nasrullah, M.; Kamyab, H.; Suzana, N.; Ab Munaim, M.S.; Ab Wahid, Z.; Ali, I.H.; Salehi, R.; Chaiprapat, S. Fouling characteristics and cleaning approach of ultrafiltration membrane during xylose reductase separation. Bioprocess Biosyst. Eng. 2022, 45, 1125–1136. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Tao, Y.; Zhang, S.; Xiao, Y.; Meng, F.; Stuckey, D.C. Size-dependent microbial diversity of sub-visible particles in a submerged anaerobic membrane bioreactor (SAnMBR): Implications for membrane fouling. Water Res. 2019, 159, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Gan, Z.; Zhou, Z.; Huang, Y.-X.; Meng, F. Carbon sources driven supernatant micro-particles differentiate in submerged anaerobic membrane bioreactors (AnMBRs). Chem. Eng. J. 2022, 430, 133020. [Google Scholar] [CrossRef]
- Nan, J.; Yao, M.; Li, Q.; Zhan, D.; Chen, T.; Wang, Z.; Li, H. The role of shear conditions on floc characteristics and membrane fouling in coagulation/ultrafiltration hybrid process—The effect of flocculation duration and slow shear force. RSC Adv. 2016, 6, 163–173. [Google Scholar] [CrossRef]
- Jermann, D.; Pronk, W.; Kägi, R.; Halbeisen, M.; Boller, M. Influence of interactions between NOM and particles on UF fouling mechanisms. Water Res. 2008, 42, 3870–3878. [Google Scholar] [CrossRef]
- Yang, H.; Li, Z.; Chen, Y.; Zhou, Z. Role of microparticles in membrane fouling from acidogenesis to methanogenesis phases in an anaerobic baffled reactor. Sci. Total Environ. 2022, 806, 150663. [Google Scholar] [CrossRef] [PubMed]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods. 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, Y.; Wang, X.C.; Zhang, Y.; Li, Y.; Chen, H.; Jin, P. Characteristics of an A2O–MBR system for reclaimed water production under constant flux at low TMP. J. Membr. Sci. 2013, 431, 156–162. [Google Scholar] [CrossRef]
- Baird, R.B.; Eaton, A.D. Standard Methods for the Examination of Water and Wastewater. Rice, E.W., Ed.; American Water Works Association: Denver, CO, USA, 2012; pp. 1496. [Google Scholar]
- Nowotny, A. Carbohydrate Determination by Phenol-Sulfuric Acid. In Basic Exercises in Immunochemistry; Nowotny, A., Ed.; Springer Berlin Heidelberg: Berlin/Heidelberg, Germany, 1979; pp. 171–173. [Google Scholar]
- Lowry, O.; Rosebrough, N.; Farr, A.L.; Randall, R. Protein Measurement with the Folin Phenol Reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Wang, S.; Li, Y.; Xiao, K.; Huang, X. Fluorescence excitation-emission matrix as a novel indicator of assimilable organic carbon in wastewater: Implication from a coal chemical wastewater study. Sci. Total Environ. 2022, 804, 150144. [Google Scholar] [CrossRef]
- Meng, Z.; Yang, F.; Zhang, X. MBR focus: Do nonwovens offer a cheaper option? Filtr. Separat. 2005, 42, 28–30. [Google Scholar] [CrossRef]
- Mahat, S.B.; Omar, R.; Che Man, H.; Md Idris, A.I.; Mustapa Kamal, S.M.; Abdullah, L.C.; Shreeshivadasan, C. Dynamic anaerobic membrane bioreactor (DAnMBR) with phase separation for food processing wastewater treatment at mesophilic temperature: Characterization of cake layer. J. Environ. Chem. Eng. 2021, 9, 105718. [Google Scholar] [CrossRef]
- Vergine, P.; Salerno, C.; Casale, B.; Berardi, G.; Pollice, A. Role of Mesh Pore Size in Dynamic Membrane Bioreactors. Int. J. Environ. Res. Public Health. 2021, 18, 1472. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Z.; Wu, Z.; Lu, F.; Tong, J.; Zang, L. Formation of dynamic membrane in an anaerobic membrane bioreactor for municipal wastewater treatment. Chem. Eng. J. 2010, 165, 175–183. [Google Scholar] [CrossRef]
- Chen, W.; Westerhoff, P.; Leenheer, J.A.; Booksh, K. Fluorescence Excitation−Emission Matrix Regional Integration to Quantify Spectra for Dissolved Organic Matter. Environ. Sci. Technol. 2003, 37, 5701–5710. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Zhang, M.; Wang, F.; Meng, F.; Liao, B.-Q.; Hong, H.; Chen, J.; Gao, W. A critical review of extracellular polymeric substances (EPSs) in membrane bioreactors: Characteristics, roles in membrane fouling and control strategies. J. Membr. Sci. 2014, 460, 110–125. [Google Scholar] [CrossRef]
- Hazrati, H.; Shayegan, J. Influence of suspended carrier on membrane fouling and biological removal of styrene and ethylbenzene in MBR. J. Taiwan Inst. Chem. Eng. 2016, 64, 59–68. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, X.C.; Sun, Q.; Ngo, H.H.; Yu, Z.; Tang, J.; Zhang, Q. Characterization of a hybrid powdered activated carbon-dynamic membrane bioreactor (PAC-DMBR) process with high flux by gravity flow: Operational performance and sludge properties. Bioresour. Technol. 2017, 223, 65–73. [Google Scholar] [CrossRef]
- Li, W.-W.; Sheng, G.-P.; Wang, Y.-K.; Liu, X.-W.; Xu, J.; Yu, H.-Q. Filtration behaviors and biocake formation mechanism of mesh filters used in membrane bioreactors. Sep. Purif. Technol. 2011, 81, 472–479. [Google Scholar] [CrossRef]
- Gao, D.; Sui, L.; Liang, H. How microbial community and membrane biofouling respond to temperature changes in an anaerobic membrane bioreactor. Environ. Technol. Inno. 2022, 28, 102675. [Google Scholar] [CrossRef]
- Chen, M.-Y.; Lee, D.-J.; Yang, Z.; Peng, X.F.; Lai, J.Y. Fluorecent Staining for Study of Extracellular Polymeric Substances in Membrane Biofouling Layers. Environ. Sci. Technol. 2006, 40, 6642–6646. [Google Scholar] [CrossRef]
- Chen, G.; Cai, D.; Huang, J.; Lu, Z.; Yu, Y.; Liu, G. Biofilm as a live and in-situ formed membrane for solids separation in bioreactors: Biofilm succession governs resistance variation demonstrated during the start-up period. J. Membr. Sci. 2020, 608, 118197. [Google Scholar] [CrossRef]
- Lu, L.; Xing, D.; Ren, N. Pyrosequencing reveals highly diverse microbial communities in microbial electrolysis cells involved in enhanced H-2 production from waste activated sludge. Water Res. 2012, 46, 2425–2434. [Google Scholar] [CrossRef]
- Cayetano, R.D.A.; Park, J.H.; Kim, S.H. Effect of shear velocity and feed concentration on the treatment of food waste in an anaerobic dynamic membrane Bioreactor: Performance Monitoring and microbial community analysis. Bioresour. Technol. 2020, 296, 122301. [Google Scholar] [CrossRef]
- Ma, J.X.; Wang, Z.W.; Zou, X.X.; Feng, J.J.; Wu, Z.C. Microbial communities in an anaerobic dynamic membrane bioreactor (AnDMBR) for municipal wastewater treatment: Comparison of bulk sludge and cake layer. Process Biochem. 2013, 48, 510–516. [Google Scholar] [CrossRef]
- Gao, D.-W.; Zhang, T.; Tang, C.-Y.Y.; Wu, W.-M.; Wong, C.-Y.; Lee, Y.H.; Yeh, D.H.; Criddle, C.S. Membrane fouling in an anaerobic membrane bioreactor: Differences in relative abundance of bacterial species in the membrane foulant layer and in suspension. J. Membr. Sci. 2010, 364, 331–338. [Google Scholar] [CrossRef]
- Cayetano, R.D.A.; Park, J.H.; Kang, S.; Kim, S.H. Food waste treatment in an anaerobic dynamic membrane bioreactor (AnDMBR): Performance monitoring and microbial community analysis. Bioresour. Technol. 2019, 280, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Wu, W.; Xu, J.; Wang, Z. An anaerobic dynamic membrane bioreactor for enhancing sludge digestion: Impact of solids retention time on digestion efficacy. Bioresour. Technol. 2021, 329, 124864. [Google Scholar] [CrossRef]
- Buakaew, T.; Ratanatamskul, C. Effects of novel anaerobic baffled biofilm membrane bioreactor configurations on membrane fouling mitigation and microbial community in treating liquor condensate. Bioresour. Technol. 2021, 335, 125310. [Google Scholar] [CrossRef]
- Zeibich, L.; Schmidt, O.; Drake, H.L. Dietary polysaccharides: Fermentation potentials of a primitive gut ecosystem. Environ. Microbiol. 2019, 21, 1436–1451. [Google Scholar] [CrossRef]
- Dereli, R.K.; Loverdou, L.; van der Zee, F.P.; van Lier, J.B. A systematic study on the effect of substrate acidification degree and acidogenic biomass on sludge filterability. Water Res. 2015, 82, 94–103. [Google Scholar] [CrossRef]
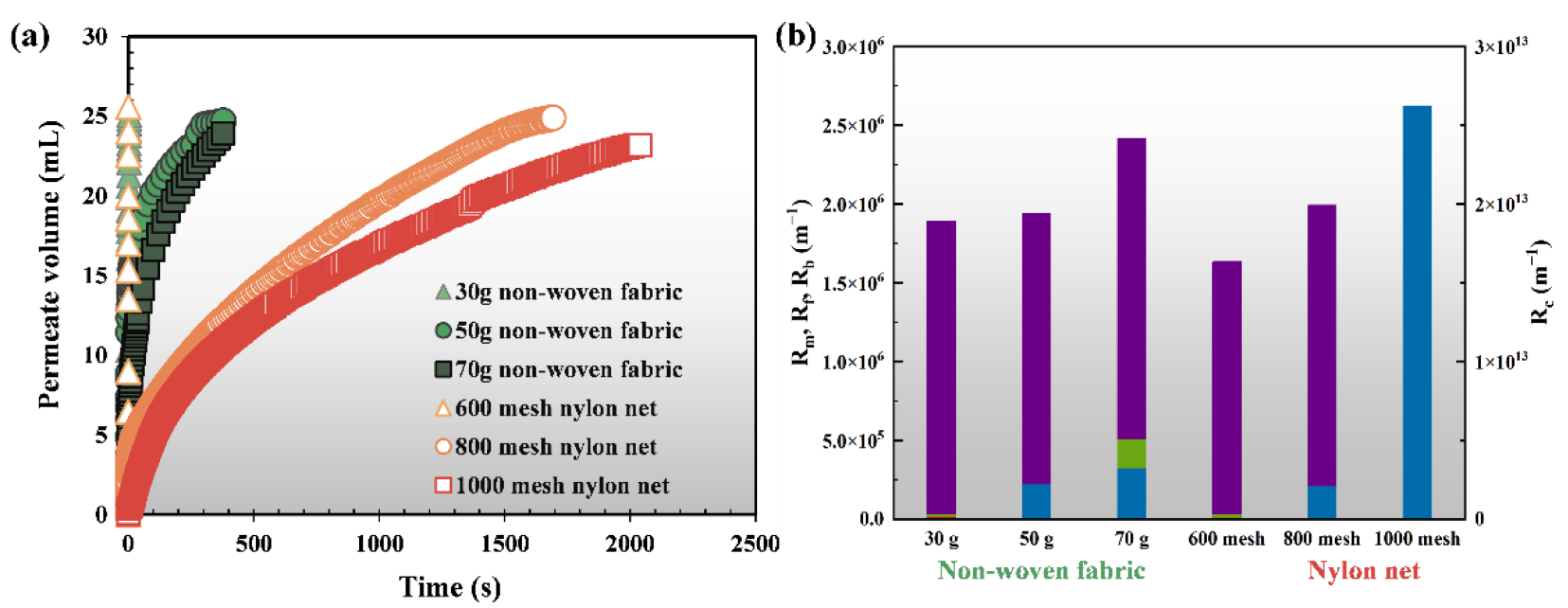


| Rm (×107 m−1) | Run (×107 m−1) | Rir (×107 m−1) | Rc (×107 m−1) | Rt (×107 m−1) | |
|---|---|---|---|---|---|
| AnGDMBR a | 0.2 (6.2%) | 0.08 (2.5%) | 0.6 (24.8%) | 2.14 (66.5%) | 3.22 (100%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pu, Y.; Fu, Z.; Li, T.; Chen, Y.; Zhou, Z. A Novel Anaerobic Gravity-Driven Dynamic Membrane Bioreactor (AnGDMBR): Performance and Fouling Characterization. Membranes 2022, 12, 683. https://doi.org/10.3390/membranes12070683
Pu Y, Fu Z, Li T, Chen Y, Zhou Z. A Novel Anaerobic Gravity-Driven Dynamic Membrane Bioreactor (AnGDMBR): Performance and Fouling Characterization. Membranes. 2022; 12(7):683. https://doi.org/10.3390/membranes12070683
Chicago/Turabian StylePu, Yingfei, Zihan Fu, Tingting Li, Yucheng Chen, and Zhongbo Zhou. 2022. "A Novel Anaerobic Gravity-Driven Dynamic Membrane Bioreactor (AnGDMBR): Performance and Fouling Characterization" Membranes 12, no. 7: 683. https://doi.org/10.3390/membranes12070683
APA StylePu, Y., Fu, Z., Li, T., Chen, Y., & Zhou, Z. (2022). A Novel Anaerobic Gravity-Driven Dynamic Membrane Bioreactor (AnGDMBR): Performance and Fouling Characterization. Membranes, 12(7), 683. https://doi.org/10.3390/membranes12070683