Xenopus Oocytes: A Tool to Decipher Molecular Specificity of Insecticides towards Mammalian and Insect GABA—A Receptors
Abstract
1. Introduction
2. Materials and Methods
2.1. Cloning of the Ap-RDL1 and Hs-a4 Receptors
2.2. RNA Preparation and Xenopus Oocyte Isolation and Injection
2.3. Electrophysiology
2.4. Solutions and Drugs
2.5. Analysis
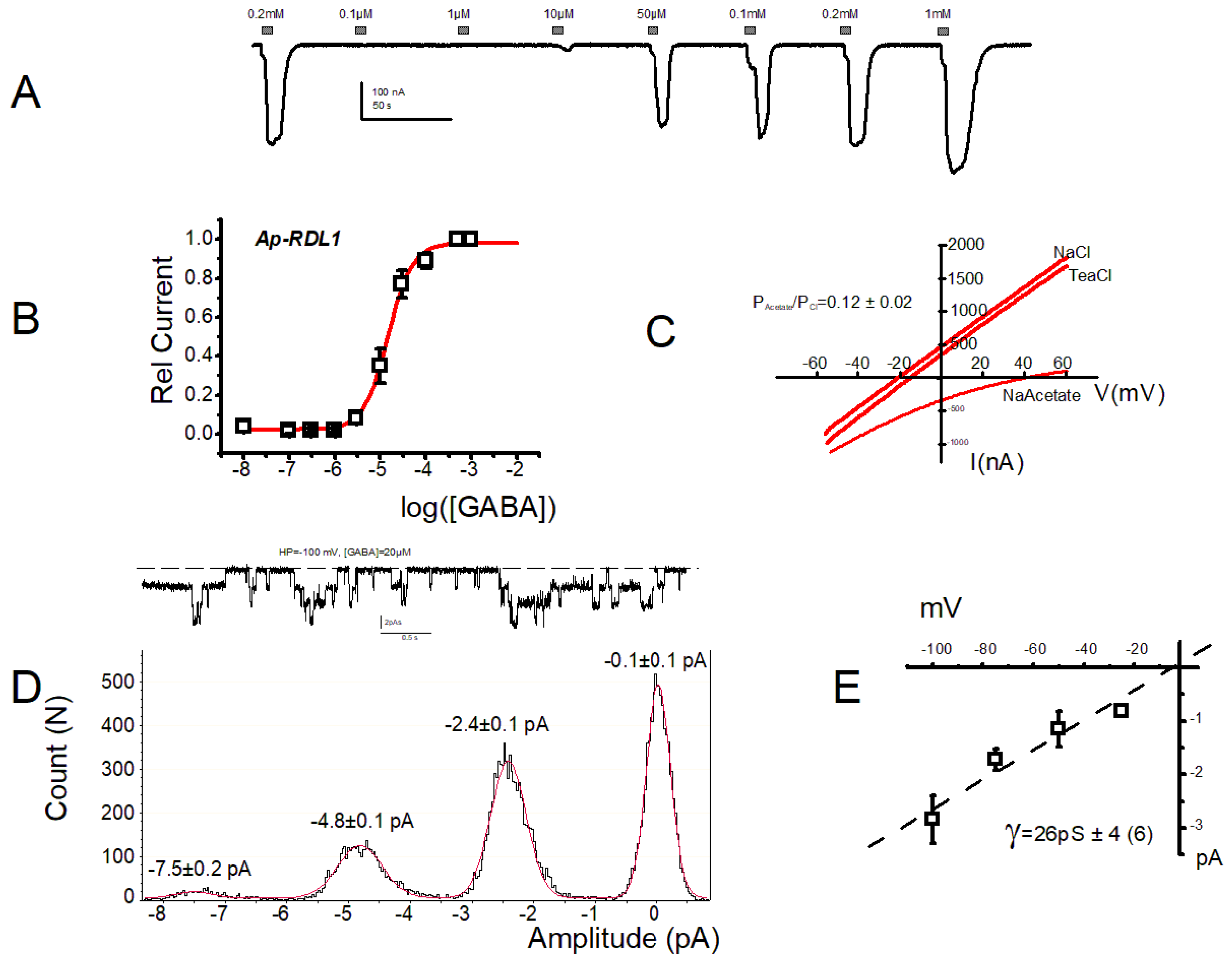
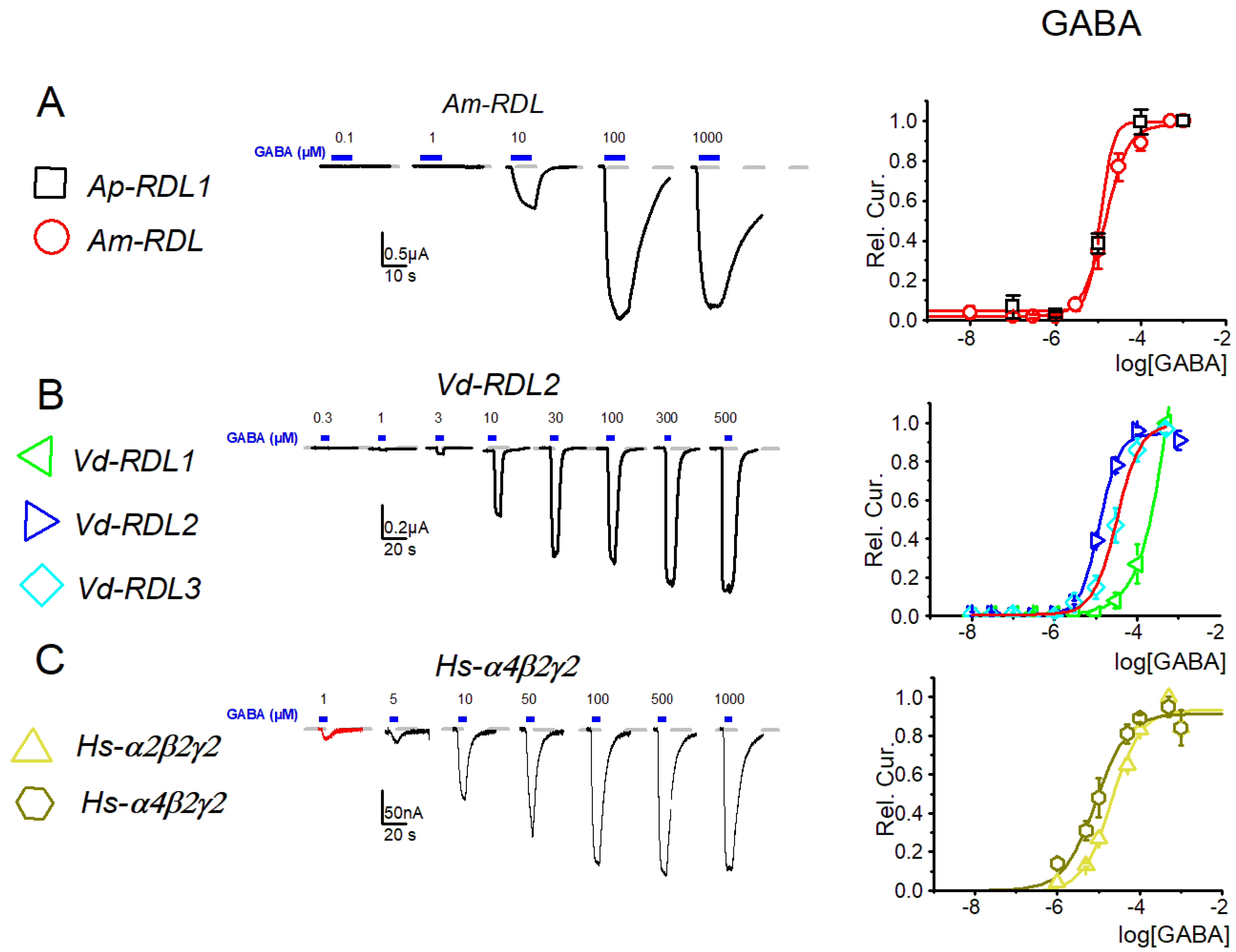
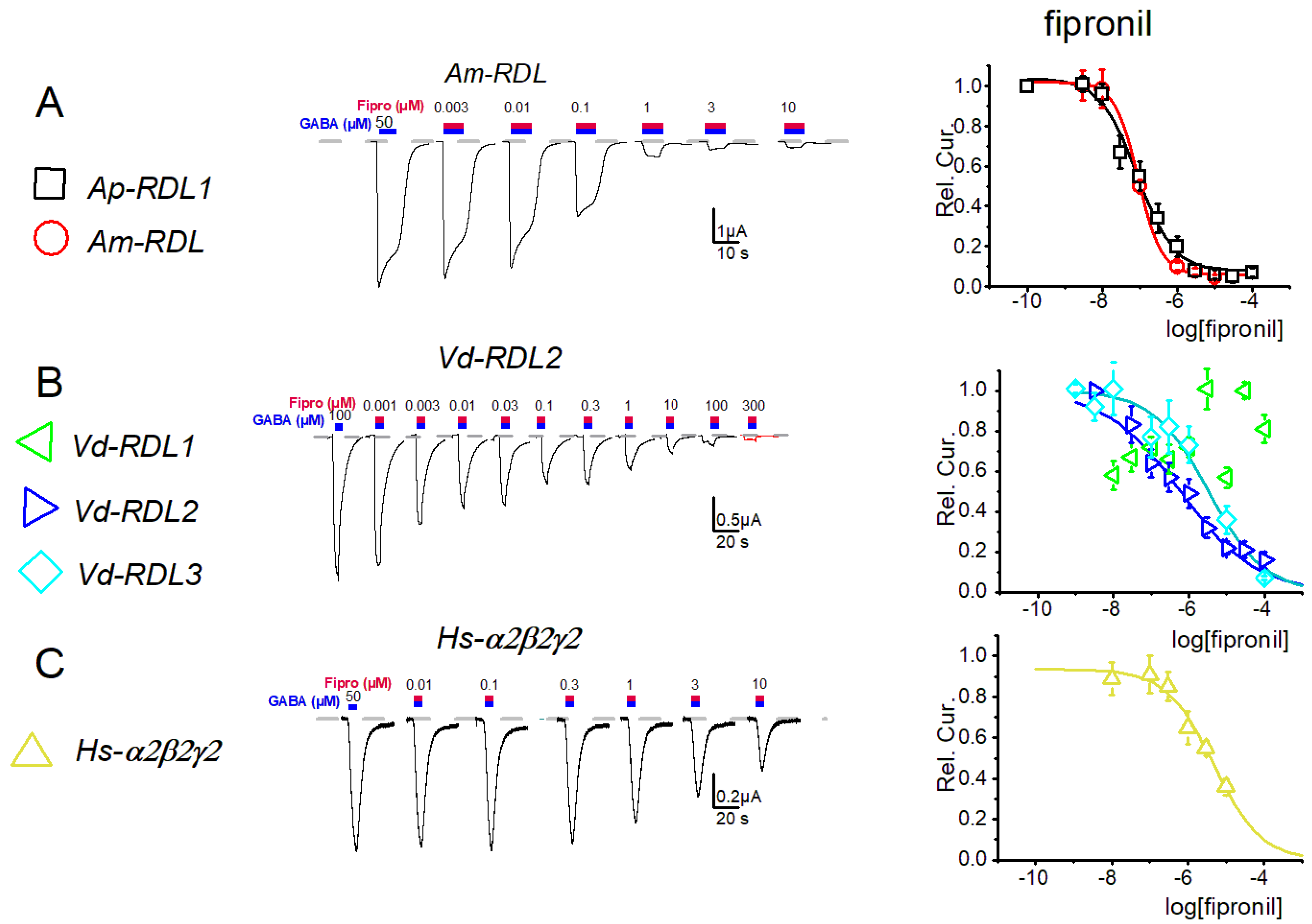
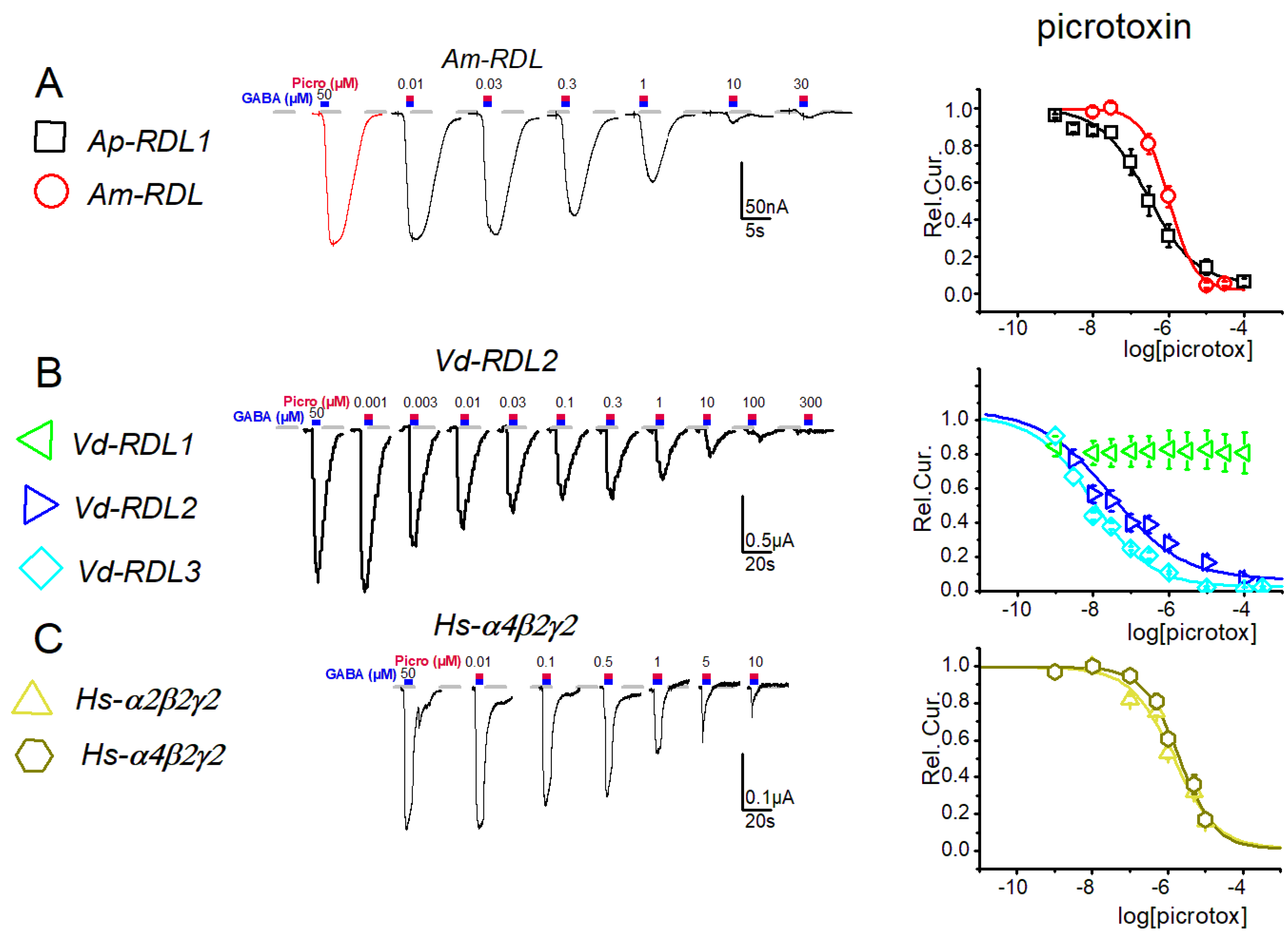
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Buckingham, S.D.; Biggin, P.C.; Sattelle, B.M.; Brown, L.A.; Sattelle, D.B. Insect GABA receptors: Splicing, editing, and targeting by antiparasitics and insecticides. Mol. Pharmacol. 2005, 68, 942–951. [Google Scholar] [CrossRef]
- Casida, J.E.; Durkin, K.A. Neuroactive Insecticides: Targets, Selectivity, Resistance, and Secondary Effects. Annu. Rev. Entomol. 2013, 58, 99–117. [Google Scholar] [CrossRef] [PubMed]
- Hosie, A.M.; Aronstein, K.; Sattelle, D.B.; Richard, H.; Aronstein, K. Molecular biology of insect neuronal GABA receptors. Trends Neurosci. 1997, 20, 578–583. [Google Scholar] [CrossRef]
- Ozoe, Y. g-Aminobutyrate- and Glutamate-gated Chloride Channels as Targets of Insecticides. In Advances in Insect Physiology; Simpson, S.J., Casas, J., Eds.; Elsevier Ltd.: Amsterdam, The Netherlands, 2013; Volume 44, pp. 211–286. [Google Scholar] [CrossRef]
- Chen, R.; Belelli, D.; Lambert, J.J.; Peters, J.A.; Reyes, A.; Lan, N.C. Cloning and functional expression of a Drosophila γ-aminobutyric acid receptor. Proc. Natl. Acad. Sci. USA 1994, 91, 6069–6073. [Google Scholar] [CrossRef] [PubMed]
- Taylor-Wells, J.; Hawkins, J.; Colombo, C.; Bermudez, I.; Jones, A.K. Cloning and functional expression of intracellular loop variants of the honey bee (Apis mellifera) RDL GABA receptor. Neurotoxicology 2017, 60, 207–213. [Google Scholar] [CrossRef]
- Ménard, C.; Folacci, M.; Brunello, L.; Charreton, M.; Collet, C.; Mary, R.; Rousset, M.; Thibaud, J.-B.; Vignes, M.; Charnet, P.; et al. Multiple combinations of RDL subunits diversify the repertoire of GABA receptors in the honey bee parasite Varroa destructor. J. Biol. Chem. 2018, 293, 19012–19024. [Google Scholar] [CrossRef]
- Del Villar, S.; Jones, A. Cloning and Functional Characterisation of the Duplicated RDL Subunits from the Pea Aphid, Acyrthosiphon pisum. Int. J. Mol. Sci. 2018, 19, 2235. [Google Scholar] [CrossRef]
- Gisselmann, G.; Plonka, J.; Pusch, H.; Hatt, H. Drosophila melanogaster GRD and LCCH3 subunits form heteromultimeric GABA-gated cation channels. Br. J. Pharmacol. 2004, 142, 409–413. [Google Scholar] [CrossRef]
- Henry, C.; Cens, T.; Charnet, P.; Cohen-Solal, C.; Collet, C.; van-Dijk, J.; Guiramand, J.; Jésus-Ferreira, M.; Menard, C.; Mokrane, N.; et al. Heterogeneous expression of GABA receptor-like subunits LCCH3 and GRD reveals functional diversity of Apis mellifera GABA receptors. Br. J. Pharmacol. 2020, 177, 3924–3940. [Google Scholar] [CrossRef]
- Jones, A.K. How Complex Can Resistance to Dieldrin, the Insect γ-Aminobutyric Acid Receptor, Get? Int. J. Insect Sci. 2018, 10, 117954331880478. [Google Scholar] [CrossRef]
- Taylor-Wells, J.; Brooke, B.D.; Bermudez, I.; Jones, A.K. The neonicotinoid imidacloprid, and the pyrethroid deltamethrin, are antagonists of the insect Rdl GABA receptor. J. Neurochem. 2015, 135, 705–713. [Google Scholar] [CrossRef]
- Sheng, C.W.; Jia, Z.Q.; Ozoe, Y.; Huang, Q.T.; Han, Z.J.; Zhao, C.Q. Molecular cloning, spatiotemporal and functional expression of GABA receptor subunits RDL1 and RDL2 of the rice stem borer Chilo suppressalis. Insect Biochem. Mol. Biol. 2018, 94, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Lees, K.; Musgaard, M.; Suwanmanee, S.; Buckingham, S.D.; Biggin, P.; Sattelle, D. Actions of Agonists, Fipronil and Ivermectin on the Predominant In Vivo Splice and Edit Variant (RDLbd, I/V) of the Drosophila GABA Receptor Expressed in Xenopus laevis Oocytes. PLoS ONE 2014, 9, e97468. [Google Scholar]
- Es-Salah, Z.; Lapied, B.; Le Goff, G.; Hamon, A. RNA editing regulates insect gamma-aminobutyric acid receptor function and insecticide sensitivity. Neuroreport 2008, 19, 939–943. [Google Scholar] [CrossRef] [PubMed]
- Nakao, T.; Banba, S.; Nomura, M.; Hirase, K. Meta-diamide insecticides acting on distinct sites of RDL GABA receptor from those for conventional noncompetitive antagonists. Insect Biochem. 2013, 43, 366–375. [Google Scholar]
- Holder, P.J.; Jones, A.; Tyler, C.R.; Cresswell, J.E. Fipronil pesticide as a suspect in historical mass mortalities of honey bees. Proc. Natl. Acad. Sci. USA 2018, 115, 13033–13038. [Google Scholar] [CrossRef]
- Cens, T.; Rousset, M.; Collet, C.; Charreton, M.; Garnery, L.; Le Conte, Y.; Chahine, M.; Sandoz, J.C.; Charnet, P. Molecular characterization and functional expression of the Apis mellifera voltage-dependent Ca2+ channels. Insect Biochem. 2015, 58, 12–27. [Google Scholar] [CrossRef]
- Dale, R.P.; Jones, A.K.; Tamborindeguy, C.; Davies, T.G.; Amey, J.S.; Williamson, S.; Wolstenholme, A.; Field, L.M.; Williamson, M.S.; Walsh, T.K.; et al. Identification of ion channel genes in the Acyrthosiphon pisum genome. Insect Mol. Biol. 2010, 19 (Suppl. 2), 141–153. [Google Scholar] [CrossRef]
- Dauvilliers, Y.; Evangelista, E.; Lopez, R.; Barateau, L.; Jaussent, I.; Cens, T.; Rousset, M.; Charnet, P. Absence of gamma-aminobutyric acid-a receptor potentiation in central hypersomnolence disorders. Ann. Neurol. 2016, 80, 259–268. [Google Scholar] [CrossRef]
- Cens, T.; Mangoni, M.E.; Richard, S.; Nargeot, J.; Charnet, P. Coexpression of the beta2 subunit does not induce voltage- dependent facilitation of the class C L-type Ca channel. Pflug. Arch. 1996, 431, 771–774. [Google Scholar]
- Lummis, S.C.R.; McGonigle, I.; Ashby, J.A.; Dougherty, D.A. Two amino acid residues contribute to a cation-π binding interaction in the binding site of an insect GABA receptor. J. Neurosci. 2011, 31, 12371–12376. [Google Scholar] [CrossRef] [PubMed]
- Ashby, J.A.; McGonigle, I.V.; Price, K.L.; Cohen, N.; Comitani, F.; Dougherty, D.A.; Molteni, C.; Lummis, S.C.R. GABA binding to an insect GABA receptor: A Molecular Dynamics and Mutagenesis Study. Biophys. J. 2012, 103, 2071–2081. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-T.; Zhang, H.-G.; Rocheleau, T.A.; Ffrench-Constant, R.H.; Jackson, M.B. Cation Permeability and Cation-Anion Interactions in a Mutant GABA-Gated Chloride Channel from Drosophila. Biophys. J. 1999, 77, 691–700. [Google Scholar] [CrossRef]
- Keramidas, A.; Moorhouse, A.J.; Schofield, P.R.; Barry, P.H. Ligand-Gated Ion Channels: Mechanisms Underlying Ion Selectivity. Prog. Biophys. Mol. Biol. 2004, 86, 161–204. [Google Scholar] [CrossRef] [PubMed]
- Charnet, P.; Labarca, C.; Leonard, R.J.; Vogelaar, N.J.; Czyzyk, L.; Gouin, A.; Davidson, N.; Lester, H.A. An open-channel blocker interacts with adjacent turns of alpha- helices in the nicotinic acetylcholine receptor. Neuron 1990, 4, 87–95. [Google Scholar] [CrossRef]
- Buckingham, S.D.; Ihara, M.; Sattelle, D.B.; Matsuda, K. Mechanisms of Action, Resistance and Toxicity of Insecticides Targeting GABA Receptors. Curr. Med. Chem. 2017, 24, 2935–2945. [Google Scholar] [CrossRef]
- Cymes, G.D.; Grosman, C. Identifying the elusive link between amino acid sequence and charge selectivity in pentameric ligand-gated ion channels. Proc. Natl. Acad. Sci. USA 2016, 113, E7106–E7115. [Google Scholar] [CrossRef]
- Comitani, F.; Cohen, N.; Ashby, J.; Botten, D.; Lummis, S.C.R.; Molteni, C. Insights into the binding of GABA to the insect RDL receptor from atomistic simulations: A comparison of models. J. Comput. Aided. Mol. Des. 2014, 28, 35–48. [Google Scholar] [CrossRef][Green Version]
- Li, P.; Akk, G. The insecticide fipronil and its metabolite fipronil sulphone inhibit the rat α1β2γ2L GABA A receptor. Br. J. Pharmacol. 2008, 155, 783–794. [Google Scholar] [CrossRef]
- Price, K.L.; Lummis, S.C. An atypical residue in the pore of Varroa destructor GABA-activated RDL receptors affects picrotoxin block and thymol modulation. Insect Biochem. 2014, 55, 19–25. [Google Scholar] [CrossRef]
- Sheng, C.W.; Casida, J.E.; Durkin, K.A.; Chen, F.; Han, Z.J.; Zhao, C.Q. Fiprole insecticide resistance of Laodelphax striatellus: Electrophysiological and molecular docking characterization of A2’N RDL GABA receptors. Pest Manag. Sci. 2018, 74, 2645–2651. [Google Scholar] [CrossRef] [PubMed]
- Charreton, M.; Decourtye, A.; Henry, M.; Rodet, G.; Sandoz, J.C.; Charnet, P.; Collet, C. A Locomotor Deficit Induced by Sublethal Doses of Pyrethroid and Neonicotinoid Insecticides in the Honeybee Apis mellifera. PLoS ONE 2015, 10, e0144879. [Google Scholar] [CrossRef] [PubMed]
- Bera, A.K.; Chatav, M.; Akabas, M.H. GABAA receptor M2-M3 loop secondary structure and changes in accessibility during channel gating. J. Biol. Chem. 2002, 277, 43002–43010. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Wu, Y.; Gao, Y.; Ju, X.; Ozoe, Y. Potential of Competitive Antagonists of Insect Ionotropic γ-Aminobutyric Acid Receptors as Insecticides. J. Agric. Food Chem. 2020, 68, 4760–4768. [Google Scholar] [CrossRef]
- Dupuis, J.P.; Bazelot, M.; Barbara, G.S.; Paute, S.; Gauthier, M.; Raymond-Delpech, V. Homomeric RDL and heteromeric RDL/LCCH3 GABA receptors in the honeybee antennal lobes: Two candidates for inhibitory transmission in olfactory processing. J. Neurophysiol. 2010, 103, 458–468. [Google Scholar] [CrossRef]
- Taylor-Wells, J.; Senan, A.; Bermudez, I.; Jones, A.K. Species specific RNA A-to-I editing of mosquito RDL modulates GABA potency and influences agonistic, potentiating and antagonistic actions of ivermectin. Insect Biochem. Mol. Biol. 2018, 93, 1–11. [Google Scholar] [CrossRef]
- Ffrench-Constant, R.H.; Williamson, M.S.; Davies, T.G.; Bass, C. Ion channels as insecticide targets. J. Neurogenet. 2016, 30, 163–177. [Google Scholar] [CrossRef]
- Berenbaum, M.R.; Johnson, R.M. Xenobiotic detoxification pathways in honey bees. Curr. Opin. Insect Sci. 2015, 10, 51–58. [Google Scholar] [CrossRef]
- Gong, Y. Current knowledge of detoxi fi cation mechanisms of xenobiotic in honey bees. Ecotoxicology 2017, 26, 1–12. [Google Scholar] [CrossRef]
- Bell-Horner, C.L.; Dibas, M.; Huang, R.Q.; Drewe, J.A.; Dillon, G.H. Influence of subunit configuration on the interaction of picrotoxin-site ligands with recombinant GABA(A) receptors. Mol. Brain Res. 2000, 76, 47–55. [Google Scholar] [CrossRef]




| Am-RDL | Ap-RDL1 | Ap-RDL2 | Hs-GABA-Aα2 | Hs-GABA-Aα4 | Vd-RDL1 | Vd-RDL2 | Vd-RDL3 | |
|---|---|---|---|---|---|---|---|---|
| Am-RDL | 85 | 86 | 35 | 33 | 59 | 62 | 58 | |
| Ap-RDL1 | 88 | 32 | 29 | 51 | 53 | 52 | ||
| Ap-RDL2 | 33 | 29 | 52 | 52 | 52 | |||
| Hs-GABA-α2 | 58 | 32 | 33 | 33 | ||||
| Hs-GABA-α4 | 28 | 28 | 26 | |||||
| Vd-RDL1 | 61 | 58 | ||||||
| Vd-RDL2 | 64 | |||||||
| Vd-RDL3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bertaud, A.; Cens, T.; Mary, R.; Rousset, M.; Arel, E.; Thibaud, J.-B.; Vignes, M.; Ménard, C.; Dutertre, S.; Collet, C.; et al. Xenopus Oocytes: A Tool to Decipher Molecular Specificity of Insecticides towards Mammalian and Insect GABA—A Receptors. Membranes 2022, 12, 440. https://doi.org/10.3390/membranes12050440
Bertaud A, Cens T, Mary R, Rousset M, Arel E, Thibaud J-B, Vignes M, Ménard C, Dutertre S, Collet C, et al. Xenopus Oocytes: A Tool to Decipher Molecular Specificity of Insecticides towards Mammalian and Insect GABA—A Receptors. Membranes. 2022; 12(5):440. https://doi.org/10.3390/membranes12050440
Chicago/Turabian StyleBertaud, Anaïs, Thierry Cens, Rosanna Mary, Matthieu Rousset, Elodie Arel, Jean-Baptiste Thibaud, Michel Vignes, Claudine Ménard, Sébastien Dutertre, Claude Collet, and et al. 2022. "Xenopus Oocytes: A Tool to Decipher Molecular Specificity of Insecticides towards Mammalian and Insect GABA—A Receptors" Membranes 12, no. 5: 440. https://doi.org/10.3390/membranes12050440
APA StyleBertaud, A., Cens, T., Mary, R., Rousset, M., Arel, E., Thibaud, J.-B., Vignes, M., Ménard, C., Dutertre, S., Collet, C., & Charnet, P. (2022). Xenopus Oocytes: A Tool to Decipher Molecular Specificity of Insecticides towards Mammalian and Insect GABA—A Receptors. Membranes, 12(5), 440. https://doi.org/10.3390/membranes12050440







