Alpha-Crystallin-Membrane Association Modulated by Phospholipid Acyl Chain Length and Degree of Unsaturation
Abstract
1. Introduction
2. Materials and Methods
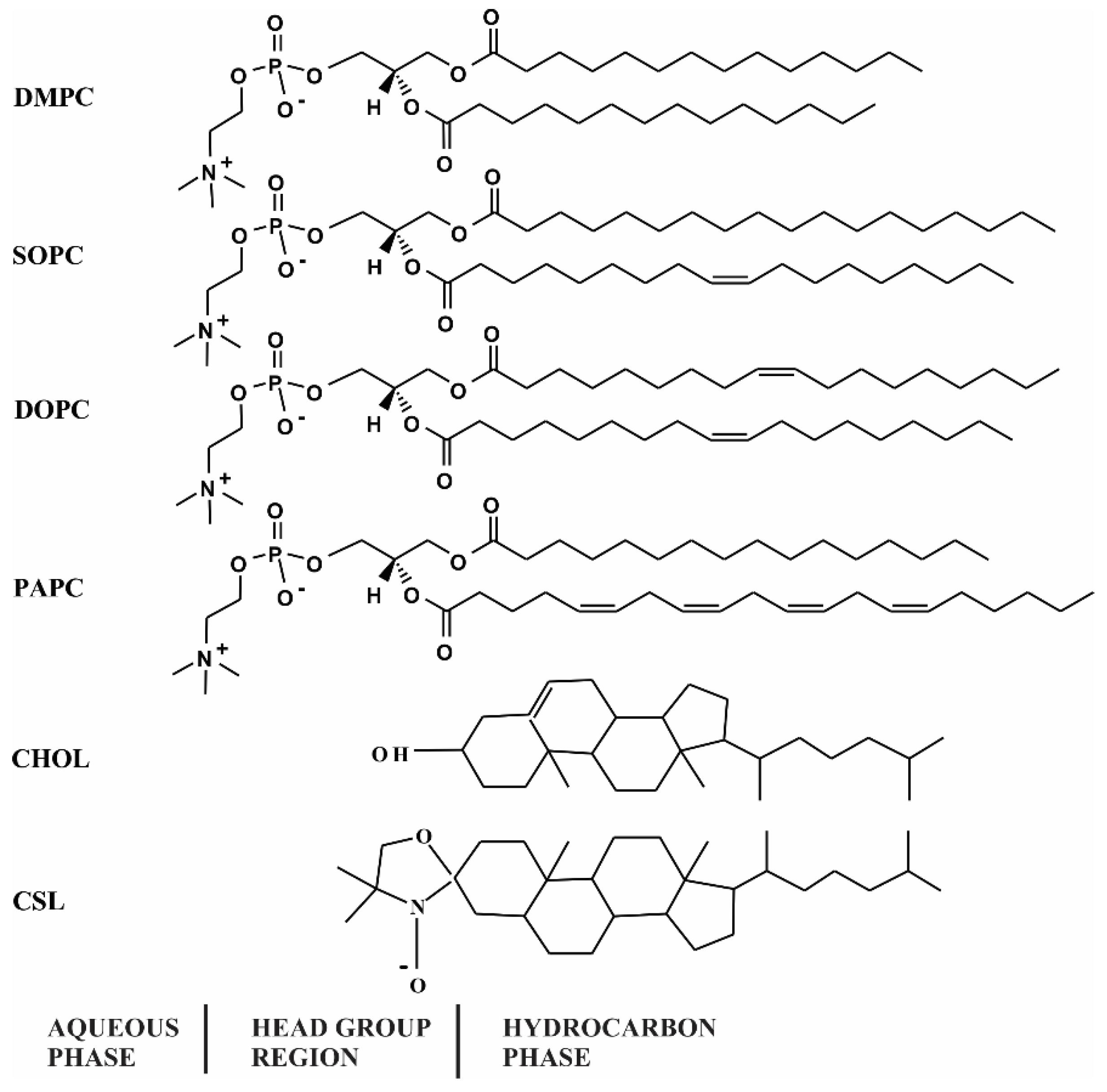
2.1. Materials
2.2. Sample Preparation for α-Crystallin-Membrane Association Studies
2.3. EPR Approach for the Investigation of the α-Crystallin-Membrane Association
2.4. Statistics
3. Results and Discussion
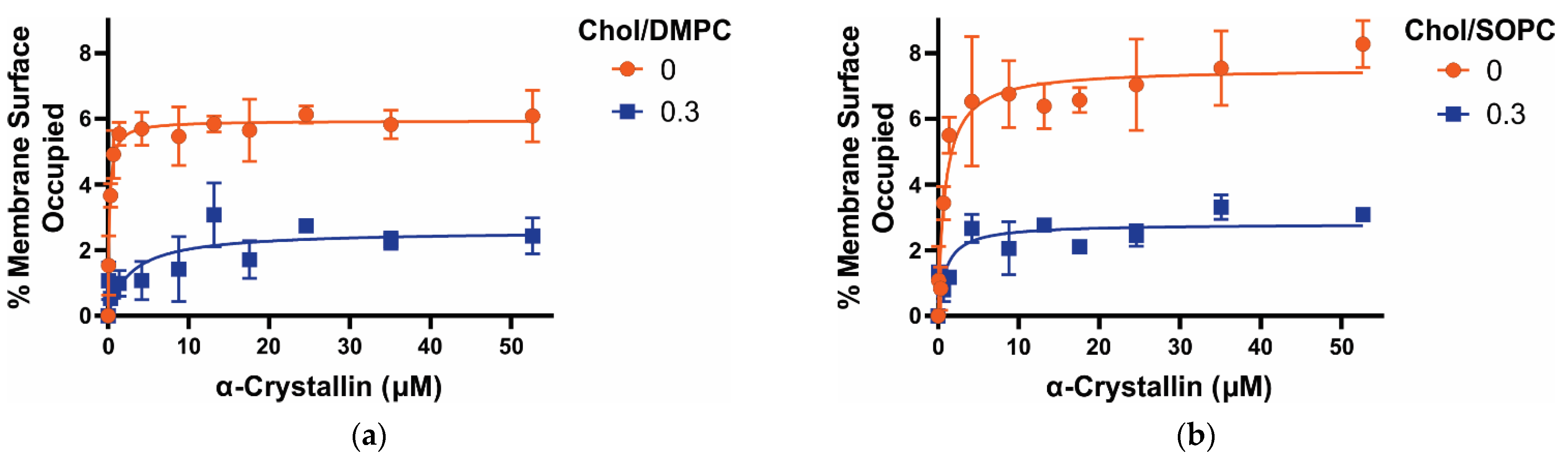
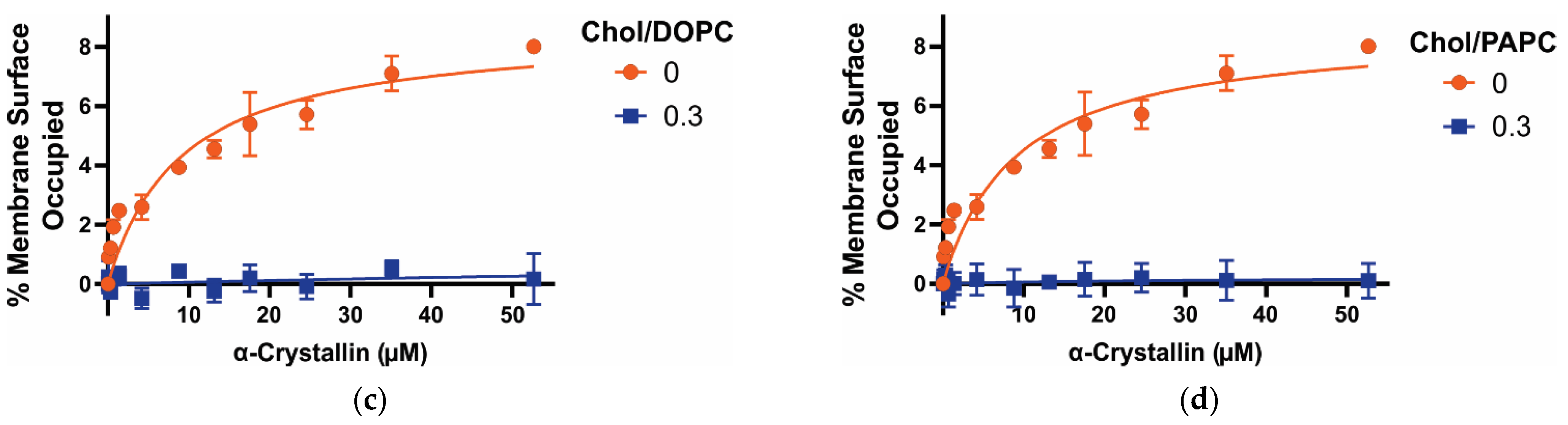
3.1. MSO by α-Crystallin on Saturated, Monounsaturated, and Polyunsaturated Membranes
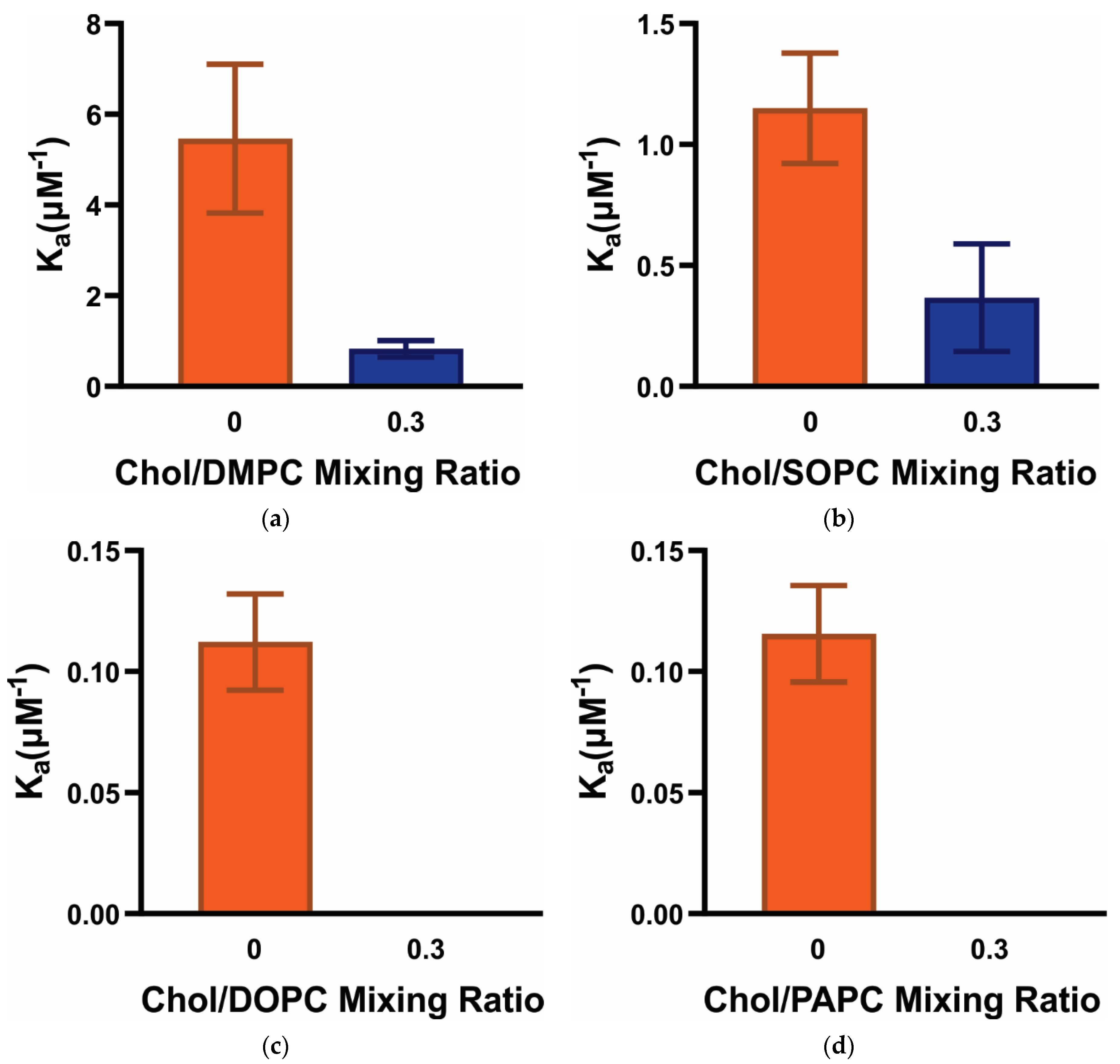
3.2. Ka of α-Crystallin Association with Saturated, Monounsaturated, and Polyunsaturated Membranes
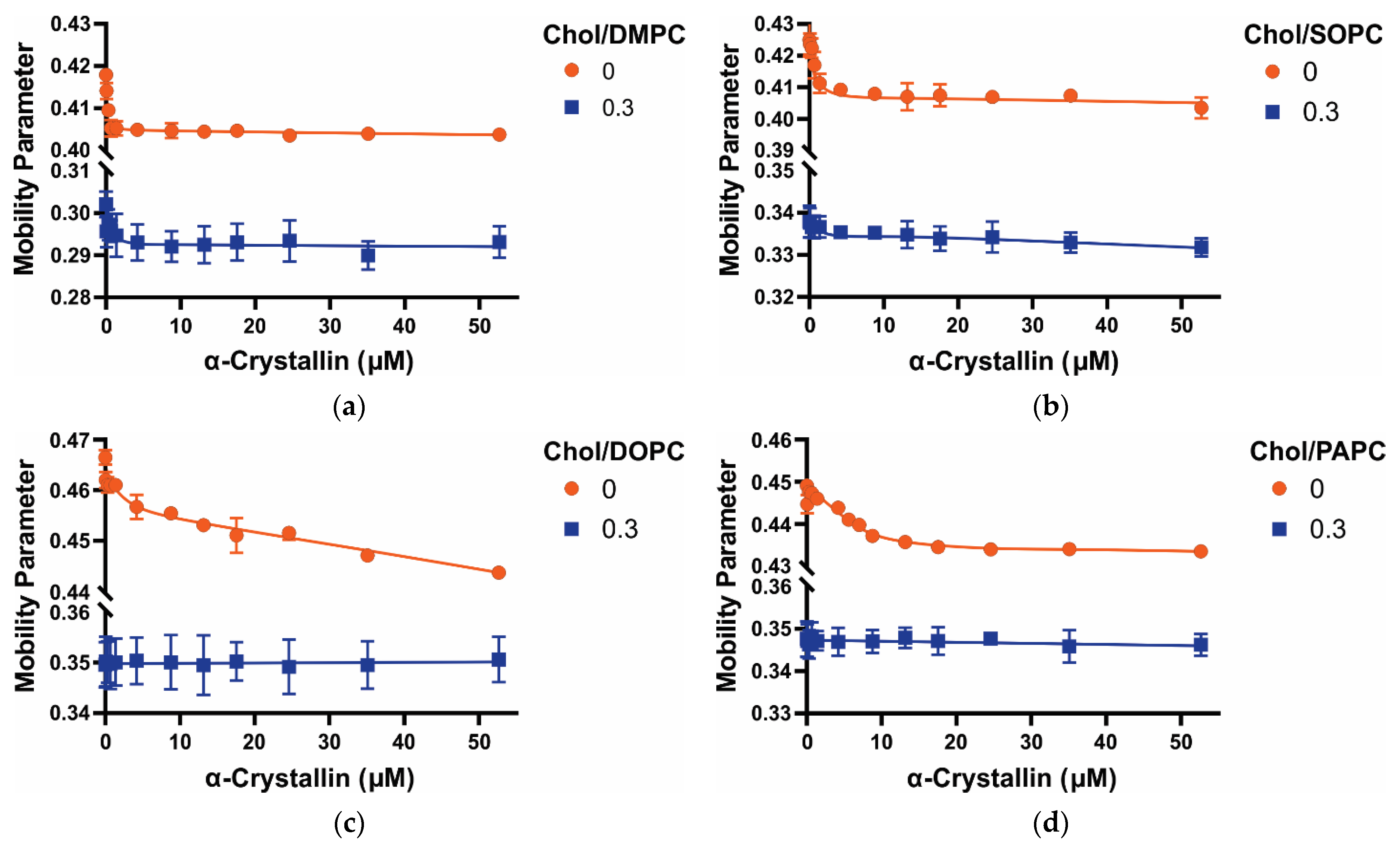
3.3. Mobility Parameter of Saturated, Monounsaturated, and Polyunsaturated Membranes with the α-Crystallin Association
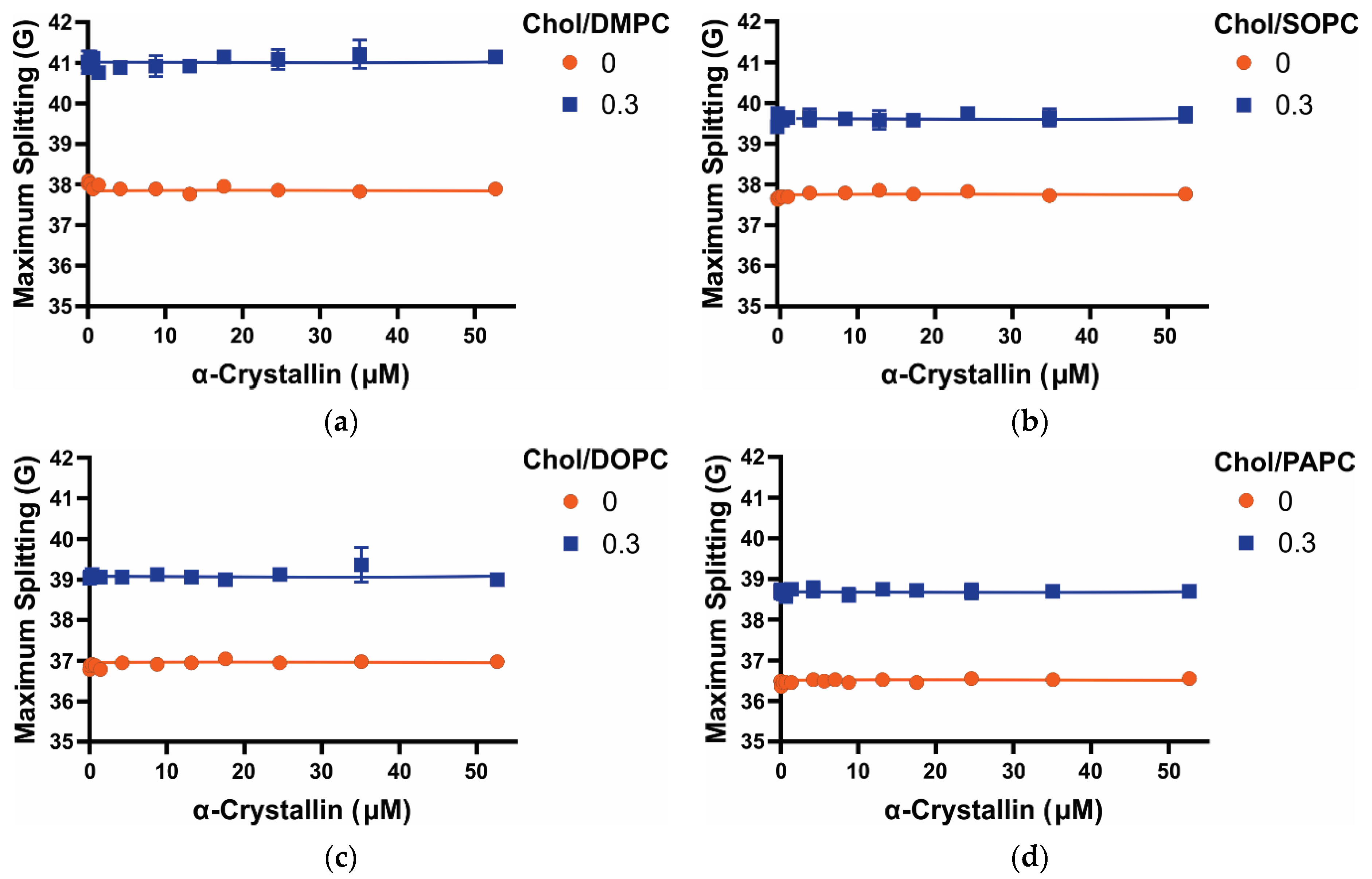
3.4. Maximum Splitting of Saturated, Monounsaturated, and Polyunsaturated Membranes with the α-Crystallin Association
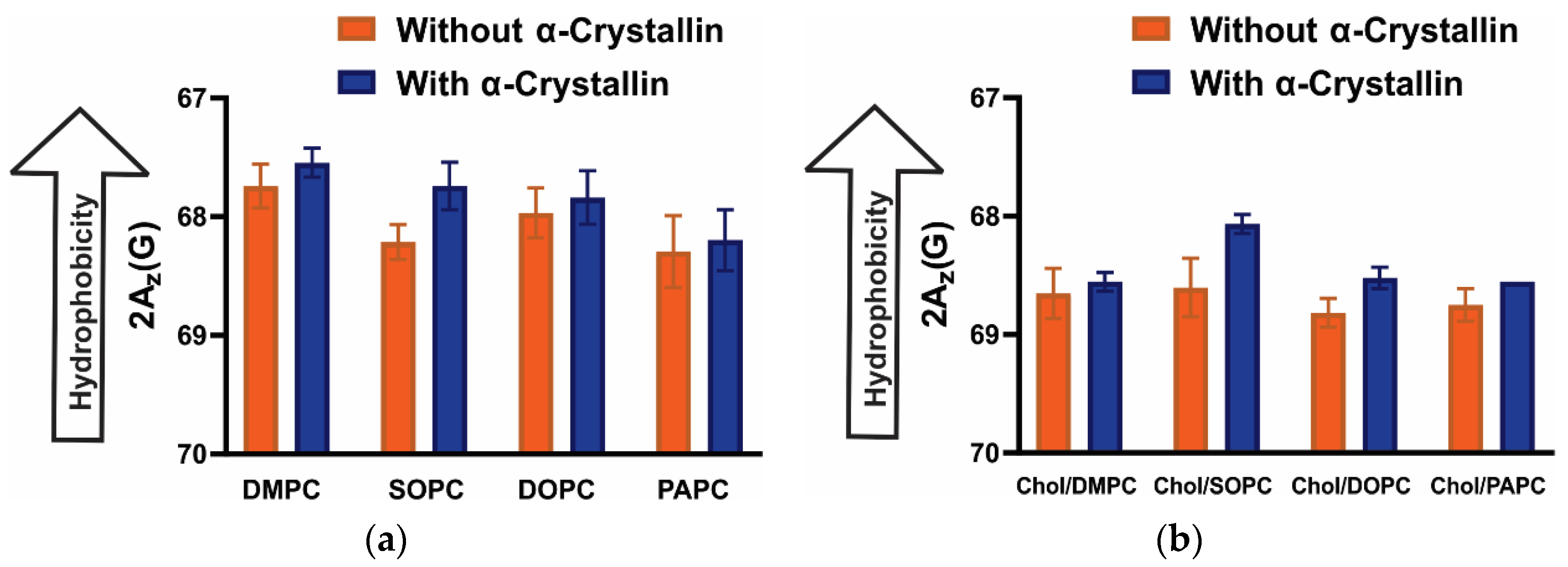
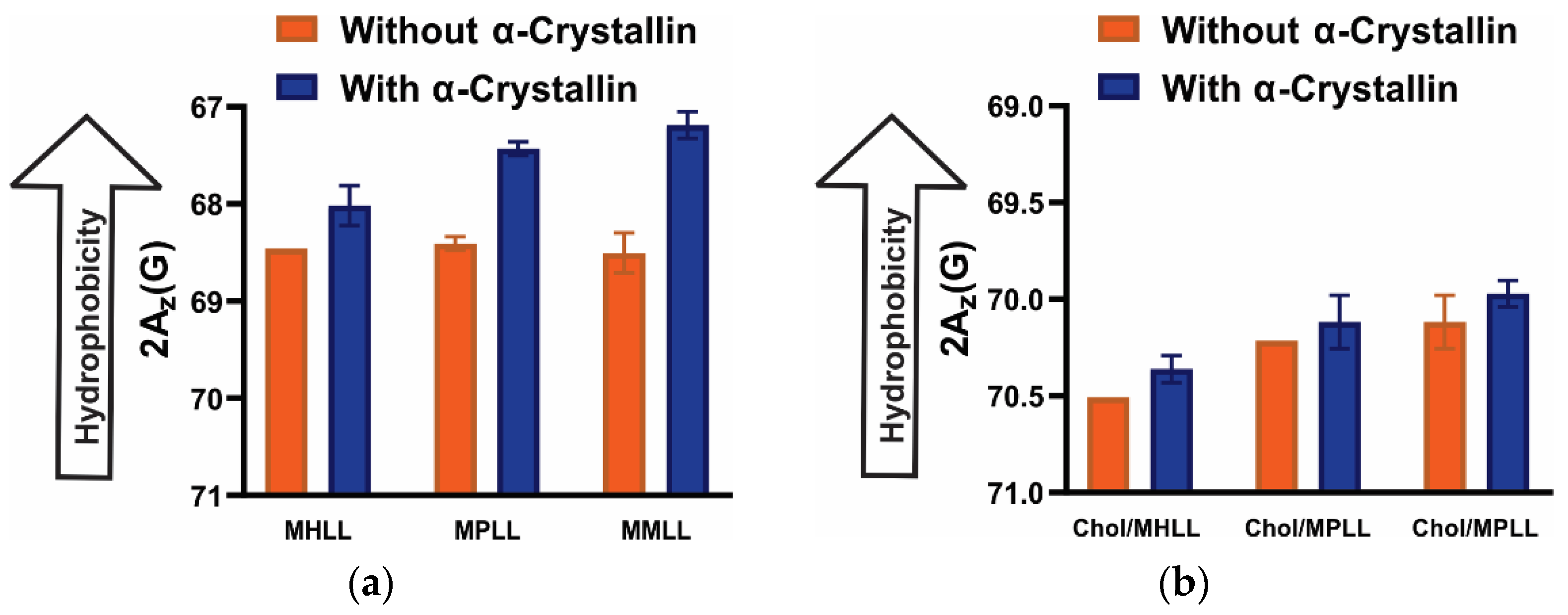
3.5. Surface Hydrophobicity of Saturated, Monounsaturated, and Polyunsaturated Membranes with the α-Crystallin Association
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hashemi, H.; Pakzad, R.; Yekta, A.; Aghamirsalim, M.; Pakbin, M.; Ramin, S.; Khabazkhoob, M. Global and Regional Prevalence of Age-Related Cataract: A Comprehensive Systematic Review and Meta-Analysis. Eye 2020, 34, 1357–1370. [Google Scholar] [CrossRef] [PubMed]
- Timsina, R.; Mainali, L. Association of Alpha-Crystallin with Fiber Cell Plasma Membrane of the Eye Lens Accompanied by Light Scattering and Cataract Formation. Membranes 2021, 11, 447. [Google Scholar] [CrossRef] [PubMed]
- Borchman, D.; Yappert, M.C. Lipids and the Ocular Lens. J. Lipid Res. 2010, 51, 2473–2488. [Google Scholar] [CrossRef] [PubMed]
- Pescosolido, N.; Barbato, A.; Giannotti, R.; Komaiha, C.; Lenarduzzi, F. Age-Related Changes in the Kinetics of Human Lenses: Prevention of the Cataract. Int. J. Ophthalmol. 2016, 9, 1506–1517. [Google Scholar] [CrossRef]
- Bloemendal, H.; de Jong, W.; Jaenicke, R.; Lubsen, N.H.; Slingsby, C.; Tardieu, A. Ageing and Vision: Structure, Stability and Function of Lens Crystallins. Prog. Biophys. Mol. Biol. 2004, 86, 407–485. [Google Scholar] [CrossRef]
- Horwitz, J. Alpha-Crystallin. Exp. Eye Res. 2003, 76, 145–153. [Google Scholar] [CrossRef]
- Horwitz, J.; Bova, M.P.; Ding, L.-L.; Haley, D.A.; Stewart, P.L. Lens α-Crystallin: Function and Structure. Eye 1999, 13, 403–408. [Google Scholar] [CrossRef]
- Bova, M.P.; McHaourab, H.S.; Han, Y.; Fung, B.K. Subunit Exchange of Small Heat Shock Proteins. Analysis of Oligomer Formation of AlphaA-Crystallin and Hsp27 by Fluorescence Resonance Energy Transfer and Site-Directed Truncations. J. Biol. Chem. 2000, 275, 1035–1042. [Google Scholar] [CrossRef]
- Bova, M.P.; Ding, L.L.; Horwitz, J.; Fung, B.K. Subunit Exchange of AlphaA-Crystallin. J. Biol. Chem. 1997, 272, 29511–29517. [Google Scholar] [CrossRef]
- Van den Oetelaar, P.J.M.; Van Someren, P.F.H.M.; Thomson, J.A.; Siezen, R.J.; Hoenders, H.J. A Dynamic Quaternary Structure of Bovine. Alpha.-Crystallin as Indicated from Intermolecular Exchange of Subunits. Biochemistry 1990, 29, 3488–3493. [Google Scholar] [CrossRef]
- Srivastava, K.; Chaves, J.M.; Srivastava, O.P.; Kirk, M. Multi-Crystallin Complexes Exist in the Water-Soluble High Molecular Weight Protein Fractions of Aging Normal and Cataractous Human Lenses. Exp. Eye Res. 2008, 87, 356–366. [Google Scholar] [CrossRef] [PubMed]
- Boyle, D.L.; Takemoto, L. EM Immunolocalization of α-Crystallins: Association with the Plasma Membrane from Normal and Cataractous Human Lenses. Curr. Eye Res. 1996, 15, 577–582. [Google Scholar] [CrossRef] [PubMed]
- Cenedella, R.J.; Fleschner, C.R. Selective Association of Crystallins with Lens “native” Membrane during Dynamic Cataractogenesis. Curr. Eye Res. 1992, 11, 801–815. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Borchman, D.; Yappert, M.C. Alpha-Crystallin/Lens Lipid Interactions Using Resonance Energy Transfer. Ophthalmic Res. 1999, 31, 452–462. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekher, G.; Cenedella, R.J. Protein Associated with Human Lens “native” Membrane during Aging and Cataract Formation. Exp. Eye Res. 1995, 60, 707–717. [Google Scholar] [CrossRef]
- Friedrich, M.G.; Truscott, R.J.W. Large-Scale Binding of α-Crystallin to Cell Membranes of Aged Normal Human Lenses: A Phenomenon That Can Be Induced by Mild Thermal Stress. Investig. Ophthalmol. Vis. Sci. 2010, 51, 5145–5152. [Google Scholar] [CrossRef]
- Cobb, B.A.; Petrash, J.M. Alpha-Crystallin Chaperone-like Activity and Membrane Binding in Age-Related Cataracts. Biochemistry 2002, 41, 483–490. [Google Scholar] [CrossRef]
- Moreau, K.L.; King, J.A. Protein Misfolding and Aggregation in Cataract Disease and Prospects for Prevention. Trends Mol. Med. 2012, 18, 273–282. [Google Scholar] [CrossRef]
- Zhao, L.; Chen, X.-J.; Zhu, J.; Xi, Y.-B.; Yang, X.; Hu, L.-D.; Ouyang, H.; Patel, S.H.; Jin, X.; Lin, D.; et al. Lanosterol Reverses Protein Aggregation in Cataracts. Nature 2015, 523, 607–611. [Google Scholar] [CrossRef]
- Datiles, M.B.; Ansari, R.R.; Yoshida, J.; Brown, H.; Zambrano, A.I.; Tian, J.; Vitale, S.; Zigler, J.S.; Ferris, F.L.; West, S.K.; et al. Longitudinal Study of Age Related Cataract Using Dynamic Light Scattering: Loss of α-Crystallin Leads to Nuclear Cataract Development. Ophthalmology 2016, 123, 248–254. [Google Scholar] [CrossRef]
- Borchman, D.; Tang, D. Binding Capacity of Alpha-Crystallin to Bovine Lens Lipids. Exp. Eye Res. 1996, 63, 407–410. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekher, G.; Cenedella, R.J. Properties of Alpha-Crystallin Bound to Lens Membrane: Probing Organization at the Membrane Surface. Exp. Eye Res. 1997, 64, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Ifeanyi, F.; Takemoto, L. Interaction of Lens Crystallins with Lipid Vesicles. Exp. Eye Res. 1991, 52, 535–538. [Google Scholar] [CrossRef]
- Borchman, D.; Byrdwell, W.C.; Yappert, M.C. Regional and Age-Dependent Differences in the Phospholipid Composition of Human Lens Membranes. Investig. Ophthalmol. Vis. Sci. 1994, 35, 3938–3942. [Google Scholar]
- Deeley, J.M.; Mitchell, T.W.; Wei, X.; Korth, J.; Nealon, J.R.; Blanksby, S.J.; Truscott, R.J.W. Human Lens Lipids Differ Markedly from Those of Commonly Used Experimental Animals. Biochim. Biophys. Acta 2008, 1781, 288–298. [Google Scholar] [CrossRef]
- Yappert, M.C.; Borchman, D. Sphingolipids in Human Lens Membranes: An Update on Their Composition and Possible Biological Implications. Chem. Phys. Lipids 2004, 129, 1–20. [Google Scholar] [CrossRef]
- Yappert, M.C.; Rujoi, M.; Borchman, D.; Vorobyov, I.; Estrada, R. Glycero- versus Sphingo-Phospholipids: Correlations with Human and Non-Human Mammalian Lens Growth. Exp. Eye Res. 2003, 76, 725–734. [Google Scholar] [CrossRef]
- Cobb, B.A.; Petrash, J.M. Factors Influencing α-Crystallin Association with Phospholipid Vesicles. Mol. Vis. 2002, 8, 85–93. [Google Scholar]
- Mainali, L.; Raguz, M.; O’Brien, W.J.; Subczynski, W.K. Properties of Membranes Derived from the Total Lipids Extracted from the Human Lens Cortex and Nucleus. Biochim. Biophys. Acta 2013, 1828, 1432–1440. [Google Scholar] [CrossRef]
- Mainali, L.; Raguz, M.; O’Brien, W.J.; Subczynski, W.K. Properties of Membranes Derived from the Total Lipids Extracted from Clear and Cataractous Lenses of 61–70-Year-Old Human Donors. Eur. Biophys. J. EBJ 2015, 44, 91–102. [Google Scholar] [CrossRef]
- Mainali, L.; Raguz, M.; O’Brien, W.J.; Subczynski, W.K. Changes in the Properties and Organization of Human Lens Lipid Membranes Occurring with Age. Curr. Eye Res. 2017, 42, 721–731. [Google Scholar] [CrossRef] [PubMed]
- Borchman, D.; Ozaki, Y.; Lamba, O.P.; Byrdwell, W.C.; Czarnecki, M.A.; Yappert, M.C. Structural Characterization of Clear Human Lens Lipid Membranes by Near-Infrared Fourier Transform Raman Spectroscopy. Curr. Eye Res. 1995, 14, 511–515. [Google Scholar] [CrossRef] [PubMed]
- Borchman, D.; Cenedella, R.J.; Lamba, O.P. Role of Cholesterol in the Structural Order of Lens Membrane Lipids. Exp. Eye Res. 1996, 62, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Paterson, C.A.; Zeng, J.; Husseini, Z.; Borchman, D.; Delamere, N.A.; Garland, D.; Jimenez-Asensio, J. Calcium ATPase Activity and Membrane Structure in Clear and Cataractous Human Lenses. Curr. Eye Res. 1997, 16, 333–338. [Google Scholar] [CrossRef]
- Tang, D.; Borchman, D.; Yappert, M.C.; Cenedella, R.J. Influence of Cholesterol on the Interaction of Alpha-Crystallin with Phospholipids. Exp. Eye Res. 1998, 66, 559–567. [Google Scholar] [CrossRef]
- Zhu, X.; Gaus, K.; Lu, Y.; Magenau, A.; Truscott, R.J.W.; Mitchell, T.W. α- and β-Crystallins Modulate the Head Group Order of Human Lens Membranes during Aging. Investig. Ophthalmol. Vis. Sci. 2010, 51, 5162–5167. [Google Scholar] [CrossRef]
- Truscott, R.J.W. Age-Related Nuclear Cataract-Oxidation Is the Key. Exp. Eye Res. 2005, 80, 709–725. [Google Scholar] [CrossRef]
- Friedrich, M.G.; Truscott, R.J.W. Membrane Association of Proteins in the Aging Human Lens: Profound Changes Take Place in the Fifth Decade of Life. Investig. Ophthalmol. Vis. Sci. 2009, 50, 4786–4793. [Google Scholar] [CrossRef]
- Bassnett, S.; Shi, Y.; Vrensen, G.F.J.M. Biological Glass: Structural Determinants of Eye Lens Transparency. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2011, 366, 1250–1264. [Google Scholar] [CrossRef]
- Reichow, S.L.; Gonen, T. Lipid-Protein Interactions Probed by Electron Crystallography. Curr. Opin. Struct. Biol. 2009, 19, 560–565. [Google Scholar] [CrossRef]
- Gonen, T.; Cheng, Y.; Sliz, P.; Hiroaki, Y.; Fujiyoshi, Y.; Harrison, S.C.; Walz, T. Lipid-Protein Interactions in Double-Layered Two-Dimensional AQP0 Crystals. Nature 2005, 438, 633–638. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, O.; Srivastava, K.; Joseph, R.; Wilson, L. Increased Association of Deamidated AA-N101D with Lens Membrane of Transgenic AAN101D vs. Wild Type AA Mice: Potential Effects on Intracellular Ionic Imbalance and Membrane Disorganization. BMC Ophthalmol. 2020, 20, 484. [Google Scholar] [CrossRef] [PubMed]
- Delamere, N.A.; Tamiya, S. Lens Ion Transport: From Basic Concepts to Regulation of Na,K-ATPase Activity. Exp. Eye Res. 2009, 88, 140–143. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, J.D.; Sanderson, J. The Mechanisms of Calcium Homeostasis and Signalling in the Lens. Exp. Eye Res. 2009, 88, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Mainali, L.; O’Brien, W.J.; Timsina, R. Interaction of Alpha-Crystallin with Phospholipid Membranes. Curr. Eye Res. 2021, 46, 185–194. [Google Scholar] [CrossRef]
- Timsina, R.; Khadka, N.K.; Maldonado, D.; Mainali, L. Interaction of Alpha-Crystallin with Four Major Phospholipids of Eye Lens Membranes. Exp. Eye Res. 2021, 202, 108337. [Google Scholar] [CrossRef]
- Timsina, R.; Trossi-Torres, G.; O’Dell, M.; Khadka, N.K.; Mainali, L. Cholesterol and Cholesterol Bilayer Domains Inhibit Binding of Alpha-Crystallin to the Membranes Made of the Major Phospholipids of Eye Lens Fiber Cell Plasma Membranes. Exp. Eye Res. 2021, 206, 108544. [Google Scholar] [CrossRef]
- Timsina, R.; Trossi-Torres, G.; Thieme, J.; O’Dell, M.; Khadka, N.K.; Mainali, L. Alpha-Crystallin Association with the Model of Human and Animal Eye Lens-Lipid Membranes Is Modulated by Surface Hydrophobicity of Membranes. Curr. Eye Res. 2022. [Google Scholar] [CrossRef]
- Cobb, B.A.; Petrash, J.M. Structural and Functional Changes in the AA-Crystallin R116C Mutant in Hereditary Cataracts. Biochemistry 2000, 39, 15791–15798. [Google Scholar] [CrossRef]
- Buboltz, J.T. A More Efficient Device for Preparing Model-Membrane Liposomes by the Rapid Solvent Exchange Method. Rev. Sci. Instrum. 2009, 80, 124301. [Google Scholar] [CrossRef]
- Mainali, L.; Raguz, M.; Subczynski, W.K. Formation of Cholesterol Bilayer Domains Precedes Formation of Cholesterol Crystals in Cholesterol/Dimyristoylphosphatidylcholine Membranes: EPR and DSC Studies. J. Phys. Chem. B 2013, 117, 8994–9003. [Google Scholar] [CrossRef] [PubMed]
- Zareba, M.; Widomska, J.; Burke, J.M.; Subczynski, W.K. Nitroxide Free Radicals Protect Macular Carotenoids against Chemical Destruction (Bleaching) during Lipid Peroxidation. Free Radic. Biol. Med. 2016, 101, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Mainali, L.; Raguz, M.; Subczynski, W.K. Phases and Domains in Sphingomyelin-Cholesterol Membranes: Structure and Properties Using EPR Spin-Labeling Methods. Eur. Biophys. J. EBJ 2012, 41, 147–159. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mainali, L.; Raguz, M.; O’Brien, W.J.; Subczynski, W.K. Properties of Fiber Cell Plasma Membranes Isolated from the Cortex and Nucleus of the Porcine Eye Lens. Exp. Eye Res. 2012, 97, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Mainali, L.; Raguz, M.; Subczynski, W.K. Phase-Separation and Domain-Formation in Cholesterol-Sphingomyelin Mixture: Pulse-EPR Oxygen Probing. Biophys. J. 2011, 101, 837–846. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Raguz, M.; Mainali, L.; Widomska, J.; Subczynski, W.K. Using Spin-Label Electron Paramagnetic Resonance (EPR) to Discriminate and Characterize the Cholesterol Bilayer Domain. Chem. Phys. Lipids 2011, 164, 819–829. [Google Scholar] [CrossRef]
- Subczynski, W.K.; Wisniewska, A.; Yin, J.-J.; Hyde, J.S.; Kusumi, A. Hydrophobic Barriers of Lipid Bilayer Membranes Formed by Reduction of Water Penetration by Alkyl Chain Unsaturation and Cholesterol. Biochemistry 1994, 33, 7670–7681. [Google Scholar] [CrossRef]
- Tang, D.; Borchman, D. Temperature Induced Structural Changes of Beta-Crystallin and Sphingomyelin Binding. Exp. Eye Res. 1998, 67, 113–118. [Google Scholar] [CrossRef]
- Mulders, J.W.; Stokkermans, J.; Leunissen, J.A.; Benedetti, E.L.; Bloemendal, H.; de Jong, W.W. Interaction of Alpha-Crystallin with Lens Plasma Membranes. Affinity for MP26. Eur. J. Biochem. 1985, 152, 721–728. [Google Scholar] [CrossRef]
- Tsvetkova, N.M.; Horváth, I.; Török, Z.; Wolkers, W.F.; Balogi, Z.; Shigapova, N.; Crowe, L.M.; Tablin, F.; Vierling, E.; Crowe, J.H.; et al. Small Heat-Shock Proteins Regulate Membrane Lipid Polymorphism. Proc. Natl. Acad. Sci. USA 2002, 99, 13504–13509. [Google Scholar] [CrossRef]
- Schreier, S.; Polnaszek, C.F.; Smith, I.C. Spin Labels in Membranes. Problems in Practice. Biochim. Biophys. Acta 1978, 515, 395–436. [Google Scholar] [CrossRef]
- Kusumi, A.; Subczynski, W.K.; Pasenkiewicz-Gierula, M.; Hyde, J.S.; Merkle, H. Spin-Label Studies on Phosphatidylcholine-Cholesterol Membranes: Effects of Alkyl Chain Length and Unsaturation in the Fluid Phase. Biochim. Biophys. Acta 1986, 854, 307–317. [Google Scholar] [CrossRef]
- Borchman, D.; Delamere, N.A.; McCauley, L.A.; Paterson, C.A. Studies on the Distribution of Cholesterol, Phospholipid, and Protein in the Human and Bovine Lens. Lens Eye Toxic. Res. 1989, 6, 703–724. [Google Scholar] [PubMed]
- Cobb, B.A.; Petrash, J.M. Characterization of Alpha-Crystallin-Plasma Membrane Binding. J. Biol. Chem. 2000, 275, 6664–6672. [Google Scholar] [CrossRef] [PubMed]
- Tjondro, H.C.; Xi, Y.-B.; Chen, X.-J.; Su, J.-T.; Yan, Y.-B. Membrane Insertion of AA-Crystallin Is Oligomer-Size Dependent. Biochem. Biophys. Res. Commun. 2016, 473, 1–7. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trossi-Torres, G.; Timsina, R.; Mainali, L. Alpha-Crystallin-Membrane Association Modulated by Phospholipid Acyl Chain Length and Degree of Unsaturation. Membranes 2022, 12, 455. https://doi.org/10.3390/membranes12050455
Trossi-Torres G, Timsina R, Mainali L. Alpha-Crystallin-Membrane Association Modulated by Phospholipid Acyl Chain Length and Degree of Unsaturation. Membranes. 2022; 12(5):455. https://doi.org/10.3390/membranes12050455
Chicago/Turabian StyleTrossi-Torres, Geraline, Raju Timsina, and Laxman Mainali. 2022. "Alpha-Crystallin-Membrane Association Modulated by Phospholipid Acyl Chain Length and Degree of Unsaturation" Membranes 12, no. 5: 455. https://doi.org/10.3390/membranes12050455
APA StyleTrossi-Torres, G., Timsina, R., & Mainali, L. (2022). Alpha-Crystallin-Membrane Association Modulated by Phospholipid Acyl Chain Length and Degree of Unsaturation. Membranes, 12(5), 455. https://doi.org/10.3390/membranes12050455







