Barrier Membrane in Regenerative Therapy: A Narrative Review
Abstract
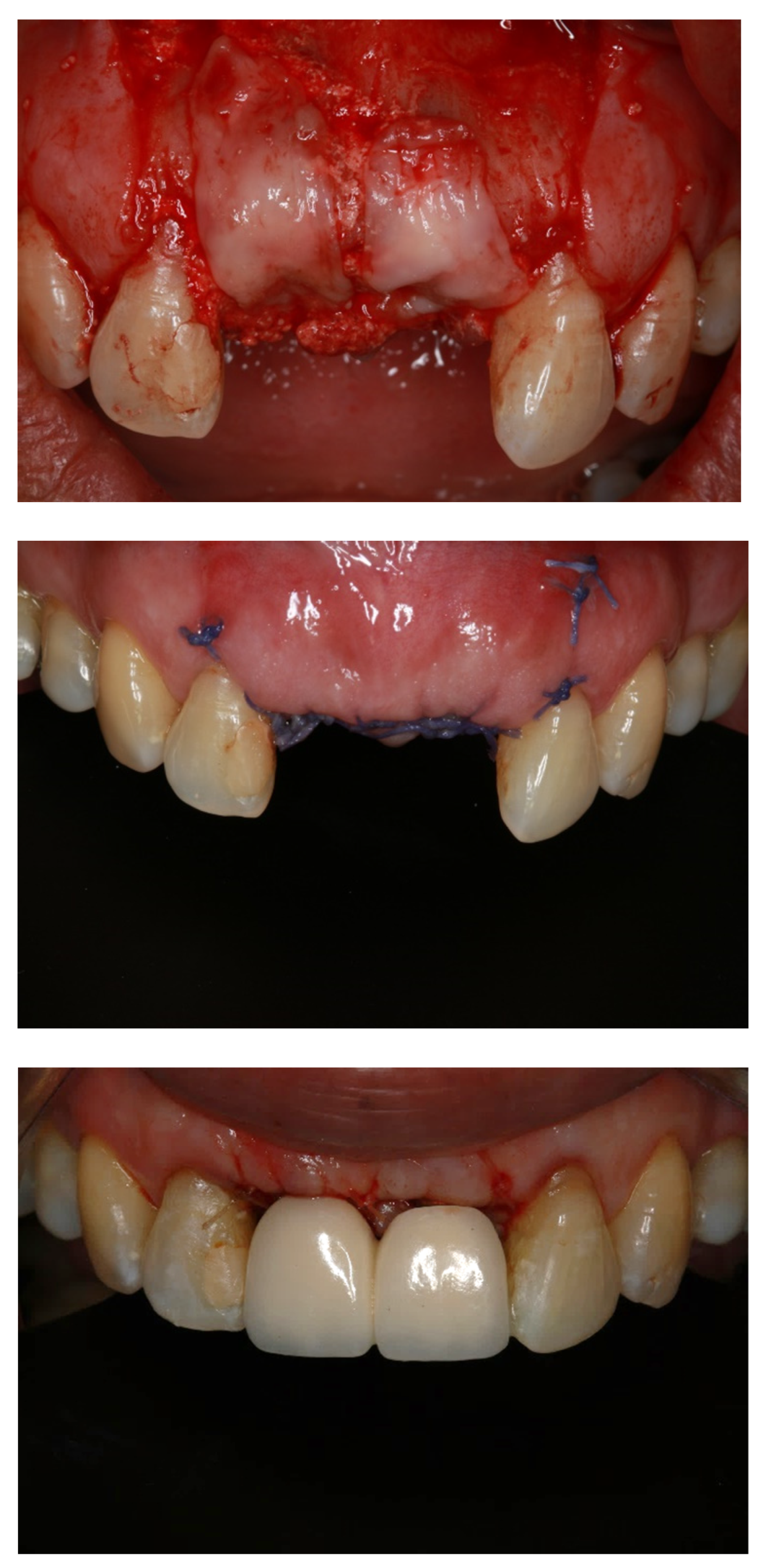
:1. Introduction
2. Materials and Methods
3. Resorbable Membranes
3.1. Collagen Membranes
3.2. Clinical Evidence
3.3. Fibrin
3.4. Placenta
3.5. Chitosan
3.6. Current Development of Resorbable Membranes
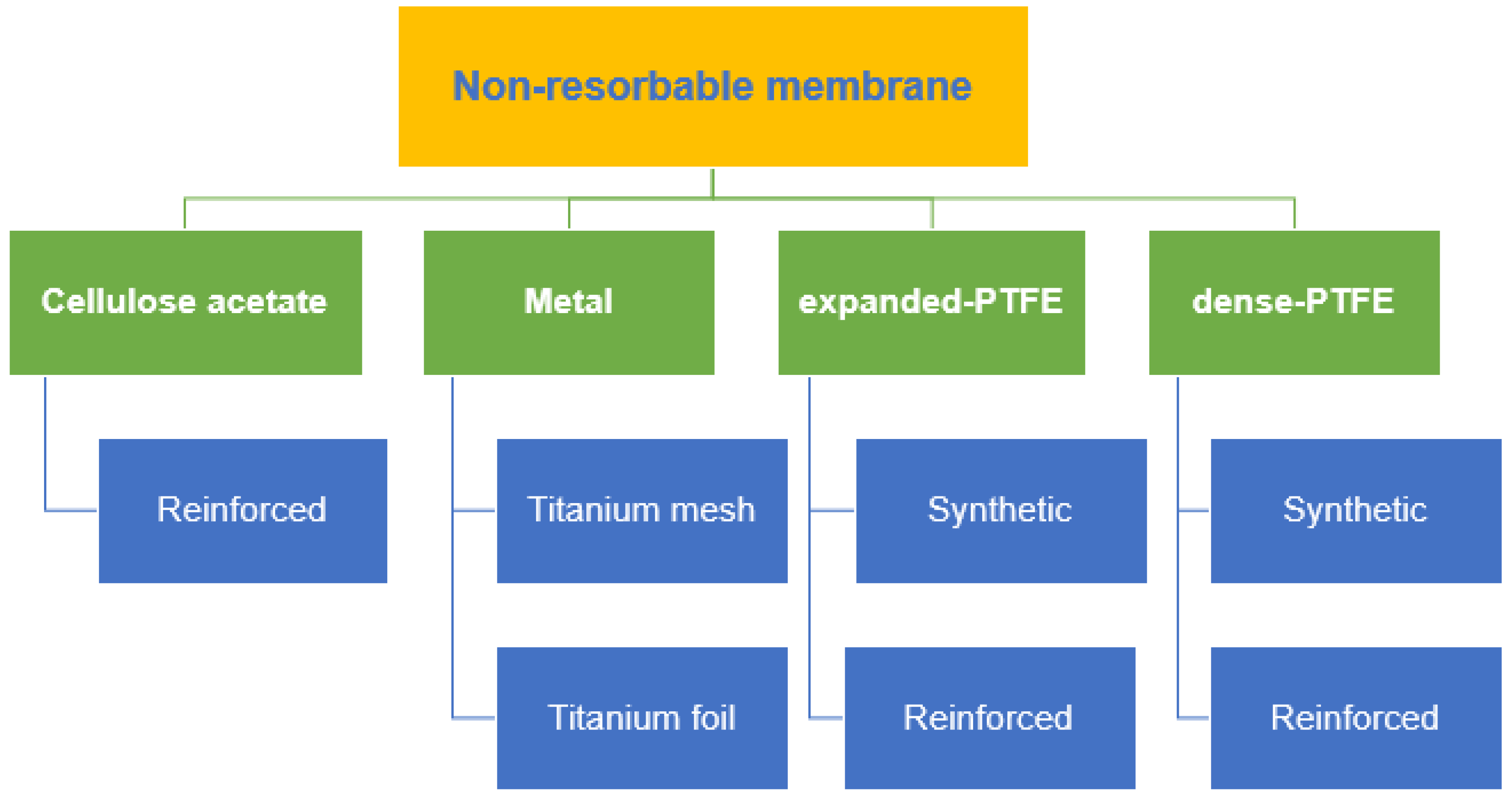
4. Non-Resorbable Membranes
5. Synthetic Membranes
6. Autologous Platelet Concentrate (APC)
Types of Autologous Platelet Concentrate
7. High-Performance Polymer
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Elgali, I.; Omar, O.; Dahlin, C.; Thomsen, P. Guided bone regeneration: Materials and biological mechanisms revisited. Eur. J. Oral Sci. 2017, 125, 315–337. [Google Scholar] [CrossRef]
- Khojasteh, A.; Kheiri, L.; Motamedian, S.R.; Khoshkam, V. Guided bone regeneration for the reconstruction of alveolar bone defects. Ann. Maxillofac. Surg. 2017, 7, 263–277. [Google Scholar] [CrossRef]
- Masquelet, A.C.; Begue, T. The Concept of Induced Membrane for Reconstruction of Long Bone Defects. Orthop. Clin. N. Am. 2010, 41, 27–37. [Google Scholar] [CrossRef]
- Liu, J.; Kerns, D.G. Mechanisms of Guided Bone Regeneration: A Review. Open Dent. J. 2014, 8, 56–65. [Google Scholar] [CrossRef] [Green Version]
- Hoornaert, A.; D’Arros, C.; Heymann, M.-F.; Layrolle, P. Biocompatibility, resorption and biofunctionality of a new synthetic biodegradable membrane for guided bone regeneration. Biomed. Mater. 2016, 11, 045012. [Google Scholar] [CrossRef]
- Caballé-Serrano, J.; Sawada, K.; Miron, R.J.; Bosshardt, D.D.; Buser, D.; Gruber, R. Collagen barrier membranes adsorb growth factors liberated from autogenous bone chips. Clin. Oral Implants Res. 2016, 28, 236–241. [Google Scholar] [CrossRef]
- Huang, H.-L.; Ma, Y.-H.; Tu, C.-C.; Chang, P.-C. Radiographic Evaluation of Regeneration Strategies for the Treatment of Advanced Mandibular Furcation Defects: A Retrospective Study. Membranes 2022, 12, 219. [Google Scholar] [CrossRef]
- Sasaki, J.-I.; Abe, G.L.; Li, A.; Thongthai, P.; Tsuboi, R.; Kohno, T.; Imazato, S. Barrier membranes for tissue regeneration in dentistry. Biomater. Investig. Dent. 2021, 8, 54–63. [Google Scholar] [CrossRef]
- Sbricoli, L.; Guazzo, R.; Annunziata, M.; Gobbato, L.; Bressan, E.; Nastri, L. Selection of collagen membranes for bone regenera-tion: A literature review. Materials 2020, 13, 786. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.L.; Boyapati, L. “PASS” principles for predictable bone regeneration. Implant Dent. 2006, 15, 8–17. [Google Scholar] [CrossRef] [Green Version]
- Gottlow, J.; Nyman, S.; Karring, T.; Lindhe, J. New attachment formation as the result of controlled tissue regeneration. J. Clin. Periodontol. 1984, 11, 494–503. [Google Scholar] [CrossRef]
- Neto, A.M.D.; Sartoretto, S.C.; Duarte, I.M.; Resende, R.F.D.B.; Alves, A.T.N.N.; Mourão, C.F.D.A.B.; Calasans-Maia, J.; Montemezzi, P.; Tristão, G.C.; Calasans-Maia, M.D. In Vivo Comparative Evaluation of Biocompatibility and Biodegradation of Bovine and Porcine Collagen Membranes. Membranes 2020, 10, 423. [Google Scholar] [CrossRef]
- Pitaru, S.; Tal, H.; Soldinger, M.; Grosskopf, A.; Noff, M. Partial regeneration of periodontal tissues using collagen barriers: Initial observations in the canine. J. Periodontol. 1988, 59, 380–386. [Google Scholar] [CrossRef]
- Annen, B.M.; Ramel, C.F.; Hämmerle, C.H.; Jung, R.E. Use of a new cross-linked collagen membrane for the treatment of pe-ri-implant dehiscence defects: A randomised controlled double-blinded clinical trial. Eur. J. Oral Implantol. 2011, 4, 87–100. [Google Scholar]
- Bunyaratavej, P.; Wang, H.-L. Collagen Membranes: A Review. J. Periodontol. 2001, 72, 215–229. [Google Scholar] [CrossRef] [Green Version]
- Quteish, D.; Singrao, S.; Dolby, A.E. Light and electron microscopic evaluation of biocompatibility, resorption and penetration characteristics of human collagen graft material. J. Clin. Periodontol. 1991, 18, 305–311. [Google Scholar] [CrossRef]
- Schlegel, A.; Möhler, H.; Busch, F.; Mehl, A. Preclinical and clinical studies of a collagen membrane (Bio-Gide®). Biomaterials 1997, 18, 535–538. [Google Scholar] [CrossRef]
- Cortellini, P.; Carnevale, G.; Sanz, M.; Tonetti, M.S. Treatment of deep and shallow intrabony defects A multicenter randomized controlled clinical trial. J. Clin. Periodontol. 1998, 25, 981–987. [Google Scholar] [CrossRef]
- Simion, M.; Rocchietta, I.; Fontana, F.; Dellavia, C. Evaluation of a resorbable collagen matrix infused with rhPDGF-BB in peri-implant soft tissue augmentation: A preliminary report with 3.5 years of observation. Int. J. Periodontics Restor. Dent. 2012, 32, 273–282. [Google Scholar]
- Gulameabasse, S.; Gindraux, F.; Catros, S.; Fricain, J.C.; Fenelon, M. Chorion and amnion/chorion membranes in oral and per-iodontal surgery: A systematic review. J. Biomed. Mater. Res. Part B Appl. Biomater. 2021, 109, 1216–1229. [Google Scholar] [CrossRef]
- Kothiwale, S.; Ajbani, J. Evaluation of anti-inflammatory effect of cho- rion membrane in periodontal pocket therapy: A clinical and biochemical study. J. Indian Soc. Periodontol. 2018, 22, 433–437. [Google Scholar] [CrossRef]
- Gupta, A.; Kediege, S.; Mittal, A.; Jain, K.; Gupta, S.; Chaudhry, S. Amnion and Chorion Membranes in the Treatment of Gingival Recession and their Effect on Gingival Biotype: A Clinical and Ultrasonographic Study. J. Clin. Diagn. Res. 2018, 12, 26–32. [Google Scholar] [CrossRef]
- Kakabadze, A.; Mardaleishvili, K.; Loladze, G.; Karalashvili, L.; Chutkerashvili, G.; Chakhunashvili, D.; Kakabadze, Z. Reconstruction of mandibular defects with autogenous bone and de-cellularized bovine bone grafts with freeze-dried bone marrow stem cell paracrine factors. Oncol. Lett. 2017, 13, 1811–1818. [Google Scholar] [CrossRef]
- Taalab, M.R.; Gamal, R.M. The effect of amniotic chorion membrane on tissue biotype, wound healing and periodontal re-generation. IOSR J. Dent. Med. Sci. 2018, 17, 61–69. [Google Scholar]
- Joshi, C.P.; D’Lima, C.B.; Samat, U.C.; Karde, P.A.; Patil, A.G.; Dani, N.H. Comparative alveolar ridge preservation using al-logenous tooth graft ver- sus free-dried bone allograft: A randomized, controlled, prospective, clinical pilot study. Contemp. Clin. Dent. 2017, 8, 211–217. [Google Scholar] [CrossRef]
- Hassan, M.; Prakasam, S.; Bain, C.; Ghoneima, A.; Liu, S.S.-Y. A randomized split-mouth clinical trial on effectiveness of am-nion-chorion membranes in alveolar ridge preservation: A clinical, radiologic, and morphometric study. Int. J. Oral Maxillofac. Implants 2017, 32, 1389–1398. [Google Scholar] [CrossRef]
- De Angelis, N.; Kassim, Z.H.; Frosecchi, M.; Signore, A. Expansion of the zone of keratinized tissue for healthy implant abutment Interface using de-epithelialized amnion/chorion allograft. Int. J. Periodontics Restor. Dent. 2019, 39, e83–e88. [Google Scholar] [CrossRef]
- Qasim, S.S.B.; Baig, M.R.; Matinlinna, J.P.; Daood, U.; Al-Asfour, A. Highly Segregated Biocomposite Membrane as a Functionally Graded Template for Periodontal Tissue Regeneration. Membranes 2021, 11, 667. [Google Scholar] [CrossRef]
- Zhang, L.; Dong, Y.; Zhang, N.; Shi, J.; Zhang, X.; Qi, C.; Midgley, A.C.; Wang, S. Potentials of sandwich-like chitosan/polycaprolactone/gelatin scaffolds for guided tissue regeneration membrane. Mater. Sci. Eng. C 2020, 109, 110618. [Google Scholar] [CrossRef]
- Qasim, S.B.; Najeeb, S.; Delaine-Smith, R.M.; Rawlinson, A.; Rehman, I.U. Potential of electrospun chitosan fibers as a surface layer in functionally graded GTR membrane for periodontal regeneration. Dent. Mater. 2017, 33, 71–83. [Google Scholar] [CrossRef] [Green Version]
- Heath, J.K.; Atkinson, S.J.; Hembry, R.M.; Reynolds, J.J.; Meikle, M.C. Bacterial antigens induce collagenase and prostaglandin E2 synthesis in human gingival fibroblasts through a primary effect on circulating mononuclear cells. Infect. Immun. 1987, 55, 2148–2154. [Google Scholar] [CrossRef] [Green Version]
- Machtei, E.E. The Effect of Membrane Exposure on the Outcome of Regenerative Procedures in Humans: A Meta-Analysis. J. Periodontol. 2001, 72, 512–516. [Google Scholar] [CrossRef]
- Chan, E.C.; Kuo, S.-M.; Kong, A.M.; Morrison, W.A.; Dusting, G.J.; Mitchell, G.M.; Lim, S.Y.; Liu, G.-S. Three Dimensional Collagen Scaffold Promotes Intrinsic Vascularisation for Tissue Engineering Applications. PLoS ONE 2016, 11, e0149799. [Google Scholar] [CrossRef] [Green Version]
- Turri, A.; Elgali, I.; Vazirisani, F.; Johansson, A.; Emanuelsson, L.; Dahlin, C.; Thomsen, P.; Omar, O. Guided bone regeneration is promoted by the molecular events in the membrane compartment. Biomaterials 2016, 84, 167–183. [Google Scholar] [CrossRef] [Green Version]
- Taguchi, Y.; Amizuka, N.; Nakadate, M.; Ohnishi, H.; Fujii, N.; Oda, K.; Nomura, S.; Maeda, T. A histological evaluation for guided bone regeneration induced by a collagenous membrane. Biomaterials 2005, 26, 6158. [Google Scholar] [CrossRef]
- Allan, B.; Ruan, R.; Landao-Bassonga, E.; Gillman, N.; Wang, T.; Gao, J.; Ruan, Y.; Xu, Y.; Lee, C.; Goonewardene, M.; et al. Collagen Membrane for Guided Bone Regeneration in Dental and Orthopedic Applications. Tissue Eng. Part A 2021, 27, 372–381. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, L.; Liu, R.; Lin, Y.; Chen, S.; Lu, S.; Lin, Z.; Chen, Z.; Wu, C.; Xiao, Y. The osteoimmunomodulatory property of a barrier collagen membrane and its manipulation via coating nanometer-sized bioactive glass to improve guided bone re-generation. Biomater. Sci. 2018, 6, 1007–1019. [Google Scholar] [CrossRef]
- Ronda, M.; Rebaudi, A.; Torelli, L.; Stacchi, C. Expanded vs. dense polytetrafluoroethylene membranes in vertical ridge augmentation around dental implants: A prospective randomized controlled clinical trial. Clin. Oral Implants Res. 2014, 25, 859–866. [Google Scholar] [CrossRef]
- Ruggiero, R.; de Almeida Carvalho, V.; da Silva, L.G.; de Magalhães, D.; Ferreira, J.A.; de Menezes, H.H.M.; de Melo, G.P.; Naves, M.M. Study of in vitro degradation of cellulose acetate membranes modified and incorporated with tetracycline for use as an adjuvant in periodontal reconstitution. Ind. Crop. Prod. 2015, 72, 2–6. [Google Scholar] [CrossRef]
- Nyman, S.; Lindhe, J.; Karring, T.; Rylander, H. New attachment following surgical treatment of human periodontal disease. J. Clin. Periodontol. 1982, 9, 290–296. [Google Scholar] [CrossRef]
- Liang, Y.; Luan, X.; Liu, X. Recent advances in periodontal regeneration: A biomaterial perspective. Bioact. Mater. 2020, 5, 297–308. [Google Scholar] [CrossRef]
- Misch, C.E.; Judy, K.W. Classification of partially edentulous arches for implant dentistry. Int. J. Oral Implantol. 1987, 4, 7–13. [Google Scholar]
- Jung, G.U.; Jeon, J.Y.; Hwang, K.G.; Park, C.J. Preliminary evaluation of a three-dimensional, customized, and preformed tita-nium mesh in peri-implant alveolar bone regeneration. J. Korean Assoc. Oral Maxillofac. Surg. 2014, 40, 181–187. [Google Scholar] [CrossRef]
- Maiorana, C.; Manfredini, M.; Beretta, M.; Signorino, F.; Bovio, A.; Poli, P.P. Clinical and Radiographic Evaluation of Simulta-neous Alveolar Ridge Augmentation by Means of Preformed Titanium Meshes at Dehiscence-Type Peri-Implant Defects: A Prospective Pilot Study. Materials 2020, 13, 2389. [Google Scholar] [CrossRef]
- Xie, Y.; Li, S.; Zhang, T.; Wang, C.; Cai, X. Titanium mesh for bone augmentation in oral implantology: Current application and progress. Int. J. Oral Sci. 2020, 12, 37. [Google Scholar] [CrossRef]
- Andreasi Bassi, M.; Andrisani, C.; Lico, S.; Ormanier, Z.; Ottria, L.; Gargari, M. Guided bone regeneration via a preformed tita-nium foil: Clinical, histological and histomorphometric outcome of a case series. Oral Implantol. 2016, 9, 164–174. [Google Scholar]
- Cucchi, A.; Ghensi, P. Vertical Guided Bone Regeneration using Titanium-reinforced d-PTFE Membrane and Prehydrated Corticocancellous Bone Graft. Open Dent. J. 2014, 8, 194–200. [Google Scholar] [CrossRef] [Green Version]
- Buser, D.; Dula, K.; Belser, U.C.; Hirt, H.P.; Berthold, H. Localized ridge augmentation using guided bone regeneration. II. Surgical procedure in the mandible. Int. J. Periodontics Restor. Dent. 1995, 15, 10–29. [Google Scholar]
- Soldatos, N.K.; Stylianou, P.; Angelov, N.; Koidou, P.; Yukna, R.; Romanos, G.E. Limitations and options using resorbable versus nonresorbable membranes for successful guided bone regeneration. Quintessence Int. 2017, 48, 131–147. [Google Scholar]
- Retzepi, M.; Donos, N. Guided Bone Regeneration: Biological principle and therapeutic applications. Clin. Oral Implants Res. 2010, 21, 567–576. [Google Scholar] [CrossRef]
- Gutta, R.; Baker, R.A.; Bartolucci, A.A.; Louis, P.J. Barrier Membranes Used for Ridge Augmentation: Is There an Optimal Pore Size? J. Oral Maxillofac. Surg. 2009, 67, 1218–1225. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Kim, Y.-K.; Yun, P.-Y.; Oh, J.-S.; Kim, S.-G. Guided bone regeneration using two types of non-resorbable barrier mem-branes. J. Korean Assoc. Oral Maxillofac. Surg. 2010, 36, 275–279. [Google Scholar] [CrossRef] [Green Version]
- Rakhmatia, Y.D.; Ayukawa, Y.; Furuhashi, A.; Koyano, K. Current barrier membranes: Titanium mesh and other membranes for guided bone regeneration in dental applications. J. Prosthodont. Res. 2013, 57, 3–14. [Google Scholar] [CrossRef] [Green Version]
- Vroom, M.; Gründemann, L. Nietresorbeerbare membranen. Tandartspraktijk 2014, 35, 8–13. [Google Scholar] [CrossRef]
- Barber, H.D.; Lignelli, J.; Smith, B.M.; Bartee, B.K. Using a Dense PTFE Membrane Without Primary Closure to Achieve Bone and Tissue Regeneration. J. Oral Maxillofac. Surg. 2007, 65, 748–752. [Google Scholar] [CrossRef]
- Rodriguez, I.A.; Selders, G.S.; Fetz, A.E.; Gehrmann, C.J.; Stein, S.H.; Evensky, J.A.; Green, M.S.; Bowlin, G.L. Barrier membranes for dental applications: A review and sweet advancement in membrane developments. Mouth Teeth 2018, 2, 1–9. [Google Scholar]
- Carbonell, J.; Martín, I.S.; Santos, A.; Pujol, A.; Sanz-Moliner, J.; Nart, J. High-density polytetrafluoroethylene membranes in guided bone and tissue regeneration procedures: A literature review. Int. J. Oral Maxillofac. Surg. 2014, 43, 75–84. [Google Scholar] [CrossRef]
- Canullo, L.; Malagnino, V.A. Vertical ridge augmentation around implants by e-PTFE titanium-reinforced membrane and bovine bone matrix: A 24- to 54-month study of 10 consecutive cases. Int. J. Oral Maxillofac. Implants 2008, 23, 858–866. [Google Scholar]
- Sun, D.-J.; Oh, Y.-A.; Yu, J.-A.; Lee, D.-W. Clinical Evaluation of Vertical Ridge Augmentation Using Titanium Reinforced PTFE membrane. Implantology 2018, 22, 2–11. [Google Scholar] [CrossRef]
- Castro, A.G.; Diba, M.; Kersten, M.; Jansen, J.A.; Beucken, J.J.V.D.; Yang, F. Development of a PCL-silica nanoparticles composite membrane for Guided Bone Regeneration. Mater. Sci. Eng. C 2018, 85, 154–161. [Google Scholar] [CrossRef]
- Wang, J.; Wang, L.; Zhou, Z.; Lai, H.; Xu, P.; Liao, L.; Wei, J. Biodegradable Polymer Membranes Applied in Guided Bone/Tissue Regeneration: A Review. Polymers 2016, 8, 115. [Google Scholar] [CrossRef] [PubMed]
- Ulery, B.D.; Nair, L.S.; Laurencin, C.T. Biomedical applications of biodegradable polymers. J. Polym. Sci. Part B Polym. Phys. 2011, 49, 832–864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iqbal, N.; Khan, A.S.; Asif, A.; Yar, M.; Haycock, J.W.; Rehman, I.U. Recent concepts in biodegradable polymers for tissue engi-neering paradigms: A critical review. Int. Mater. Rev. 2019, 64, 91–126. [Google Scholar] [CrossRef] [Green Version]
- Casalini, T.; Rossi, F.; Castrovinci, A.; Perale, G. A Perspective on Polylactic Acid-Based Polymers Use for Nanoparticles Synthesis and Applications. Front. Bioeng. Biotechnol. 2019, 7, 259. [Google Scholar] [CrossRef] [PubMed]
- Farah, S.; Anderson, D.G.; Langer, R. Physical and mechanical properties of PLA, and their functions in widespread appli-cations—A comprehensive review. Adv. Drug Deliv. Rev. 2016, 107, 367–392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xue, J.; He, M.; Liang, Y.; Crawford, A.; Coates, P.; Chen, D.; Shi, R.; Zhang, L. Fabrication and evaluation of electrospun PCL–gelatin micro-/nanofiber membranes for anti-infective GTR implants. J. Mater. Chem. B 2014, 2, 6867–6877. [Google Scholar] [CrossRef]
- Farnezi Bassi, A.P.; Ferreira Bizelli, V.; Mello Francatti, T.; Rezende de Moares Ferreira, A.C.; Carvalho Pereira, J.; Al-Sharani, H.M.; de Almeida Lucas, F.; Perez Faverani, L. Bone Regeneration Assessment of Polycaprolactone Membrane on Critical-Size Defects in Rat Calvaria. Membranes 2021, 11, 124. [Google Scholar] [CrossRef]
- Alauddin, M.S.; Ramli, H. Management of Membrane Exposure Utilizing Concentrated Growth Factor (CFG) in Guided Bone Regeneration: A Clinical Report. Open Dent. J. 2020, 14, 763–768. [Google Scholar] [CrossRef]
- Alauddin, M.S.; Yusof, N.M.; Adnan, A.S.; Said, Z. Preliminary Novel Analysis on Antimicrobial Properties of Concentrated Growth Factor against Bacteria-Induced Oral Diseases. Eur. J. Dent. 2022, 1–239. [Google Scholar] [CrossRef]
- Dohan, D.M.; Choukroun, J.; Diss, A.; Dohan, S.L.; Dohan, A.; Mouhyi, J.; Gogly, B. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part I: Technological concepts and evolution. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2006, 101, e37–e44. [Google Scholar] [CrossRef]
- Dohan Ehrenfest, D.M.; Rasmusson, L.; Albrektsson, T. Classification of platelet concentrates: From pure platelet-rich plasma (P-PRP) to leucocyte- and platelet-rich fibrin (L-PRF). Trends Biotechnol. 2009, 27, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Rodella, L.F.; Favero, G.; Boninsegna, R.; Buffoli, B.; Labanca, M.; Scarì, G.; Sacco, L.; Batani, T.; Rezzani, R. Growth factors, CD34 positive cells, and fibrin network analysis in concentrated growth factors fraction. Microsc. Res. Tech. 2011, 74, 772–777. [Google Scholar] [CrossRef] [PubMed]
- Isobe, K.; Watanabe, T.; Kawabata, H.; Kitamura, Y.; Okudera, T.; Okudera, H.; Uematsu, K.; Okuda, K.; Nakata, K.; Tanaka, T.; et al. Mechanical and degradation properties of advanced platelet-rich fibrin (A-PRF), concentrated growth factors (CGF), and platelet-poor plasma-derived fibrin (PPTF). Int. J. Implant Dent. 2017, 3, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Panda, S.; Sankari, M.; Satpathy, A.; Jayakumar, D.; Mozzati, M.; Mortellaro, C.; Gallesio, G.; Taschieri, S.; Del Fabbro, M. Adjunctive Effect of Autologus Platelet-Rich Fibrin to Barrier Membrane in the Treatment of Periodontal Intrabony Defects. J. Craniofac. Surg. 2016, 27, 691–696. [Google Scholar] [CrossRef]
- Miron, R.J.; Moraschini, V.; Fujioka-Kobayashi, M.; Zhang, Y.; Kawase, T.; Cosgarea, R.; Jepsen, S.; Bishara, M.; Canullo, L.; Shirakata, Y.; et al. Use of platelet-rich fibrin for the treatment of periodontal intrabony defects: A systematic review and me-ta-analysis. Clin. Oral Investig. 2021, 25, 2461–2478. [Google Scholar] [CrossRef]
- Tanuja, B.; Kondareddy, K.M.; Ramesh, A.; Rajesh, N.; Prakash, R. Efficacy of Bovine Hydroxyapatite and Collagen Along with Platelet-Rich Fibrin as a Scaffold and Human Chorion as a Membrane for Ridge Preservation: A Case-Control Study. Cureus 2022, 14, e21362. [Google Scholar]
- Kurtz, S.M.; Devine, J.N. PEEK biomaterials in trauma, orthopedic, and spinal implants. Biomaterials 2007, 28, 4845–4869. [Google Scholar] [CrossRef] [Green Version]
- Schwitalla, A.; Müller, W.-D. PEEK Dental Implants: A Review of the Literature. J. Oral Implant. 2013, 39, 743–749. [Google Scholar] [CrossRef]
- Toth, J.M.; Wang, M.; Estes, B.T.; Scifert, J.L.; Seim, H.B., III; Turner, A.S. Polyetheretherketone as a biomaterial for spinal applica-tions. Biomaterials 2006, 27, 324–334. [Google Scholar] [CrossRef] [Green Version]
- Santing, H.J.; Meijer, H.J.; Raghoebar, G.M.; Özcan, M. Fracture strength and failure mode of maxillary implant-supported provisional single crowns: A comparison of composite resin crowns fabricated directly over PEEK abutments and solid titanium abutments. Clin. Implant Dent. Relat. Res. 2012, 14, 882–889. [Google Scholar] [CrossRef]
- Tannous, F.; Steiner, M.; Shahin, R.; Kern, M. Retentive forces and fatigue resistance of thermoplastic resin clasps. Dent. Mater. 2012, 28, 273–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costa-Palau, S.; Torrents-Nicolas, J.; Barberà, M.B.-D.; Cabratosa-Termes, J. Use of polyetheretherketone in the fabrication of a maxillary obturator prosthesis: A clinical report. J. Prosthet. Dent. 2014, 112, 680–682. [Google Scholar] [CrossRef] [PubMed]
- Aldhuwayhi, S.; Alauddin, M.S.; Martin, N. The Structural Integrity and Fracture Behaviour of Teeth Restored with PEEK and Lithium-Disilicate Glass Ceramic Crowns. Polymers 2022, 14, 1001. [Google Scholar] [CrossRef] [PubMed]
- Papia, E.; Brodde, S.A.; Becktor, J.P. Deformation of polyetheretherketone, PEEK, with different thicknesses. J. Mech. Behav. Biomed. Mater. 2022, 125, 104928. [Google Scholar] [CrossRef]
- Alauddin, M.S. A Review of Polymer Crown Materials: Biomechanical and Material Science. J. Clin. Diagn. Res. 2019, 13, ZE01–ZE05. [Google Scholar] [CrossRef]
- Najeeb, S.; Zafar, M.S.; Khurshid, Z.; Siddiqui, F. Applications of polyetheretherketone (PEEK) in oral implantology and prosthodontics. J. Prosthodont. Res. 2016, 60, 12–19. [Google Scholar] [CrossRef]
- Alauddin, M.S.; Baharuddin, A.S.; Mohd Ghazali, M.I. The modern and digital transformation of oral health care: A mini review. Healthcare 2021, 9, 118. [Google Scholar] [CrossRef]
- El Morsy, O.A.; Barakat, A.; Mekhemer, S.; Mounir, M. Assessment of 3-dimensional bone augmentation of severely atrophied maxillary alveolar ridges using patient-specific poly ether-ether ketone (PEEK) sheets. Clin. Implant Dent. Relat. Res. 2020, 22, 148–155. [Google Scholar] [CrossRef]
- Mounir, M.; Shalash, M.; Mounir, S.; Nassar, Y.; El Khatib, O. Assessment of three dimensional bone augmentation of severely atrophied maxillary alveolar ridges using prebent titanium mesh vs customized poly-ether-ether-ketone (PEEK) mesh: A randomized clinical trial. Clin. Implant Dent. Relat. Res. 2019, 21, 960–967. [Google Scholar] [CrossRef]
- Alqurashi, H.; Khurshid, Z.; Syed, A.U.; Habib, S.R.; Rokaya, D.; Zafar, M.S. Polyetherketoneketone (PEKK): An emerging bio-material for oral implants and dental prostheses. J. Adv. Res. 2021, 28, 87–95. [Google Scholar] [CrossRef]
| Product (Company) | Material |
|---|---|
| Ti- Micromesh (ACE) | Titanium mesh |
| Tocksystem (MeshTM) | Titanium mesh |
| Millipore | Cellulose acetate |
| Gore-Tex® | ePTFE |
| Cytoplast™ | dPTFE |
| Ti-Reinforced Gore-Tex® | Titanium-reinforced ePTFE |
| Cytoplast™ Ti-Reinforced 250 | Titanium-reinforced dPTFE |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alauddin, M.S.; Abdul Hayei, N.A.; Sabarudin, M.A.; Mat Baharin, N.H. Barrier Membrane in Regenerative Therapy: A Narrative Review. Membranes 2022, 12, 444. https://doi.org/10.3390/membranes12050444
Alauddin MS, Abdul Hayei NA, Sabarudin MA, Mat Baharin NH. Barrier Membrane in Regenerative Therapy: A Narrative Review. Membranes. 2022; 12(5):444. https://doi.org/10.3390/membranes12050444
Chicago/Turabian StyleAlauddin, Muhammad Syafiq, Nur Ayman Abdul Hayei, Muhammad Annurdin Sabarudin, and Nor Haliza Mat Baharin. 2022. "Barrier Membrane in Regenerative Therapy: A Narrative Review" Membranes 12, no. 5: 444. https://doi.org/10.3390/membranes12050444
APA StyleAlauddin, M. S., Abdul Hayei, N. A., Sabarudin, M. A., & Mat Baharin, N. H. (2022). Barrier Membrane in Regenerative Therapy: A Narrative Review. Membranes, 12(5), 444. https://doi.org/10.3390/membranes12050444






