Preparation of Nanofiltration Membrane Modified with Sawdust-Derived Cellulose Nanocrystals for Removal of Nitrate from Drinking Water
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Membrane Synthesis
2.3. Membrane Characterization
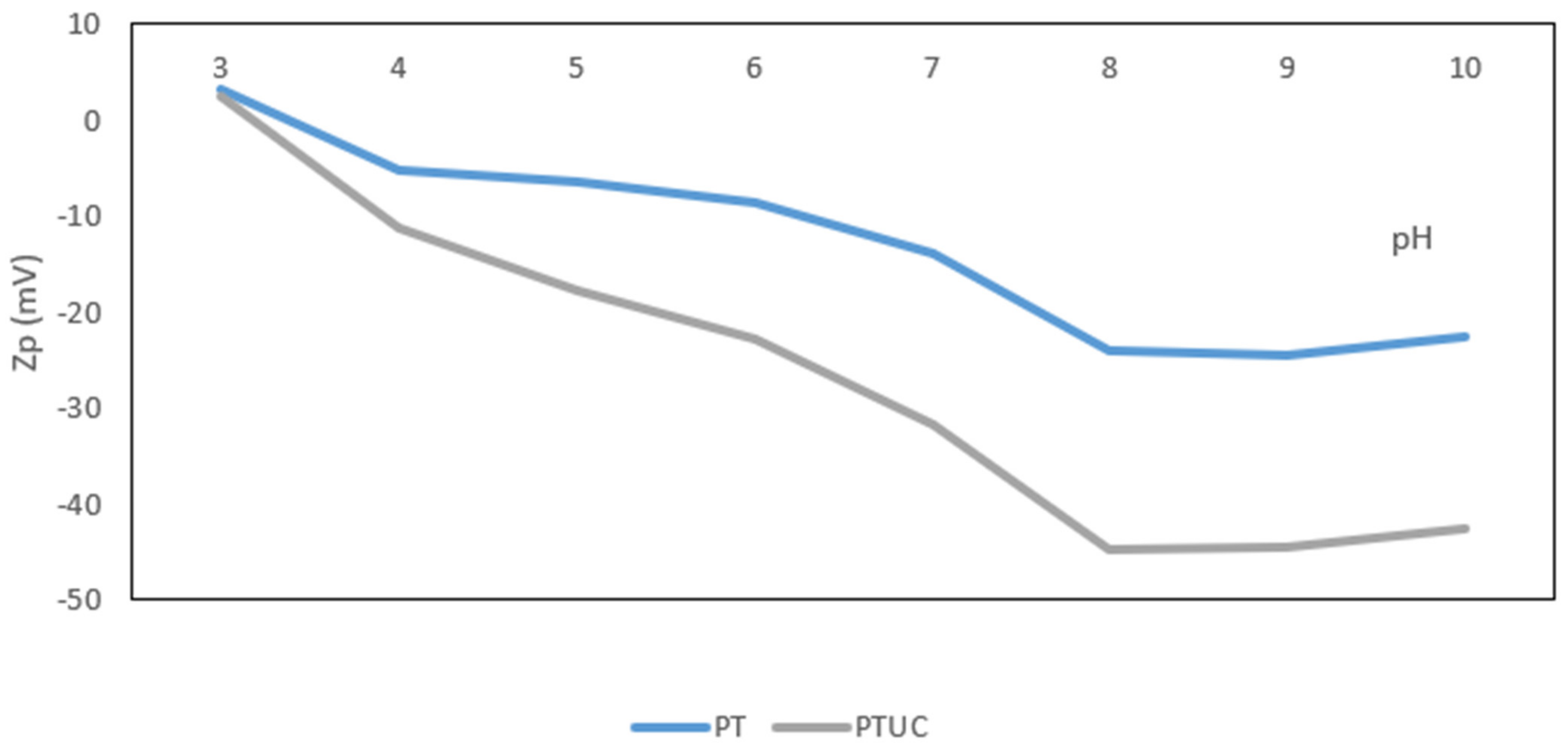
Zeta Potential Measurement
2.4. Water Treatment Performance Tests
3. Results and Discussion
3.1. Membrane Characteristics
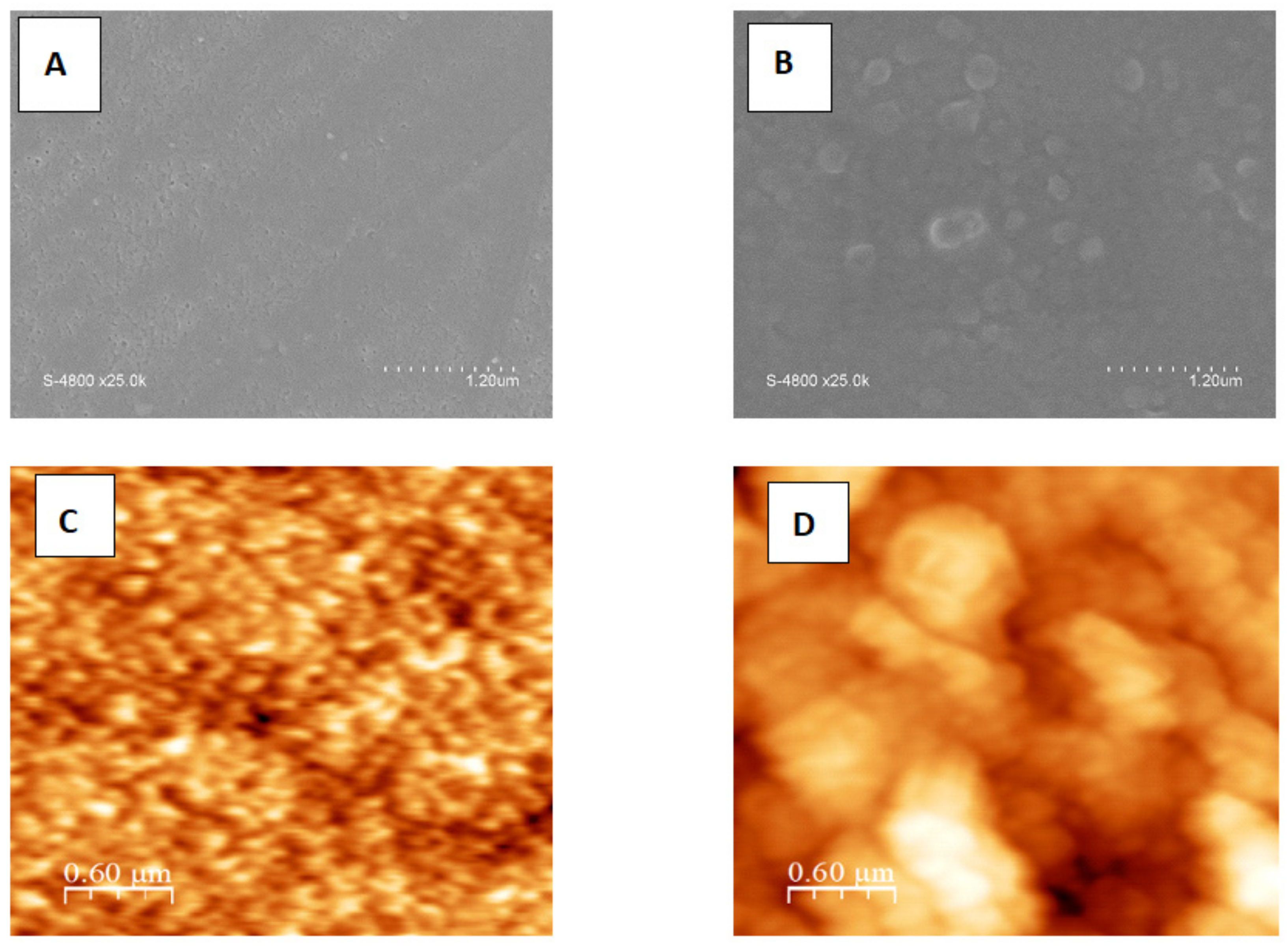
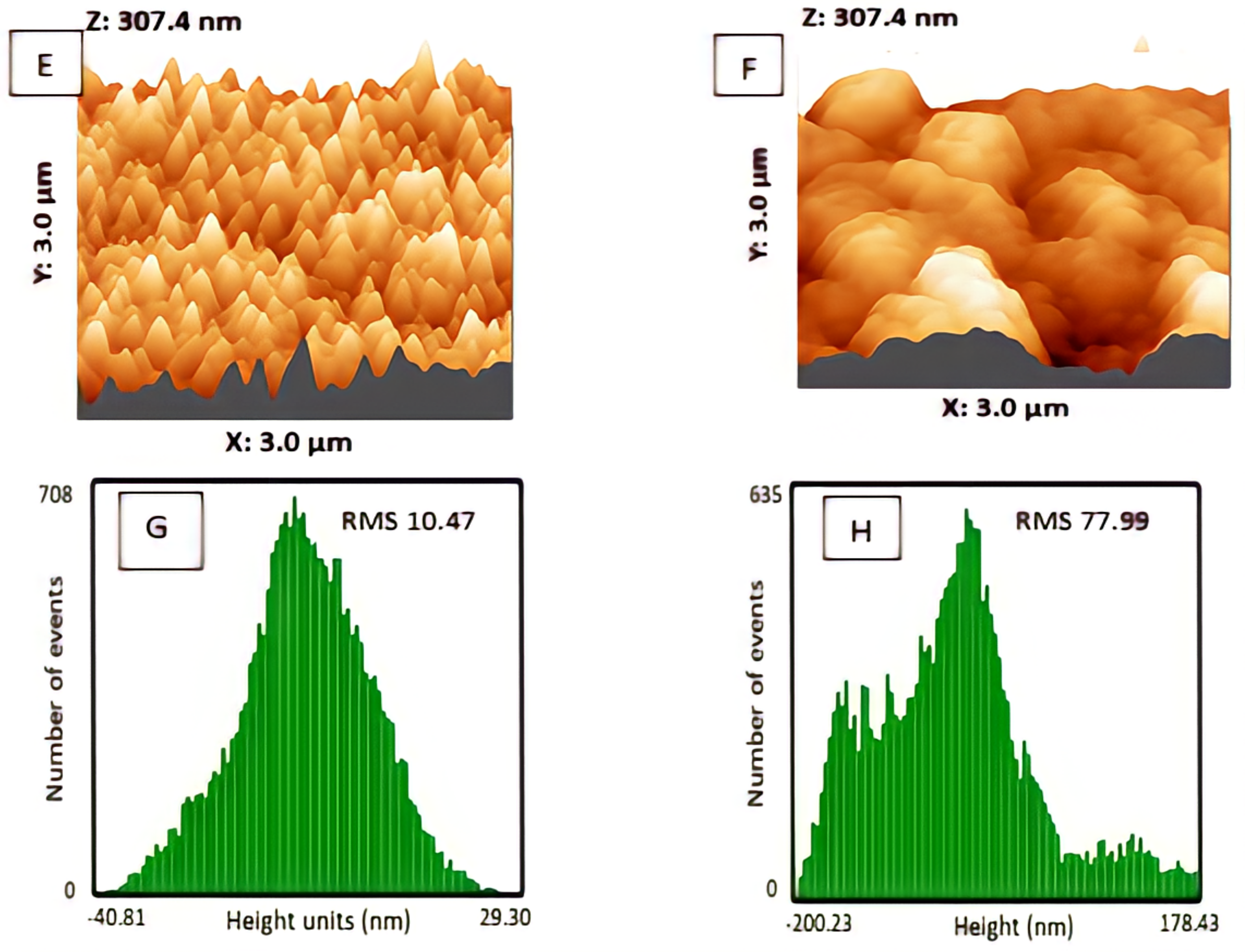
3.1.1. Morphology and Surface Structure
3.1.2. Surface Charge
3.1.3. Atomic Concentration
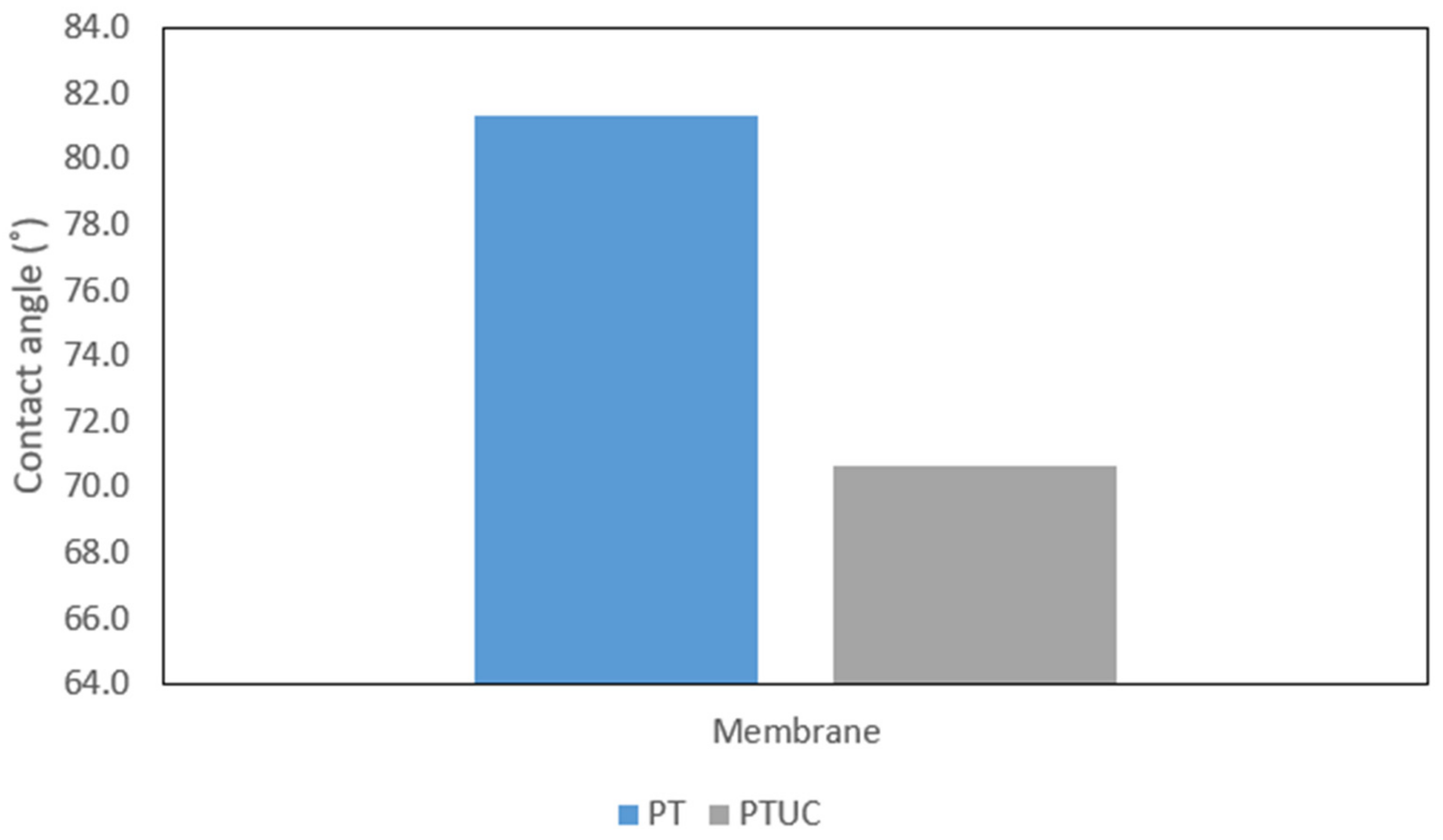
3.1.4. Hydrophilicity
3.2. Comparing the Performance of the Membranes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ravindra, K.; Thind, P.S.; Mor, S.; Singh, T.; Mor, S. Evaluation of groundwater contamination in Chandigarh: Source identification and health risk assessment. Environ. Pollut. 2019, 255, 113062. [Google Scholar] [CrossRef] [PubMed]
- Nieder, R.; Benbi, D.K.; Reichl, F.X. Reactive water-soluble forms of nitrogen and phosphorus and their impacts on environment and human health. In Soil Components and Human Health; Springer: Berlin/Heidelberg, Germany, 2018; pp. 223–255. [Google Scholar]
- Li, P.; He, X.; Guo, W. Spatial groundwater quality and potential health risks due to nitrate ingestion through drinking water: A case study in Yan’an City on the Loess Plateau of northwest China. Hum. Ecol. Risk Assess. Int. J. 2019, 25, 11–31. [Google Scholar] [CrossRef]
- Helmecke, M.; Fries, E.; Schulte, C. Regulating water reuse for agricultural irrigation: Risks related to organic micro-contaminants. Environ. Sci. Eur. 2020, 32, 4. [Google Scholar] [CrossRef] [Green Version]
- Mateo-Sagasta, J.; Zadeh, S.M.; Turral, H.; Burke, J. Water Pollution from Agriculture: A Global Review. Executive Summary; Food and Agriculture Organization of the United Nations: Roma, Italy, 2017. [Google Scholar]
- Pokhrel, S. A Study On Agricultural Effluents: A Havoc to Water Bodies. Environ. Contam. Rev. (ECR) 2020, 3, 92–96. [Google Scholar]
- Zhou, Z.; Ansems, N.; Torfs, P. A global assessment of nitrate contamination in groundwater. In International Groundwater Resources Assessment Center Internship Report; Wageningen University: Wageningen, The Netherlands, 2015. [Google Scholar]
- Edokpayi, J.N.; Rogawski, E.T.; Kahler, D.M.; Hill, C.L.; Reynolds, C.; Nyathi, E.; Smith, J.A.; Odiyo, J.O.; Samie, A.; Bessong, P. Challenges to sustainable safe drinking water: A case study of water quality and use across seasons in rural communities in Limpopo province, South Africa. Water 2018, 10, 159. [Google Scholar] [CrossRef] [Green Version]
- Ntshangase, S.N. Assessment of Nitrate Pollution in Groundwater (Chaneng Village, Rustenburg); North-West University: Potchefstroom, South Africa, 2019. [Google Scholar]
- Yang, Z.; Zhou, Y.; Feng, Z.; Rui, X.; Zhang, T.; Zhang, Z. A review on reverse osmosis and nanofiltration membranes for water purification. Polymers 2019, 11, 1252. [Google Scholar] [CrossRef] [Green Version]
- Ji, Y.; Qian, W.; Yu, Y.; An, Q.; Liu, L.; Zhou, Y.; Gao, C. Recent developments in nanofiltration membranes based on nanomaterials. Chin. J. Chem. Eng. 2017, 25, 1639–1652. [Google Scholar] [CrossRef]
- Adeniyi, A.; Oyewo, A.O.; Sadiku, R.; Onyango, M.S. Incorporation of Cellulose Nanomaterials into Membrane Materials for Water Treatment. In Handbook of Nanomaterials and Nanocomposites for Energy and Environmental Applications; Springer: Berlin/Heidelberg, Germany, 2021; pp. 3581–3601. [Google Scholar]
- Yang, Z.; Guo, H.; Yao, Z.-k.; Mei, Y.; Tang, C.Y. Hydrophilic silver nanoparticles induce selective nanochannels in thin film nanocomposite polyamide membranes. Environ. Sci. Technol. 2019, 53, 5301–5308. [Google Scholar] [CrossRef]
- Zahid, M.; Rashid, A.; Akram, S.; Rehan, Z.; Razzaq, W. A comprehensive review on polymeric nano-composite membranes for water treatment. J. Membr. Sci. Technol. 2018, 8, 1–20. [Google Scholar] [CrossRef]
- Ang, M.B.M.Y.; Huang, S.-H.; Tsai, S.-J.; De Guzman, M.R.; Lee, K.-R.; Lai, J.-Y. Embedding hollow silica nanoparticles of varying shapes and dimensions in nanofiltration membranes for optimal performance. J. Membr. Sci. 2020, 611, 118333. [Google Scholar] [CrossRef]
- Soria, R.B.; Zhu, J.; Gonza, I.; Van der Bruggen, B.; Luis, P. Effect of (TiO2: ZnO) ratio on the anti-fouling properties of bio-inspired nanofiltration membranes. Sep. Purif. Technol. 2020, 251, 117280. [Google Scholar] [CrossRef]
- Li, T.; Xiao, Y.; Guo, D.; Shen, L.; Li, R.; Jiao, Y.; Xu, Y.; Lin, H. In-situ coating TiO2 surface by plant-inspired tannic acid for fabrication of thin film nanocomposite nanofiltration membranes toward enhanced separation and antibacterial performance. J. Colloid Interface Sci. 2020, 572, 114–121. [Google Scholar] [CrossRef]
- Polisetti, V.; Ray, P. Nanoparticles modified Polyacrylonitrile/Polyacrylonitrile–Polyvinylidenefluoride blends as substrate of high flux anti-fouling nanofiltration membranes. J. Appl. Polym. Sci. 2021, 138, 50228. [Google Scholar] [CrossRef]
- Shojaeiarani, J.; Bajwa, D.S.; Chanda, S. Cellulose Nanocrystal Based Composites: A Review. Compos. Part C Open Access 2021, 5, 100164. [Google Scholar] [CrossRef]
- Zulu, B.; Oyewo, O.A.; Sithole, B.; Leswifi, T.Y.; Onyango, M.S. Functionalized sawdust-derived cellulose nanocrystalline adsorbent for efficient removal of vanadium from aqueous solution. Front. Environ. Sci. 2020, 8, 56. [Google Scholar] [CrossRef]
- Bai, L.; Liu, Y.; Ding, A.; Ren, N.; Li, G.; Liang, H. Fabrication and characterization of thin-film composite (TFC) nanofiltration membranes incorporated with cellulose nanocrystals (CNCs) for enhanced desalination performance and dye removal. Chem. Eng. J. 2019, 358, 1519–1528. [Google Scholar] [CrossRef]
- Bai, L.; Liu, Y.; Bossa, N.; Ding, A.; Ren, N.; Li, G.; Liang, H.; Wiesner, M.R. Incorporation of cellulose nanocrystals (CNCs) into the polyamide layer of thin-film composite (TFC) nanofiltration membranes for enhanced separation performance and antifouling properties. Environ. Sci. Technol. 2018, 52, 11178–11187. [Google Scholar] [CrossRef]
- Ghaee, A.; Zerafat, M.; Askari, P.; Sabbaghi, S.; Sadatnia, B. Fabrication of polyamide thin-film nanocomposite membranes with enhanced surface charge for nitrate ion removal from water resources. Environ. Technol. 2017, 38, 772–781. [Google Scholar] [CrossRef]
- Zou, L.; Zhang, S.; Liu, J.; Cao, Y.; Qian, G.; Li, Y.-Y.; Xu, Z.P. Nitrate removal from groundwater using negatively charged nanofiltration membrane. Environ. Sci. Pollut. Res. 2019, 26, 34197–34204. [Google Scholar] [CrossRef]
- Tian, J.; Chang, H.; Gao, S.; Zhang, R. How to fabricate a negatively charged NF membrane for heavy metal removal via the interfacial polymerization between PIP and TMC? Desalination 2020, 491, 114499. [Google Scholar] [CrossRef]
- Adeniyi, A.; Gonzalez-Ortiz, D.; Pochat-Bohatier, C.; Oyewo, O.; Sithole, B.; Onyango, M. Incorporation of Cellulose Nanocrystals (CNC) derived from sawdust into polyamide thin-film composite membranes for enhanced water recovery. Alex. Eng. J. 2020, 59, 4201–4210. [Google Scholar] [CrossRef]
- Sadler, R.; Maetam, B.; Edokpolo, B.; Connell, D.; Yu, J.; Stewart, D.; Park, M.-J.; Gray, D.; Laksono, B. Health risk assessment for exposure to nitrate in drinking water from village wells in Semarang, Indonesia. Environ. Pollut. 2016, 216, 738–745. [Google Scholar] [CrossRef] [PubMed]
- Adeniyi, A.; Onyango, M.; Bopape, M. Impact of nodular structure in characterization of three thin film composite membranes made from 1, 2-benzisothiazol-3 (2H)-one, sodium salt. J. Mater. Environ. Sci. 2019, 10, 213–223. [Google Scholar]
- Stinson-Bagby, K.L.; Roberts, R.; Foster, E.J. Effective cellulose nanocrystal imaging using transmission electron microscopy. Carbohydr. Polym. 2018, 186, 429–438. [Google Scholar] [CrossRef] [PubMed]
- Rho, H.; Chon, K.; Cho, J. Surface charge characterization of nanofiltration membranes by potentiometric titrations and electrophoresis: Functionality vs. zeta potential. Desalination 2018, 427, 19–26. [Google Scholar] [CrossRef]
- Ang, M.B.M.Y.; Tang, C.-L.; De Guzman, M.R.; Maganto, H.L.C.; Caparanga, A.R.; Huang, S.-H.; Tsai, H.-A.; Hu, C.-C.; Lee, K.-R.; Lai, J.-Y. Improved performance of thin-film nanofiltration membranes fabricated with the intervention of surfactants having different structures for water treatment. Desalination 2020, 481, 114352. [Google Scholar] [CrossRef]
- Al-Nahari, A.; Li, S.; Su, B. Negatively charged nanofiltration membrane with high performance via the synergetic effect of benzidinedisulfonic acid and trimethylamine during interfacial polymerization. Sep. Purif. Technol. 2022, 291, 120947. [Google Scholar] [CrossRef]
- Aziz, M.; Ismail, A. X-ray photoelectron spectroscopy (XPS). In Membrane Characterization; Elsevier: Amsterdam, The Netherlands, 2017; pp. 81–93. [Google Scholar]
- Ariza, M.; Benavente, J.; Rodriguez-Castellon, E.; Palacio, L. Effect of hydration of polyamide membranes on the surface electrokinetic parameters: Surface characterization by X-ray photoelectronic spectroscopy and atomic force microscopy. J. Colloid Interface Sci. 2002, 247, 149–158. [Google Scholar] [CrossRef] [Green Version]
- Bai, L.; Bossa, N.; Qu, F.; Winglee, J.; Li, G.; Sun, K.; Liang, H.; Wiesner, M.R. Comparison of hydrophilicity and mechanical properties of nanocomposite membranes with cellulose nanocrystals and carbon nanotubes. Environ. Sci. Technol. 2017, 51, 253–262. [Google Scholar] [CrossRef]
- Azad, H.; Mohsennia, M. A novel free-standing polyvinyl butyral-polyacrylonitrile/ZnAl-layered double hydroxide nanocomposite membrane for enhanced heavy metal removal from wastewater. J. Membr. Sci. 2020, 615, 118487. [Google Scholar] [CrossRef]
- Suhalim, N.S.; Kasim, N.; Mahmoudi, E.; Shamsudin, I.J.; Mohammad, A.W.; Mohamed Zuki, F.; Jamari, N.L.-A. Rejection Mechanism of Ionic Solute Removal by Nanofiltration Membranes: An Overview. Nanomaterials 2022, 12, 437. [Google Scholar] [CrossRef]
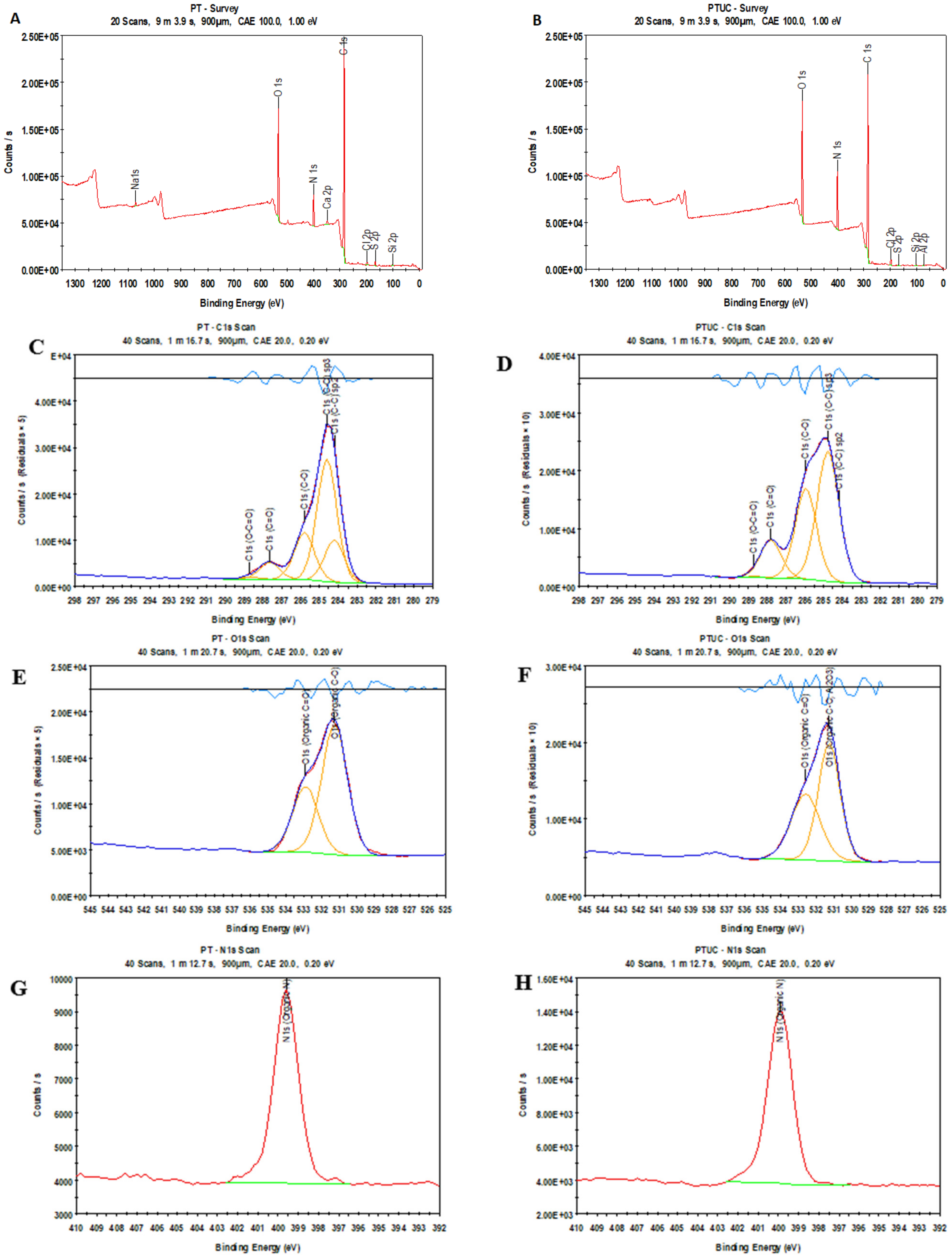

| Membrane | Elemental Composition | O/C | N/C | N/O | ||
|---|---|---|---|---|---|---|
| C | O | N | ||||
| PT | 76.00 | 16.1 | 5.9 | 0.21 | 0.08 | 0.37 |
| PTUC | 73.06 | 14.92 | 12.02 | 0.20 | 0.16 | 0.81 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adeniyi, A.; Gonzalez-Ortiz, D.; Pochat-Bohatier, C.; Mbakop, S.; Onyango, M.S. Preparation of Nanofiltration Membrane Modified with Sawdust-Derived Cellulose Nanocrystals for Removal of Nitrate from Drinking Water. Membranes 2022, 12, 670. https://doi.org/10.3390/membranes12070670
Adeniyi A, Gonzalez-Ortiz D, Pochat-Bohatier C, Mbakop S, Onyango MS. Preparation of Nanofiltration Membrane Modified with Sawdust-Derived Cellulose Nanocrystals for Removal of Nitrate from Drinking Water. Membranes. 2022; 12(7):670. https://doi.org/10.3390/membranes12070670
Chicago/Turabian StyleAdeniyi, Amos, Danae Gonzalez-Ortiz, Céline Pochat-Bohatier, Sandrine Mbakop, and Maurice Stephen Onyango. 2022. "Preparation of Nanofiltration Membrane Modified with Sawdust-Derived Cellulose Nanocrystals for Removal of Nitrate from Drinking Water" Membranes 12, no. 7: 670. https://doi.org/10.3390/membranes12070670
APA StyleAdeniyi, A., Gonzalez-Ortiz, D., Pochat-Bohatier, C., Mbakop, S., & Onyango, M. S. (2022). Preparation of Nanofiltration Membrane Modified with Sawdust-Derived Cellulose Nanocrystals for Removal of Nitrate from Drinking Water. Membranes, 12(7), 670. https://doi.org/10.3390/membranes12070670






