A Review on Removal and Destruction of Per- and Polyfluoroalkyl Substances (PFAS) by Novel Membranes
Abstract
1. Background on PFAS
2. Current Treatment Approaches
2.1. Adsorption and Ion Exchange
2.2. Membrane Separation
3. Factors Controlling PFAS Separation by Membranes
4. Novel Membranes for PFAS Rejection and Removal
4.1. Polymeric Membranes
4.2. Ceramic Membranes
4.3. Polyamide-Modified Thin Film Composite Membranes
4.4. Modified Silica Membrane
4.5. Graphene Oxide (GO)-Nanofiltration-Membranes
4.6. Metal Organic Framework (MOF)-Based Membranes
4.7. Functionalized-MXene Hollow Fiber Membranes
5. Coupled Membrane Technology
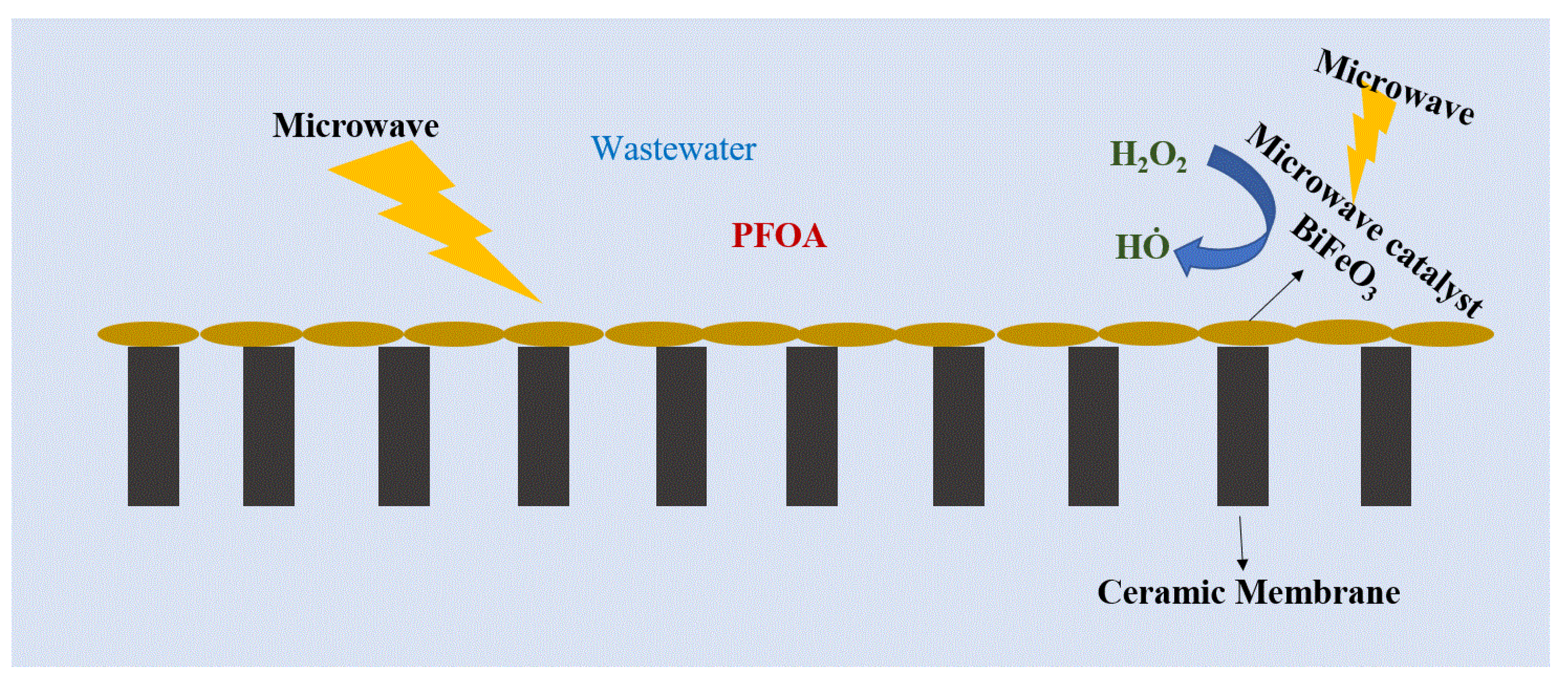
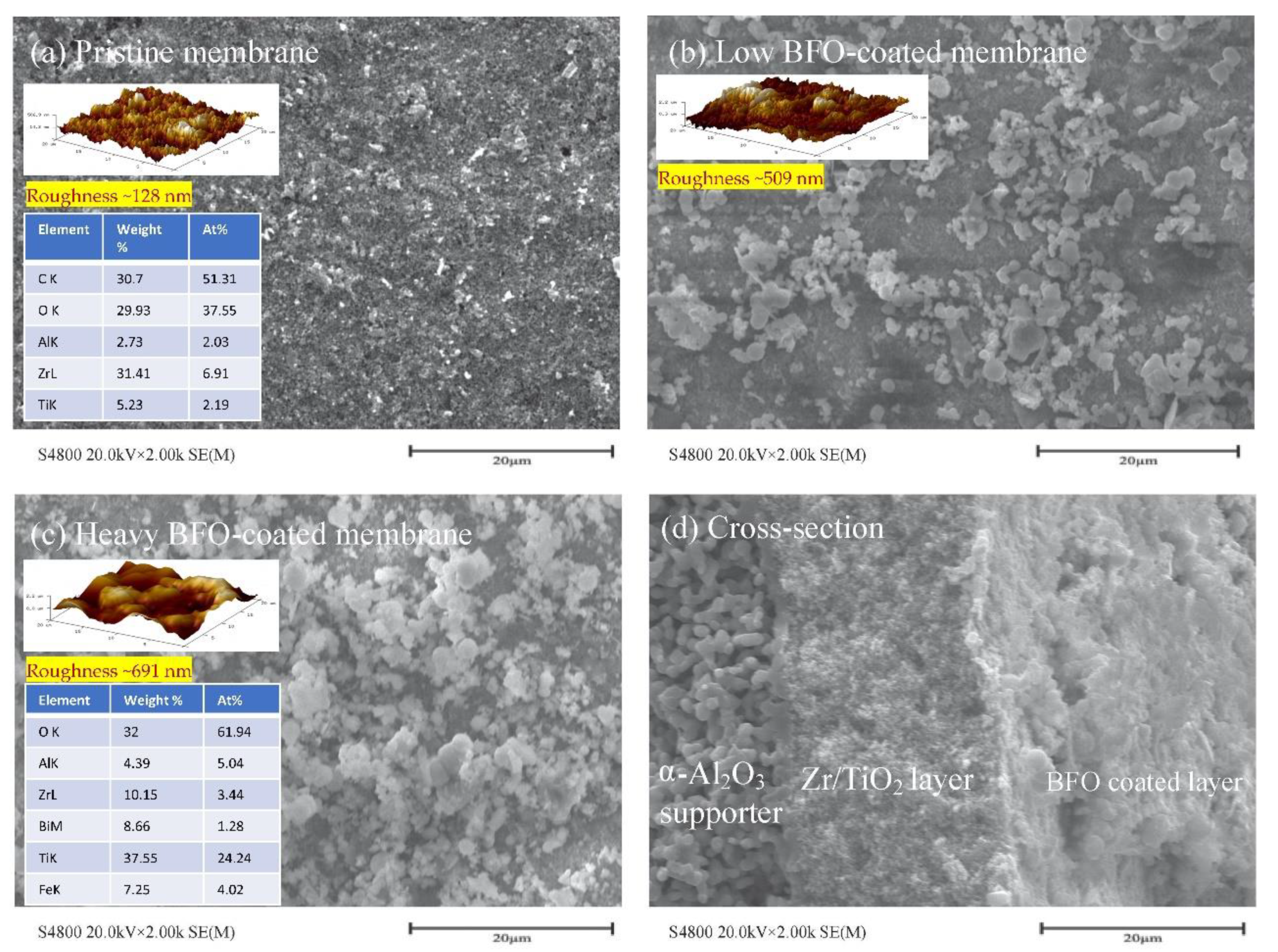
5.1. Electromagnetic Ceramic Membrane
5.2. Reactive Electrochemical Membrane
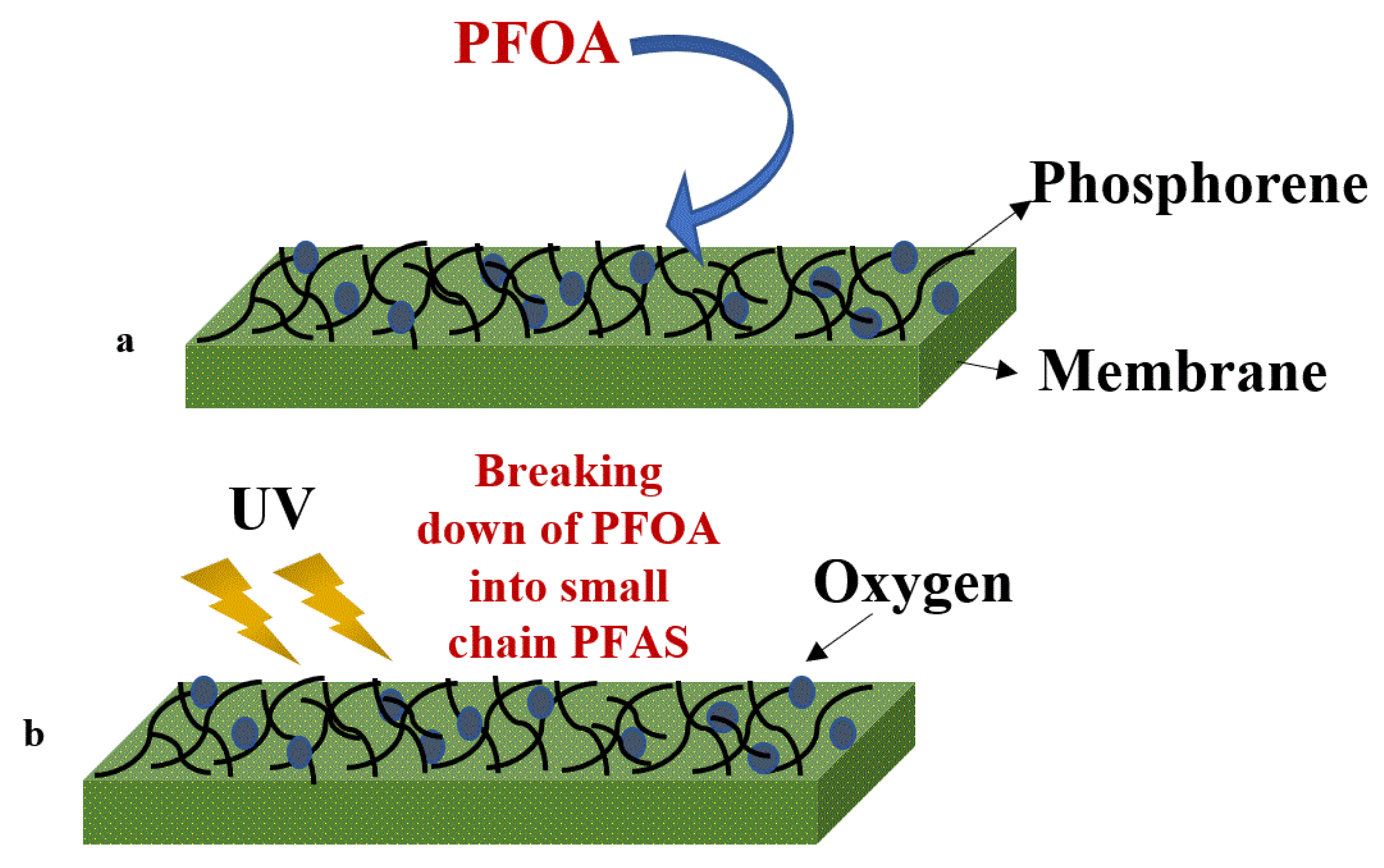
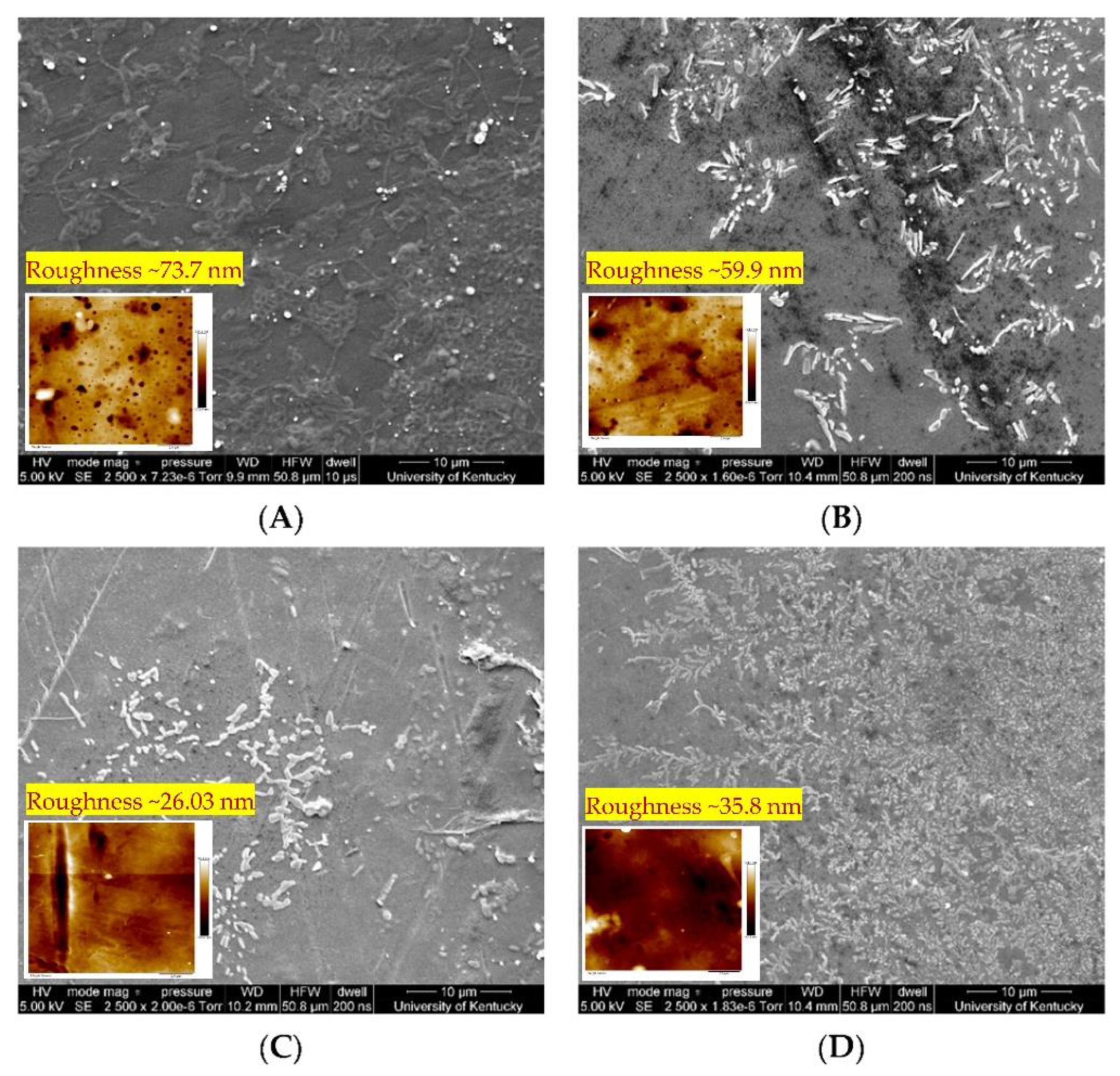
5.3. Phosphorene Nanocomposite Membranes
6. Cost Analysis of the PFAS Treatment Technologies
7. Future Directions
8. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PFAS | Per and Poly-fluoroalkyl Substances |
| PFHxS | Perfluorohexane sulfonate |
| PFOS | Perfluorooctanesulfonic acid |
| PFHpA | Perfluoroheptanoic acid |
| PFOA | Perfluorooctanoic acid |
| PFNA | Perfluorononanoic acid |
| PFDA | Perfluorodecanoic acid |
| PFUnDA | Perfluoroundecanoic acid |
| PFDoDA | Perfluorododecanoic acid |
| PFTA | Perfluorotetradecanoic acid |
| PFPeA | Perfluoropentanoate |
| PFHxA | Perfluorohexanoate |
| PFPrS | Perfluoropropane sulfonate |
| PFBA | Perfluorobutanoate |
| PFNA | Perfluorononanoic acid |
| FPeSA | Perfluoropentane sulfonamide |
| FBSA | Perfluorobutane sulfonamide |
| FPrSA | Perfluoropropane sulfonamide |
| FOSA | Perfluoroalkyl sulfonamide |
| FTSA | 6:2 Fluorotelomer sulfonate |
| PFBS | Perfluorobutane sulfonate |
| PFPeS | Perfluoropentane sulfonate |
| PFHpS | Perfluoroheptane sulfonate |
| PFDS | Perfluorodecane sulfonate |
| GO | Graphene oxide |
| GAC | Granular activated carbon |
| PAC | Powder activated carbon |
| PMPA | Perfluoro-2-(perfluoromethoxy) propanoic acid |
| PEI | Polyethyleneimine |
| PVDF | Polyvinylidene fluoride |
| AFFF | Aqueous Film-Forming Foam |
| UF | Ultrafiltration |
| MF | Microfiltration |
| RO | Reverse Osmosis |
| NF | Nanofiltration |
| MD | Membrane Distillation |
| MOF | Metal Organic Frameworks |
| COF | Covalent Organic Frameworks |
| AOPs | Advanced Oxidation Processes |
| ARPs | Advanced Reduction Processes |
| UV | Ultraviolet |
References
- Glüge, J.; Scheringer, M.; Cousins, I.T.; Dewitt, J.C.; Goldenman, G.; Herzke, D.; Lohmann, R.; Ng, C.A.; Trier, X.; Wang, Z. An overview of the uses of per- and polyfluoroalkyl substances (PFAS). Environ. Sci. Process. Impacts 2020, 22, 2345–2373. [Google Scholar] [CrossRef] [PubMed]
- Son, H.; Kim, T.; Yoom, H.; Zhao, D.; An, B. The Adsorption Selectivity of Short and Long Per- and Polyfluoroalkyl Substances (PFASs) from Surface Water Using Powder-Activated Carbon. Water 2020, 12, 3287. [Google Scholar] [CrossRef]
- Kwiatkowski, C.F.; Andrews, D.Q.; Birnbaum, L.S.; Bruton, T.A.; Dewitt, J.C.; Knappe, D.R.U.; Maffini, M.V.; Miller, M.F.; Pelch, K.E.; Reade, A.; et al. Scientific Basis for Managing PFAS as a Chemical Class. Environ. Sci. Technol. Lett. 2020, 7, 532–543. [Google Scholar] [CrossRef] [PubMed]
- Death, C.; Bell, C.; Champness, D.; Milne, C.; Reichman, S.; Hagen, T. Per-and polyfluoroalkyl substances (PFAS) in livestock and game species: A review. Sci. Total Environ. 2021, 774, 144795. [Google Scholar] [CrossRef] [PubMed]
- Domazet, S.L.; Jensen, T.K.; Wedderkopp, N.; Nielsen, F.; Andersen, L.B.; Grøntved, A. Exposure to perfluoroalkylated substances (PFAS) in relation to fitness, physical activity, and adipokine levels in childhood: The european youth heart study. Environ. Res. 2020, 191, 110110. [Google Scholar] [CrossRef]
- Blake, B.E.; Fenton, S.E. Early life exposure to per-and polyfluoroalkyl substances (PFAS) and latent health outcomes: A review including the placenta as a target tissue and possible driver of peri- and postnatal effects. Toxicology 2020, 443, 152565. [Google Scholar] [CrossRef]
- De Silva, A.O.; Armitage, J.M.; Bruton, T.A.; Dassuncao, C.; Heiger-Bernays, W.; Hu, X.C.; Kärrman, A.; Kelly, B.; Ng, C.; Robuck, A.; et al. PFAS Exposure Pathways for Humans and Wildlife: A Synthesis of Current Knowledge and Key Gaps in Understanding. Environ. Toxicol. Chem. 2021, 40, 631–657. [Google Scholar] [CrossRef]
- Ebrahimi, F.; Lewis, A.J.; Sales, C.M.; Suri, R.; McKenzie, E.R. Linking PFAS partitioning behavior in sewage solids to the solid characteristics, solution chemistry, and treatment processes. Chemosphere 2021, 271, 129530. [Google Scholar] [CrossRef]
- Pétré, M.A.; Genereux, D.P.; Koropeckyj-Cox, L.; Knappe, D.R.U.; Duboscq, S.; Gilmore, T.E.; Hopkins, Z.R. Per- And Polyfluoroalkyl Substance (PFAS) Transport from Groundwater to Streams near a PFAS Manufacturing Facility in North Carolina, USA. Environ. Sci. Technol. 2021, 55, 5848–5856. [Google Scholar] [CrossRef]
- Bai, X.; Son, Y. Perfluoroalkyl substances (PFAS) in surface water and sediments from two urban watersheds in Nevada, USA. Sci. Total Environ. 2021, 751, 141622. [Google Scholar] [CrossRef]
- Gebbink, W.A.; van Leeuwen, S.P.J. Environmental contamination and human exposure to PFASs near a fluorochemical production plant: Review of historic and current PFOA and GenX contamination in the Netherlands. Environ. Int. 2020, 137, 105583. [Google Scholar] [CrossRef] [PubMed]
- Kostianoy, A.G.; De Boer, J.; Garrigues, P.; Gu, J.; Jones, K.C.; Knepper, T.P.; Newton, A.; Sparks, D.L. The Handbook of Environmental Chemistry: Polyfluorinated Chemicals and Transformation Products; Springer: Berlin/Heidelberg, Germany, 2012; ISBN 9783642218712. [Google Scholar]
- Cordner, A.; De La Rosa, V.Y.; Schaider, L.A.; Rudel, R.A.; Richter, L.; Brown, P. Guideline levels for PFOA and PFOS in drinking water: The role of scientific uncertainty, risk assessment decisions, and social factors. J. Expo. Sci. Environ. Epidemiol. 2019, 29, 157–171. [Google Scholar] [CrossRef] [PubMed]
- Cáñez, T.T.; Guo, B.; McIntosh, J.C.; Brusseau, M.L. Perfluoroalkyl and Polyfluoroalkyl substances (PFAS) in Groundwater at a Reclaimed Water Recharge Facility. Sci. Total Environ. 2021, 791, 147906. [Google Scholar] [CrossRef] [PubMed]
- Kurwadkar, S.; Dane, J.; Kanel, S.R.; Nadagouda, M.N.; Cawdrey, R.W.; Ambade, B.; Struckhoff, G.C.; Wilkin, R. Per- and polyfluoroalkyl substances in water and wastewater: A critical review of their global occurrence and distribution. Sci. Total Environ. 2022, 809, 151003. [Google Scholar] [CrossRef]
- Boone, J.S.; Vigo, C.; Boone, T.; Byrne, C.; Ferrario, J.; Benson, R.; Donohue, J.; Simmons, J.E.; Kolpin, D.W.; Furlong, E.T.; et al. Per- and polyfluoroalkyl substances in source and treated drinking waters of the United States. Sci. Total Environ. 2019, 653, 359–369. [Google Scholar] [CrossRef]
- Liou, J.S.C.; Szostek, B.; DeRito, C.M.; Madsen, E.L. Investigating the biodegradability of perfluorooctanoic acid. Chemosphere 2010, 80, 176–183. [Google Scholar] [CrossRef]
- Ross, I.; McDonough, J.; Miles, J.; Storch, P.; Thelakkat Kochunarayanan, P.; Kalve, E.; Hurst, J.; Dasgupta, S.S.; Burdick, J. A review of emerging technologies for remediation of PFASs. Remediation 2018, 28, 101–126. [Google Scholar] [CrossRef]
- Krug, J.D.; Lemieux, P.M.; Lee, C.-W.; Ryan, J.V.; Kariher, P.H.; Shields, E.P.; Wickersham, L.C.; Denison, M.K.; Davis, K.A.; Swensen, D.A.; et al. Combustion of C 1 and C 2 PFAS: Kinetic modeling and experiments. J. Air Waste Manag. Assoc. 2022, 72, 1–15. [Google Scholar] [CrossRef]
- Gomez-Ruiz, B.; Ribao, P.; Diban, N.; Rivero, M.J.; Ortiz, I.; Urtiaga, A. Photocatalytic degradation and mineralization of perfluorooctanoic acid (PFOA) using a composite TiO2 −rGO catalyst. J. Hazard. Mater. 2018, 344, 950–957. [Google Scholar] [CrossRef]
- Wu, Y.; Li, Y.; Fang, C.; Li, C. Highly Efficient Degradation of Perfluorooctanoic Acid over a MnOx-Modified Oxygen-Vacancy-Rich In2O3 Photocatalyst. ChemCatChem 2019, 11, 2297–2303. [Google Scholar] [CrossRef]
- Singh, R.K.; Fernando, S.; Baygi, S.F.; Multari, N.; Thagard, S.M.; Holsen, T.M. Breakdown Products from Perfluorinated Alkyl Substances (PFAS) Degradation in a Plasma-Based Water Treatment Process. Environ. Sci. Technol. 2019, 53, 2731–2738. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Zhang, W.; Wang, C.; Liang, Y. Sonochemical degradation of poly- and perfluoroalkyl substances—A review. Ultrason. Sonochem. 2020, 69, 105245. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Vanangamudi, A.; Wang, J.; Jegatheesan, J.; Mishra, V.; Sharma, R.; Gray, S.R.; Kujawa, J.; Kujawski, W.; Wicaksana, F.; et al. Direct contact membrane distillation for effective concentration of perfluoroalkyl substances—Impact of surface fouling and material stability. Water Res. 2020, 182, 116010. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.C.; Vatankhah, H.; McDonough, C.A.; Nickerson, A.; Hedtke, T.T.; Cath, T.Y.; Higgins, C.P.; Bellona, C.L. Removal of per- and polyfluoroalkyl substances using super-fine powder activated carbon and ceramic membrane filtration. J. Hazard. Mater. 2019, 366, 160–168. [Google Scholar] [CrossRef]
- Soriano, Á.; Gorri, D.; Biegler, L.T.; Urtiaga, A. An optimization model for the treatment of perfluorocarboxylic acids considering membrane preconcentration and BDD electrooxidation. Water Res. 2019, 164, 114954. [Google Scholar] [CrossRef]
- Duan, L.; Wang, B.; Heck, K.; Guo, S.; Clark, C.A.; Arredondo, J.; Wang, M.; Senftle, T.P.; Westerhoff, P.; Wen, X.; et al. Efficient Photocatalytic PFOA Degradation over Boron Nitride. Environ. Sci. Technol. Lett. 2020, 7, 613–619. [Google Scholar] [CrossRef]
- Jin, T.; Peydayesh, M.; Joerss, H.; Zhou, J.; Bolisetty, S.; Mezzenga, R. Amyloid fibril-based membranes for PFAS removal from water. Environ. Sci. Water Res. Technol. 2021, 7, 1873–1884. [Google Scholar] [CrossRef]
- Ko, J.S.; Le, N.Q.; Schlesinger, D.R.; Johnson, J.K.; Xia, Z. Novel niobium-doped titanium oxide towards electrochemical destruction of forever chemicals. Sci. Rep. 2021, 11, 18020. [Google Scholar] [CrossRef]
- Shrestha, B.; Ezazi, M.; Ajayan, S.; Kwon, G. Reversible adsorption and desorption of PFAS on inexpensive graphite adsorbents: Via alternating electric field. RSC Adv. 2021, 11, 34652–34659. [Google Scholar] [CrossRef]
- Woodard, S.; Berry, J.; Newman, B. Ion exchange resin for PFAS removal and pilot test comparison to GAC. Remediation 2017, 27, 19–27. [Google Scholar] [CrossRef]
- Appleman, T.D.; Higgins, C.P.; Quiñones, O.; Vanderford, B.J.; Kolstad, C.; Zeigler-Holady, J.C.; Dickenson, E.R.V. Treatment of poly- and perfluoroalkyl substances in U.S. full-scale water treatment systems. Water Res. 2014, 51, 246–255. [Google Scholar] [CrossRef] [PubMed]
- Belkouteb, N.; Franke, V.; McCleaf, P.; Köhler, S.; Ahrens, L. Removal of per- and polyfluoroalkyl substances (PFASs) in a full-scale drinking water treatment plant: Long-term performance of granular activated carbon (GAC) and influence of flow-rate. Water Res. 2020, 182, 115913. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Xie, Z.; Dorian, B.; Gray, S.; Zhang, J. Comparative study of PFAS treatment by UV, UV/ozone, and fractionations with air and ozonated air. Environ. Sci. Water Res. Technol. 2019, 5, 1897–1907. [Google Scholar] [CrossRef]
- Stebel, E.K.; Pike, K.A.; Nguyen, H.; Hartmann, H.A.; Klonowski, M.J.; Lawrence, M.G.; Collins, R.M.; Hefner, C.E.; Edmiston, P.L. Absorption of short-chain to long-chain perfluoroalkyl substances using swellable organically modified silica. Environ. Sci. Water Res. Technol. 2019, 5, 1854–1866. [Google Scholar] [CrossRef]
- Murray, C.C.; Marshall, R.E.; Liu, C.J.; Vatankhah, H.; Bellona, C.L. PFAS treatment with granular activated carbon and ion exchange resin: Comparing chain length, empty bed contact time, and cost. J. Water Process Eng. 2021, 44, 102342. [Google Scholar] [CrossRef]
- Pramanik, B.K.; Pramanik, S.K.; Suja, F. A comparative study of coagulation, granular- and powdered-activated carbon for the removal of perfluorooctane sulfonate and perfluorooctanoate in drinking water treatment. Environ. Technol. 2015, 36, 2610–2617. [Google Scholar] [CrossRef]
- Franke, V.; Ullberg, M.; McCleaf, P.; Wålinder, M.; Köhler, S.J.; Ahrens, L. The Price of Really Clean Water: Combining Nanofiltration with Granular Activated Carbon and Anion Exchange Resins for the Removal of Per- And Polyfluoralkyl Substances (PFASs) in Drinking Water Production. ACS ES&T Water 2021, 1, 782–795. [Google Scholar] [CrossRef]
- McCleaf, P.; Englund, S.; Östlund, A.; Lindegren, K.; Wiberg, K.; Ahrens, L. Removal efficiency of multiple poly- and perfluoroalkyl substances (PFASs) in drinking water using granular activated carbon (GAC) and anion exchange (AE) column tests. Water Res. 2017, 120, 77–87. [Google Scholar] [CrossRef]
- Sukeesan, S.; Boontanon, S.K.; Boontanon, N.; Fujii, S. Regeneration of Ion-Exchange Resins and Granular Activated Carbon with the Sonochemical Technique for Enabling Adsorption of Aqueous Per- and Polyfluoroalkyl Substances. IOP Conf. Ser. Earth Environ. Sci. 2022, 973, 012004. [Google Scholar] [CrossRef]
- Liu, Y.L.; Sun, M. Ion exchange removal and resin regeneration to treat per- and polyfluoroalkyl ether acids and other emerging PFAS in drinking water. Water Res. 2021, 207, 117781. [Google Scholar] [CrossRef]
- Yu, Q.; Zhang, R.; Deng, S.; Huang, J.; Yu, G. Sorption of perfluorooctane sulfonate and perfluorooctanoate on activated carbons and resin: Kinetic and isotherm study. Water Res. 2009, 43, 1150–1158. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Ching, C.; Dichtel, W.R.; Helbling, D.E. Evaluating the Removal of Per- And Polyfluoroalkyl Substances from Contaminated Groundwater with Different Adsorbents Using a Suspect Screening Approach. Environ. Sci. Technol. Lett. 2020, 7, 954–960. [Google Scholar] [CrossRef]
- Ateia, M.; Arifuzzaman, M.; Pellizzeri, S.; Attia, M.F.; Tharayil, N.; Anker, J.N.; Karanfil, T. Cationic polymer for selective removal of GenX and short-chain PFAS from surface waters and wastewaters at ng/L levels. Water Res. 2019, 163, 114874. [Google Scholar] [CrossRef] [PubMed]
- Olimattel, K.; Zhai, L.; Sadmani, A.H.M.A. Enhanced removal of perfluorooctane sulfonic acid and perfluorooctanoic acid via polyelectrolyte functionalized ultrafiltration membrane: Effects of membrane modification and water matrix. J. Hazard. Mater. Lett. 2021, 2, 100043. [Google Scholar] [CrossRef]
- Boonya-atichart, A.; Boontanon, S.K.; Boontanon, N. Study of hybrid membrane filtration and photocatalysis for removal of perfluorooctanoic acid (PFOA) in groundwater. Water Sci. Technol. 2017, 2017, 561–569. [Google Scholar] [CrossRef]
- Pica, N.E.; Funkhouser, J.; Yin, Y.; Zhang, Z.; Ceres, D.M.; Tong, T.; Blotevogel, J. Electrochemical Oxidation of Hexafluoropropylene Oxide Dimer Acid (GenX): Mechanistic Insights and Efficient Treatment Train with Nanofiltration. Environ. Sci. Technol. 2019, 53, 12602–12609. [Google Scholar] [CrossRef]
- Ochoa-Herrera, V.; Sierra-Alvarez, R. Removal of perfluorinated surfactants by sorption onto granular activated carbon, zeolite and sludge. Chemosphere 2008, 72, 1588–1593. [Google Scholar] [CrossRef]
- Deng, S.; Niu, L.; Bei, Y.; Wang, B.; Huang, J.; Yu, G. Adsorption of perfluorinated compounds on aminated rice husk prepared by atom transfer radical polymerization. Chemosphere 2013, 91, 124–130. [Google Scholar] [CrossRef]
- Hu, X.; Liu, Y.; Yang, R.; Jiang, Y.; Meng, M.; Liu, Z.; Ni, L.; Wu, W.; Liu, H. Adsorption for perfluorooctanoic acid with graphitic-phase carbon nitride and its HPLC fluorescence determination. Can. J. Chem. Eng. 2020, 98, 394–403. [Google Scholar] [CrossRef]
- Li, R.; Alomari, S.; Islamoglu, T.; Farha, O.K.; Fernando, S.; Thagard, S.M.; Holsen, T.M.; Wriedt, M. Systematic Study on the Removal of Per-and Polyfluoroalkyl Substances from Contaminated Groundwater Using Metal-Organic Frameworks. Environ. Sci. Technol. 2021, 55, 15162–15171. [Google Scholar] [CrossRef]
- Li, R.; Alomari, S.; Stanton, R.; Wasson, M.C.; Islamoglu, T.; Farha, O.K.; Holsen, T.M.; Thagard, S.M.; Trivedi, D.J.; Wriedt, M. Efficient Removal of Per-and Polyfluoroalkyl Substances from Water with Zirconium-Based Metal-Organic Frameworks. Chem. Mater. 2021, 33, 3276–3285. [Google Scholar] [CrossRef]
- Ji, W.; Xiao, L.; Ling, Y.; Ching, C.; Matsumoto, M.; Bisbey, R.P.; Helbling, D.E.; Dichtel, W.R. Removal of GenX and Perfluorinated Alkyl Substances from Water by Amine-Functionalized Covalent Organic Frameworks. J. Am. Chem. Soc. 2018, 140, 12677–12681. [Google Scholar] [CrossRef] [PubMed]
- Elanchezhiyan, S.S.; Preethi, J.; Rathinam, K.; Njaramba, L.K.; Park, C.M. Synthesis of magnetic chitosan biopolymeric spheres and their adsorption performances for PFOA and PFOS from aqueous environment. Carbohydr. Polym. 2021, 267, 118165. [Google Scholar] [CrossRef] [PubMed]
- Sörengård, M.; Östblom, E.; Köhler, S.; Ahrens, L. Adsorption behavior of per- And polyfluoralkyl substances (PFASs) to 44 inorganic and organic sorbents and use of dyes as proxies for PFAS sorption. J. Environ. Chem. Eng. 2020, 8, 103744. [Google Scholar] [CrossRef]
- Hassan, M.; Liu, Y.; Naidu, R.; Du, J.; Qi, F. Adsorption of Perfluorooctane sulfonate (PFOS) onto metal oxides modified biochar. Environ. Technol. Innov. 2020, 19, 100816. [Google Scholar] [CrossRef]
- Omo-Okoro, P.N.; Curtis, C.J.; Marco, A.M.; Melymuk, L.; Okonkwo, J.O. Removal of per- and polyfluoroalkyl substances from aqueous media using synthesized silver nanocomposite-activated carbons. J. Environ. Health Sci. Eng. 2021, 19, 217–236. [Google Scholar] [CrossRef]
- Wu, C.; Klemes, M.J.; Trang, B.; Dichtel, W.R.; Helbling, D.E. Exploring the factors that influence the adsorption of anionic PFAS on conventional and emerging adsorbents in aquatic matrices. Water Res. 2020, 182, 115950. [Google Scholar] [CrossRef]
- Gao, X.; Chorover, J. Adsorption of perfluorooctanoic acid and perfluorooctanesulfonic acid to iron oxide surfaces as studied by flow-through ATR-FTIR spectroscopy. Environ. Chem. 2012, 9, 148–157. [Google Scholar] [CrossRef]
- Wang, F.; Shih, K. Adsorption of perfluorooctanesulfonate (PFOS) and perfluorooctanoate (PFOA) on alumina: Influence of solution pH and cations. Water Res. 2011, 45, 2925–2930. [Google Scholar] [CrossRef]
- Siriwardena, D.P.; James, R.; Dasu, K.; Thorn, J.; Iery, R.D.; Pala, F.; Schumitz, D.; Eastwood, S.; Burkitt, N. Regeneration of per- and polyfluoroalkyl substance-laden granular activated carbon using a solvent based technology. J. Environ. Manag. 2021, 289, 112439. [Google Scholar] [CrossRef]
- Gagliano, E.; Falciglia, P.P.; Zaker, Y.; Karanfil, T.; Roccaro, P. Microwave regeneration of granular activated carbon saturated with PFAS. Water Res. 2021, 198, 117121. [Google Scholar] [CrossRef] [PubMed]
- Sonmez Baghirzade, B.; Zhang, Y.; Reuther, J.F.; Saleh, N.B.; Venkatesan, A.K.; Apul, O.G. Thermal Regeneration of Spent Granular Activated Carbon Presents an Opportunity to Break the Forever PFAS Cycle. Environ. Sci. Technol. 2021, 55, 5608–5619. [Google Scholar] [CrossRef]
- Campos-Pereira, H.; Kleja, D.B.; Sjöstedt, C.; Ahrens, L.; Klysubun, W.; Gustafsson, J.P. The Adsorption of Per- And Polyfluoroalkyl Substances (PFASs) onto Ferrihydrite Is Governed by Surface Charge. Environ. Sci. Technol. 2020, 54, 15722–15730. [Google Scholar] [CrossRef] [PubMed]
- Ching, C.; Klemes, M.J.; Trang, B.; Dichtel, W.R.; Helbling, D.E. β-Cyclodextrin Polymers with Different Cross-Linkers and Ion-Exchange Resins Exhibit Variable Adsorption of Anionic, Zwitterionic, and Nonionic PFASs. Environ. Sci. Technol. 2020, 54, 12693–12702. [Google Scholar] [CrossRef] [PubMed]
- Maimaiti, A.; Deng, S.; Meng, P.; Wang, W.; Wang, B.; Huang, J.; Wang, Y.; Yu, G. Competitive adsorption of perfluoroalkyl substances on anion exchange resins in simulated AFFF-impacted groundwater. Chem. Eng. J. 2018, 348, 494–502. [Google Scholar] [CrossRef]
- Gao, P.; Cui, J.; Deng, Y. Direct regeneration of ion exchange resins with sulfate radical-based advanced oxidation for enabling a cyclic adsorption—Regeneration treatment approach to aqueous perfluorooctanoic acid (PFOA). Chem. Eng. J. 2021, 405, 126698. [Google Scholar] [CrossRef]
- Víctor-Ortega, M.D.; Ochando-Pulido, J.M.; Martínez-Ferez, A. Impacts of main parameters on the regeneration process efficiency of several ion exchange resins after final purification of olive mill effluent. Sep. Purif. Technol. 2017, 173, 1–8. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, Y.; Li, Y.; Chang, H. Electro-assisted regeneration of ion exchange resins. Front. Environ. Sci. Eng. China 2008, 2, 410–414. [Google Scholar] [CrossRef]
- Dillmann, S.; Kaushik, S.A.; Stumme, J.; Ernst, M. Characterization and performance of lbl-coated multibore membranes: Zeta potential, mwco, permeability and sulfate rejection. Membranes 2020, 10, 412. [Google Scholar] [CrossRef]
- Manawi, Y.; Kochkodan, V.; Mahmoudi, E.; Johnson, D.J.; Mohammad, A.W.; Atieh, M.A. Characterization and Separation Performance of a Novel Polyethersulfone Membrane Blended with Acacia Gum. Sci. Rep. 2017, 7, 15831. [Google Scholar] [CrossRef]
- Rezaee, R.; Nasseri, S.; Mahvi, A.H.; Nabizadeh, R.; Mousavi, S.A.; Rashidi, A.; Jafari, A.; Nazmara, S. Fabrication and characterization of a polysulfone-graphene oxide nanocomposite membrane for arsenate rejection from water. J. Environ. Health Sci. Eng. 2015, 13, 61. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Shi, Y.; Jegatheesan, V.; Ul Haq, I. A Review on the Mechanism, Impacts and Control Methods of Membrane Fouling in MBR System. Membranes 2020, 10, 24. [Google Scholar] [CrossRef] [PubMed]
- Agenson, K.O.; Urase, T. Change in membrane performance due to organic fouling in nanofiltration (NF)/reverse osmosis (RO) applications. Sep. Purif. Technol. 2007, 55, 147–156. [Google Scholar] [CrossRef]
- Zeng, C.; Tanaka, S.; Suzuki, Y.; Yukioka, S.; Fujii, S. Rejection of trace level perfluorohexanoic acid (PFHxA) in pure water by loose nanofiltration membrane. J. Water Environ. Technol. 2017, 15, 120–127. [Google Scholar] [CrossRef]
- Anvari, A.; Kekre, K.M.; Azimi Yancheshme, A.; Yao, Y.; Ronen, A. Membrane distillation of high salinity water by induction heated thermally conducting membranes. J. Memb. Sci. 2019, 589, 117253. [Google Scholar] [CrossRef]
- Anvari, A.; Azimi Yancheshme, A.; Kekre, K.M.; Ronen, A. State-of-the-art methods for overcoming temperature polarization in membrane distillation process: A review. J. Memb. Sci. 2020, 616, 118413. [Google Scholar] [CrossRef]
- Franke, V.; McCleaf, P.; Lindegren, K.; Ahrens, L. Efficient removal of per- And polyfluoroalkyl substances (PFASs) in drinking water treatment: Nanofiltration combined with active carbon or anion exchange. Environ. Sci. Water Res. Technol. 2019, 5, 1836–1843. [Google Scholar] [CrossRef]
- Patterson, C.; Burkhardt, J.; Schupp, D.; Krishnan, E.R.; Dyment, S.; Merritt, S.; Zintek, L.; Kleinmaier, D. Effectiveness of point-of-use/point-of-entry systems to remove per- and polyfluoroalkyl substances from drinking water. AWWA Water Sci. 2019, 1, e1131. [Google Scholar] [CrossRef] [PubMed]
- Alsawaftah, N.; Abuwatfa, W.; Darwish, N.; Husseini, G. A Comprehensive Review on Membrane Fouling: Mathematical. Water 2021, 13, 1327. [Google Scholar] [CrossRef]
- Tang, C.Y.; Fu, Q.S.; Criddle, C.S.; Leckie, J.O. Effect of flux (transmembrane pressure) and membrane properties on fouling and rejection of reverse osmosis and nanofiltration membranes treating perfluorooctane sulfonate containing wastewater. Environ. Sci. Technol. 2007, 41, 2008–2014. [Google Scholar] [CrossRef]
- Flores, C.; Ventura, F.; Martin-Alonso, J.; Caixach, J. Occurrence of perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA) in N.E. Spanish surface waters and their removal in a drinking water treatment plant that combines conventional and advanced treatments in parallel lines. Sci. Total Environ. 2013, 461–462, 618–626. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.Y.; Fu, Q.S.; Robertson, A.P.; Criddle, C.S.; Leckie, J.O. Use of reverse osmosis membranes to remove perfluorooctane sulfonate (PFOS) from semiconductor wastewater. Environ. Sci. Technol. 2006, 40, 7343–7349. [Google Scholar] [CrossRef] [PubMed]
- Steinle-Darling, E.; Reinhard, M. Nanofiltration for trace organic contaminant removal: Structure, solution, and membrane fouling effects on the rejection of perfluorochemicals. Environ. Sci. Technol. 2008, 42, 5292–5297. [Google Scholar] [CrossRef] [PubMed]
- Żyłła, R.; Foszpańczyk, M.; Olak-Kucharczyk, M.; Marszałek, J.; Ledakowicz, S. Removal of Organic Compounds with an Amino Group during the Nanofiltration Process. Membranes 2022, 12, 58. [Google Scholar] [CrossRef] [PubMed]
- Alhweij, H.; Anna, E.; Emanuelsson, C.; Shahid, S.; Wenk, J. Journal of Environmental Chemical Engineering Organic matter removal and antifouling performance of sulfonated polyaniline nanofiltration ( S-PANI NF ) membranes. J. Environ. Chem. Eng. 2022, 10, 107906. [Google Scholar] [CrossRef]
- Wang, Q.; Luo, L.; Huang, N.; Wang, W.; Rong, Y.; Wang, Z.; Yuan, Y.; Xu, A.; Xiong, J.; Wu, Q.; et al. Evolution of low molecular weight organic compounds during ultrapure water production process: A pilot-scale study. Sci. Total Environ. 2022, 830, 154713. [Google Scholar] [CrossRef]
- Soriano, Á.; Gorri, D.; Urtiaga, A. Selection of High Flux Membrane for the Effective Removal of Short-Chain Perfluorocarboxylic Acids. Ind. Eng. Chem. Res. 2019, 58, 3329–3338. [Google Scholar] [CrossRef]
- Zhao, C.; Zhang, J.; He, G.; Wang, T.; Hou, D.; Luan, Z. Perfluorooctane sulfonate removal by nanofiltration membrane the role of calcium ions. Chem. Eng. J. 2013, 233, 224–232. [Google Scholar] [CrossRef]
- Wang, T.; Zhao, C.; Li, P.; Li, Y.; Wang, J. Fabrication of novel poly(m-phenylene isophthalamide) hollow fiber nanofiltration membrane for effective removal of trace amount perfluorooctane sulfonate from water. J. Memb. Sci. 2015, 477, 74–85. [Google Scholar] [CrossRef]
- Jin, T.; Peydayesh, M.; Mezzenga, R. Membrane-based technologies for per- and poly-fluoroalkyl substances (PFASs) removal from water: Removal mechanisms, applications, challenges and perspectives. Environ. Int. 2021, 157, 106876. [Google Scholar] [CrossRef]
- Golledge, R.G. The solution-diffusion model: A review. J. Memb. Sci. 1995, 107, 1–21. [Google Scholar] [CrossRef]
- Chuntanalerg, P.; Bureekaew, S.; Klaysom, C.; Lau, W.J.; Faungnawakij, K. Nanomaterial-Incorporated Nanofiltration Membranes for Organic Solvent Recovery; Elsevier Inc.: Amsterdam, The Netherlands, 2018. [Google Scholar] [CrossRef]
- Shahmirzadi, M.A.A.; Kargari, A. Nanocomposite Membranes; Elsevier Inc.: Oxford, UK, 2018. [Google Scholar] [CrossRef]
- Meng, P.; Deng, S.; Maimaiti, A.; Wang, B.; Huang, J.; Wang, Y.; Cousins, I.T.; Yu, G. Efficient removal of perfluorooctane sulfonate from aqueous film-forming foam solution by aeration-foam collection. Chemosphere 2018, 203, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Appleman, T.D.; Dickenson, E.R.V.; Bellona, C.; Higgins, C.P. Nanofiltration and granular activated carbon treatment of perfluoroalkyl acids. J. Hazard. Mater. 2013, 260, 740–746. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.J.; Strathmann, T.J.; Bellona, C. Rejection of per- and polyfluoroalkyl substances (PFASs) in aqueous film-forming foam by high-pressure membranes. Water Res. 2021, 188, 116546. [Google Scholar] [CrossRef]
- Eschauzier, C.; Beerendonk, E.; Scholte-Veenendaal, P.; De Voogt, P. Impact of treatment processes on the removal of perfluoroalkyl acids from the drinking water production chain. Environ. Sci. Technol. 2012, 46, 1708–1715. [Google Scholar] [CrossRef]
- Kucharzyk, K.H.; Darlington, R.; Benotti, M.; Deeb, R.; Hawley, E. Novel treatment technologies for PFAS compounds: A critical review. J. Environ. Manag. 2017, 204, 757–764. [Google Scholar] [CrossRef]
- Quiñones, O.; Snyder, S.A. Occurrence of perfluoroalkyl carboxylates and sulfonates in drinking water utilities and related waters from the United States. Environ. Sci. Technol. 2009, 43, 9089–9095. [Google Scholar] [CrossRef]
- Lipp, P.; Sacher, F.; Baldauf, G. Removal of organic micro-pollutants during drinking water treatment by nanofiltration and reverse osmosis. Desalin. Water Treat. 2010, 13, 226–237. [Google Scholar] [CrossRef]
- Boo, C.; Wang, Y.; Zucker, I.; Choo, Y.; Osuji, C.O.; Elimelech, M. High Performance Nanofiltration Membrane for Effective Removal of Perfluoroalkyl Substances at High Water Recovery. Environ. Sci. Technol. 2018, 52, 7279–7288. [Google Scholar] [CrossRef]
- Yang, Z.; Peng, H.; Wang, W.; Liu, T. Effect of Membrane Pore Size on the pH-Sensitivity of Polyethersulfone Hollow Fiber Ultrafiltration Membrane. J. Appl. Polym. Sci. 2010, 116, 2658–2667. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, Y.; Esparra-Alvarado, M.; Wang, X.; Yang, H.; Xie, Y. Effects of pH and temperature on forward osmosis membrane flux using rainwater as the makeup for cooling water dilution. Desalination 2014, 351, 70–76. [Google Scholar] [CrossRef]
- Zeng, C.; Tanaka, S.; Suzuki, Y.; Fujii, S. Impact of feed water pH and membrane material on nanofiltration of perfluorohexanoic acid in aqueous solution. Chemosphere 2017, 183, 599–604. [Google Scholar] [CrossRef] [PubMed]
- Pensini, E.; Dinardo, A.; Lamont, K.; Longstaffe, J.; Elsayed, A.; Singh, A. Effect of salts and pH on the removal of perfluorooctanoic acid (PFOA) from aqueous solutions through precipitation and electroflocculation. Can. J. Civ. Eng. 2019, 46, 881–886. [Google Scholar] [CrossRef]
- Cai, W.; Navarro, D.A.; Du, J.; Ying, G.; Yang, B.; McLaughlin, M.J.; Kookana, R.S. Increasing ionic strength and valency of cations enhance sorption through hydrophobic interactions of PFAS with soil surfaces. Sci. Total Environ. 2022, 817, 152975. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Xia, X.; Dong, J.; Xia, N.; Jiang, X.; Li, Y.; Zhu, Y. Short- and long-chain perfluoroalkyl substances in the water, suspended particulate matter, and surface sediment of a turbid river. Sci. Total Environ. 2016, 568, 57–65. [Google Scholar] [CrossRef]
- Wang, J.; Wang, L.; Xu, C.; Zhi, R.; Miao, R.; Liang, T.; Yue, X.; Lv, Y.; Liu, T. Perfluorooctane sulfonate and perfluorobutane sulfonate removal from water by nanofiltration membrane: The roles of solute concentration, ionic strength, and macromolecular organic foulants. Chem. Eng. J. 2018, 332, 787–797. [Google Scholar] [CrossRef]
- Lath, S.; Knight, E.R.; Navarro, D.A.; Kookana, R.S.; McLaughlin, M.J. Sorption of PFOA onto different laboratory materials: Filter membranes and centrifuge tubes. Chemosphere 2019, 222, 671–678. [Google Scholar] [CrossRef]
- Shih, K.; Wang, F. Adsorption Behavior of Perfluorochemicals (PFCs) on Boehmite: Influence of Solution Chemistry. Procedia Environ. Sci. 2013, 18, 106–113. [Google Scholar] [CrossRef]
- Le, T.; Jamshidi, E.; Beidaghi, M.; Esfahani, M.R. Functionalized-MXene Thin-Film Nanocomposite Hollow Fiber Membranes for Enhanced PFAS Removal from Water. ACS Appl. Mater. Interfaces 2022, 14, 25397–25408. [Google Scholar] [CrossRef]
- Eke, J.; Banks, L.; Mottaleb, M.A.; Morris, A.J.; Tsyusko, O.V.; Escobar, I.C. Dual-functional phosphorene nanocomposite membranes for the treatment of perfluorinated water: An investigation of perfluorooctanoic acid removal via filtration combined with ultraviolet irradiation or oxygenation. Membranes 2021, 11, 18. [Google Scholar] [CrossRef]
- Lee, T.; Speth, T.F.; Nadagouda, M.N. High-pressure membrane filtration processes for separation of Per- and polyfluoroalkyl substances (PFAS). Chem. Eng. J. 2022, 431, 134023. [Google Scholar] [CrossRef]
- Johnson, J.K.; Hoffman, C.M.; Smith, D.A.; Xia, Z. Advanced Filtration Membranes for the Removal of Perfluoroalkyl Species from Water. ACS Omega 2019, 4, 8001–8006. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Luo, X.B.; Ding, L.; Luo, S.L. Application of Nanotechnology in the Removal of Heavy Metal From Water; Elsevier Inc.: Amsterdam, The Netherlands, 2018. [Google Scholar] [CrossRef]
- Lee, M.; Li, K. Microstructured Ceramic Hollow Fiber Membranes and Their Applications; Elsevier B.V.: London, UK, 2017. [Google Scholar] [CrossRef]
- Bagnato, G.; Sanna, A. Membrane Considerations and Plant Design for Pre-Combustion CO2 Capture; Elsevier Inc.: Amsterdam, The Netherlands, 2018. [Google Scholar] [CrossRef]
- Sonawane, S.; Thakur, P.; Sonawane, S.H.; Bhanvase, B.A. Nanomaterials for Membrane Synthesis: Introduction, Mechanism, and Challenges for Wastewater Treatment; Elsevier Inc.: Amsterdam, The Netherlands, 2021. [Google Scholar] [CrossRef]
- Hsieh, H.P.; Liu, P.K.T.; Dillman, T.R. Microporous ceramic membranes. Polym. J. 1991, 23, 407–415. [Google Scholar] [CrossRef][Green Version]
- Nadagouda, M.N.; Lee, T. Cross-Flow Treatment of PFAS in Water: Materials Challenges and Potential Solutions. Accounts Mater. Res. 2021, 2, 129–133. [Google Scholar] [CrossRef]
- Zhou, Y.; He, Z.; Tao, Y.; Xiao, Y.; Zhou, T.; Jing, T.; Zhou, Y.; Mei, S. Preparation of a functional silica membrane coated on Fe3O4 nanoparticle for rapid and selective removal of perfluorinated compounds from surface water sample. Chem. Eng. J. 2016, 303, 156–166. [Google Scholar] [CrossRef]
- El Meragawi, S.; Akbari, A.; Hernandez, S.; Mirshekarloo, M.S.; Bhattacharyya, D.; Tanksale, A.; Majumder, M. Enhanced permselective separation of per-fluorooctanoic acid in graphene oxide membranes by a simple PEI modification. J. Mater. Chem. A 2020, 8, 24800–24811. [Google Scholar] [CrossRef]
- Li, Y.; Gao, C.; Jiao, J.; Cui, J.; Li, Z.; Song, Q. Selective Adsorption of Metal-Organic Framework toward Methylene Blue: Behavior and Mechanism. ACS Omega 2021, 6, 33961–33968. [Google Scholar] [CrossRef]
- Zheng, Y.; Rao, F.; Zhang, M.; Li, J.; Huang, W. Efficient, selective, and reusable metal–organic framework-based adsorbent for the removal of Pb(II) and Cr(VI) heavy-metal pollutants from wastewater. Clean. Eng. Technol. 2021, 5, 100344. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, Z.; Gao, L.; Gray, S.; Xie, Z. Study of MOF incorporated dual layer membrane with enhanced removal of ammonia and per-/poly-fluoroalkyl substances (PFAS) in landfill leachate treatment. Sci. Total Environ. 2022, 806, 151207. [Google Scholar] [CrossRef]
- Cui, D.; Li, X.; Quinete, N. Occurrence, fate, sources and toxicity of PFAS: What we know so far in Florida and major gaps. TrAC—Trends Anal. Chem. 2020, 130, 115976. [Google Scholar] [CrossRef]
- Londhe, K.; Lee, C.-S.; Zhang, Y.; Grdanovska, S.; Kroc, T.; Cooper, C.A.; Venkatesan, A.K. Energy Evaluation of Electron Beam Treatment of Perfluoroalkyl Substances in Water: A Critical Review. ACS ES&T Eng. 2021, 1, 827–841. [Google Scholar] [CrossRef]
- Speth, T.; Crimi, M.; Chowdhury, Z.; Dickenson, E.; Guelfo, J.; Knappe, D.; Liu, J.; Leeson, A. PFAS are forever? The state of the science and research needs for analyzing and treating PFAS-laden water. AWWA Water Sci. 2022, 4, e1276. [Google Scholar] [CrossRef]
- Krause, M.J.; Thoma, E.; Sahle-Damesessie, E.; Crone, B.; Whitehill, A.; Shields, E.; Gullett, B. Supercritical Water Oxidation as an Innovative Technology for PFAS Destruction. J. Environ. Eng. 2022, 148, 05021006. [Google Scholar] [CrossRef]
- Morganti, M.; Polesello, S.; Pascariello, S.; Ferrario, C.; Rubolini, D.; Valsecchi, S.; Parolini, M. Exposure assessment of PFAS-contaminated sites using avian eggs as a biomonitoring tool: A frame of reference and a case study in the Po River valley (Northern Italy). Integr. Environ. Assess. Manag. 2021, 17, 733–745. [Google Scholar] [CrossRef] [PubMed]
- Olatunde, O.C.; Kuvarega, A.T.; Onwudiwe, D.C. Photo enhanced degradation of polyfluoroalkyl and perfluoroalkyl substances. Heliyon 2020, 6, e05614. [Google Scholar] [CrossRef]
- Wanninayake, D.M. Comparison of currently available PFAS remediation technologies in water: A review. J. Environ. Manag. 2021, 283, 111977. [Google Scholar] [CrossRef]
- Liu, F.; Hua, L.; Zhang, W. Influences of microwave irradiation on performances of membrane filtration and catalytic degradation of perfluorooctanoic acid (PFOA). Environ. Int. 2020, 143, 105969. [Google Scholar] [CrossRef]
- Le, T.X.H.; Haflich, H.; Shah, A.D.; Chaplin, B.P. Energy-Efficient Electrochemical Oxidation of Perfluoroalkyl Substances Using a Ti4O7 Reactive Electrochemical Membrane Anode. Environ. Sci. Technol. Lett. 2019, 6, 504–510. [Google Scholar] [CrossRef]
- Zhuo, Q.; Deng, S.; Yang, B.; Huang, J.; Wang, B.; Zhang, T.; Yu, G. Degradation of perfluorinated compounds on a boron-doped diamond electrode. Electrochim. Acta 2012, 77, 17–22. [Google Scholar] [CrossRef]
- Soriano, Á.; Gorri, D.; Urtiaga, A. Membrane preconcentration as an efficient tool to reduce the energy consumption of perfluorohexanoic acid electrochemical treatment. Sep. Purif. Technol. 2019, 208, 160–168. [Google Scholar] [CrossRef]
- Soriano, A.; Schaefer, C.; Urtiaga, A. Enhanced treatment of perfluoroalkyl acids in groundwater by membrane separation and electrochemical oxidation. Chem. Eng. J. Adv. 2020, 4, 100042. [Google Scholar] [CrossRef]
- Shi, H.; Wang, Y.; Li, C.; Pierce, R.; Gao, S.; Huang, Q. Degradation of Perfluorooctanesulfonate by Reactive Electrochemical Membrane Compose of Magnéli Phase Titanium Suboxie. Environ. Sci. Technol. 2019, 53, 14528–14537. [Google Scholar] [CrossRef]
- Leonello, D.; Fendrich, M.A.; Parrino, F.; Patel, N.; Orlandi, M.; Miotello, A. Light-induced advanced oxidation processes as pfas remediation methods: A review. Appl. Sci. 2021, 11, 8458. [Google Scholar] [CrossRef]
- Kowald, C.; Brorman, E.; Shankar, S.; Klemashevich, C.; Staack, D.; Pillai, S.D. PFOA and PFOS breakdown in experimental sand, laboratory-grade water, investigation-derived groundwater and wastewater effluent samples at 50 kGy electron beam dose. Radiat. Phys. Chem. 2021, 180, 109323. [Google Scholar] [CrossRef]
- Chaplin, B.P. The Prospect of Electrochemical Technologies Advancing Worldwide Water Treatment. Acc. Chem. Res. 2019, 52, 596–604. [Google Scholar] [CrossRef]
- Stoiber, T.; Evans, S.; Naidenko, O.V. Disposal of products and materials containing per- and polyfluoroalkyl substances (PFAS): A cyclical problem. Chemosphere 2020, 260, 127659. [Google Scholar] [CrossRef] [PubMed]
- Winchell, L.J.; Ross, J.J.; Wells, M.J.M.; Fonoll, X.; Norton, J.W.; Bell, K.Y. Per- and polyfluoroalkyl substances thermal destruction at water resource recovery facilities: A state of the science review. Water Environ. Res. 2021, 93, 826–843. [Google Scholar] [CrossRef] [PubMed]
- Lim, X. Can Microbes Save Us from PFAS? ACS Cent. Sci. 2021, 7, 3–6. [Google Scholar] [CrossRef]
- Presentato, A.; Lampis, S.; Vantini, A.; Manea, F.; Daprà, F.; Zuccoli, S.; Vallini, G. On the ability of perfluorohexane sulfonate (PFHxS) bioaccumulation by two Pseudomonas sp. strains isolated from PFAS-contaminated environmental matrices. Microorganisms 2020, 8, 92. [Google Scholar] [CrossRef]
- Shahsavari, E.; Rouch, D.; Khudur, L.S.; Thomas, D.; Aburto-Medina, A.; Ball, A.S. Challenges and Current Status of the Biological Treatment of PFAS-Contaminated Soils. Front. Bioeng. Biotechnol. 2021, 8, 602040. [Google Scholar] [CrossRef]
- Mastropietro, T.F.; Bruno, R.; Pardo, E.; Armentano, D. Reverse osmosis and nanofiltration membranes for highly efficient PFASs removal: Overview, challenges and future perspectives. Dalt. Trans. 2021, 50, 5398–5410. [Google Scholar] [CrossRef] [PubMed]
- Kamble, A.R.; Patel, C.M.; Murthy, Z.V.P. A review on the recent advances in mixed matrix membranes for gas separation processes. Renew. Sustain. Energy Rev. 2021, 145, 111062. [Google Scholar] [CrossRef]
- Mallya, D.S.; Dumée, L.F.; Muthukumaran, S.; Lei, W.; Baskaran, K. 2D nanosheet enabled thin film nanocomposite membranes for freshwater production—A review. Mater. Adv. 2021, 2, 3519–3537. [Google Scholar] [CrossRef]
- Backe, W.J.; Day, T.C.; Field, J.A. Zwitterionic, cationic, and anionic fluorinated chemicals in aqueous film forming foam formulations and groundwater from U.S. military bases by nonaqueous large-volume injection HPLC-MS/MS. Environ. Sci. Technol. 2013, 47, 5226–5234. [Google Scholar] [CrossRef]
- Garcia-Segura, S.; Qu, X.; Alvarez, P.J.J.; Chaplin, B.P.; Chen, W.; Crittenden, J.C.; Feng, Y.; Gao, G.; He, Z.; Hou, C.H.; et al. Opportunities for nanotechnology to enhance electrochemical treatment of pollutants in potable water and industrial wastewater-a perspective. Environ. Sci. Nano 2020, 7, 2178–2194. [Google Scholar] [CrossRef]
- Deng, S.; Nie, Y.; Du, Z.; Huang, Q.; Meng, P.; Wang, B.; Huang, J.; Yu, G. Enhanced Adsorption of Perfluorooctane Sulfonate and Perfluorooctanoate by Bamboo-Derived Granular Activated Carbon. J. Hazard. Mater. 2015, 282, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Fagbayigbo, B.O.; Opeolu, B.O.; Fatoki, O.S.; Akenga, T.A.; Olatunji, O.S. Removal of PFOA and PFOS from Aqueous Solutions Using Activated Carbon Produced from Vitis Vinifera Leaf Litter. Environ. Sci. Pollut. Res. 2017, 24, 13107–13120. [Google Scholar] [CrossRef]
- Lundquist, N.A.; Sweetman, M.J.; Scroggie, K.R.; Worthington, M.J.H.; Esdaile, L.J.; Alboaiji, S.F.K.; Plush, S.E.; Hayball, J.D.; Chalker, J.M. Polymer Supported Carbon for Safe and Effective Remediation of PFOA-and PFOS-Contaminated Water. ACS Sustain. Chem. Eng. 2019, 7, 11044–11049. [Google Scholar] [CrossRef]
- Xu, J.; Liu, Z.; Zhao, D.; Gao, N.; Fu, X. Enhanced Adsorption of Perfluorooctanoic Acid (PFOA) from Water by Granular Activated Carbon Supported Magnetite Nanoparticles. Sci. Total Environ. 2020, 723, 137757. [Google Scholar] [CrossRef] [PubMed]
- Van den Bergh, M.; Krajnc, A.; Voorspoels, S.; Tavares, S.R.; Mullens, S.; Beurroies, I.; Maurin, G.; Mali, G.; De Vos, D.E. Highly Selective Removal of Perfluorinated Contaminants by Adsorption on All-Silica Zeolite Beta. Angew. Chemie - Int. Ed. 2020, 59, 14086–14090. [Google Scholar] [CrossRef] [PubMed]
- Sini, K.; Bourgeois, D.; Idouhar, M.; Carboni, M.; Meyer, D. Metal-Organic Framework Sorbents for the Removal of Perfluorinated Compounds in an Aqueous Environment. New J. Chem. 2018, 42, 17889–17894. [Google Scholar] [CrossRef]
- Liu, K.; Zhang, S.; Hu, X.; Zhang, K.; Roy, A.; Yu, G. Understanding the Adsorption of PFOA on MIL-101(Cr)-Based Anionic-Exchange Metal-Organic Frameworks: Comparing DFT Calculations with Aqueous Sorption Experiments. Environ. Sci. Technol. 2015, 49, 8657–8665. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Pang, H.; Gray, S.; Ma, S.; Xie, Z.; Gao, L. PFAS Removal from Wastewater by In-Situ Formed Ferric Nanoparticles: Solid Phase Loading and Removal Efficiency. J. Environ. Chem. Eng. 2021, 9, 105452. [Google Scholar] [CrossRef]
- Dixit, F.; Barbeau, B.; Mostafavi, S.G.; Mohseni, M. PFOA and PFOS Removal by Ion Exchange for Water Reuse and Drinking Applications: Role of Organic Matter Characteristics. Environ. Sci. Water Res. Technol. 2019, 5, 1782–1795. [Google Scholar] [CrossRef]
- Gao, Y.; Deng, S.; Du, Z.; Liu, K.; Yu, G. Adsorptive Removal of Emerging Polyfluoroalky Substances F-53B and PFOS by Anion-Exchange Resin: A Comparative Study. J. Hazard. Mater. 2017, 323, 550–557. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Deng, S.; Chen, Y.; Wang, B.; Huang, J.; Wang, Y.; Yu, G. Removal of Perfluorinated Carboxylates from Washing Wastewater of Perfluorooctanesulfonyl Fluoride Using Activated Carbons and Resins. J. Hazard. Mater. 2015, 286, 136–143. [Google Scholar] [CrossRef]
- Deng, S.; Yu, Q.; Huang, J.; Yu, G. Removal of Perfluorooctane Sulfonate from Wastewater by Anion Exchange Resins: Effects of Resin Properties and Solution Chemistry. Water Res. 2010, 44, 5188–5195. [Google Scholar] [CrossRef]
- Choe, H.-S.; Kim, K.Y.; Oh, J.-E.; Kim, J.-H. Parallel Study on Removal Efficiency of Pharmaceuticals and PFASs in Advanced Water Treatment Processes: Ozonation, GAC Adsorption, and RO Processes. Environ. Eng. Res. 2020, 27, 200509. [Google Scholar] [CrossRef]
- Franke, V.; Schäfers, M.D.; Lindberg, J.J.; Ahrens, L. Removal of Per- And Polyfluoroalkyl Substances (PFASs) from Tap Water Using Heterogeneously Catalyzed Ozonation. Environ. Sci. Water Res. Technol. 2019, 5, 1887–1896. [Google Scholar] [CrossRef]
- Trojanowicz, M.; Bojanowska-Czajka, A.; Bartosiewicz, I.; Kulisa, K. Advanced Oxidation/Reduction Processes Treatment for Aqueous Perfluorooctanoate (PFOA) and Perfluorooctanesulfonate (PFOS)—A Review of Recent Advances. Chem. Eng. J. 2018, 336, 170–199. [Google Scholar] [CrossRef]
- Radjenovic, J.; Duinslaeger, N.; Avval, S.S.; Chaplin, B.P. Facing the Challenge of Poly- And Perfluoroalkyl Substances in Water: Is Electrochemical Oxidation the Answer? Environ. Sci. Technol. 2020, 54, 14815–14829. [Google Scholar] [CrossRef]
- Nzeribe, B.N.; Crimi, M.; Mededovic Thagard, S.; Holsen, T.M. Physico-Chemical Processes for the Treatment of Per- And Polyfluoroalkyl Substances (PFAS): A Review. Crit. Rev. Environ. Sci. Technol. 2019, 49, 866–915. [Google Scholar] [CrossRef]
- Schaefer, C.E.; Andaya, C.; Maizel, A.; Higgins, C.P. Assessing Continued Electrochemical Treatment of Groundwater Impacted by Aqueous Film-Forming Foams. J. Environ. Eng. 2019, 145, 06019007. [Google Scholar] [CrossRef]
- Lacasa, E.; Cotillas, S.; Saez, C.; Lobato, J.; Cañizares, P.; Rodrigo, M.A. Environmental Applications of Electrochemical Technology. What Is Needed to Enable Full-Scale Applications? Curr. Opin. Electrochem. 2019, 16, 149–156. [Google Scholar] [CrossRef]
- Guo, L.; Jing, Y.; Chaplin, B.P. Development and Characterization of Ultrafiltration TiO2 Magnéli Phase Reactive Electrochemical Membranes. Environ. Sci. Technol. 2016, 50, 1428–1436. [Google Scholar] [CrossRef]
- Ensch, M.; Rusinek, C.A.; Becker, M.F.; Schuelke, T. A Combined Current Density Technique for the Electrochemical Oxidation of Perfluorooctanoic Acid (PFOA) with Boron-Doped Diamond. Water Environ. J. 2021, 35, 158–165. [Google Scholar] [CrossRef]
- Pétrier, C.; Torres-Palma, R.; Combet, E.; Sarantakos, G.; Baup, S.; Pulgarin, C. Enhanced Sonochemical Degradation of Bisphenol-A by Bicarbonate Ions. Ultrason. Sonochem. 2010, 17, 111–115. [Google Scholar] [CrossRef]
- Moriwaki, H.; Takagi, Y.; Tanaka, M.; Tsuruho, K.; Okitsu, K.; Maeda, Y. Sonochemical Decomposition of Perfluorooctane Sulfonate and Perfluorooctanoic Acid. Environ. Sci. Technol. 2005, 39, 3388–3392. [Google Scholar] [CrossRef]
- Gole, V.L.; Fishgold, A.; Sierra-Alvarez, R.; Deymier, P.; Keswani, M. Treatment of Perfluorooctane Sulfonic Acid (PFOS) Using a Large-Scale Sonochemical Reactor. Sep. Purif. Technol. 2018, 194, 104–110. [Google Scholar] [CrossRef]
- Campbell, T.Y.; Vecitis, C.D.; Mader, B.T.; Hoffmann, M.R. Perfluorinated Surfactant Chain-Length Effects on Sonochemical Kinetics. J. Phys. Chem. A 2009, 113, 9834–9842. [Google Scholar] [CrossRef]
- Lee, Y.C.; Chen, M.J.; Huang, C.P.; Kuo, J.; Lo, S.L. Efficient Sonochemical Degradation of Perfluorooctanoic Acid Using Periodate. Ultrason. Sonochem. 2016, 31, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.C.; Lo, S.L.; Hu, C.Y.; Lee, Y.C.; Kuo, J. Enhanced Sonochemical Degradation of Perfluorooctanoic Acid by Sulfate Ions. Ultrason. Sonochem. 2015, 22, 542–547. [Google Scholar] [CrossRef]
- Rodriguez-Freire, L.; Abad-Fernández, N.; Sierra-Alvarez, R.; Hoppe-Jones, C.; Peng, H.; Giesy, J.P.; Snyder, S.; Keswani, M. Sonochemical Degradation of Perfluorinated Chemicals in Aqueous Film-Forming Foams. J. Hazard. Mater. 2016, 317, 275–283. [Google Scholar] [CrossRef]
- Campbell, T.; Hoffmann, M.R. Sonochemical Degradation of Perfluorinated Surfactants: Power and Multiple Frequency Effects. Sep. Purif. Technol. 2015, 156, 1019–1027. [Google Scholar] [CrossRef]
- James Wood, R.; Sidnell, T.; Ross, I.; McDonough, J.; Lee, J.; Bussemaker, M.J. Ultrasonic Degradation of Perfluorooctane Sulfonic Acid (PFOS) Correlated with Sonochemical and Sonoluminescence Characterisation. Ultrason. Sonochem. 2020, 68, 105196. [Google Scholar] [CrossRef] [PubMed]
- Tenorio, R.; Liu, J.; Xiao, X.; Maizel, A.; Higgins, C.P.; Schaefer, C.E.; Strathmann, T.J. Destruction of Per-and Polyfluoroalkyl Substances (PFASs) in Aqueous Film-Forming Foam (AFFF) with UV-Sulfite Photoreductive Treatment. Environ. Sci. Technol. 2020, 54, 6957–6967. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Wei, Z.; He, K.; Blaney, L.; Cheng, X.; Xu, T.; Liu, W.; Zhao, D. A Concentrate-and-Destroy Technique for Degradation of Perfluorooctanoic Acid in Water Using a New Adsorptive Photocatalyst. Water Res. 2020, 185, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Zhou, J.L.; Altaee, A.; Ahmed, M.B.; Johir, M.A.H.; Ren, J.; Li, X. Improved Photocatalysis of Perfluorooctanoic Acid in Water and Wastewater by Ga2O3/UV System Assisted by Peroxymonosulfate. Chemosphere 2020, 239, 124722. [Google Scholar] [CrossRef]
- Gu, P.; Zhang, C.; Sun, Z.; Zhang, H.; Zhou, Q.; Lin, S.; Rong, J.; Hoffmann, M.R. Enhanced Photoreductive Degradation of Perfluorooctanesulfonate by UV Irradiation in the Presence of Ethylenediaminetetraacetic Acid. Chem. Eng. J. 2020, 379. [Google Scholar] [CrossRef]
- Azarpira, H.; Abtahi, M.; Sadani, M.; Rezaei, S.; Atafar, Z.; bay, A.; Mohseni, S.M.; Sarkhosh, M.; Shanbedi, M.; Alidadi, H.; et al. Photo-Catalytic Degradation of Trichlorophenol with UV/Sulfite/ZnO Process, Simultaneous Usage of Homogeneous Reductive and Heterogeneous Oxidative Agents Generator as a New Approach of Advanced Oxidation/Reduction Processes (AO/RPs). J. Photochem. Photobiol. A Chem. 2019, 374, 43–51. [Google Scholar] [CrossRef]
- Xu, T.; Zhu, Y.; Duan, J.; Xia, Y.; Tong, T.; Zhang, L.; Zhao, D. Enhanced Photocatalytic Degradation of Perfluorooctanoic Acid Using Carbon-Modified Bismuth Phosphate Composite: Effectiveness, Material Synergy and Roles of Carbon. Chem. Eng. J. 2020, 395. [Google Scholar] [CrossRef]
- Maga, D.; Aryan, V.; Bruzzano, S. Environmental Assessment of Various End-of-Life Pathways for Treating Per- and Polyfluoroalkyl Substances in Spent Fire-Extinguishing Waters. Environ. Toxicol. Chem. 2021, 40, 947–957. [Google Scholar] [CrossRef]
- Ahmed, M.B.; Alam, M.M.; Zhou, J.L.; Xu, B.; Johir, M.A.H.; Karmakar, A.K.; Rahman, M.S.; Hossen, J.; Hasan, A.T.M.K.; Moni, M.A. Advanced Treatment Technologies Efficacies and Mechanism of Per- and Poly-Fluoroalkyl Substances Removal from Water. Process Saf. Environ. Prot. 2020, 136, 1–14. [Google Scholar] [CrossRef]
- Solo-Gabriele, H.M.; Jones, A.S.; Lindstrom, A.B.; Lang, J.R. Waste Type, Incineration, and Aeration Are Associated with per- and Polyfluoroalkyl Levels in Landfill Leachates. Waste Manag. 2020, 107, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Dixit, F.; Dutta, R.; Barbeau, B.; Berube, P.; Mohseni, M. PFAS Removal by Ion Exchange Resins: A Review. Chemosphere 2021, 272, 129777. [Google Scholar] [CrossRef]
- Mahinroosta, R.; Senevirathna, L. A Review of the Emerging Treatment Technologies for PFAS Contaminated Soils. J. Environ. Manag. 2020, 255, 109896. [Google Scholar] [CrossRef]
- Lu, D.; Sha, S.; Luo, J.; Huang, Z.; Zhang Jackie, X. Treatment Train Approaches for the Remediation of Per- and Polyfluoroalkyl Substances (PFAS): A Critical Review. J. Hazard. Mater. 2020, 386, 121963. [Google Scholar] [CrossRef]
- Feng, M.; Gao, R.; Staack, D.; Pillai, S.D.; Sharma, V.K. Degradation of Perfluoroheptanoic Acid in Water by Electron Beam Irradiation. Environ. Chem. Lett. 2021, 19, 2689–2694. [Google Scholar] [CrossRef]
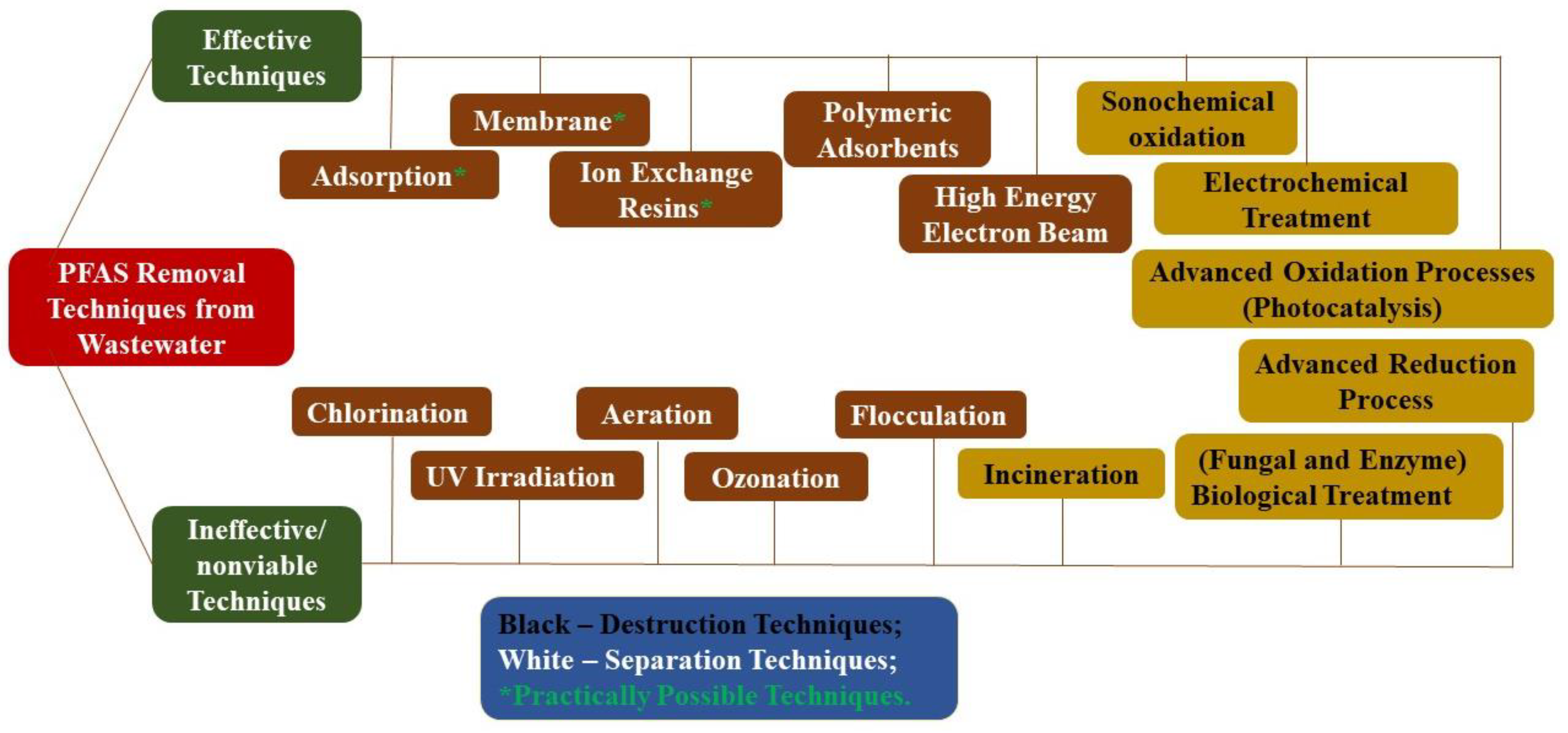
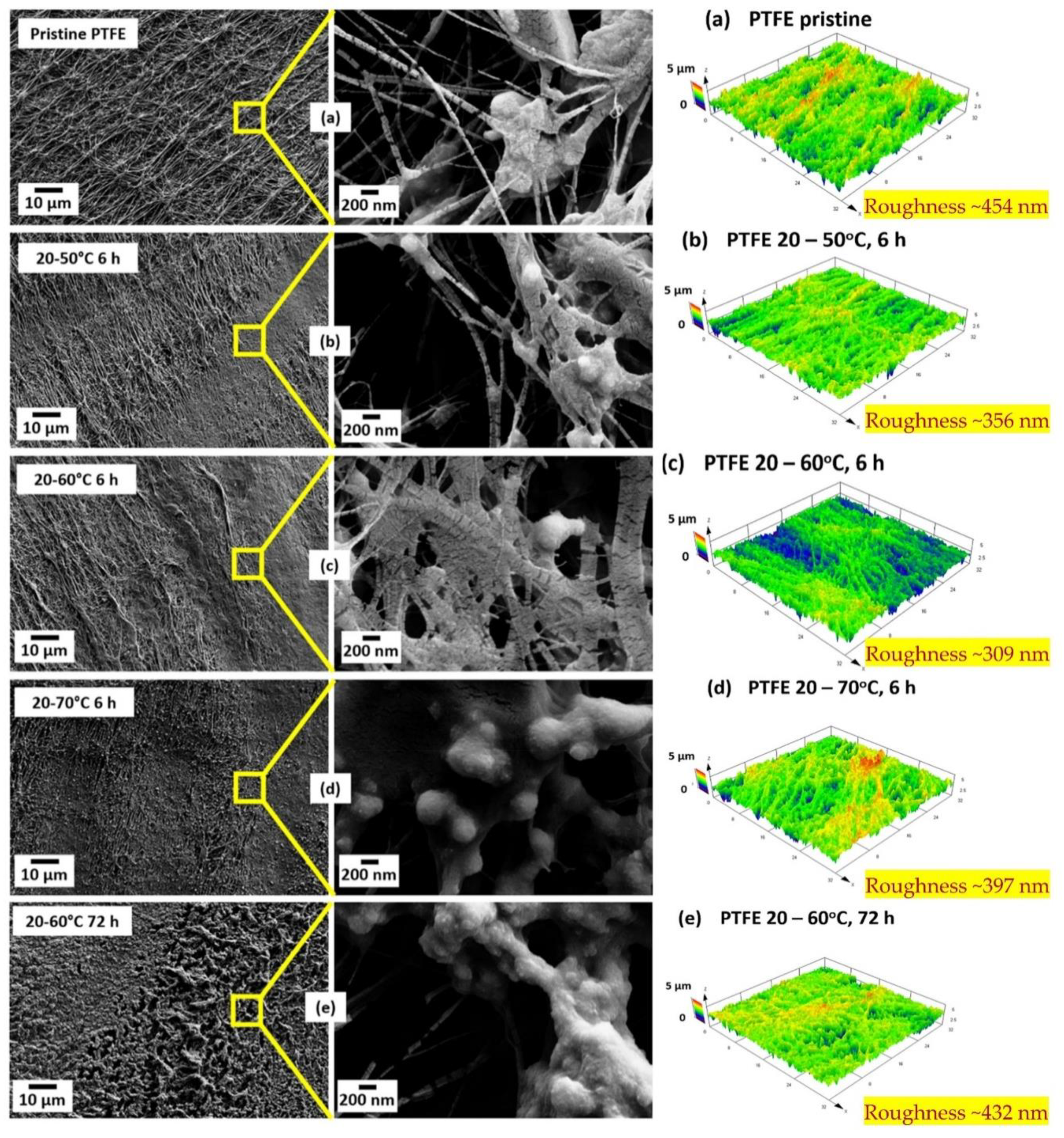
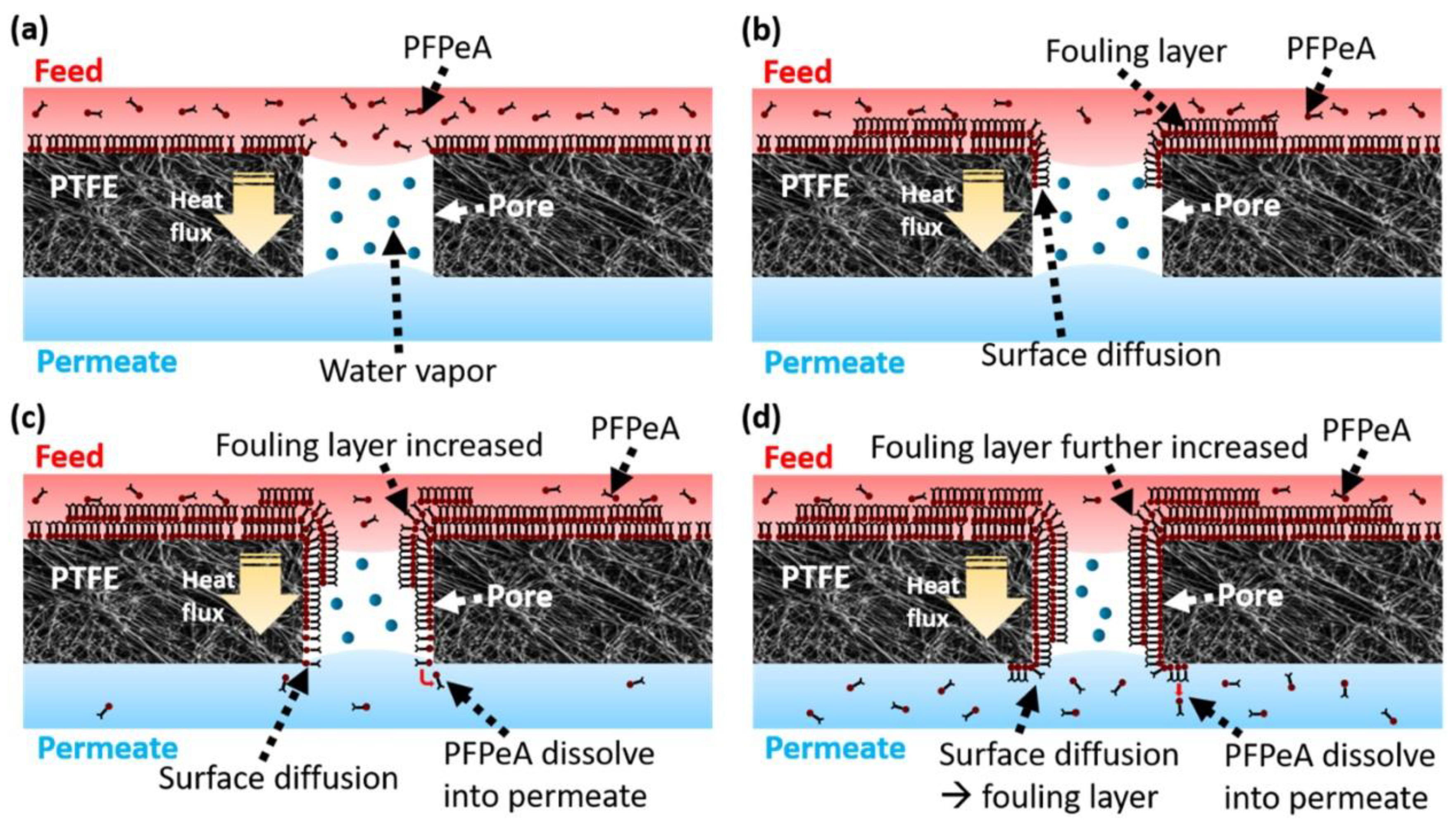
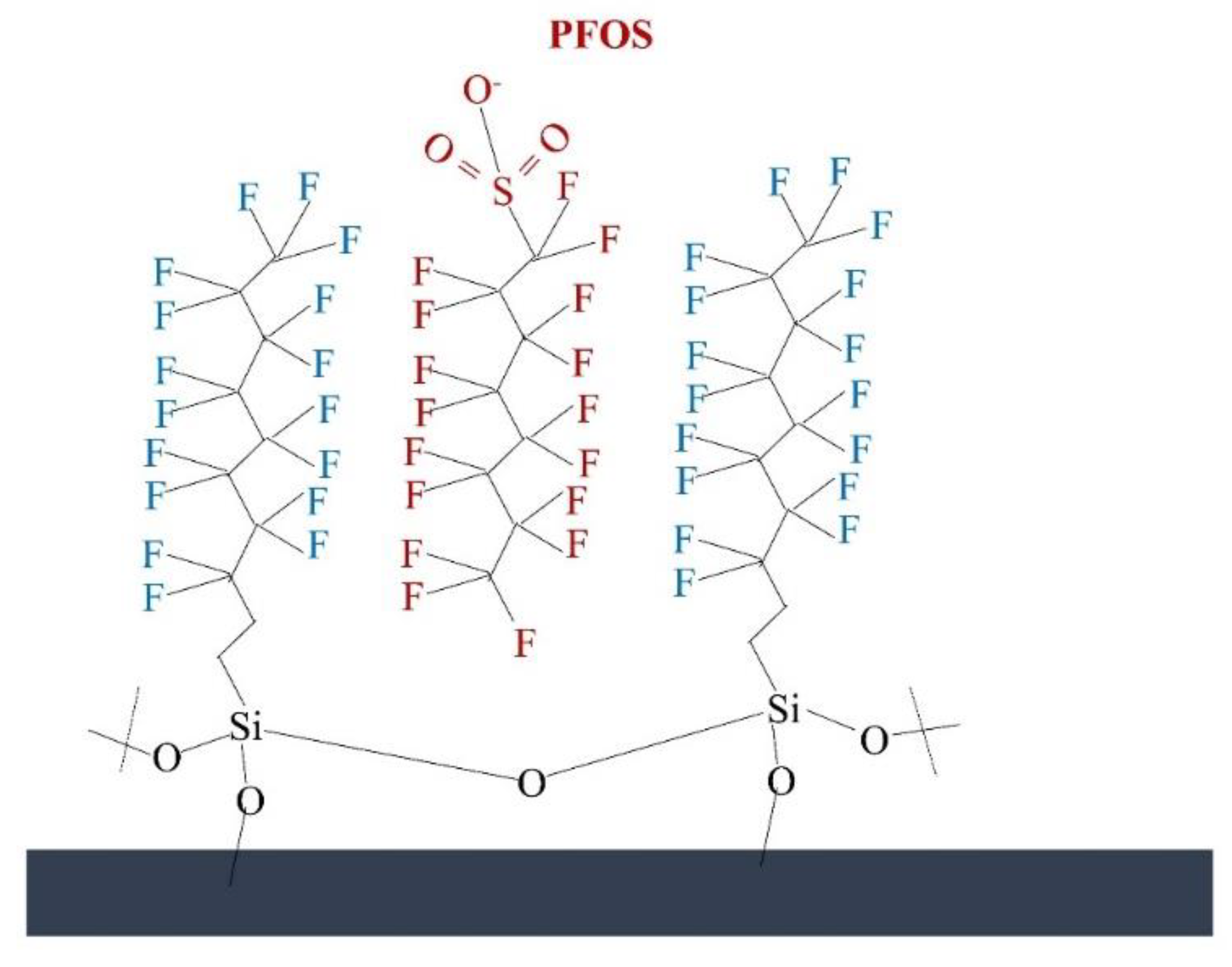
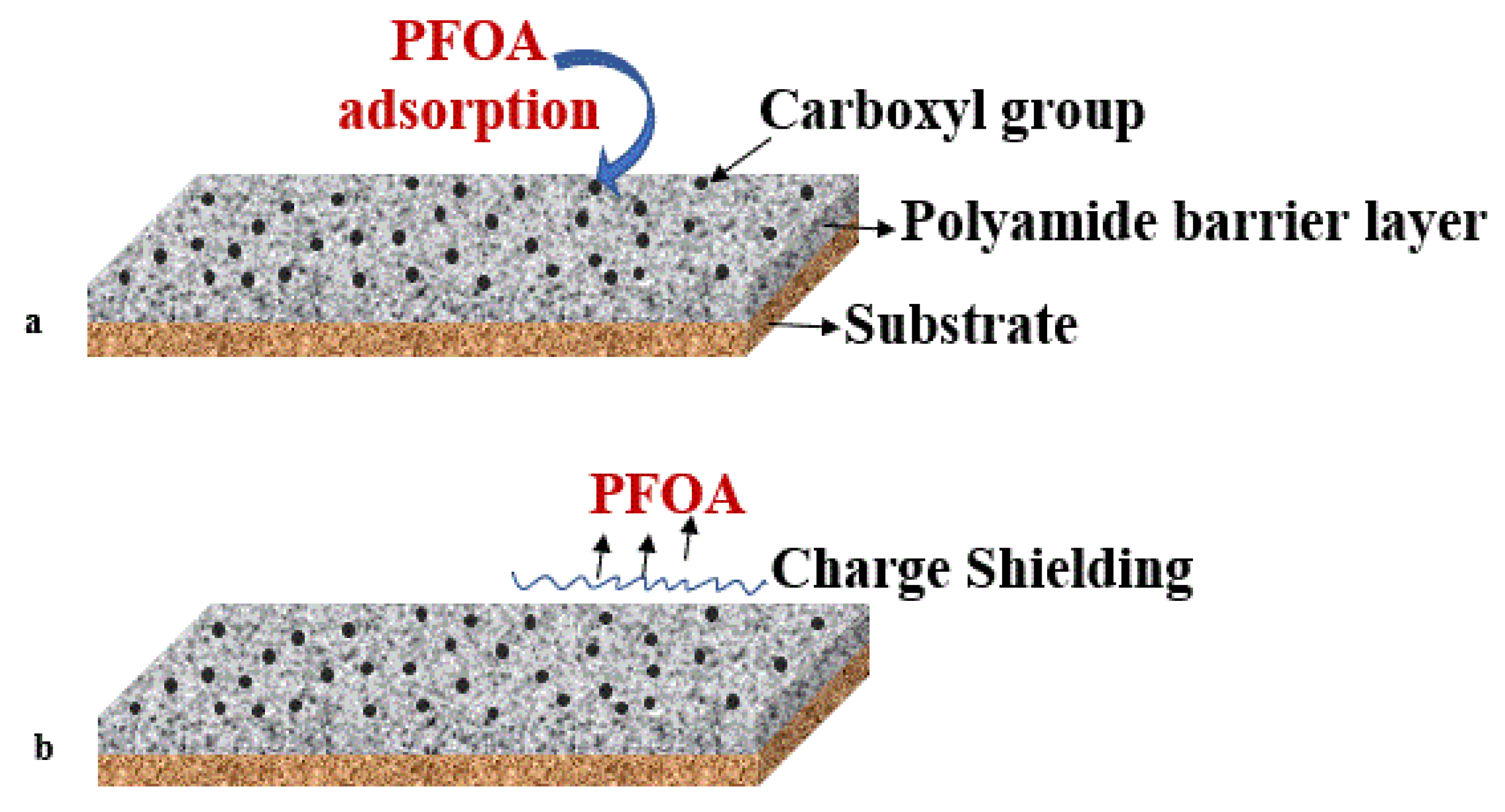
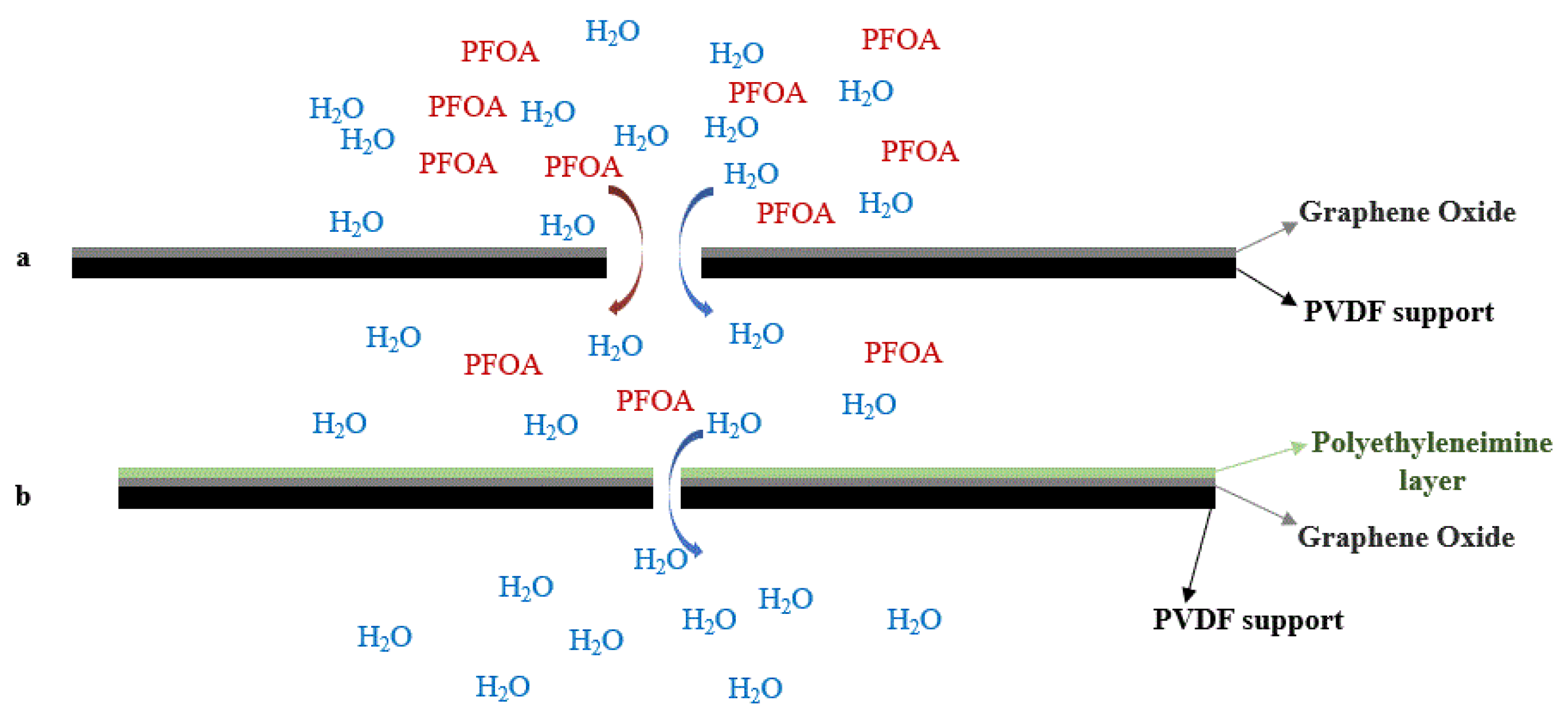

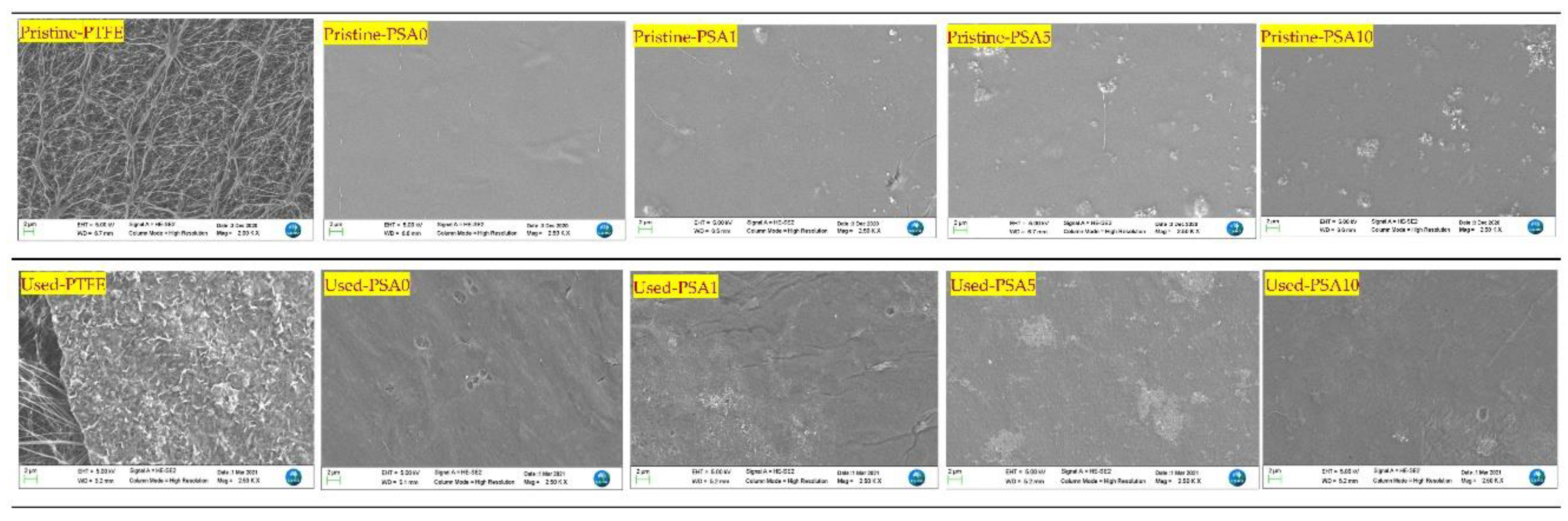
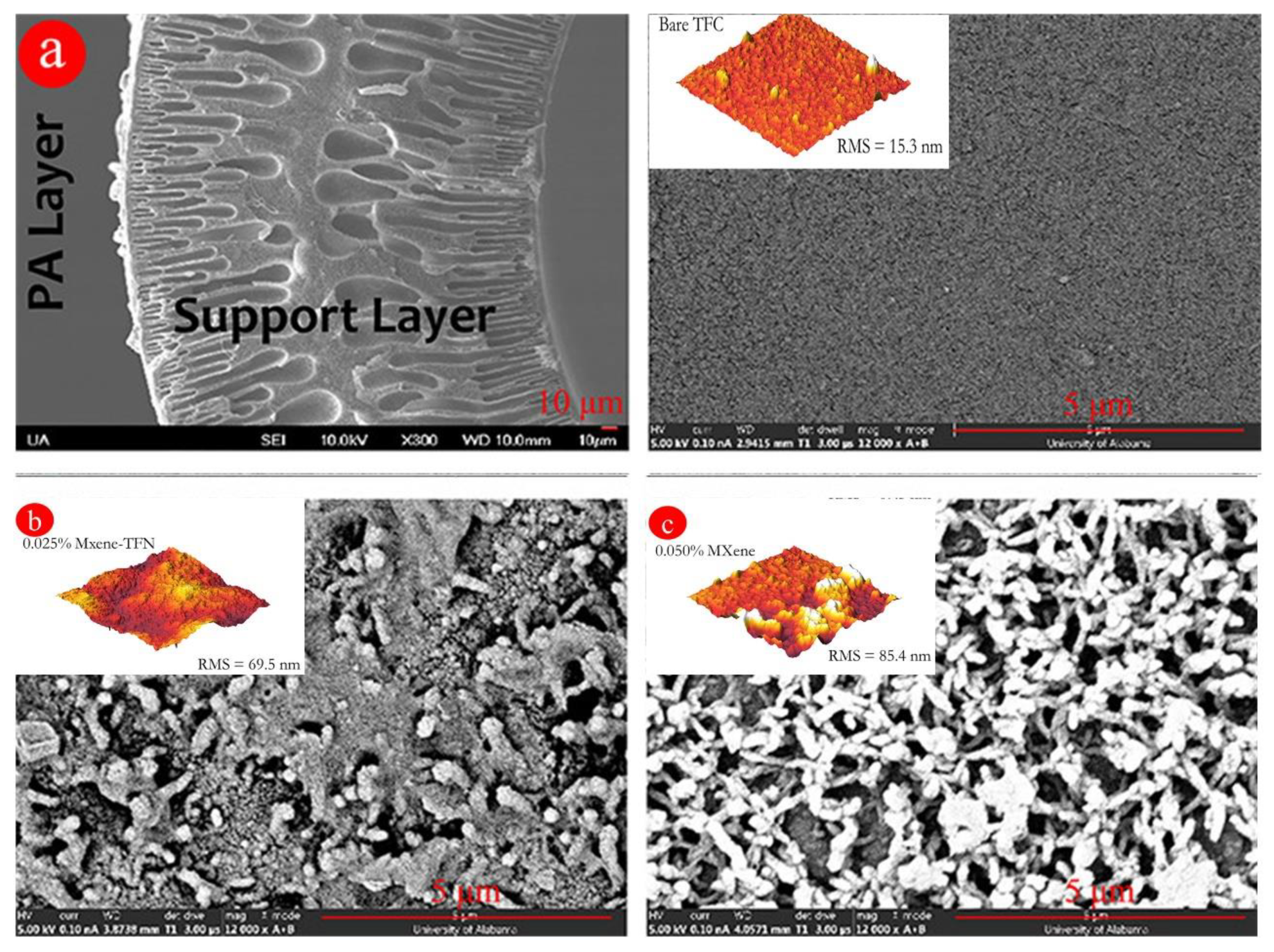
| Advantages | Limitations |
|---|---|
| Granular activated carbon (GAC) or powder activated carbon (PAC) | |
| Can remove low concentrations (ng/L) from drinking water [33] compared to other methods (UV [34], Ozone [34], modified silica [35], etc.). Long-chain PFAS (e.g., legacy PFAS as PFOA and PFOS) are efficiently (>90%) removed by GAC or PAC depending on the flow rate of the water, carbon bed depth, empty bed contact time, the temperature of the medium, and the presence of other organic matters [33,36,37]. Relatively low cost (0.093–0.12 $/m3) [33,38]. | Inefficient for removal of short-chain PFAS due to weak (hydrophobic) interaction [36,39]. The presence of organic compounds reduces adsorption efficiency [25]. Regeneration and reuse are energy-intensive (0.78 $/kg) [40]. |
| Ion-exchange resin | |
| Efficient for removal of anionic and long-chain PFAS (even for ng/L concentrations) [41]. Adsorption capacity is higher compared to GAC or PAC. Fast adsorption kinetics [36,42]. Operating cost is about 60% of GAC and PAC [38]. | Less efficient for water containing organic or inorganic matter [38]. limited removal of short-chain PFAS (efficiency ratio PFOS (C8):PFPrS (C3) = 82) [43]. Requires expensive regeneration [40]. |
| Membrane separation | |
| Effective for short-chain as well as long-chain PFAS [44]. Other organic and inorganic impurities are also removed [45]. High removal rate and efficiency (discharge goal 10–75 ng/L) [44]. Time-efficient compared to adsorption technique as no adsorption is required [38]. | Fouling of membranes due to inorganic, organic, biological, and colloidal impurities may result in limited efficiency [24]. Requires brine management, which can be overcome by partnering it with a destruction process [46,47]. The energy requirement for membrane wastewater treatment is high compared to adsorption or ion exchange resin (~0.12 $/m3 permeate) [38]. |
| Pollutant (Concentration, ppm) | Membrane Technology Used | Conditions | Water Matrix | Rejection | Ref. |
|---|---|---|---|---|---|
| PFOS: 0.5–1500 | RO | pH 4 25 °C 200 psi 24 h | Real wastewater | >99% | [83] |
| Perfluorobutanoic acid (PFBA), perfluorobutane sulfonate (PFBS), perfluorooctanoic acid (PFOA), and perfluorooctane sulfonate (PFOS): 0.001 | NF and RO | 87–116 psi 22–28 °C pH 7.4 | Tap water | 95–99.9% | [101] |
| PFXxA: 0.0001–0.0003 | RO, NF, and UF | pH 7 | MilliQ water | 69–99.2% | [75] |
| 9 types of PFAS | NF | pH 6.7 18 °C 125 psi | Artificial ground water | 95–99% | [96] |
| PFOA: 1 | NF (negatively charged) | pH ~7 25 °C 100 psi | Simulated groundwater | ∼90% | [102] |
| Membrane Type | Pollutant (Concentration, ppm) | Experimental Conditions | Water Matrix | Rejection | Reference |
|---|---|---|---|---|---|
| Polymeric | PFOS and PFOA: 0.00086 and 0.00039 | pH 7.5 Room temperature Flux: 1223 LMH Pressure drop: 0.04–0.07 bar Time 0.5 h | DI water | >90% | [115] |
| PFOA: 100 | pH 7 Pressure: 2.06 bar Room temperature Flux: 123–145 LMH Time: 3.34–4.67 h | DI water | 99% | [113] | |
| 15 different PFAS (PFBA, PFPeA, PFHxA, PFHpA, PFOA, PFNA, PFDA, PFUnDA, PFDoDA, PFBS, PFHxS, PFOS, PFDS, FOSA, FTSA) | pH ~7.7 Temperature: 8.5 °C Water flow rate: 2.3 m3/h | Wastewater | 99% | [78] | |
| PFOS and AFFF: 0.06 and 100 | pH ~7 Temperature: 20 ± 2 °C Flux: 7–50 LMH Pressure: 4.14 bar Time: continuous operation for 13 days | DI water | >98% | [97] | |
| Ceramic | 12 different PFAS (PFPeA, PFHxA, PFHpA, PFOA, PFNA, PFPrS, PFBS, PFPeS, PFHxS, PFHpS, PFOS, and PFDS): 1.18 × 10−6–55.7 × 10−6 | Flux: 60–65 LMH Time: 42–200 h | Real wastewater | ~10% specific water flux | [25] |
| Silica membrane | 9 different PFAS (PFHxS, PFOS, PFHpA, PFOA, PFNA, PFDA, PFUnDA, PFDoDA, and PFTA) 0.2 mg mL−1 | Room temperature, Time: 24 h, pH 3 | DI water and real wastewater | 8.6–99.17% removal efficiency. | [122] |
| Processes | Materials/Approach | Conditions | Water Matrix | Remarks | References |
|---|---|---|---|---|---|
| Membrane-adsorption/Ion exchange resin | NF membrane (NF90-400), Granular activated carbon (Filtrasorb® 400), and anion exchange resins (Resin A600) | pH ~7.7, Temperature: 8.5 °C, Water flow rate: 2.3 m3/h. | Real wastewater (contains other impurities as well) | Combining the technologies worked in favor of the efficient removal of PFAS from wastewater. | [78] |
| Membrane-adsorption-Ion exchange resin | NF270 membrane, Granular activated carbon (Filtrasorb 400 and Norit 1240 W), and anion exchange resins (Purolite A600 and Purofine PFA694) | pH ~7.8, 8.5 °C, Pressure: 5–8 bar, Feedwater flow rate: 8 m3/h. | Real wastewater (32 different PFAS: 0.0001–0.0002 ppm) | This study expands knowledge of cost-efficient PFAS removal technology based on the pollutant concentration present in wastewater. | [38] |
| Membrane-adsorption | NF270 membrane and Granular activated carbon (Filtrasorb 300, Filtrasorb 600, and AquaCarb 1240C) | pH 6.7, 18 °C, Pressure: 1.7–9.6 bar, Permeate flow rate: 4.5–20.5 mL/min. | Artificial groundwater (PFAAs: 0.001 ppm) | This bench-scale study demonstrates the effective removal of long-chain PFAS (by adsorbents) and short-chain PFAS (by NF) from the wastewater, but further work is needed before it is implemented for large-scale application. | [96] |
| Membrane-adsorption | Adsorbents: Chemviron F-400 (density 440 kg/m3; 12 filters), Norit ROW 0.8 (density 381 kg/m3; 2 filters) and Norit 1240 EN. | - | Real wastewater | The combined process effectively removed >86% pollutants (present in ppt-range) from the wastewater. | [82] |
| Membrane-UV/O2 | The membrane was a polymeric blend of polysulfone and poly ether ketone; oxygen flowrate 3 L/min, UV lamp intensity 365 nm | Pressure 2.06 bar, Room temperature, pH 7, Flux: 123–145 LMH, Time: 3.34–4.67 h | Synthetic wastewater (PFOA) | 99% PFOA rejection. | [113] |
| Membrane-photocatalysis | NF membrane (2540-ACM5-TSF) and nano zero-valent iron as a photocatalyst (20–100 mg/L) | pH–11, Temperature: 2–45 °C, Feed flow rate: 1.4 m3/h, Flux: 70–150 LMH, Pressure: 3–41 bar | Synthetic wastewater (PFOA: 0.1 ppm) | In this coupling technology, Nanofiltration alone efficiently removed >99% PFOA, and the PFOA concentrated rejected water was photocatalytically degraded (~60%). This type of coupled technology needs more attention since it can first remove the pollutants and then destroy them successfully. | [46] |
| Activated carbon/Ceramic membrane | Ceramic microfiltration membrane (nominal pore size of 0.1 μm) and super-fine powder activated carbon (particle diameter < 1 μm) | Flux: 60–65 LMH, Time: 42–200 h | Real wastewater (12 different PFAS: 1.18 × 10−6–55.7 × 10−6 ppm). | ~10% specific water flux | [25] |
| Membrane-Electrochemical technology | NF90 membrane | Pressure: 10.3–17.2 bar, Time: 10 min, crossflow velocity: 21.3 cm/s | Simulated wastewater (Hexafluoropropylene oxide dimer acid: 1 ppm) | The electrochemical treatment after membrane treatment appeared to be cost-efficient compared to direct electrochemical oxidation. | [47] |
| Membrane -electrochemical treatment | NF90 and NF270 membranes | Feed flow rate: 3.6 m3/h, Pressure: 10 bar, Temperature: 20 °C, Other ions present in the feed water (SO42−, Cl−, Ca2+, and Na+ with concentrations of 321, 19.8, 172, and 24.9 ppm, respectively) | Simulated wastewater (PFHxA: 204 ppm) | Energy savings with NF90 membrane was 60–71% for 99% and 90% removal ratio. | [137] |
| Membrane-electrooxidation | NF90 and NF270 membranes | Flow rate: 3.2 m3/h, Permeability: 6.98–9.4 LMH/bar, Other ions: Na+ (162 ppm), SO42− (338 ppm); Feed volume: 10 m3; pressure: 10 bar; Temperature: 25 °C | Simulated wastewater (Perflurohexanoic acid: 100 ppm) | The treatment cost can be reduced further by replacing boron-doped diamond electrodes. | [26] |
| Membrane-electrooxidation | NF90 and BW30 membranes | Pressure: 10 bar, Crossflow velocity: 24.7 cm/s, Other salts present: NaCl and CaSO4 | Simulated wastewater (mixture of PFOA, PFHpA, PFHxA, PFPeA, and PFBA with initial concentrations of 0.01 ppm each) | Efficiently removed PFAS to the below level set by the USEPA. | [138] |
| Technology | Membrane Used | Effectiveness | Remarks/(Rejection/Removal) | References |
|---|---|---|---|---|
| Removal | UF | Not effective | Works better with surface modification (10–75%). | [45] |
| MD | To some extent | Not effective for short-chain PFAS (58–85%). | [24] | |
| NF | Highly efficient | May suffer from scale formation (~90–99%). | [78,84,96,97,102] | |
| RO | Highly efficient | May suffer from fouling and scale formation (>99%). | [83,98,99,100] | |
| FO | Not reported | - | - | |
| GO-nanofiltration-membrane | Reasonable | Increases membrane stability (74.3%). | [123] | |
| Ceramic membrane | Effective | Irreversible change on the membrane surface can reduce the performance of the membrane. | [25,116] | |
| Nanoparticle coated silica membrane | Highly effective | Membrane is stable and reusable (8.67–99.17%). | [122] | |
| Destruction | Reactive electrochemical membrane | Highly effective | Reduction in operating cost is possible without compromising the final concentration of PFAS to the safe limit, but further work is needed with real wastewater (98.3%). | [135,139] |
| Phosphorene Nanocomposite membrane | Highly effective | Destruction of fluorine compound after membrane treatment was removed by UV photolysis and liquid aerobic oxidation, which can also negatively affect the membrane surface (99%). | [113] | |
| Electromagnetic (microwave) membrane | Effective to some extent | Further improvement needed (65.9% degraded). | [134] |
| Processes | Materials | Treatment Cost/Energy Requirement | References |
|---|---|---|---|
| Adsorption | GAC (~$1.2–2.75/kg) | 0.084–0.11 $/m3 wastewater for 10 ng/L treatment goal 0.021–0.025 $/m3 wastewater for 85 ng/L treatment goal | [33,36] |
| Ion exchange resins (~$17.6–20.35/kg) | 1.2–8.9 $/m3 wastewater for 25 ng/L discharge goal | [36] | |
| GAC and Ion exchange resins combined | 0.84–3.28 $/m3 for 25 ng/L discharge goal~3.78 × 106 L/day | [36] | |
| Membrane | NF | 0.016–0.16 $/m3 permeate | [28,38] |
| Membrane-Adsorption | - | ~0.28 $/m3 for 90 ng/L discharge goal ~0.87 $/m3 for 25 ng/L discharge goal ~1.31 $/m3 for 4 ng/L discharge goal | [38,83] |
| Membrane-electrochemical oxidation | - | 2.7–13.1$/m3 (High energy requirement) | [26,47,137,138] |
| Photocatalysis | Indium Oxides@254 nm light source | (Mostly depends on the catalyst); energy requirement 2106 KWh/m3, $295/m3, time required >11 h, ~89% removal efficiency | [140] |
| Pt-TiO2@365 nm light source | Energy requirement 1458 KWh/m3, time required >7 h, 100% removal efficiency | [135] | |
| Electron-beam | - | 98% PFOA and 99.99% PFOS removal at 1500 kGy (~$295/m3) | [141] |
| Electrochemical treatment | Ti4O7 electrode (∼$0.36/m2) Boron doped diamond ($7000/m2) | 5–32 KWh/m3 (high electrode cost and energy requirement) | [136,142] |
| Incineration | For regeneration of GAC or Ion exchange resins | ~0.751$/kg | [143,144] |
| Biological treatment (cost not reported) | - | Selection of a proper biological entity, pre-treatment; additionally, the process takes a longer time, which increases the operating cost | [145,146,147] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Das, S.; Ronen, A. A Review on Removal and Destruction of Per- and Polyfluoroalkyl Substances (PFAS) by Novel Membranes. Membranes 2022, 12, 662. https://doi.org/10.3390/membranes12070662
Das S, Ronen A. A Review on Removal and Destruction of Per- and Polyfluoroalkyl Substances (PFAS) by Novel Membranes. Membranes. 2022; 12(7):662. https://doi.org/10.3390/membranes12070662
Chicago/Turabian StyleDas, Suman, and Avner Ronen. 2022. "A Review on Removal and Destruction of Per- and Polyfluoroalkyl Substances (PFAS) by Novel Membranes" Membranes 12, no. 7: 662. https://doi.org/10.3390/membranes12070662
APA StyleDas, S., & Ronen, A. (2022). A Review on Removal and Destruction of Per- and Polyfluoroalkyl Substances (PFAS) by Novel Membranes. Membranes, 12(7), 662. https://doi.org/10.3390/membranes12070662





