Solution-Processed Silicon Doped Tin Oxide Thin Films and Thin-Film Transistors Based on Tetraethyl Orthosilicate
Abstract
1. Introduction
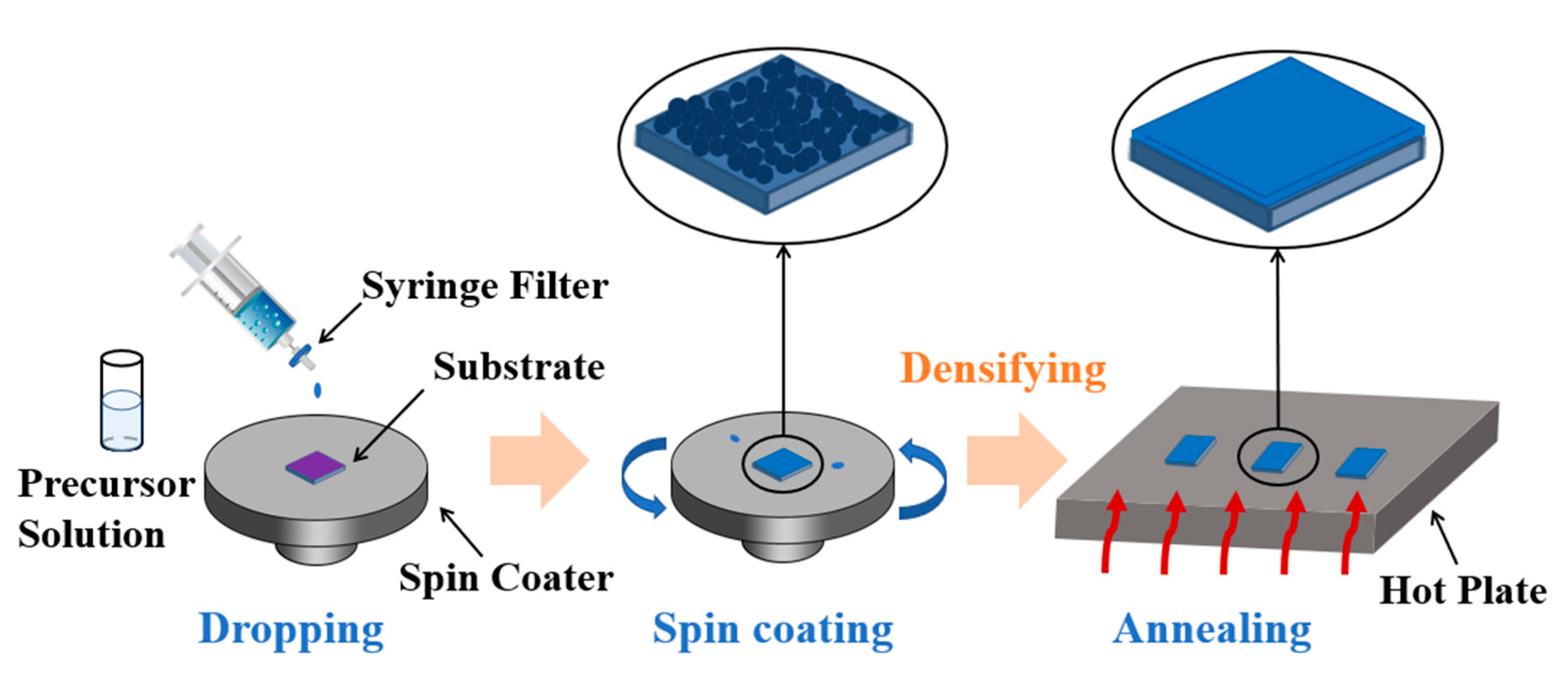
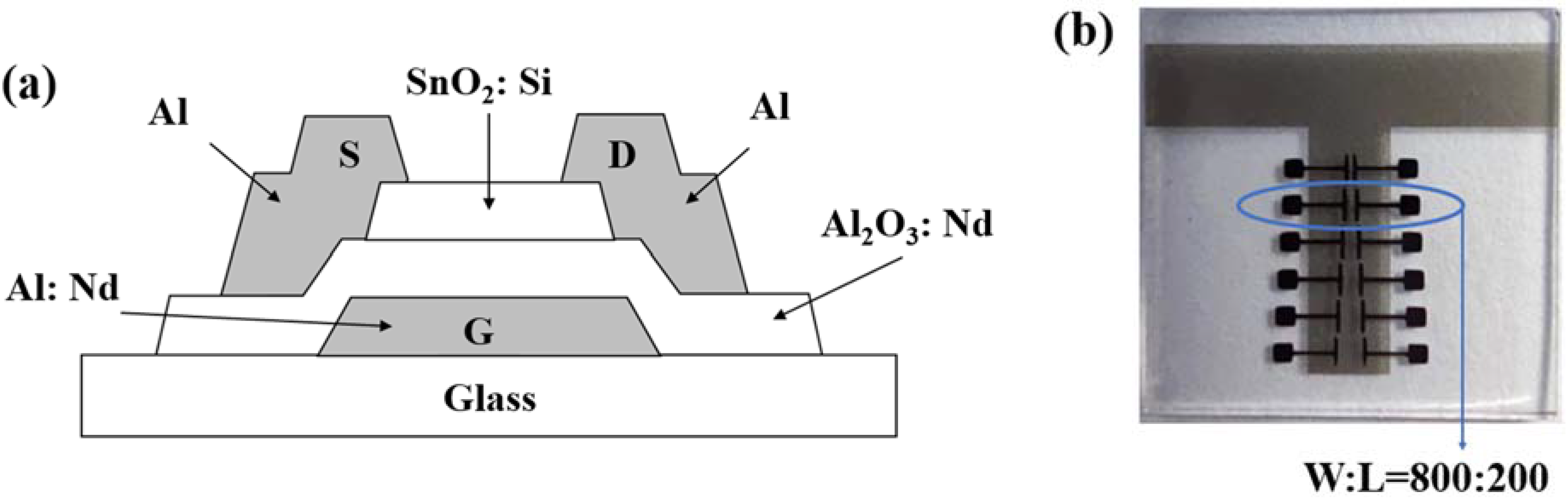
2. Materials and Methods
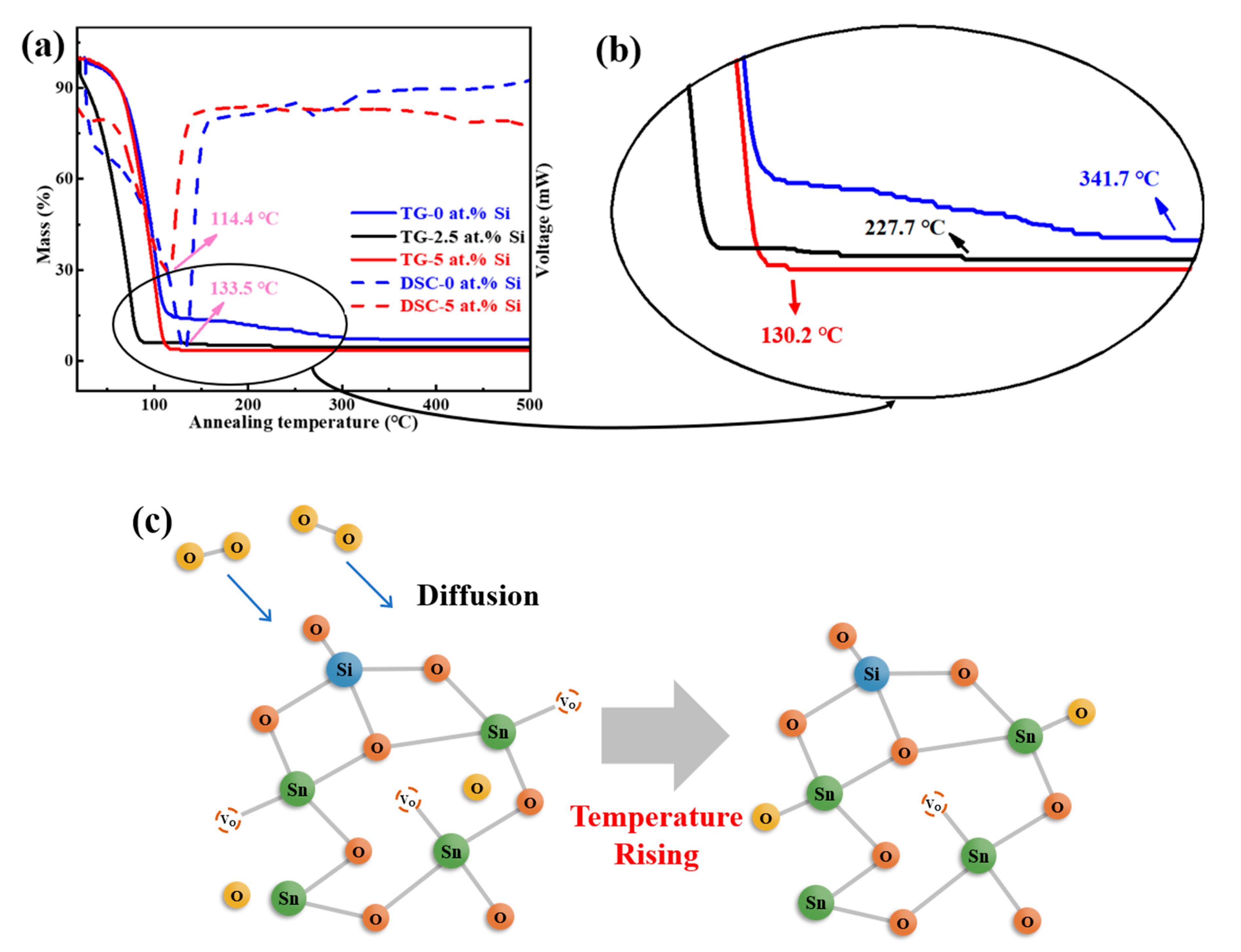
3. Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Jeong, J.K. The status and perspectives of metal oxide thin- film transistors for active matrix flexible displays. Semicond. Sci. Technol. 2011, 26, 34008. [Google Scholar] [CrossRef]
- Ahn, B.D.; Jeon, H.; Sheng, J.; Park, J.; Park, J. A review on the recent developments of solution processes for oxide thin film transistors. Semicond. Sci. Technol. 2015, 30, 64001. [Google Scholar] [CrossRef]
- Fukuda, K.; Takeda, Y.; Mizukami, M.; Kumaki, D.; Tokito, S. Fully solution-processed flexible organic thin film transistor arrays with high mobility and exceptional uniformity. Sci. Rep. 2015, 4, 3947. [Google Scholar] [CrossRef] [PubMed]
- Wager, J.F. Oxide TFTs: A progress report. Frontline Technol. 2016, 32, 16–21. [Google Scholar] [CrossRef]
- Yu, X.; Marks, T.J.; Facchetti, A. Metal oxides for optoelectronic applications. Nat. Mater. 2016, 15, 383–396. [Google Scholar] [CrossRef] [PubMed]
- Jenifer, K.; Arulkumar, S.; Parthiban, S.; Kwon, J.Y. A review on the recent advancements in tin oxide-based thin-film transistors for large-area electronics. J. Electron. Mater. 2020, 49, 7098–7111. [Google Scholar] [CrossRef]
- Nomura, K.; Hiromichi, O.; Akihiro, T.; Toshio, K.; Hosono, M.H.H. Room-temperature fabrication of transparent flexible thin-film. Nature 2004, 432, 488–492. [Google Scholar] [CrossRef]
- Hosono, H. Ionic amorphous oxide semiconductors: Material design, carrier transport, and device application. J. Non-Cryst. Solids 2006, 352, 851–858. [Google Scholar] [CrossRef]
- Saji, K.J.; Mary, A.P.R. Tin oxide based p and n-type thin film transistors developed by RF sputtering. ECS J. Solid State Sci. Technol. 2015, 4, Q101–Q104. [Google Scholar] [CrossRef]
- Jadhav, H.S.S.; Suryawanshi, M.A. Pulsed laser deposition of tin oxide thin films for field emission studies. Appl. Surf. Sci. 2017, 419, 764–769. [Google Scholar] [CrossRef]
- Park, J.; Oh, K.T.; Kim, D.H.; Jeong, H.J.; Park, Y.C.; Kim, H.S.; Park, J.S. High-performance zinc tin oxide semiconductor grown by atmospheric-pressure mist-CVD and the associated thin-film transistor properties. ACS Appl. Mater. Interfaces 2017, 9, 20656–20663. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Zheng, H.; Li, L.; Chen, S. Highly conducting and transparent antimony doped tin oxide thin films: The role of sputtering power density. Ceram. Int. 2017, 43, 5654–5660. [Google Scholar] [CrossRef]
- Kamiya, T.; Hosono, H. Material characteristics and applications of transparent amorphous oxide semiconductors. NPG Asia Mater. 2010, 2, 15–22. [Google Scholar] [CrossRef]
- Kuo, Y. Thin film transistor technology—Past, present, and future. Interface Mag. 2013, 22, 55–61. [Google Scholar] [CrossRef]
- Thomas, S.R.; Pattanasattayavong, P.; Anthopoulos, T.D. Solution-processable metal oxide semiconductors for thin-film transistor applications. Chem. Soc. Rev. 2013, 42, 6910. [Google Scholar] [CrossRef]
- Younis, A.; Chu, D.; Li, S. Voltage sweep modulated conductance quantization in oxide nanocomposites. J. Mater. Chem. C 2014, 2, 10291–10297. [Google Scholar] [CrossRef]
- Du, H.; Wang, Y.; Arandiyan, H.; Younis, A.; Scott, J.; Qu, B.; Wan, T.; Lin, X.; Chen, J.; Chu, D. Design and synthesis of CeO2 nanowire/MnO2 nanosheet heterogeneous structure for enhanced catalytic properties. Mater. Today Commun. 2017, 11, 103–111. [Google Scholar] [CrossRef]
- Tsay, C.; Liang, S. Fabrication of p-type conductivity in SnO2 thin films through Ga doping. J. Alloys Compd. 2015, 622, 644–650. [Google Scholar] [CrossRef]
- Liu, X.; Ning, H.; Chen, J.; Cai, W.; Hu, S.; Tao, R.; Zeng, Y.; Zheng, Z.; Yao, R.; Xu, M.; et al. High-performance back-channel-etched thin-film transistors with amorphous Si-incorporated SnO2 active layer. Appl. Phys. Lett. 2016, 108, 112106. [Google Scholar] [CrossRef]
- Jo, K.; Moon, S.; Cho, W. Fabrication of high-performance ultra-thin-body SnO2 thin-film transistors using microwave-irradiation post-deposition annealing. Appl. Phys. Lett. 2015, 106, 43501. [Google Scholar] [CrossRef]
- Zhang, L.; Zhu, D.; Han, S.; Lu, Y.; Fang, M.; Liu, W.; Cao, P.; Xu, W. Aqueous solution deposition of amorphous gallium tin oxide for thin-film transistors applications. Ceram. Int. 2020, 46, 19557–19563. [Google Scholar] [CrossRef]
- Cojocaru, B.; Avram, D.; Kessler, V.; Parvulescu, V.; Seisenbaeva, G.; Tiseanu, C. Nanoscale insights into doping behavior, particle size and surface effects in trivalent metal doped SnO2. Sci. Rep. 2017, 7, 9598. [Google Scholar] [CrossRef] [PubMed]
- Kiisk, V.; Kangur, T.; Paalo, M.; Tätte, T.; Lange, S.; Pikker, S.; Sildos, I. Structural and luminescence characteristics of SnO2: Eu and SnO2: Eu, Sb nanophosphors upon annealing at high temperatures. Mater. Chem. Phys. 2011, 130, 293–298. [Google Scholar] [CrossRef]
- García-Tecedor, M.; Maestre, D.; Cremades, A.; Piqueras, J. Influence of Cr doping on the morphology and luminescence of SnO2 nanostructures. J. Phys. Chem. C 2016, 120, 22028–22034. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, X.; Ning, H.; Yuan, W.; Deng, Y.; Zhang, X.; Wang, S.; Wang, J.; Yao, R.; Peng, J. Characterization studies of the structure and properties of Zr-doped SnO2 thin films by spin-coating technique. Superlattices Microstruct. 2018, 123, 330–337. [Google Scholar] [CrossRef]
- Lee, C.; Lee, W.; Lee, H.; Ha, S.; Bae, J.; Kang, I.; Kang, H.; Kim, K.; Jang, J. Sol-gel processed yttrium-doped SnO2 thin film transistors. Electronics 2020, 9, 254. [Google Scholar] [CrossRef]
- Corsino, D.C.; Bermundo, J.P.S.; Kulchaisit, C.; Fujii, M.N.; Ishikawa, Y.; Ikenoue, H.; Uraoka, Y. High-performance fully solution-processed oxide thin-film transistors via photo-assisted role tuning of InZnO. ACS Appl. Electron. Mater. 2020, 2, 2398–2407. [Google Scholar] [CrossRef]
- Kang, I.; Park, C.H.; Chong, E.; Lee, S.Y. Role of Si as carrier suppressor in amorphous Zn-Sn-O. Curr. Appl. Phys. 2012, 12, S12–S16. [Google Scholar] [CrossRef]
- Tricoli, A.; Graf, M.; Pratsinis, S.E. Optimal doping for enhanced SnO2 sensitivity and thermal stability. Adv. Funct. Mater. 2008, 18, 1969–1976. [Google Scholar] [CrossRef]
- Liu, X.; Ning, H.; Zhang, X.; Deng, Y.; Guo, D.; Wang, Y.; Wang, X.; Yuan, W.; Yao, R.; Peng, J. Flexible thin-film transistors application of amorphous tin oxide-based semiconductors. J. Soc. Inf. Disp. 2019, 27, 769–775. [Google Scholar] [CrossRef]
- Fortunato, E.; Barquinha, P.; Martins, R. Oxide semiconductor thin-film transistors: A review of recent advances. Adv. Mater. 2012, 24, 2945–2986. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Luo, D.; Li, M.; Xu, M.; Zou, J.; Tao, H.; Lan, L.; Wang, L.; Peng, J.; Cao, Y. A flexible AMOLED display on the PEN substrate driven by oxide thin-film transistors using anodized aluminium oxide as dielectric. J. Mater. Chem. C 2014, 2, 1255–1259. [Google Scholar] [CrossRef]
- Kim, G.H.; Shin, H.S.; Ahn, B.D.; Kim, K.H.; Park, W.J.; Kim, H.J. Formation mechanism of solution-processed nanocrystalline InGaZnO thin film as active channel layer in thin-Film transistor. J. Electrochem. Soc. 2009, 156, H7. [Google Scholar] [CrossRef]
- Van Der Vis, M.G.M.; Cordfunke, E.; Konings, R. The thermodynamic properties of tetraethoxysilane (TEOS) and an infrared study of its thermal decomposition. J. Phys. IV 1993, 3, C3–C75. [Google Scholar] [CrossRef][Green Version]
- Nurkowski, D.; Buerger, P.; Akroyd, J.; Kraft, M. A detailed kinetic study of the thermal decomposition of tetraethoxysilane. Proc. Combust. Inst. 2015, 35, 2291–2298. [Google Scholar] [CrossRef]
- Liu, M.; Yu, L.; Zhang, Y.; Zhang, S.; Yang, Y.; Liu, R. Preparation and characterization of superparamagnetic α-Fe2O3/Fe3O4 @ SiO2 nanocomposites via a citric-TEOS-ethanol solution combustion process. Mater. Res. Express 2021, 8, 15013. [Google Scholar] [CrossRef]
- Serwickab, H.M. Laponite-derived porous clay heterostructures Synthesis and physicochemical characterization. Microporous Mesoporous Mater. 2010, 127, 228–236. [Google Scholar]
- Yasuno, S.; Kugimiya, T.; Morita, S.; Miki, A.; Ojima, F.; Sumie, S. Correlation of photoconductivity response of amorphous In-Ga-Zn-O films with transistor performance using microwave photoconductivity decay method. Appl. Phys. Lett. 2011, 98, 102107. [Google Scholar] [CrossRef]
- Goto, H.; Tao, H.; Morita, S.; Takanashi, Y.; Hino, A.; Kishi, T.; Ochi, M.; Hayashi, K.; Kugimiya, T. In-line process monitoring for amorphous oxide semiconductor TFT fabrication using microwave-detected photoconductivity decay technique. IEICE Trans. Electron. 2014, 97, 1055–1062. [Google Scholar] [CrossRef]
- Dong, C.; Qu, Z.; Jiang, X.; Ren, Y. Tuning oxygen vacancy concentration of MnO2 through metal doping for improved toluene oxidation. J. Hazard. Mater. 2020, 391, 122181. [Google Scholar] [CrossRef]
- Singhal, R.K.; Samariya, A.; Kumar, S.; Xing, Y.T.; Jain, D.C.; Dolia, S.N.; Deshpande, U.P.; Shripathi, T.; Saitovitch, E.B. Study of defect-induced ferromagnetism in hydrogenated anatase TiO2: Co. J. Appl. Phys. 2010, 107, 113916. [Google Scholar] [CrossRef]
- Yan, Y.; Wei, S. Doping asymmetry in wide-bandgap semiconductors: Origins and solutions. Phys. Status Solidi B 2008, 245, 641–652. [Google Scholar] [CrossRef]
- Dutta, D.; Bahadur, D. Influence of confinement regimes on magnetic property of pristine SnO2 quantum dots. J. Mater. Chem. 2012, 22, 24545. [Google Scholar] [CrossRef]
- Wang, Z.; Zheng, J.; Li, M.; Wu, Q.; Huang, B.; Chen, C.; Wu, J.; Liu, C. Solution-based SnGaO thin-film transistors for Zn- and In-free oxide electronic devices. Appl. Phys. Lett. 2018, 113, 122101. [Google Scholar] [CrossRef]
- Gunawan, C.; Lord, M.S.; Lovell, E.; Wong, R.J.; Jung, M.S.; Oscar, D.; Mann, R.; Amal, R. Oxygen-vacancy engineering of cerium-oxide nanoparticles for antioxidant activity. ACS Omega 2019, 4, 9473–9479. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Tyagi, B.P. On-current modeling of polycrystalline silicon thin-film transistors. Phys. Scr. 2005, 72, 339. [Google Scholar] [CrossRef]
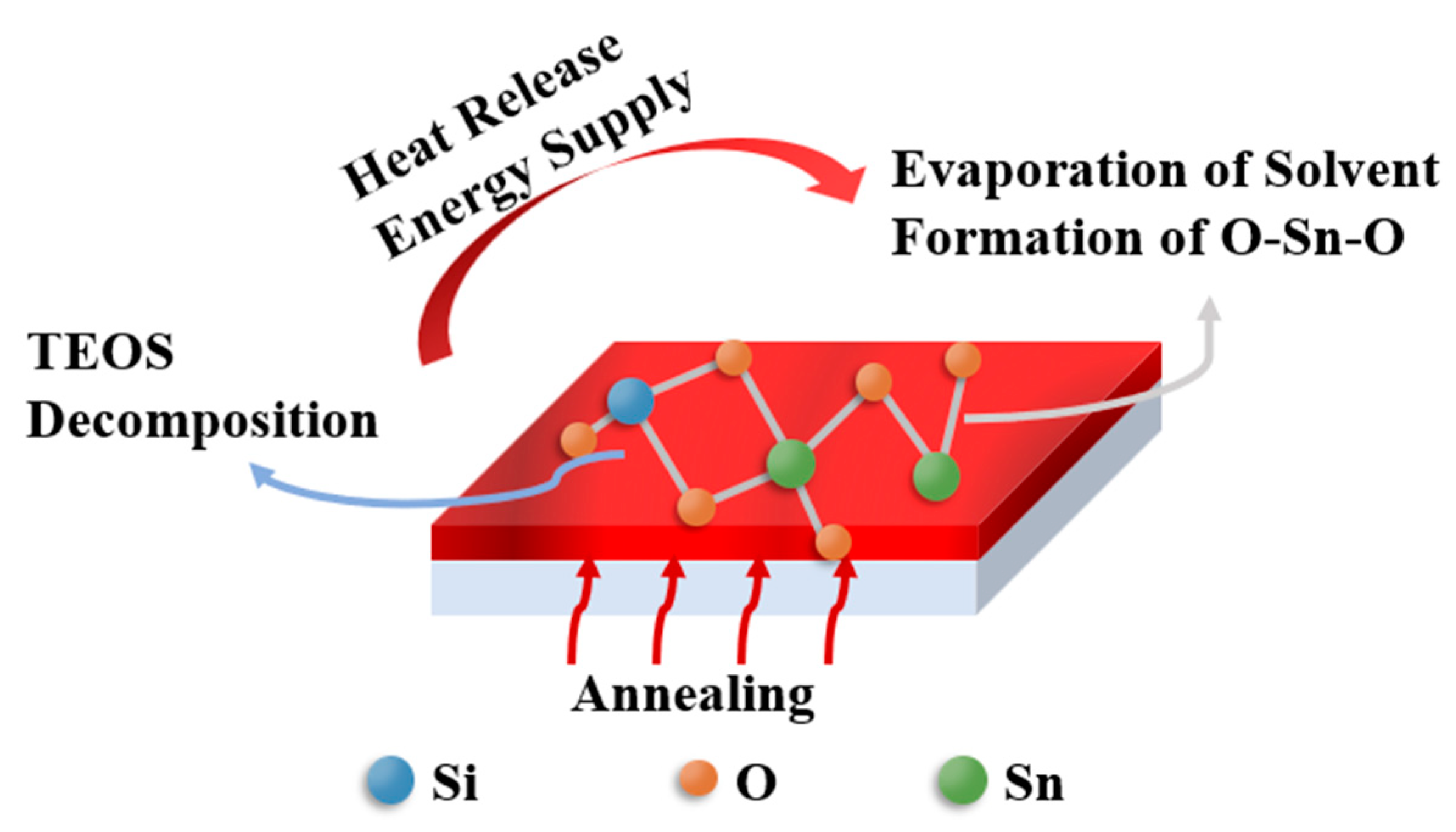
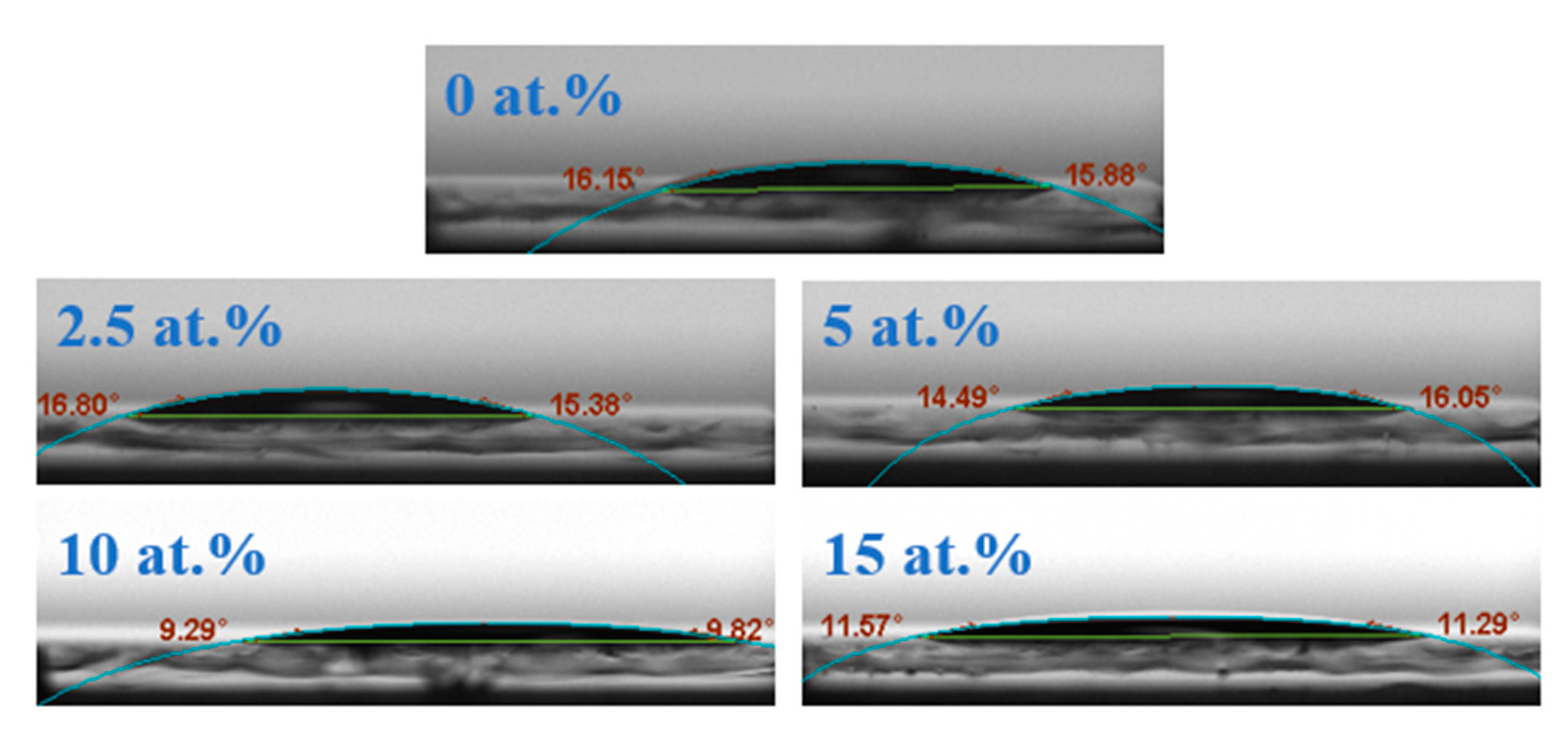
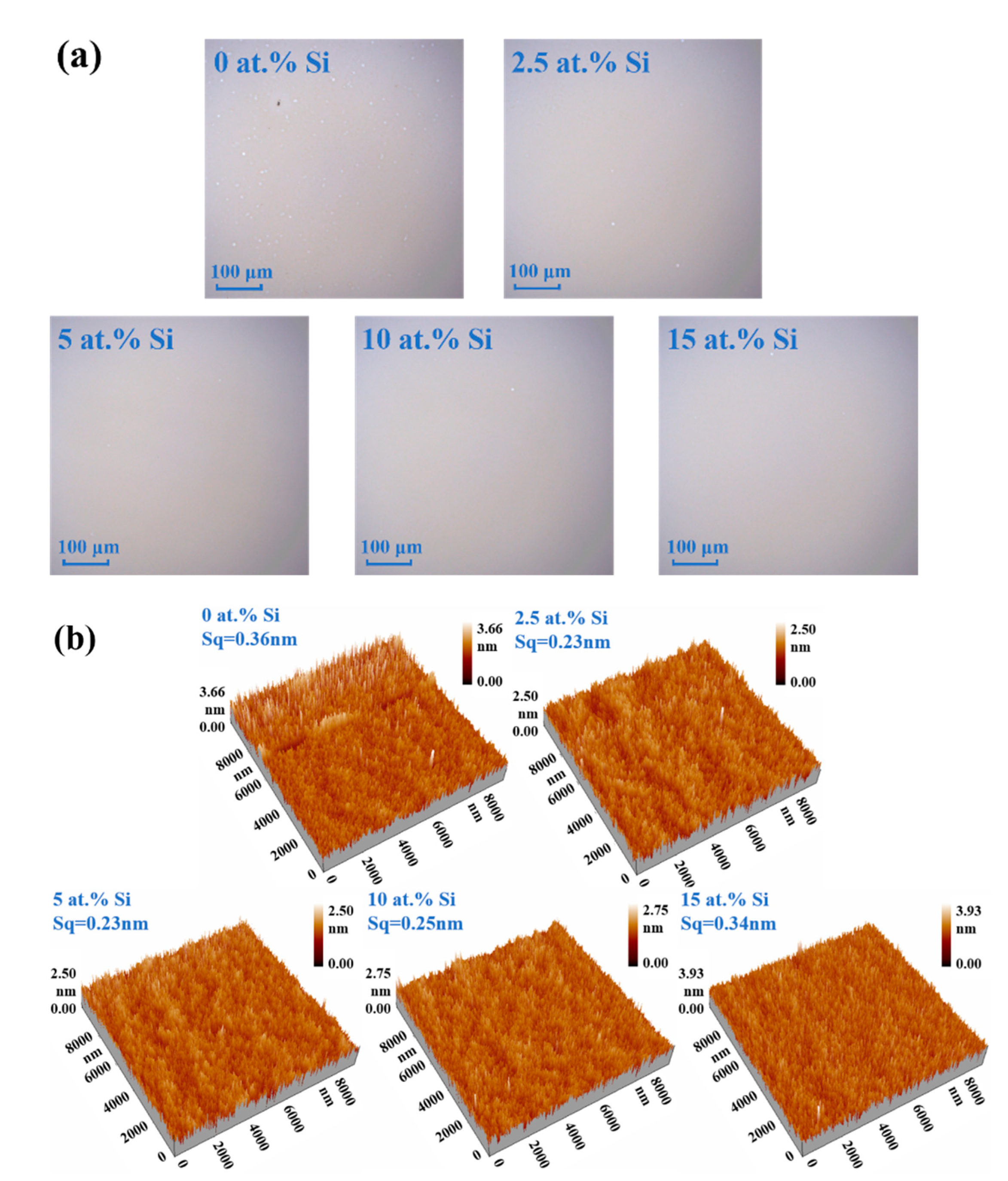
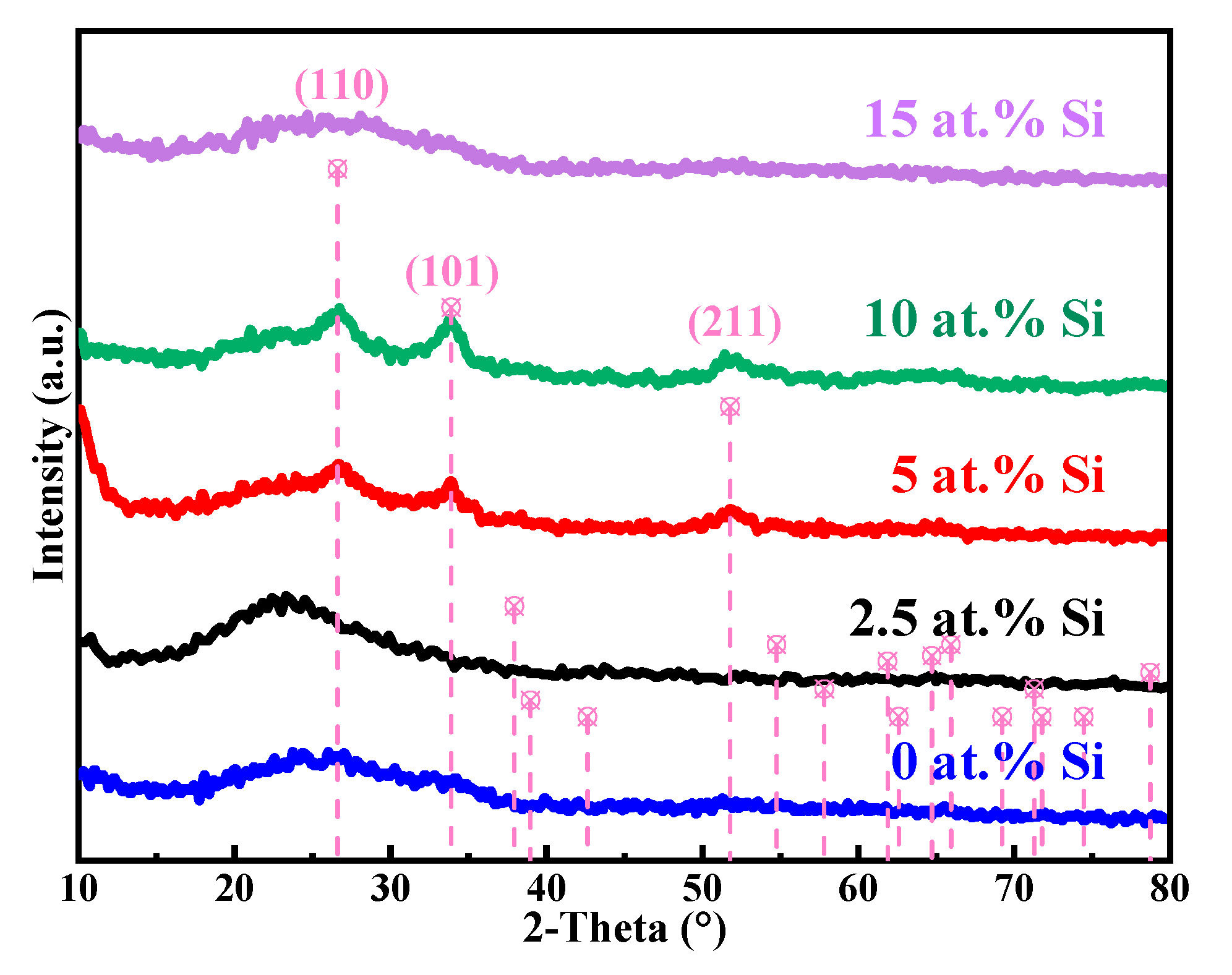
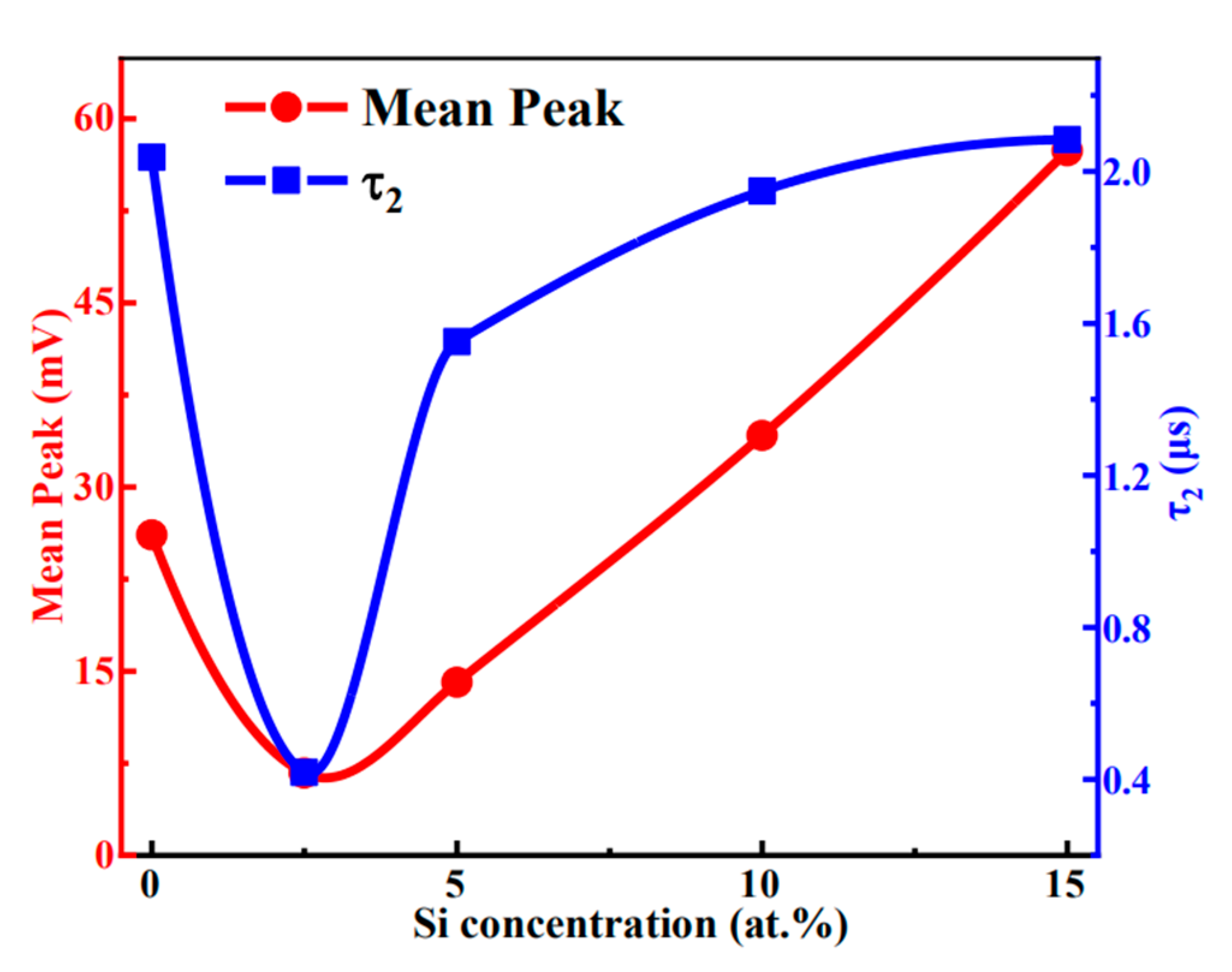
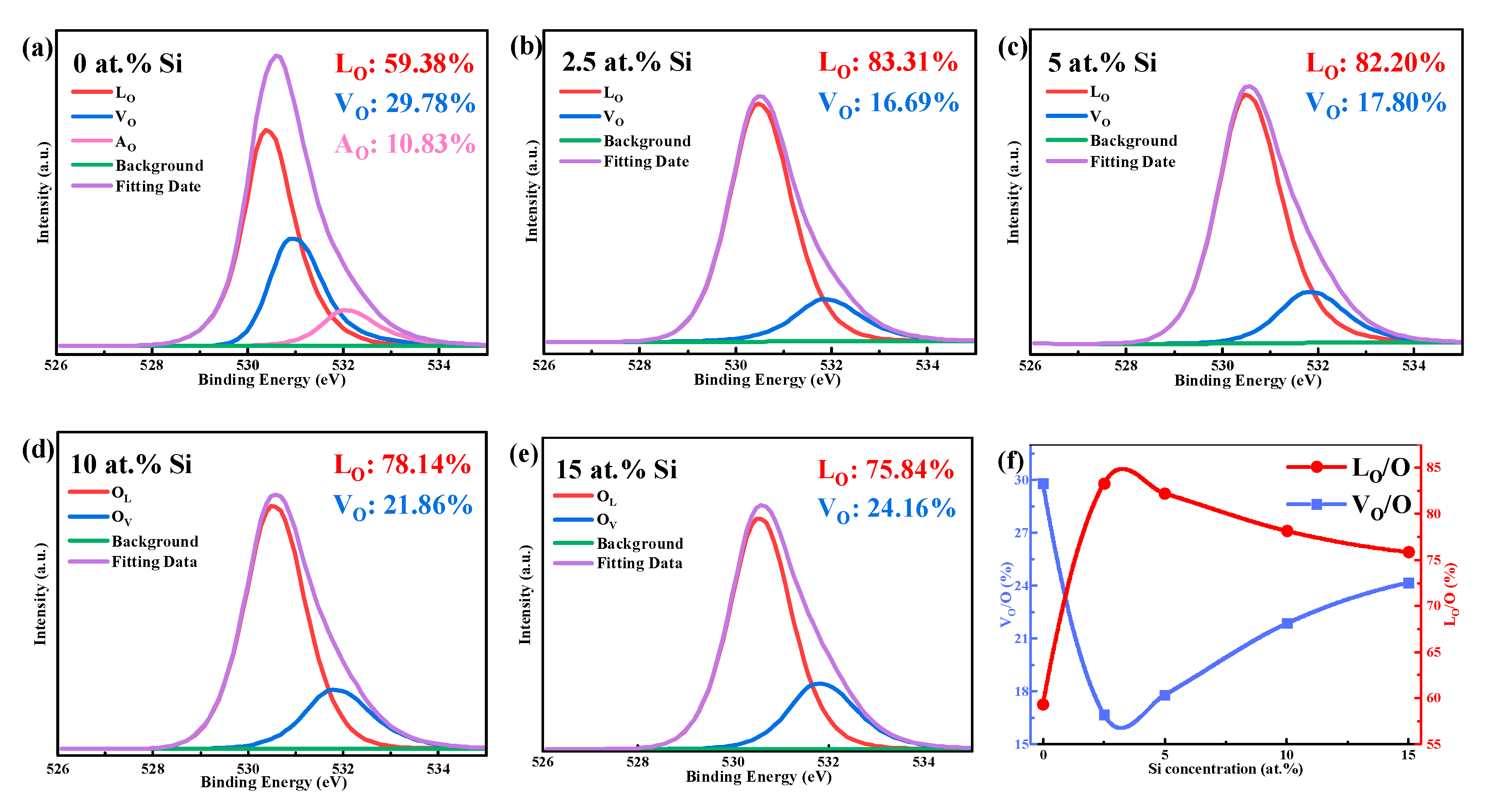
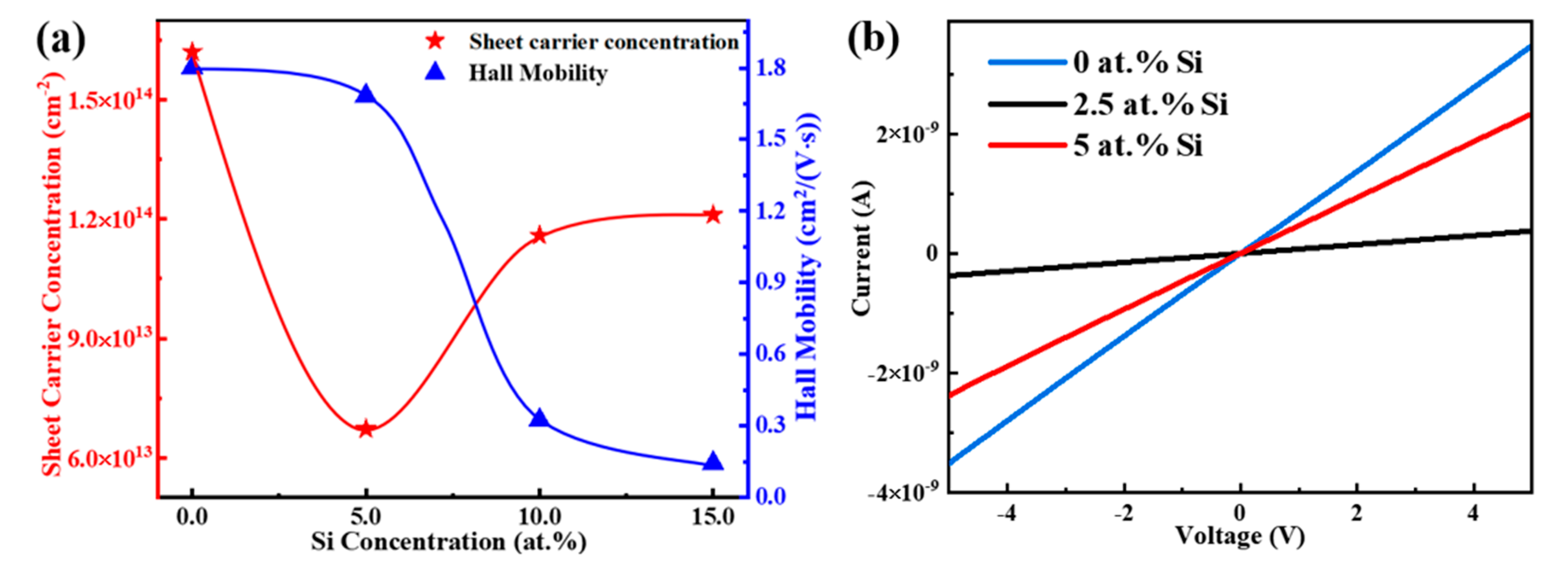
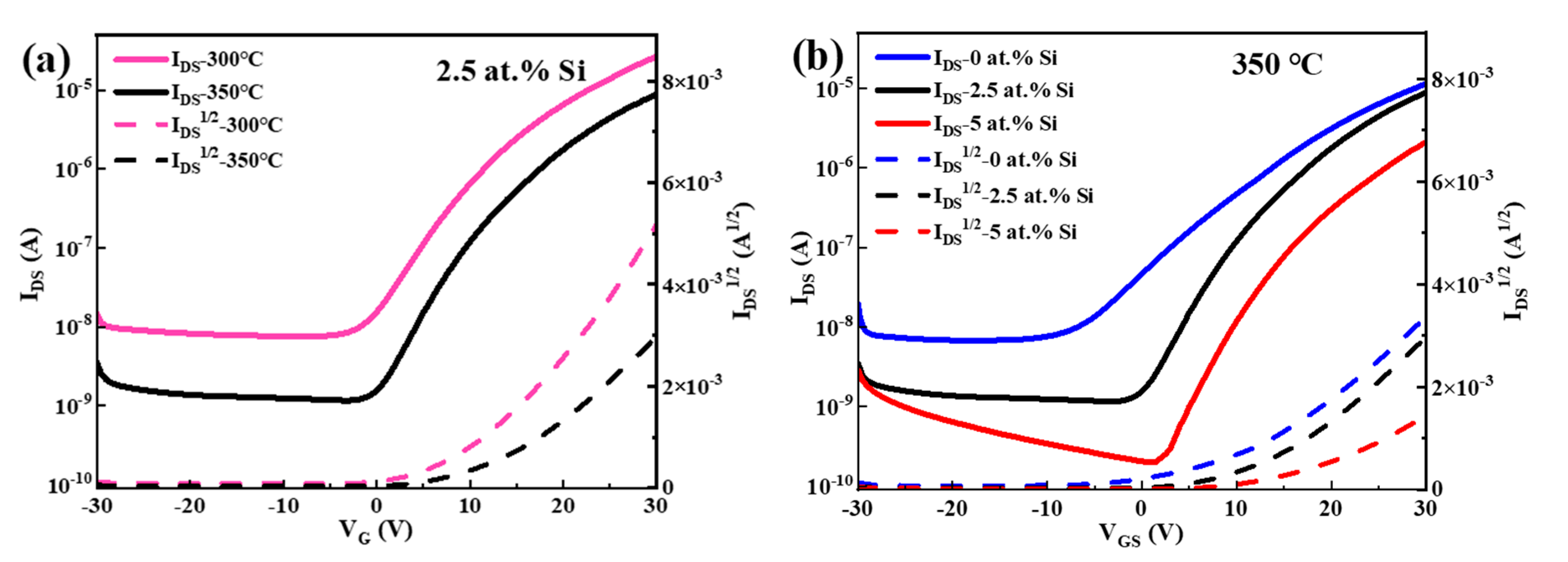
| Si (at.%) | Ion (A) | Ioff (A) | Ion/Ioff | Von (V) | μ (cm2/(V·s)) | SS (V/Dec) |
|---|---|---|---|---|---|---|
| 0.0 | 1.04 × 10−5 | 6.76 × 10−9 | 1.54 × 103 | −17.22 | 0.05 | 8.73 |
| 2.5 | 8.84 × 10−6 | 1.19 × 10−9 | 7.43 × 103 | −2.00 | 0.32 | 4.24 |
| 5.0 | 2.10 × 10−6 | 2.01 × 10−10 | 1.04 × 104 | 1.51 | 0.13 | 3.48 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Z.; Zhang, X.; Wei, X.; Luo, D.; Ning, H.; Ye, Q.; Wu, R.; Guo, Y.; Yao, R.; Peng, J. Solution-Processed Silicon Doped Tin Oxide Thin Films and Thin-Film Transistors Based on Tetraethyl Orthosilicate. Membranes 2022, 12, 590. https://doi.org/10.3390/membranes12060590
He Z, Zhang X, Wei X, Luo D, Ning H, Ye Q, Wu R, Guo Y, Yao R, Peng J. Solution-Processed Silicon Doped Tin Oxide Thin Films and Thin-Film Transistors Based on Tetraethyl Orthosilicate. Membranes. 2022; 12(6):590. https://doi.org/10.3390/membranes12060590
Chicago/Turabian StyleHe, Ziyan, Xu Zhang, Xiaoqin Wei, Dongxiang Luo, Honglong Ning, Qiannan Ye, Renxu Wu, Yao Guo, Rihui Yao, and Junbiao Peng. 2022. "Solution-Processed Silicon Doped Tin Oxide Thin Films and Thin-Film Transistors Based on Tetraethyl Orthosilicate" Membranes 12, no. 6: 590. https://doi.org/10.3390/membranes12060590
APA StyleHe, Z., Zhang, X., Wei, X., Luo, D., Ning, H., Ye, Q., Wu, R., Guo, Y., Yao, R., & Peng, J. (2022). Solution-Processed Silicon Doped Tin Oxide Thin Films and Thin-Film Transistors Based on Tetraethyl Orthosilicate. Membranes, 12(6), 590. https://doi.org/10.3390/membranes12060590








