Virtual and Artificial Cardiorespiratory Patients in Medicine and Biomedical Engineering
Abstract
:1. Introduction
2. Approaches to Cardiovascular and Respiratory Systems Modelling
3. Cardiovascular and Respiratory Models Elaborated by the Authors
3.1. Preliminary
3.2. Numerical Components
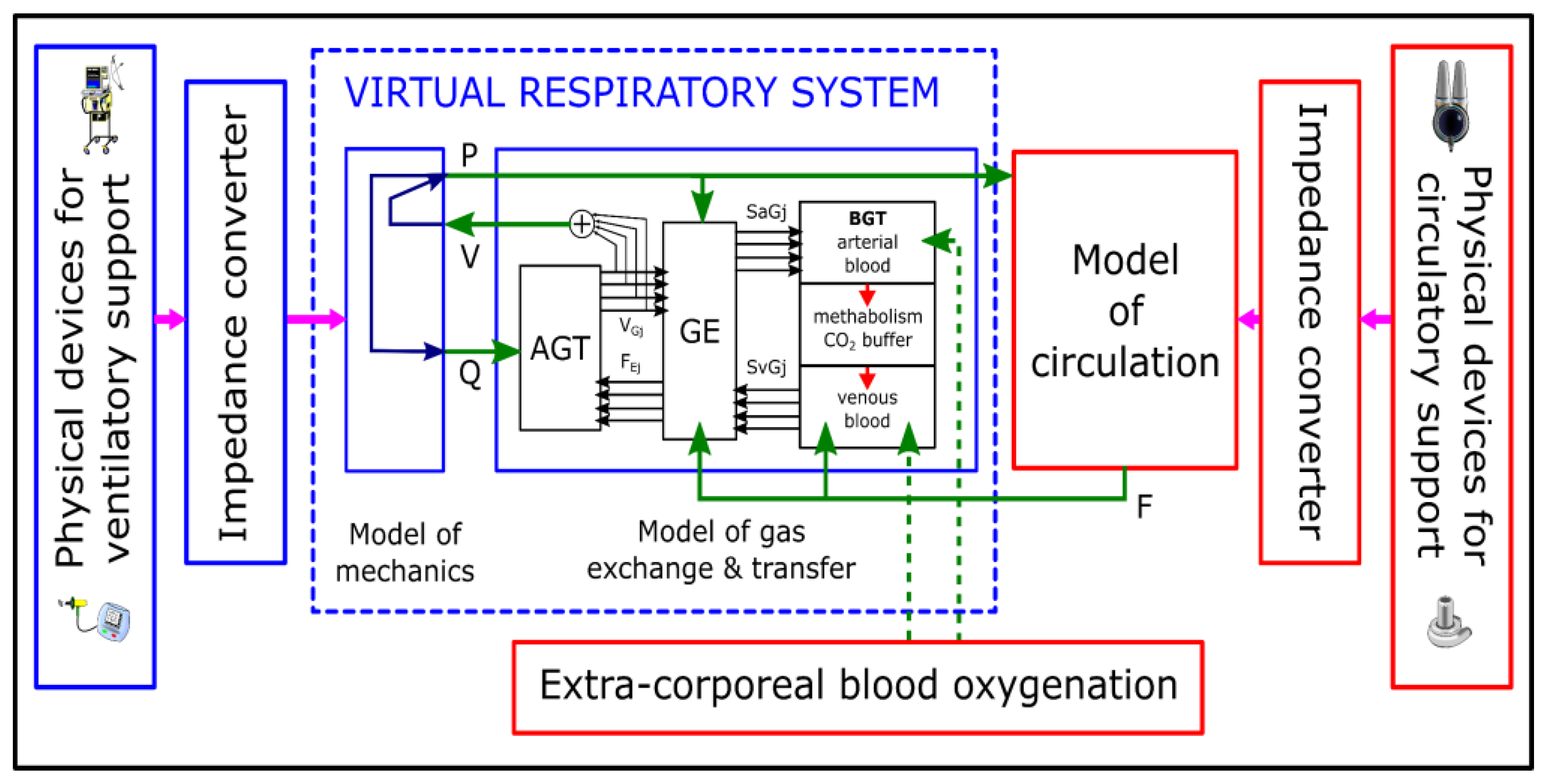
3.2.1. Virtual Patient
3.2.2. Cardiovascular System Models
3.3. Hardware and Physical Components
3.3.1. Respiratory System
3.3.2. Cardiovascular System
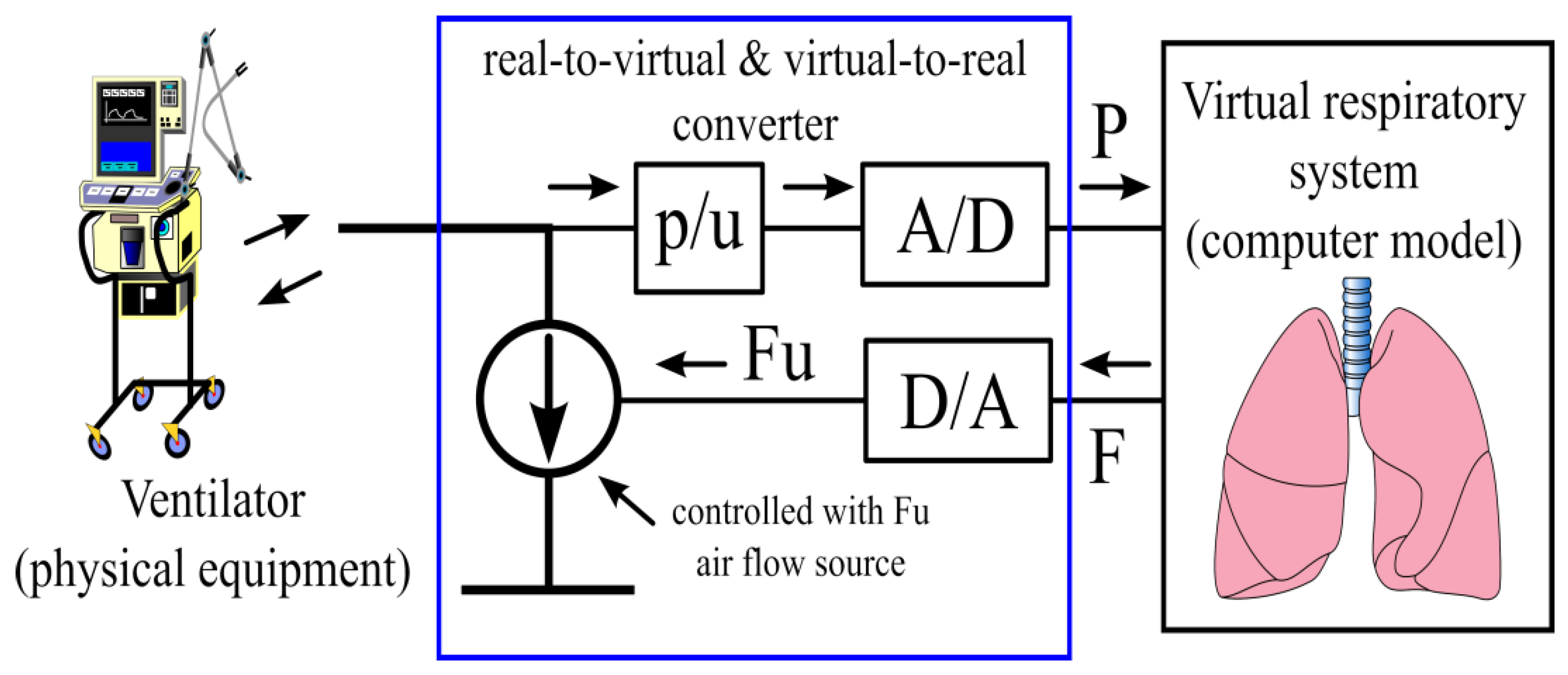
3.3.3. Numerical—Physical Interface
3.4. Summary
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
Appendix A.1. Tests of Physical Equipment
Appendix A.2. E-Learning

Appendix A.3. E-Support of Medical Decisions and Treatment Optimization (Patient-Specific Approach)
Appendix A.4. Cardiopulmonary Interaction (e.g., during Mechanical Ventilation)
Appendix A.5. Interpretation of Physiological Phenomena (e.g., Observed during Therapeutic Thoracentesis)
Appendix A.6. Mechanical Ventilation in Obstructive Lung Diseases


| RR | TV | TV/IBW | PEEPi | WOB | VD/TV | FRC | RV | SpO2 | PaO2 | PaCO2 |
|---|---|---|---|---|---|---|---|---|---|---|
| [1/min] | [mL] | [mL/kg] | [cmH2O] | [J/L] | [L] | [L] | [%] | [mmHg] | [mmHg] | |
| 28 | 190 | 2.96 | 4.6 | 0.68 | 0.73 | 4.9 | 4.0 | 79 | 49 | 63.6 |
| BiPAP | PSV | CPAP | ||||
|---|---|---|---|---|---|---|
| Sim | Lit | Sim | Lit | Sim | Lit | |
| WOB [J/L] | 1.54 | 0.46 ÷ 1.6 | 0.61 | 0.15 ÷ 1.09 | 0.7 | 0.8 ÷ 1.8 |
| PEEPI [cmH2O] | 6.05 | 1.14 ÷ 7.14 | 4.32 | 0.1 ÷ 6.1 | 3.31 | 1.3 ÷ 5.3 |
| TV [L] | 0.35 | 0.21 ÷ 0.59 | 0.48 | 0.33 ÷ 0.59 | 0.3 | 0.25 ÷ 0.49 |
| Ti/Ttot | 0.42 | 0.36 ÷ 0.44 | 0.35 | 0.31 ÷ 0.41 | 0.4 | 0.34 ÷ 0.44 |
| PaO2 [mmHg] | 119 | 80 ÷ 132 | 125 | 75 ÷ 121 | 97 | 81 ÷ 131 |
| PaCO2 [mmHg] | 38 | 33 ÷ 61 | 35 | 32 ÷ 55 | 58 | 36 ÷ 59 |
References
- Viceconti, M.; Clapworthy, G.; Jan, S.V.S. The virtual physiological human—A European Initiative for in silico human modelling. J. Physiol. Sci. 2008, 58, 441–446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mincarone, P.; Bodini, A.; Sabina, S.; Colella, R.; Tumolo, M.R.; Fawdry, M.; Fotiadis, D.I.; Leo, C.G. Simulated versus physical bench tests: The economic evaluation of the InSilc platform for designing, developing, and assessing vascular scaffolds. Medicine 2021, 100, e26198. [Google Scholar] [CrossRef] [PubMed]
- Brogi, S.; Ramalho, T.C.; Kuca, K.; Medina-Franco, J.L.; Valko, M. Editorial: In silico methods for drug design and discovery. Front. Chem. 2020, 8, 612. [Google Scholar] [CrossRef]
- Ramalho, T.C.; Rocha, M.V.J.; da Cunha, E.F.F.; Oliveira, L.C.A.; Carvalho, K.T.G. Understanding the molecular behavior of organotin compounds to design their effective use as agrochemicals: Exploration via quantum chemistry and experiments. J. Biomol. Struct. Dyn. 2010, 28, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Brossier, D.; Sauthier, M.; Alacoque, X.; Masse, B.; Eltaani, R.; Guillois, B.; Jouvet, P. Perpetual and virtual patients for cardi-orespiratory physiological studies. J. Pediatr. Intensiv. Care 2016, 5, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Pillon, M.; Duffour, H.; Jufer, M. In vitro experiments: Circulatory assist device interaction with a virtual cardiovascular system. In Proceedings of the 14th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Paris, France, 29 October–1 November 1992; pp. 740–741. [Google Scholar] [CrossRef]
- Verbraak, A.F.M.; Beneken, J.E.W.; Bogaard, J.M.; Versprille, A. Computer-controlled mechanical lung model for application in pulmonary function studies. Med. Biol. Eng. Comput. 1995, 33, 776–783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zielinski, K.; Darowski, M.; Kozarski, M.; Ferrari, G. The need for hybrid modeling in analysis of cardiovascular and respiratory support. Int. J. Artif. Organs 2016, 39, 265–271. [Google Scholar] [CrossRef]
- Oda, H.; Hamaji, M.; Motoyama, H.; Ikeda, T.; Minatoya, K.; Nakajima, D.; Chen-Yoshikawa, T.F.; Date, H. Use of a three-dimensional model in lung transplantation for a patient with giant pulmonary aneurysm. Ann. Thorac. Surg. 2020, 109, e183–e185. [Google Scholar] [CrossRef]
- Jacob, S.; Pooley, R.A.; Thomas, M. Three-dimensional–printed model as a template for chest wall reconstruction. Heart Lung Circ. 2020, 29, 1566–1570. [Google Scholar] [CrossRef]
- Nia, P.S.; Olsthoorn, J.R.; Heuts, S.; Maessen, J.G. Interactive 3D reconstruction of pulmonary anatomy for preoperative planning, virtual simulation, and intraoperative guiding in video-assisted thoracoscopic lung surgery. Innovations 2019, 14, 17–26. [Google Scholar] [CrossRef]
- Myers, B.; Obr, C. Preparing for cardiopulmonary bypass: A simulation scenario for anesthesia providers. MedEdPORTAL 2017, 13, 10578. [Google Scholar] [CrossRef] [PubMed]
- Copploe, A.; Vatani, M.; Choi, J.-W.; Tavana, H. A Three-dimensional model of human lung airway tree to study therapeutics delivery in the lungs. Ann. Biomed. Eng. 2019, 47, 1435–1445. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-W.; Huang, S.X.; De Carvalho, A.L.R.T.; Ho, S.-H.; Islam, M.N.; Volpi, S.; Notarangelo, L.D.; Ciancanelli, M.; Casanova, J.-L.; Bhattacharya, J.; et al. A three-dimensional model of human lung development and disease from pluripotent stem cells. Nat. Cell Biol. 2017, 19, 542–549. [Google Scholar] [CrossRef]
- Lee, W.; Jung, E. A multiscale model of cardiovascular system including an immersed whole heart in the cases of normal and ventricular septal defect (VSD). Bull. Math. Biol. 2015, 77, 1349–1376. [Google Scholar] [CrossRef] [PubMed]
- Zieliński, K.; Stecka, A.; Gólczewski, T. VirRespir—An application for virtual pneumonological experimentation and clinical training. In Proceedings of the World Congress on Medical Physics and Biomedical Engineering, Prague, Czech Republic, 3–8 June 2018; Lhotska, L., Sukupova, L., Lacković, I., Ibbott, G., Eds.; Springer: Singapore, 2018; Volume 68/1, pp. 697–701. [Google Scholar] [CrossRef]
- Stecka, A.M.; Gólczewski, T.; Grabczak, E.M.; Zieliński, K.; Michnikowski, M.; Zielińska-Krawczyk, M.; Korczyński, P.; Krenke, R. The use of a virtual patient to follow changes in arterial blood gases associated with therapeutic thoracentesis. Int. J. Artif. Organs 2018, 41, 690–697. [Google Scholar] [CrossRef]
- Golczewski, T. Gas exchange in a virtual respiratory system—Simulation of ventilation without lung movement. Int. J. Artif. Organs 2007, 30, 1047–1056. [Google Scholar] [CrossRef] [PubMed]
- Golczewski, T.; Darowski, M. Virtual respiratory system for education and research: Simulation of expiratory flow limitation for spirometry. Int. J. Artif. Organs 2006, 29, 961–972. [Google Scholar] [CrossRef]
- Serna, L.Y.; Mananas, M.A.; Hernandez, A.M.; Rabinovich, R.A. An improved dynamic model for the respiratory response to exercise. Front. Physiol. 2018, 9, 69. [Google Scholar] [CrossRef] [Green Version]
- Tran, C.; Toth-Pal, E.; Ekblad, S.; Fors, U.; Salminen, H. A virtual patient model for students’ interprofessional learning in primary healthcare. PLoS ONE 2020, 15, e0238797. [Google Scholar] [CrossRef]
- Arnal, J.-M.; Garnero, A.; Saoli, M.; Chatburn, R.L. Parameters for simulation of adult subjects during mechanical ventilation. Respir. Care 2018, 63, 158–168. [Google Scholar] [CrossRef] [Green Version]
- Tomalak, W.; Golczewski, T.; Michnikowski, M.; Darowski, M. Virtual respiratory system for interactive e-learning of spirometry. Eur. Respir. Rev. 2008, 17, 36–38. [Google Scholar] [CrossRef] [Green Version]
- Zieliński, K.; Kozarski, M.; Fresiello, L.; Di Molfetta, A.; Ferrari, G.; Peristeris, S.; Verde, A.; Darowski, M. A hybrid cardiovascular simulator for VAD training. Int. J. Artif. Organs 2014, 37, 625. [Google Scholar]
- Welcome to Harvi. Available online: https://harvi.online/site/welcome/ (accessed on 25 April 2022).
- Pahuja, M.; Schrage, B.; Westermann, D.; Basir, M.B.; Garan, A.R.; Burkhoff, D. Hemodynamic effects of mechanical circulatory support devices in ventricular septal defect. Circ. Heart Fail. 2019, 12, e005981. [Google Scholar] [CrossRef] [PubMed]
- Karmonik, C.; Partovi, S.; Rengier, F.; Meredig, H.; Farag, M.B.; Müller-Eschner, M.; Arif, R.; Popov, A.-F.; Kauczor, H.-U.; Karck, M.; et al. Hemodynamic assessment of partial mechanical circulatory support: Data derived from computed tomography angiographic images and computational fluid dynamics. Cardiovasc. Diagn. Ther. 2015, 5, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Capoccia, M.; Singh, S.A.; De Lazzari, C. The role of simulation for preoperative planning in patients requiring mechanical circulatory support. In Proceedings of the Joint Conference of the European Medical and Biological Engineering Conference (EMBEC) and the Nordic-Baltic Conference on Biomedical Engineering and Medical Physics (NBC), Tampere, Finland, 11–15 June 2017; Eskola, H., Väisänen, O., Viik, J., Hyttinen, J., Eds.; Springer: Singapore, 2018; Volume 65. [Google Scholar] [CrossRef]
- Capoccia, M.; Marconi, S.; Singh, S.A.; Pisanelli, D.M.; De Lazzari, C. Simulation as a preoperative planning approach in advanced heart failure patients. A retrospective clinical analysis. Biomed. Eng. Online 2018, 17, 52. [Google Scholar] [CrossRef]
- Chase, J.G.; Preiser, J.-C.; Dickson, J.L.; Pironet, A.; Chiew, Y.S.; Pretty, C.G.; Shaw, G.M.; Benyo, B.; Moeller, K.; Safaei, S.; et al. Next-generation, personalised, model-based critical care medicine: A state-of-the art review of in silico virtual patient models, methods, and cohorts, and how to validation them. Biomed. Eng. Online 2018, 17, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gray, R.A.; Pathmanathan, P. Patient-specific cardiovascular computational modeling: Diversity of personalization and chal-lenges. J. Cardiovasc. Transl. Res. 2018, 11, 80–88. [Google Scholar] [CrossRef] [Green Version]
- Ladjal, H.; Giroux, M.; Beuve, M.; Giraud, P.; Shariat, B. Patient-specific physiological model of the respiratory system based on inverse finite element analysis: A comparative study. Comput. Methods Biomech. Biomed. Eng. 2019, 22, S45–S47. [Google Scholar] [CrossRef]
- Niederer, S.A.; Aboelkassem, Y.; Cantwell, C.D.; Corrado, C.; Coveney, S.; Cherry, E.M.; Delhaas, T.; Fenton, F.H.; Panfilov, A.; Pathmanathan, P.; et al. Creation and application of virtual patient cohorts of heart models. Philos. Trans. Math. Phys. Eng. Sci. 2020, 378, 20190558. [Google Scholar] [CrossRef]
- Pałko, K.J.; Gólczewski, T.; Zieliński, K.; Kozarski, M.; Darowski, M. Validation of the added compliance and resistance method for lung function tests on a population of artificial patients. Int. J. Artif. Organs 2012, 35, 606. [Google Scholar]
- Kung, E.; Farahmand, M.; Gupta, A. A hybrid experimental-computational modeling framework for cardiovascular device testing. J. Biomech. Eng. 2019, 141, 051012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Molfetta, A.; Zielinski, K.; Ferrari, G.; Kozarski, M.; Okrzeja, P.; Iacobelli, R.; Filippelli, S.; Perri, G.; Darowski, M.; Massetti, M.; et al. Is the new infant Jarvik 2015 suitable for patients <8 kg? In vitro study using a hybrid simulator. Artif. Organs 2019, 43, E1–E8. [Google Scholar] [CrossRef] [PubMed]
- Dexter, A.; McNinch, N.; Kaznoch, D.; Volsko, T.A. Validating lung models using the ASL 5000 breathing simulator. Simul. Healthc. 2018, 13, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Baldoli, I.; Cuttano, A.; Scaramuzzo, R.T.; Tognarelli, S.; Ciantelli, M.; Cecchi, F.; Gentile, M.; Sigali, E.; Laschi, C.; Ghirri, P.; et al. A novel simulator for mechanical ventilation in newborns: MEchatronic REspiratory System SImulator for neonatal applications. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2015, 229, 581–591. [Google Scholar] [CrossRef]
- Ochsner, G.; Amacher, R.; Amstutz, A.; Plass, A.; Daners, M.S.; Tevaearai, H.; Vandenberghe, S.; Wilhelm, M.J.; Guzzella, L. A novel interface for hybrid mock circulations to evaluate ventricular assist devices. IEEE Trans. Biomed. Eng. 2013, 60, 507–516. [Google Scholar] [CrossRef] [PubMed]
- Petrou, A.; Granegger, M.; Meboldt, M.; Daners, M.S. A versatile hybrid mock circulation for hydraulic investigations of active and passive cardiovascular implants. ASAIO J. 2019, 65, 495–502. [Google Scholar] [CrossRef] [Green Version]
- Mirzaei, E.; Farahmand, M.; Kung, E. An algorithm for coupling multibranch in vitro experiment to numerical physiology simulation for a hybrid cardiovascular model. Int. J. Numer. Methods Biomed. Eng. 2019, 36, e3289. [Google Scholar] [CrossRef] [Green Version]
- Thomas, F.; Chung, S.; Holt, D.W. Effects of ECMO simulations and protocols on patient safety. J. Extra-Corpor. Technol. 2019, 51, 12–19. [Google Scholar]
- Mahmoud, A.; Alsalemi, A.; Bensaali, F.; Hssain, A.A.; Hassan, I. A review of human circulatory system simulation: Bridging the gap between engineering and medicine. Membranes 2021, 11, 744. [Google Scholar] [CrossRef]
- Alhomsi, Y.; Alsalemi, A.; Noorizadeh, M.; Bensaali, F.; Meskin, N.; Hssain, A. A modular approach for a patient unit for extracorporeal membrane oxygenation simulator. Membranes 2021, 11, 424. [Google Scholar] [CrossRef]
- Colasanti, S.; Piemonte, V.; Devolder, E.; Zieliński, K.; Vandendriessche, K.; Meyns, B.; Fresiello, L. Development of a computational simulator of the extracorporeal membrane oxygenation and its validation with in vitro measurements. Artif. Organs 2021, 45, 399–410. [Google Scholar] [CrossRef] [PubMed]
- Zieliński, K.; Okrzeja, P.; Stecka, A.; Kozarski, M.; Darowski, M. A hybrid cardio-pulmonary simulation platform—An application for extracorporeal assist devices. In Proceedings of the World Congress on Medical Physics and Biomedical Engineering, Prague, Czech Republic, 3–8 June 2018; Lhotska, L., Sukupova, L., Lacković, I., Ibbott, G., Eds.; Springer: Singapore, 2018; Volume 68/1, pp. 703–706. [Google Scholar] [CrossRef]
- Walter, M.; Eisenbrand, S.; Kopp, R.; Leonhardt, S. Hardware-in-the-loop test bench for artificial lungs. AIP Conf. Proc. 2019, 2140, 020078. [Google Scholar] [CrossRef] [Green Version]
- De Lazzari, B.; Iacovoni, A.; Mottaghy, K.; Capoccia, M.; Badagliacca, R.; Vizza, C.D.; De Lazzari, C. ECMO Assistance during mechanical ventilation: Effects induced on energetic and haemodynamic variables. Comput. Methods Programs Biomed. 2021, 202, 106003. [Google Scholar] [CrossRef] [PubMed]
- Eynde, J.V.D.; Kutty, S.; Danford, D.A.; Manlhiot, C. Artificial intelligence in pediatric cardiology: Taking baby steps in the big world of data. Curr. Opin. Cardiol. 2022, 37, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Fresiello, L.; Zieliński, K.; Jacobs, S.; Di Molfetta, A.; Pałko, K.J.; Bernini, F.; Martin, M.; Claus, P.; Ferrari, G.; Trivella, M.G.; et al. Reproduction of continuous flow left ventricular assist device experimental data by means of a hybrid cardiovascular model with baroreflex control. Artif. Organs 2014, 38, 456–468. [Google Scholar] [CrossRef]
- Ferrari, G.; Kozarski, M.; De Lazzari, C.; Górczyńska, K.; Mimmo, R.; Guaragno, M.; Tosti, G.; Darowski, M. Modelling of cardiovascular system: Development of a hybrid (numerical-physical) model. Int. J. Artif. Organs 2003, 26, 1104–1114. [Google Scholar] [CrossRef]
- Gólczewski, T.; Kozarski, M.; Darowski, M. The respirator as a user of virtual lungs. Biocybern. Biomed. Eng. 2003, 23, 57–66. [Google Scholar]
- Stankiewicz, B.; Pałko, K.J.; Darowski, M.; Kozarski, M. How to ventilate preterm infants with lung compliance close to circuit compliance: Real-time simulations on an infant hybrid respiratory simulator. Med. Biol. Eng. Comput. 2020, 58, 357–372. [Google Scholar] [CrossRef]
- Gólczewski, T.; Stecka, A.M.; Michnikowski, M.; Grabczak, E.M.; Korczyński, P.; Krenke, R. The use of a virtual patient to follow pleural pressure changes associated with therapeutic thoracentesis. Int. J. Artif. Organs 2017, 40, 690–695. [Google Scholar] [CrossRef] [Green Version]
- Lubiński, W.; Gólczewski, T. Physiologically interpretable prediction equations for spirometric indexes. J. Appl. Physiol. 2010, 108, 1440–1446. [Google Scholar] [CrossRef] [Green Version]
- Gólczewski, T.; Zieliński, K.; Ferrari, G.; Pałko, K.J.; Darowski, M. Influence of ventilation mode on blood oxygenation—Investigation with Polish Virtual Lungs and Italian model of circulation. Biocybern. Biomed. Eng. 2010, 30, 17–30. [Google Scholar]
- Ferrari, G.; Kozarski, M.; Zieliński, K.; Fresiello, L.; Di Molfetta, A.; Górczyńska, K.; Pałko, K.J.; Darowski, M. A modular computational circulatory model applicable to VAD testing and training. J. Artif. Organs 2012, 15, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Di Molfetta, A.; Santini, L.; Forleo, G.B.; Cesario, M.; Tota, C.; Sgueglia, M.; Sergi, D.; Ferrari, G.; Romeo, F. Use of a comprehensive numerical model to improve biventricular pacemaker temporization in patients affected by heart failure undergoing to CRT-D therapy. Med. Biol. Eng. Comput. 2010, 48, 755–764. [Google Scholar] [CrossRef] [PubMed]
- Darowski, M.; Ferrari, G. Comprehensive Models of Cardiovascular and Respiratory Systems: Their Mechanical Support and Interactions, 1st ed.; Nova Science: New York, NY, USA, 2010; p. 249. [Google Scholar]
- Fresiello, L.; Zieliński, K. Hemodynamic modelling and simulations for mechanical circulatory support. In Mechanical Support for Heart Failure; Springer: Cham, Switzerland, 2020; pp. 429–447. [Google Scholar] [CrossRef]
- Tortora, G.; Fontana, R.; Fresiello, L.; Di Molfetta, A.; Silvestri, M.; Vatteroni, M.; Zielinski, K.; Kozarski, M.; Dario, P.; Trivella, M.G.; et al. Experimental integration of autoregulation unit for left ventricular assist devices in a cardiovascular hybrid simu-lator. In Proceedings of the 36th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Chicago, IL, USA, 26–30 August 2014; pp. 282–285. [Google Scholar] [CrossRef]
- Fresiello, L.; Najar, A.; Ignell, N.B.; Zieliński, K.; Rocchi, M.; Meyns, B.; Perkins, I.L. Hemodynamic characterization of the Realheart® total artificial heart with a hybrid cardiovascular simulator. Artif. Organs 2022, 39, E202–E212. [Google Scholar] [CrossRef] [PubMed]
- Di Molfetta, A.; Filippelli, S.; Ferrari, G.; Secinaro, A.; Zielinski, K.; Amodeo, A. Berlin heart EXCOR ventricular assist device: Multilayer membrane rupture in a pediatric patient. Ann. Thorac. Surg. 2016, 102, e129–e130. [Google Scholar] [CrossRef] [Green Version]
- Tzallas, A.T.; Katertsidis, N.S.; Karvounis, E.C.; Tsipouras, M.G.; Rigas, G.; Goletsis, Y.; Zielinski, K.; Fresiello, L.; Di Molfetta, A.; Ferrari, G.; et al. Modeling and simulation of speed selection on left ventricular assist devices. Comput. Biol. Med. 2014, 51, 128–139. [Google Scholar] [CrossRef] [Green Version]
- Mahmood, S.S.; Pinsky, M.R. Heart-lung interactions during mechanical ventilation: The basics. Ann. Transl. Med. 2018, 6, 349. [Google Scholar] [CrossRef]
- Cabello, B.; Mancebo, J. Work of breathing. Intensiv. Care Med. 2006, 32, 1311–1314. [Google Scholar] [CrossRef]
- Clive, A.O.; Jones, H.; Bhatnagar, R.; Preston, N.; Maskell, N. Interventions for the management of malignant pleural effusions: A network meta-analysis. Cochrane Database Syst. Rev. 2016, 5, CD010529. [Google Scholar] [CrossRef]
- Reddy, R.M.; Guntupalli, K.K. Review of ventilatory technics to optimise mechanical ventilation in acute exacerbation of chronic obstructive pulmonary disease. Int. J. Chron. Obstruct. Pulmon. Dis. 2007, 2, 441–452. [Google Scholar]
- O’Donnell, D.E.; Laveneziana, P. Physiology and consequences of lung hyperinflation in COPD. Eur. Respir. Rev. 2006, 15, 61–67. [Google Scholar] [CrossRef]
- Karcz, M.; Vitkus, A.; Papadakos, P.J.; Schwaiberger, D.; Lachmann, B. State-of-the-art mechanical ventilation. J. Cardiothorac. Vasc. Anesth. 2012, 26, 486–506. [Google Scholar] [CrossRef] [PubMed]
- Katz-Papatheophilou, E.; Heindl, W.; Gelbmann, H.; Hollaus, P.; Neumann, M. Effects of biphasic positive airway pressure in patients with chronic obstructive pulmonary disease. Eur. Respir. J. 2000, 15, 498–504. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zieliński, K.; Gólczewski, T.; Kozarski, M.; Darowski, M. Virtual and Artificial Cardiorespiratory Patients in Medicine and Biomedical Engineering. Membranes 2022, 12, 548. https://doi.org/10.3390/membranes12060548
Zieliński K, Gólczewski T, Kozarski M, Darowski M. Virtual and Artificial Cardiorespiratory Patients in Medicine and Biomedical Engineering. Membranes. 2022; 12(6):548. https://doi.org/10.3390/membranes12060548
Chicago/Turabian StyleZieliński, Krzysztof, Tomasz Gólczewski, Maciej Kozarski, and Marek Darowski. 2022. "Virtual and Artificial Cardiorespiratory Patients in Medicine and Biomedical Engineering" Membranes 12, no. 6: 548. https://doi.org/10.3390/membranes12060548
APA StyleZieliński, K., Gólczewski, T., Kozarski, M., & Darowski, M. (2022). Virtual and Artificial Cardiorespiratory Patients in Medicine and Biomedical Engineering. Membranes, 12(6), 548. https://doi.org/10.3390/membranes12060548






