Ionomer Membranes Produced from Hexaarylbenzene-Based Partially Fluorinated Poly(arylene ether) Blends for Proton Exchange Membrane Fuel Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. General Methods
2.2. General Procedure for Producing Ionomers
2.3. Preparation of Blend Membranes
2.4. Measurements
2.4.1. Oxidative Stability
2.4.2. Water Uptake and Dimensional Stability
2.4.3. Ion Exchange Capacity
2.4.4. Proton Conductivity
2.4.5. Microstructure Analysis
2.4.6. Single-cell Performance
2.5. Simulation Method
- The aromatic ring has a low rate of sulfonation substitution because of the deactivation effect in the presence of an electron-withdrawing group (e.g., −CF3 and −SO3H) [53].
- Under mild reaction conditions, the substitution reaction on the benzene ring tends to be monosubstitution or substitution at specific positions (e.g., at the 2,7 position on the fluorene ring [46]).
- The active position has high steric hindrance and a low reaction rate [53].
3. Results and Discussion
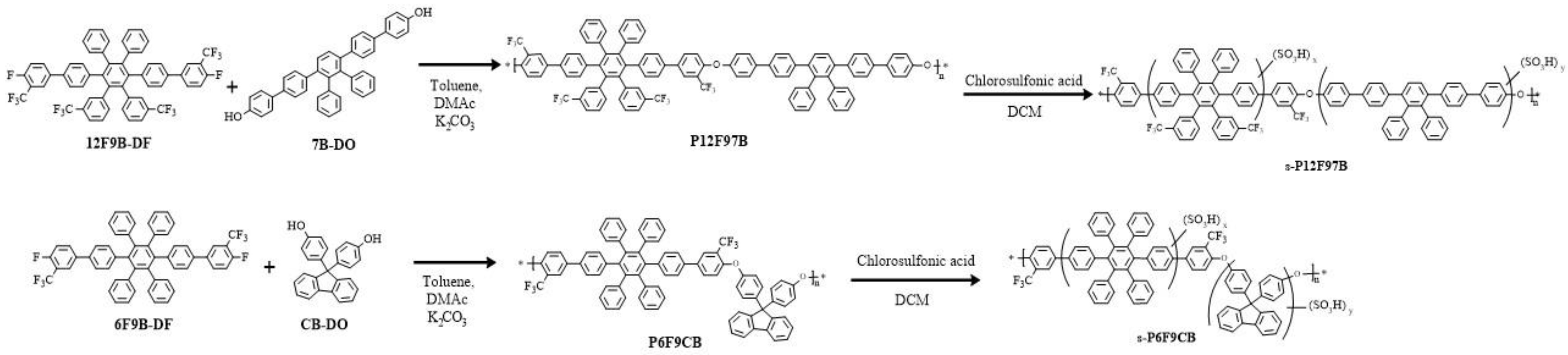
3.1. Synthesis and Characterization of the Monomers and Polymers
3.2. Thermal Stability
3.3. Mechanical Properties
3.4. Oxidative Stability
3.5. Hydration Behavior
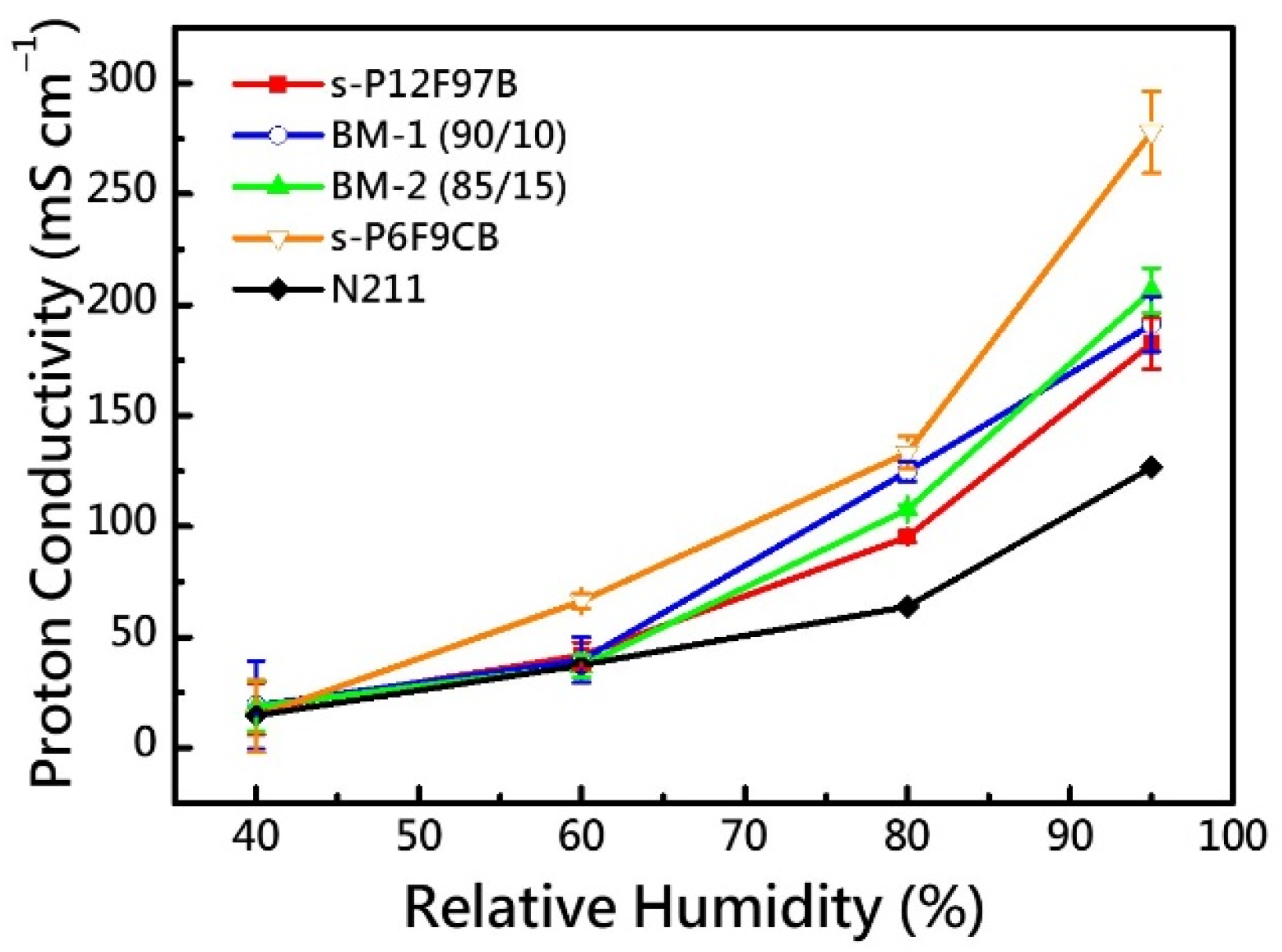
3.6. Proton Conductivity
3.7. Microstructure Analysis
3.8. Fuel Cell Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kusoglu, A.; Weber, A.Z. New Insights into Perfluorinated Sulfonic-Acid lonomers. Chem. Rev. 2017, 117, 987–1104. [Google Scholar] [CrossRef] [PubMed]
- Dodds, P.E.; Staffell, L.; Hawkes, A.D.; Li, F.; Grunewald, P.; McDowall, W.; Ekins, P. Hydrogen and fuel cell technologies for heating: A review. Int. J. Hydrog. Energy 2015, 40, 2065–2083. [Google Scholar] [CrossRef]
- Kraytsberg, A.; Ein-Eli, Y. Review of Advanced Materials for Proton Exchange Membrane Fuel Cells. Energy Fuels 2014, 28, 7303–7330. [Google Scholar] [CrossRef]
- Zhang, J. PEM Fuel Cell Electrocatalysts and Catalyst Layers: Fundamentals and Applications; Springer: Berlin/Heidelberg, Germany, 2008. [Google Scholar]
- Wu, Z.; Tan, P.; Chen, B.; Cai, W.; Chen, M.; Xu, X.; Zhang, Z.; Ni, M. Dynamic modeling and operation strategy of an NG-fueled SOFC-WGS-TSA-PEMFC hybrid energy conversion system for fuel cell vehicle by using MATLAB/SIMULINK. Energy 2019, 175, 567–579. [Google Scholar] [CrossRef]
- Tanç, B.; Arat, H.T.; Baltacıoğlu, E.; Aydın, K. Overview of the next quarter century vision of hydrogen fuel cell electric vehicles. Int. J. Hydrog. Energy 2019, 44, 10120–10128. [Google Scholar] [CrossRef]
- Johnston, B.; Mayo, M.C.; Khare, A. Hydrogen: The energy source for the 21st century. Technovation 2005, 25, 569–585. [Google Scholar] [CrossRef]
- Miller, A.I. Heavy water: A manufacturers’ guide for the hydrogen century. Can. Nucl. Soc. Bull. 2001, 22, 1–14. [Google Scholar]
- Kim, S.; Melnyk, A.; Andrienko, D.; Suzuki, Y. Solid-State Electron Affinity Analysis of Amorphous Fluorinated Polymer Electret. J. Phys. Chem. B 2020, 124, 10507–10513. [Google Scholar] [CrossRef]
- Li, Y.T.; Zhang, X.L.; He, G.H.; Zhang, F.X. Sulfonated poly(phenylene sulfide) grafted polysulfone proton exchange membrane with improved stability. Int. J. Hydrog. Energy 2017, 42, 2360–2369. [Google Scholar] [CrossRef]
- Pirali-Hamedani, M.; Mehdipour-Ataei, S. Effect of sulfonation degree on molecular weight, thermal stability, and proton conductivity of poly(arylene ether sulfone)s membrane. Des. Monomers Polym. 2017, 20, 54–65. [Google Scholar] [CrossRef]
- Andujar, J.M.; Segura, F. Fuel cells: History and updating. A walk along two centuries. Renew. Sustain. Energy Rev. 2009, 13, 2309–2322. [Google Scholar] [CrossRef]
- Alashkar, A.; Al-Othman, A.; Tawalbeh, M.; Qasim, M. A Critical Review on the Use of Ionic Liquids in Proton Exchange Membrane Fuel Cells. Membranes 2022, 12, 178. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Tan, Y.; Li, B.; Ming, P.; Xiao, Q.; Zhang, C. A Review of the Transition Region of Membrane Electrode Assembly of Proton Exchange Membrane Fuel Cells: Design, Degradation, and Mitigation. Membranes 2022, 12, 306. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.-Y.; Zhang, H.; Luo, X.-D.; Chen, N. A comparative study of Nafion and sulfonated poly (ether ether ketone) membrane performance for iron-chromium redox flow battery. Ionics 2019, 25, 4219–4229. [Google Scholar] [CrossRef]
- Mendil-Jakani, H.; López, I.Z.; Mareau, V.; Gonon, L. Optimization of hydrophilic/hydrophobic phase separation in sPEEK membranes by hydrothermal treatments. Phys. Chem. Chem. Phys. 2017, 19, 16013–16022. [Google Scholar] [CrossRef] [PubMed]
- Slack, J.; Halevi, B.; McCool, G.; Li, J.; Pavlicek, R.; Wycisk, R.; Mukerjee, S.; Pintauro, P. Electrospun Fiber Mat Cathode with Platinum-Group-Metal-Free Catalyst Powder and Nafion/PVDF Binder. ChemElectroChem 2018, 5, 1537–1542. [Google Scholar] [CrossRef]
- Padilha, J.C.; Basso, J.; Da Trindade, L.G.; Martini, E.M.; De Souza, M.O.; De Souza, R.F. Ionic liquids in proton exchange membrane fuel cells: Efficient systems for energy generation. J. Power Sources 2010, 195, 6483–6485. [Google Scholar] [CrossRef]
- Lee, S.; Mukerjee, S.; McBreen, J.; Rho, Y.; Kho, Y.; Lee, T. Effects of Nafion impregnation on performances of PEMFC electrodes. Electrochim. Acta 1998, 43, 3693–3701. [Google Scholar] [CrossRef]
- Peron, J.; Mani, A.; Zhao, X.; Edwards, D.; Adachi, M.; Soboleva, T.; Shi, Z.; Xie, Z.; Navessin, T.; Holdcroft, S. Properties of Nafion® NR-211 membranes for PEMFCs. J. Membr. Sci. 2010, 356, 44–51. [Google Scholar] [CrossRef]
- Li, G.; Pickup, P.G. Ionic conductivity of PEMFC electrodes: Effect of Nafion loading. J. Electrochem. Soc. 2003, 150, C745. [Google Scholar] [CrossRef]
- Ramani, V.; Kunz, H.; Fenton, J. Investigation of Nafion®/HPA composite membranes for high temperature/low relative humidity PEMFC operation. J. Membr. Sci. 2004, 232, 31–44. [Google Scholar] [CrossRef]
- Lin, J.; Liu, Y.; Zhang, Q. Charge dynamics and bending actuation in Aquivion membrane swelled with ionic liquids. Polymer 2011, 52, 540–546. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Boaretti, C.; Pasquini, L.; Sood, R.; Giancola, S.; Donnadio, A.; Roso, M.; Modesti, M.; Cavaliere, S. Mechanically stable nanofibrous sPEEK/Aquivion® composite membranes for fuel cell applications. J. Membr. Sci. 2018, 545, 66–74. [Google Scholar] [CrossRef]
- D’urso, C.; Oldani, C.; Baglio, V.; Merlo, L.; Aricò, A. Towards fuel cell membranes with improved lifetime: Aquivion® Perfluorosulfonic Acid membranes containing immobilized radical scavengers. J. Power Sources 2014, 272, 753–758. [Google Scholar] [CrossRef]
- Siracusano, S.; Baglio, V.; Stassi, A.; Merlo, L.; Moukheiber, E.; Arico, A. Performance analysis of short-side-chain Aquivion® perfluorosulfonic acid polymer for proton exchange membrane water electrolysis. J. Membr. Sci. 2014, 466, 1–7. [Google Scholar] [CrossRef]
- Yoshida, N.; Ishisaki, T.; Watakabe, A.; Yoshitake, M. Characterization of Flemion® membranes for PEFC. Electrochim. Acta 1998, 43, 3749–3754. [Google Scholar] [CrossRef]
- Savadogo, O.; Tian, H. Composite Membranes Cast from Silicotungstic Acid (STA), Nafion, or Flemion in Dimethylformamide for Polymer Electrolyte Fuel Cells (PEMFCs). In ECS Meeting Abstracts; IOP Publishing: Bristol, UK, 2006; p. 969. [Google Scholar]
- Endoh, E. Development of highly durable PFSA membrane and MEA for PEMFC under high temperature and low humidity conditions. Ecs Trans. 2008, 16, 1229. [Google Scholar] [CrossRef]
- Kusoglu, A.; Dursch, T.J.; Weber, A.Z. Nanostructure/Swelling Relationships of Bulk and Thin-Film PFSA Ionomers. Adv. Funct. Mater. 2016, 26, 4961–4975. [Google Scholar] [CrossRef]
- Clark, J.K., II; Paddison, S.J.; Hamrock, S.J. The effect of hydrogen bond reorganization and equivalent weight on proton transfer in 3M perfluorosulfonic acid ionomers. Phys. Chem. Chem. Phys. 2012, 14, 16349–16359. [Google Scholar] [CrossRef]
- Yandrasits, M.; Lindell, M.; Schaberg, M.; Kurkowski, M. Increasing fuel cell efficiency by using ultra-low equivalent weight ionomers. Electrochem. Soc. Interface 2017, 26, 49. [Google Scholar] [CrossRef]
- Liu, Y.; Horan, J.L.; Schlichting, G.J.; Caire, B.R.; Liberatore, M.W.; Hamrock, S.J.; Haugen, G.M.; Yandrasits, M.A.; Seifert, S.n.; Herring, A.M. A small-angle X-ray scattering study of the development of morphology in films formed from the 3M perfluorinated sulfonic acid ionomer. Macromolecules 2012, 45, 7495–7503. [Google Scholar] [CrossRef]
- Zuo, Z.; Fu, Y.; Manthiram, A. Novel blend membranes based on acid-base interactions for fuel cells. Polymers 2012, 4, 1627–1644. [Google Scholar] [CrossRef]
- Campagne, B.; David, G.; Ameduri, B.; Jones, D.J.; Roziere, J.; Roche, I. Novel blend membranes of partially fluorinated copolymers bearing azole functions with sulfonated PEEK for PEMFC operating at low relative humidity: Influence of the nature of the N-heterocycle. Macromolecules 2013, 46, 3046–3057. [Google Scholar] [CrossRef]
- Şengül, E.; Erdener, H.; Akay, R.G.; Yücel, H.; Bac, N.; Eroğlu, İ. Effects of sulfonated polyether-etherketone (SPEEK) and composite membranes on the proton exchange membrane fuel cell (PEMFC) performance. Int. J. Hydrog. Energy 2009, 34, 4645–4652. [Google Scholar] [CrossRef]
- Guo, Z.; Xiu, R.; Lu, S.; Xu, X.; Yang, S.; Xiang, Y. Submicro-pore containing poly (ether sulfones)/polyvinylpyrrolidone membranes for high-temperature fuel cell applications. J. Mater. Chem. A 2015, 3, 8847–8854. [Google Scholar] [CrossRef]
- Hagesteijn, K.F.; Jiang, S.; Ladewig, B.P. A review of the synthesis and characterization of anion exchange membranes. J. Mater. Sci. 2018, 53, 11131–11150. [Google Scholar] [CrossRef]
- Xu, T. Ion exchange membranes: State of their development and perspective. J. Membr. Sci. 2005, 263, 1–29. [Google Scholar] [CrossRef]
- Xiang, Y.; Yang, M.; Guo, Z.; Cui, Z. Alternatively chitosan sulfate blending membrane as methanol-blocking polymer electrolyte membrane for direct methanol fuel cell. J. Membr. Sci. 2009, 337, 318–323. [Google Scholar] [CrossRef]
- Wang, J.; Liao, J.; Yang, L.; Zhang, S.; Huang, X.; Ji, J. Highly compatible acid–base blend membranes based on sulfonated poly (ether ether ketone) and poly (ether ether ketone-alt-benzimidazole) for fuel cells application. J. Membr. Sci. 2012, 415, 644–653. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, Y.; Deng, X.; Zhou, G.; Khalid, S.; Sun, X.; Sun, W.; Zhou, Q.; Lu, G. Dissipative particle dynamics and molecular dynamics simulations on mesoscale structure and proton conduction in a SPEEK/PVDF-g-PSSA membrane. RSC Adv. 2017, 7, 39676–39684. [Google Scholar] [CrossRef]
- Dong, T.; Hu, J.; Ueda, M.; Wu, Y.; Zhang, X.; Wang, L. Enhanced proton conductivity of multiblock poly (phenylene ether ketone) s via pendant sulfoalkoxyl side chains with excellent H 2/air fuel cell performance. J. Mater. Chem. A 2016, 4, 2321–2331. [Google Scholar] [CrossRef]
- Komarov, P.; Veselov, I.; Chu, P.; Khalatur, P.; Khokhlov, A. Atomistic and mesoscale simulation of polymer electrolyte membranes based on sulfonated poly (ether ether ketone). Chem. Phys. Lett. 2010, 487, 291–296. [Google Scholar] [CrossRef]
- Du, C.M.; Ji, Y.J.; Xue, J.W.; Hou, T.J.; Tang, J.X.; Lee, S.T.; Li, Y.Y. Morphology and Performance of Polymer Solar Cell Characterized by DPD Simulation and Graph Theory. Sci. Rep. 2015, 5, 16854. [Google Scholar] [CrossRef]
- Miyatake, K.; Bae, B.; Watanabe, M. Fluorene-containing cardo polymers as ion conductive membranes for fuel cells. Polym. Chem. 2011, 2, 1919–1929. [Google Scholar] [CrossRef]
- Devibala, P.; Balambiga, B.; Noureen, S.; Nagarajan, S. Hexaarylbenzene based high-performance p-channel molecules for electronic applications. RSC Adv. 2021, 11, 11672–11701. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.S.; Hsieh, T.L.; Lai, C.C.; Wen, H.Y.; Huang, W.Y.; Chang, M.Y. Highly Proton-Conducting Membranes Based on Poly(arylene ether)s with Densely Sulfonated and Partially Fluorinated Multiphenyl for Fuel Cell Applications. Membranes 2021, 11, 626. [Google Scholar] [CrossRef]
- Liaw, B.R.; Huang, W.Y.; Huang, P.T.; Chang, M.Y.; Han, Y.K. Novel poly(arylene ether)s containing multi-substituted pentaphenylene moiety. Polymer 2007, 48, 7087–7097. [Google Scholar] [CrossRef]
- Schmidt-Rohr, K.; Chen, Q. Parallel cylindrical water nanochannels in Nafion fuel-cell membranes. Nat. Mater. 2008, 7, 75–83. [Google Scholar] [CrossRef]
- Sun, C.; Negro, E.; Nale, A.; Pagot, G.; Vezzù, K.; Zawodzinski, T.A.; Meda, L.; Gambaro, C.; Di Noto, V. An efficient barrier toward vanadium crossover in redox flow batteries: The bilayer [Nafion/(WO3) x] hybrid inorganic-organic membrane. Electrochim. Acta 2021, 378, 138133. [Google Scholar] [CrossRef]
- Robert, M.; El Kaddouri, A.; Perrin, J.C.; Mozet, K.; Daoudi, M.; Dillet, J.; Morel, J.Y.; Andre, S.; Lottin, O. Effects of conjoint mechanical and chemical stress on perfluorosulfonic-acid membranes for fuel cells. J. Power Sources 2020, 476, 228662. [Google Scholar] [CrossRef]
- Dhara, M.G.; Banerjee, S. Fluorinated high-performance polymers: Poly(arylene ether)s and aromatic polyimides containing trifluoromethyl groups. Prog. Polym. Sci. 2010, 35, 1022–1077. [Google Scholar] [CrossRef]
- Sun, C.; Negro, E.; Vezzù, K.; Pagot, G.; Cavinato, G.; Nale, A.; Bang, Y.H.; Di Noto, V. Hybrid inorganic-organic proton-conducting membranes based on SPEEK doped with WO3 nanoparticles for application in vanadium redox flow batteries. Electrochim. Acta 2019, 309, 311–325. [Google Scholar] [CrossRef]
- Gruger, A.; Régis, A.; Schmatko, T.; Colomban, P. Nanostructure of Nafion® membranes at different states of hydration: An IR and Raman study. Vib. Spectrosc. 2001, 26, 215–225. [Google Scholar] [CrossRef]
- Monroe, C.W.; Romero, T.; Mérida, W.; Eikerling, M. A vaporization-exchange model for water sorption and flux in Nafion. J. Membr. Sci. 2008, 324, 1–6. [Google Scholar] [CrossRef]
- Takenaka, M.; Kimura, Y.; Ohara, H. Influence of decomposition temperature of aromatic sulfonic acid catalysts on the molecular weight and thermal stability of poly (L-lactic acid) prepared by melt/solid state polycondenstaion. Polymer 2018, 155, 218–224. [Google Scholar] [CrossRef]
- Liu, B.; Robertson, G.P.; Kim, D.-S.; Guiver, M.D.; Hu, W.; Jiang, Z. Aromatic poly (ether ketone) s with pendant sulfonic acid phenyl groups prepared by a mild sulfonation method for proton exchange membranes. Macromolecules 2007, 40, 1934–1944. [Google Scholar] [CrossRef]
- Tan, S.; Bélanger, D. Characterization and transport properties of Nafion/polyaniline composite membranes. J. Phys. Chem. B 2005, 109, 23480–23490. [Google Scholar] [CrossRef]
- Tsai, J.-C.; Lin, C.-K. Effect of PTFE content in gas diffusion layer based on Nafion®/PTFE membrane for low humidity proton exchange membrane fuel cell. J. Taiwan Inst. Chem. Eng. 2011, 42, 945–951. [Google Scholar] [CrossRef]
- Adamski, M.; Skalski, T.J.G.; Schibli, E.M.; Killer, M.; Wu, Y.; Peressin, N.; Frisken, B.J.; Holdcroft, S. Molecular branching as a simple approach to improving polymer electrolyte membranes. J. Membr. Sci. 2020, 595, 117539. [Google Scholar] [CrossRef]
- Bauer, F.; Denneler, S.; Willert-Porada, M. Influence of temperature and humidity on the mechanical properties of Nafion (R) 117 polymer electrolyte membrane. J. Polym. Sci. Part B-Polym. Phys. 2005, 43, 786–795. [Google Scholar] [CrossRef]
- Tsuneda, T.; Singh, R.K.; Iiyama, A.; Miyatake, K. Theoretical investigation of the H2o2-induced degradation mechanism of hydrated Nafion membrane via ether-linkage dissociation. ACS Omega 2017, 2, 4053–4064. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Tang, H.; Pan, M.; Li, D. Ex situ investigation of the proton exchange membrane chemical decomposition. Int. J. Hydrog. Energy 2008, 33, 2283–2288. [Google Scholar] [CrossRef]
- Mohanty, A.K.; Mistri, E.A.; Ghosh, A.; Banerjee, S. Synthesis and characterization of novel fluorinated poly(arylene ether sulfone)s containing pendant sulfonic acid groups for proton exchange membrane materials. J. Membr. Sci. 2012, 409, 145–155. [Google Scholar] [CrossRef]
- Kreuer, K.D.; Paddison, S.J.; Spohr, E.; Schuster, M. Transport in proton conductors for fuel-cell applications: Simulations, elementary reactions, and phenomenology. Chem. Rev. 2004, 104, 4637–4678. [Google Scholar] [CrossRef]
- Huang, Y.C.; Lee, H.F.; Tseng, Y.C.; Lee, C.C.; Chang, M.Y.; Huang, W.Y. Synthesis of novel sulfonated poly(arylene ether)s containing a tetra-trifluoromethyl side chain and multi-phenyl for proton exchange membrane fuel cell application. Rsc Adv. 2017, 7, 33068–33077. [Google Scholar] [CrossRef]
- Karimi, M.B.; Mohammadi, F.; Hooshyari, K. Recent approaches to improve Nafion performance for fuel cell applications: A review. Int. J. Hydrog. Energy 2019, 44, 28919–28938. [Google Scholar] [CrossRef]
- Lafitte, B.; Jannasch, P. Proton-conducting aromatic polymers carrying hypersulfonated side chains for fuel cell applications. Adv. Funct. Mater. 2007, 17, 2823–2834. [Google Scholar] [CrossRef]
- Feng, S.; Wang, G.; Zhang, H.; Pang, J. Graft octa-sulfonated poly (arylene ether) for high performance proton exchange membrane. J. Mater. Chem. A 2015, 3, 12698–12708. [Google Scholar] [CrossRef]


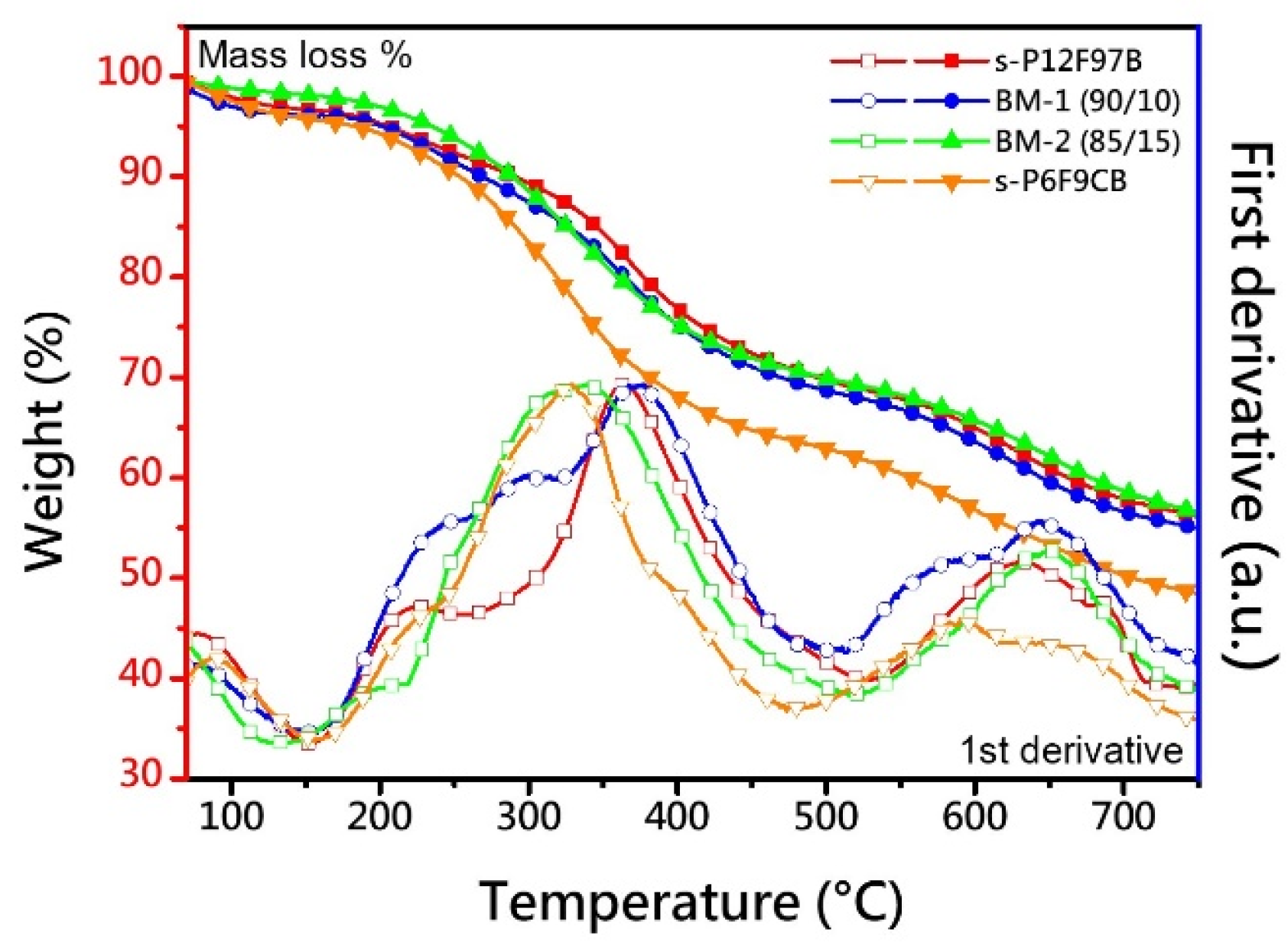
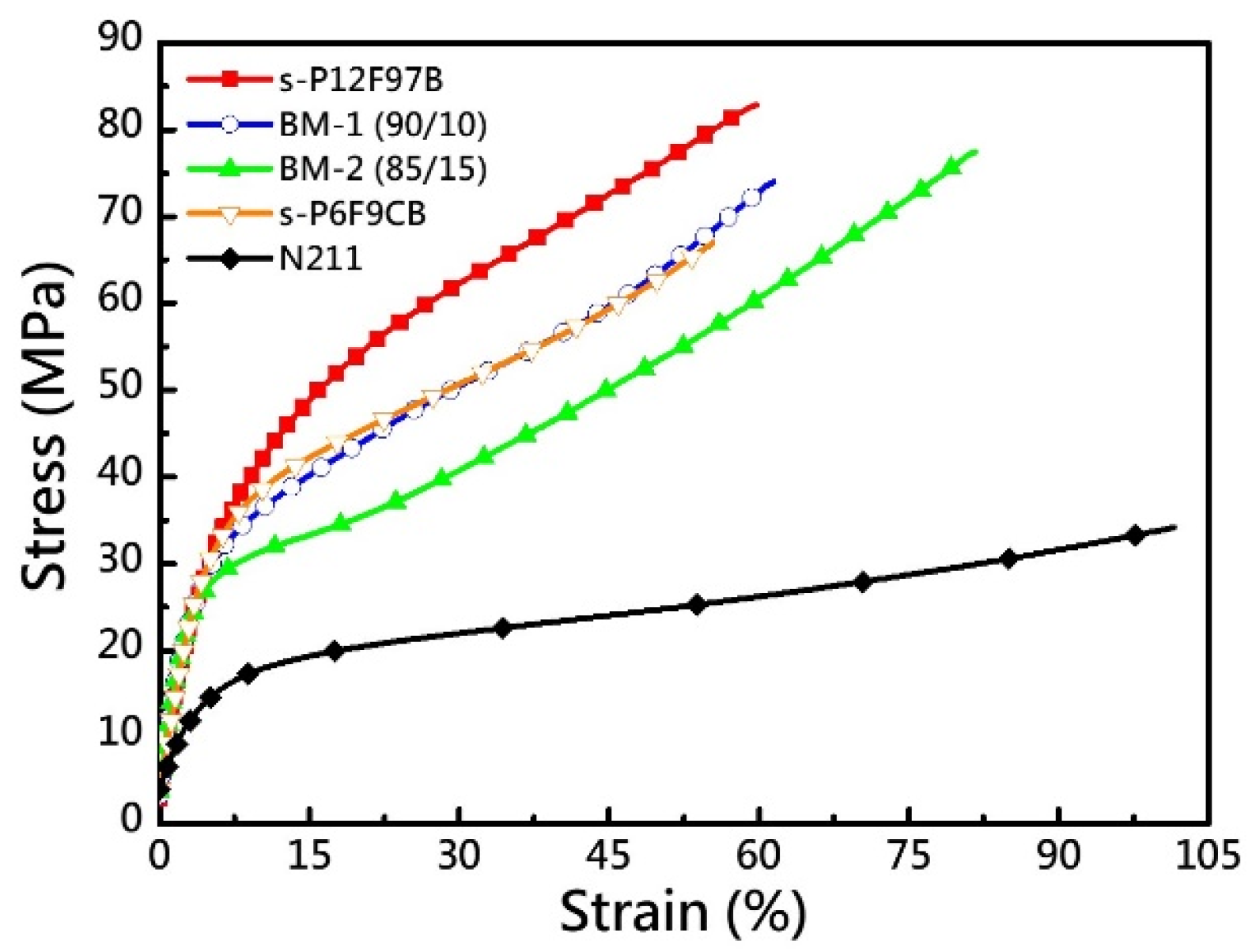

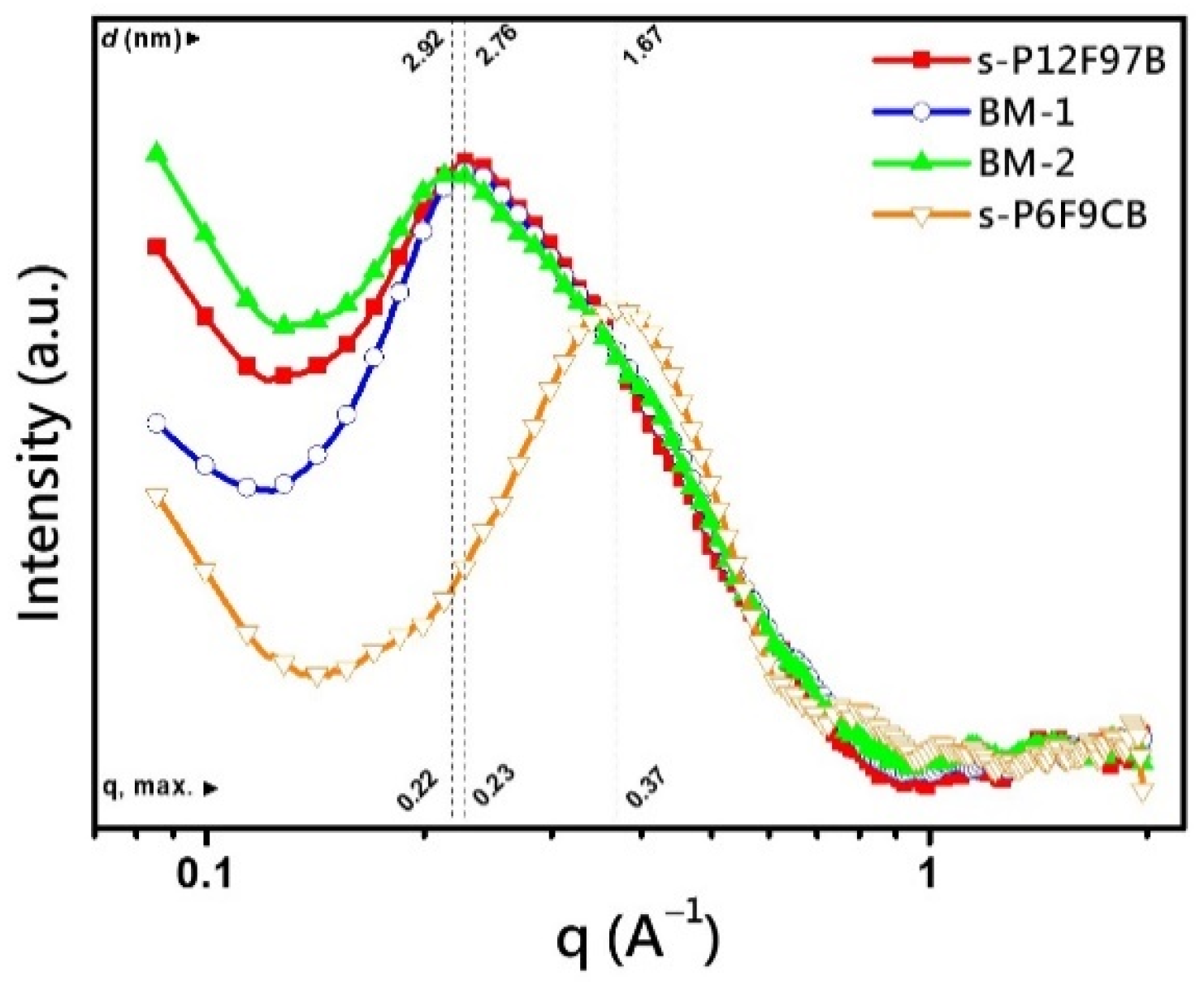
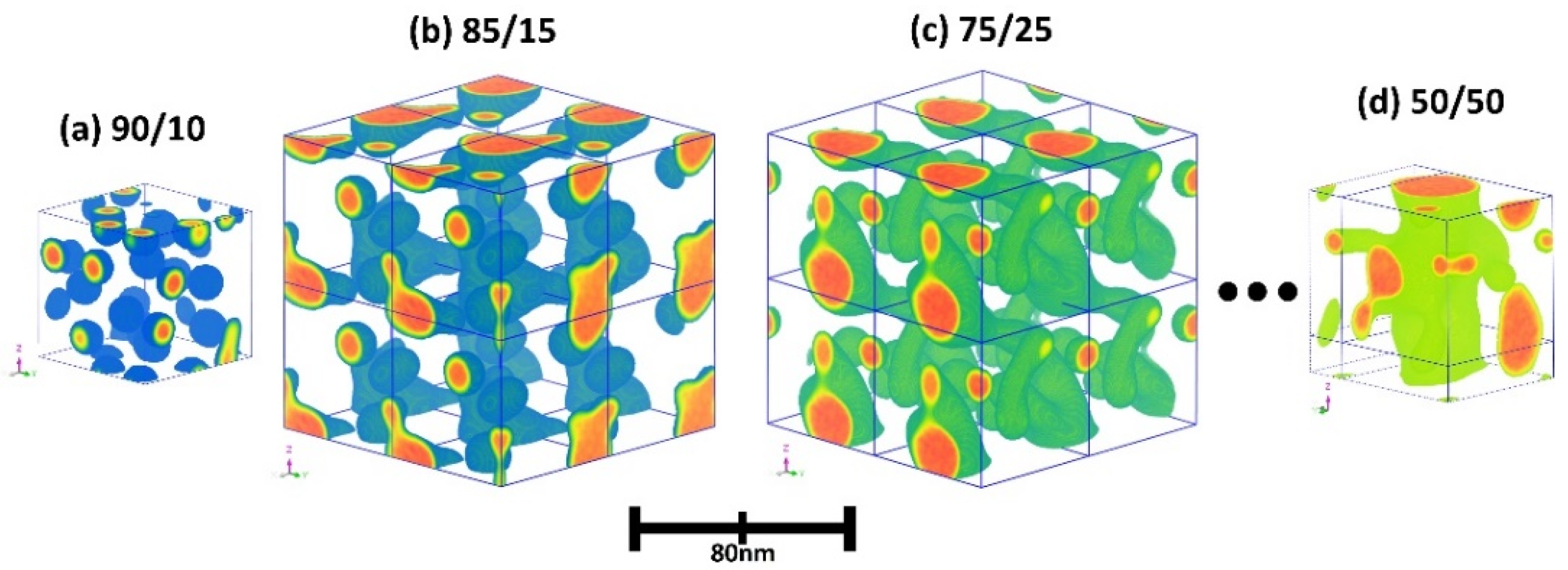
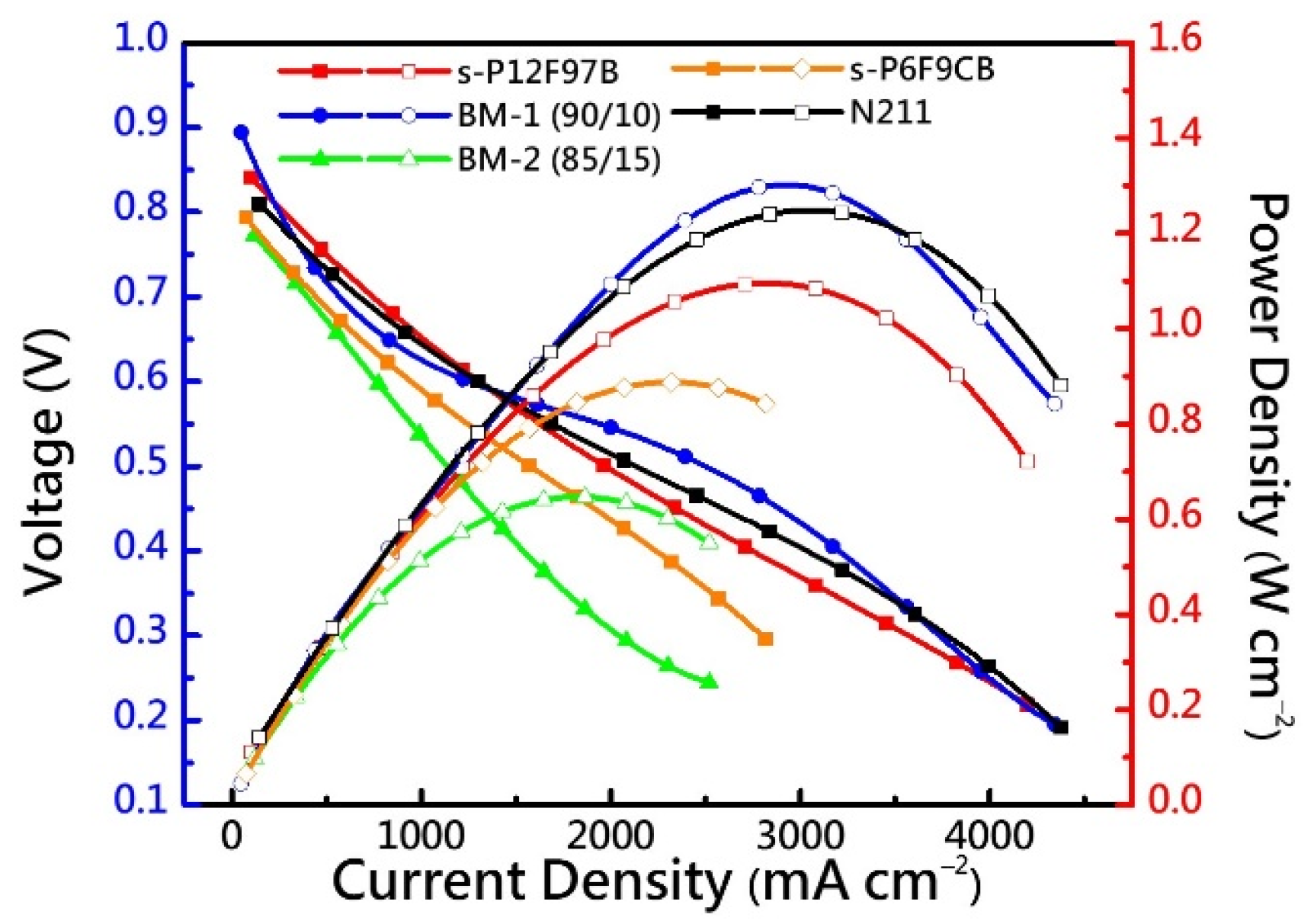
| Membrane | IEC a | Td,5% | Td,CR | YM b | TS b | EB b | OS c |
|---|---|---|---|---|---|---|---|
| (mmol g−1) | (°C) | (°C) | (GPa) | (MPa) | (%) | (%) | |
| s-P12F97B | 2.84 | 205.8 | 254.9 | 0.80 | 82.8 | 59.6 | 94.4 |
| BM-1 | 2.89 | 201.7 | 251.9 | 0.84 | 73.8 | 61.1 | 79.4 |
| BM-2 | 2.92 | 239.2 | 254.7 | 0.78 | 77.4 | 81.7 | 28.3 |
| s-P6F9CB | 3.23 | 187.0 | 238.1 | 0.67 | 66.9 | 55.4 | - d |
| N211 | 0.91 | - | - | 0.26 | 34.1 | 101.1 | 98.6 |
| Membrane | IEC a | WU (%) | ΔA (%) | σion @ 80 °C | |||
|---|---|---|---|---|---|---|---|
| (mmol g−1) | 30 °C | 80 °C | 30 °C | 80 °C | 60% RH | 95% RH | |
| s-P12F97B | 2.84 | 49.6 | 76.7 | 26.8 | 33.1 | 41.7 | 182.6 |
| BM-1 | 2.89 | 57.6 | 91.2 | 24.4 | 34.5 | 39.8 | 191.2 |
| BM-2 | 2.92 | 63.0 | 83.5 | 32.9 | 51.2 | 37.0 | 206.4 |
| s-P6F9CB | 3.23 | 273.9 | - b | 158.1 | - b | 66.4 | 277.9 |
| N211 | 0.91 | 12.7 | 30.3 | - c | 8.2 | 37.5 | 126.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, T.-S.; Wen, H.-Y.; Chen, Y.-Y.; Hung, P.-H.; Hsieh, T.-L.; Huang, W.-Y.; Chang, M.-Y. Ionomer Membranes Produced from Hexaarylbenzene-Based Partially Fluorinated Poly(arylene ether) Blends for Proton Exchange Membrane Fuel Cells. Membranes 2022, 12, 582. https://doi.org/10.3390/membranes12060582
Huang T-S, Wen H-Y, Chen Y-Y, Hung P-H, Hsieh T-L, Huang W-Y, Chang M-Y. Ionomer Membranes Produced from Hexaarylbenzene-Based Partially Fluorinated Poly(arylene ether) Blends for Proton Exchange Membrane Fuel Cells. Membranes. 2022; 12(6):582. https://doi.org/10.3390/membranes12060582
Chicago/Turabian StyleHuang, Tzu-Sheng, Hsin-Yi Wen, Yi-Yin Chen, Po-Hao Hung, Tung-Li Hsieh, Wen-Yao Huang, and Mei-Ying Chang. 2022. "Ionomer Membranes Produced from Hexaarylbenzene-Based Partially Fluorinated Poly(arylene ether) Blends for Proton Exchange Membrane Fuel Cells" Membranes 12, no. 6: 582. https://doi.org/10.3390/membranes12060582
APA StyleHuang, T.-S., Wen, H.-Y., Chen, Y.-Y., Hung, P.-H., Hsieh, T.-L., Huang, W.-Y., & Chang, M.-Y. (2022). Ionomer Membranes Produced from Hexaarylbenzene-Based Partially Fluorinated Poly(arylene ether) Blends for Proton Exchange Membrane Fuel Cells. Membranes, 12(6), 582. https://doi.org/10.3390/membranes12060582









