Plasma Polymerized Coatings on Hollow Fiber Membranes-Applications and Their Aging Characteristics in Different Media
Abstract
:1. Introduction
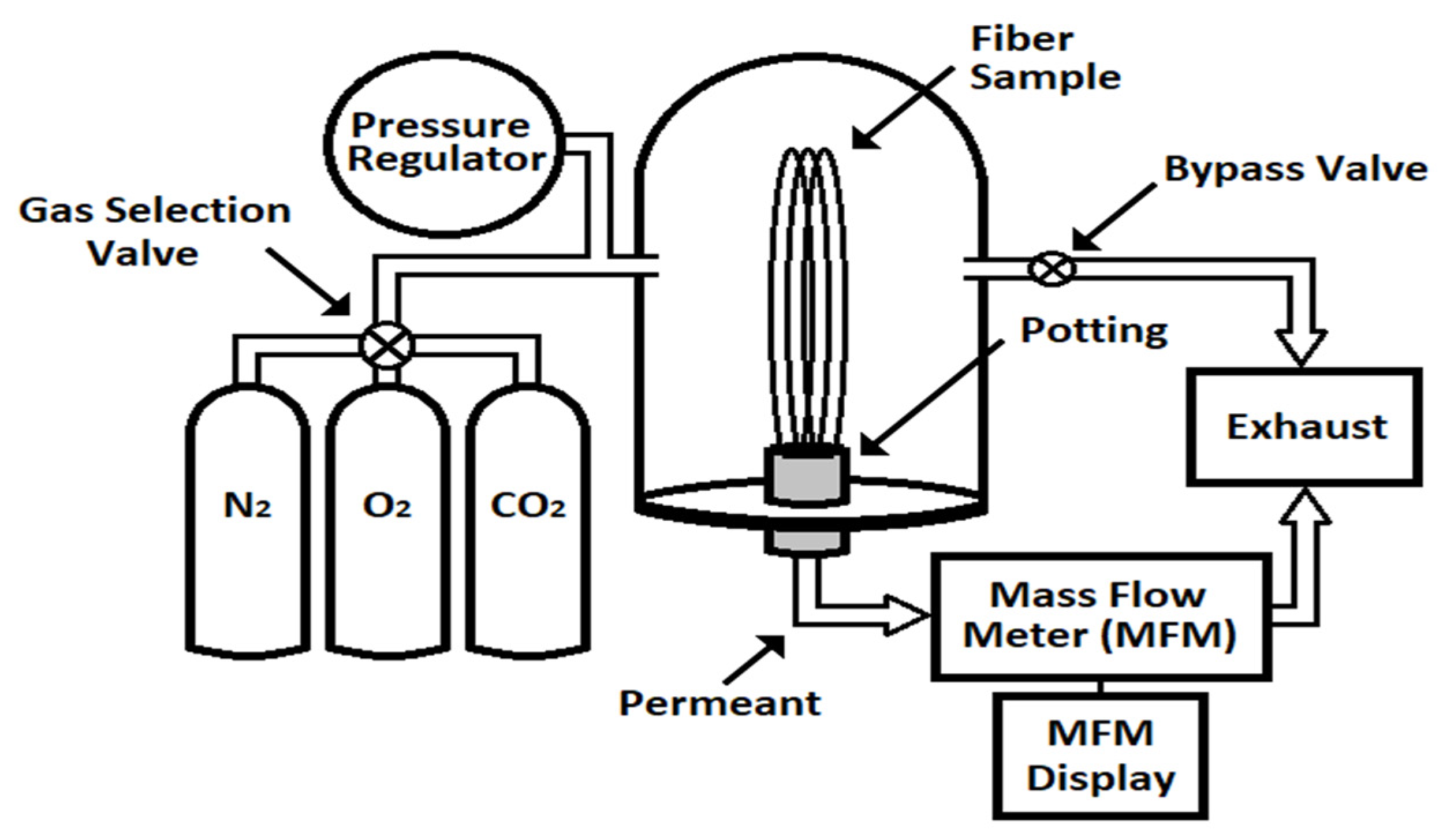
2. Experimental
2.1. Materials and Methods
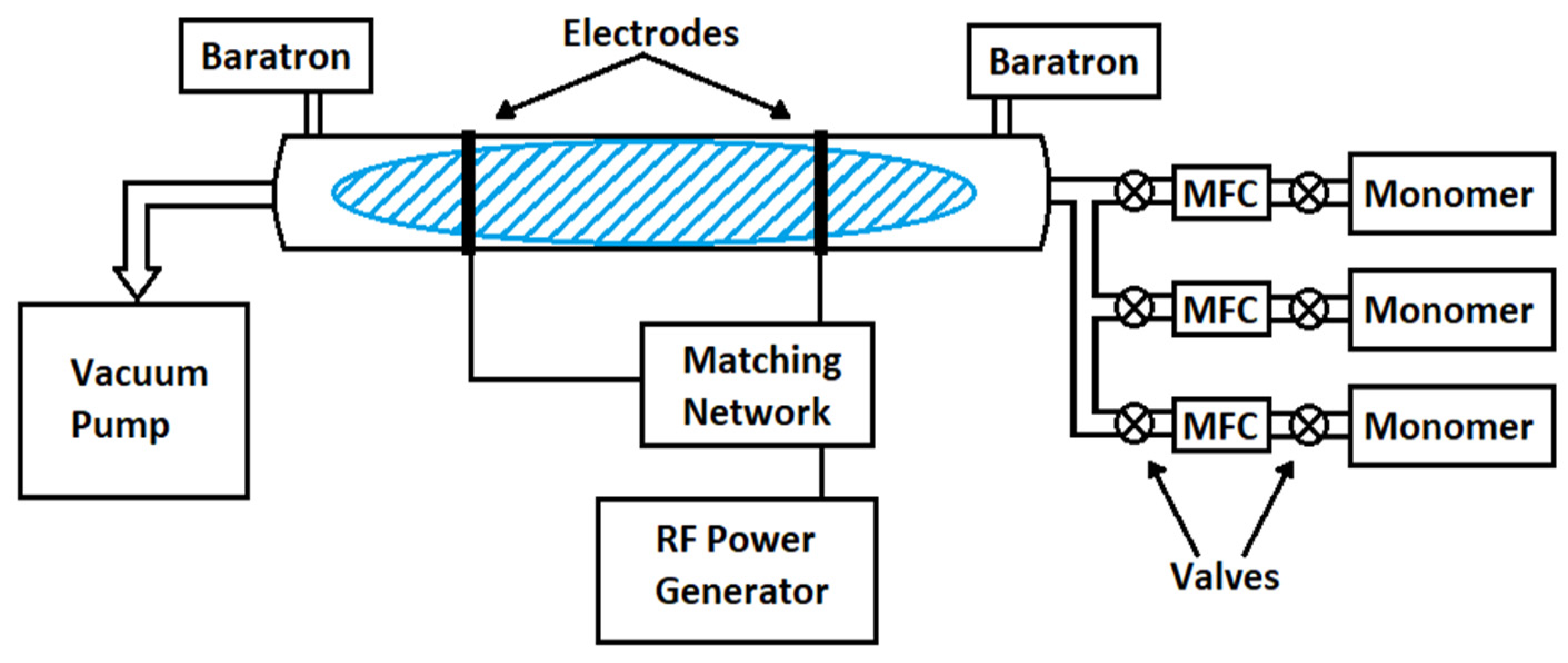
2.2. Plasma Polymerization Process
3. Results and Discussion
3.1. Pore Size Control Using Plasma Polymerization
3.2. Thickness of Plasma Polymer Coatings
3.3. Results of Field Emission Scanning Electron Microscopy (FESEM)
3.4. Contact Angle with Water
3.5. Tensile Properties
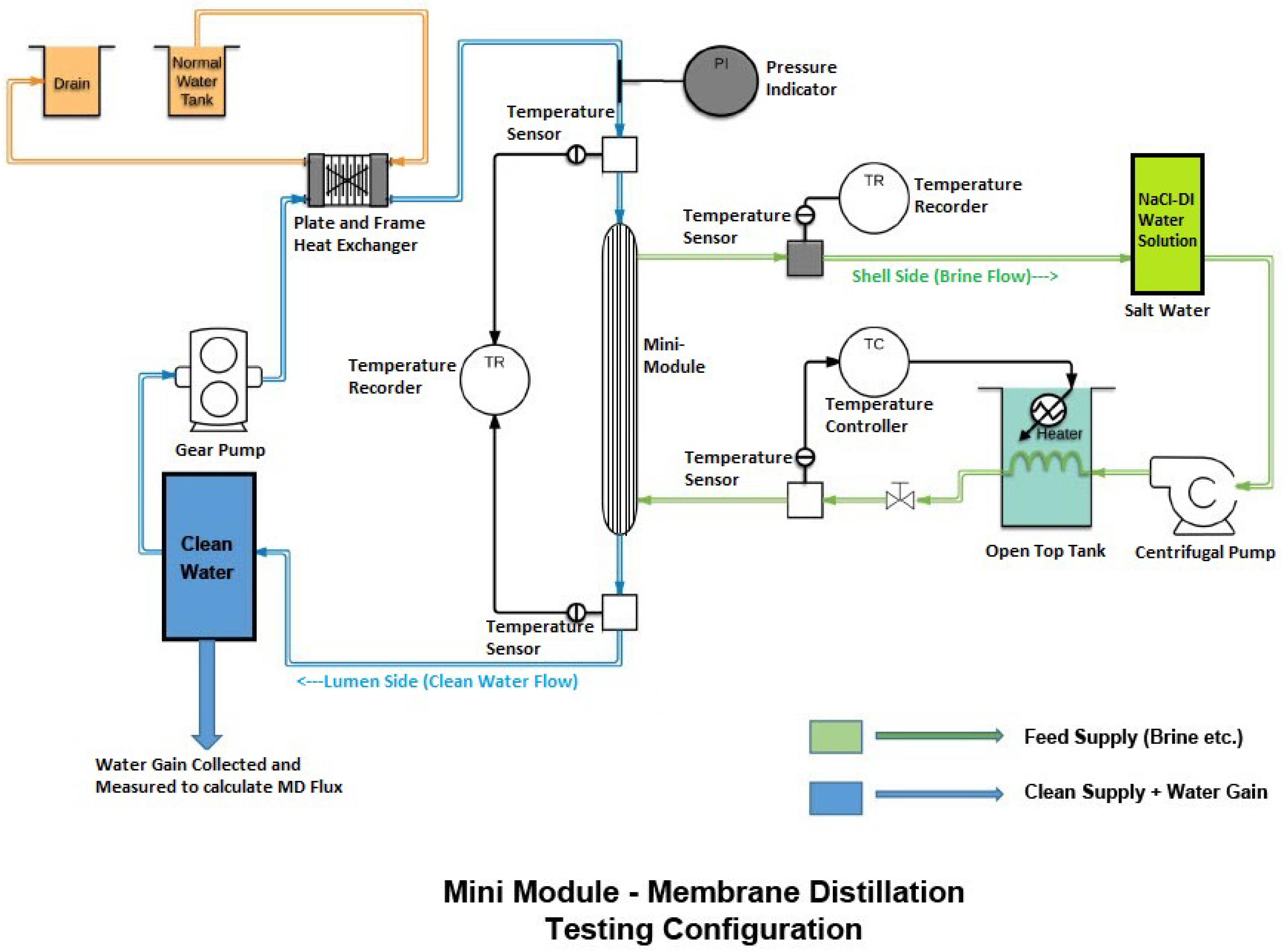
3.6. Development of Membranes for VOC Removal, Pervaporation and Direct Contact Membrane Distillation (DCMD)
3.7. Semipermeable Membranes from Plasma Polymerization and Their Aging Characteristics in Air
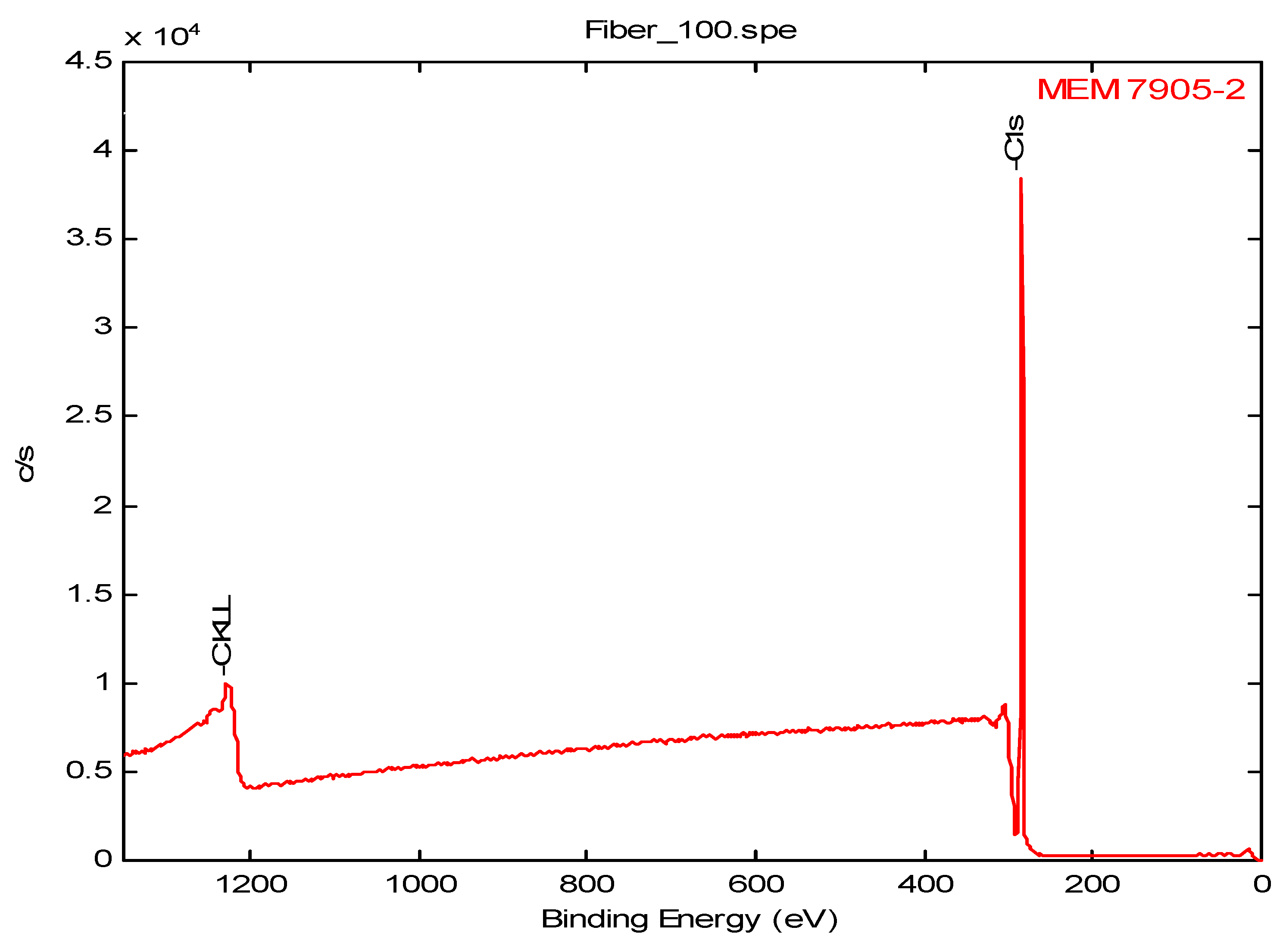
3.8. XPS Data
3.9. Heat Aging
3.10. Aging in Contact with Chemicals
3.10.1. Aging in Buffered Saline
3.10.2. Aging in Contact with Alkaline solutions and Organics
3.11. Aging in Ionized Air
4. Concluding Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yasuda, H. Glow Discharge Polymerization. J. Polym. Sci. Macromol. Rev. 1981, 16, 199–293. [Google Scholar] [CrossRef]
- Inagaki, N. Plasma Surface Modification and Plasma Polymerization; CRC Press: Lancaster, PA, USA; Technomic Publishing Co Inc.: Lancaster, PA, USA, 1996; ISBN 978-156676-337-0. [Google Scholar]
- Akhavan, B.; Jarvis, K.; Majewski, P. Hydrophobic Plasma Polymer Coated Silica Particles for Petroleum Hydrocarbon Removal. ACS Appl. Mater. Interfaces 2013, 5, 8563–8571. [Google Scholar] [CrossRef] [PubMed]
- Zang, Z. Surface Modification by Plasma Polymerization and Application on Plasma Polymers as Biomaterials. Ph.D. Thesis, Johanneses Gutenberg University of Mainz, Mainz, Germany, 2003. [Google Scholar]
- Hegemann, D. Plasma polymerization and its application in textiles. Indian J. Fiber Text. 2006, 31, 99–115. [Google Scholar]
- Zheng, Z.; Wang, W.; Xin, H.; Wenling, F.; Lei, L. Surface modification of polysulfone hollow fiber membrane oxygenator using low-temperature plasma treatment. Plasma Process. Polym. 2017, 15, 1700122. [Google Scholar] [CrossRef]
- Saavedra-Romero, R.; Paz, F.; Litell, J.M.; Weinkauf, J.; Benson, C.C.; Tindell, L.; Williams, K. Treatment of Severe Hypercapnic Respiratory Failure caused by SARS-CoV2 Lung Injury with ECCO2R using the Hemolung Respiratory Assist System. Case Rep. Crit. Care 2021, 2021, 9958343. [Google Scholar] [CrossRef]
- Naito, N.; Ukita, R.; Wilbs, J.; Wu, K.; Lin, X.; Carleton, N.M.; Roberts, K.; Jiang, S.; Heinis, C.; Cook, K.E. Combination of polycarboxybetaine coating and factor XII inhibitor reduces clot formation while preserving normal tissue coagulation during extracorporeal life support. Biomaterials 2021, 272, 120778. [Google Scholar] [CrossRef]
- Mangindaan, D.; Kuo, W.-H.; Kurniawan, H.; Wang, M.-J. Creation of biofunctionalized plasma polymerized allylamine gradients. J. Polym. Sci. Part B Polym. Phys. 2013, 51, 1361–1367. [Google Scholar] [CrossRef]
- Thongsukmak, A.; Sirkar, K. Pervaporation membranes highly selective for solvents present in fermentation broths. J. Membr. Sci. 2007, 302, 45–58. [Google Scholar] [CrossRef]
- Yared, I.; Wang, S.-L.; Wang, M.-J. Effects of Oxygen Plasma and Dopamine Coating on Poly(Vinylidene Fluoride) Microfiltration Membrane for the Resistance to Protein Fouling. IEEE Trans. Plasma Sci. 2014, 42, 3847–3857. [Google Scholar] [CrossRef]
- Yang, X.; Fane, A.G.; Wang, R. Membrane Distillation: Now and Future. In Desalination: Water from Wate, 2nd ed.; Kucera, J., Ed.; Scrivener Publishing LLC: Beverly, MA, USA, 2018; pp. 329–386. [Google Scholar]
- Gao, Z.F.; Shi, G.M.; Cui, Y.; Chung, T.S. Organic solvent nanofiltration (OSN) membranes made from plasma grafting of polyethylene glycol on cross-linked polyamide ultrafiltration substrates. J. Membr. Sci. 2018, 565, 169–178. [Google Scholar] [CrossRef]
- Qin, Y.; Sirkar, K.K. Pervaporation Membranes That Are Highly Selective for Acetic Acid over Water. Ind. Eng. Chem. Res. 2003, 42, 582–595. [Google Scholar] [CrossRef]
- Li, B.; Sirkar, K.K. Novel Membrane and Device for Direct Contact Membrane Distillation-Based Desalination Process. Ind. Eng. Chem. Res. 2004, 43, 5300–5309. [Google Scholar] [CrossRef]
- Obuskovic, G.; Majumdar, S.; Sirkar, K. Highly VOC-selective hollow fiber membranes for separation by vapor permeation. J. Membr. Sci. 2003, 217, 99–116. [Google Scholar] [CrossRef]
- Thongsukmak, A.; Sirkar, K. Extractive pervaporation to separate ethanol from its dilute aqueous solutions characteristic of ethanol-producing fermentation processes. J. Membr. Sci. 2009, 329, 119–129. [Google Scholar] [CrossRef]
- Sirkar, K.K. Pervaporation Membranes Highly Selective for Volatile Solvents Present in Fermentation Broths. U.S. Patent 9085476 B2, 21 July 2015. [Google Scholar]
- Dai, L.; Griesser, H.J.; Mau, A.W.H. Surface modification by plasma etching and patterning. J. Phys. Chem. B 1997, 101, 9548–9554. [Google Scholar] [CrossRef]
- Mantovani, D.; Castonguay, M.; Pageau, J.F.; Fiset, M.; Laroche, G. Ammonia RF-Plasma Treatment of Tubular ePTFE Vascular Prostheses. Plasmas Polym. 1999, 4, 207–228. [Google Scholar] [CrossRef]
- Yasuda, H.; Hsu, T. Some aspects of Plasma Polymerization investigated by pulsed R F Discharge. J. Polym. Sci. Part A Polym. Chem. 1977, 15, 81–97. [Google Scholar] [CrossRef]
- Sharma, A.K.; Millich, F.; Hellmuth, E.W. Propylene Glow Discharge Polymerization in the Presence of Bromotrichloromethane; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 1979; Volume 108, Chapter 3; pp. 53–64. [Google Scholar]
- Oldfield, F.F.; Cowan, D.L.; Yasuda, H. ESR Studies of the Plasma Polymerization pf Trimethylsilane and Methane. Plasmas Polym. 2000, 5, 235–253. [Google Scholar] [CrossRef]
- Kobayashi, H.; Bell, A.T.; Shen, M. Formation of an amorphous powder during polymerization of ethylene in a radio-frequency discharge. J. Appl. Polym. Sci. 1973, 17, 885–892. [Google Scholar] [CrossRef]
- Yasuda, H.; Sharma, A.K. Effect of orientation and mobility of polymer molecules at surfaces on contact angle and its hysteresis. J. Polym. Sci. Polym. Phys. Ed. 1981, 19, 1285–1291. [Google Scholar] [CrossRef]
- Sharma, A.K.; Millich, F.; Hellmuth, E.W. Glow Discharge Polymer of Caprolactam—A synthetic peptide copolymer. In Abstracts of Papers of The American Chemical Society; American Chemical Society: Washington, DC, USA, 1979; Volume 20, pp. 624–627. [Google Scholar]
- Bell, A.T. Fundamentals of Plasma Polymerization. J. Macromol. Sci. Chem. 1976, 10, 369. [Google Scholar] [CrossRef]
- Kobayashi, H.; Bell, A.T.; Shen, M. Plasma Polymerization of Saturated and Unsaturated Hydrocarbons. Macromolecules 1974, 7, 277–283. [Google Scholar] [CrossRef]
- Sharma, A.K.; Yasuda, H. Polymerization of Methane. J. Appl. Polym. Sci. 1989, 38, 751–754. [Google Scholar] [CrossRef]
- Konig, U.; Nitschke, M.; Menning, A.; Eberth, G.; Pilz, M.; Arnold, C.; Simon, F.; Adam, G.; Werner, C. Durable surface modification of poly(tetrafluoroethylene) by low pressure water plasma treatment followed by acrylic acid graft polymerization. Colloids Surf. B Biointerfaces 2002, 24, 63–71. [Google Scholar] [CrossRef]
- Coulson, S. Method for Liquid Proofing an Item by Plasma Graft Polymerization. U.S. Patent 2010/0203347A1, 12 August 2010. [Google Scholar]
- Chawla, A.S. Evaluation of plasma polymerized hexamethylcyclotrisiloxane biomaterials towards adhesion of canine platelets and leucocytes. Biomaterials 1981, 2, 83–88. [Google Scholar] [CrossRef]
- Sarmadi, A.M.; Ying, T.; Denes, F. HMDSO-plasma modification of polypropylene fabrics. Eur. Polym. J. 1995, 31, 847–857. [Google Scholar] [CrossRef]
- Egghe, T.; Ghobeira, R.; Tabaei, P.S.E.; Morent, R.; Hoogenboom, R.; De Geyter, N. Silanization of Plasma-Activated Hexamethyldisiloxane-Based Plasma Polymers for Substrate-Independent Deposition of Coatings with Controlled Surface Chemistry. ACS Appl. Mater. Interfaces 2022, 14, 4620–4636. [Google Scholar] [CrossRef]
- Hirotsu, T.; Tagaki, C.; Partridge, A. Plasma copolymerization of acrylic acid with hexamethyldisilazane. Plasmas Polym. 2002, 7, 353–366. [Google Scholar] [CrossRef]
- Lecoq, E.; Duday, D.; Bulou, S.; Frache, G.; Hilt, F.; Maurau, R.; Choquet, P. Plasma Polymerization of APTES to Elaborate Nitrogen Containing Organosilicon Thin Films: Influence of Process Parameters and Discussion about the Growing Mechanisms. Plasma Process. Polym. 2012, 10, 250–261. [Google Scholar] [CrossRef]
- Cornelius, D.J.; Monroe, C.M. The unique properties of silicone and fluorosilicone elastomers. Polym. Eng. Sci. 1985, 25, 467–473. [Google Scholar] [CrossRef]
- AMT Inc Internal Test Method, AMT-TM-8069. Gas Permeability Measurement of HFM on Shepherd Hooks.
- Nomura, H. Gas Permselective Composite Membrane Prepared by Plasma Polymerization Coating Techniques. U.S. Patent 4824444, 25 April 1989. [Google Scholar]
- Sharma, A.K. Organosiloxane Films for Gas Separation. U.S. Patent 9339770 B2, 17 May 2016. [Google Scholar]
- Sirkar, K. Separation of Molecules, Macromolecules and Particles: Principles, Phenomena and Processes; Cambridge Series in Chemical Engineering; Cambridge University Press: Cambridge, UK, 2014; p. 185. ISBN 978-0-521-89573-6. [Google Scholar]
- Song, L.; Ma, Z.; Liao, X.; Kosaraju, P.B.; Irish, J.R.; Sirkar, K.K. Pilot plant studies of novel membranes and devices for direct contact membrane distillation. J. Membr. Sci. 2008, 323, 257–270. [Google Scholar] [CrossRef]
- Alkhudhiri, A.; Darwish, N.; Hilal, N. Membrane distillation: A comprehensive review. Desalination 2012, 287, 2–18. [Google Scholar] [CrossRef]
- Enny, R.; Putu, T.P.; Nurul, F.; Anita, K.K.; Khoiruddin, G.; Kadja, T.M.; Nicholaus, P.; Wenten, I.G. Membrane Oxygenator for Extracorporeal Blood Oxygenation. J. Eng. Technol. Sci. 2021, 53, 210502. [Google Scholar] [CrossRef]
- Batchinsky, A.I.; Jordan, B.S.; Regen, D.; Nessoiu, C.; Federspiel, W.J.; Morris, M.J.; Cancio, L.C. Respiratory Dialysis: Reduction in dependence on mechanical ventilation by venovenous extracorporeal CO2 removal. Crit. Care Med. 2011, 39, 1382–1387. [Google Scholar] [CrossRef] [Green Version]
- Sharma, A.K.; Yasuda, H. Plasma polymerization of tetramethyldislioxane by a magnetron glow discharge. Thin Solid Film. 1983, 110, 171–184. [Google Scholar] [CrossRef]
- Sharma, A.; Juelfs, A.; Colling, C.; Sharma, S.; Conover, S.; Puranik, A.; Chau, J.; Rodrigues, L.; Sirkar, K. Porous Hydrophobic–Hydrophilic Composite Hollow Fiber and Flat Membranes Prepared by Plasma Polymerization for Direct Contact Membrane Distillation. Membranes 2021, 11, 120. [Google Scholar] [CrossRef]






| PROPERTIES | X30-240 | X30-150 | PP50/200 | PP50/280 | PP150/330 |
|---|---|---|---|---|---|
| Polymer | PP | PP | PP | PP | PP |
| OD (μm) | 300 ± 6 | 200 ± 7 | 300 ± 20 | 380 ± 20 | 630 ± 50 |
| ID (μm) | 233–255 | 140–160 | 200 | 280 | 330 ± 50 |
| Wall Thickness (μm) | 28 ± 2 | 25 ± 3 | 50 ± 10 | 50 ± 10 | 150 ± 25 |
| Pore Size(μm) | 0.04 (0.04 × 0.10) | - | ≤0.2 | ≤0.2 | 0.60 |
| Porosity (%) | 40% | 40% | - | 50–55% | |
| Resistance to Air Flow (Gurley * sec) | 25–45 | 49–76 | - | - | - |
| Nitrogen Permeance (cm3 cm−2 s−1 cm Hg−1) | - | 1.67 ± 0.67 ×10−2 | 1.67 ± 0.67 ×10−2 | - | |
| Tensile Strength at Break | ≥175 g/fil | ≥100 g/fil | 153 g/fil (150 cN) ** | 153 g/fil (150 cN) | - |
| Burst Strength | 220 psi | 200 psi | - | - | - |
| Bubble Point | - | - | 45 psig in IPA *** | 44 psig in IPA | 15 psig in IPA |
| Solvent Residue | - | - | ≤100 ppm | ≤ 100 ppm | - |
| Elongation at Break | ≥50% | - | 400% | 400% | |
| Explosion Pressure | - | - | 44 psig | 44 psig | - |
| Implosion Pressure | - | - | 51 psig | 51 psig | - |
| Suggested Applications | Blood Oxygenators, Gas Separation, Liquid Degassing, Biotechnology, etc. | Cardiac Therapy, Gas separation, and other Industrial applications | Biological separations, Blood Oxygenation, and other medical applications | Gas Contactors, Air filtration, Water filtration, Food and Beverage | Industrial oxygenation, Membrane Distillation, Waste water treatment, etc. |
| Sample No. | TMDSO Flow Rate (SCCM) | Reaction Time (sec) | N2 Permeance * (10−3 cm3 cm−2 s−1 cm Hg−1) | Effective Pore Diameter (μm) |
|---|---|---|---|---|
| Untreated PP 50/280 | - | - | 12.1 | 0.200 |
| 3069 | 6.1 | 17 | 11.6 | 0.191 |
| 3070 | 10.1 | 17 | 10.6 | 0.178 |
| 3071 | 14.9 | 17 | 8.3 | 0.164 |
| 3119 | 6.1 | 25 | 10.1 | 0.177 |
| 3120 | 14.9 | 25 | 4.1 | 0.112 |
| 3121 | 22.3 | 25 | 2.9 | 0.095 |
| Sample No. | TMDSO MFR (SCCM) | Reaction Time (Sec) | N2 Permeance (10−3 cm3 cm−2 s−1 cm Hg−1) | Effective Pore Diameter (μm) |
|---|---|---|---|---|
| Untreated PP 50/200 | - | - | 12.00 | 0.200 |
| 2394 | 2.0 | 12 | 10.40 | 0.186 |
| 2393 | 3.4 | 12 | 9.90 | 0.181 |
| 2392 | 6.1 | 12 | 9.30 | 0.176 |
| 2391 | 6.1 | 17 | 8.38 | 0.167 |
| 2390 | 6.1 | 25 | 4.81 | 0.127 |
| 2426 | 10.1 | 25 | 1.17 | 0.063 |
| 2417 | 12.2 | 25 | 0.70 | 0.048 |
| 2418 | 15.5 | 25 | 0.28 | 0.031 |
| 2420 | 18.3 | 25 | 0.15 | 0.022 |
| Sample No. | TMDSO MFR (SCCM) | Reaction Time (Sec) | N2 Permeance (10−3 cm3 cm−2 s−1 cm Hg−1) | Effective Pore Diameter (μm) |
|---|---|---|---|---|
| Untreated PP 150/330 | - | - | 73.7 | 0.600 |
| 5803 | 6.1 | 25 | 61.4 | 0.548 |
| 5804 | 12.2 | 25 | 59.7 | 0.540 |
| 5805 | 22.3 | 25 | 56.2 | 0.524 |
| Sample No. | PFM | θ H2O at t = 0 | θ H2O at t = 3 year | θ H2O at t = 48 h in Water |
|---|---|---|---|---|
| 6090 | TMDSO | 103° | 100° | 98° |
| 6093 | TMDSO/HFE | 102° | 101° | 91° |
| 6573 | TMDSO/PFHX | 105° | 106° | 107° |
| 6245 | HFE | 32° | - | 17° |
| Sample No. | TMDSO Flow Rate (SCCM) | W/FM (106 J kg−1) | Load (Standard Deviation) (N/fiber) | Elongation (Standard Deviation) (%) |
|---|---|---|---|---|
| Untreated Membrane | - | 1.58 (1.77) | 578.1 (30.05) | |
| #2390 | 6.1 | 132.2 | 1.53 (3.79) | 637.5 (16.58) |
| #2395 | 8.1 | 99.8 | 1.56 (3.26) | 659.5 (15.21) |
| #2396 | 10.2 | 78.2 | 1.59 (2.89) | 617.5 (18.76) |
| #2401 | 14.3 | 56.6 | 1.54 (2.89) | 571.5 (26.26) |
| Sample No. | TMDSO Flow Rate (SCCM) | W/FM (106 J kg−1) | Load (Standard Deviation) (N/fiber) | Elongation (Standard Deviation) (%) |
|---|---|---|---|---|
| Untreated X30-240 | - | - | 2.35 (0.04) | 117.7 (7.0) |
| 2285 * | 14.85 | 47.2 | 3.63 (0.01) | 76.0 (6.0) |
| 2286 ** | 14.85 | 47.2 | 3.62 (0.01) | 73.9 (7.3) |
| 2287 ** | 14.85 | 53.9 | 3.53 (0.02) | 70.0 (5.3) |
| Sample No. | PFMs (Monomers) | Brine Temp. (°C) | Cold Water Temp. (°C) | Water Flux (kg m−2 h−1) | Product Water Conductivity (μS cm−1) |
|---|---|---|---|---|---|
| Uncoated | 70 | 23 | 11.9 | 3–4 | |
| 6108 | TMDSO/HFE | 70 | 23 | 13.4 | 5–10 |
| 6179 | TMCTS/HFE | 70 | 23 | 15.2 | 4–9 |
| 6199 | TMDSO/PFFM | 70 | 23 | 17.5 | 4–8 |
| 6199 * | TMDSO/PFFM | 70 | 23 | 17.0 | 2–4 |
| 6199 ** | TMDSO/PFFM | 70 | 23 | 18.2 | 2–3 |
| Sample No. | PFMs | Brine Temp. (°C) | Cold Water Temp. (°C) | Water Flux (kg m−2 h−1) | Product Water Conductivity (μS cm−1) |
|---|---|---|---|---|---|
| 6199 | TMDSO/PFFM | 50 | 23 | 7.4 | 1.9–2.2 |
| 6199 | TMDSO/PFFM | 70 | 23 | 18.3 | 1.8–2.1 |
| 6179 | TMCTS/PFFM | 81 | 23 | 55.6 | 2.1–2.4 |
| Sample No. | PFM | Decrease in CO2 Permeance at Different Time Intervals | ||||
|---|---|---|---|---|---|---|
| 7 days | 14 days | 28 days | 60 days | 120 days | ||
| 5978 | TMDSO | 9% | 11% | 15% | 18% | 22% |
| 6244 | TMCTS | 6% | 10% | 17% | 28% | |
| 5979/5978 * | TMSAA | 3% | 5% | 7% | 10% | |
| 6081 | HMTSO | 2% | 3% | 5% | - | 7% |
| Sample No. | Age of Sample (Day) | Gas Permeance (10−4 cm3 cm−2 s−1 cm Hg−1) | Selectivity | ||
|---|---|---|---|---|---|
| N2 | O2 | CO2 | CO2/O2 | ||
| 2991 | 1 | 1.86 | 3.77 | 14.8 | 3.94 |
| 2991 * | 3405 | 1.52 | 3.13 | 13.1 | 4.19 |
| 2991 ** | 3405 | 1.65 | 3.45 | 14.2 | 4.10 |
| Sample No. | Age of Sample (Day) | Gas Permeance (10−4 cm3 cm−2 s−1 cm Hg−1) | Selectivity | ||
|---|---|---|---|---|---|
| N2 | O2 | CO2 | CO2/O2 | ||
| 2985 | 1 | 1.74 | 3.36 | 13.10 | 3.90 |
| 2985 * | 515 | 1.86 | 3.06 | 10.20 | 3.32 |
| 2985 ** | 4650 | 1.67 | 2.75 | 11.0 | 3.96 |
| 2985 *** | 4830 | 1.67 | 2.74 | 10.3 | 3.75 |
| Sample No. | PFM | Age of Sample | %C | %Si | %O | %F | C/Si | O/Si Ratio |
|---|---|---|---|---|---|---|---|---|
| 6348 | TMDSO | 33 Days | 38.39 | 27.68 | 33.92 | - | 1.39 | 1.225 |
| 6348 | TMDSO | 52 Days | 38.04 | 27.07 | 34.89 | - | 1.40 | 1.289 |
| 3175-6 | TMDSO/HFE | 320 Days | 33.30 | 19.50 | 31.50 | 15.7 | 1.71 | 1.615 |
| 7905 * | - | - | 100.0 | - | - | - | - | - |
| Sample No. | PFM | RT (sec) | CO2 Gas Permeance (10−4 cm3 cm−2 s−1 cm Hg−1) | % Change | Selectivity CO2/O2 | ||
|---|---|---|---|---|---|---|---|
| Before | After | Before | After | ||||
| 6620 | TMDSO | 14 | 6.48 | 5.53 | (−14.7%) | 4.40 | 4.60 |
| 6621 | TMDSO | 20 | 4.31 | 3.39 | (−21.4%) | 4.85 | 5.63 |
| 7011 | HMTSO | 12 | 24.1 | 17.1 | (−29.0%) | 4.25 | 4.45 |
| 7011 | HMTSO | 12 | 24.1 | 20.1 * | (−16.6%) | 4.25 | 4.45 |
| Exposure Time | Sample No. | Gas Permeance and Selectivity before Exposure (10−4 cm3 cm−2 s−1 cm Hg−1) | Gas Permeance and Selectivity after Exposure (10−4 cm3 cm−2 s−1 cm Hg−1) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| N2 | O2 | CO2 | CO2/O2 | N2 | O2 | CO2 | CO2/O2 | ||
| 7 days | 6258-7A | 2.30 | 4.00 | 15.70 | 3.93 | 1.70 | 3.26 | 13.99 | 4.29 |
| 6258-7B | 2.47 | 4.09 | 15.40 | 3.77 | 1.81 | 3.33 | 13.96 | 4.19 | |
| 6258-7C | 2.36 | 4.08 | 16.16 | 3.96 | 1.90 | 3.48 | 14.63 | 4.20 | |
| 14 days | 6258-14A | 2.47 | 4.18 | 16.27 | 3.89 | 1.68 | 3.27 | 14.24 | 4.35 |
| 6258-14B | 2.12 | 3.83 | 15.90 | 4.15 | 1.54 | 3.12 | 14.00 | 4.49 | |
| 6258-14C | 2.38 | 4.10 | 16.35 | 3.98 | 1.75 | 3.33 | 14.37 | 4.31 | |
| 21 days | 6258-21A | 2.32 | 4.00 | 15.86 | 3.96 | 1.56 | 3.10 | 13.57 | 4.38 |
| 6258-21B | 2.34 | 4.03 | 15.87 | 3.94 | 1.63 | 3.17 | 13.76 | 4.34 | |
| 6258-21C | 2.47 | 4.21 | 16.25 | 3.86 | 1.63 | 3.19 | 14.07 | 4.41 | |
| 28 days | 6258-28A | 2.31 | 4.11 | 16.61 | 4.05 | 1.37 | 2.81 | 12.75 | 4.53 |
| 6258-28B | 2.43 | 4.20 | 16.53 | 3.94 | 1.42 | 2.85 | 12.62 | 4.42 | |
| 6258-28C | 2.18 | 3.94 | 14.79 | 3.76 | 1.38 | 2.82 | 12.28 | 4.36 | |
| Exposure Time | Sample No. | Gas Permeance and Selectivity Before Exposure (10−4 cm3 cm−2 s−1 cm Hg−1) | Gas Permeance and Selectivity After Exposure (10−4 cm3 cm−2 s−1 cm Hg−1) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| N2 | O2 | CO2 | CO2/O2 | N2 | O2 | CO2 | CO2/O2 | ||
| 7 days | 6281-7A | 0.66 | 1.78 | 8.44 | 4.74 | 0.68 | 1.64 | 7.63 | 4.65 |
| 6281-7B | 0.68 | 1.83 | 8.67 | 4.73 | 0.64 | 1.64 | 7.66 | 4.68 | |
| 6281-7C | 0.72 | 1.90 | 9.07 | 4.78 | 0.84 | 1.90 | 8.16 | 4.31 | |
| 14 days | 6281-14A | 0.67 | 1.85 | 9.00 | 4.86 | 0.64 | 1.64 | 7.62 | 4.65 |
| 6281-14B | 0.67 | 1.79 | 8.37 | 4.67 | 0.57 | 1.51 | 7.21 | 4.78 | |
| 6281-14C | 0.66 | 1.78 | 8.30 | 4.67 | 1.37 | 2.29 | 7.66 | 3.35 | |
| 21 days | 6281-21A | 0.66 | 1.78 | 8.46 | 4.75 | 0.70 | 1.65 | 7.30 | 4.41 |
| 6281-21B | 0.64 | 1.71 | 8.31 | 4.85 | 0.64 | 1.56 | 7.17 | 4.60 | |
| 6281-21C | 0.72 | 1.89 | 8.94 | 4.73 | 0.89 | 1.87 | 7.96 | 4.25 | |
| 28 days | 6281-28A | 0.70 | 1.86 | 8.89 | 4.79 | 0.74 | 1.70 | 7.52 | 4.42 |
| 6281-28B | 0.66 | 1.79 | 8.67 | 4.83 | 0.61 | 1.61 | 7.53 | 4.68 | |
| 6281-28C | 0.68 | 1.81 | 8.74 | 4.82 | 0.57 | 1.44 | 6.78 | 4.69 | |
| Sample No. | Chemicals | Gas Permeance and Selectivity before Exposure (10−4 cm3 cm−2 s−1 cm Hg−1) | Gas Permeance and Selectivity after Exposure (10−4 cm3 cm−2 s−1 cm Hg−1) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| N2 | O2 | CO2 | CO2/O2 | N2 | O2 | CO2 | CO2/O2 | ||
| 5229-4 | NH4OH 0.45 M | 1.07 | 2.35 | 9.62 | 4.09 | 0.98 | 2.19 | 8.91 (−7.90%) | 4.06 (−0.7%) |
| 5229-5 | NH4OH 0.90 M | 1.08 | 2.37 | 9.68 | 4.08 | 0.96 | 2.18 | 8.96 (−7.40%) | 4.10 (+0.5%) |
| 5229-9 | DEA 20% H2O | 1.12 | 2.36 | 9.39 | 3.97 | 1.06 | 2.15 | 8.27 (−12.0%) | 3.85 (−3.0%) |
| 5229-6 | TOA 50% EtOH | 1.05 | 2.26 | 9.16 | 4.06 | 0.63 | 1.94 | 7.43 (−18.9%) | 3.83 (−5.6%) |
| 0.66 * | 1.92 * | 7.99 * (−16.8%) | 4.17 * (+2.7%) | ||||||
| 5229-7 | DC 200 50% EtOH | 1.02 | 2.28 | 9.23 | 4.05 | 0.94 | 2.04 | 7.85 (−15.0%) | 3.85 (−5.0%) |
| 5229-8 | Hexane | 1.02 | 2.28 | 9.36 | 4.11 | 0.85 | 2.11 | 7.91 (−15.5%) | 3.75 (−8.7%) |
| AJ1033 | 2-PrOH | 4.37 | 5.48 | 13.7 | 2.50 | 3.38 | 4.59 | 13.3 (−3.0%) | 2.89 (+15.6%) |
| AJ1031 | DCM | 4.58 | 5.71 | 14.2 | 2.48 | 2.65 | 3.67 | 11.2 (−22.2%) | 3.04 (+22.6%) |
| Sample No. | W/FM (106 J kg−1) | Chemicals | Gas Permeance and Selectivity before Exposure (10−4 cm3 cm−2 s−1 cm Hg−1) | Gas Permeance and Selectivity after Exposure (10−4 cm3 cm−2 s−1 cm Hg−1) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N2 | O2 | CO2 | CO2/O2 | N2 | O2 | CO2 | CO2/O2 | |||
| 5292-2 | 94.4 | Hexane | 0.47 | 1.22 | 5.76 | 4.73 | 0.35 | 0.93 | 4.42 (−23%) | 4.74 (+0.2%) |
| 5300-1 | 47.2 | Hexane | 0.76 | 1.61 | 6.70 | 4.16 | 0.35 | 0.93 | 4.40 (−34%) | 4.74 (+5.8%) |
| 5292-1 | 94.4 | Toluene | 0.47 | 1.22 | 5.79 | 4.75 | 0.28 | 0.81 | 3.84 (−33%) | 4.72 (+0.6%) |
| 5300-3 | 47.2 | Toluene | 0.75 | 1.58 | 7.01 | 4.44 | 0.46 | 1.00 | 4.36 (−38%) | 4.38 (−1.4%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, A.K.; Conover, S.P.; Sirkar, K.K. Plasma Polymerized Coatings on Hollow Fiber Membranes-Applications and Their Aging Characteristics in Different Media. Membranes 2022, 12, 656. https://doi.org/10.3390/membranes12070656
Sharma AK, Conover SP, Sirkar KK. Plasma Polymerized Coatings on Hollow Fiber Membranes-Applications and Their Aging Characteristics in Different Media. Membranes. 2022; 12(7):656. https://doi.org/10.3390/membranes12070656
Chicago/Turabian StyleSharma, Ashok K., Stephen P. Conover, and Kamalesh K. Sirkar. 2022. "Plasma Polymerized Coatings on Hollow Fiber Membranes-Applications and Their Aging Characteristics in Different Media" Membranes 12, no. 7: 656. https://doi.org/10.3390/membranes12070656
APA StyleSharma, A. K., Conover, S. P., & Sirkar, K. K. (2022). Plasma Polymerized Coatings on Hollow Fiber Membranes-Applications and Their Aging Characteristics in Different Media. Membranes, 12(7), 656. https://doi.org/10.3390/membranes12070656








