Construction of PPSU-MoS2/PA-MIL-101(Cr) Membrane with Highly Enhanced Permeance and Stability for Organic Solvent Nanofiltration
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials and Reactants
2.2. Synthesis of MIL-101(Cr)
2.3. Preparation of PPSU-MoS2 Substrate
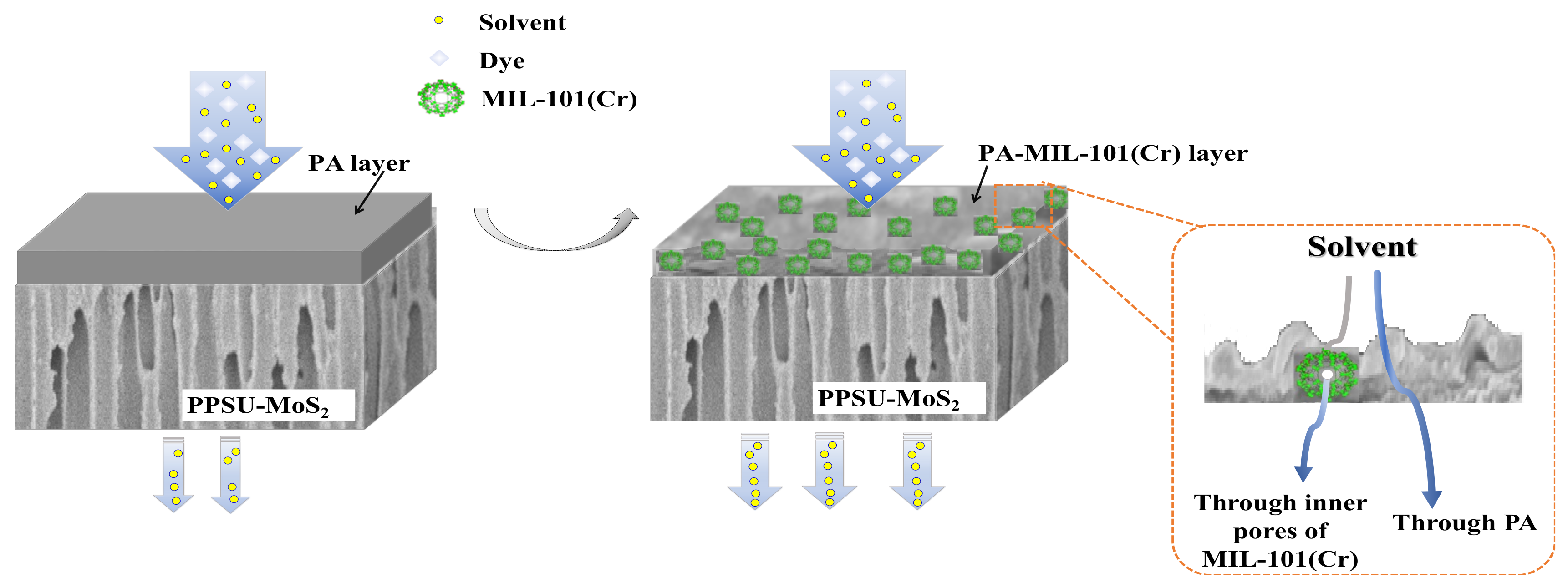
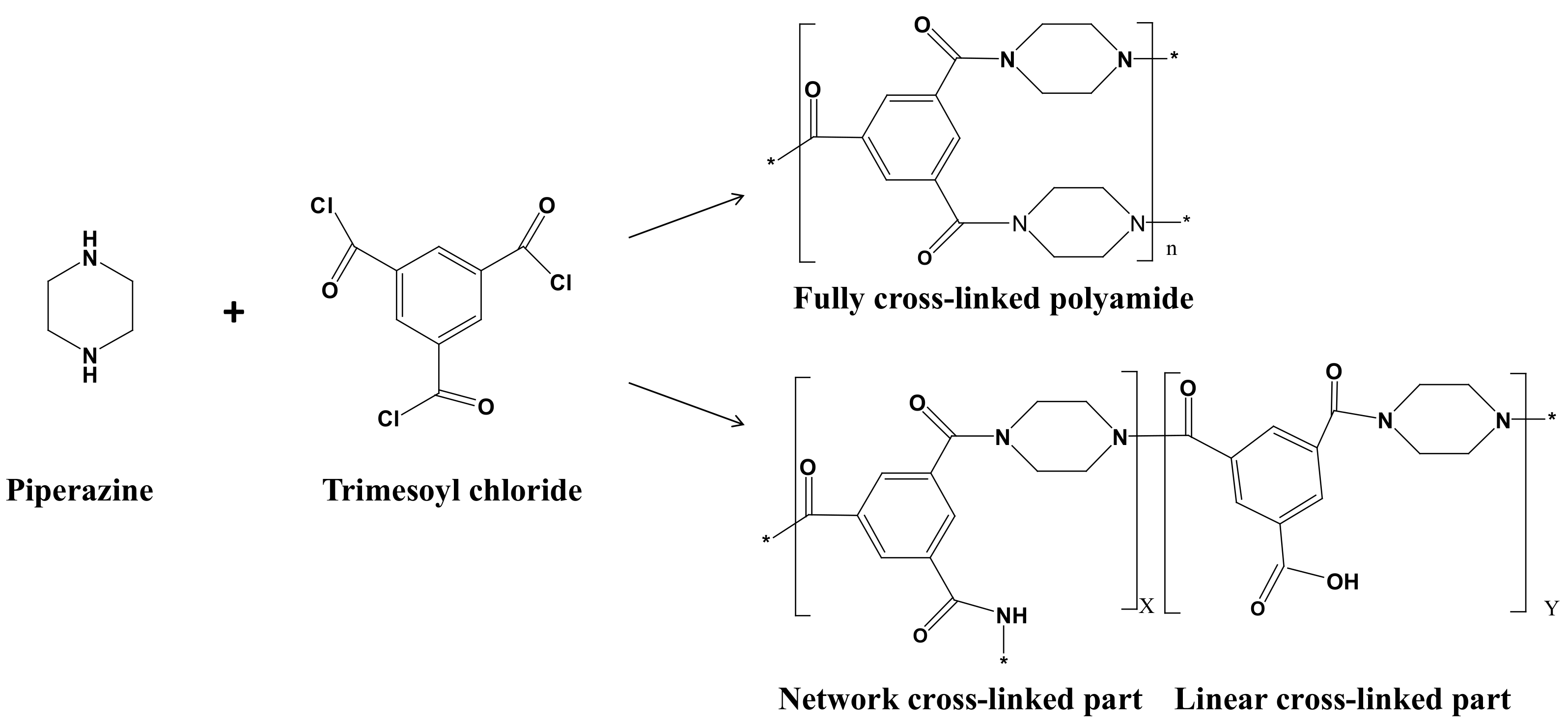
2.4. Preparation of PPSU-MoS2/PA-MIL-101(Cr) Membrane
2.5. Characterization of MIL-101(Cr)
2.6. Characterization of Membranes
2.7. Density and Cross-linking Degree of Membranes
2.8. Characterization of Membrane Performance
3. Results and Discussion
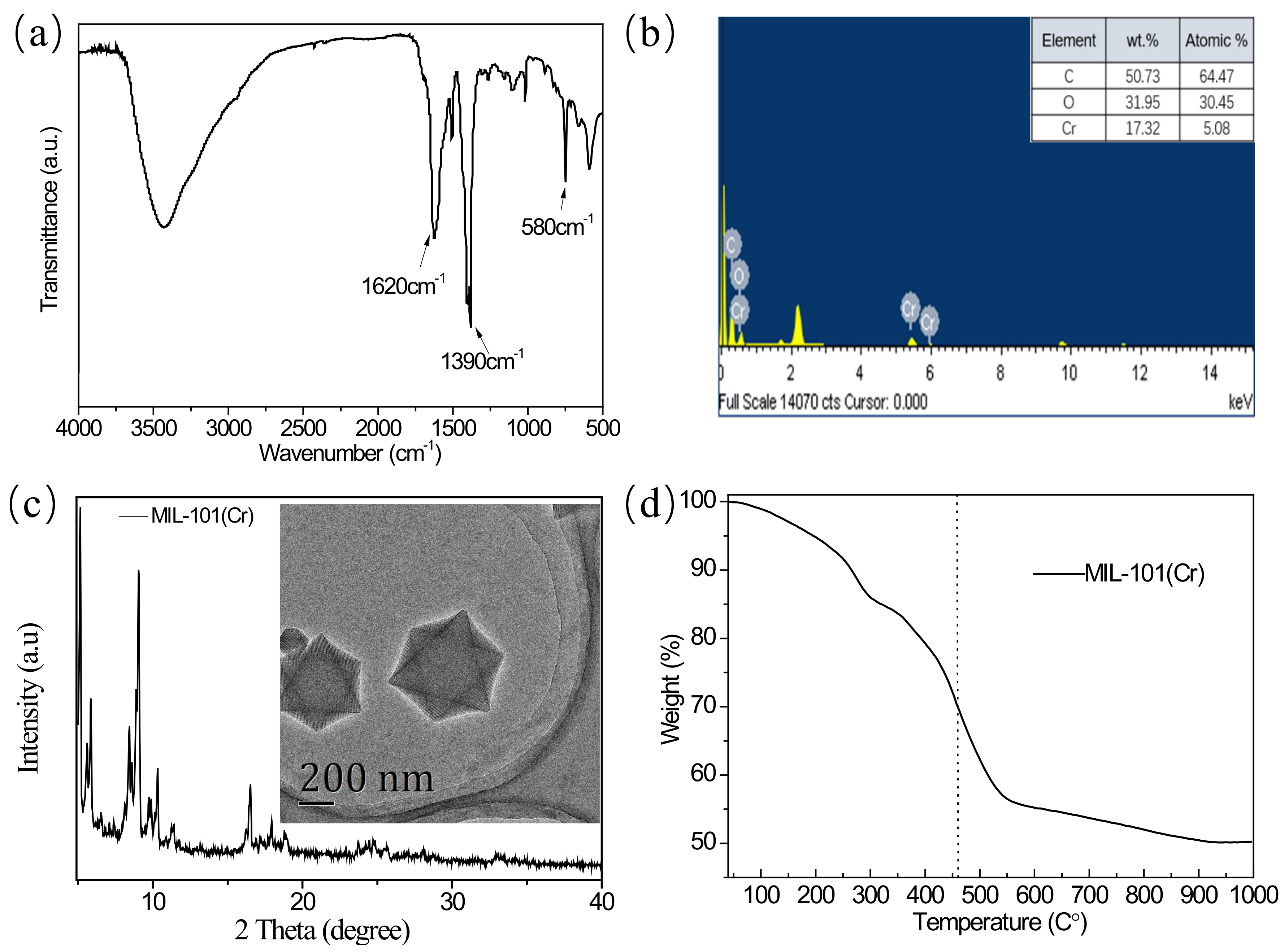
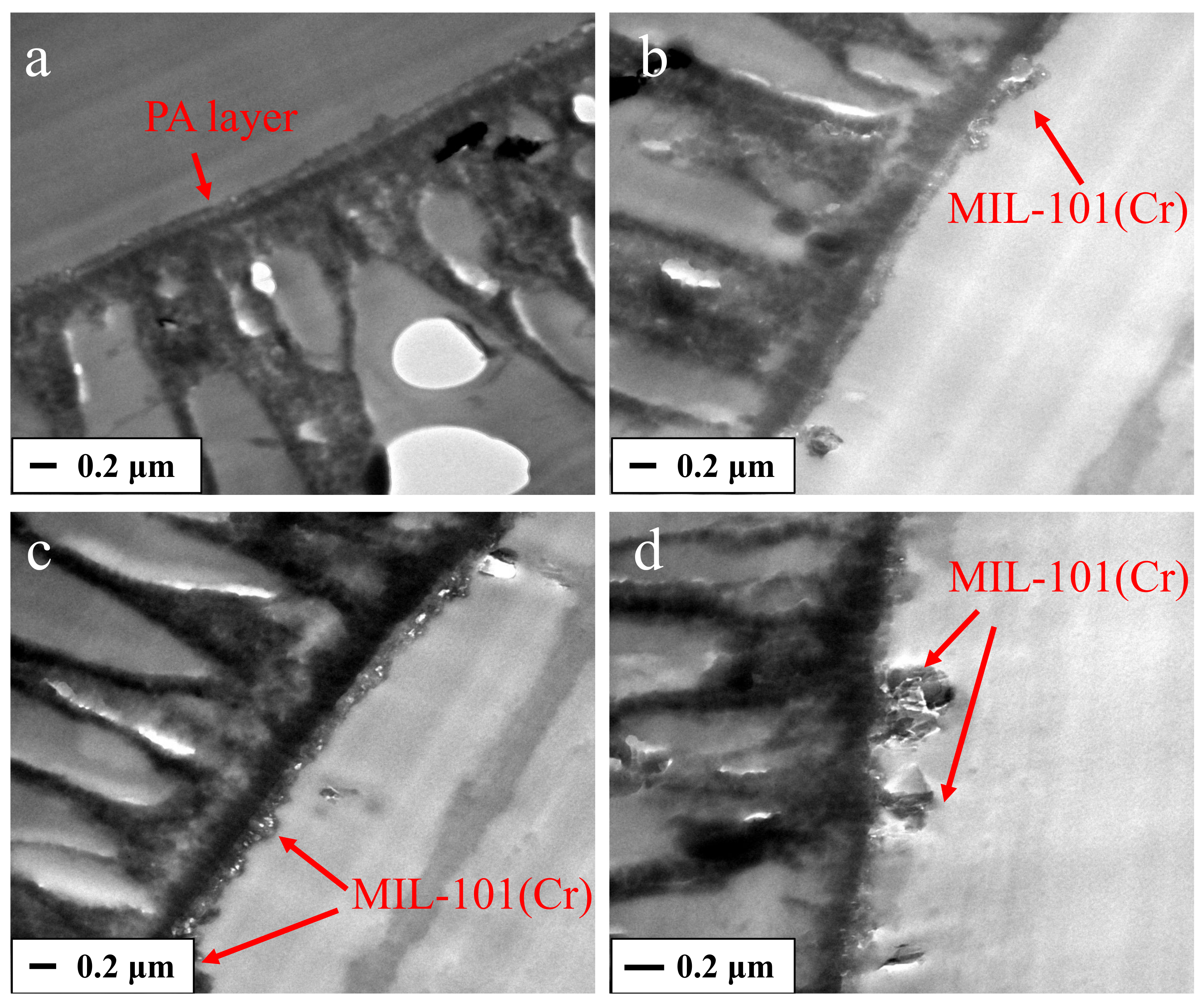
3.1. MIL-101(Cr) Characterization
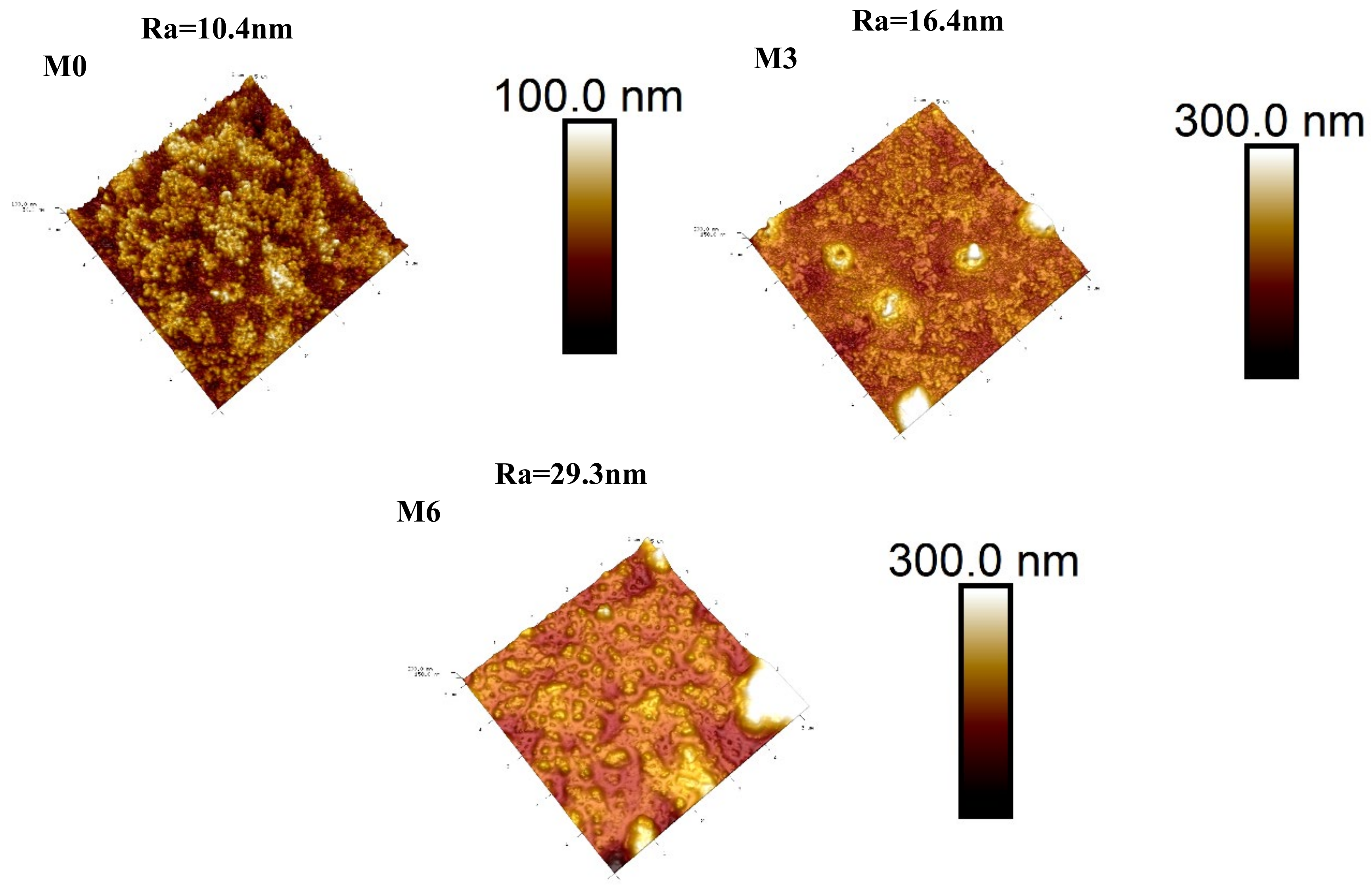
3.2. Characterization of Membranes
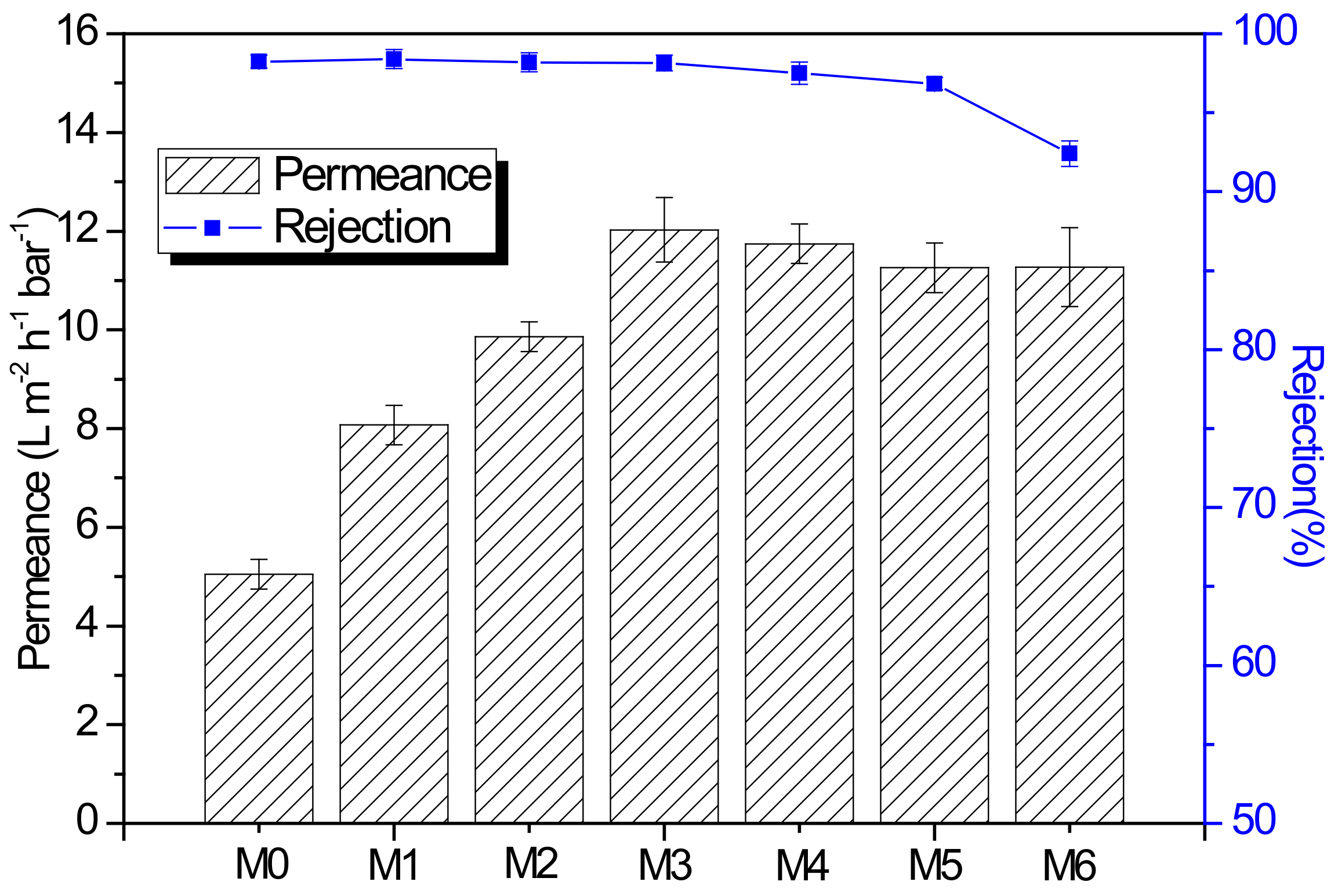
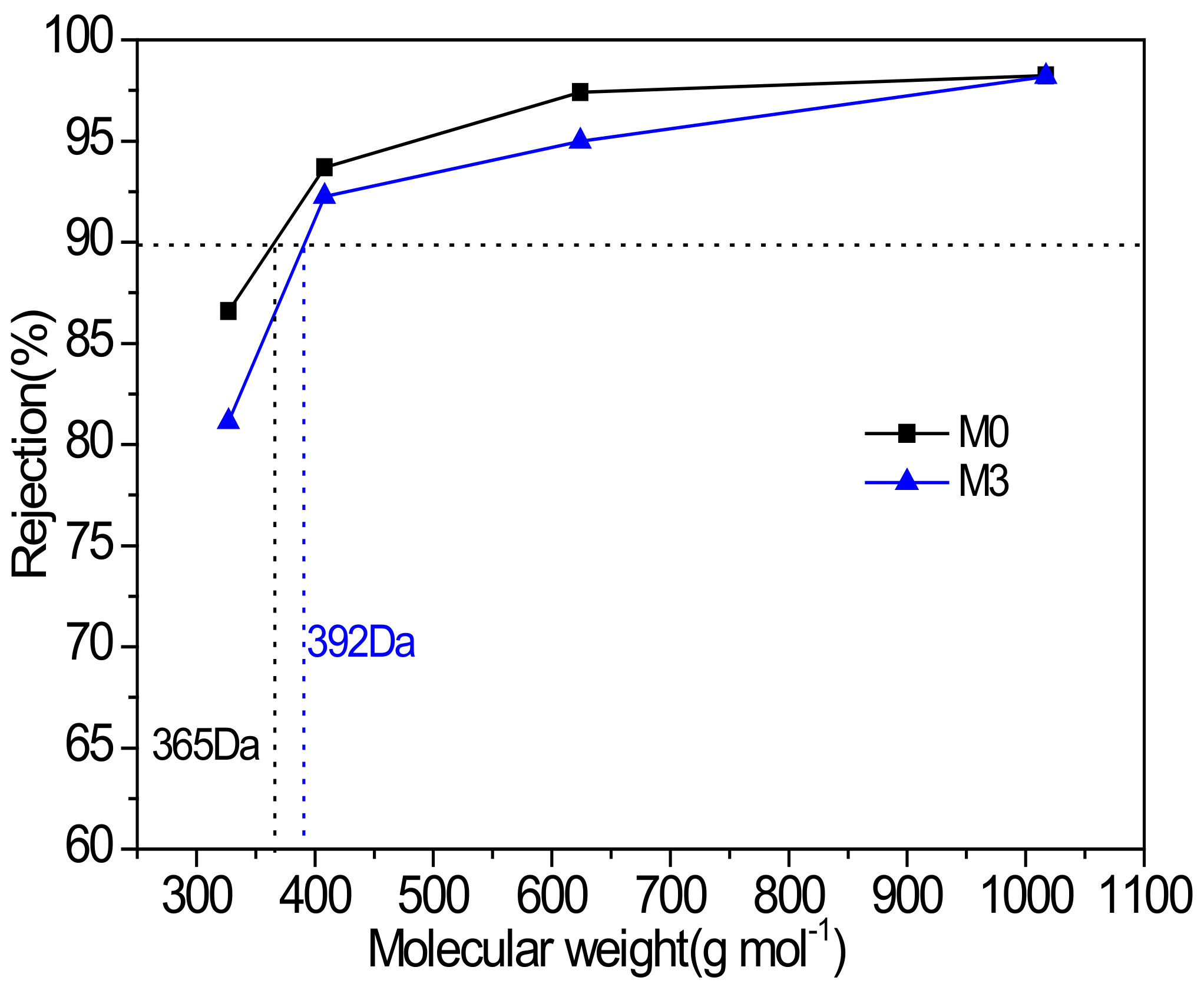
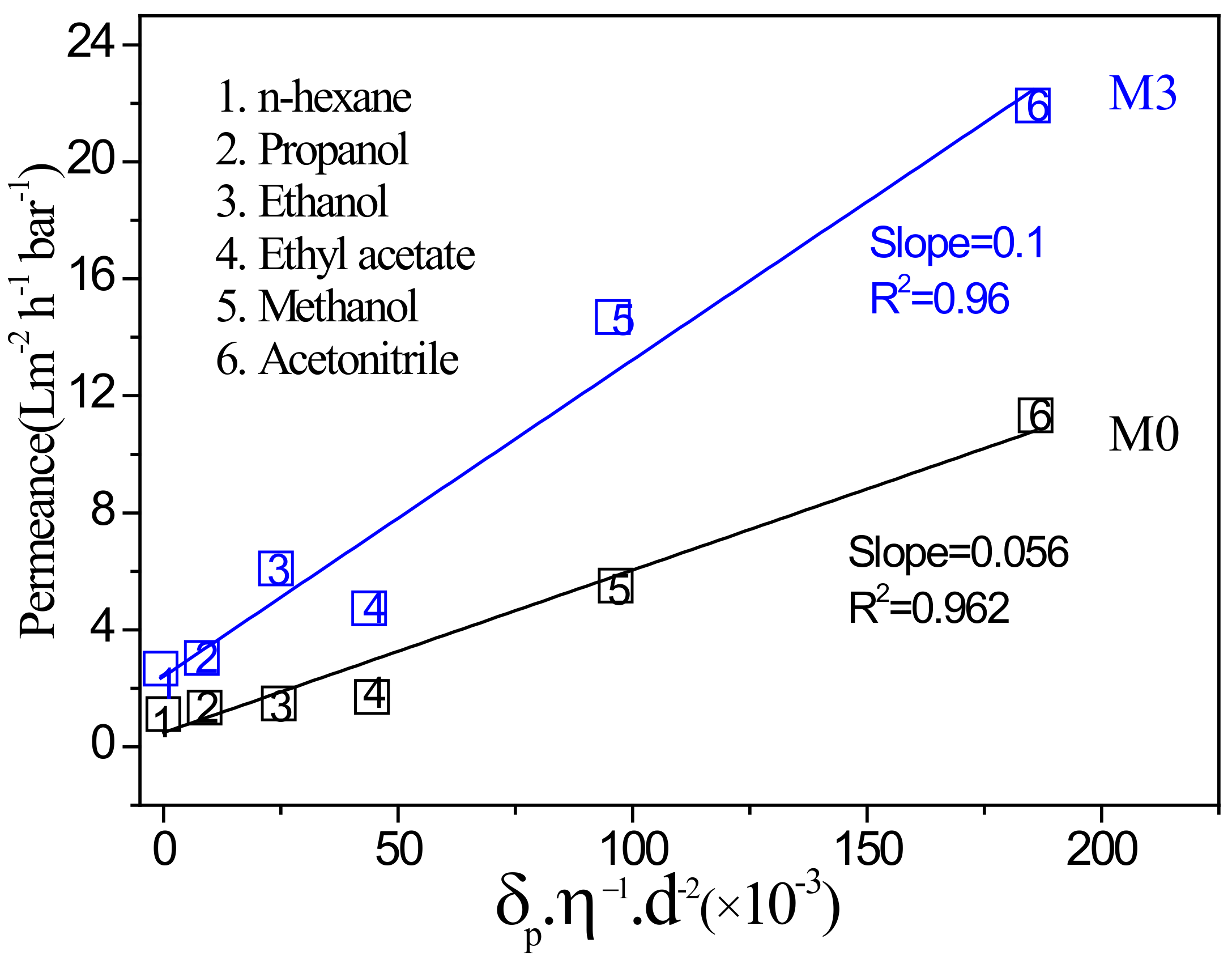
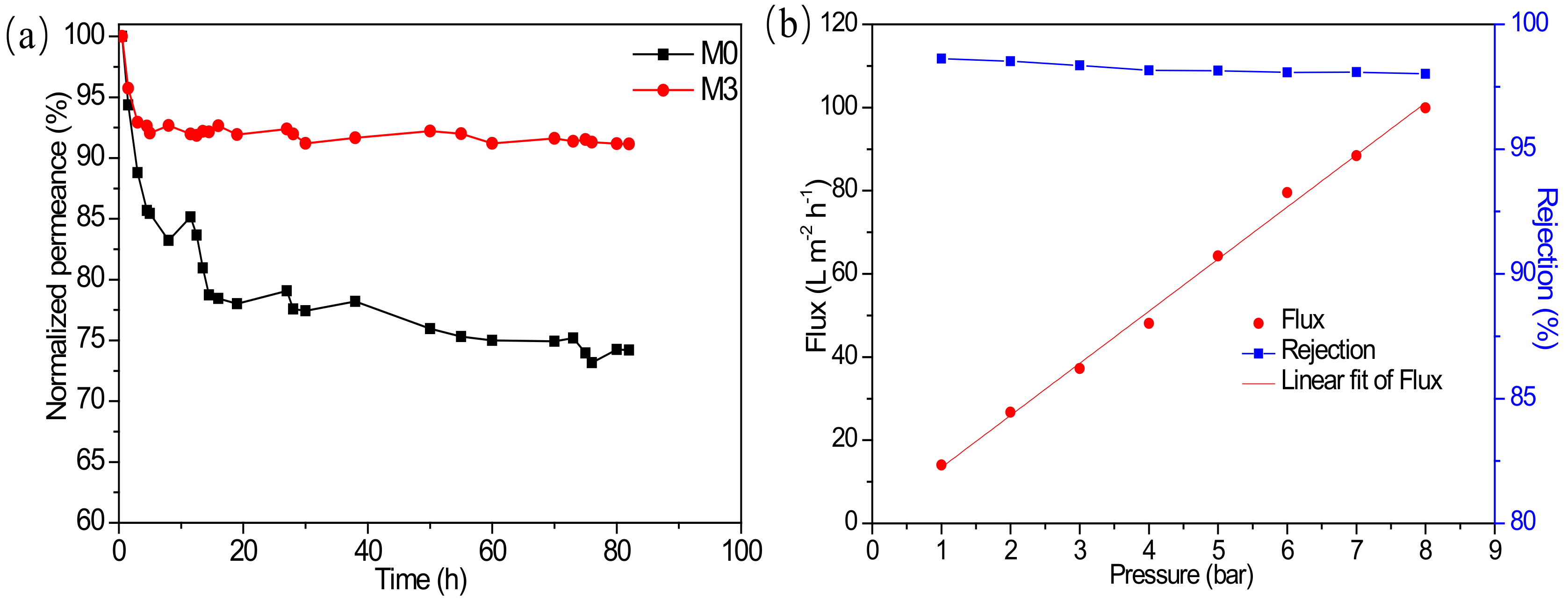
3.3. Organic Solvent Nanofiltration Performance of Membranes
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vandezande, P.; Gevers, L.E.; Vankelecom, I.F. Solvent resistant nanofiltration: Separating on a molecular level. Chem. Soc. Rev. 2008, 37, 365–405. [Google Scholar] [CrossRef] [PubMed]
- Ding, R.; Zhang, H.; Li, Y.; Wang, J.; Shi, B.; Mao, H.; Dang, J.; Liu, J. Graphene oxide-embedded nanocomposite membrane for solvent resistant nanofiltration with enhanced rejection ability. Chem. Eng. Sci. 2015, 138, 227–238. [Google Scholar] [CrossRef]
- Fritsch, D.; Merten, P.; Heinrich, K.; Lazar, M.; Priske, M. High performance organic solvent nanofiltration membranes: Development and thorough testing of thin film composite membranes made of polymers of intrinsic microporosity (PIMs). J. Membr. Sci. 2012, 401–402, 222–231. [Google Scholar] [CrossRef]
- Soroko, I.; Bhole, Y.; Livingston, A.G. Environmentally friendly route for the preparation of solvent resistant polyimide nanofiltration membranes. Green Chem. 2011, 13, 162–168. [Google Scholar] [CrossRef]
- Vanherck, K.; Vandezande, P.; Aldea, S.O.; Vankelecom, I.F.J. Cross-linked polyimide membranes for solvent resistant nanofiltration in aprotic solvents. J. Membr. Sci. 2008, 320, 468–476. [Google Scholar] [CrossRef]
- Li, Y.; Mao, H.; Zhang, H.; Yang, G.; Ding, R.; Wang, J. Tuning the microstructure and permeation property of thin film nanocomposite membrane by functionalized inorganic nanospheres for solvent resistant nanofiltration. Sep. Purif. Technol. 2016, 165, 60–70. [Google Scholar] [CrossRef]
- Yuan, Z.; Wu, X.; Jiang, Y.; Li, Y.; Huang, J.; Hao, L.; Zhang, J.; Wang, J. Carbon dots-incorporated composite membrane towards enhanced organic solvent nanofiltration performance. J. Membr. Sci. 2018, 549, 1–11. [Google Scholar] [CrossRef]
- Peyravi, M.; Rahimpour, A.; Jahanshahi, M. Thin film composite membranes with modified polysulfone supports for organic solvent nanofiltration. J. Membr. Sci. 2012, 423–424, 225–237. [Google Scholar] [CrossRef]
- Liu, Q.; Wu, X.; Zhang, K. Polysulfone/Polyamide-SiO2 Composite Membrane with High Permeance for Organic Solvent Nanofiltration. Membranes 2018, 8, 89. [Google Scholar] [CrossRef] [Green Version]
- Golpour, M.; Pakizehm, M. Preparation and characterization of new PA-MOF/PPSU-GO membrane for the separation of KHI from water. Chem. Eng. J. 2018, 88, 132–141. [Google Scholar] [CrossRef]
- Hwang, L.L.; Tseng, H.H.; Chen, J.C. Fabrication of polyphenylsulfone/polyetherimide blend membranes for ultrafiltration applications: The effects of blending ratio on membrane properties and humic acid removal performance. J. Membr. Sci. 2011, 384, 72–81. [Google Scholar] [CrossRef]
- Liu, S.; Fang, F.; Wu, J.; Zhang, K. The anti-biofouling properties of thin-film composite nanofiltration membranes grafted with biogenic silver nanoparticles. Desalination 2015, 375, 121–128. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.; Field, R.W.; Wu, J.J.; Zhang, K. Polyvinylpyrrolidone modified graphene oxide as a modifier for thin film composite forward osmosis membranes. J. Membr. Sci. 2017, 540, 251–260. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Li, S.; Zhang, K. Influence of hydrophilic carbon dots on polyamide thin film nanocomposite reverse osmosis membranes. J. Membr. Sci. 2017, 537, 42–53. [Google Scholar] [CrossRef]
- Li, Y.; Yang, S.; Zhang, K.; Bart, V. Thin film nanocomposite reverse osmosis membrane modified by two dimensional laminar MoS2 with improved desalination performance and fouling-resistant characteristics. Desalination 2018, 454, 48–58. [Google Scholar] [CrossRef]
- Kwak, S.Y.; Kim, S.H.; Kim, S.S. Hybrid organic/inorganic reverse osmosis (RO) membrane for bactericidal anti-fouling. 1. Preparation and characterization of TiO2 nanoparticle self-assembled aromatic polyamide thin-film-composite (TFC) membrane. Environ. Sci. Technol. 2001, 35, 2388–2394. [Google Scholar] [CrossRef]
- Yang, H.; Wang, N.; Wang, L.; Liu, H.-X.; An, Q.-F.; Ji, S. Vacuum-assisted assembly of ZIF-8@GO composite membranes on ceramic tube with enhanced organic solvent nanofiltration performance. J. Membr. Sci. 2018, 545, 158–166. [Google Scholar] [CrossRef]
- Ploegmakers, J.; Japip, S.; Nijmeijer, K. Mixed matrix membranes containing MOFs for ethylene/ethane separation Part A: Membrane preparation and characterization. J. Membr. Sci. 2013, 428, 445–453. [Google Scholar] [CrossRef]
- Emadzadeh, D.; Lau, W.J.; Matsuura, T.; Ismail, A.F.; Rahbari-Sisakht, M. Synthesis and characterization of thin film nanocomposite forward osmosis membrane with hydrophilic nanocomposite support to reduce internal concentration polarization. J. Membr. Sci. 2014, 449, 74–85. [Google Scholar] [CrossRef]
- Niu, Q.; Liu, M.; Xiao, Z.; Yuan, X.; Wu, J. In situ sulfonic acid-functionalized MIL-101(Cr) catalyzed liquid-phase Beckmann rearrangement of cyclohexanone oxime. Microporous Mesoporous Mater. 2020, 297, 110031. [Google Scholar] [CrossRef]
- Hong, W.Y.; Perera, S.P.; Burrows, A.D. Comparison of MIL-101(Cr) metal-organic framework and 13X zeolite monoliths for CO2 capture. Microporous Mesoporous Mater. 2020, 308, 110525. [Google Scholar] [CrossRef]
- Wang, J.; Muhammad, Y.; Gao, Z.; Jalil Shah, S.; Nie, S.; Kuang, L.; Zhao, Z.; Qiao, Z.; Zhao, Z. Implanting polyethylene glycol into MIL-101(Cr) as hydrophobic barrier for enhancing toluene adsorption under highly humid environment. Chem. Eng. J. 2021, 404, 126562. [Google Scholar] [CrossRef]
- Paz, F.A.; Klinowski, J.; Vilela, S.M.; Tome, J.P.; Cavaleiro, J.A.; Rocha, J. Ligand design for functional metal-organic frameworks. Chem. Soc. Rev. 2012, 41, 1088–1110. [Google Scholar]
- Vo, T.K.; Kim, J.-H.; Kwon, H.T.; Kim, J. Cost-effective and eco-friendly synthesis of MIL-101(Cr) from waste hexavalent chromium and its application for carbon monoxide separation. J. Ind. Eng. Chem. 2019, 80, 345–351. [Google Scholar] [CrossRef]
- Elsayed, E.; Al-Dadah, R.; Mahmoud, S.; Anderson, P.A.; Elsayed, A.; Youssef, P.G. CPO-27(Ni), aluminium fumarate and MIL-101(Cr) MOF materials for adsorption water desalination. Desalination 2017, 406, 25–36. [Google Scholar] [CrossRef]
- Song, N.; Sun, Y.; Xie, X.; Wang, D.; Shao, F.; Yu, L.; Dong, L. Doping MIL-101(Cr)@GO in polyamide nanocomposite membranes with improved water flux. Desalination 2020, 492, 114601. [Google Scholar] [CrossRef]
- Song, N.; Xie, X.; Chen, D.; Li, G.; Dong, H.; Yu, L.; Dong, L. Tailoring nanofiltration membrane with three-dimensional turing flower protuberances for water purification. J. Membr. Sci. 2021, 621, 118985. [Google Scholar] [CrossRef]
- Xu, Y.; Gao, X.; Wang, X.; Wang, Q.; Ji, Z.; Wang, X.; Wang, T.; Cao, C. Highly and stably water permeable thin film nanocomposite membranes doped with mil-101 (cr) nanoparticles for reverse osmosis application. Materials 2016, 9, 870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferey, G.; Mellot-Draznieks, C.; Serre, C.; Millange, F.; Dutour, J.; Surble, S.; Margiolaki, I. A Chromium Terephthalate-Based Solid with Unusually Large Pore Volumes and Surface Area. Science 2005, 309, 2040–2042. [Google Scholar] [CrossRef] [PubMed]
- Karan, S.; Jiang, Z.; Livingston, A.G. Sub–10 nm polyamide nanofilms with ultrafast solvent transport for molecular separation. Science 2015, 348, 1347–1351. [Google Scholar] [CrossRef] [PubMed]
- Maksimchuk, N.; Timofeeva, M.; Melgunov, M.; Shmakov, A.; Chesalov, Y.; Dybtsev, D.; Fedin, V.; Kholdeeva, O. Heterogeneous selective oxidation catalysts based on coordination polymer MIL-101 and transition metal-substituted polyoxometalates. J. Catal. 2008, 257, 315–323. [Google Scholar] [CrossRef]
- Bayazit, Ş.S.; Yildiz, M.; Aşçi, Y.S.; Şahin, M.; Bener, M.; Eğlence, S.; Abdel Salam, M. Rapid adsorptive removal of naphthalene from water using graphene nanoplatelet/MIL-101 (Cr) nanocomposite. J. Alloy. Compd. 2017, 701, 740–749. [Google Scholar] [CrossRef]
- Chang, Q.; Wang, R.; Wang, J.; Muhammad, Y.; Zhao, Z.; Feng, Z.; Huang, Z.; Zhang, Y.; Zhao, Z. Nitrogen-Doped Hollow Copolymer Tube via Template-Free Asynchronous Polymerization with Highly Selective Separation of Hydrophilic Dipeptide for Enhancing Inhibitory Activity of Angiotensin Converting Enzyme. ACS Appl. Mater. Interfaces 2019, 11, 31700–31708. [Google Scholar] [CrossRef]
- Hong, D.-Y.; Hwang, Y.K.; Serre, C.; Férey, G.; Chang, J.-S. Porous Chromium Terephthalate MIL-101 with Coordinatively Unsaturated Sites: Surface Functionalization, Encapsulation, Sorption and Catalysis. Adv. Funct. Mater. 2009, 19, 1537–1552. [Google Scholar] [CrossRef]
- Tan, Z.; Chen, S.; Peng, X.; Zhang, L.; Gao, C. Polyamide membraneswith nanoscale Turing structures for water purification. Science 2018, 360, 518–521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freger, V. Kinetics of Film Formation by Interfacial Polycondensation. Langmuir 2005, 21, 1884–1894. [Google Scholar] [CrossRef] [PubMed]
- Ukrainsky, B.; Ramon, G.Z. Temperature measurement of the reaction zone during polyamide film formation by interfacial polymerization. J. Membr. Sci. 2018, 566, 329–335. [Google Scholar] [CrossRef]
- Duan, J.; Pan, Y.; Pacheco, F.; Litwiller, E.; Lai, Z.; Pinnau, I. High-performance polyamide thin-film-nanocomposite reverse osmosis membranes containing hydrophobic zeolitic imidazolate framework-8. J. Membr. Sci. 2015, 476, 303–310. [Google Scholar] [CrossRef]
- Wang, Y.; Li, X.; Zhao, S.; Fang, Z.; Ng, D.; Xie, C.; Wang, H.; Xie, Z. Thin-Film Composite Membrane with Interlayer Decorated Metal–Organic Framework UiO-66 toward Enhanced Forward Osmosis Performance. Ind. Eng. Chem. Res. 2018, 58, 195–206. [Google Scholar] [CrossRef]
- Tang, C.; Kwon, Y.; Leckie, J. Probing the nano- and micro-scales of reverse osmosis membranes—A comprehensive characterization of physiochemical properties of uncoated and coated membranes by XPS, TEM, ATR-FTIR, and streaming potential measurements. J. Membr. Sci. 2007, 287, 146–156. [Google Scholar] [CrossRef]


| Membranes | Organic Solution | |
|---|---|---|
| TMC (w/v%) | MIL-101(Cr) (w/v%) | |
| M0 | 0.35 | 0 |
| M1 | 0.35 | 0.003 |
| M2 | 0.35 | 0.004 |
| M3 | 0.35 | 0.005 |
| M4 | 0.35 | 0.007 |
| M5 | 0.35 | 0.01 |
| M6 | 0.35 | 0.02 |
| Membranes | C (%) | N (%) | O (%) | Cr (%) | O/Na (%) | O/Nb (%) | Cross-linking Degree c (%) |
|---|---|---|---|---|---|---|---|
| M0 | 67.66 | 14.55 | 17.79 | - | 1.223 | 1.223 | 69.91 |
| M1 | 67.95 | 14.12 | 17.90 | 0.03 | 1.268 | 1.255 | 66.08 |
| M3 | 67.35 | 14.05 | 18.51 | 0.09 | 1.317 | 1.279 | 63.27 |
| M6 | 68.73 | 13.18 | 18.02 | 0.07 | 1.367 | 1.335 | 56.92 |
| Solute | Molecular-Weight (g mol−1) | Chemical Structure | 3D Molecuar Structure | Wavelength (nm) MeOH |
|---|---|---|---|---|
| Rose Bengal (RB) | 1017 | 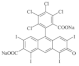 |  | 556 |
| Bromothymol blue (BTB) | 624 | 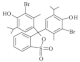 |  | 420 |
| Crystal Violet (CV) | 408 |  |  | 583 |
| Methyl orange (MO) | 327 |  |  | 421 |
| Solvent | d (nm) | Molar Volume (cm−3 mol−1) | Viscosity (cP) | Hansen Solubility Parameter (δ, (MPa1/2)) |
|---|---|---|---|---|
| n-hexane | 86.18 | 1304 | 0.307 | 0 |
| Acetonitrile | - | - | 0.302 | 18 |
| Ethyl acetate | 88.1 | 98 | 0.44 | 5.3 |
| MeOH | 32 | 40.7 | 0.55 | 12.3 |
| EtOH | 46.1 | 58.5 | 1.1 | 8.8 |
| IPA | 60.1 | 76.9 | 2 | 6.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Q.; Wu, X.; Xie, Z.; Zhang, K. Construction of PPSU-MoS2/PA-MIL-101(Cr) Membrane with Highly Enhanced Permeance and Stability for Organic Solvent Nanofiltration. Membranes 2022, 12, 639. https://doi.org/10.3390/membranes12070639
Liu Q, Wu X, Xie Z, Zhang K. Construction of PPSU-MoS2/PA-MIL-101(Cr) Membrane with Highly Enhanced Permeance and Stability for Organic Solvent Nanofiltration. Membranes. 2022; 12(7):639. https://doi.org/10.3390/membranes12070639
Chicago/Turabian StyleLiu, Qin, Xing Wu, Zongli Xie, and Kaisong Zhang. 2022. "Construction of PPSU-MoS2/PA-MIL-101(Cr) Membrane with Highly Enhanced Permeance and Stability for Organic Solvent Nanofiltration" Membranes 12, no. 7: 639. https://doi.org/10.3390/membranes12070639
APA StyleLiu, Q., Wu, X., Xie, Z., & Zhang, K. (2022). Construction of PPSU-MoS2/PA-MIL-101(Cr) Membrane with Highly Enhanced Permeance and Stability for Organic Solvent Nanofiltration. Membranes, 12(7), 639. https://doi.org/10.3390/membranes12070639








