Applications of Polymeric Membranes with Carbon Nanotubes: A Review
Abstract
:1. Introduction
2. Types of CNT-Based Membranes
3. Detailed Applications of CNT Membranes
3.1. CNT Membranes in Water Purification
3.2. CNT Membranes in Desalination Technology
3.3. CNT Membranes for Energy Storage
3.4. CNT Membranes for Gas Separations
4. Challenges and Future of CNT-Based Membranes
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Niu, J.J.; Wang, J.N.; Jiang, Y.; Su, L.F.; Ma, J. An approach to carbon nanotubes with high surface area and large pore volume. Microporous Mesoporous Mater. 2007, 100, 1–5. [Google Scholar] [CrossRef]
- Dai, H. Carbon nanotubes: Opportunities and challenges. Surf. Sci. 2002, 500, 218–241. [Google Scholar] [CrossRef]
- Mukiibi, M.; Feathers, R. Membrane Technology: A Breakthrough in Water Treatment; WCP. 2009. Available online: https://wcponline.com/2009/02/10/membrane-technology-break-water-treatment/ (accessed on 21 March 2022).
- Drioli, E.; Curcio, E. Membrane engineering for process intensification: A perspective. J. Chem. Technol. Biotechnol. Int. Res. Process Environ. Clean Technol. 2007, 82, 223–227. [Google Scholar] [CrossRef]
- Peng, F.; Hu, C.; Jiang, Z. Novel ploy(vinyl alcohol)/carbon nanotube hybrid membranes for pervaporation separation of benzene/cyclohexane mixtures. J. Membr. Sci. 2007, 297, 236–242. [Google Scholar] [CrossRef]
- Fan, X.; Zhao, H.; Liu, Y.; Quan, X.; Yu, H.; Chen, S. Enhanced Permeability, Selectivity, and Antifouling Ability of CNTs/Al2O3 Membrane under Electrochemical Assistance. Environ. Sci. Technol. 2015, 49, 2293–2300. [Google Scholar] [CrossRef] [PubMed]
- Dervin, S.; Dionysiou, D.D.; Pillai, S.C. 2D nanostructures for water purification: Graphene and beyond. Nanoscale 2016, 8, 15115–15131. [Google Scholar] [CrossRef]
- Yang, Z.; Zhou, Y.; Feng, Z.; Rui, X.; Zhang, T.; Zhang, Z. A Review on Reverse Osmosis and Nanofiltration Membranes for Water Purification. Polymers 2019, 11, 1252. [Google Scholar] [CrossRef] [Green Version]
- Tanudjaja, H.J.; Hejase, C.A.; Tarabara, V.V.; Fane, A.G.; Chew, J.W. Membrane-based separation for oily wastewater: A practical perspective. Water Res. 2019, 156, 347–365. [Google Scholar] [CrossRef]
- Nasibulin, A.G.; Kaskela, A.; Mustonen, K.; Anisimov, A.S.; Ruiz, V.; Kivistö, S.; Rackauskas, S.; Timmermans, M.Y.; Pudas, M.; Aitchison, B.; et al. Multifunctional Free-Standing Single-Walled Carbon Nanotube Films. ACS Nano 2011, 5, 3214–3221. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, Y.; Ma, X.Y.D.; Ang, J.; Zeng, Z.; Ng, B.F.; Wan, M.P.; Wong, S.-C.; Lu, X. Polymer/MOF-derived multilayer fibrous membranes for moisture-wicking and efficient capturing both fine and ultrafine airborne particles. Sep. Purif. Technol. 2020, 235, 116183. [Google Scholar] [CrossRef]
- Dharupaneedi, S.P.; Nataraj, S.K.; Nadagouda, M.; Reddy, K.R.; Shukla, S.S.; Aminabhavi, T.M. Membrane-based separation of potential emerging pollutants. Sep. Purif. Technol. 2019, 210, 850–866. [Google Scholar] [CrossRef] [PubMed]
- Che, G.L.; Lakshmi, B.B.; Fisher, E.R.; Martin, C.R. Carbon nanotubule membranes for electrochemical energy storage and production. Nature 1998, 393, 346–349. [Google Scholar] [CrossRef]
- Shin, M.; Song, W.-J.; Bin Son, H.; Yoo, S.; Kim, S.; Song, G.; Choi, N.-S.; Park, S. Highly Stretchable Separator Membrane for Deformable Energy-Storage Devices. Adv. Energy Mater. 2018, 8, 1801025. [Google Scholar] [CrossRef]
- Xu, Y.; Goh, K.; Wang, R.; Bae, T.-H. A review on polymer-based membranes for gas-liquid membrane contacting processes: Current challenges and future direction. Sep. Purif. Technol. 2019, 229, 115791. [Google Scholar] [CrossRef]
- He, Z.; Lyu, Z.; Gu, Q.; Zhang, L.; Wang, J. Ceramic-based membranes for water and wastewater treatment. Colloids Surf. A Physicochem. Eng. Asp. 2019, 578, 123513. [Google Scholar] [CrossRef]
- Guizard, C.; Bac, A.; Barboiu, M.; Hovnanian, N. Hybrid organic-inorganic membranes with specific transport properties. Sep. Purif. Technol. 2001, 25, 167–180. [Google Scholar] [CrossRef]
- Shao, P.; Huang, R.Y.M. Polymeric membrane pervaporation. J. Membr. Sci. 2007, 287, 162–179. [Google Scholar] [CrossRef]
- Siddiqui, M.U.; Arif, A.F.M.; Bashmal, S. Permeability-Selectivity Analysis of Microfiltration and Ultrafiltration Membranes: Effect of Pore Size and Shape Distribution and Membrane Stretching. Membranes 2016, 6, 40. [Google Scholar] [CrossRef] [Green Version]
- Zuo, H.-R.; Shi, P.; Duan, M. A review on thermally stable membranes for water treatment: Material, fabrication, and application. Sep. Purif. Technol. 2020, 236, 116223. [Google Scholar] [CrossRef]
- Sianipar, M.; Kim, S.H.; Khoiruddin, K.; Iskandar, F.; Wenten, I.G. Functionalized carbon nanotube (CNT) membrane: Progress and challenges. RSC Adv. 2017, 7, 51175–51198. [Google Scholar] [CrossRef] [Green Version]
- Van Der Bruggen, B. The Separation Power of Nanotubes in Membranes: A Review. ISRN Nanotechnol. 2012, 2012, 693485. [Google Scholar] [CrossRef]
- Das, R.; Ali, M.E.; Hamid, S.B.A.; Ramakrishna, S.; Chowdhury, Z.Z. Carbon nanotube membranes for water purification: A bright future in water desalination. Desalination 2014, 336, 97–109. [Google Scholar] [CrossRef]
- Ahn, C.H.; Baek, Y.; Lee, C.; Kim, S.O.; Kim, S.; Lee, S.; Kim, S.-H.; Bae, S.S.; Park, J.; Yoon, J. Carbon nanotube-based membranes: Fabrication and application to desalination. J. Ind. Eng. Chem. 2012, 18, 1551–1559. [Google Scholar] [CrossRef]
- Lee, J.; Jeong, S.; Liu, Z. Progress and challenges of carbon nanotube membrane in water treatment. Crit. Rev. Environ. Sci. Technol. 2016, 46, 999–1046. [Google Scholar] [CrossRef]
- Yu, M.; Funke, H.H.; Falconer, J.L.; Noble, R.D. High Density, Vertically-Aligned Carbon Nanotube Membranes. Nano Lett. 2009, 9, 225–229. [Google Scholar] [CrossRef] [PubMed]
- Bisignano, F.; Mattia, D.; De Luca, G. Selectivity-permeability optimization of functionalised CNT–polymer membranes for water treatment: A modeling study. Sep. Purif. Technol. 2015, 146, 235–242. [Google Scholar] [CrossRef] [Green Version]
- Ong, Y.T.; Tan, S.H. Synthesis of the novel symmetric buckypaper supported ionic liquid membrane for the dehydration of ethylene glycol by pervaporation. Sep. Purif. Technol. 2015, 143, 135–145. [Google Scholar] [CrossRef]
- Dumée, L.F.; Sears, K.; Schutz, J.; Finn, N.; Huynh, C.; Hawkins, S.; Duke, M.; Gray, S. Characterization and evaluation of carbon nanotube Bucky-Paper membranes for direct contact membrane distillation. J. Membr. Sci. 2010, 351, 36–43. [Google Scholar] [CrossRef] [Green Version]
- Dumée, L.; Germain, V.; Sears, K.; Schutz, J.; Finn, N.; Duke, M.; Cerneaux, S.; Cornu, D.; Gray, S. Enhanced durability and hydrophobicity of carbon nanotube bucky paper membranes in membrane distillation. J. Membr. Sci. 2011, 376, 241–246. [Google Scholar] [CrossRef]
- Constantopoulos, K.T.; Shearer, C.J.; Ellis, A.V.; Voelcker, N.H.; Shapter, J.G. Carbon Nanotubes Anchored to Silicon for Device Fabrication. Adv. Mater. 2010, 22, 557–571. [Google Scholar] [CrossRef]
- Hinds, B.J.; Chopra, N.; Rantell, T.; Andrews, R.; Gavalas, V.; Bachas, L.G. Aligned Multiwalled Carbon Nanotube Membranes. Science 2004, 303, 62–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mittal, G.; Dhand, V.; Rhee, K.Y.; Park, S.-J.; Lee, W.R. A review on carbon nanotubes and graphene as fillers in reinforced polymer nanocomposites. J. Ind. Eng. Chem. 2014, 21, 11–25. [Google Scholar] [CrossRef]
- Nieto, A.; Agarwal, A.; Lahiri, D.; Bisht, A.; Bakshi, S.R. Carbon Nanotubes: Reinforced Metal Matrix Composites, 2nd ed.; Series: Nanomaterials and their applications; CRC Press: Boca Raton, FL, USA, 2021. [Google Scholar] [CrossRef]
- Choi, J.-H.; Jegal, J.; Kim, W.-N. Fabrication and characterization of multi-walled carbon nanotubes/polymer blend membranes. J. Membr. Sci. 2006, 284, 406–415. [Google Scholar] [CrossRef]
- Li, W.; Pan, F.; Song, Y.; Wang, M.; Wang, H.; Walker, S.; Wu, H.; Jiang, Z. Construction of molecule-selective mixed matrix membranes with confined mass transfer structure. Chin. J. Chem. Eng. 2017, 25, 1563–1580. [Google Scholar] [CrossRef]
- Mauter, M.S.; Elimelech, M. Environmental Applications of Carbon-Based Nanomaterials. Environ. Sci. Technol. 2008, 42, 5843–5859. [Google Scholar] [CrossRef] [PubMed]
- Arora, B.; Attri, P. Carbon Nanotubes (CNTs): A Potential Nanomaterial for Water Purification. J. Compos. Sci. 2020, 4, 135. [Google Scholar] [CrossRef]
- Das, R.; Leo, B.F.; Murphy, F. The Toxic Truth About Carbon Nanotubes in Water Purification: A Perspective View. Nanoscale Res. Lett. 2018, 13, 183–192. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Drinking-Water. Available online: https://www.who.int/news-room/fact-sheets/detail/drinking-water (accessed on 21 March 2022).
- Qasem, N.A.A.; Mohammed, R.H.; Lawal, D.U. Removal of heavy metal ions from wastewater: A comprehensive and critical review. Npj Clean Water 2021, 4, 1–15. [Google Scholar] [CrossRef]
- Abdullah, N.; Yusof, N.; Lau, W.J.; Jaafar, J.; Ismail, A.F. Recent trends of heavy metal removal from water/wastewater by membrane technologies. J. Ind. Eng. Chem. 2019, 76, 17–38. [Google Scholar] [CrossRef]
- Pabby, A.K.; Swain, B.; Sonar, N.L.; Mittal, V.K.; Valsala, T.P.; Ramsubramanian, S.; Sathe, D.B.; Bhatt, R.B.; Pradhan, S. Radioactive waste processing using membranes: State of the art technology, challenges and perspectives. Sep. Purif. Rev. 2021, 51, 143–173. [Google Scholar] [CrossRef]
- Baek, Y.; Kim, C.; Seo, D.K.; Kim, T.; Lee, J.S.; Kim, Y.H.; Ahn, K.H.; Bae, S.S.; Lee, S.C.; Lim, J.; et al. High performance and antifouling vertically aligned carbon nanotube membrane for water purification. J. Membr. Sci. 2014, 460, 171–177. [Google Scholar] [CrossRef]
- Kang, S.; Herzberg, M.; Rodrigues, D.F.; Elimelech, M. Antibacterial Effects of Carbon Nanotubes: Size Does Matter! Langmuir 2008, 24, 6409–6413. [Google Scholar] [CrossRef] [PubMed]
- Rajavel, K.; Gomathi, R.; Manian, S.; Kumar, R.T.R. In Vitro Bacterial Cytotoxicity of CNTs: Reactive Oxygen Species Mediate Cell Damage Edges over Direct Physical Puncturing. Langmuir 2014, 30, 592–601. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Mikoryak, C.; Li, S.; Bushdiecker, I.D.; Musselman, I.H.; Pantano, P.; Draper, R.K. Cytotoxicity Screening of Single-Walled Carbon Nanotubes: Detection and Removal of Cytotoxic Contaminants from Carboxylated Carbon Nanotubes. Mol. Pharm. 2011, 8, 1351–1361. [Google Scholar] [CrossRef] [Green Version]
- Tiraferri, A.; Vecitis, C.D.; Elimelech, M. Covalent Binding of Single-Walled Carbon Nanotubes to Polyamide Membranes for Antimicrobial Surface Properties. ACS Appl. Mater. Interfaces 2011, 3, 2869–2877. [Google Scholar] [CrossRef]
- Chen, Z.; Ziegler, K.J.; Shaver, J.; Hauge, R.H.; Smalley, R.E. Cutting of Single-Walled Carbon Nanotubes by Ozonolysis. J. Phys. Chem. B 2006, 110, 11624–11627. [Google Scholar] [CrossRef]
- Li, M.; Boggs, M.; Beebe, T.P.; Huang, C. Oxidation of single-walled carbon nanotubes in dilute aqueous solutions by ozone as affected by ultrasound. Carbon 2008, 46, 466–475. [Google Scholar] [CrossRef]
- Wang, W.; Zhu, L.; Shan, B.; Xie, C.; Liu, C.; Cui, F.; Li, G. Preparation and characterization of SLS-CNT/PES ultrafiltration membrane with antifouling and antibacterial properties. J. Membr. Sci. 2018, 548, 459–469. [Google Scholar] [CrossRef]
- Shi, H.; Liu, H.; Luan, S.; Shi, D.; Yan, S.; Liu, C.; Li, R.K.Y.; Yin, J. Effect of polyethylene glycol on the antibacterial properties of polyurethane/carbon nanotube electrospun nanofibers. RSC Adv. 2016, 6, 19238–19244. [Google Scholar] [CrossRef]
- Greenlee, L.F.; Lawler, D.F.; Freeman, B.D.; Marrot, B.; Moulin, P. Reverse osmosis desalination: Water sources, technology, and today’s challenges. Water Res. 2009, 43, 2317–2348. [Google Scholar] [CrossRef]
- Wu, Y.-L.; Li, Q.-W.; Zhang, X.-L.; Chen, X.; Wang, X.-M. Glucose biosensor based on new carbon nanotube–gold–titania nano-composites modified glassy carbon electrode. Chin. Chem. Lett. 2013, 24, 1087–1090. [Google Scholar] [CrossRef]
- Xu, Q.; Liu, Y.; Wang, Y.; Song, Y.; Zhao, C.; Han, L. Synergistic oxidation-filtration process of electroactive peroxydisulfate with a cathodic composite CNT-PPy/PVDF ultrafiltration membrane. Water Res. 2022, 210, 117971. [Google Scholar] [CrossRef] [PubMed]
- Yanez, J.E.Y.; Wang, Z.; Lege, S.; Obst, M.; Roehler, S.; Burkhardt, C.J.; Zwiener, C. Application and characterization of electroactive membranes based on carbon nanotubes and zerovalent iron nanoparticles. Water Res. 2017, 108, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wu, P.; Liu, F.; Li, F.; An, X.; Liu, J.; Wang, Z.; Shen, C.; Sand, W. Electroactive Modified Carbon Nanotube Filter for Simultaneous Detoxification and Sequestration of Sb(III). Environ. Sci. Technol. 2019, 53, 1527–1535. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, F.; Ding, N.; Shen, C.; Li, F.; Dong, L.; Huang, M.; Yang, B.; Wang, Z.; Sand, W. Boosting Cr(VI) detoxification and sequestration efficiency with carbon nanotube electrochemical filter functionalized with nanoscale polyaniline: Performance and mechanism. Sci. Total Environ. 2019, 695, 133926. [Google Scholar] [CrossRef]
- Gong, K.; Hu, Q.; Xiao, Y.; Cheng, X.; Liu, H.; Wang, N.; Qiu, B.; Guo, Z. Triple layered core–shell ZVI@carbon@polyaniline composite enhanced electron utilization in Cr(vi) reduction. J. Mater. Chem. A 2018, 6, 11119–11128. [Google Scholar] [CrossRef]
- Ding, J.; Pu, L.; Wang, Y.; Wu, B.; Yu, A.; Zhang, X.; Pan, B.; Zhang, Q.; Gao, G. Adsorption and Reduction of Cr(VI) Together with Cr(III) Sequestration by Polyaniline Confined in Pores of Polystyrene Beads. Environ. Sci. Technol. 2018, 52, 12602–12611. [Google Scholar] [CrossRef]
- Sheikholeslami, R.; Bright, J. Silica and metals removal by pretreatment to prevent fouling of reverse osmosis membranes. Desalination 2002, 143, 255–267. [Google Scholar] [CrossRef]
- Al-Abri, M.; Al-Ghafri, B.; Bora, T.; Dobretsov, S.; Dutta, J.; Castelletto, S.; Rosa, L.; Boretti, A. Chlorination disadvantages and alternative routes for biofouling control in reverse osmosis desalination. Npj Clean Water 2019, 2, 2. [Google Scholar] [CrossRef] [Green Version]
- Trivedi, S.; Alameh, K. Effect of vertically aligned carbon nanotube density on the water flux and salt rejection in desalination membranes. SpringerPlus 2016, 5, 1158. [Google Scholar] [CrossRef] [Green Version]
- Cavas, L.; Yildiz, P.G.; Mimigianni, P.; Sapalidis, A.; Nitodas, S. Reinforcement effects of multiwall carbon nanotubes and graphene oxide on PDMS marine coatings. J. Coat. Technol. Res. 2018, 15, 105–120. [Google Scholar] [CrossRef]
- Sekitani, T.; Zschieschang, U.; Klauk, H.; Someya, T. Flexible organic transistors and circuits with extreme bending stability. Nat. Mater. 2010, 9, 1015–1022. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.; Corry, B. A computational assessment of the permeability and salt rejection of carbon nanotube membranes and their application to water desalination. Philos. Trans. R. Soc. London. Ser. A Math. Phys. Eng. Sci. 2016, 374, 20150020. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Lee, B.; Kim, Y. High performance reverse osmosis membrane with carbon nanotube support layer. J. Membr. Sci. 2019, 592, 117358. [Google Scholar] [CrossRef]
- Corry, B. Water and ion transport through functionalised carbon nanotubes: Implications for desalination technology. Energy Environ. Sci. 2011, 4, 751–759. [Google Scholar] [CrossRef]
- Lee, H.D.; Kim, H.W.; Cho, Y.H.; Park, H.B. Experimental Evidence of Rapid Water Transport through Carbon Nanotubes Embedded in Polymeric Desalination Membranes. Small 2014, 10, 2653–2660. [Google Scholar] [CrossRef]
- Tortello, M.; Bianco, S.; Ijeri, V.; Spinelli, P.; Tresso, E. Nafion membranes with vertically-aligned CNTs for mixed proton and electron conduction. J. Membr. Sci. 2012, 415–416, 346–352. [Google Scholar] [CrossRef]
- Luo, C.; Zuo, X.; Wang, L.; Wang, E.; Song, S.; Wang, J.; Wang, J.; Fan, C.; Cao, Y. Flexible Carbon Nanotube−Polymer Composite Films with High Conductivity and Superhydrophobicity Made by Solution Process. Nano Lett. 2008, 8, 4454–4458. [Google Scholar] [CrossRef]
- Pilgrim, G.A.; Leadbetter, J.W.; Qiu, F.; Siitonen, A.J.; Pilgrim, S.M.; Krauss, T.D. Electron Conductive and Proton Permeable Vertically Aligned Carbon Nanotube Membranes. Nano Lett. 2014, 14, 1728–1733. [Google Scholar] [CrossRef]
- Wang, S.; Li, X.; Wu, H.; Tian, Z.; Xin, Q.; He, G.; Peng, D.; Chen, S.; Yin, Y.; Jiang, Z.; et al. Advances in high permeability polymer-based membrane materials for CO2 separations. Energy Environ. Sci. 2016, 9, 1863–1890. [Google Scholar] [CrossRef]
- Lin, H.; He, Z.; Sun, Z.; Vu, J.; Ng, A.; Mohammed, M.; Kniep, J.; Merkel, T.C.; Wu, T.; Lambrecht, R.C. CO2-selective membranes for hydrogen production and CO2 capture–Part I: Membrane development. J. Membr. Sci. 2014, 457, 149–161. [Google Scholar] [CrossRef]
- Song, Q.; Jiang, S.; Hasell, T.; Liu, M.; Sun, S.; Cheetham, A.K.; Sivaniah, E.; Cooper, A.I. Porous Organic Cage Thin Films and Molecular-Sieving Membranes. Adv. Mater. 2016, 28, 2629–2637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sears, K.; Dumée, L.; Schütz, J.; She, M.; Huynh, C.; Hawkins, S.; Duke, M.; Gray, S. Recent Developments in Carbon Nanotube Membranes for Water Purification and Gas Separation. Materials 2010, 3, 127–149. [Google Scholar] [CrossRef] [Green Version]
- Yang, D.; Tian, D.; Cheng, C.; Liu, Y.; Zhao, Z.; Liu, Y.; Bao, Y.; Xue, C. Carbon nanotube arrays hybrid membrane with excellent separation performance and conductivity. J. Membr. Sci. 2020, 620, 118874. [Google Scholar] [CrossRef]
- Li, L.; Song, C.; Jiang, D.; Wang, T. Preparation and enhanced gas separation performance of Carbon/Carbon nanotubes (C/CNTs) hybrid membranes. Sep. Purif. Technol. 2017, 188, 73–80. [Google Scholar] [CrossRef]
- Zhang, H.; Guo, R.; Hou, J.; Wei, Z.; Li, X. Mixed-Matrix Membranes Containing Carbon Nanotubes Composite with Hydrogel for Efficient CO2 Separation. ACS Appl. Mater. Interfaces 2016, 8, 29044–29051. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.M.; Filiz, V.; Bengtson, G.; Shishatskiy, S.; Rahman, M.; Abetz, V. Functionalized carbon nanotubes mixed matrix membranes of polymers of intrinsic microporosity for gas separation. Nanoscale Res. Lett. 2012, 7, 504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guiver, M.D.; Yahia, M.; Dal-Cin, M.M.; Robertson, G.P.; Garakani, S.S.; Du, N.; Tavajohi, N. Gas Transport in a Polymer of Intrinsic Microporosity (PIM-1) Substituted with Pseudo-Ionic Liquid Tetrazole-Type Structures. Macromolecules 2020, 53, 8951–8959. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, S.; Wang, C.; Chang, H.-C.; Guo, R. Carbon nanotube-based mixed-matrix membranes with supramolecularly engineered interface for enhanced gas separation performance. J. Membr. Sci. 2019, 598, 117794. [Google Scholar] [CrossRef]
- Robeson, L.M. The upper bound revisited. J. Membr. Sci. 2008, 320, 390–400. [Google Scholar] [CrossRef]
- Jia, G.; Wang, H.; Yan, L.; Wang, X.; Pei, R.; Yan, T.; Zhao, Y.; Guo, X. Cytotoxicity of Carbon Nanomaterials: Single-Wall Nanotube, Multi-Wall Nanotube, and Fullerene. Environ. Sci. Technol. 2005, 39, 1378–1383. [Google Scholar] [CrossRef] [PubMed]
- Koyama, S.; Endo, M.; Kim, Y.A.; Hayashi, T.; Yanagisawa, T.; Osaka, K.; Koyama, H.; Haniu, H.; Kuroiwa, N. Role of systemic T-cells and histopathological aspects after subcutaneous implantation of various carbon nanotubes in mice. Carbon 2006, 44, 1079–1092. [Google Scholar] [CrossRef] [Green Version]
- Francis, A.P.; Devasena, T. Toxicity of carbon nanotubes: A review. Toxicol. Ind. Health 2018, 34, 200–210. [Google Scholar] [CrossRef] [PubMed]
- Berber, M.R. Current Advances of Polymer Composites for Water Treatment and Desalination. J. Chem. 2020, 2020, 7608423. [Google Scholar] [CrossRef] [Green Version]
- Ghadiri, M.; Fakhri, S.; Shirazian, S. Modeling of water transport through nanopores of membranes in direct-contact membrane distillation process. Polym. Eng. Sci. 2013, 54, 660–666. [Google Scholar] [CrossRef]
- Roy, K.; Mukherjee, A.; Maddela, N.R.; Chakraborty, S.; Shen, B.; Li, M.; Du, D.; Peng, Y.; Lu, F.; Cruzatty, L.C.G. Outlook on the bottleneck of carbon nanotube in desalination and membrane-based water treatment—A review. J. Environ. Chem. Eng. 2020, 8, 103572. [Google Scholar] [CrossRef]
- Mehra, N.K.; Jain, A.K.; Lodhi, N.; Raj, R.; Dubey, V.; Mishra, D.; Nahar, M.; Jain, N.K. Challenges in the use of carbon nanotubes for biomedical applications. Crit. Rev. Ther. Drug Carr. Syst. 2008, 25, 169–206. [Google Scholar] [CrossRef]
- Tofighy, M.A.; Shirazi, Y.; Mohammadi, T.; Pak, A. Salty water desalination using carbon nanotubes membrane. Chem. Eng. J. 2011, 168, 1064–1072. [Google Scholar] [CrossRef]
- Pei, B.; Wang, W.; Dunne, N.; Li, X. Applications of Carbon Nanotubes in Bone Tissue Regeneration and Engineering: Superiority, Concerns, Current Advancements, and Prospects. Nanomaterials 2009, 9, 1501. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Hu, X.; Jiang, S.; Wang, Y.; Parungao, R.; Zheng, S.; Nie, Y.; Liu, T.; Song, K. The Application of Multi-Walled Carbon Nanotubes in Bone Tissue Repair Hybrid Scaffolds and the Effect on Cell Growth In Vitro. Polymers 2019, 11, 230. [Google Scholar] [CrossRef] [Green Version]
- Ma, L.; Dong, X.; Chen, M.; Zhu, L.; Wang, C.; Yang, F.; Dong, Y. Fabrication and Water Treatment Application of Carbon Nanotubes (CNTs)-Based Composite Membranes: A Review. Membranes 2017, 7, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, M.; Ando, Y. Chemical Vapor Deposition of Carbon Nanotubes: A Review on Growth Mechanism and Mass Production. J. Nanosci. Nanotechnol. 2010, 10, 3739–3758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.; Fornasiero, F.; Park, H.G.; Bin In, J.; Meshot, E.; Giraldo, G.; Stadermann, M.; Fireman, M.; Shan, J.; Grigoropoulos, C.P.; et al. Fabrication of flexible, aligned carbon nanotube/polymer composite membranes by in-situ polymerization. J. Membr. Sci. 2014, 460, 91–98. [Google Scholar] [CrossRef]
- Bassyouni, M.; Abdel-Aziz, M.H.; Zoromba, M.S.; Abdel-Hamid, S.M.S.; Drioli, E. A review of polymeric nanocomposite membranes for water purification. J. Ind. Eng. Chem. 2019, 73, 19–46. [Google Scholar] [CrossRef]
- Li, X.; Sotto, A.; Li, J.; Van der Bruggen, B. Progress and perspectives for synthesis of sustainable antifouling composite membranes containing in situ generated nanoparticles. J. Membr. Sci. 2017, 524, 502–528. [Google Scholar] [CrossRef]
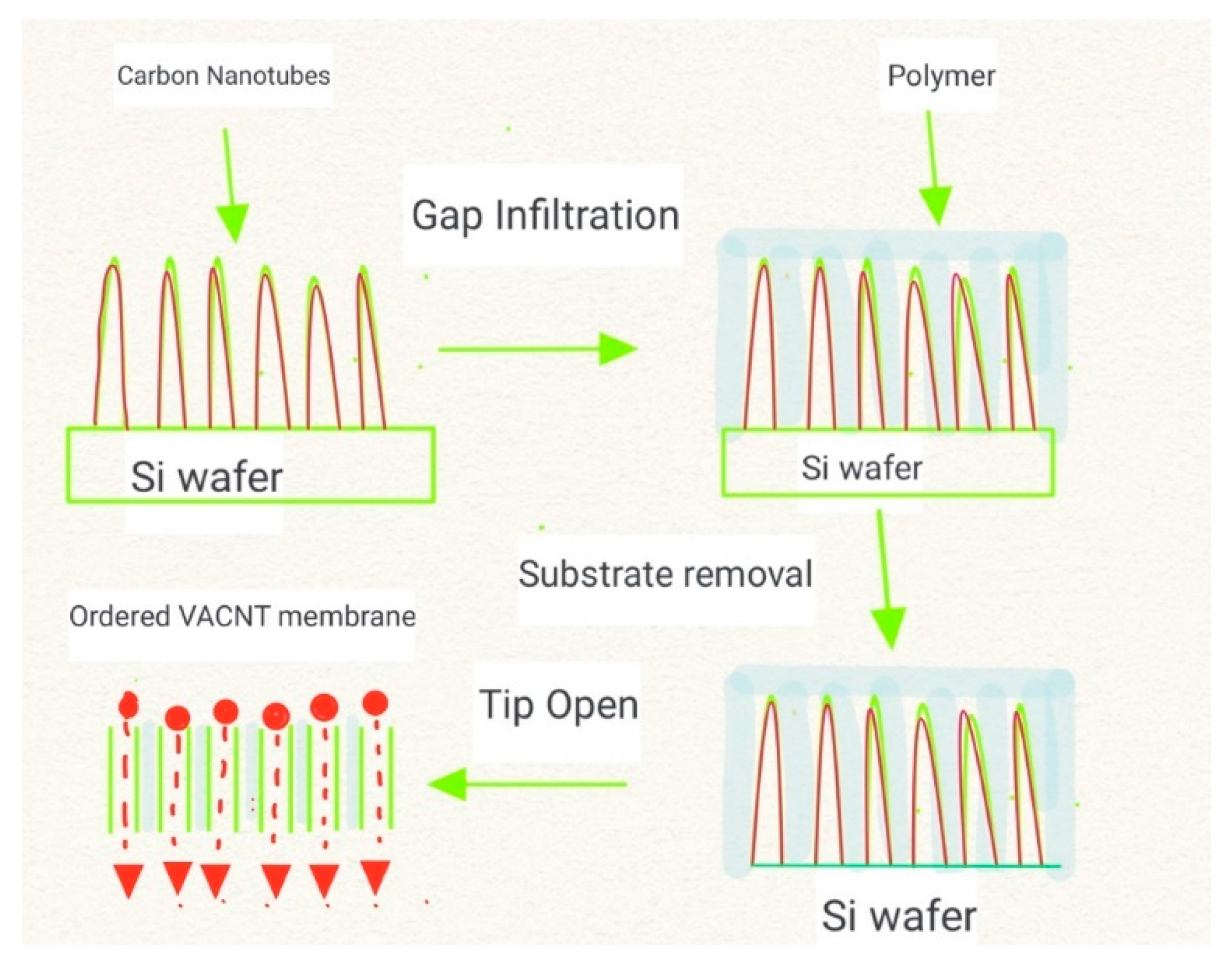
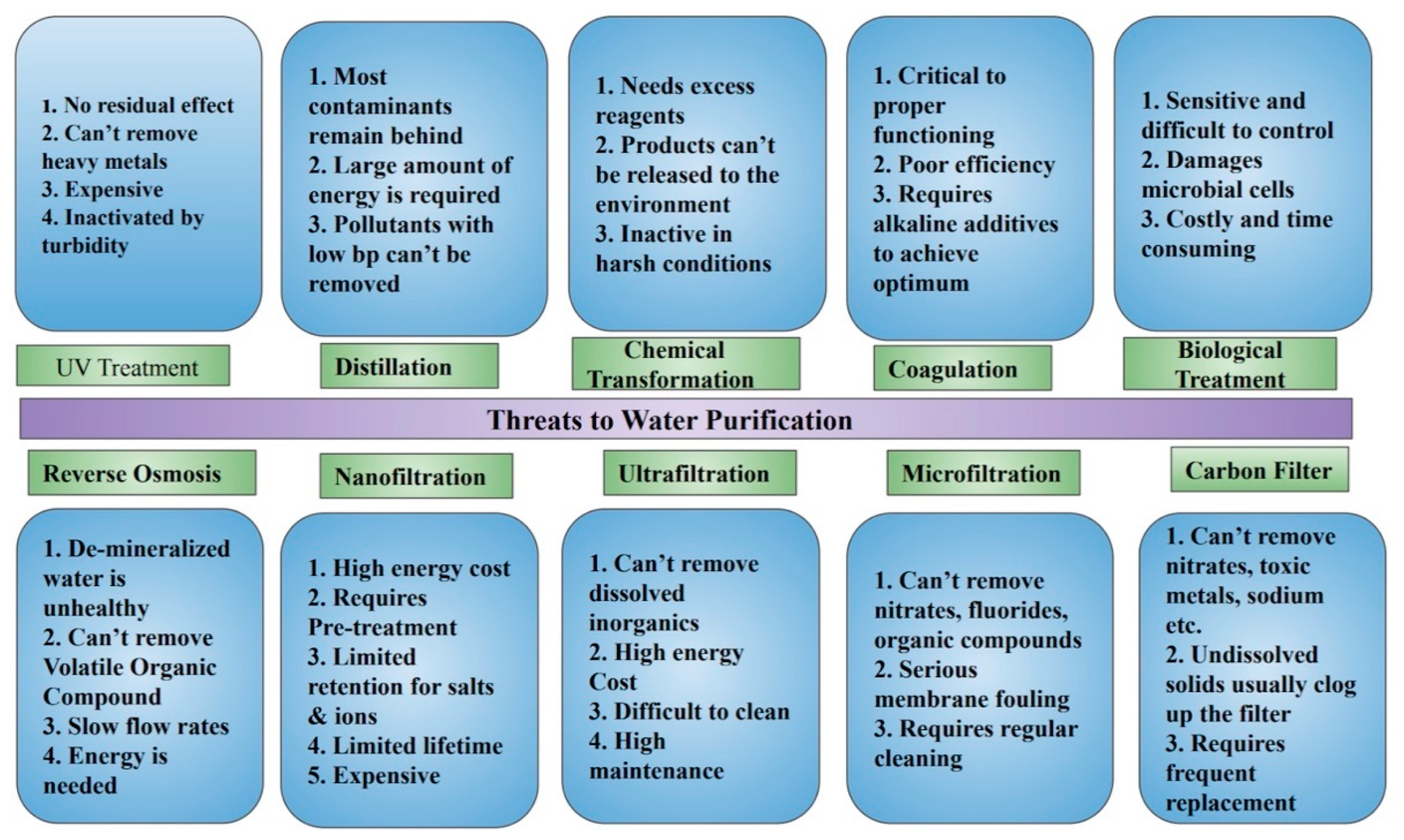

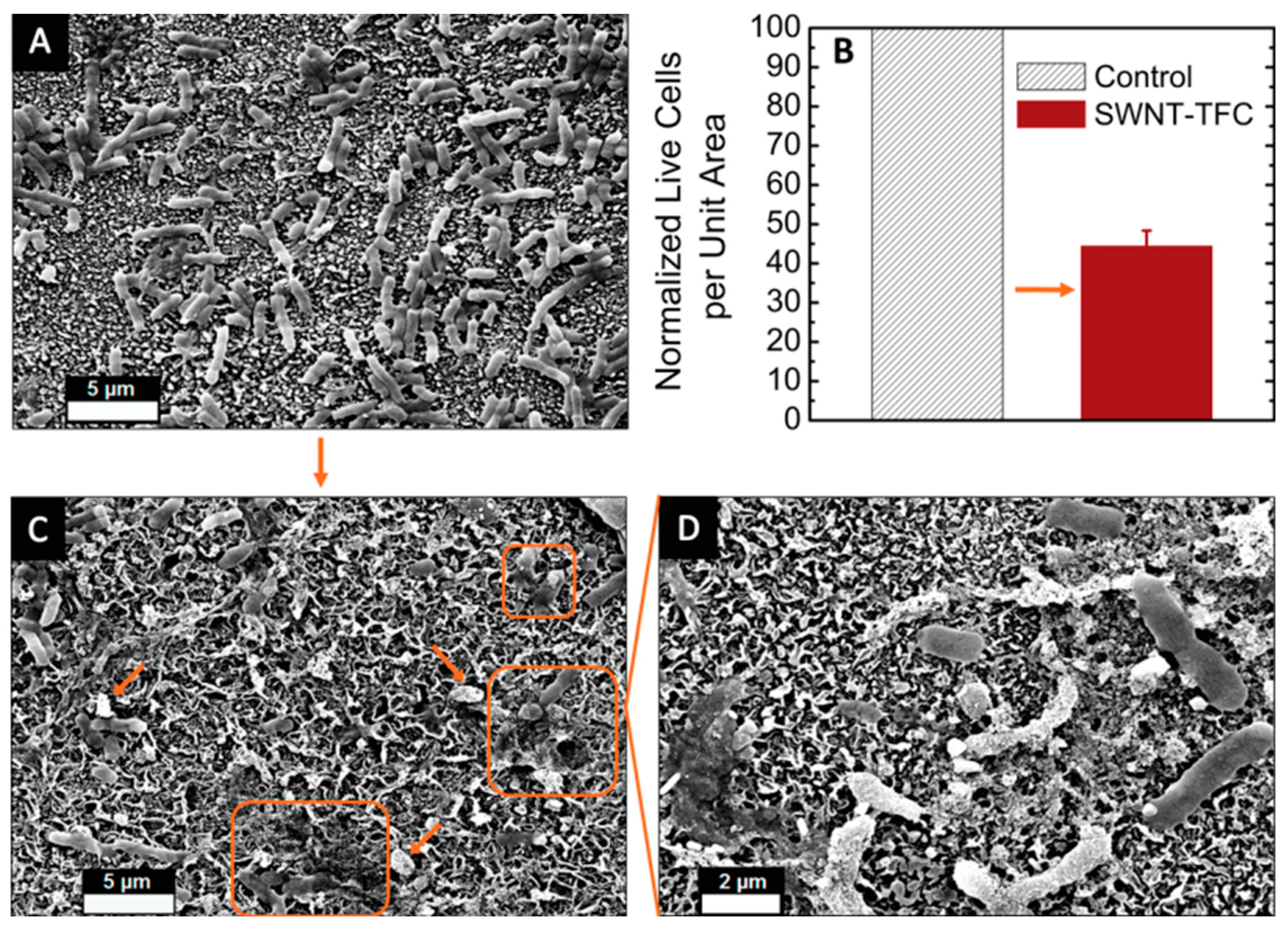
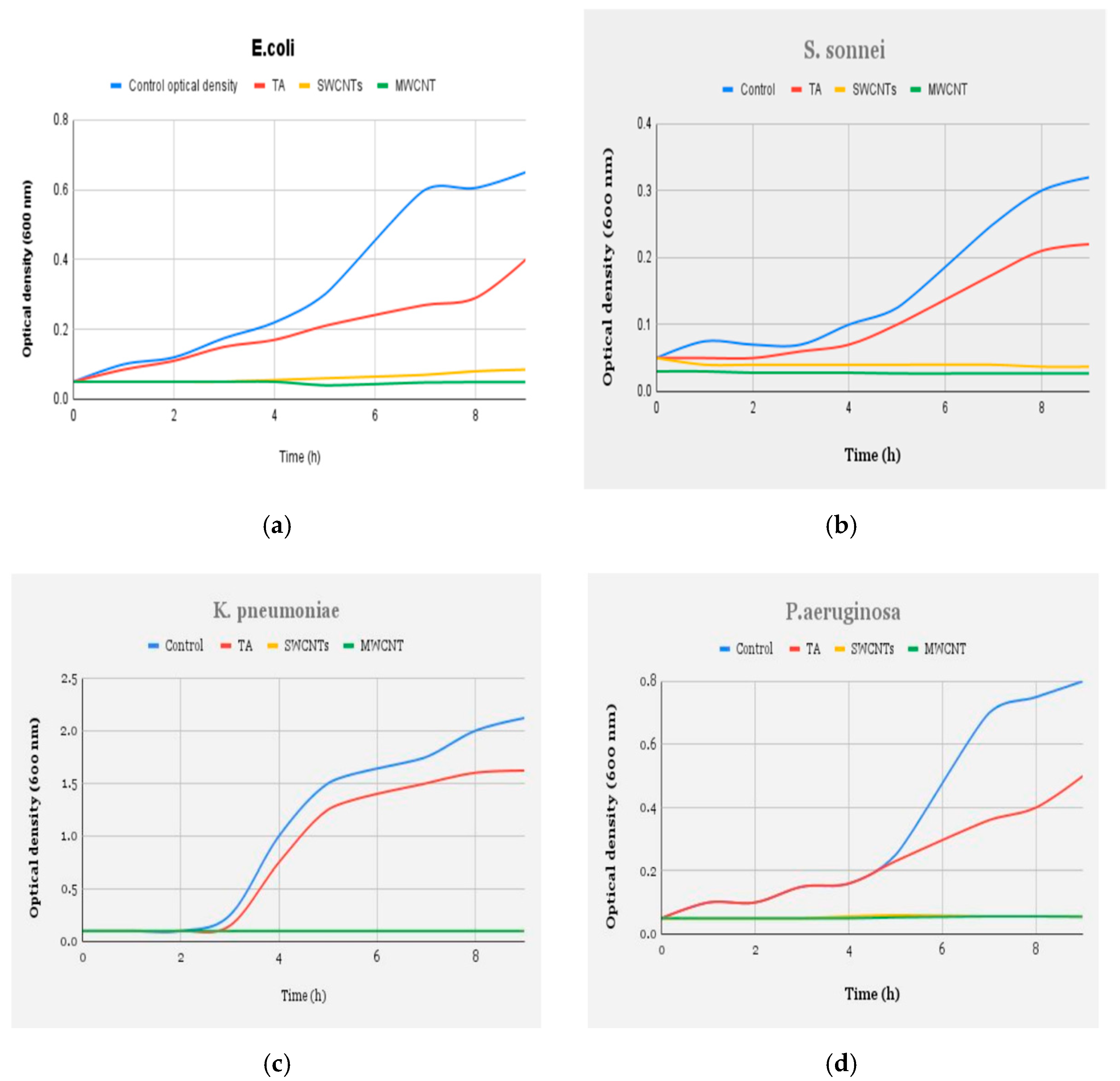
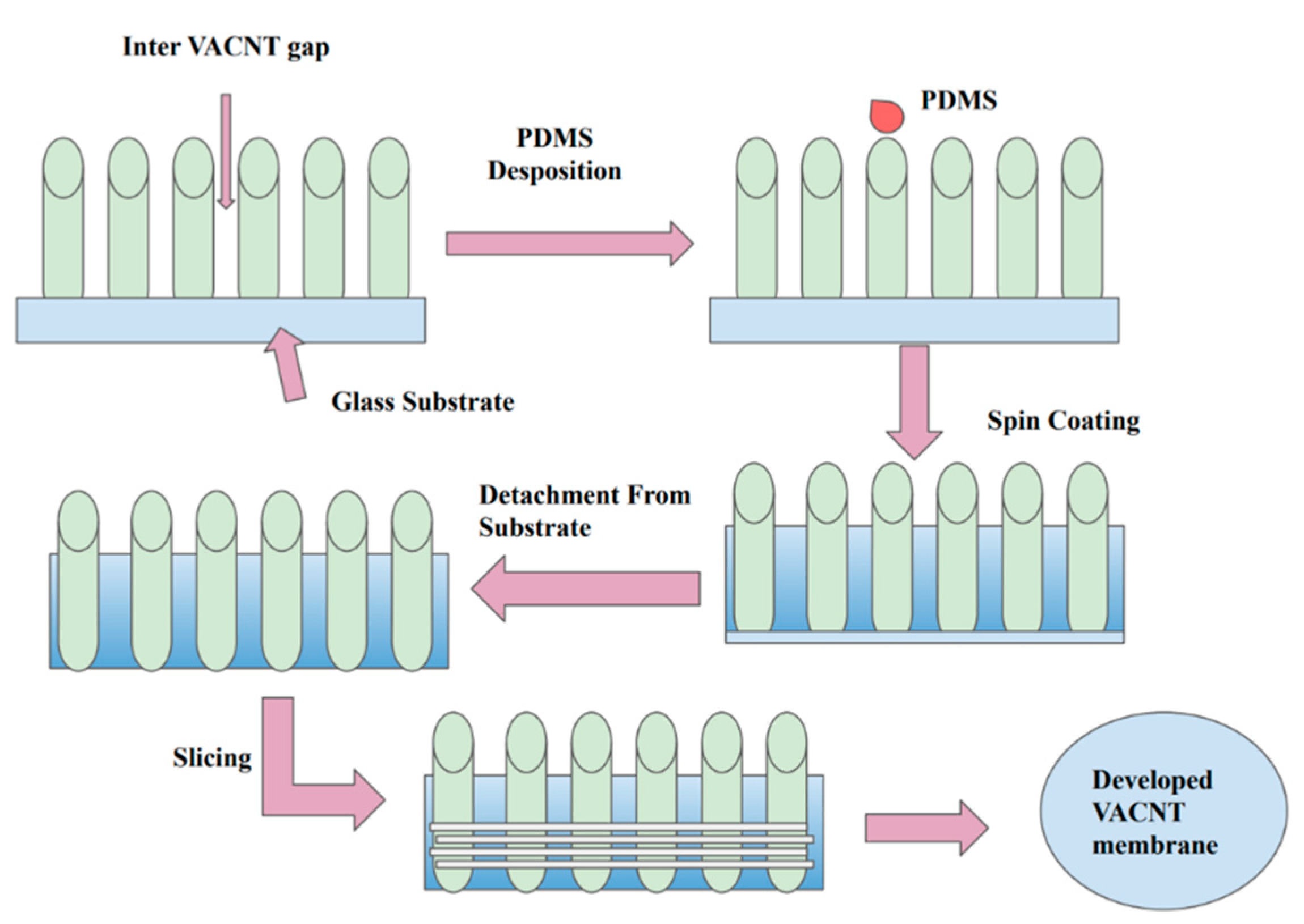
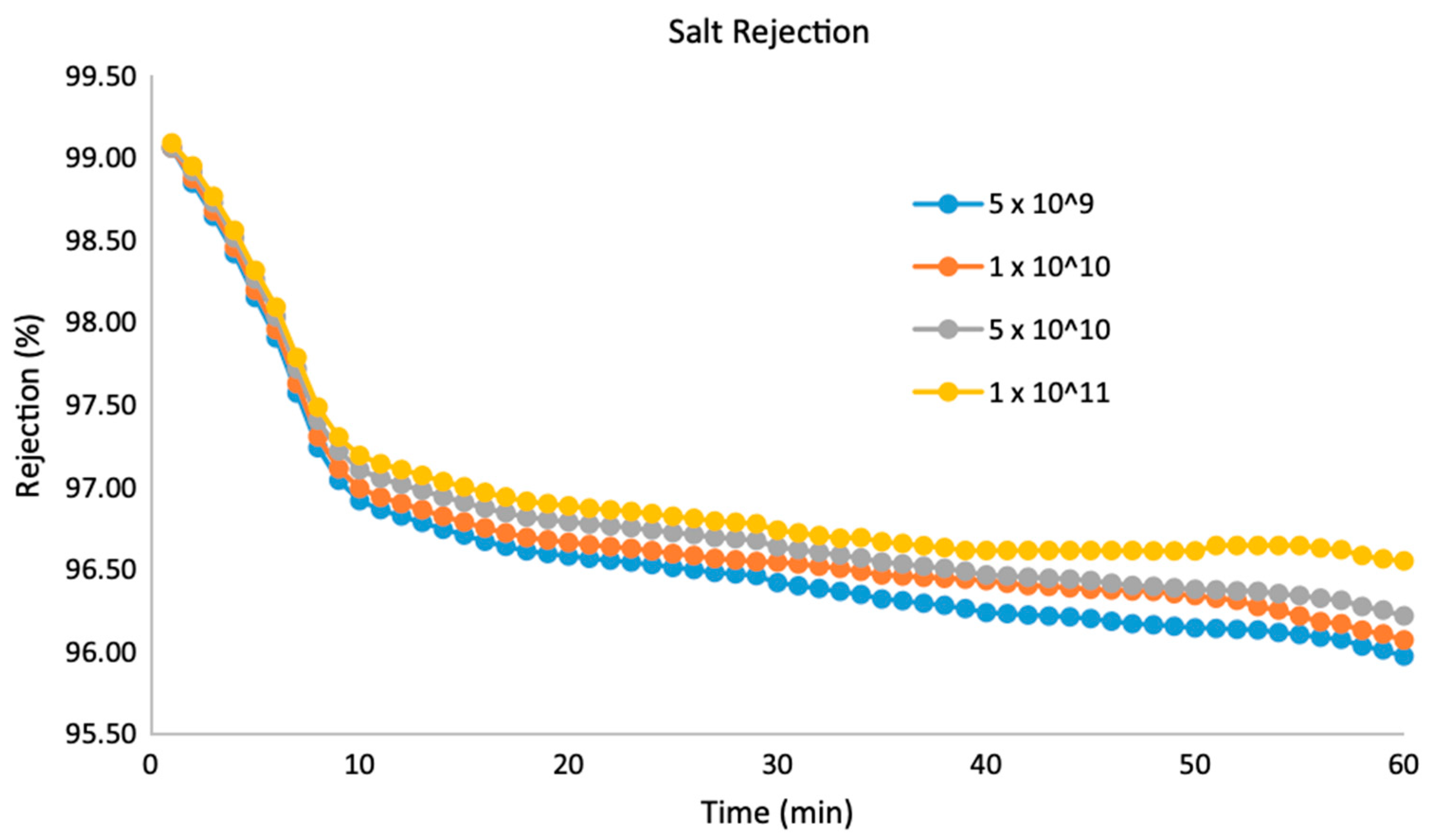
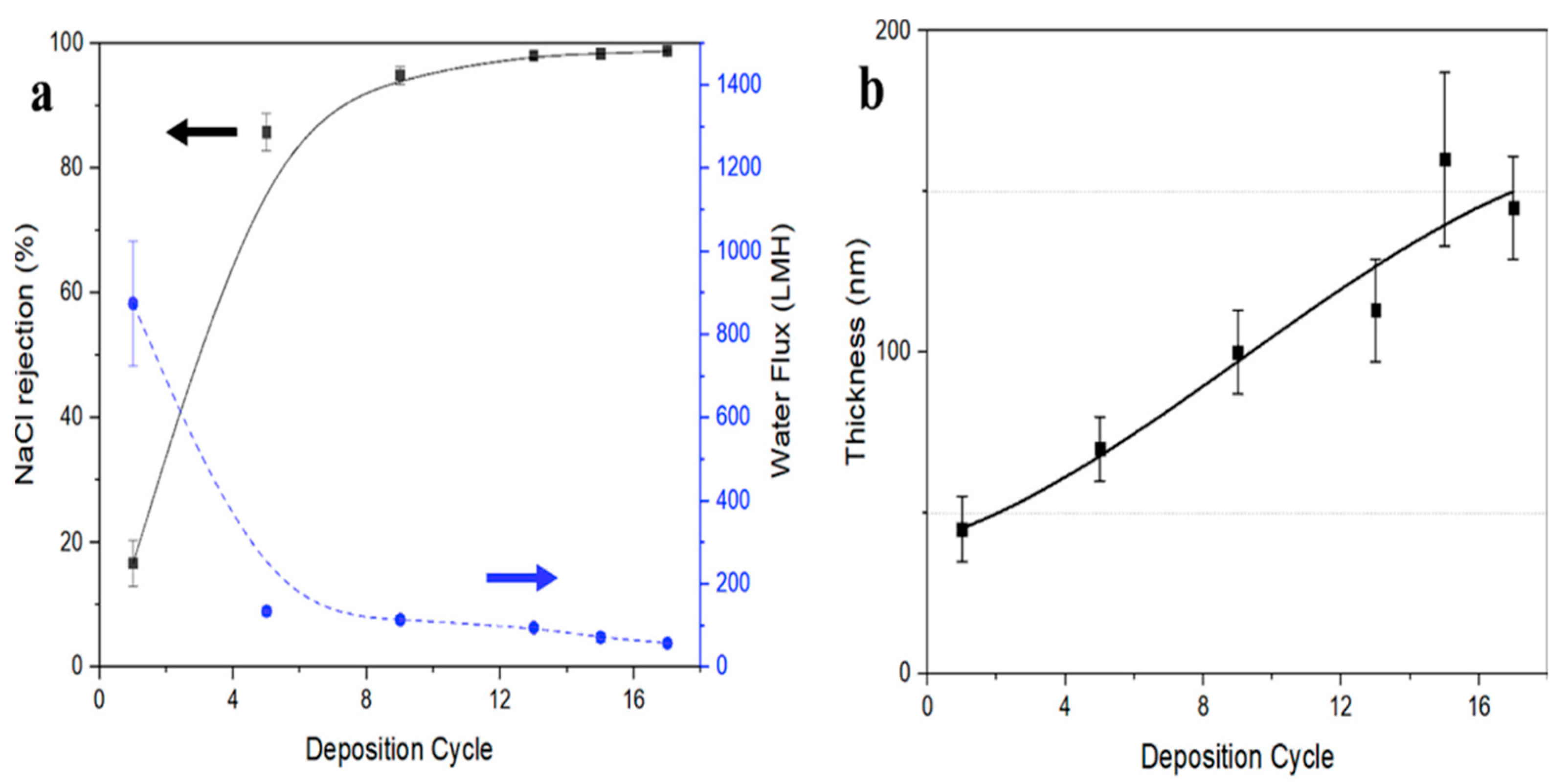
| Pure CNT (VA-CNT) | Mixed-Matrix CNT Membrane |
|---|---|
| Vertical CNT arrangement | Mixed CNT arrangement |
| Compact CNT network | CNT network loosely fit |
| The water flux rate is high | Water flux rate is moderately high |
| Complicated fabrication | Simple fabrication |
| VA-CNT Membrane | UF Membrane | |
|---|---|---|
| Material | CNT + epoxy | Polysulfone |
| Avg. pore diameter | 4.8 ± 0.9 | 5.7 ± 2.5 |
| Pore density (#/cm2) | 6.8 × 10¹⁰ | 8.8 × 10¹⁰ |
| Thickness (μm) | ∼200 | ∼0.1 |
| Electron Conductivity by EIS (mS/cm) | Electron Conductivity by EIS (Wet Condition) | Proton Conductivity by EIS (mS/cm) | Proton Conductivity by EIS (Wet Condition) | |
|---|---|---|---|---|
| Nafion/CNTS nanocomposite, CNTs 5 wt% | 0.364 | 0.197 | 2 | 5.1 |
| VACNTs/Nafion, CNT 7 wt% | 0.568 | 0.315 | 2 | 8.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nitodas, S.F.; Das, M.; Shah, R. Applications of Polymeric Membranes with Carbon Nanotubes: A Review. Membranes 2022, 12, 454. https://doi.org/10.3390/membranes12050454
Nitodas SF, Das M, Shah R. Applications of Polymeric Membranes with Carbon Nanotubes: A Review. Membranes. 2022; 12(5):454. https://doi.org/10.3390/membranes12050454
Chicago/Turabian StyleNitodas, Steve F., Mrinaleni Das, and Raj Shah. 2022. "Applications of Polymeric Membranes with Carbon Nanotubes: A Review" Membranes 12, no. 5: 454. https://doi.org/10.3390/membranes12050454
APA StyleNitodas, S. F., Das, M., & Shah, R. (2022). Applications of Polymeric Membranes with Carbon Nanotubes: A Review. Membranes, 12(5), 454. https://doi.org/10.3390/membranes12050454








