Preparation of Electrospun Active Molecules Membrane Application to Atmospheric Free Radicals
Abstract
:1. Introduction
2. Materials and Methods
2.1. Apparatus and Reagents
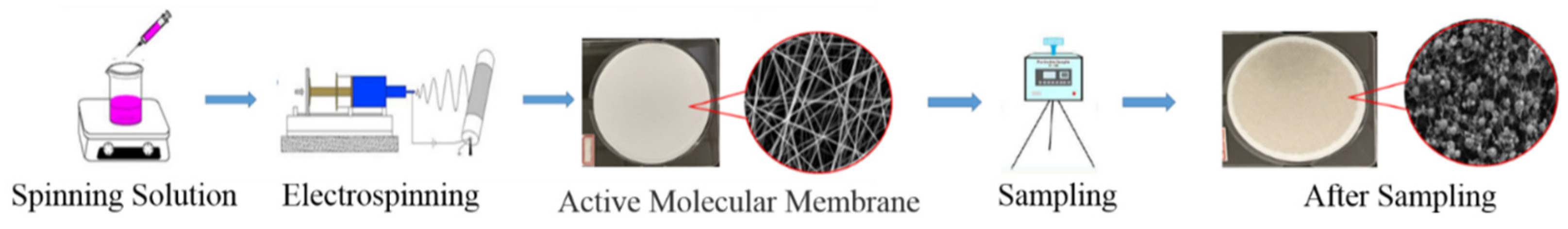
2.2. Preparation of Electrospinning Membrane of Active Molecules
2.3. Fluorescence Emission Spectroscopy Detection
2.4. Reaction Capacity and Free Radical Concentration Calculation
2.5. Fluorescence Detection Precision Experiment of Active Molecular Electrospinning Membrane
2.6. Generation and Trapping of ROS Radicals
2.7. Generation and Trapping of OH Radicals
2.8. Ozone Generation and Trapping
3. Results and Discussion
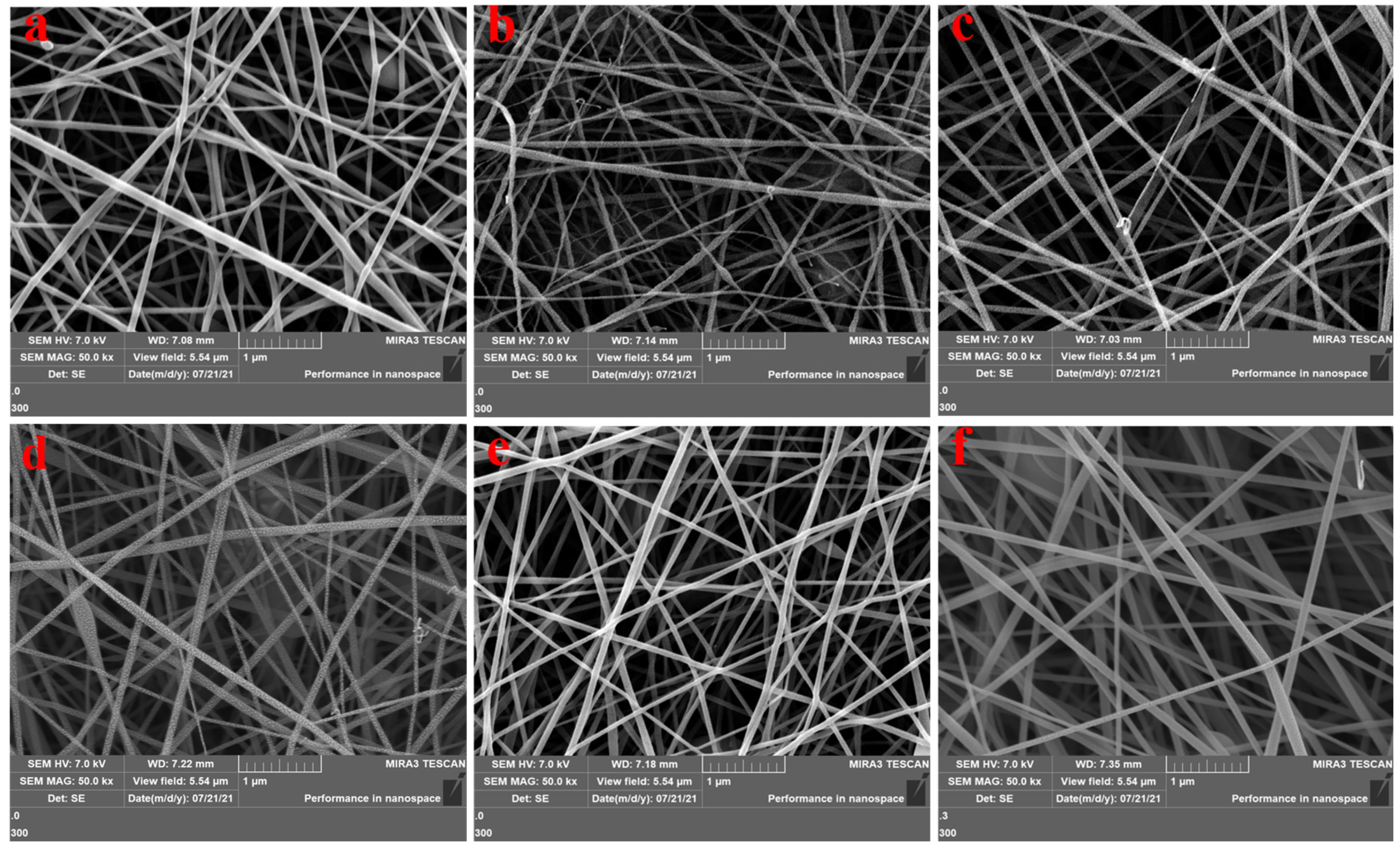
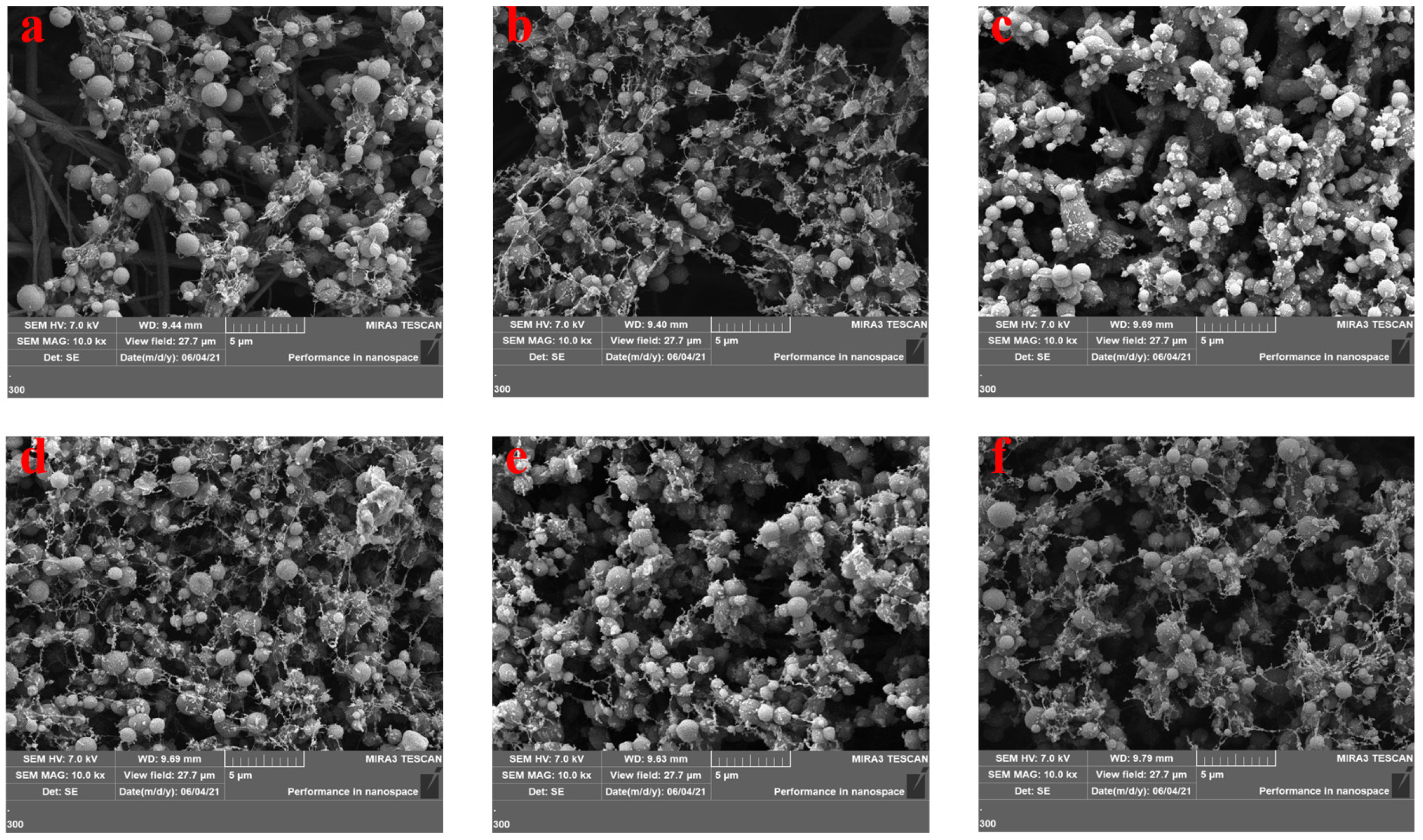
3.1. Scanning Electron Microscopy Characterization of Active Molecular Membranes
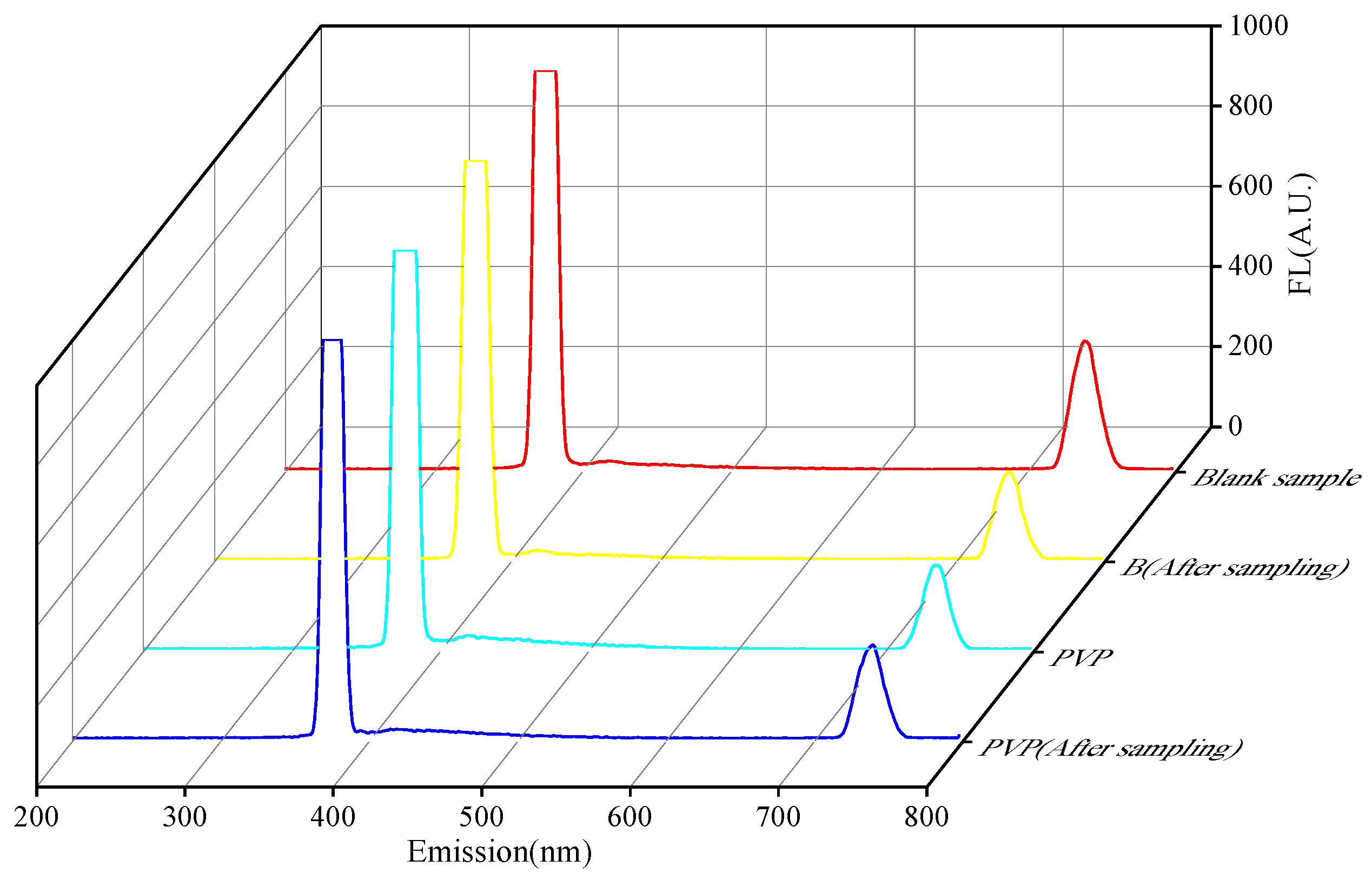
3.2. Fluorescence Detection of Active Molecular Membranes
3.3. Analysis of Simulated ROS Radical Trapping in the Chamber of Activated Molecular Membrane
3.4. Analysis of Atmospheric ROS Radical Trapping by Reactive Molecular Membrane Environment
3.5. The Comparison Analysis of Experimental Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pardo, M.; Xu, F.; Shemesh, M.; Qiu, X.; Barak, Y.; Zhu, T.; Rudich, Y. Nrf2 protects against diverse PM2.5 components-induced mitochondrial oxidative damage in lung cells. Sci. Total Environ. 2019, 669, 303–313. [Google Scholar] [CrossRef]
- Qiu, T.A.; Gallagher, M.J.; Hudson-Smith, N.V.; Wu, J.; Krause, M.O.P.; Fortner, J.D.; Haynes, C.L. Research highlights: Unveiling the mechanisms underlying nanoparticle-induced ROS generation and oxidative stress. Environ. Sci. Nano 2016, 3, 940–945. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, S.; Li, J.; Han, L.; He, Q.; Wang, R.; Wang, X.; Liu, K. Developmental toxicity induced by PM2.5 through endoplasmic reticulum stress and autophagy pathway in zebrafish embryos. Chemosphere 2018, 197, 611–621. [Google Scholar] [CrossRef]
- Wu, B.; Dong, Y.; Wang, M.; Yang, W.; Hu, L.; Zhou, D.; Lv, J.; Chai, T. Pathological damage, immune-related protein expression, and oxidative stress in lungs of BALB/c mice induced by haze PM2.5 biological components exposure. Atmos. Environ. 2020, 223. [Google Scholar] [CrossRef]
- Brown, R.A.; Stevanovic, S.; Bottle, S.; Wang, H.; Hu, Z.; Wu, C.; Wang, B.; Ristovski, Z. Relationship between Atmospheric PM-Bound Reactive Oxygen Species, Their Half-Lives, and Regulated Pollutants: Investigation and Preliminary Model. Environ. Sci. Technol. 2020, 54, 4995–5002. [Google Scholar] [CrossRef]
- Zhou, J.; Bruns, E.A.; Zotter, P.; Stefenelli, G.; Prévôt, A.S.H.; Baltensperger, U.; El-Haddad, I.; Dommen, J. Development, characterization and first deployment of an improved online reactive oxygen species analyzer. Atmos. Meas. Tech. 2018, 11, 65–80. [Google Scholar] [CrossRef] [Green Version]
- Fuller, S.J.; Wragg, F.P.H.; Nutter, J.; Kalberer, M. Comparison of on-line and off-line methods to quantify reactive oxygen species (ROS) in atmospheric aerosols. Atmos. Environ. 2014, 92, 97–103. [Google Scholar] [CrossRef] [Green Version]
- Huang, W.; Zhang, Y.; Zhang, Y.; Zeng, L.; Dong, H.; Huo, P.; Fang, D.; Schauer, J.J. Development of an automated sampling-analysis system for simultaneous measurement of reactive oxygen species (ROS) in gas and particle phases: GAC-ROS. Atmos. Environ. 2016, 134, 18–26. [Google Scholar] [CrossRef]
- Chen, X.; Wang, T.; Le, W.; Huang, X.; Gao, M.; Chen, Q.; Xu, S.; Yin, D.; Fu, Q.; Shao, C.; et al. Smart Sorting of Tumor Phenotype with Versatile Fluorescent Ag Nanoclusters by Sensing Specific Reactive Oxygen Species. Theranostics 2020, 10, 3430–3450. [Google Scholar] [CrossRef]
- Liu, S.; Oshita, S.; Kawabata, S.; Makino, Y.; Yoshimoto, T. Identification of ROS Produced by Nanobubbles and Their Positive and Negative Effects on Vegetable Seed Germination. Langmuir 2016, 32, 11295–11302. [Google Scholar] [CrossRef]
- Fattibene, P.; Trompier, F.; Wieser, A.; Brai, M.; Ciesielski, B.; de Angelis, C.; della Monaca, S.; Garcia, T.; Gustafsson, H.; Hole, E.O.; et al. EPR Dosimetry Intercomparison Using Smart Phone Touch Screen Glass. In Proceedings of the Radiation and Environmental Biophysics; Springer: New York, NY, USA, 2014; Volume 53, pp. 311–320. [Google Scholar]
- Trompier, F.; Burbidge, C.; Bassinet, C.; Baumann, M.; Bortolin, E.; De Angelis, C.; Eakins, J.; Della Monaca, S.; Fattibene, P.; Quattrini, M.C.; et al. Overview of physical dosimetry methods for triage application integrated in the new European network RENEB. Int. J. Radiat. Biol. 2017, 93, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Mrakic-Sposta, S.; Gussoni, M.; Montorsi, M.; Porcelli, S.; Vezzoli, A. Assessment of a Standardized ROS Production Profile in Humans by Electron Paramagnetic Resonance. Oxidative Med. Cell. Longev. 2012, 2012, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Suzen, S.; Gurer-Orhan, H.; Saso, L. Detection of Reactive Oxygen and Nitrogen Species by Electron Paramagnetic Resonance (EPR) Technique. Molecules 2017, 22, 181. [Google Scholar] [CrossRef] [PubMed]
- King, L.E.; Weber, R.J. Development and testing of an online method to measure ambient fine particulate reactive oxygen species (ROS) based on the 2′,7′-dichlorofluorescin (DCFH) assay. Atmos. Meas. Tech. 2013, 6, 1647–1658. [Google Scholar] [CrossRef] [Green Version]
- Huang, W.; Zhang, Y.; Zhang, Y.; Fang, D.; Schauer, J.J. Optimization of the Measurement of Particle-Bound Reactive Oxygen Species with 2′,7′-dichlorofluorescin (DCFH). Water Air Soil Pollut. 2016, 227, 1–10. [Google Scholar] [CrossRef]
- Salimi, R.; Yener, N.; Safari, R. Use and Evaluation of Newly Synthesized Fluorescence Probes to Detect Generated OH• Radicals in Fibroblast Cells. J. Fluoresc. 2016, 26, 919–924. [Google Scholar] [CrossRef]
- Zhang, W.; Hao, L.; Huang, J.; Xia, L.; Cui, M.; Zhang, X.; Gu, Y.; Wang, P. Chemiluminescence chitosan hydrogels based on the luminol analog L-012 for highly sensitive detection of ROS. Talanta 2019, 201, 455–459. [Google Scholar] [CrossRef]
- Su, Y.; Song, H.; Lv, Y. Recent advances in chemiluminescence for reactive oxygen species sensing and imaging analysis. Microchem. J. 2019, 146, 83–97. [Google Scholar] [CrossRef]
- Zhang, Y.; Dai, M.; Yuan, Z. Methods for the detection of reactive oxygen species. Anal. Methods 2018, 10, 4625–4638. [Google Scholar] [CrossRef]
- Wang, G.; Iradukunda, Y.; Shi, G.; Sanga, P.; Niu, X.; Wu, Z. Hydroxyl, hydroperoxyl free radicals determination methods in atmosphere and troposphere. J. Environ. Sci. 2021, 99, 324–335. [Google Scholar] [CrossRef]
- Yang, X.; Wang, J.; Guo, H.; Liu, L.; Xu, W.; Duan, G. Structural Design toward Functional Materials by Electrospinning: A Review. E-Polymers 2020, 20, 682–712. [Google Scholar] [CrossRef]
- Chen, S.; Jia, F.; Zhao, L.; Qiu, F.; Jiang, S.; Ji, J.; Fu, G. Electrospun fiber membrane with asymmetric NO release for the differential regulation of cell growth. Bio-Design Manuf. 2021, 4, 469–478. [Google Scholar] [CrossRef]
- Wang, G.; Su, Y.; Yu, J.; Li, R.; Ma, S.; Niu, X.; Shi, G. Preparation of Electrospun Active Molecular Membrane and Atmospheric Free Radicals Capture. Molecules 2019, 24, 3037. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Zou, W.; Liu, L.; Wang, M.; Li, F.; Shen, W. Characterization and bacteriostatic effects of β-cyclodextrin/quercetin inclusion compound nanofilms prepared by electrospinning. Food Chem. 2021, 338, 127980. [Google Scholar] [CrossRef] [PubMed]
- Alamir, M.A.; Alarifi, I.M.; Khan, W.A.; Khan, W.S.; Asmatulu, R. Electrospun Nanofibers: Preparation, Characterization and Atmospheric Fog Capturing Capabilities. Fibers Polym. 2019, 20, 2090–2098. [Google Scholar] [CrossRef]
- Ren, B.; Pi, H.; Zhao, X.; Hu, M.; Zhang, X.; Wang, R.; Wu, J. Janus membrane with novel directional water transport capacity for efficient atmospheric water capture. Nanoscale 2021, 13, 9354–9363. [Google Scholar] [CrossRef]
- Goel, R.; Bitzer, Z.; Reilly, S.M.; Bhangu, G.; Trushin, N.; Elias, R.J.; Foulds, J.; Muscat, J.; Richie, J.P. Effect of Charcoal in Cigarette Filters on Free Radicals in Mainstream Smoke. Chem. Res. Toxicol. 2018, 31, 745–751. [Google Scholar] [CrossRef]
- Vaida, V. Atmospheric radical chemistry revisited Sunlight may directly drive previously unknown organic reactions at environmental surfaces. Science 2016, 353, 650. [Google Scholar]
- Limaye, V.; Knowlton, K. Shining New Light on Long-term Ozone Harms. JAMA Intern. Med. 2020, 180, 115. [Google Scholar] [CrossRef]
- Zhang, J.J.; Wei, Y.; Fang, Z. Ozone Pollution: A Major Health Hazard Worldwide. Front. Immunol. 2019, 10, 2518. [Google Scholar] [CrossRef] [Green Version]
- Iradukunda, Y.; Wang, G.; Li, X.; Shi, G.; Albashir, A.I.M.; Dusengemungu, L.; Hu, Y.; Luo, F.; Yi, K.; Niu, X.; et al. Multifunctional flexible porous liquefied bio-carbon nanofibers prepared from the combination of mangosteen (Garcinia mangostana) peels and monohydroxybenzene for supercapacitors applications. J. Electroanal. Chem. 2021, 890, 115228. [Google Scholar] [CrossRef]
- Wang, G.; Liu, Y.; Zhao, Q.; Chen, X.; Hu, S.; Li, L.; Jiang, X.; Liu, C.; Jia, S.; Tian, H.; et al. Preparation of Electrostatic Spinning Composite Film Loaded with Polyvinylpyrrolidone for the Detection of Free Radicals in Polluted Air. Int. J. Electrochem. Sci. 2018, 13, 11466–11479. [Google Scholar] [CrossRef]
- Mishra, R.K.; Mishra, P.; Verma, K.; Mondal, A.; Chaudhary, R.G.; Abolhasani, M.M.; Loganathan, S. Electrospinning production of nanofibrous membranes. Environ. Chem. Lett. 2019, 17, 767–800. [Google Scholar] [CrossRef]
- Abideen, Z.U.; Kim, J.-H.; Lee, J.-H.; Kim, J.-Y.; Mirzaei, A.; Kim, H.W.; Kim, S.S. Electrospun Metal Oxide Composite Nanofibers Gas Sensors: A Review. J. Korean Ceram. Soc. 2017, 54, 366–379. [Google Scholar] [CrossRef] [Green Version]
- Korotcenkov, G. Electrospun Metal Oxide Nanofibers and Their Conductometric Gas Sensor Application. Part 1: Nanofibers and Features of Their Forming. Nanomaterials 2021, 11, 1544. [Google Scholar] [CrossRef]
- Korotcenkov, G. Electrospun Metal Oxide Nanofibers and Their Conductometric Gas Sensor Application. Part 2: Gas Sensors and Their Advantages and Limitations. Nanomaterials 2021, 11, 1555. [Google Scholar] [CrossRef]
- Sassi, A.; Maatouk, M.; El Gueder, D.; Bzéouich, I.M.; Hatira, S.A.-B.; Jemni-Yacoub, S.; Ghedira, K.; Chekir-Ghedira, L. Chrysin, a natural and biologically active flavonoid suppresses tumor growth of mouse B16F10 melanoma cells: In vitro and In vivo study. Chem. Interactions 2018, 283, 10–19. [Google Scholar] [CrossRef]
- Mani, R.; Natesan, V. Chrysin: Sources, beneficial pharmacological activities, and molecular mechanism of action. Phytochemistry 2018, 145, 187–196. [Google Scholar] [CrossRef]
- Oh, M.C.; Piao, M.J.; Fernando, P.M.D.J.; Han, X.; Hewage, S.R.K.M.; Park, J.E.; Ko, M.S.; Jung, U.; Kim, I.G.; Hyun, J.W. Baicalein Protects Human Skin Cells against Ultraviolet B-Induced Oxidative Stress. Biomol. Ther. 2016, 24, 616–622. [Google Scholar] [CrossRef] [Green Version]
- Son, S.-H.; Kang, J.; Ahn, M.; Nam, S.; Jung, Y.W.; Lee, K.Y.; Jeon, Y.H.; Byun, Y.; Lee, K. Synthesis and Biochemical Evaluation of Baicalein Prodrugs. Pharmaceutics 2021, 13, 1516. [Google Scholar] [CrossRef]
- Javad, S.; Cristina, Q.; Muhammad, I.; Abdur, R.; Muhammad, N.; Aslam, G.T.; Bashir, A.; Muhammad, A.; Mohammad, S.M.; Oksana, S.; et al. Genistein: An Integrative Overview of Its Mode of Action, Pharmacological Properties, and Health Benefits. Oxidative Med. Cell. Longev. 2021, 2021, 3268136. [Google Scholar]
- Song, X.; Wang, Y.; Gao, L. Mechanism of antioxidant properties of quercetin and quercetin-DNA complex. J. Mol. Model. 2020, 26, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Imai, K.; Nakanishi, I.; Ohkubo, K.; Ohba, Y.; Arai, T.; Mizuno, M.; Fukuzumi, S.; Matsumoto, K.-I.; Fukuhara, K. Synthesis of methylated quercetin analogues for enhancement of radical-scavenging activity. RSC Adv. 2017, 7, 17968–17979. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Y.-Z.; Deng, G.; Zhang, Y.-C. Multiple free radical scavenging reactions of flavonoids. Dyes Pigment. 2022, 198, 109877. [Google Scholar] [CrossRef]
- Kuwabara, K.; Nakano, R.; Sakurai, Y.; Yamaguchi, T.; Miyake, Y.; Kanaori, K.; Tajima, K. Stopped-flow-optical Absorption and -electron Spin Resonance Studies on Short-lived Quercetin Semiquinone Radical Produced by Redox Reactions with O2−• Radical in DMSO. Chem. Lett. 2020, 49, 260–263. [Google Scholar] [CrossRef]



| The Number of Polymers Added (g) | Spinning Voltage (kV) | Receiving Distance (cm) | Push Injection Speed (mm/min) | |
|---|---|---|---|---|
| Chrysin | 0.1700 | 16.00 | 15.00 | 0.180 |
| Baicalein | 0.1700 | 14.00 | 15.00 | 0.180 |
| Scutellarein | 0.1700 | 16.00 | 17.00 | 0.155 |
| Genistein | 0.1500 | 16.00 | 15.00 | 0.130 |
| Quercetin | 0.1500 | 16.00 | 15.00 | 0.230 |
| Baicalin | 0.1700 | 16.00 | 15.00 | 0.130 |
| Chrysin | Baicalein | Scutellarein | Genistein | Quercetin | Baicalin | |
|---|---|---|---|---|---|---|
| Fluorescence emission wavelength (nm) | 364 | 350 | 358 | 340 | 360 | 365 |
| Before Sampling | ROS | OH | O3 | Atmospheric ROS | |
|---|---|---|---|---|---|
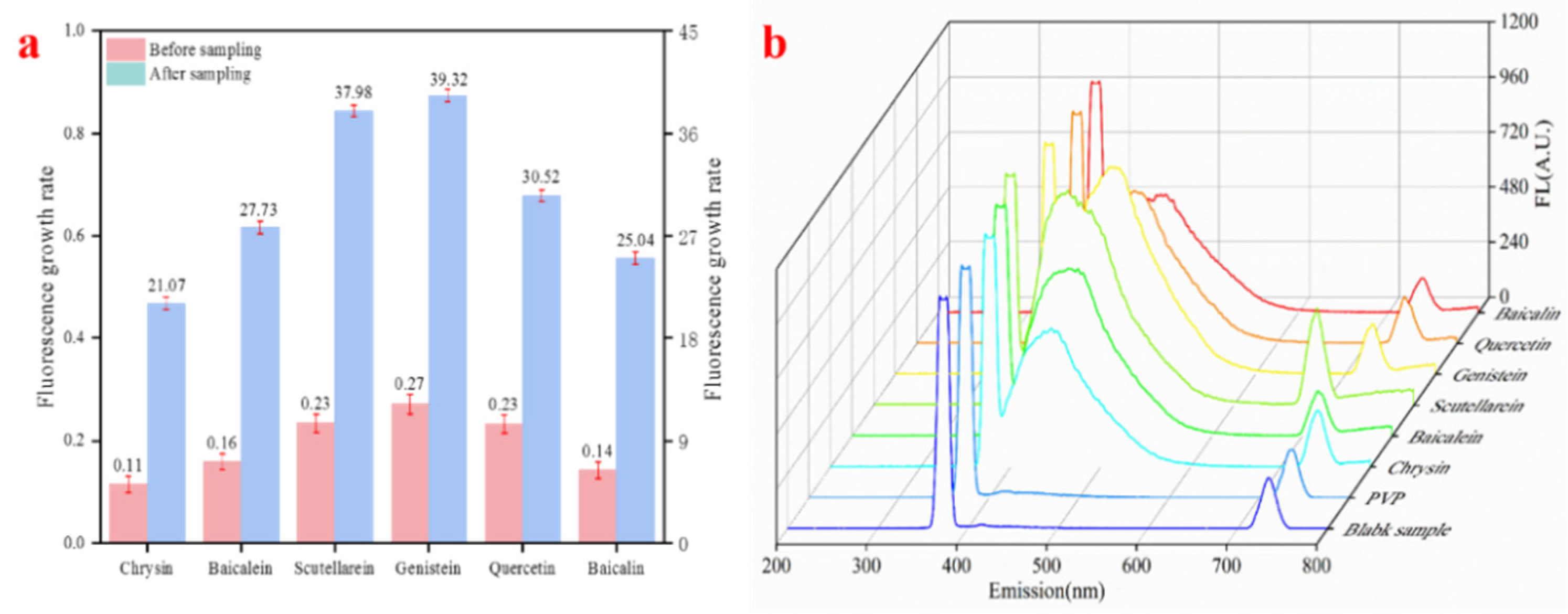
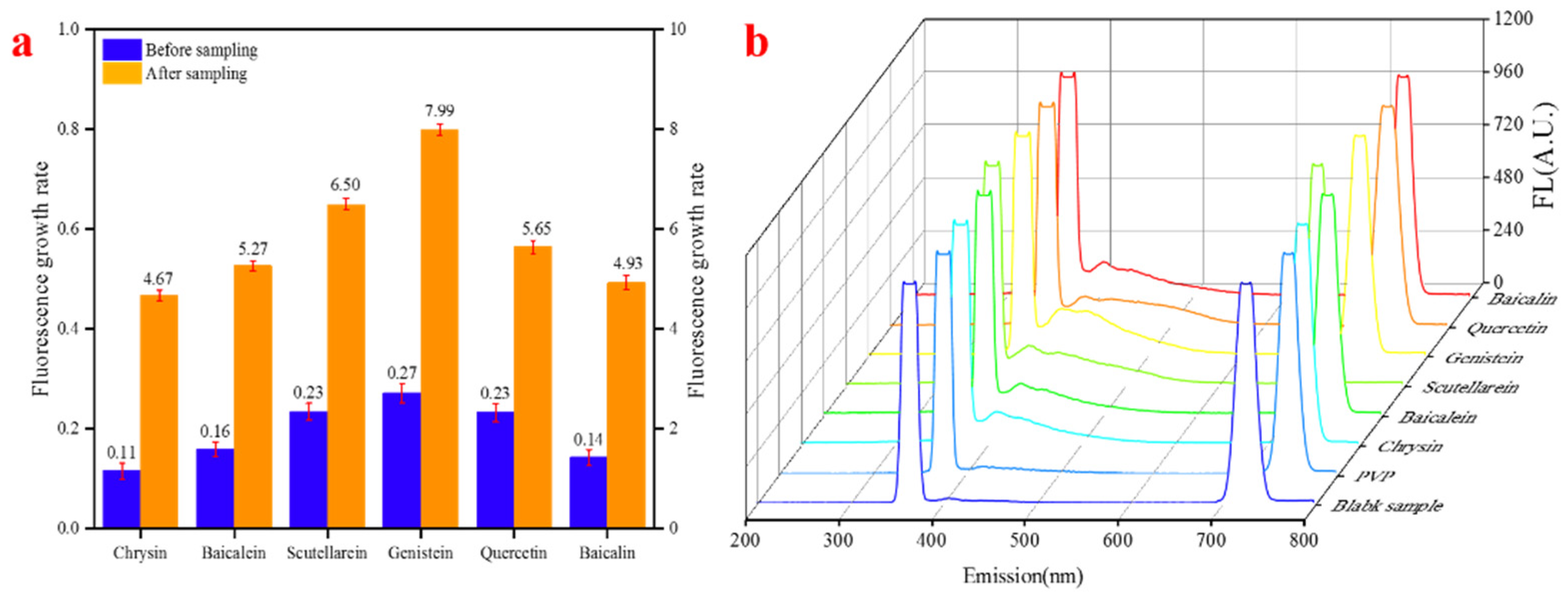
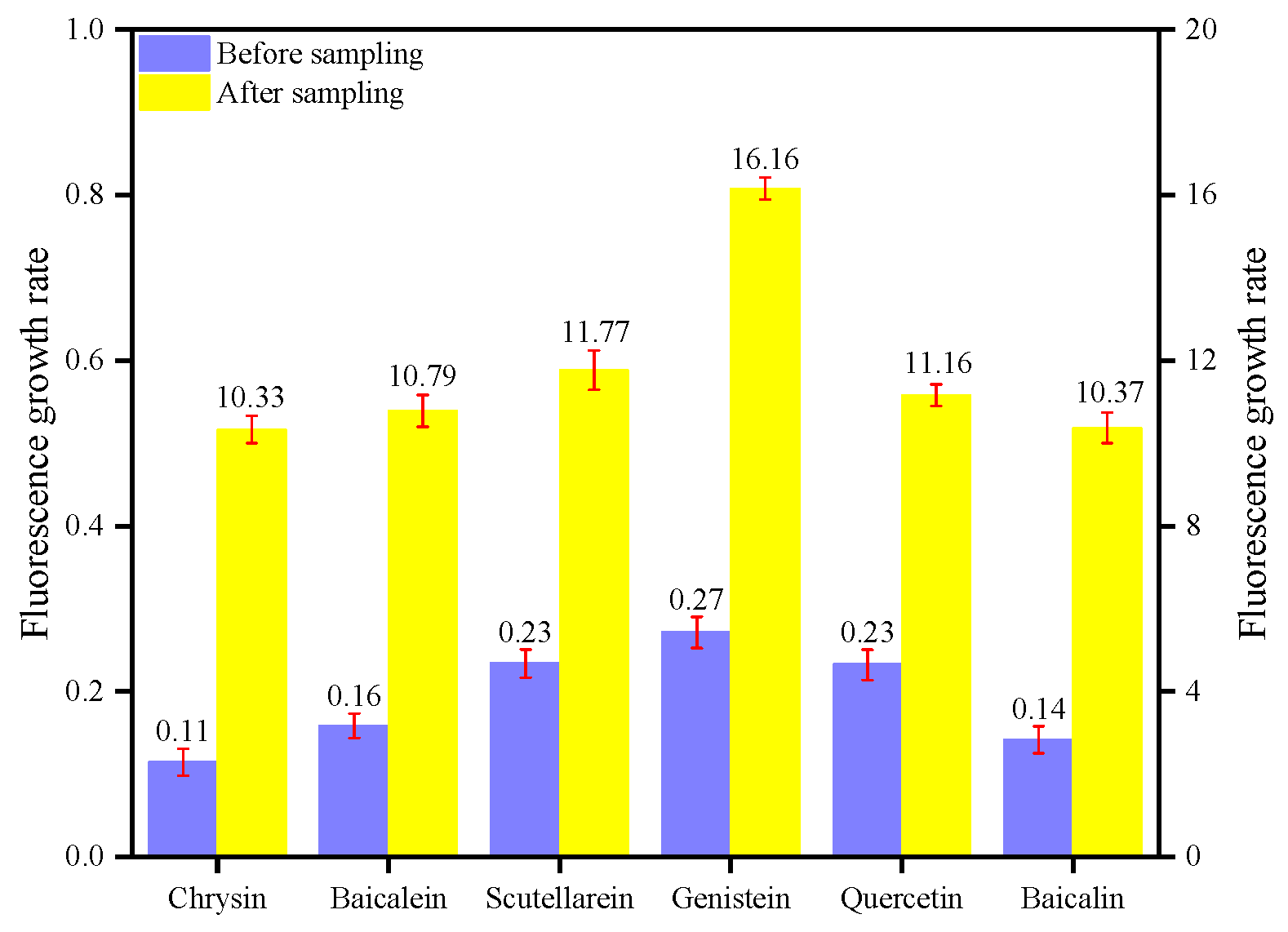
| Chrysin | 0.11 | 21.07 | 4.67 | 6.03 | 10.33 |
| Baicalein | 0.16 | 27.73 | 5.27 | 6.34 | 10.79 |
| Scutellarein | 0.23 | 37.98 | 6.50 | 8.26 | 11.77 |
| Before Sampling | ROS | OH | O3 | Atmospheric ROS | |
|---|---|---|---|---|---|
| Baicalein | 0.16 | 27.73 | 5.27 | 6.34 | 10.79 |
| Scutellarein | 0.23 | 37.98 | 6.50 | 8.26 | 11.77 |
| Genistein | 0.27 | 39.32 | 7.99 | 11.92 | 16.16 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Wang, G.; Li, X.; Iradukunda, Y.; Liu, F.; Li, Z.; Gao, H.; Shi, G. Preparation of Electrospun Active Molecules Membrane Application to Atmospheric Free Radicals. Membranes 2022, 12, 480. https://doi.org/10.3390/membranes12050480
Yang Y, Wang G, Li X, Iradukunda Y, Liu F, Li Z, Gao H, Shi G. Preparation of Electrospun Active Molecules Membrane Application to Atmospheric Free Radicals. Membranes. 2022; 12(5):480. https://doi.org/10.3390/membranes12050480
Chicago/Turabian StyleYang, Yang, Guoying Wang, Xin Li, Yves Iradukunda, Fengshuo Liu, Zhiqian Li, Hongli Gao, and Gaofeng Shi. 2022. "Preparation of Electrospun Active Molecules Membrane Application to Atmospheric Free Radicals" Membranes 12, no. 5: 480. https://doi.org/10.3390/membranes12050480
APA StyleYang, Y., Wang, G., Li, X., Iradukunda, Y., Liu, F., Li, Z., Gao, H., & Shi, G. (2022). Preparation of Electrospun Active Molecules Membrane Application to Atmospheric Free Radicals. Membranes, 12(5), 480. https://doi.org/10.3390/membranes12050480






