Graphene-Coated PVDF Membranes: Effects of Multi-Scale Rough Structure on Membrane Distillation Performance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of PVDF Membranes
2.3. WET-Filtration Method
2.4. Membrane Characterization
2.5. Membrane Distillation Tests
3. Results and Discussion
3.1. Chemical and Morphological Features of Membranes
3.2. Wetting Properties of Membranes
3.3. Membrane Distillation Experiments
- (a)
- Large flux is obtained at lower feed temperature and using small temperature differences across the membranes, resulting in a cheaper process.
- (b)
- A simpler and inexpensive DCMD configuration can be used to get the best-performing processes for productivity and selectivity under softer working conditions without the necessity to increase electric energy inputs like the VMD configuration.
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pendergast, M.M.; Hoek, E.M.V. A Review of Water Treatment Membrane Nanotechnologies. Energy Environ. Sci. 2011, 4, 1946. [Google Scholar] [CrossRef] [Green Version]
- Lee, A.; Darling, S.B.; Elam, J.W. Membrane Materials for Water Purification: Design, Development, and Application. Environ. Sci. Water Res. Technol. 2016, 2, 17–42. [Google Scholar] [CrossRef]
- Speranza, V.; Trotta, F.; Drioli, E.; Gugliuzza, A. High-Definition Polymeric Membranes: Construction of 3D Lithographed Channel Arrays through Control of Natural Building Blocks Dynamics. ACS Appl. Mater. Interfaces 2010, 2, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Perrotta, M.L.; Saielli, G.; Casella, G.; Macedonio, F.; Giorno, L.; Drioli, E.; Gugliuzza, A. An Ultrathin Suspended Hydrophobic Porous Membrane for High-Efficiency Water Desalination. Appl. Mater. Today 2017, 9, 1–9. [Google Scholar] [CrossRef]
- Frappa, M.; Del Rio Castillo, A.E.; Macedonio, F.; Politano, A.; Drioli, E.; Bonaccorso, F.; Pellegrini, V.; Gugliuzza, A. A Few-Layer Graphene for Advanced Composite PVDF Membranes Dedicated to Water Desalination: A Comparative Study. Nanoscale Adv. 2020, 2, 4728–4739. [Google Scholar] [CrossRef]
- Gugliuzza, A.; Basile, A. Membrane Contactors: Fundamentals, Membrane Materials and Key Operations. In Handbook of Membrane Reactors; Elsevier: Amsterdam, The Netherlands, 2013; pp. 54–106. [Google Scholar]
- Gontarek-Castro, E.; Castro-Muñoz, R.; Lieder, M. New Insights of Nanomaterials Usage toward Superhydrophobic Membranes for Water Desalination via Membrane Distillation: A Review. Crit. Rev. Environ. Sci. Technol. 2021, 52, 2104–2149. [Google Scholar] [CrossRef]
- Gontarek, E.; Macedonio, F.; Militano, F.; Giorno, L.; Lieder, M.; Politano, A.; Drioli, E.; Gugliuzza, A. Adsorption-Assisted Transport of Water Vapour in Super-Hydrophobic Membranes Filled with Multilayer Graphene Platelets. Nanoscale 2019, 11, 11521–11529. [Google Scholar] [CrossRef]
- Mao, Y.; Huang, Q.; Meng, B.; Zhou, K.; Liu, G.; Gugliuzza, A.; Drioli, E.; Jin, W. Roughness-Enhanced Hydrophobic Graphene Oxide Membrane for Water Desalination via Membrane Distillation. J. Memb. Sci. 2020, 611, 118364. [Google Scholar] [CrossRef]
- Tang, N.; Jia, Q.; Zhang, H.; Li, J.; Cao, S. Preparation and Morphological Characterization of Narrow Pore Size Distributed Polypropylene Hydrophobic Membranes for Vacuum Membrane Distillation via Thermally Induced Phase Separation. Desalination 2010, 256, 27–36. [Google Scholar] [CrossRef]
- Zhu, H.; Wang, H.; Wang, F.; Guo, Y.; Zhang, H.; Chen, J. Preparation and Properties of PTFE Hollow Fiber Membranes for Desalination through Vacuum Membrane Distillation. J. Memb. Sci. 2013, 446, 145–153. [Google Scholar] [CrossRef]
- Devi, S.; Ray, P.; Singh, K.; Singh, P.S. Preparation and Characterization of Highly Micro-Porous PVDF Membranes for Desalination of Saline Water through Vacuum Membrane Distillation. Desalination 2014, 346, 9–18. [Google Scholar] [CrossRef]
- Tijing, L.D.; Woo, Y.C.; Choi, J.S.; Lee, S.; Kim, S.H.; Shon, H.K. Fouling and Its Control in Membrane Distillation-A Review. J. Memb. Sci. 2015, 475, 215–244. [Google Scholar] [CrossRef]
- Kullab, A.; Martin, A. Membrane Distillation and Applications for Water Purification in Thermal Cogeneration Plants. Sep. Purif. Technol. 2011, 76, 231–237. [Google Scholar] [CrossRef]
- Hausmann, A.; Sanciolo, P.; Vasiljevic, T.; Weeks, M.; Schroën, K.; Gray, S.; Duke, M. Fouling of Dairy Components on Hydrophobic Polytetrafluoroethylene (PTFE) Membranes for Membrane Distillation. J. Memb. Sci. 2013, 442, 149–159. [Google Scholar] [CrossRef] [Green Version]
- Meng, S.; Ye, Y.; Mansouri, J.; Chen, V. Fouling and Crystallisation Behaviour of Superhydrophobic Nano-Composite PVDF Membranes in Direct Contact Membrane Distillation. J. Memb. Sci. 2014, 463, 102–112. [Google Scholar] [CrossRef]
- Perrotta, M.L.; Macedonio, F.; Tocci, E.; Giorno, L.; Drioli, E.; Gugliuzza, A. Graphene Stimulates the Nucleation and Growth Rate of NaCl Crystals from Hypersaline Solution via Membrane Crystallization. Environ. Sci. Water Res. Technol. 2020, 6, 1723–1736. [Google Scholar] [CrossRef]
- Gugliuzza, A.; Aceto, M.C.; Drioli, E. Interactive Functional Poly(Vinylidene Fluoride) Membranes with Modulated Lysozyme Affinity: A Promising Class of New Interfaces for Contactor Crystallizers. Polym. Int. 2009, 58, 1452–1464. [Google Scholar] [CrossRef]
- Frappa, M.; Castillo, A.E.D.R.; Macedonio, F.; Di Luca, G.; Drioli, E.; Gugliuzza, A. Exfoliated Bi2Te3-Enabled Membranes for New Concept Water Desalination: Freshwater Production Meets New Routes. Water Res. 2021, 203, 117503. [Google Scholar] [CrossRef]
- Han, Y.; Giorno, L.; Gugliuzza, A. Photoactive Gel for Assisted Cleaning during Olive Mill Wastewater Membrane Microfiltration. Membranes 2017, 7, 66. [Google Scholar] [CrossRef] [Green Version]
- Gugliuzza, A.; Fabiano, R.; Garavaglia, M.G.; Spisso, A.; Drioli, E. Study of the Surface Character as Responsible for Controlling Interfacial Forces at Membrane-Feed Interface. J. Colloid Interface Sci. 2006, 303, 388–403. [Google Scholar] [CrossRef]
- Bhadra, M.; Roy, S.; Mitra, S. Desalination across a Graphene Oxide Membrane via Direct Contact Membrane Distillation. Desalination 2016, 378, 37–43. [Google Scholar] [CrossRef]
- Shirtcliffe, N.J.; McHale, G.; Atherton, S.; Newton, M.I. An Introduction to Superhydrophobicity. Adv. Colloid Interface Sci. 2010, 161, 124–138. [Google Scholar] [CrossRef] [Green Version]
- De Luca, G.; Gugliuzza, A.; Drioli, E. Competitive hydrogen-bonding interactions in modified polymer membranes: A density functional theory investigation. J. Phys. Chem. B. 2009, 113, 5473–5477. [Google Scholar] [CrossRef]
- Wang, M.; Liu, G.; Yu, H.; Lee, S.H.; Wang, L.; Zheng, J.; Wang, T.; Yun, Y.; Lee, J.K. ZnO Nanorod Array Modified PVDF Membrane with Superhydrophobic Surface for Vacuum Membrane Distillation Application. ACS Appl. Mater. Interfaces 2018, 10, 13452–13461. [Google Scholar] [CrossRef] [PubMed]
- Cassie, A.B.D.; Baxter, S. Wettability of Porous Surfaces. Trans. Faraday Soc. 1944, 40, 546. [Google Scholar] [CrossRef]
- Shan, H.; Liu, J.; Li, X.; Li, Y.; Tezel, F.H.; Li, B.; Wang, S. Nanocoated Amphiphobic Membrane for Flux Enhancement and Comprehensive Anti-Fouling Performance in Direct Contact Membrane Distillation. J. Memb. Sci. 2018, 567, 166–180. [Google Scholar] [CrossRef]
- Zheng, R.; Chen, Y.; Wang, J.; Song, J.; Li, X.M.; He, T. Preparation of Omniphobic PVDF Membrane with Hierarchical Structure for Treating Saline Oily Wastewater Using Direct Contact Membrane Distillation. J. Memb. Sci. 2018, 555, 197–205. [Google Scholar] [CrossRef]
- Cai, X.; Lei, T.; Sun, D.; Lin, L. A Critical Analysis of the α, β and γ Phases in Poly(Vinylidene Fluoride) Using FTIR. RSC Adv. 2017, 7, 15382–15389. [Google Scholar] [CrossRef] [Green Version]
- Kabir, E.; Khatun, M.; Nasrin, L.; Raihan, M.J.; Rahman, M. Pure β-Phase Formation in Polyvinylidene Fluoride (PVDF)-Carbon Nanotube Composites. J. Phys. D Appl. Phys. 2017, 50, 163002. [Google Scholar] [CrossRef]
- Sun, G.; Li, X.; Qu, Y.; Wang, X.; Yan, H.; Zhang, Y. Preparation and Characterization of Graphite Nanosheets from Detonation Technique. Mater. Lett. 2008, 62, 703–706. [Google Scholar] [CrossRef]
- Bello, R.H.; Coelho, L.A.; Becker, D. Role of Chemical Funcionalization of Carbon Nanoparticles in Epoxy Matrices. J. Compos. Mater. 2018, 52, 449–464. [Google Scholar] [CrossRef]
- Hong, G.; Han, Y.; Schutzius, T.M.; Wang, Y.; Pan, Y.; Hu, M.; Jie, J.; Sharma, C.S.; Müller, U.; Poulikakos, D. On the Mechanism of Hydrophilicity of Graphene. Nano Lett. 2016, 16, 4447–4453. [Google Scholar] [CrossRef] [PubMed]
- Belyaeva, L.A.; van Deursen, P.M.G.; Barbetsea, K.I.; Schneider, G.F. Hydrophilicity of Graphene in Water through Transparency to Polar and Dispersive Interactions. Adv. Mater. 2018, 30, 1703274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.; Zhang, Y.; Abidi, N.; Cabrales, L. Wettability and Surface Free Energy of Graphene Films. Langmuir 2009, 25, 11078–11081. [Google Scholar] [CrossRef] [PubMed]
- Taherian, F.; Marcon, V.; Van Der Vegt, N.F.A.; Leroy, F. What Is the Contact Angle of Water on Graphene? Langmuir 2013, 29, 1457–1465. [Google Scholar] [CrossRef]
- Yu, Y.S.; Wang, Z.; Zhao, Y.P. Experimental and Theoretical Investigations of Evaporation of Sessile Water Droplet on Hydrophobic Surfaces. J. Colloid Interface Sci. 2012, 365, 254–259. [Google Scholar] [CrossRef] [Green Version]
- Xu, W.; Leeladhar, R.; Kang, Y.T.; Choi, C.H. Evaporation Kinetics of Sessile Water Droplets on Micropillared Superhydrophobic Surfaces. Langmuir 2013, 29, 6032–6041. [Google Scholar] [CrossRef]
- Birdi, K.S.; Vu, D.T. Wettability and the evaporation rates of fluids from solid surfaces. J. Adhes. Sci. Technol. 1993, 7, 485–493. [Google Scholar] [CrossRef]
- Extrand, C.W. Contact Angles and Hysteresis on Surfaces with Chemically Heterogeneous Islands. Langmuir 2003, 19, 3793–3796. [Google Scholar] [CrossRef]
- Shin, D.H.; Lee, S.H.; Jung, J.Y.; Yoo, J.Y. Evaporating Characteristics of Sessile Droplet on Hydrophobic and Hydrophilic Surfaces. Microelectron. Eng. 2009, 86, 1350–1353. [Google Scholar] [CrossRef]
- Liu, T.; Kim, C.-J. Turning a Surface Superrepellent Even to Completely Wetting Liquids. Science 2014, 346, 1096–1100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.M.; Reinhoudt, D.; Crego-Calama, M. What Do We Need for a Superhydrophobic Surface? A Review on the Recent Progress in the Preparation of Superhydrophobic Surfaces. Chem. Soc. Rev. 2007, 36, 1350–1368. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Wang, R.; Fane, A.G. Fabrication of Bioinspired Composite Nanofiber Membranes with Robust Superhydrophobicity for Direct Contact Membrane Distillation. Environ. Sci. Technol. 2014, 48, 6335–6341. [Google Scholar] [CrossRef] [PubMed]
- Razmjou, A.; Arifin, E.; Dong, G.; Mansouri, J.; Chen, V. Superhydrophobic Modification of TiO2 Nanocomposite PVDF Membranes for Applications in Membrane Distillation. J. Memb. Sci. 2012, 415–416, 850–863. [Google Scholar] [CrossRef]
- Palacio, I.; Lauwaet, K.; Vázquez, L.; Palomares, F.J.; González-Herrero, H.; Martínez, J.I.; Aballe, L.; Foerster, M.; García-Hernández, M.; Martín-Gago, J.Á. Ultra-Thin NaCl Films as Protective Layers for Graphene. Nanoscale 2019, 11, 16767–16772. [Google Scholar] [CrossRef]
- An, A.K.; Lee, E.J.; Guo, J.; Jeong, S.; Lee, J.G.; Ghaffour, N. Enhanced Vapor Transport in Membrane Distillation via Functionalized Carbon Nanotubes Anchored into Electrospun Nanofibres. Sci. Rep. 2017, 7, 41562. [Google Scholar] [CrossRef] [Green Version]
- Grasso, G.; Galiano, F.; Yoo, M.J.; Mancuso, R.; Park, H.B.; Gabriele, B.; Figoli, A.; Drioli, E. Development of Graphene-PVDF Composite Membranes for Membrane Distillation. J. Memb. Sci. 2020, 604, 118017. [Google Scholar] [CrossRef]
- Jafari, A.; Kebria, M.R.S.; Rahimpour, A.; Bakeri, G. Graphene Quantum Dots Modified Polyvinylidenefluride (PVDF) Nanofibrous Membranes with Enhanced Performance for Air Gap Membrane Distillation. Chem. Eng. Process. Process Intensif. 2018, 126, 222–231. [Google Scholar] [CrossRef]
- Leaper, S.; Abdel-Karim, A.; Faki, B.; Luque-Alled, J.M.; Alberto, M.; Vijayaraghavan, A.; Holmes, S.M.; Szekely, G.; Badawy, M.I.; Shokri, N.; et al. Flux-Enhanced PVDF Mixed Matrix Membranes Incorporating APTS-Functionalized Graphene Oxide for Membrane Distillation. J. Memb. Sci. 2018, 554, 309–323. [Google Scholar] [CrossRef]

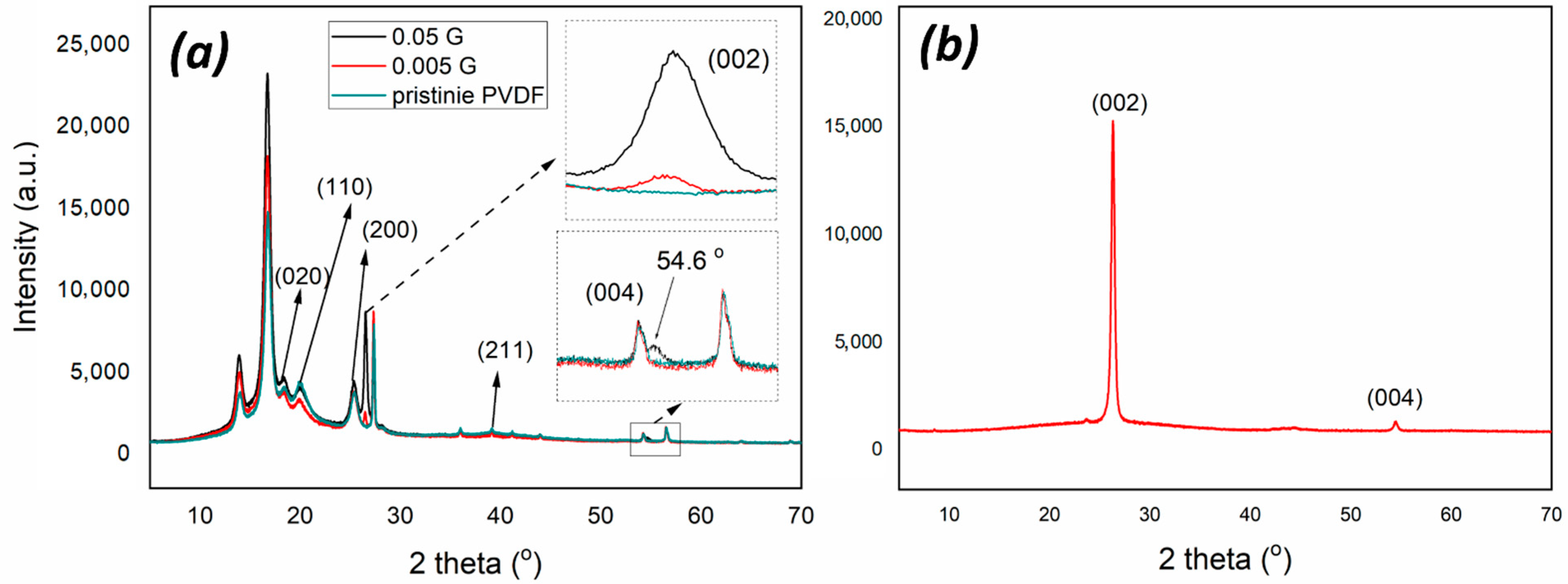
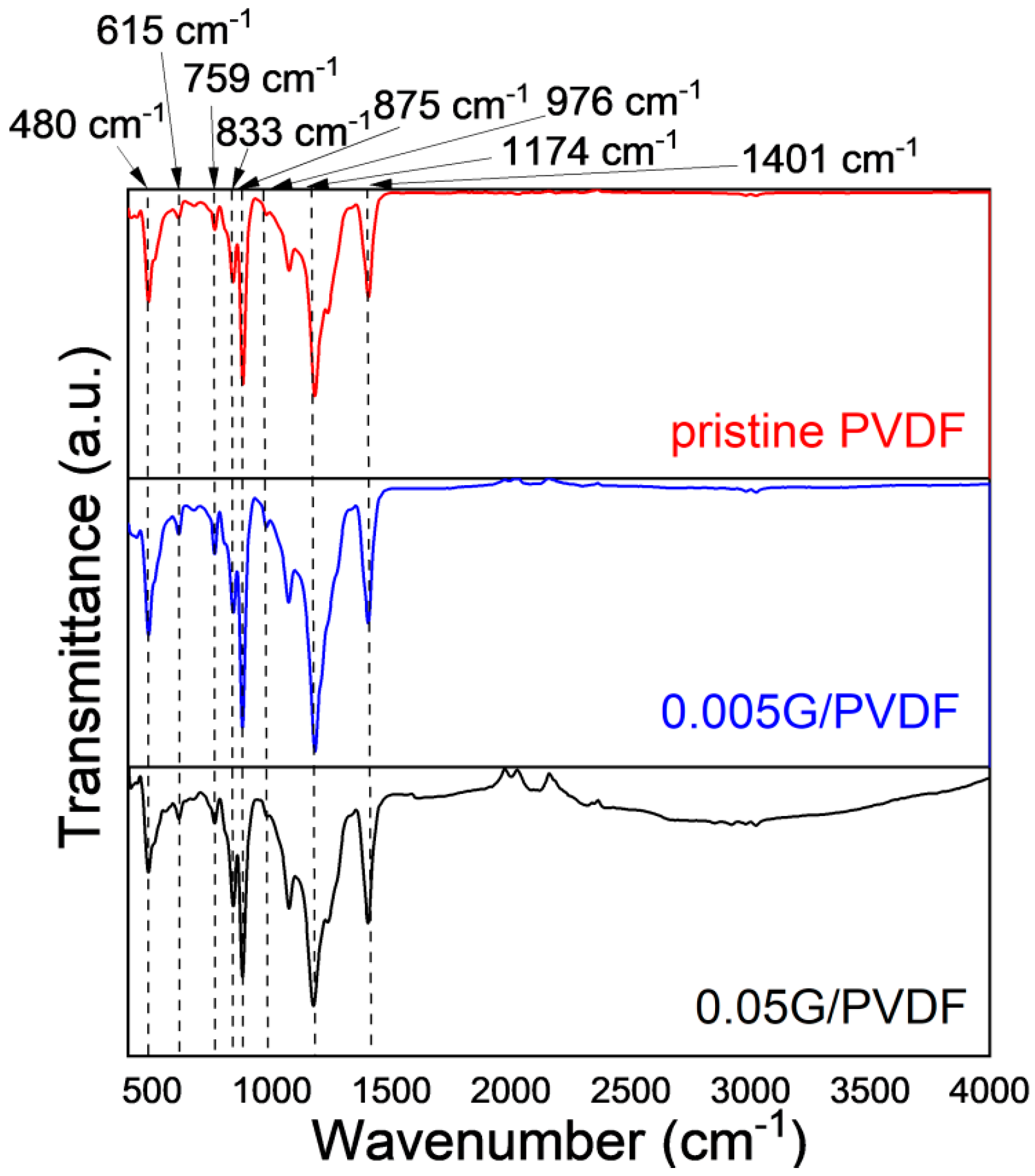
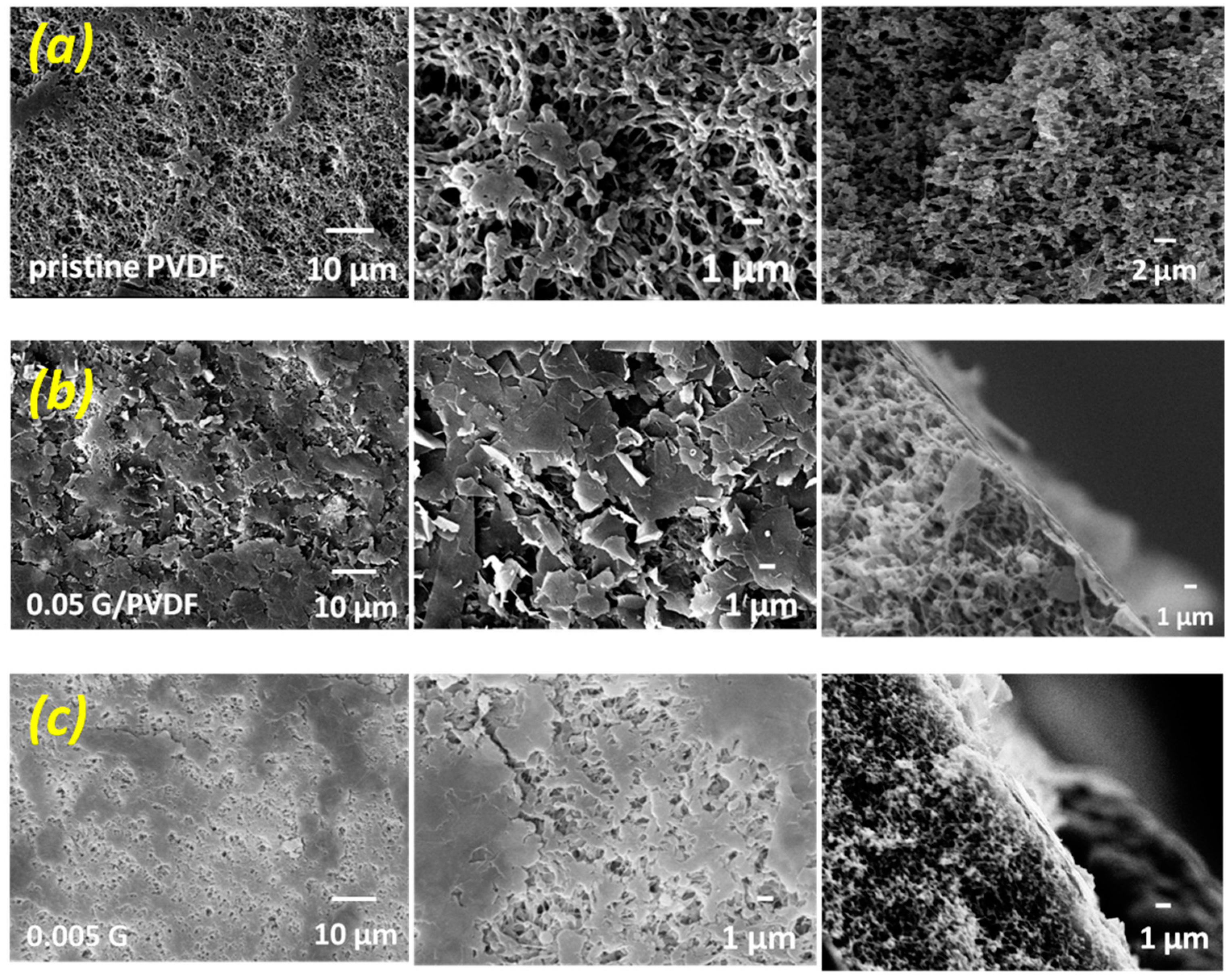



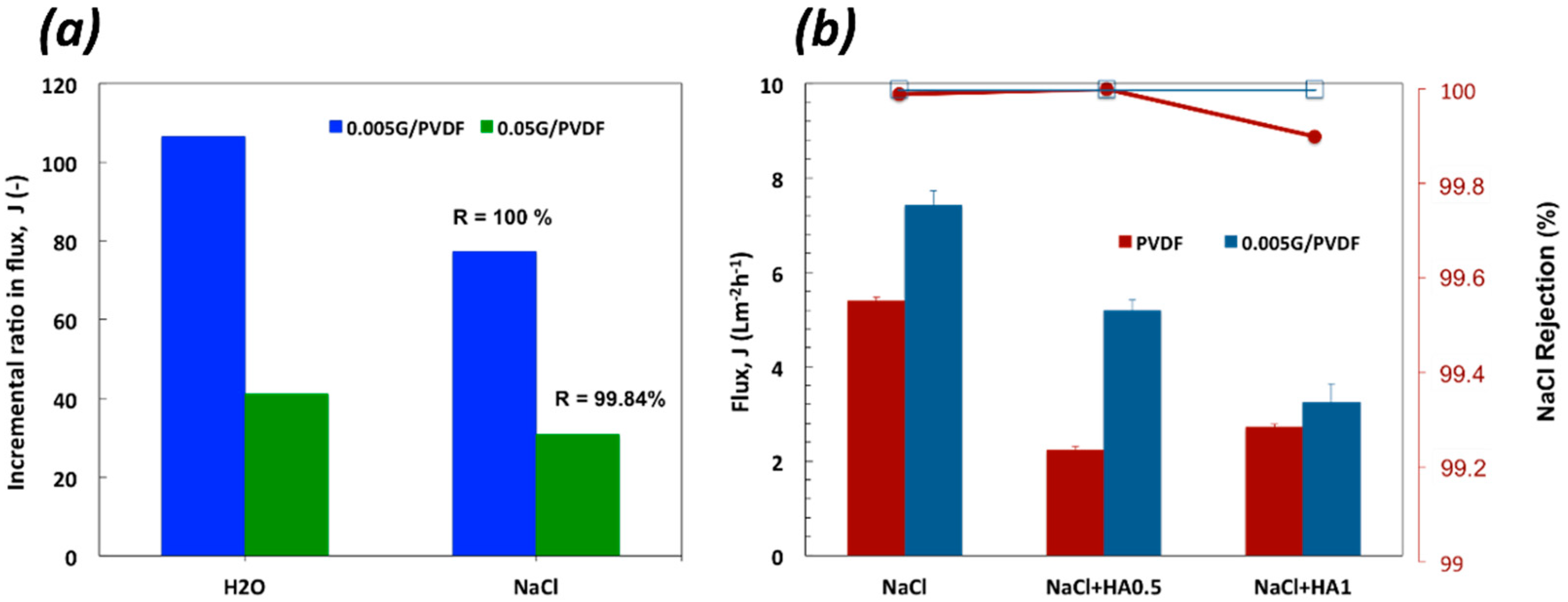
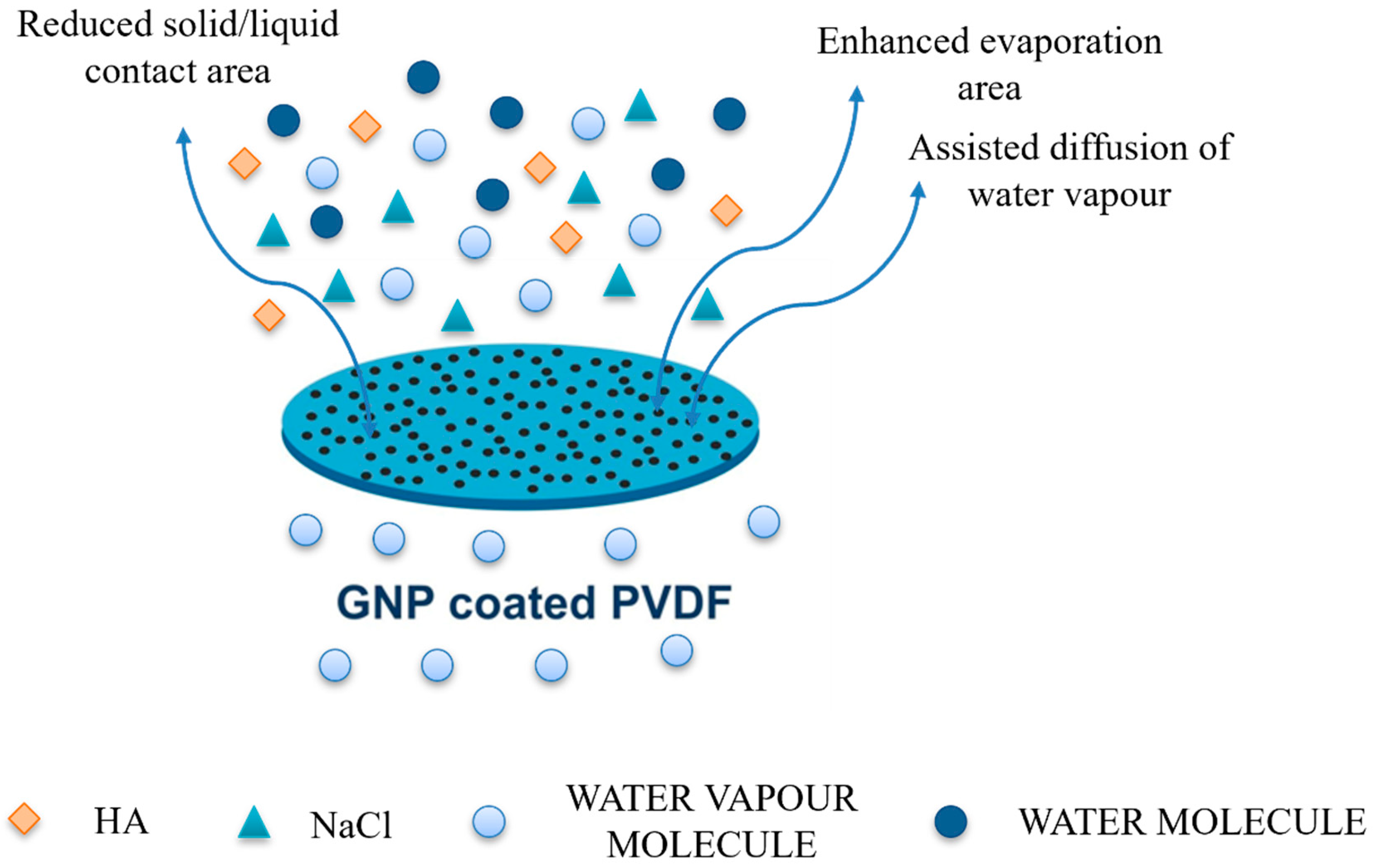

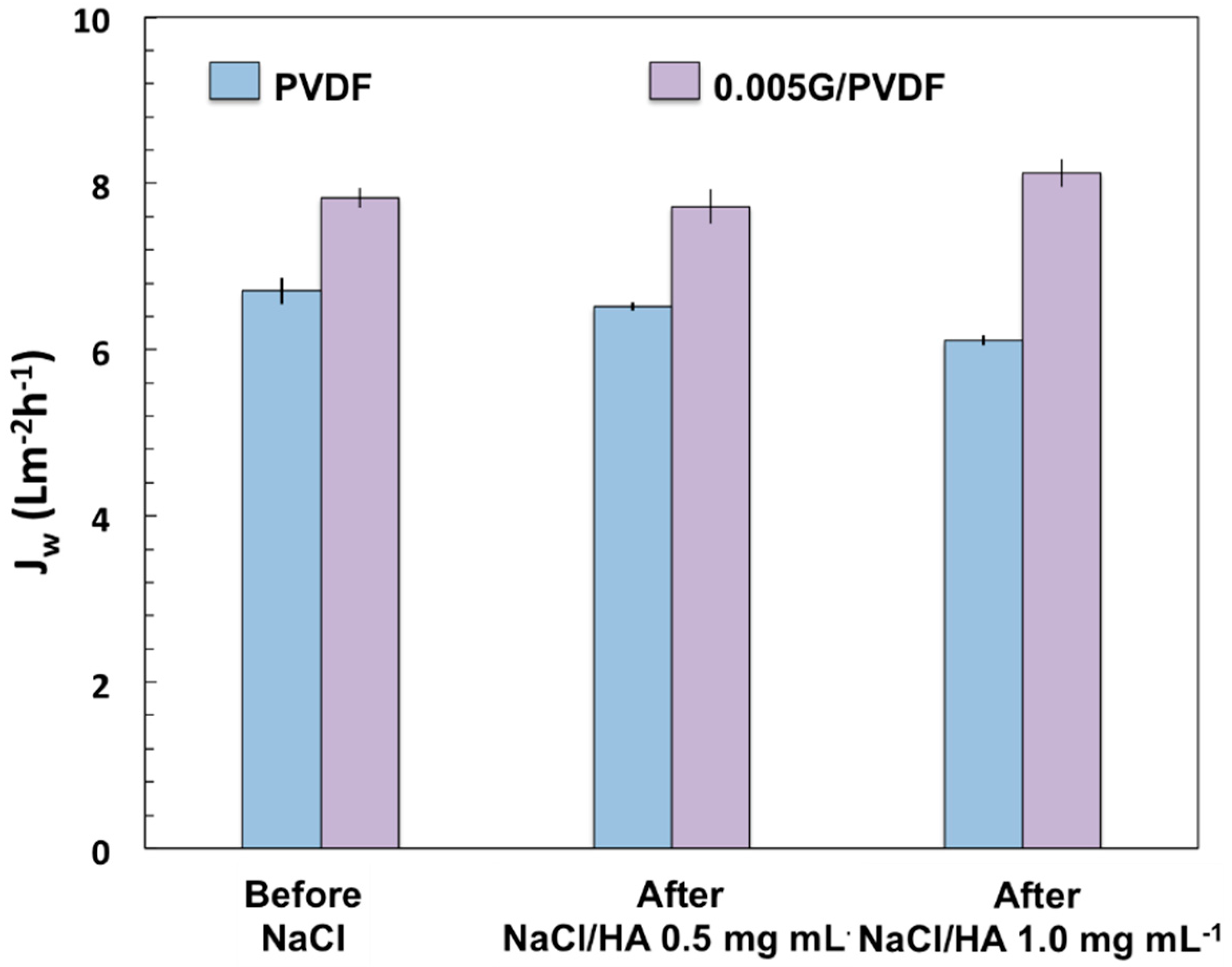
| Sample | Thickness (µm) | Porosity (%) | Largest Pore Size (µm) | Mean Pore Size (µm) |
|---|---|---|---|---|
| PVDF | 50 ± 1 | 65 ± 4 | 0.7 ± 0.1 | 0.48 ± 0.09 |
| 0.05G/PVDF | 50 ± 1 | 62 ± 2 | 0.43 ± 0.09 | 0.24 ± 0.01 |
| 0.005G/PVDF | 50 ± 4 | 63 ± 2 | 0.80 ± 0.06 | 0.49 ± 0.01 |
| Sample | Ra (nm) | Rq (nm) | Rz (nm) |
|---|---|---|---|
| PVDF | 214 ± 19 | 272 ± 10 | 499 ± 21 |
| 0.05G/PVDF | 97 ± 7 | 133 ± 15 | 180 ± 16 |
| 0.005G/PVDF | 272 ± 10 | 327 ± 6 | 767 ± 73 |
| Membrane | MD Configuration | Process Parameters | Flux (L/m2 h) | Rejection | Reference |
|---|---|---|---|---|---|
| PVDF + GNP (0.5 wt%) | DCMD | 0.6 M NaCl Tf: 56 °C Tp: 15 °C | ~9 | 99.9% | [8] |
| PVDF-f-G | DCMD | 0.5 M NaCl Tf: 70 °C Tp: 20 °C | ~3 | 99.9% | [48] |
| PVDF + GQDs (0.3) wt% | AGMD | 0.6 M NaCl Tf: 60 °C Tp: 20 °C | 18 | 99.8% | [49] |
| PVDF + GO- APTS 0.3% | AGMD | 0.6 M NaCl Tf: 85 °C Tp: 23 °C | 5.4 | 99.6% | [50] |
| PVDF + GO- APTS 0.3% | AGMD | 0.6 M NaCl Tf: 85 °C Tp: 25 °C | 6.25 | 99.9% | [50] |
| Coated 0.005G/PVDF | DCMD | 0.6 M NaCl Tf: 40 °C Tp: 16 °C | 7.5 | 100% | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gontarek-Castro, E.; Di Luca, G.; Lieder, M.; Gugliuzza, A. Graphene-Coated PVDF Membranes: Effects of Multi-Scale Rough Structure on Membrane Distillation Performance. Membranes 2022, 12, 511. https://doi.org/10.3390/membranes12050511
Gontarek-Castro E, Di Luca G, Lieder M, Gugliuzza A. Graphene-Coated PVDF Membranes: Effects of Multi-Scale Rough Structure on Membrane Distillation Performance. Membranes. 2022; 12(5):511. https://doi.org/10.3390/membranes12050511
Chicago/Turabian StyleGontarek-Castro, Emilia, Giuseppe Di Luca, Marek Lieder, and Annarosa Gugliuzza. 2022. "Graphene-Coated PVDF Membranes: Effects of Multi-Scale Rough Structure on Membrane Distillation Performance" Membranes 12, no. 5: 511. https://doi.org/10.3390/membranes12050511
APA StyleGontarek-Castro, E., Di Luca, G., Lieder, M., & Gugliuzza, A. (2022). Graphene-Coated PVDF Membranes: Effects of Multi-Scale Rough Structure on Membrane Distillation Performance. Membranes, 12(5), 511. https://doi.org/10.3390/membranes12050511







