Surface Modification of ETFE Membrane and PTFE Membrane by Atmospheric DBD Plasma
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
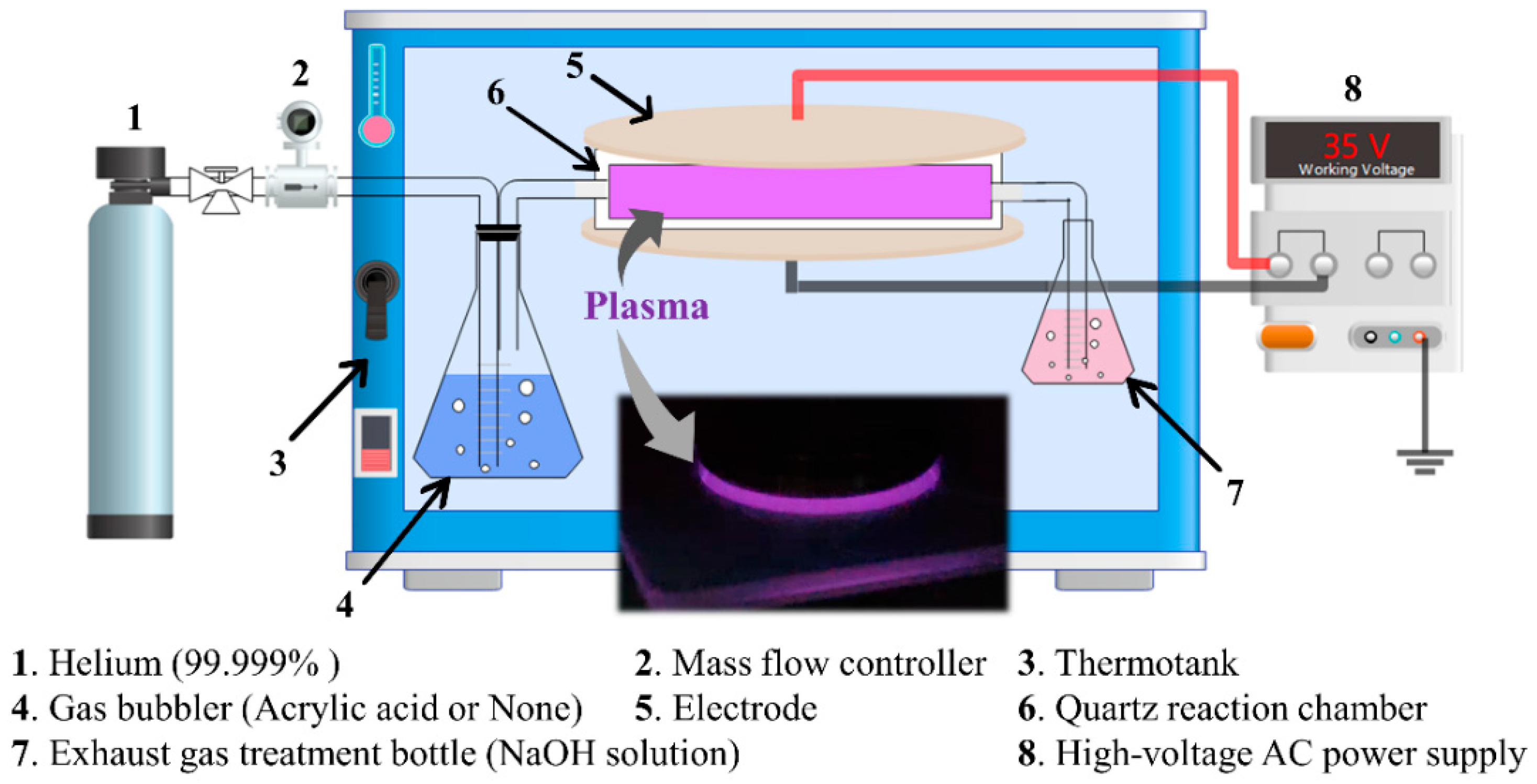
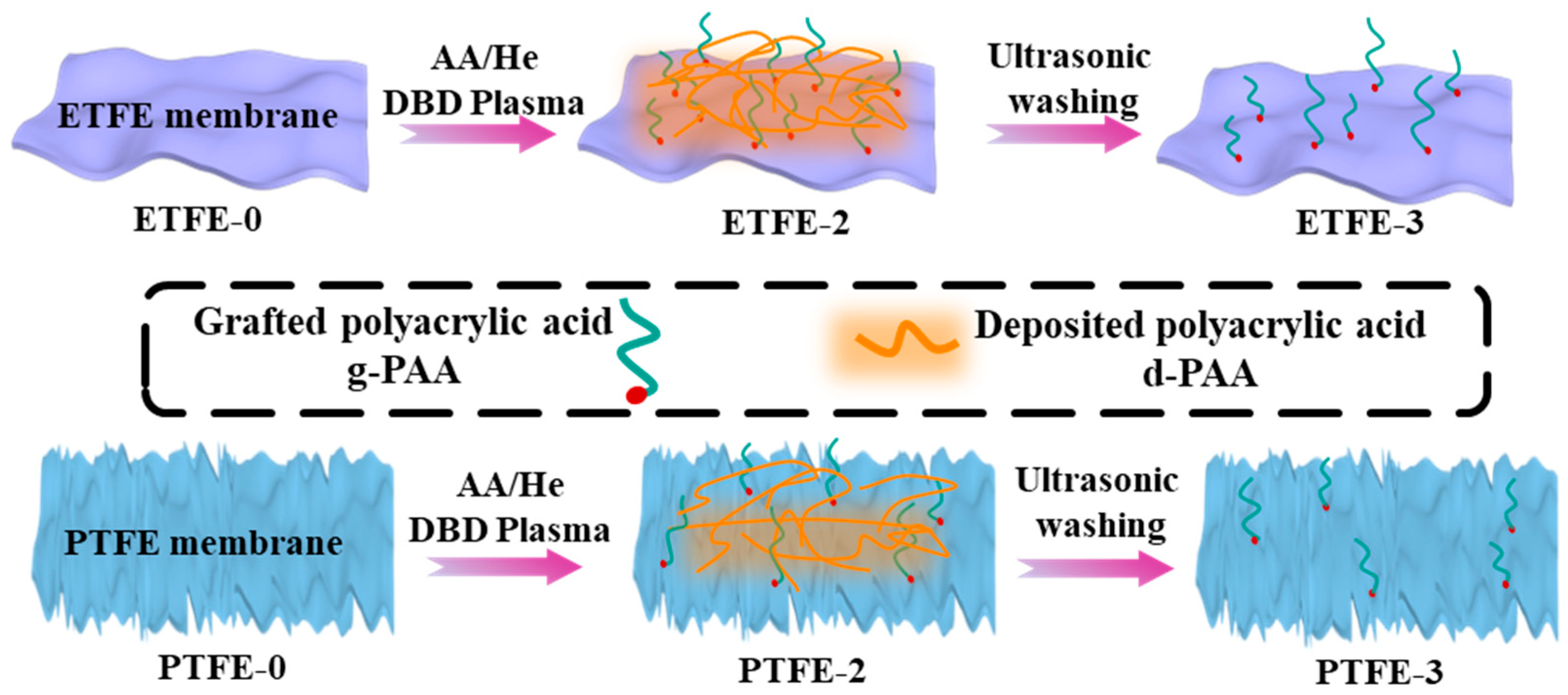
2.2. Plasma Treatment
2.3. Characterization
3. Results and Discussion
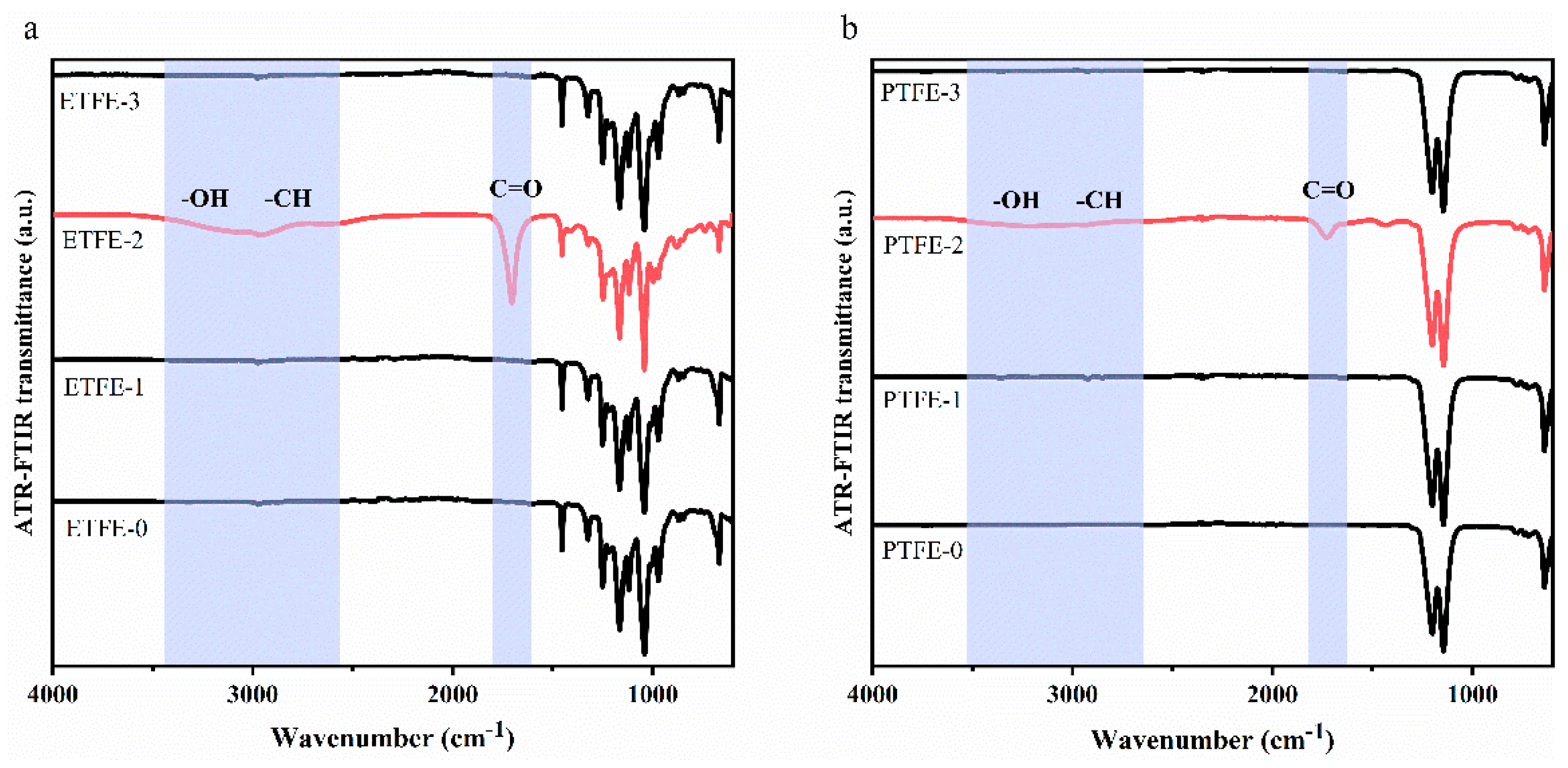
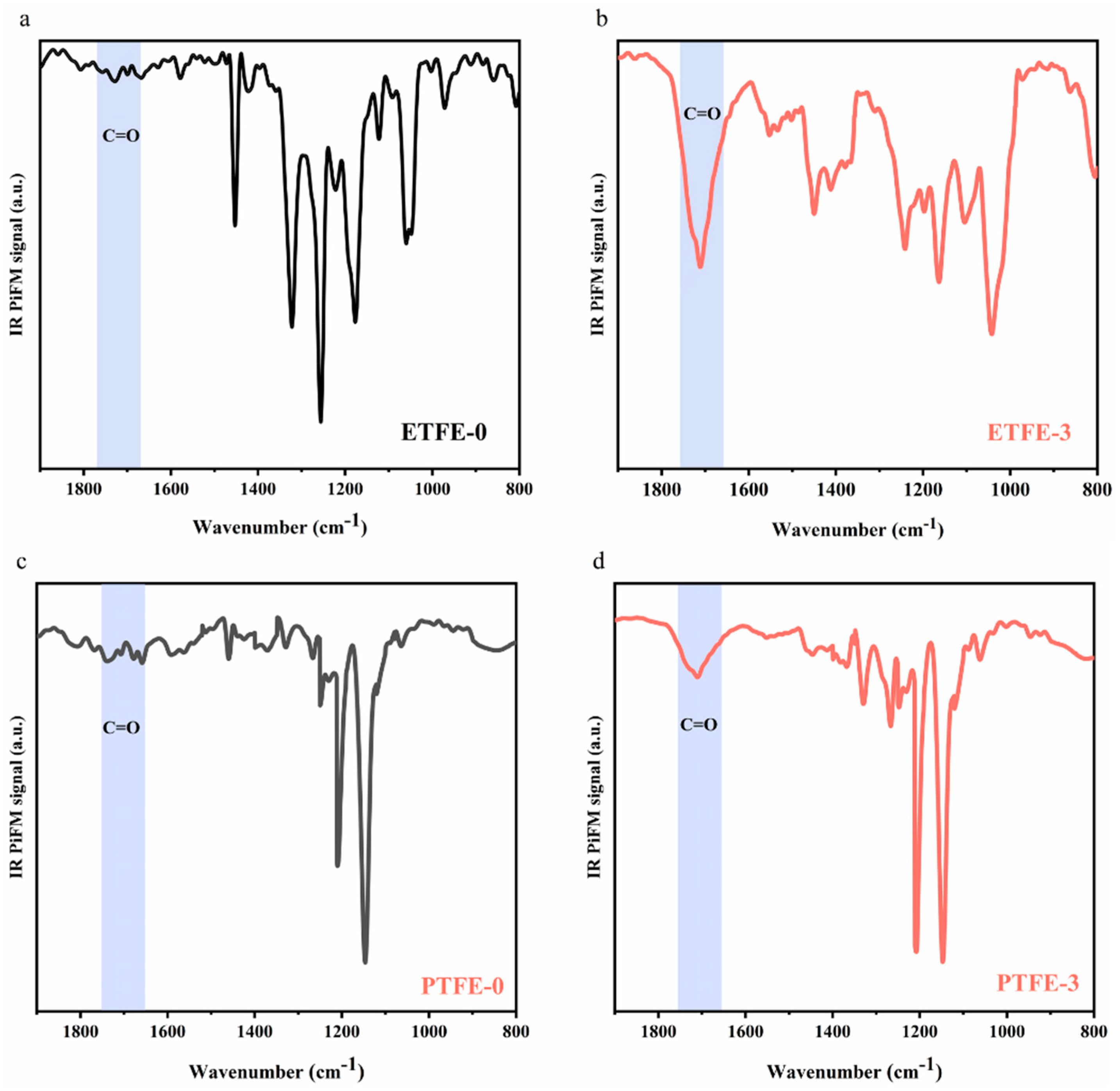
3.1. ATR-FTIR and IR PiFM Analysis
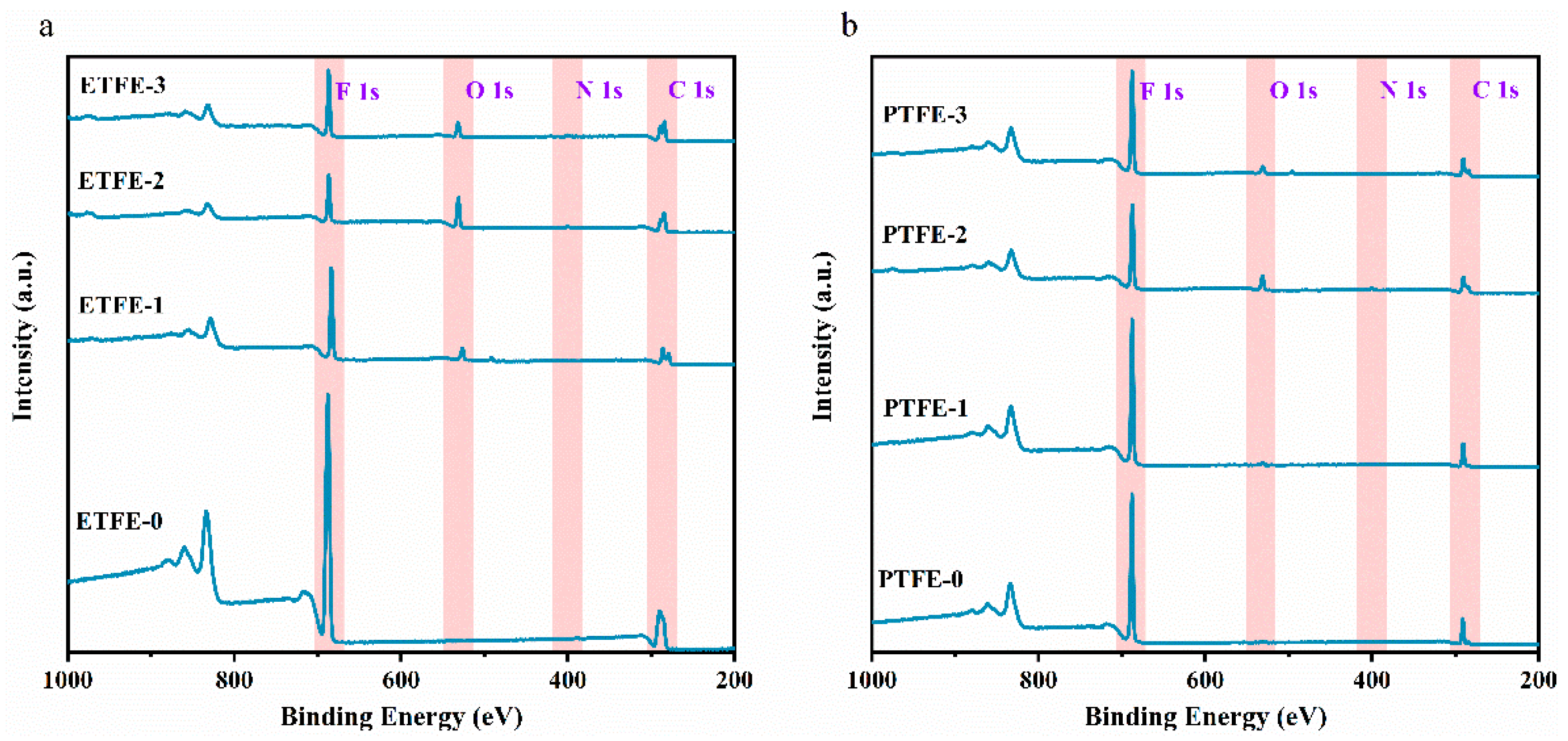
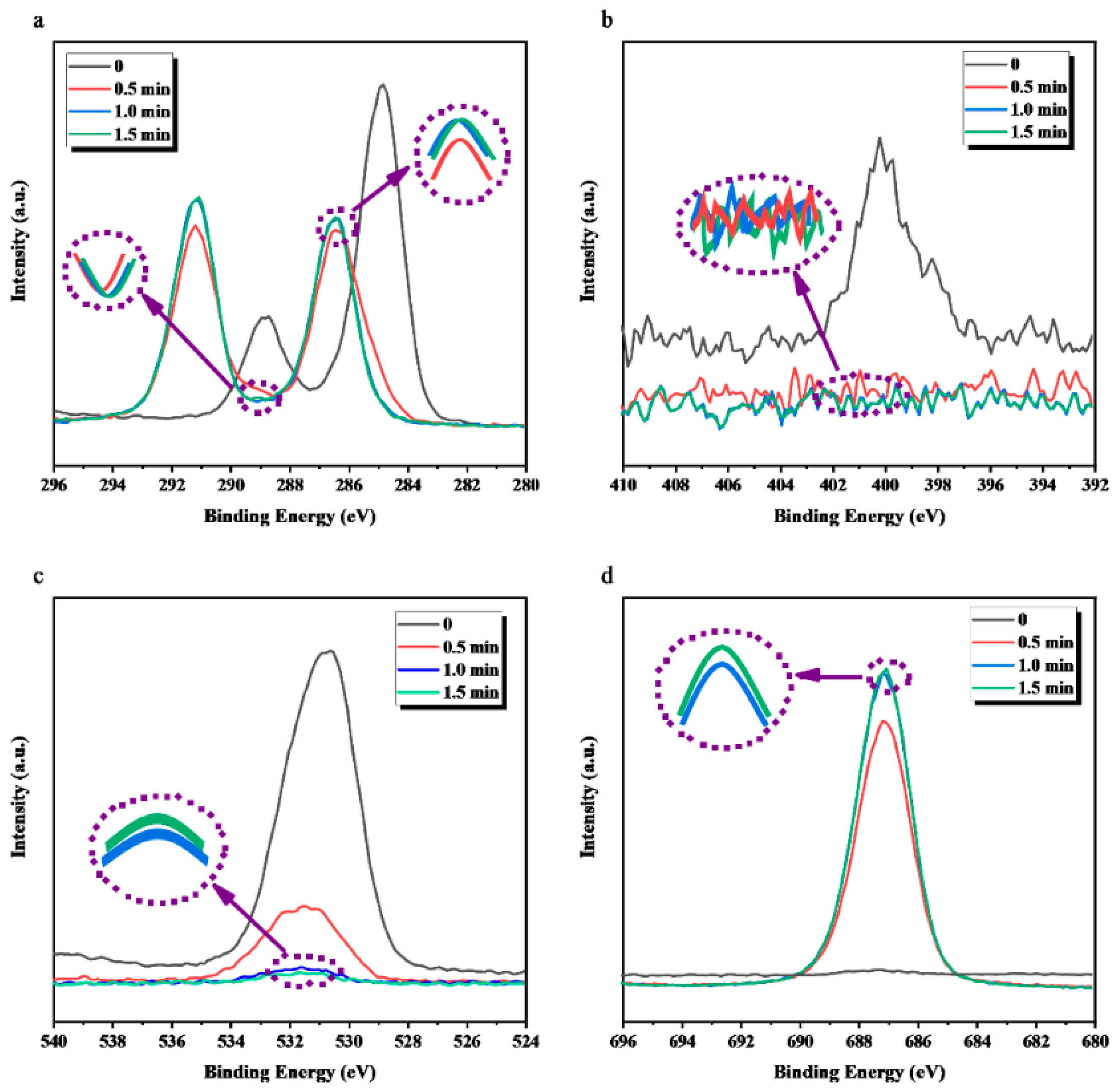
3.2. XPS Analysis
3.3. Surface Morphlogy Analysis
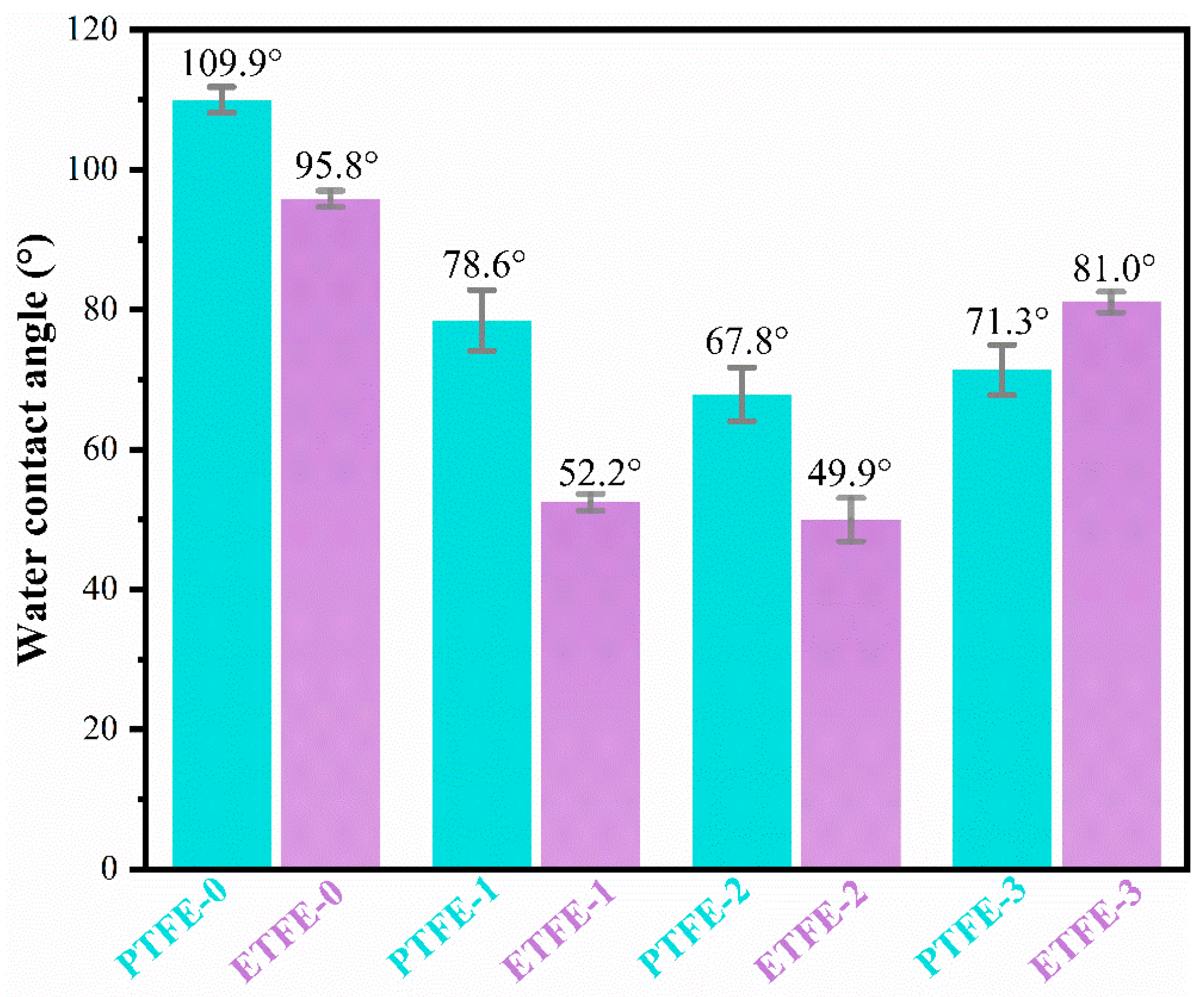
3.4. Hydrophilicity Analysis
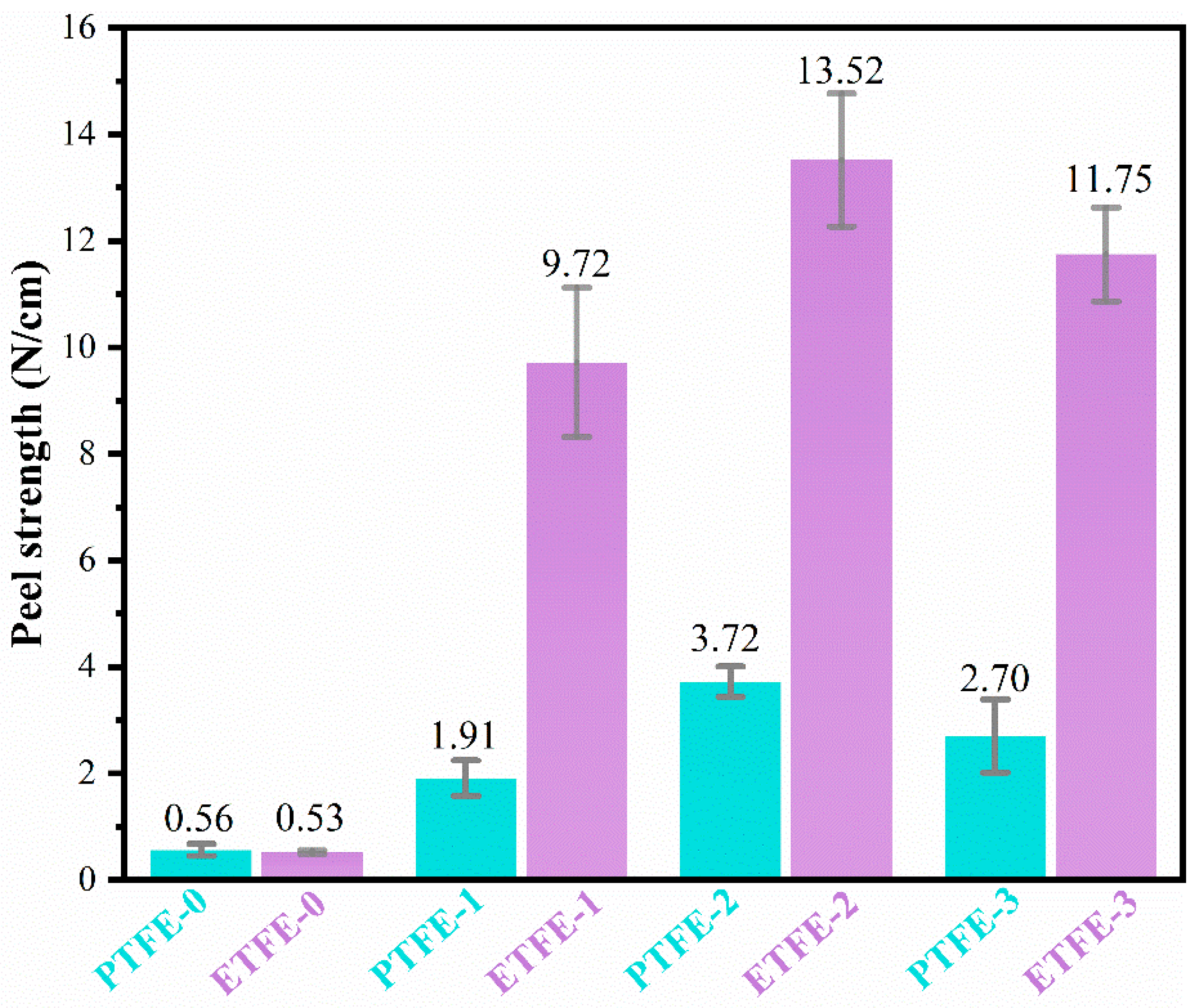
3.5. Adhesion Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stock, B. Industrial Guide to Chemical and Drug Safety; Dikshith, T.S.S., Diwan, P.V., Eds.; Angewandte Chemie International Edition: Hoboken, NJ, USA, 2003. [Google Scholar]
- Zhao, Y.; Wang, X.; Wang, D.; Li, H.; Li, L.; Zhang, S.; Zhou, C.; Zheng, X.; Men, Q.; Zhong, J.; et al. Preparation and Chemical Protective Clothing Application of PVDF Based Sodium Sulfonate Membrane. Membranes 2020, 10, 190. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, S.; Joshi, R.; Chughtai, A.A.; Macintyre, C.R. Graphene Modified Multifunctional Personal Protective Clothing. Adv. Mater. Interfaces 2019, 6, 1900622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhuiyan, M.A.R.; Wang, L.; Shaid, A.; Shanks, R.A.; Ding, J. Advances and applications of chemical protective clothing system. J. Ind. Text. 2018, 49, 97–138. [Google Scholar] [CrossRef]
- Sciarratta, V.; Vohrer, U.; Hegemann, D.; Müller, M.; Oehr, C. Plasma functionalization of polypropylene with acrylic acid. Surf. Coat. Technol. 2003, 174–175, 805–810. [Google Scholar] [CrossRef]
- Chen, X.; Huang, G.; An, C.; Feng, R.; Yao, Y.; Zhao, S.; Huang, C.; Wu, Y. Plasma-induced poly(acrylic acid)-TiO2 coated polyvinylidene fluoride membrane for produced water treatment: Synchrotron X-Ray, optimization, and insight studies. J. Clean. Prod. 2019, 227, 772–783. [Google Scholar] [CrossRef]
- Cheng, C.-Y.; Chung, F.-Y.; Chou, P.-Y.; Huang, C. Surface Modification of Polytetrafluoroethylene by Atmospheric Pressure Plasma-Grafted Polymerization. Plasma Chem. Plasma Process. 2020, 40, 1507–1523. [Google Scholar] [CrossRef]
- Abu-Saied, M.; Fahmy, A.; Morgan, N.; Qutop, W.; Abdelbary, H.; Friedrich, J.F. Enhancement of Poly(vinyl chloride) Electrolyte Membrane by Its Exposure to an Atmospheric Dielectric Barrier Discharge Followed by Grafting with Polyacrylic Acid. Plasma Chem. Plasma Process. 2019, 39, 1499–1517. [Google Scholar] [CrossRef]
- Demaude, A.; Poleunis, C.; Goormaghtigh, E.; Viville, P.; Lazzaroni, R.; Delcorte, A.; Gordon, M.; Reniers, F. Atmospheric Pressure Plasma Deposition of Hydrophilic/Phobic Patterns and Thin Film Laminates on Any Surface. Langmuir 2019, 35, 9677–9683. [Google Scholar] [CrossRef] [Green Version]
- Yin, M.; Huang, J.; Yu, J.; Chen, G.; Qu, S.; Wang, X.; Li, C. The polypropylene membrane modified by an atmospheric pressure plasma jet as a separator for lithium-ion button battery. Electrochim. Acta 2018, 260, 489–497. [Google Scholar] [CrossRef]
- Ward, L.J.; Schofield, W.; Badyal, J.; Goodwin, A.J.; Merlin, P.J. Atmospheric Pressure Plasma Deposition of Structurally Well-Defined Polyacrylic Acid Films. Chem. Mater. 2003, 15, 1466–1469. [Google Scholar] [CrossRef]
- Basarir, F.; Choi, E.; Moon, S.; Song, K.; Yoon, T. Electrochemical properties of PP membranes with plasma polymer coatings of acrylic acid. J. Membr. Sci. 2005, 260, 66–74. [Google Scholar] [CrossRef]
- Mahmoud Nasef, M.; Saidi, H.; Ahmad, A.; Ahmad Ali, A. Optimization and kinetics of phosphoric acid doping of poly(1-vinylimidazole)-graft-poly(ethylene-co-tetrafluorethylene) proton conducting membrane precursors. J. Membr. Sci. 2013, 446, 422–432. [Google Scholar] [CrossRef]
- Schmidt, C.; Schmidt-Naake, G. Proton Conducting Membranes Obtained by Doping Radiation-Grafted Basic Membrane Matrices with Phosphoric Acid. Macromol. Mater. Eng. 2007, 292, 1164–1175. [Google Scholar] [CrossRef]
- Badiei, Y.M.; Traba, C.; Rosales, R.; Rojas, A.L.; Amaya, C.; Shahid, M.; Vera-Rolong, C.; Concepcion, J.J. Plasma-Initiated Graft Polymerization of Acrylic Acid onto Fluorine-Doped Tin Oxide as a Platform for Immobilization of Water-Oxidation Catalysts. ACS Appl. Mater. Interfaces 2021, 13, 14077–14090. [Google Scholar] [CrossRef]
- Qiu, J.; Zhai, M.; Chen, J.; Wang, Y.; Peng, J.; Xu, L.; Li, J.; Wei, G. Performance of vanadium redox flow battery with a novel amphoteric ion exchange membrane synthesized by two-step grafting method. J. Membr. Sci. 2009, 342, 215–220. [Google Scholar] [CrossRef]
- Fahmy, A.; Abu-Saied, M.; Morgan, N.; Qutop, W.; Abdelbary, H.; Salama, T. Surface modification of polyvinyl chloride by polyacrylic acid graftas a polyelectrolyte membrane using Ar plasma. Turk. J. Chem. 2019, 43, 1686–1696. [Google Scholar] [CrossRef]
- Świtała-Żeliazkow, M. Thermal degradation of copolymers of styrene with dicarboxylic acidsI. Alternating styrene-maleic acid copolymer. Polym. Degrad. Stab. 2001, 74, 579–584. [Google Scholar] [CrossRef]
- Chen, G.; He, S.; Shi, G.; Ma, Y.; Ruan, C.; Jin, X.; Chen, Q.; Liu, X.; Dai, H.; Chen, X.; et al. In-situ immobilization of ZIF-67 on wood aerogel for effective removal of tetracycline from water. Chem. Eng. J. 2021, 423, 130184. [Google Scholar] [CrossRef]
- Wang, L.; Jakob, D.S.; Wang, H.; Apostolos, A.; Pires, M.M.; Xu, X.G. Generalized Heterodyne Configurations for Photoinduced Force Microscopy. Anal. Chem. 2019, 91, 13251–13259. [Google Scholar] [CrossRef]
- Almajhadi, M.A.; Uddin, S.M.A.; Wickramasinghe, H.K. Observation of nanoscale opto-mechanical molecular damping as the origin of spectroscopic contrast in photo induced force microscopy. Nat. Commun. 2020, 11, 5691. [Google Scholar] [CrossRef]
- Kim, B.; Jahng, J.; Sifat, A.; Lee, E.S.; Potma, E.O. Monitoring Fast Thermal Dynamics at the Nanoscale through Frequency Domain Photoinduced Force Microscopy. J. Phys. Chem. C 2021, 125, 7276–7286. [Google Scholar] [CrossRef]
- Gu, K.L.; Zhou, Y.; Morrison, W.A.; Park, K.; Park, S.; Bao, Z. Nanoscale Domain Imaging of All-Polymer Organic Solar Cells by Photo-Induced Force Microscopy. ACS Nano 2018, 12, 1473–1481. [Google Scholar] [CrossRef]

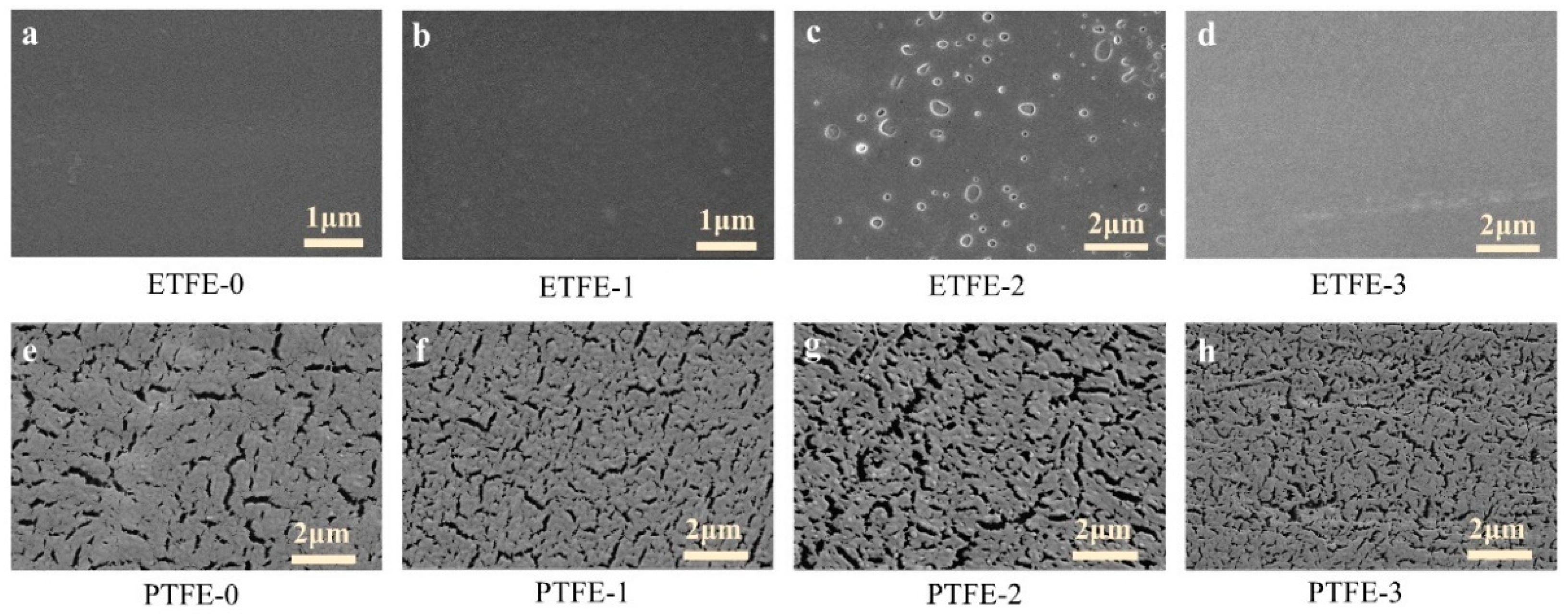
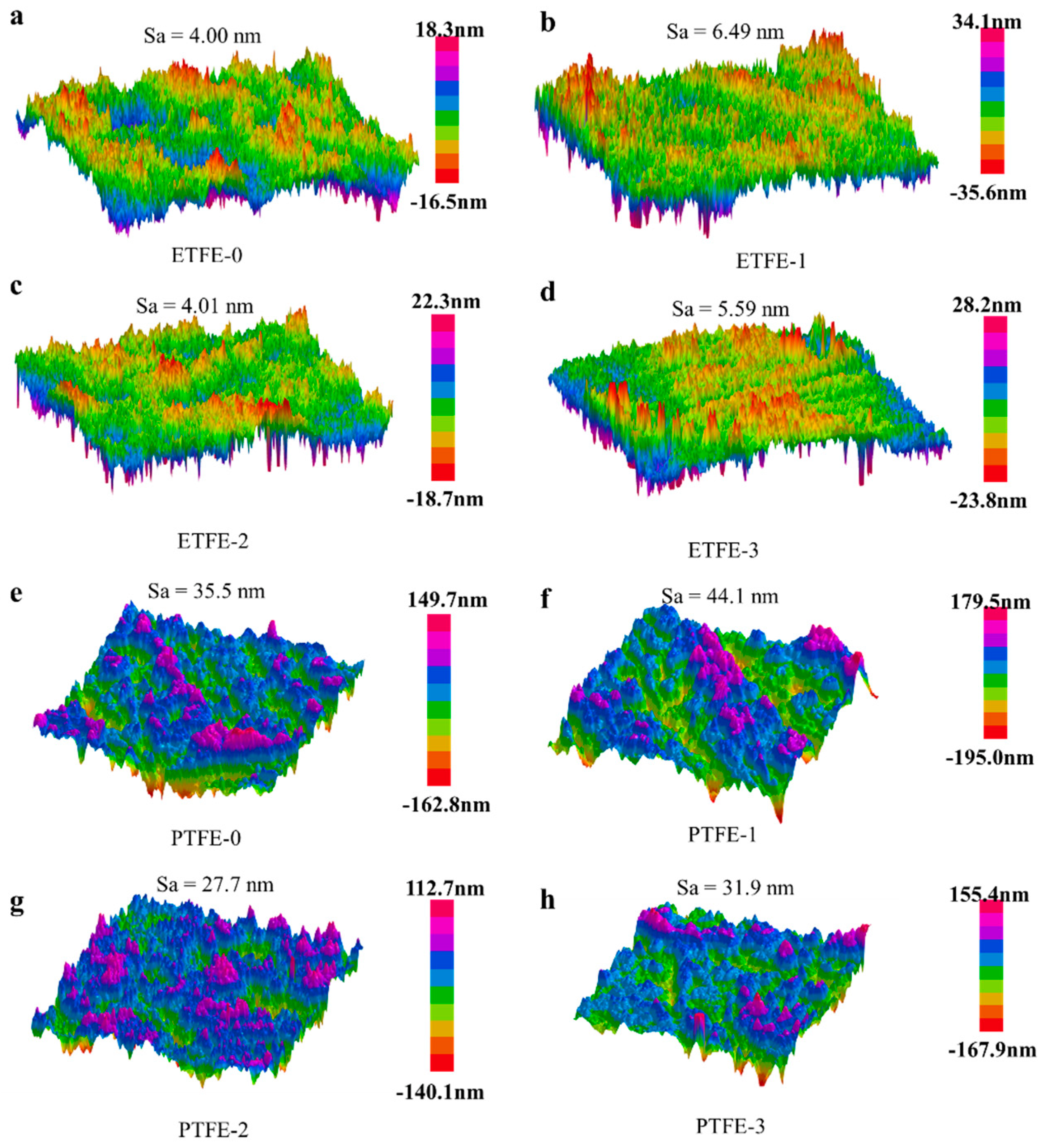
| Untreated | He Plasma Treatment | He/AA Plasma Treatment | He/AA Plasma Treatment + Acetone Ultrasonic | |
|---|---|---|---|---|
| ETFE | ETFE-0 | ETFE-1 | ETFE-2 | ETFE-3 |
| PTFE | PTFE-0 | PTFE-1 | PTFE-2 | PTFE-3 |
| C (%) | N (%) | O (%) | F (%) | F/C | O/C | |
|---|---|---|---|---|---|---|
| ETFE-0 | 48.83 | 0.04 | 0.17 | 50.96 | 1.0436 | 0.0035 |
| ETFE-1 | 49.31 | 0.16 | 8.52 | 42.01 | 0.8519 | 0.1727 |
| ETFE-2 | 56.00 | 2.17 | 20.3 | 21.54 | 0.3846 | 0.3625 |
| ETFE-3 | 52.51 | 0.09 | 8.24 | 39.16 | 0.7457 | 0.1569 |
| PTFE-0 | 35.65 | 0.02 | 0.54 | 63.79 | 1.7893 | 0.0151 |
| PTFE-1 | 35.78 | 0.22 | 1.47 | 62.54 | 1.7479 | 0.0410 |
| PTFE-2 | 41.73 | 2.25 | 10.72 | 45.31 | 1.0857 | 0.2568 |
| PTFE-3 | 40.57 | 0.71 | 5.79 | 52.93 | 1.3046 | 0.1427 |
| Sputtering Time | C (%) | N (%) | O (%) | F (%) | F/C | O/C |
|---|---|---|---|---|---|---|
| ETFE-2 0 min | 56.00 | 2.17 | 20.29 | 21.54 | 0.3846 | 0.3625 |
| ETFE-2 0.5 min | 49.72 | 1.59 | 22.34 | 26.35 | 0.5299 | 0.4493 |
| ETFE-2 1.0 min | 50.43 | 1.34 | 17.91 | 30.32 | 0.6012 | 0.3551 |
| ETFE-2 1.5 min | 50.51 | 1.00 | 13.92 | 34.57 | 0.6844 | 0.2755 |
| PTFE-2 0 min | 41.73 | 2.25 | 10.72 | 45.31 | 1.0857 | 0.2568 |
| PTFE-2 1.5 min | 40.24 | 1.12 | 7.93 | 50.71 | 1.2601 | 0.1970 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, Z.; Zhao, Y.; Zhang, M.; Li, X.; Li, H. Surface Modification of ETFE Membrane and PTFE Membrane by Atmospheric DBD Plasma. Membranes 2022, 12, 510. https://doi.org/10.3390/membranes12050510
Ji Z, Zhao Y, Zhang M, Li X, Li H. Surface Modification of ETFE Membrane and PTFE Membrane by Atmospheric DBD Plasma. Membranes. 2022; 12(5):510. https://doi.org/10.3390/membranes12050510
Chicago/Turabian StyleJi, Zuohui, Yue Zhao, Min Zhang, Xiaopeng Li, and Heguo Li. 2022. "Surface Modification of ETFE Membrane and PTFE Membrane by Atmospheric DBD Plasma" Membranes 12, no. 5: 510. https://doi.org/10.3390/membranes12050510
APA StyleJi, Z., Zhao, Y., Zhang, M., Li, X., & Li, H. (2022). Surface Modification of ETFE Membrane and PTFE Membrane by Atmospheric DBD Plasma. Membranes, 12(5), 510. https://doi.org/10.3390/membranes12050510







