Carbon Nanotube Enhanced Filtration and Dewatering of Kerosene
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Methods
2.2. CNIM Fabrication Process
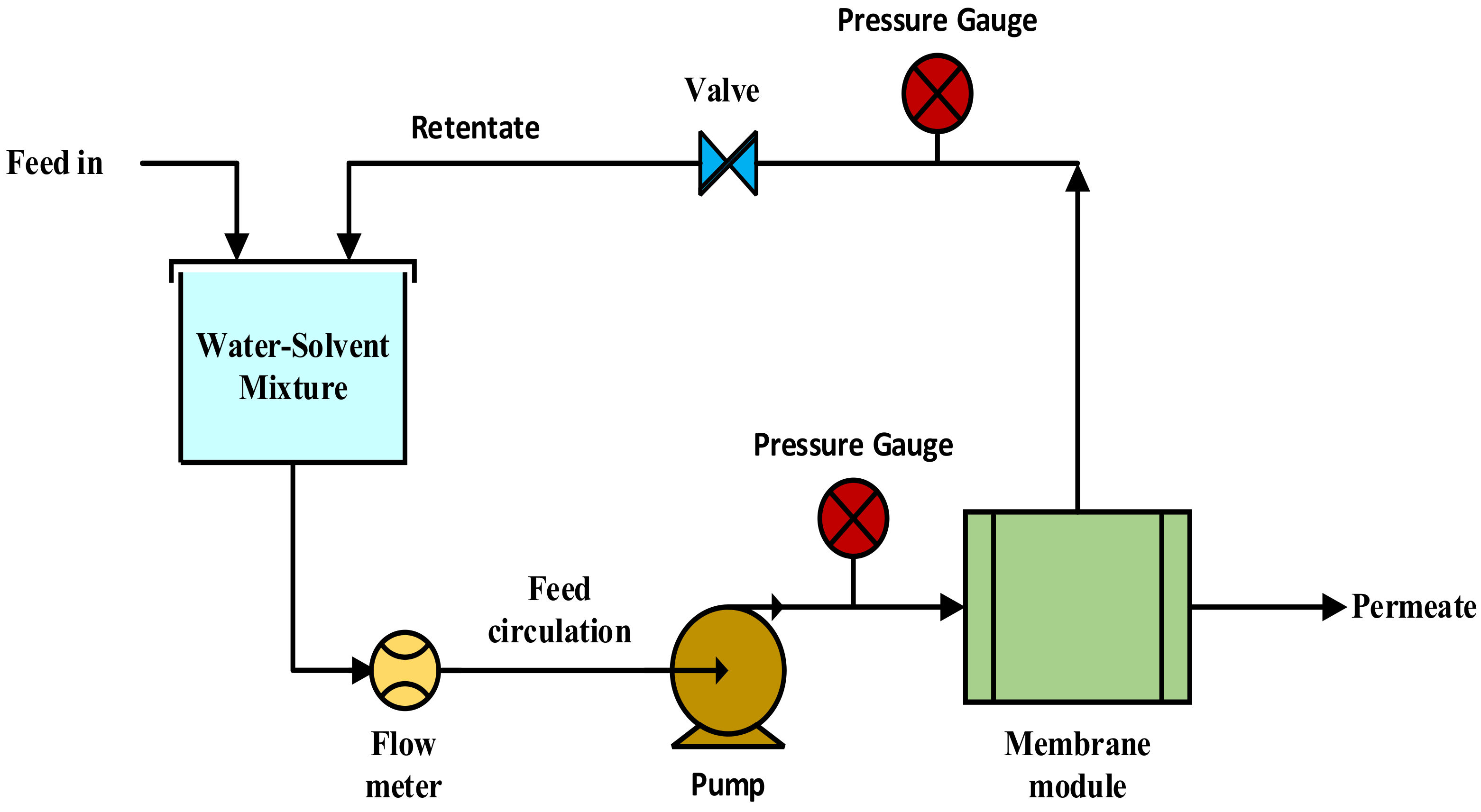
2.3. Filtration Procedure
3. Results and Discussion
3.1. Scanning Electron Microscope of the Membranes
3.2. Contact Angle Measurements
3.3. Membrane Porosity
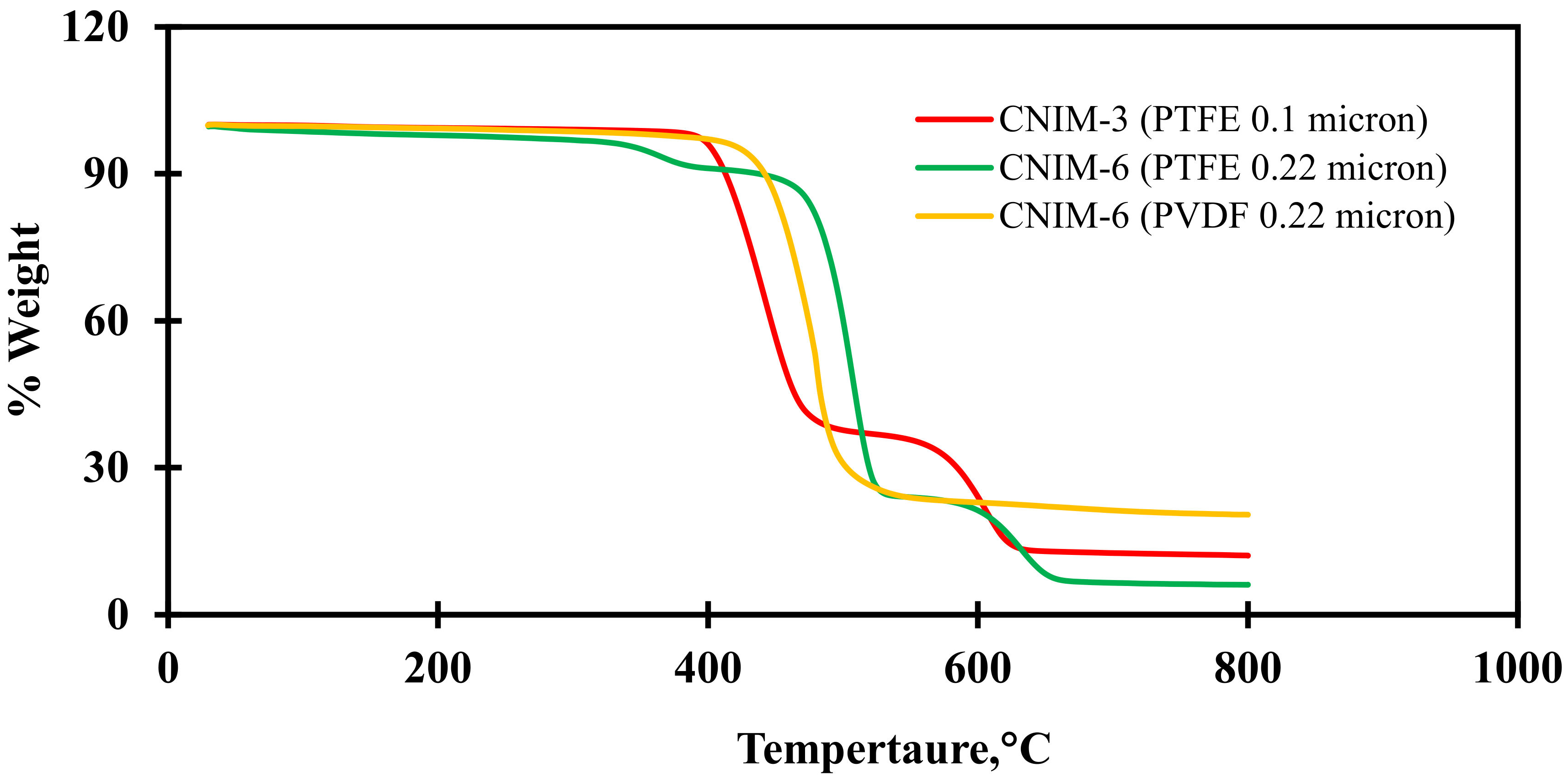
3.4. Thermal Gravimetric Analysis (TGA)
3.5. Optimisation of CNTs Concentrations
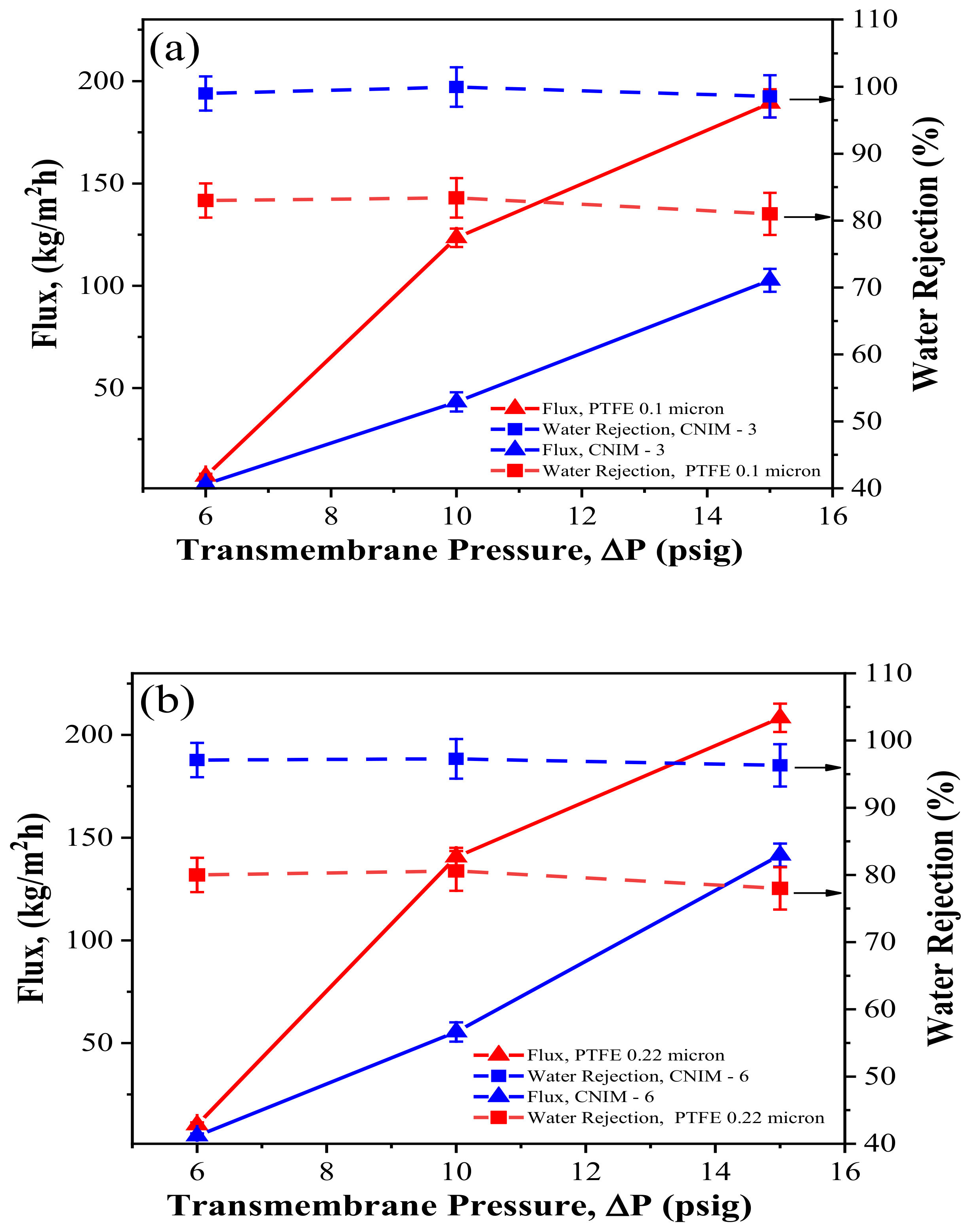
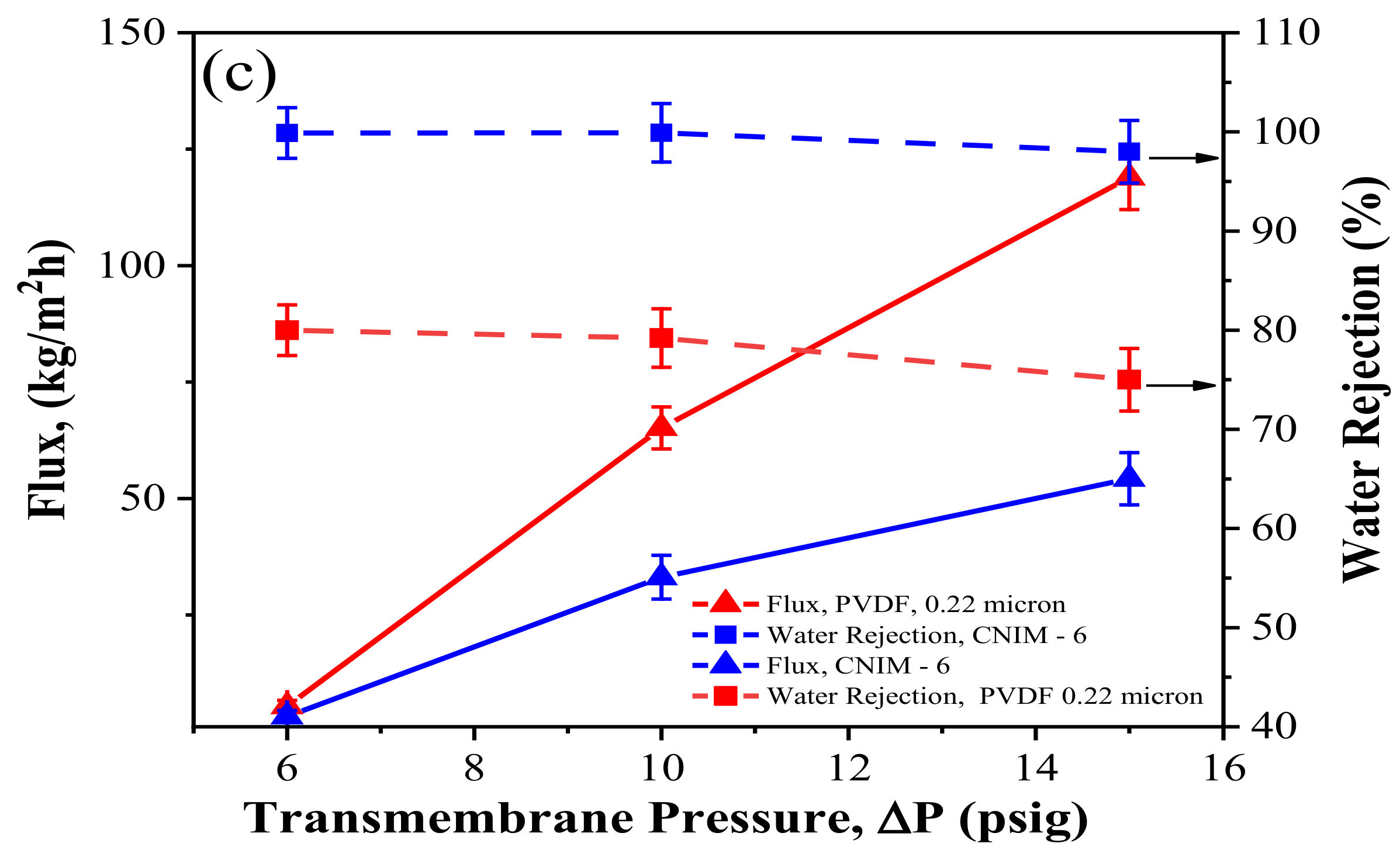
3.6. Effect of Transmembrane Pressure
3.7. Effect of Water Concentration
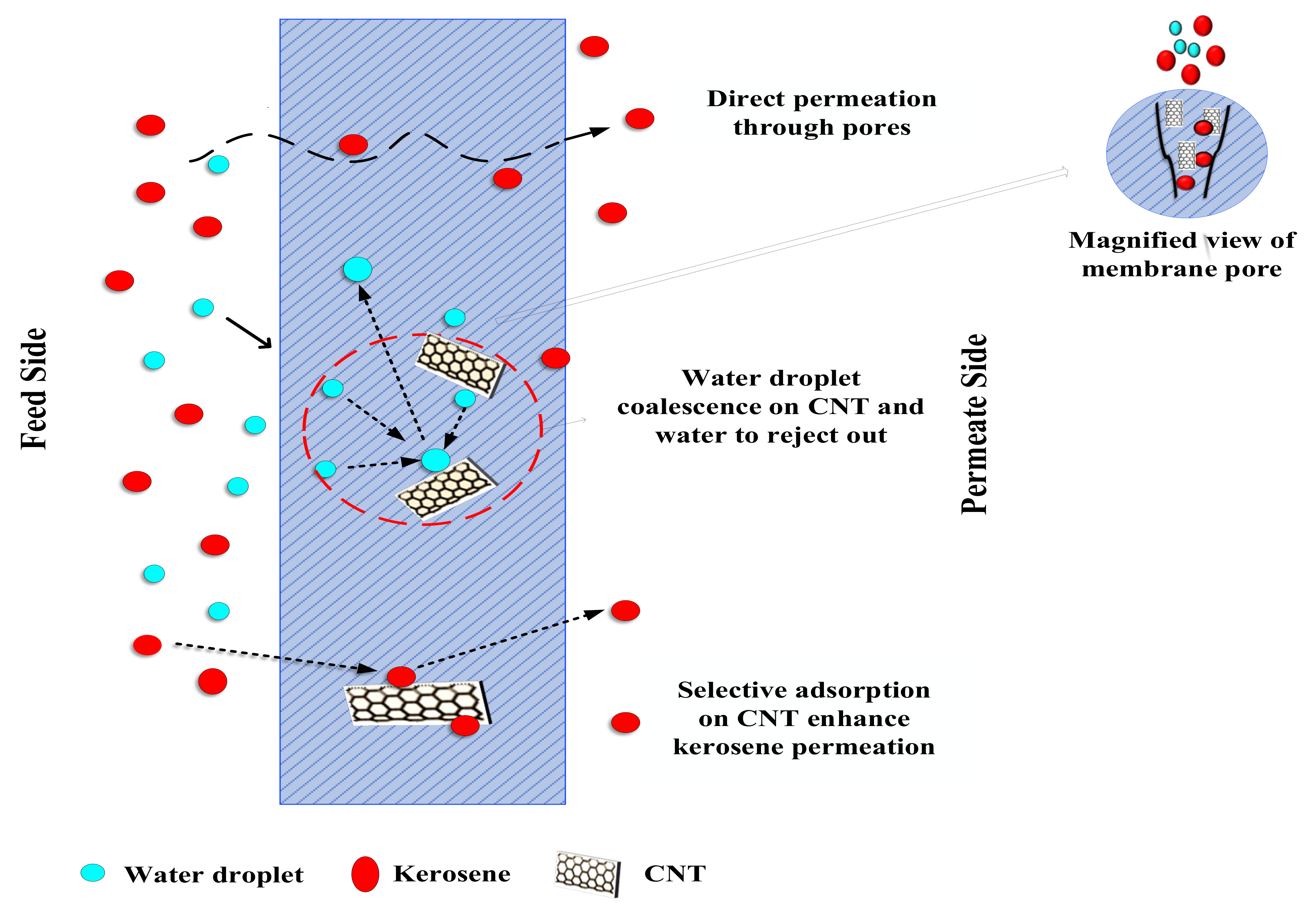
4. Proposed Mechanism
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Korotney, D. Water phase separation in oxygenated gasoline. Recuper. El 1995, 24. [Google Scholar]
- Tadros, T.; Izquierdo, P.; Esquena, J.; Solans, C. Formation and stability of nano-emulsions. Adv. Colloid Interface Sci. 2004, 108, 303–318. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, P.; Jimenez Solomon, M.F.; Szekely, G.; Livingston, A.G. Molecular separation with organic solvent nanofiltration: A critical review. Chem. Rev. 2014, 114, 10735–10806. [Google Scholar] [CrossRef] [PubMed]
- Gebreslase, G.A. Review on Membranes for the Filtration of Aqueous Based Solution: Oil in Water Emulsion. J. Membr. Sci. Technol. 2018, 8. [Google Scholar] [CrossRef]
- Wang, H.; Hu, X.; Ke, Z.; Du, C.Z.; Zheng, L.; Wang, C.; Yuan, Z. Review: Porous metal filters and membranes for oil-water separation. Nanoscale Res. Lett. 2018, 13, 284. [Google Scholar] [CrossRef]
- Aboagye, E.A.; Chea, J.D.; Yenkie, K.M. Systems level roadmap for solvent recovery and reuse in industries. iScience 2021, 24, 103114. [Google Scholar] [CrossRef]
- Bhattacharya, M. Polymer nanocomposites—A Comparison between carbon nanotubes, graphene, and clay as nanofillers. Materials 2016, 9, 262. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, D.; Jiang, L.; Jin, J. Recent progress in developing advanced membranes for emulsified oil/water separation. NPG Asia Mater. 2014, 6, e101. [Google Scholar] [CrossRef]
- Saji, V.S. Carbon nanostructure-based superhydrophobic surfaces and coatings. Nanotechnol. Rev. 2021, 10, 518–571. [Google Scholar] [CrossRef]
- Mittal, G.; Dhand, V.; Rhee, K.Y.; Park, S.-J.; Lee, W.R. A review on carbon nanotubes and graphene as fillers in reinforced polymer nanocomposites. J. Ind. Eng. Chem. 2015, 21, 11–25. [Google Scholar] [CrossRef]
- Madaeni, S.S.; Zinadini, S.; Vatanpour, V. Preparation of superhydrophobic nanofiltration membrane by embedding multiwalled carbon nanotube and polydimethylsiloxane in pores of microfiltration membrane. Sep. Purif. Technol. 2013, 111, 98–107. [Google Scholar] [CrossRef]
- Lee, C.H.; Johnson, N.; Drelich, J.; Yap, Y.K. The performance of superhydrophobic and superoleophilic carbon nanotube meshes in water-oil filtration. Carbon 2011, 49, 669–676. [Google Scholar] [CrossRef]
- Paul, S.; Bhoumick, M.C.; Roy, S.; Mitra, S. Carbon nanotube enhanced membrane filtration for trace level dewatering of hydrocarbons. Sep. Purif. Technol. 2022, 292, 121047. [Google Scholar] [CrossRef]
- Intrchom, W.; Roy, S.; Mitra, S. Removal and recovery of methyl tertiary butyl ether (MTBE) from water using carbon nanotube and graphene oxide immobilized membranes. Nanomaterials 2020, 10, 578. [Google Scholar] [CrossRef] [Green Version]
- Humoud, M.S.; Roy, S.; Mitra, S. Scaling reduction in carbon nanotube-immobilized membrane during membrane distillation. Water 2019, 11, 2588. [Google Scholar] [CrossRef] [Green Version]
- Bhoumick, M.C.; Roy, S.; Mitra, S. Reduction and Elimination of Humic Acid Fouling in Air Sparged Membrane Distillation Using Nanocarbon Immobilized Membrane. Molecules 2022, 27, 2896. [Google Scholar] [CrossRef]
- Gupta, I.; Chakraborty, J.; Roy, S.; Farinas, E.T.; Mitra, S. Nanocarbon immobilized membranes for generating bacteria and endotoxin free water via membrane distillation. Sep. Purif. Technol. 2021, 259, 118133. [Google Scholar] [CrossRef]
- Bhoumick, M.C.; Roy, S.; Mitra, S. Synergistic effect of air sparging in direct contact membrane distillation to control membrane fouling and enhancing flux. Sep. Purif. Technol. 2021, 272, 118681. [Google Scholar] [CrossRef]
- Bhoumick, M.C.; Roy, S.; Mitra, S. Enrichment of 1, 4-dioxane from water by sweep gas membrane distillation on nano-carbon immobilized membranes. Sep. Purif. Technol. 2021, 276, 119360. [Google Scholar]
- Xu, B.Q.; Rao, C.-Q.; Cui, S.-F.; Wang, J.; Wang, J.-L.; Liu, L.-P. Determination of trace water contents of organic solvents by gas chromatography-mass spectrometry-selected ion monitoring. J. Chromatogr. A 2018, 1570, 109–115. [Google Scholar] [CrossRef]
- Saadati, J.; Pakizeh, M. Separation of oil/water emulsion using a new PSf/pebax/F-MWCNT nanocomposite membrane. J. Taiwan Inst. Chem. Eng. 2017, 71, 265–276. [Google Scholar] [CrossRef]
- Gupta, O.; Roy, S.; Mitra, S. Low temperature recovery of acetone-butanol-ethanol (ABE) fermentation products via microwave induced membrane distillation on carbon nanotube immobilized membranes. Sustain. Energy Fuels 2020, 4, 3487–3499. [Google Scholar] [CrossRef]
- Gupta, O.; Roy, S.; Mitra, S. Microwave Induced Membrane Distillation for Enhanced Ethanol–Water Separation on a Carbon Nanotube Immobilized Membrane. Ind. Eng. Chem. Res. 2019, 58, 18313–18319. [Google Scholar] [CrossRef]
- Ragunath, S.; Roy, S.; Mitra, S. Carbon nanotube immobilized membrane with controlled nanotube incorporation via phase inversion polymerization for membrane distillation based desalination. Sep. Purif. Technol. 2018, 194, 249–255. [Google Scholar] [CrossRef]
- Hong, A.; Fane, A.G.; Burford, R. Factors affecting membrane coalescence of stable oil-in-water emulsions. J. Membr. Sci. 2003, 222, 19–39. [Google Scholar] [CrossRef]
- Lipp, P.; Lee, C.H.; Fane, A.G.; Fell, C.J.D. A fundamental study of the ultrafiltration of oil-water emulsions. J. Membr. Sci. 1988, 36, 161–177. [Google Scholar] [CrossRef]
- Qin, Z.; Xiang, H.; Liu, J.; Zeng, X. High-performance oil-water separation polytetrafluoroethylene membranes prepared by picosecond laser direct ablation and drilling. Mater. Des. 2019, 184, 108200. [Google Scholar] [CrossRef]
- Nazzal, F.F.; Wiesner, M.R. Microfiltration of oil-in-water emulsions. Water Environ. Res. 1996, 68, 1187–1191. [Google Scholar] [CrossRef] [Green Version]
- Gethard, K.; Sae-Khow, O.; Mitra, S. Carbon nanotube enhanced membrane distillation for simultaneous generation of pure water and concentrating pharmaceutical waste. Sep. Purif. Technol. 2012, 90, 239–245. [Google Scholar] [CrossRef]
- Gupta, I.; Azizighannad, S.; Farinas, E.T.; Mitra, S. Antiviral properties of select carbon nanostructures and their functionalized analogs. Mater. Today Commun. 2021, 29, 102743. [Google Scholar] [CrossRef]
- Gupta, O.; Roy, S.; Mitra, S. Nanocarbon-immobilized membranes for separation of tetrahydrofuran from water via membrane distillation. ACS Appl. Nano Mater. 2020, 3, 6344–6353. [Google Scholar] [CrossRef]
- Su, F.; Lu, C. Adsorption kinetics, thermodynamics and desorption of natural dissolved organic matter by multiwalled carbon nanotubes. J. Environ. Sci. Health Part A 2007, 42, 1543–1552. [Google Scholar] [CrossRef] [PubMed]
- Hussain, C.M.; Saridara, C.; Mitra, S. Self-assembly of carbon nanotubes via ethanol chemical vapor deposition for the synthesis of gas chromatography columns. Anal. Chem. 2010, 82, 5184–5188. [Google Scholar] [CrossRef] [PubMed]
- Di, Z.; Chen, H.; Yin, Z.; Cui, C.; Qian, W.; Han, M. Carbon nanotube-alumina strips as robust, rapid, reversible adsorbents of organics. RSC Adv. 2018, 8, 10715–10718. [Google Scholar] [CrossRef] [Green Version]
- Ji, D.; Xiao, C.; An, S.; Liu, H.; Chen, K.; Hao, J.; Zhang, T. Preparation of PSF/FEP mixed matrix membrane with super hydrophobic surface for efficient water-in-oil emulsion separation. RSC Adv. 2018, 8, 10097–10106. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Wang, J.; Han, X.; Li, L.; Liu, E.; Lu, C. Carbon Nanotubes and Polydopamine Modified Poly(dimethylsiloxane) Sponges for Efficient Oil-Water Separation. Materials 2021, 14, 2431. [Google Scholar] [CrossRef]
- Xu, H.; Chang, C.; Yi, N.; Tao, P.; Song, C.; Wu, J.; Deng, T.; Shang, W. Coalescence, spreading, and rebound of two water droplets with different temperatures on a superhydrophobic surface. ACS Omega 2019, 4, 17615–17622. [Google Scholar] [CrossRef]
- Li, Z.; Wang, B.; Qin, X.; Wang, Y. Superhydrophobic/superoleophilic polycarbonate/carbon nanotubes porous monolith for selective oil adsorption from water. ACS Sustain. Chem. Eng. 2018, 6, 13747–13755. [Google Scholar] [CrossRef]


| Membrane | Contact Angle | % Water Rejection * |
|---|---|---|
| 0.1-micron PTFE | 122 ± 2° | 83.40 |
| 0.22-micron PTFE | 112 ± 2° | 80.60 |
| 0.22-micron PVDF | 92 ± 2° | 79.00 |
| CNIM-3 (0.1-micron PTFE) | 133 ± 2° | 99.97 |
| CNIM-6 (0.22-micron PTFE) | 130 ± 2° | 97.27 |
| CNIM-6 (0.22-micron PVDF) | 130 ± 2° | 99.9 |
| Membrane Performance | PTFE 0.1 Micron | PTFE 0.22 Micron | PVDF 0.22 Micron | ||||||
|---|---|---|---|---|---|---|---|---|---|
| CNIM-2 | CNIM-3 | CNIM-6 | CNIM-3 | CNIM-6 | CNIM-8 | CNIM-3 | CNIM-6 | CNIM-8 | |
| Water Contact Angle (°) | 130 ± 2 | 133 ± 2 | 134 ± 2 | 125 ± 2 | 130 ± 2 | 132 ± 2 | 123 ± 2 | 130 ± 2 | 131 ± 2 |
| Flux (kg/m2 h) | 56.18 | 43.221 | 36.83 | 62.76 | 55.44 | 43.12 | 37.65 | 33.12 | 21.12 |
| Water rejection (%) | 95.34 | 99.97 | 100 | 94.10 | 97.27 | 98.00 | 93.00 | 99.90 | 99.97 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paul, S.; Bhoumick, M.C.; Roy, S.; Mitra, S. Carbon Nanotube Enhanced Filtration and Dewatering of Kerosene. Membranes 2022, 12, 621. https://doi.org/10.3390/membranes12060621
Paul S, Bhoumick MC, Roy S, Mitra S. Carbon Nanotube Enhanced Filtration and Dewatering of Kerosene. Membranes. 2022; 12(6):621. https://doi.org/10.3390/membranes12060621
Chicago/Turabian StylePaul, Sumona, Mitun Chandra Bhoumick, Sagar Roy, and Somenath Mitra. 2022. "Carbon Nanotube Enhanced Filtration and Dewatering of Kerosene" Membranes 12, no. 6: 621. https://doi.org/10.3390/membranes12060621
APA StylePaul, S., Bhoumick, M. C., Roy, S., & Mitra, S. (2022). Carbon Nanotube Enhanced Filtration and Dewatering of Kerosene. Membranes, 12(6), 621. https://doi.org/10.3390/membranes12060621









