Abstract
The mass production of lithium-ion batteries and lithium-rich e-products that are required for electric vehicles, energy storage devices, and cloud-connected electronics is driving an unprecedented demand for lithium resources. Current lithium production technologies, in which extraction and purification are typically achieved by hydrometallurgical routes, possess strong environmental impact but are also energy-intensive and require extensive operational capabilities. The emergence of selective membrane materials and associated electro-processes offers an avenue to reduce these energy and cost penalties and create more sustainable lithium production approaches. In this review, lithium recovery technologies are discussed considering the origin of the lithium, which can be primary sources such as minerals and brines or e-waste sources generated from recycling of batteries and other e-products. The relevance of electro-membrane processes for selective lithium recovery is discussed as well as the potential and shortfalls of current electro-membrane methods.
1. Introduction
Lithium (Li) metal is a unique element exhibiting the most negative redox potential, equal to −3.014 V compared to a standard hydrogen electrode, making it extraordinarily reactive and valuable across multiple electrochemical applications [1]. Li is also the lightest non-iron metal, with a density of 0.53 g/cm3, and has the third-highest specific heat capacity (Cp = 3.6 J g−1 K−1). These exceptional properties have supported the emergence of Li-based devices in applications where higher power densities and the long-lasting life of energy storage devices are required [2].
Lithium resources can be divided into either primary or secondary sources. Primary sources include mineral rocks [3], saline lakes [4], brines [5], sea water [6], underground water [7], and groundwater [8], while secondary sources are typically lithium obtained from recycling lithium-containing devices and components, such as batteries, capacitors [9], or general e-wastes [10]. The world’s primary reserves of lithium are estimated at over 250 billion tons [1], of which 230 billion tons are present within oceans, while the remaining amount exists as ores or continental brines. The demand for Li metal has increased exponentially over the past 20 years and was 33,300 tons in 2015. The global production of lithium in 2017 [3] primarily originated from Australia, China, Argentina, and Chile (Figure 1).
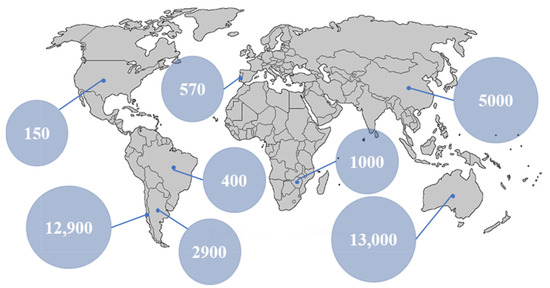
Figure 1.
World production of lithium in 2017 calculated in ton/year. Reprinted based on the open access license [11].
Driven by current demands for lithium and limited resources, spot lithium carbonate prices in China have increased by 300% and, recently, briefly exceeded USD 20,000 per ton due to an acute but temporary shortage of imported spodumene from Australia. However, this is an indicator of future long-term market sensitivity and trends. Although Li compounds are used in glass and ceramics manufacturing (30%), as a component during lubricants synthesis (8%), and in air purification (3%) and polymer production (5%), its largest application is in batteries and capacitors (39%) [5]. This use will increase exponentially in future years due to sustainability-focused policies and the increasing dominance of electric vehicles [12]. The consumption of lithium will also be stimulated by emerging applications for Li such as rocket fuel, where high energy densities and specific impulses are required for take-off, and in Li-based alloys and lithium hydride production [13,14]. Based on the current price at USD 100,000 per ton, the market for Li-metal would reach USD 500 billion by 2050 [15].
Current commercial lithium production strategies from primary sources are primarily based on continental (59%) and geothermal brines (3%), as well as ores in the form of hard rock (25%) and hectorite (7%) minerals. Over 130 minerals containing lithium have been identified and exploited at industrial scales, including silicates and phosphate-based ores [16,17]. Although spodumene, pegmatites, petalites, lepidolite, amblygonite, zinnwaldite, and eucryptite offer theoretical lithium contents between 3 and 5.53% [18], the achieved concentrations rarely exceed 0.5 to 2% (Table 1) [5,18].

Table 1.
Chemical composition with the percentage of lithium in minerals [16,17].
Lithium may also be extracted from surface and ocean waters (Table 2). Although ocean waters contain between 0.1 and 0.2 ppm Li, there is no cost-effective technology available now to extract Li at such low levels. On the order hand, the Li concentration of 10 to 20 ppm within geothermal brines is much more attractive, but challenges related to the presence of other highly concentrated metals ions, such as arsenic, mercury, or boron, render selective extraction challenging [19,20]. Salt lake brines are amongst the most concentrated naturally occurring sources of Li ions, ranging from a few hundred to thousands of ppm. However, a critical challenge in extracting lithium from this source also relates to the presence of interfering ions which contribute to water hardness, such as calcium and magnesium. The ratio of Mg2+/Li+ is typically larger than 40 and can be as high as 200 in some extreme cases.

Table 2.
The most common lithium salt pools [17,18].
Secondary sources of lithium arise from the recycling of e-waste materials, including batteries and capacitors. The amount of Li across such electronic parts dramatically varies based on brands and fabrication technologies, but these materials still typically represent a significant component. There is also an imperative to re-use spent Li-containing components rather than sending environmentally damaging material to waste given the current global focus on the Circular Economy. In 2015, at least 5600 million LIB cells were sold worldwide, and the LIB market size is forecasted to increase by another 10.6% from 2016 to 2024, reaching a market value of USD 56 billion by 2024 [21]. LIBs contain 2 to 7 wt% of Li, a concentration significantly higher than that present in natural ores or within marine sources, making extraction from spent batteries attractive as an essential secondary source of Li (Table 3).

Table 3.
The weight percentage of Li in each part of LIB material [22].
Conventional LIB materials include LiCoO2 [23], LiMn2O2 [24], LiNi0.33Mn0.33Co0.33O2, or LiFePO4 [25]. One ton of spent LIBs cathode battery waste represents approximately USD 8500 of Li and USD 7200 of Co [9]. The LIBs electrolyte, whose role is to support the rapid transportation of carrier ions across the electrodes, is typically composed of LiPF6 [26] with additives such as NaPF6 [27] or LiBF4 [28].
The previous review published in 2021 dealt with lithium recovery through green electrochemical-battery approaches [29]. The authors focused on challenges for lithium extraction from battery wastes by the application of an electrochemical battery system employed with a lithium-capturing electrode for Li recovery [30]. Another review, which was also published in 2021, dealt with challenges for lithium supply, focusing on the life cycle of lithium and its recovery following circular economy rules [29]. Moreover, Kader et al. summarized the techniques of lithium recycling from lithium-ion batteries [31]
From these reviews, it is clear that challenges in efficient and cost-effective separation are still limiting the cost-effectiveness of Li production, and advances in techniques for the selective speciation of Li from complex brines and effluents are required. This review discusses the potentials of electro-membrane processes to support mining and hydrometallurgical operations, as well as the recycling and recovery of Li from used items and devices. The application of electro-membrane processes supporting the speciation of Li will be presented and critically discussed in terms of ion selectivity, Li recovery efficiency, the theory of specific capturing Li, and techno-economical aspects. A circular Li economy will only arise from the synergistic development of intensive and integrated technologies trains. The prospects for electro-membrane processes to contribute to this technology paradigm will be discussed.
2. Benchmark Lithium Compounds Production Technologies
The current methods of producing lithium compounds vary with the origin of the feedstock, whereby Li-ions, as well as other valuable metal ions, are extracted. In the following sections, technologies are therefore divided into methods applicable to mineral rocks, brines, and lixiviate from e-waste digestion. This section will briefly benchmark existing commercial technologies to enable subsequent comparisons with electro-membrane processes.
2.1. Conventional Recovery of Li from Ores
In extractive metallurgy, Li is recovered chemically or through a combination of chemical and pyro-metallurgical processes. Two different processes, namely, roasting and calcination or chlorination and leaching, are reported to support Li recovery from ores. These processes involve calcination or roasting followed by leaching to dissolve lithium and transfer it into an aqueous phase. Following typical ore processing techniques, such as grinding, filtration of slurries, and water recovery processes, Li may be selectively produced and extracted from mineral ores by leaching processes either in acidic or alkaline aqueous solutions [5,11,17,18]. The first stage of the chemical processing on hard rock Li mineral-bearing ores these days typically involves the sulfuric acid pug roasting of the mineral ore at a temperature between 250 and 400 °C to support the decomposition of the silica mesostructure and convert the Li contained in the minerals into a water-soluble form [32]. Alkaline processes, whereby minerals are reacted with a mixture of calcium sulfate and calcium oxide/hydroxide at ~250 °C to convert the silicate into water soluble Li aluminate can also be performed to yield LiOH or Li2CO3 salts [33]. Ion exchange processes are sometimes required to support the extraction of undesired components and increase the purity of the Li product liquor [6,34,35].
During acid/sulfonation processes, alkali metal sulfates, sulfuric acid, or SO3 gas mixed with water and oxygen are employed as reagents to produce highly water-soluble Li sulfates that are less prone to precipitation compared to other Li compounds. However, drawbacks include the large volumes of reagent chemicals required and challenges in producing high-purity Li carbonates from such brines resulting from the capacity of sulfate reagents to bind to Al, Na, Mg, Fe, and K [2,36]. The sulfate roasting of lepidolite followed by water leaching has been studied widely using Na2SO4/K2SO4/CaO, Na2SO4, and FeSO4 and has yielded Li extraction extents up to 99.5% at 1000 °C [37,38,39]. The use of Na2SO4 and H2SO4 with the zinnwaldite, petalite, and montmorillonite ores has yielded Li extraction extents up to 90, 97.3, and 90%, respectively [5,37,40,41]. However, this approach normally requires sodium carbonate dosing to precipitate Li carbonates [5].
The alkaline Li extraction process is a more economical and more environmentally benign process that involves Li extraction from minerals with lime as an active leaching reagent. The roasting of Li ores in the temperature ranges 100–205 °C and 825–1050 °C will convert Li ores to LiO2, a precursor to LiOH. The lithium hydroxide produced can be further converted to LiCl or LiCO3 by a reaction with hydrochloric acid or carbon dioxide [5]. The lithium precipitates may be further upgraded while the mother liquor, such as the liquor obtained after lithium crystallization, is looped to the first stage of the process.
The chlorination of lithium concentrates takes place between 800 and 1100 °C in the presence of hydrochloric acid, sodium chloride, calcium chloride, or chlorine gas, depending on the original ore chemistry. The process is used to convert the lithium compounds into lithium chloride (LiCl), which can be solubilized in water and thus purified. As an example, an acid baking process involving roasting of β-spodumene with Cl2 gas at 1100 °C for 2.5 h resulted in almost complete extraction of Li as LiCl2 [42]. The systems utilized for lithium recovery from minerals are summarized in Table 4.

Table 4.
Comparison of leaching processes for lithium extraction from minerals [5,36,43].
2.2. Conventional Recovery of Li from Brines
The comparison the different type of conventional method of lithium extraction from brines are summarized in the Table 5.
Brines have become one of the most popular sources of Li ions since Li extraction requires fewer pre-treatments than from ores and a large variety of Li salts are available, as well as the relatively high concentration of Li in brines, as well as from the ratio of rare earths and alkaline metals to lithium ions, supporting the co-regeneration of various valuable compounds. Brines may be divided into three types, including brines generated during the evaporation processes, directly extracted from geothermal and underground sources, and aqueous liquors produced from oil/petroleum fields [6]. The traditional methods of production of Li compounds from brines include evaporation, column adsorption, and diffusion dialysis, which leads to Li-enriched solutions that are further augmented in Li by ion exchange, sequential adsorption, or solvent extraction [34].
Ion exchange (IEX) resins are amongst the most used technologies to extract Li from brines. Commercial IEX materials including MC50 (Chemie AG, Bitterfeld-Wolfen, Germany), TP207 (Bayer AG), and Y80-N Chemie AG (Chemie AG, Bitterfeld-Wolfen, Germany) have been used for the separation of Li from synthetic brines [44]. Li extraction from the Dead Sea waters using ionic liquids such as triisobutyl phosphate [45] and liquid chromatography using polyactylamide Bio-Gel P-2 and Blue Dextran 2000 were also demonstrated [6], supporting selective extraction against Mg2+ and Ca2+ ions. Hybrid ion exchangers based on inorganic adsorbents or aluminate salts were also tried to effectively capture Li ions from brines. The inorganic ion exchanger H2TiO3 was used to separate lithium from the Uyuani lake in Bolivia, where the Li-ion adsorption capacity was estimated at 32.6 mg/g (4.8 mmol/g) at a pH of 6.5 [46]. It was possible to apply the cation exchanger titanium (IV) antimonate to reduce the content of K+, Mg2+, and Ca2+.
An attractive set of technologies to generate Li cost-effectively from aqueous solutions involve membrane processes, including pressure-driven processes. Reverse osmosis (RO) and nanofiltration (NF) have been used to concentrate and separate lithium ions selectively [47]. NF90 membranes yielded 85 wt% separation of Li+ from Mg2+ salts with a relatively low desalination range of about 15 wt% of lithium. These membrane processes may be intensified towards the speciation of mixed Li and boron from geothermal water by combining membrane technologies with adsorbents [20]. Dowex XUS-43594 combined with λ-MnO2 ion exchange resins supported the selective extraction of Li and boron at 100% and 83%, respectively [20]. Membrane distillation coupled to crystallization processes has also been considered for Li recovery. Direct contact membrane distillation and osmotic membrane distillation processes achieved a degree of saturation of LiCl in an aqueous solution. Electro-membrane processes based on electrodialysis and capacitive deionization have been developed and demonstrated and will be discussed in more detail in Section 3.

Table 5.
Comparison of processes for lithium extraction from brines [48,49,50,51,52].
Table 5.
Comparison of processes for lithium extraction from brines [48,49,50,51,52].
| Process | Adsorption | Membrane-Type Technologies | Thermal Technologies |
|---|---|---|---|
| Active reagents | Ion exchange resins, sorbents such as activated carbon or spinel-type materials | Ion exchange membranes, porous and nonporous membranes, asymmetrical with active thin layer | Thermal energy from sun light |
| Time | 12–24 h | 12–24 h | >45 days |
| Temperature | 25 °C | 25 °C | Depends on the region of evaporation (25–35 °C) |
| Disadvantages | Sorption and desorption operation are required; batch operation; column package consumes a lot of resin (>0.5 kg); pretreatment is required | Fouling of membranes; stack of member to be effective; costs of membranes; required the separation and concentration nexus; pretreatment is required; required driven forces | Long-lasting process; small amount of brine rich in Li+ salts; low selective method |
| Advantages | Flexibility of application depends on the type of resin; high selective; long-lasting time of using | High selectivity; continuous operations; flexibility of application | High concentrations of Li salts are obtained |
2.3. Conventional Li Recovery from e-Waste Products and Process Liquors
Li-ion extraction from recycling of e-waste materials can be achieved through hydrometallurgical and pyro-metallurgical methods [21]. Mechanical pre-processing is required to generate individual streams of Li-rich waste.
Pyro-metallurgical methods involve high-temperature operations, where redox reactions are activated to smelt and purify valuable metals. Pyro-metallurgical methods are typically combined with hydrometallurgical methods, which involve the leaching of valuable elements from a solid matrix and their subsequent precipitation by solvent-phase separation [21].
Pyro-metallurgical processes are performed at a temperature range between 800 and 1000 °C [53]. LiCoO2 with commercially required properties was generated by pyro-metallurgical processing of crushed LIBs calcined in air at 850–950 °C for 12 h [54]. Oxygen-free roasting combined with wet magnetic separation and the regeneration of cobalt and lithium carbonates was also performed at 1000 °C, resulting in the recovery of 95.72% and 98.93%of Co and Li2CO3, respectively [55]. Vacuum metallurgy was also used for LIB waste processing, and both Li2CO3 and Mn3O4 were obtained by heating at 800 °C under vacuum conditions, yielding purities of 91.3% and 95.1%, respectively [56].
Hydrometallurgical processes involve the extraction by leaching valuable metals from the LIBs and the subsequent recovery of the dissolved metal ions, including Li from the generated liquors. This is a mature technology, and a number of optimization studies of the leaching conditions such as reagent type and dosage, leaching rate and duration time, pulp density, and temperature have been performed. Such leaching processes may be performed in various alkali or acid leaching systems under different redox conditions. Alkali leaching is typically more selective and reduces the number of purification steps required. For example, ammonia-based systems are utilized since ammonia may form stable and selective complexes with transition metal ions [57,58]. Different behavior is exhibited by manganese, where the success of the complexation reaction is strongly related to the concentration of the ammonia agent [58]. The acidic extraction systems from LIB wastes remain prevalent compared to alkaline ones, as they often offer high recovery efficiencies. However, the use of strong inorganic acids may lead to product contamination, which is difficult to remediate. The most efficient inorganic acids leaching agents are HCl [59], H2SO4 [60], and HNO3 [61], while organic acid leaching agents include citric [62], ascorbic, oxalic [63], and formic acids [64]. The choice of leaching agent has a strong influence on economic aspects of the process, as well as on environmental aspects and the production and/or reduction of by-products [61]. However, typically, hydrometallurgical technology is characterized by high recovery efficacy, low energy requirements, and high reaction rates.
Biometallurgy or bioleaching is a recently developed technology for the extraction of valuable metals from spent LIBs, whereby microbial metabolism or microbial acid production processes are used to extract the metals from effluents. Bacteria digestion will generate inorganic acid, while fungi digestion may form organic acids. A key drawback of bioleaching is the long culturing time and the susceptibility of the biological agents to contamination and poisoning. An indirect, non-contact bio-hydrometallurgy process for polymetallic waste processing was proposed, whereby biological reagents, produced by Acidithiobacillus ferrooxidans DSM 14882T and Acidithiobacillus thiooxidans DSM 14887T, were mixed with 100 mM H2SO4 into a biogenic ferric solution to achieve leaching yields of 53.2% for Co, 60.0% for Li, 48.7% for Ni, 81.8% for Mn, and 74.4% for Cu [65]. The traditional techniques of LIB leaching by sulfuric acid applied the 2 M of H2SO4 (T = 80 °C, t = 60 min). The following recovery efficiencies could be attained: 98.7% for Ni, 97.1% for Mn, 98.2% for Co, and 81.0% for Li under optimized experimental conditions [66]. The biometallurgy method compared with the traditional method obtain a lower efficiency of extraction metals from LIBs. Considering the 20 times lower concentration of extractant, the results with biological reagents are promising.
The metal recoveries from the use of pyro-metallurgy, hydrometallurgy, and bio-metallurgy for spent LIBs recycling are shown in Table 6.

Table 6.
Comparison of different processes for recycling spent LIBs [67].
3. Electro-Membrane Processes for Lithium Recovery
Electro-membrane processes, including electrodialysis (ED) and capacitive deionization (CDI), are described and discussed in terms of Li recovery and extraction efficiencies in this section. The relationships between the source materials’ intrinsic properties and their response to electrical current and voltage applications are also presented.
3.1. Lithium Extraction from Brines by Electrodialysis
Electrodialysis (ED) is a mature membrane-based separation process, developed in the 1950s, allowing for the specific ions’ speciation across ion-exchange membranes. ED was primarily applied and scaled up for the purification of industrial wastewaters, fine chemical broth deionization, as well as ultra-pure water production. The permeation of cations and anions across the respective cation and anion exchange membranes is achieved upon application of an electrical potential difference [68,69,70].
3.1.1. Principle of Electrodialysis and Materials Considerations
The ED membranes may be designed from a single type of ion exchange material to create charge- or valence-selective membranes, or they can be assembled in layers of alternating cation and anion exchange components, leading to bipolar membrane systems, used extensively in fuel cells for selective proton transfers [71]. In a typical process, flat-sheet membranes are assembled in stacks between two electrodes that are used to generate a potential difference leading to ion diffusion within the diluate and membrane materials. The general operation of electrodialysis is illustrated in Figure 2.
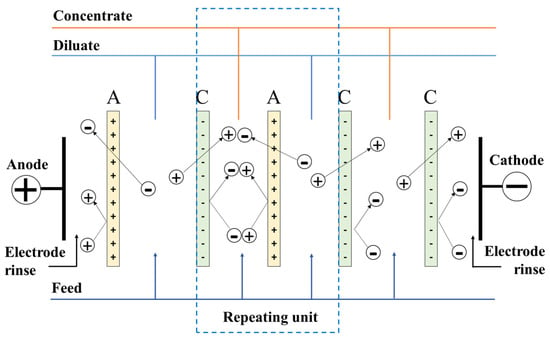
Figure 2.
Schematic diagram of principles of classic electrodialysis.
The process relies on the effect that in high-saline aqueous systems, the mass transfer is significantly affected by the complexity of ions, where the main role in the transfer is due to the steric hindrance and charge effect. The hydration radius of monovalent cations is smaller than divalent cations, which leads to the ability to attract free water molecules to the ionic center [8]. Furthermore, considering the hydration potential, which indicates how strongly an ion would its lose water shell, the influence sequence of coexisting cations was explained legitimately. The decline in water shell envelope cations is strongly dependent on the concentration and existing co-ions in aqueous solution.
Ion selectivity across ion exchange membranes is directly related to the chemistry and morphology of the micropores within the material. An electric double layer (EDL) will be formed across the surface of the pores within ion exchange membranes, which is characterized by the Debye length, a variable depending on the ionic strength of the solution and the distance between the surface and charge species. The EDL, the depth of which may vary from ~1 nm to a few tens of nano-meters, consists of the Stern and Helmholtz layers, which correspond to either polarized or diffuse layers, respectively [72]. The electrical attraction generated by the diffuse layer is weaker than that of the polarized layer, which means that counter ions can diffuse with limited resistances. These interactions are typically measured in terms of the streaming or zeta potential, which decay exponentially concerning the inverse of the distance from the surface [72]. The ion selectivity within the pores may therefore be explained by accounting for differences between the hydration free energy of the ion and the energy of interaction between the ion and the charged site within the micropores [73,74]. The anionic field strength of the binding sites is the critical factor determining the selectivity sequence of the micropores for a series of cations. A typical selectivity sequence ranges from Li+ > Na+ > K+ > Rb+ > Cs+, while at the lowest anionic field strength, the micropores, corresponding to the free volume between the macromolecular chains of the ion exchange resins, may reverse the selectivity sequence as follows: Li+ < Na+ < K+ < Rb+ < Cs+ (Figure 3a).
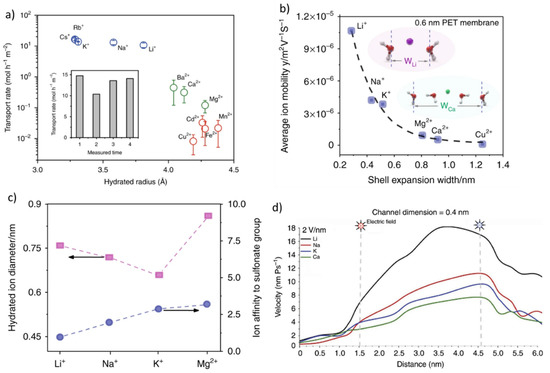
Figure 3.
Effect of nanochannel size of Li-ion selectivity (a). The ion mobility vs. hydration shell (b). The hydration ions diameter of light metal cations (c). Comparison of velocity of Li+, Na+, K+, and Ca2+ in 0.4 nm vermiculite nanochannel (d) Reprinted based on the open access license from [72,75].
The size of the free volume, the charge of the surface, and the external driving forces applied across the membrane stack will influence the rate of diffusion and the perm-selectivity of Li ions diffusion compared to other cations across membranes [72]. The dimension of the micropores and the loss of the hydration shells of the ions upon entering the channels are crucial to diffusion since these critical dimensions are typically smaller than the hydrated radii of most alkali metal ions (Figure 3b). The charge distribution and densities across the micro-channels will also dictate the rate of ion transfer and negatively charged moieties, and polymer backbones should be used for cation diffusion and to repel anions. The impact of the pendant cation exchange groups across ion exchange resins was evaluated to optimize Li+ ion perm-selectivity. Sulphonate [76,77], carboxylic [78], as well as hydroxide groups were found to offer weak interactions supporting ion hopping (Figure 3c). Ion affinity to -SO3− was found to follow the trend Mg2+ > K+ > Na+ > Li+, thus promoting the facile release of Li ions.
The application of the external electric field during ED promotes the migration and depletion of ions from the feed side across the membrane stack. The enrichment of ions across the membranes and on the permeate side results in a strong polarization. This mechanism, which creates electro-convection at the membrane surface but also water stripping, may lead to changes in ion selectivity. Such limiting considerations in designing Li-ion-selective membranes are created due to the high diffusivity and response rate in Li to variations in current densities (Figure 3d) [72].
3.1.2. Li extraction Case Studies with ED
Lithium extraction from brines by ED has been demonstrated from model solutions, industrial wastewaters, and natural lake waters. The impact of the Mg2+/Li+ ratio, feed temperature (15 to 30 °C), feed flow rate, solution residence time, and current densities across the membrane stack (5.9–13.8 A/m2) were systematically investigated. The speciation between Li+ and Mg2+ was achieved at high Mg2+/Li+ ratios. The Mg/Li mass ratio decreased as high as 21.8 times for the mixture with initial mass ratio of a Mg/Li of 400 [79]. In this research the commercial ion exchange membranes Asahi Glass Selemion CSO and ASA were applied. The influence of cations other than lithium ones affected the separation efficiency at different concentrations of Na+, Mg2+, and sulfates. The specific transfer mechanism of lithium could be related to the presence of sulfate ions. The mass transfer through the ion-exchange membrane of each ion species was determined by its dominant existing form [80].
The influence of the presence of coexisting species on the speciation of Li ions across cation exchange membranes was studied. Neosepta CIMS membranes were used for selective extraction of Li ions in mixed liquors containing other ions. The results showed sequences of coexisting cations, in the series K+ > Na+ > Ca2+ > Mg2+, directly affected separation behaviors of lithium. Interestingly, the higher the concentration of the mixed competing monovalent cations, the lower the selectivity for Li-ion was reported. The presence of sulfate and carbonate anions promoted Li over Mg fractionation. Furthermore, the presence of the coexisting anions affected the migration of Mg2+ [4].
The extraction of Li+ ions from lithium bromide solutions contaminated with Na+ ions was demonstrated for industrial liquors where lithium bromide is used as a working liquid within absorption chillers [81]. Although the feed solution contained ~13 g/L of Li ions and 1.35 g/L of Na ions, concentration factors of 88 were achieved for Li/Na. The ratio for fresh and unpolluted lithium bromide solution was 58 [81]. The separation of Mg2+ from Li+ ions was evaluated in terms of separation efficiency and economic benefit, with monovalent ion-exchange membranes. At an optimal applied ED cell voltage of 5 V and a pH range of 4–5, the Li-ions recovery reached 75.44% [82]. The modification of commercial membranes to improve lithium transport with ionic liquids (N,N,N-trimethyl-N-propylammonium–bis(trifluoromethanesulfonyl) imide (TMPA–TFSI) the Selemion CMV) was evaluated [83]. The application of selective cation exchange membranes was also evaluated. The electrodialysis voltage was 2–3 V, and the process was run for up to 15 h. After this time, 63% of the lithium was separated from the Li, Na, Mg, and K ions mixture [84].
Spent battery effluents were treated by ED to support Li-ions extraction [85]. The solution was first purified and lithium precipitated with phosphate to obtain Li3PO4. The selective separation of lithium over phosphor was achieved [85]. Li-ion recovery from spent battery effluents containing Co ions was performed with multi-stage metal-ion chelation and the ED process. Ethylenediaminetetraacetic acid (EDTA) was added to cause the selective chelation of Co ions and to increase the concentration of Li ions in the permeate stream [86]. Lithium and cobalt separations with monovalent selective ion exchange membranes such as PC-MVK were demonstrated. The value of the applied potential did not influence significantly the separation efficiency: the rise in voltage from 5 V to 15 V turned the separation factor from 98.6 to 99.4% [87]. The cobalt ion concentration in the feed solution affected the selectivity of the monovalent ion exchange membrane. Some reports on the use of electrodialysis for lithium recovery are summarized in Table 7.

Table 7.
Electrodialysis process for lithium separation from aqueous solutions.
3.2. Capacitive Deionization (CDI)
Capacitive Deionization is an electro-adsorption technique developed in the late 1970s for the removal of ions from aqueous solutions by electrosorption on porous material [90,91]. CDI has been primarily applied to seawater and brackish water desalination, sewage remediation, as well as in the softening of drinking water [92,93].
3.2.1. Operation of CDI Systems
Electro-active adsorbent materials, such as those used in CDI, mostly involve physisorption at the solid–liquid interface to support ultra-selective extraction of resources [90,92,94,95,96]. Typical CDI configurations are shown in Figure 4.
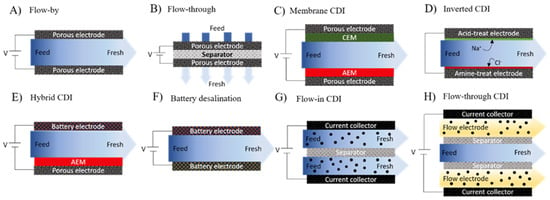
Figure 4.
Cell composition of CDI family cells.
The most popular material for CDI electrodes is activated carbon (AC) due to its low cost, high electrical conductivity, and large specific surface area. However, AC does not exhibit any selectivity toward ions due to the absence of selective sites (Figure 5a). To generate Li+ ion selectivity, the surface of activated carbon could be modified by selective moieties such as α-MnO2 [96].
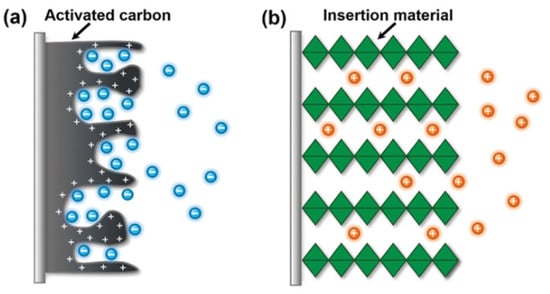
Figure 5.
Two type of ions accumulation in (a) activated carbon according with EDLs mechanism and (b) intercalation and de-intercalation ions according with Faradaic reactions. Reprinted based on the open access license from [95].
The ion insertion reaction across AC is due to the diffusion into interstitial sites of the electrode material through a Faradaic charge-transfer process (Figure 5b). The MnO2 materials can store lithium ions via two types of electrochemical processes, namely, surface-dependent Faradaic reaction, or pseudo-capacitance, and insertion into the bulk material. Highly crystallized MnO2 materials have 1D-, 2D-, or 3D-type tunnels built from MnO6 octa-hedric assemblies that support the intercalation. Their spacing may be controlled by doping such metal as Ti or Fe [97,98]. Electrochemical lithium recovery was introduced to extract lithium from Li+ ions from geothermal or industrial brines. The method requires lithium-selective materials to recover lithium, such as LiFePO4/FePO4, MnO2, and lithium manganese oxides [99].
The use of nickel hexacyanoferrate (KNiFe(CN)6) as a Li+ exclusion electrode material [100] was considered since nickel hexacyanoferrate has a higher affinity toward such ions as Na+ or K+ rather than for Li+. With this strategy, seawater can be used as a recovery solution and reduces the consumption of freshwater. By optimizing the CDI process variables, lithium enriched streams were obtained, and a lithium recovery of 73% was obtained. The process was characterized by an extremely high salt adsorption capacity of 800 mg/g and total energy consumption of 0.183 W h/g of adsorbed salt [7]. The development of lithium iron manganese oxide electrodes as selective materials to facilitate Li+ release [101] was evaluated, and it obtained an over 70% Li recovery. The ratio Na:K:Li changed from 227:1.1:1 in feed to 2.9:0:1 after one cycle of separation [101].
3.2.2. Performance/Materials Relationships for Li Recovery in CDI Systems
In a classic CDI system, the cell is composed of a pair of porous membrane electrodes sandwiched between a separator that facilitates the flow of liquids and prevents electrode physical contact of electrodes [102,103]. A typical CDI system is composed of a porous carbon electrode and a current collector. The surface of the CDI electrode may be further functionalized with specific chemical moieties or polymers to enhance ion selectivity [83,84]. The addition of ion-exchange species on porous electrodes enables selective ion capturing and prevents re-adsorption during the discharge of the electrode [80]. The nature of the functional groups located on the CDI scaffold affects the electrostatic interactions with ions [85,86], reduces energy requirements [87], and improves the process stability [85].
Coating of carbon electrodes with LiMn2O4 was performed to support Li recovery from lithium hydroxide solutions. The desorbed lithium ions from the modified MCDI system were found to be 8.7 mg/g at a constant voltage of 3.5 V, and this was lower (by approximately 45%) than the desorption for the conventional process with acidic solution [104]. A cathode composed only of LiMn2O4 was also developed [105]. The maximum salt adsorption capacity (SAC) was estimated at 24 mg of lithium per 1 g of electrode material. Moreover, this process did not require the use of the acidic solution in the desorption process [105].
Selective lithium recovery from multi-component aqueous solutions (Li+, Na+, K+, Ca2+, and Mg2+) reached 0.22 μg/g (with applied 1 V constant voltage electric mode) and was 7 times higher than that from a control physio-sorption process running (without application external electrical field) in similar experimental conditions during the CDI process. During application 1 V, the recovery amount of Li+ reached 350 µmol/gadsorbent. When the electrical field was not applied, the recovery reached only 50 µmol/gadsorbent. The energy required for the recovery was estimated to be 23.3 W h/g of lithium. Moreover, manganese dissolution was not observed during five consecutive recovery cycles supporting the scalability and reproducibility of the process [106]. Monovalent selective cation exchange membranes (Neosepta CIMS, Astom Corporation, Japan) were tested for various rates of lithium over magnesium ions in feed solutions. The maximum performance was found to be 38.4% of recovered Li with an energy consumption 0.36 W h/g of lithium [107].
A hybrid capacitive deionization (HCDI) process has been performed with lithium titanium manganese oxide as the cathode material. The anode material was modified by adding polypyrrole (PPy) to increase the conductivity of the material. An electro-sorption capacity for LiCl of 36.9 mg/g was achieved while the corresponding capacities for NaCl and KCl were 18.09 mg/g and 9.07 mg/g, respectively [108]. Modified anion exchange membrane (AEM) was produced by chemical grafting of poly(vinyl chloride) (PVC) with aliphatic amines. The extraction of 40 mg/g of LiCl was obtained in comparison to 10 mg/g for NaCl. A recovery of about 50% of lithium was noted [94,109]. Poly(vinylidene fluoride) materials were evaluated as a supporting polymer for the AEM preparation. The salt adsorption capacity of the optimized materials was estimated at 30 mg/g with 0.9 current efficiencies and 96% of desorption efficiency [110].
The active cathode material is the next important element of the CDI system. Lithium–manganese–titanium oxide (LMTO) with varying concentrations of titanium dioxide (TiO2) has been tested as a cathode [98]. The best-performing material, which contained 5% of TiO2, had a sorption capacity of 36 mg/g, and the uptakes of KCl and NaCl were 16 and 11 mg/g, respectively. Additionally, this adsorbent needed two times less energy for the recovery of lithium chloride than other monovalent salts [98]. That adsorbent was used for selective recovery of Li from geothermal waters of the Western Carpathian Mountains region [7]. Lithium recovery of 73% was achieved with an extremely high salt adsorption capacity of 800 mg/g and total energy consumption of 0.183 W h/g [7]. Lithium–iron–manganese adsorbents, with varied ratios of Li/Mn and Li/Fe, were tested for a similar feed [101]. The adsorbents with the molar ratios of Li/Mn and Li/Fe of 1.5:1 showed the best salt adsorption capacity for LiCl. Moreover, 32 mg/g of lithium, 16 mg/g of sodium, and 0 mg/g of potassium was found. The use of a modified electrical protocol, with double stage of desorption, as found to be a good method for lithium recovery from solution. The recovery reached the efficiency of 76% and reduced the Na:K:Li-ions ratio from 227:1.1:1 at the feed to 2.9:0:1.
The CDI process with flowing electrodes (FCDI) was investigated with adsorbing materials developed from reduced graphene oxide and mixed metal oxides and activated carbon. The suspension of fine particles of reduced graphene oxide formed the cathode, and the suspension of the activated carbon formed the anode [106]. A lithium extraction efficiency of 13.684 mg/g was obtained, and the energy consumption per lithium was as small as 0.22 W h/g of Li. The overall process led to 93% of lithium-ion recovery from the model brines, which indicated that the investigated materials could be promising in the recovery of lithium from natural and battery leachate solutions.
A summary of CDI processes for lithium recovery is presented in Table 8.

Table 8.
Comparison of CDI techniques for lithium removal from brines.
3.3. Hybrid Membrane Systems Involving Electro-Membrane Processes
Greater lithium-ion recoveries from any feed source may be achieved through a combination of processes into the treatment trains. Such approaches may not only support a more cost-effective Li extraction but also support higher product purity, lower energy consumption, and safer operation resulting in the more sustainable technologies. The comparison of described methods is presented in Table 9.

Table 9.
Comparison of the hybrid process of lithium extraction.
3.3.1. Electrodialysis (ED)–Reverse Osmosis (RO)
Integration of reverse osmosis and electrodialysis was used for lithium recovery from wastewater [113]. The RO concentrate was used as feed for the ED process. It was noted energy reduction from 26.67 to 7.81 kW h/m3 was achieved. An obtained enrichment concentration factor of 12.32 showed the feasibility for the use of this approach to produce high-volume concentrate.
3.3.2. Ion Exchange Adsorption–Ultrafiltration (UF)
A process coupling ion-exchange adsorption and UF was developed to support Li recovery from geothermal waters. λ-MnO2 was produced from spinel-type lithium manganese dioxide, grounded to fine particles, and used in a concentration of 1.5 g adsorbent/L. The authors identified advantages of the use of ion exchange–UF hybrid for the separation process of lithium from geothermal water [20,114].
3.3.3. Adsorptive Ion Exchange Membranes
Another approach for selective Li-ion extraction is to combine mass transfer through ion exchange membranes and adsorption within an adsorptive lithium-selective membrane [115]. This type of material enables the separation of Li ions from brines at enrichments concentration factors up to 62,000 compared to less than 100 for other metal ions. This type of membrane adsorbent may concentrate Li-ions efficiently from seawater even though the native Li-ions concentration in such effluents is very small in comparison to Na+, K+, Mg2+, or Ca2+ ions. Most of the metal ions adsorbed on the membrane were desorbed to the solution by the treatment with a 0.75 M HCl solution. The desorbed fractions contained 95%, 95%, 93%, and 93% of Na+, K+, Mg2+, and Ca2+ ions, respectively [115].
3.3.4. Membrane Distillation Crystallization
The process employed a membrane distillation (MD) and crystallization process is called membrane distillation crystallization (MDC). Compared with the traditional crystallization process, the MDC displays rapid crystallization and well-controlled nucleation kinetics. The MDC was investigated to recover salt crystals from a single-salt LiCl solution. The required concentration of precipitate the LiCl should be over 14 M. The MDC reached only 10 M. The required concentration level is possible by applying the vacuum membrane distillation [116].
3.3.5. Leaching–Flotation–Precipitation Process
The stepwise leaching–flotation–precipitation process was adopted to separate the Li/Fe/Mn from batteries [117]. First, the cathode material was leached according to the acid leaching procedure. Then, the Fe3+ cations are selectively floated and recovered as a FeCl3 in the flotation step. Finally, the Mn2+/Mn3+ and Li+ cations are precipitated and separated as MnO2/Mn2O3 and Li3PO4 using saturated KMnO4 solution and Na3PO4, respectively. As a result, the total recovery of Li, Fe, and Mn is ~81%, ~85%, and ~81%, respectively. Hence, that stepwise process could be considered an alternative way to separate and recover metals from spent Li-ion batteries effectively.
3.3.6. Membrane Electrolysis
The membrane electrolysis was investigated to crystallize lithium carbonate from lithium-rich brines. The three-compartment reactor was applied. The brines were introduced in the middle compartment, separated from the anolyte and catholyte compartment outside. When a current is applied, anions and cations selectively migrate into the anionic and cathodic compartments, respectively. Water reduction increases the pH of the catholyte, which is recirculated in a crystallizer where CO2 is bubbled and converted to carbonate, precipitating Li2CO3 with a purity of at least 93.8 wt%. The method allows recovering as much as 90% of the lithium-containing solution volume as low salinity water, with up to 99.7% less total dissolved solids than the processed brine, in marked contrast with current practice [118].
3.3.7. Membrane with Incorporated Metal–Organic Frameworks (MOF-on-MOF)
The distinguished research was conducted on adapting the biological ion channels features to the alkali metal ions recovery (Na+, K+, and Li+). The main concept was the fabrication of monovalent ion-selective membranes with asymmetrical sub-nanometer pores dedicated to transportation lithium cations. The ionic current measurements exhibit an unprecedented ionic current rectification ratio of above 100 with exceptionally high selectivity ratios of 84 and 80 for K+/Li+ and Na+/ Li+, respectively (1.14 Li+ mol m−2 h−1) [119].
3.3.8. Pervaporation
The modified by incorporating graphene oxide (GO) into polypropylene hollow fiber membranes (Accurel PP S6/2, from Membrana GmbH, Germany). With a high initial feed concentration (>200 g/L of salt) the GO composite pervaporation membrane increased lithium concentration from 0.3 to 1.27 g/L (73% feed volume reduction) [120].
4. Economical Aspects of Lithium Recovery with Electro-Driven Membrane Processes
Techno-economic analysis of lithium production based on three main sources of lithium: Namely, minerals, brines, and e-waste, is also discussed in the following sections. Relationships between the operating conditions and the required performance are developed to shed light on the energy requirements for each source of lithium ions and the results for traditional hydrometallurgical technologies and electro-driven membrane processes or hybrid solutions are compared.
4.1. Lithium Recovery from Minerals
Traditionally, for the extraction of lithium salt to produce lithium carbonate from minerals the leaching acid, alkaline, chlorination, and a combination of these techniques are applied. The cost factors include mine and concentrator development and construction. The distribution of cost among the individual components is shown in Figure 6a.
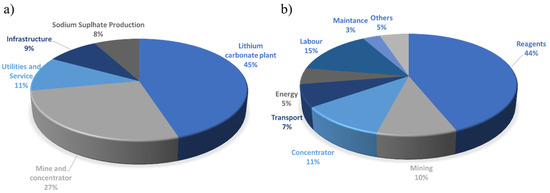
Figure 6.
The distribution of shares of the individual components for lithium recovery from minerals (a) and brines (b) [121].
The pie chart shows that 45% of lithium recovery costs are related to the lithium carbonate plate cost. The second most expensive component is the mine and concentrator. The dominating costs within the lithium carbonate plant are reagents, labor, and energy costs [121].
4.2. Lithium Recovery from Brines
For lithium recovery from brines, the evaporation methods were chosen as the main technology at the industrial scale. Within this technique, the evaporation ponds costs play an important role. The second place takes lithium carbonate plate with its utilities and infrastructure (Figure 6b) [121].
From the operational cost, the reagents’ costs seem to be the most important part. Among them, sodium carbonate (28%), calcium oxide (12%), sodium hydroxide (7%), carbon dioxide (4%), and hydrochloric acid (1%) should be mentioned [121].
4.3. Lithium Recovery from e-Waste Brines
The lower cost of process utilization battery spent solutions is expected to be the main driver for recycling end-of-life LIBs. From a typical economic analysis of LIB recycling delivery, the total cost of recycling 3974 tons of LIBs was USD 22,824,666. The operation cost was USD 8,941,500 (2250 USD/ton), transportation was USD12,078,970 (USD 3039.5/ton), and material handling was USD 1,804,196 (454 USD/ton) [34]. Environmental and social aspects could also contribute to the need for more recycling of end-of-life batteries. The key areas for the reduction in cost are related to energy and greenhouse gas emissions. Figure 7 summarizes some values from several studies comparing processes involving leaching acid, pyrometallurgy, hydrometallurgy, and electro-membrane processes [122,123,124].
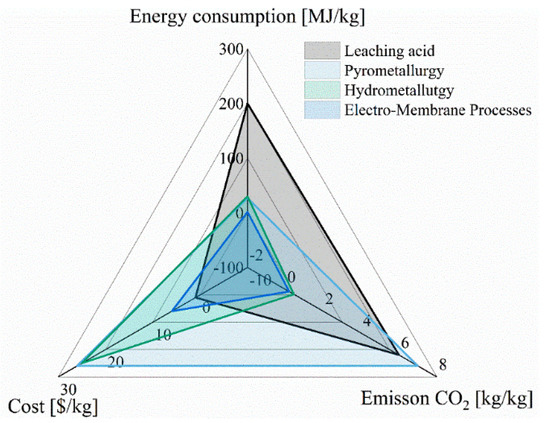
Figure 7.
Economic analysis for methods of recycling of LIBs.
Based on the data from these studies, the most efficient method, with the lowest carbon footprint, is offered by the electro-membrane processes. On the other hand, the most expensive technologies for lithium recovery are pyrometallurgy and hydrometallurgy. This is due to the high energy requirements for heating and extraction.
The comparison of all of the above-mentioned methods is difficult due to the usability of various operations, materials, and energy requirements.
5. Summary and Prospects
Lithium’s unique properties make it a critical metal for a wide range of applications. The demand for Li compounds in the commodity market over the next decade and beyond is expected to increase dramatically according to the rising use of portable energy storage devices. At this stage, there are already some industrial-scale or laboratory-established technologies for recovering lithium from minerals, brines, and lithium-ion batteries. The process of lithium recovery from minerals and clays is expensive at the both mining costs and energy consumption. The main source of Li from minerals is spodumene. Spodumene has a high energy requirement to convert α-spodumene into β-spodumene, which is more readily leachable. The extraction of lithium from brines and seawater reveals that a very long duration is necessary for evaporation and concentration. That process has a serious drawback and is seriously affected by climate. The recycling process of the LIBs mainly consists of dismantling for the removal of plastic and iron scraps, the separation of cathode and anode materials, the leaching of the electrode, the removal of unwanted metal impurities in the leachate, separation, and the recovery of metals from the solutions by solvent extraction, ion-exchange, and precipitation. Electro-membrane processes could be applied for lithium removal from brines and spent batteries. For brines and groundwater, capacitive deionization can be efficiently applied. By using CDI and HCDI, it is possible to reduce energy consumption, as well as intensify removal operations with high selectivity. For releasing lithium from e-wastes, the ED process can be used. By applying ED, it is possible to reduce recycling costs and energy consumption. The additional benefit of ED over other technologies is the extraction of is the extraction of lithium in higher-grades. However, despite the promise of electro-membrane processes for lithium recovery, there is a need to continue research on the development of sustainable technologies that can effectively recover all valuable metals from both primary and secondary resources, simplify the recycling process, and make the recycling costs lower.
Author Contributions
A.S.: Conceptualization, methodology, validation, investigation, resources, writing—original draft, writing—review and editing, visualisation, funding acquisition; M.B.: writing—original draft, writing—review and editing; A.R.; writing—original draft, writing—review and editing; W.K.: writing—original draft, writing—review and editing; A.N.N.: writing—original draft; writing—review and editing; L.F.D.: supervision, project administration, writing—original draft, writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Foundation for Polish Science (START) grant number 075.2021 and by Khalifa University by grant number RC2-2019-007.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
A.S. would like to thank the Department of Process Engineering and Technology of Polymeric and Carbon Materials, Wroclaw University of Science and Technology, for the financial support from the ministerial subsidy. A.S. is supported by the Polish Ministry of Education and Science under the program for outstanding young scientists. A.S. is supported by the Foundation for Polish Science (START) under project number 075.2021. L.F.D. acknowledges the support from Khalifa University through project RC2-2019-007.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zhang, X.; Han, A.; Yang, Y. Review on the production of high-purity lithium metal. J. Mater. Chem. A 2020, 8, 22455–22466. [Google Scholar] [CrossRef]
- Choubey, P.K.; Kim, M.S.; Srivastava, R.R.; Lee, J.C.; Lee, J.Y. Advance review on the exploitation of the prominent energy-storage element: Lithium. Part I: From mineral and brine resources. Miner. Eng. 2016, 89, 119–137. [Google Scholar] [CrossRef]
- Brandt, F.; Haus, R. New concepts for lithium minerals processing. Miner. Eng. 2010, 23, 659–661. [Google Scholar] [CrossRef]
- Ji, P.Y.; Ji, Z.Y.; Chen, Q.B.; Liu, J.; Zhao, Y.Y.; Wang, S.Z.; Li, F.; Yuan, J.S. Effect of coexisting ions on recovering lithium from high Mg2+/Li+ ratio brines by selective-electrodialysis. Sep. Purif. Technol. 2018, 207, 1–11. [Google Scholar] [CrossRef]
- Meshram, P.; Pandey, B.D.; Mankhand, T.R. Extraction of lithium from primary and secondary sources by pre-treatment, leaching and separation: A comprehensive review. Hydrometallurgy 2014, 150, 192–208. [Google Scholar] [CrossRef]
- Swain, B. Recovery and recycling of lithium: A review. Sep. Purif. Technol. 2017, 172, 388–403. [Google Scholar] [CrossRef]
- Siekierka, A.; Tomaszewska, B.; Bryjak, M. Lithium capturing from geothermal water by hybrid capacitive deionization. Desalination 2018, 436, 8–14. [Google Scholar] [CrossRef]
- Chen, Q.B.; Ji, Z.Y.; Liu, J.; Zhao, Y.Y.; Wang, S.Z.; Yuan, J.S. Development of recovering lithium from brines by selective-electrodialysis: Effect of coexisting cations on the migration of lithium. J. Memb. Sci. 2018, 548, 408–420. [Google Scholar] [CrossRef]
- Georgi-Maschler, T.; Friedrich, B.; Weyhe, R.; Heegn, H.; Rutz, M. Development of a recycling process for Li-ion batteries. J. Power Sources 2012, 207, 173–182. [Google Scholar] [CrossRef]
- Zeng, X.; Li, J. Spent rechargeable lithium batteries in e-waste: Composition and its implications. Front. Environ. Sci. Eng. 2014, 8, 792–796. [Google Scholar] [CrossRef]
- Nguyen, T.; Lee, M. A Review on the Separation of Lithium Ion from Leach Liquors of Primary and Secondary Resources by Solvent Extraction with Commercial Extractants. Processes 2018, 6, 55. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.; Liang, J.; Yang, Z.; Li, G. A review of lithium-ion battery for electric vehicle applications and beyond. Energy Procedia 2019, 158, 4363–4368. [Google Scholar] [CrossRef]
- Schiemann, M.; Bergthorson, J.; Fischer, P.; Scherer, V.; Taroata, D.; Schmid, G. A review on lithium combustion. Appl. Energy 2016, 162, 948–965. [Google Scholar] [CrossRef]
- Xu, W.; Birbilis, N.; Sha, G.; Wang, Y.; Daniels, J.E.; Xiao, Y.; Ferry, M. A high-specific-strength and corrosion-resistant magnesium alloy. Nat. Mater. 2015, 14, 1229–1235. [Google Scholar] [CrossRef]
- Schmuch, R.; Wagner, R.; Hörpel, G.; Placke, T.; Winter, M. Performance and cost of materials for lithium-based rechargeable automotive batteries. Nat. Energy 2018, 3, 267–278. [Google Scholar] [CrossRef]
- National Minerals Information Center. Lithium (Data in Metric Tons of Lithium Content Unless Otherwise Noted); National Minerals Information Center: Reston, WV, USA, 2019.
- Vikström, H.; Davidsson, S.; Höök, M. Lithium availability and future production outlooks. Appl. Energy 2013, 110, 252–266. [Google Scholar] [CrossRef] [Green Version]
- Mohr, S.H.; Mudd, G.M.; Giurco, D. Lithium Resources and Production: Critical Assessment and Global Projections. Minerals 2012, 2, 65–84. [Google Scholar] [CrossRef]
- Bunani, S.; Kabay, N.; Bunani, S.; Arda, M.; Yoshizuka, K.; Nishihama, S.; Bunani, S. Effect of process conditions on recovery of lithium and boron from water using bipolar membrane electrodialysis (BMED). Desalination 2017, 416, 10–15. [Google Scholar] [CrossRef]
- Recepoğlu, Y.K.; Kabay, N.; Yılmaz-Ipek, İ.; Arda, M.; Yüksel, M.; Yoshizuka, K.; Nishihama, S. Elimination of boron and lithium coexisting in geothermal water by adsorption-membrane filtration hybrid process. Sep. Sci. Technol. 2018, 53, 856–862. [Google Scholar] [CrossRef]
- Velázquez-Martínez, O.; Valio, J.; Santasalo-Aarnio, A.; Reuter, M.; Serna-Guerrero, R. A Critical Review of Lithium-Ion Battery Recycling Processes from a Circular Economy Perspective. Batteries 2019, 5, 68. [Google Scholar] [CrossRef] [Green Version]
- Qadir, R.; Gulshan, F. Reclamation of Lithium Cobalt Oxide from Waste Lithium Ion Batteries to Be Used as Recycled Active Cathode Materials. Mater. Sci. Appl. 2018, 09, 142–154. [Google Scholar] [CrossRef] [Green Version]
- Lin, S.; Zhao, J. Functional Electrolyte of Fluorinated Ether and Ester for Stabilizing Both 4.5 v LiCoO2 Cathode and Lithium Metal Anode. ACS Appl. Mater. Interfaces 2020, 12, 8316–8323. [Google Scholar] [CrossRef]
- Dominko, R.; Gaberscek, M.; Drofenik, J.; Bele, M.; Pejovnik, S.; Jamnik, J. The role of carbon black distribution in cathodes for Li ion batteries. J. Power Sources 2003, 119–121, 770–773. [Google Scholar] [CrossRef]
- Naumann, M.; Spingler, F.; Jossen, A. Analysis and modeling of cycle aging of a commercial LiFePO4/graphite cell. J. Power Sources 2020, 451, 227666. [Google Scholar] [CrossRef]
- Yamada, Y. Concentrated Battery Electrolytes: Developing New Functions by Manipulating the Coordination States. Bull. Chem. Soc. Jpn. 2020, 93, 109–118. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Y.; Liu, F.; Cheng, F.; Chen, J. Improving metallic lithium anode with NaPF6 additive in LiPF6-carbonate electrolyte. J. Energy Chem. 2020, 42, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Sun, Y.; Zhou, Y.; Hai, C.; Hu, S.; Zeng, J.; Shen, Y.; Dong, S.; Qi, G.; Li, F. Investigation of the synergetic effects of LiBF4 and LiODFB as wide-temperature electrolyte salts in lithium-ion batteries. Ionics 2018, 24, 2995–3004. [Google Scholar] [CrossRef]
- Alessia, A.; Alessandro, B.; Maria, V.G.; Carlos, V.A.; Francesca, B. Challenges for sustainable lithium supply: A critical review. J. Clean. Prod. 2021, 300, 126954. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, W.; Xu, R.; Wang, L.; Tang, H. Lithium extraction from water lithium resources through green electrochemical-battery approaches: A comprehensive review. J. Clean. Prod. 2021, 285, 124905. [Google Scholar] [CrossRef]
- Kader, Z.A.; Marshall, A.; Kennedy, J. A review on sustainable recycling technologies for lithium-ion batteries. Emergent Mater. 2021, 4, 725–735. [Google Scholar] [CrossRef]
- Jandová, J.; Vu, H.N.; Belková, T.; Dvořák, P.; Kondás, J. Obtaining Li2CO3 from Zinnwaldite Wastes. Ceram. Silikáty 2009, 53, 108–112. [Google Scholar]
- Guo, H.; Kuang, G.; Yang, J.X.; Hu, S. Fundamental Research on a New Process to Remove Al3+ as Potassium Alum during Lithium Extraction from Lepidolite. Metall. Mater. Trans. B Process. Metall. Mater. Process. Sci. 2016, 47, 3557–3564. [Google Scholar] [CrossRef]
- Choubey, P.K.; Chung, K.S.; Kim, M.S.; Lee, J.C.; Srivastava, R.R. Advance review on the exploitation of the prominent energy-storage element Lithium. Part II: From sea water and spent lithium ion batteries (LIBs). Miner. Eng. 2017, 110, 104–121. [Google Scholar] [CrossRef]
- Talens Peiró, L.; Villalba Méndez, G.; Ayres, R.U. Lithium: Sources, production, uses, and recovery outlook. JOM 2013, 65, 986–996. [Google Scholar] [CrossRef] [Green Version]
- Fosu, A.Y.; Kanari, N.; Vaughan, J.; Chagnes, A. Literature Review and Thermodynamic Modelling of Roasting Processes for Lithium Extraction from Spodumene. Metals 2020, 10, 1312. [Google Scholar] [CrossRef]
- Siame, E.; Pascoe, R.D. Extraction of lithium from micaceous waste from china clay production. Miner. Eng. 2011, 24, 1595–1602. [Google Scholar] [CrossRef]
- Luong, V.T.; Kang, D.J.; An, J.W.; Dao, D.A.; Kim, M.J.; Tran, T. Iron sulphate roasting for extraction of lithium from lepidolite. Hydrometallurgy 2014, 141, 8–16. [Google Scholar] [CrossRef]
- Luong, V.T.; Kang, D.J.; An, J.W.; Kim, M.J.; Tran, T. Factors affecting the extraction of lithium from lepidolite. Hydrometallurgy 2013, 134–135, 54–61. [Google Scholar] [CrossRef]
- Amer, A.M. The hydrometallurgical extraction of lithium from Egyptian montmorillonite-type clay. JOM 2008, 60, 55–57. [Google Scholar] [CrossRef]
- Sitando, O.; Crouse, P.L. Processing of a Zimbabwean petalite to obtain lithium carbonate. Int. J. Miner. Process. 2012, 102–103, 45–50. [Google Scholar] [CrossRef] [Green Version]
- Barbosa, L.I.; Valente, G.; Orosco, R.P.; González, J.A. Lithium extraction from β-spodumene through chlorination with chlorine gas. Miner. Eng. 2014, 56, 29–34. [Google Scholar] [CrossRef]
- Salakjani, N.K.; Singh, P.; Nikoloski, A.N. Production of Lithium—A Literature Review Part 1: Pretreatment of Spodumene. Miner. Process. Extr. Metall. Rev. 2019, 41, 335–348. [Google Scholar] [CrossRef]
- Bukowsky, H.; Uhlemann, E.; Steinborn, D. The recovery of pure lithium chloride from “brines” containing higher contents of calcium chloride and magnesium chloride. Hydrometallurgy 1991, 27, 317–325. [Google Scholar] [CrossRef]
- Zhou, Z.; Qin, W.; Fei, W. Extraction equilibria of lithium with tributyl phosphate in three diluents. J. Chem. Eng. Data 2011, 56, 3518–3522. [Google Scholar] [CrossRef]
- Chitrakar, R.; Makita, Y.; Ooi, K.; Sonoda, A. Lithium recovery from salt lake brine by H2TiO3. Dalton Trans. 2014, 43, 8933–8939. [Google Scholar] [CrossRef]
- Somrani, A.; Hamzaoui, A.H.; Pontie, M. Study on lithium separation from salt lake brines by nanofiltration (NF) and low pressure reverse osmosis (LPRO). Desalination 2013, 317, 184–192. [Google Scholar] [CrossRef]
- Warren, P. Techno-Economic Analysis of Lithium Extraction from Geothermal Brines. 2019. Available online: www.nrel.gov/publications (accessed on 11 March 2022).
- Lalasari, L.H.; Andriyah, L.; Arini, T.; Sulistiyono, E.; Prasetyo, A.B.; Firdiyono, F.; Natasha, N.C. Lithium extraction from brine water Tirtasanita Bogor, Indonesia by evaporation method. J. Phys. Conf. Ser. 2020, 1450, 012013. [Google Scholar] [CrossRef]
- Nishihama, S.; Onishi, K.; Yoshizuka, K. Selective recovery process of lithium from seawater using integrated ion exchange methods. Solvent Extr. Ion Exch. 2011, 29, 421–431. [Google Scholar] [CrossRef]
- Arroyo, F.; Morillo, J.; Usero, J.; Rosado, D.; El Bakouri, H. Lithium recovery from desalination brines using specific ion-exchange resins. Desalination 2019, 468, 114073. [Google Scholar] [CrossRef]
- Xu, S.; Song, J.; Bi, Q.; Chen, Q.; Zhang, W.M.; Qian, Z.; Zhang, L.; Xu, S.; Tang, N.; He, T. Extraction of lithium from Chinese salt-lake brines by membranes: Design and practice. J. Memb. Sci. 2021, 635, 119441. [Google Scholar] [CrossRef]
- Siqi, Z.; Guangming, L.; Wenzhi, H.; Juwen, H.; Haochen, Z. Recovery methods and regulation status of waste lithium-ion batteries in China: A mini review. Waste Manag. Res. 2019, 37, 1142–1152. [Google Scholar] [CrossRef] [PubMed]
- Nie, H.; Xu, L.; Song, D.; Song, J.; Shi, X.; Wang, X.; Zhang, L.; Yuan, Z. LiCoO2: Recycling from spent batteries and regeneration with solid state synthesis. Green Chem. 2015, 17, 1276–1280. [Google Scholar] [CrossRef]
- Li, J.; Wang, G.; Xu, Z. Environmentally-friendly oxygen-free roasting/wet magnetic separation technology for in situ recycling cobalt, lithium carbonate and graphite from spent LiCoO2/graphite lithium batteries. J. Hazard. Mater. 2016, 302, 97–104. [Google Scholar] [CrossRef]
- Xiao, J.; Li, J.; Xu, Z. Recycling metals from lithium ion battery by mechanical separation and vacuum metallurgy. J. Hazard. Mater. 2017, 338, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Gao, W.; Zhang, X.; He, M.; Lin, X.; Cao, H.; Zhang, Y.; Sun, Z. Spent lithium-ion battery recycling—Reductive ammonia leaching of metals from cathode scrap by sodium sulphite. Waste Manag. 2017, 60, 680–688. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, N.; Hu, F.; Ye, L.; Xi, Y.; Yang, S. Thermal treatment and ammoniacal leaching for the recovery of valuable metals from spent lithium-ion batteries. Waste Manag. 2018, 75, 469–476. [Google Scholar] [CrossRef]
- Barik, S.P.; Prabaharan, G.; Kumar, L. Leaching and separation of Co and Mn from electrode materials of spent lithium-ion batteries using hydrochloric acid: Laboratory and pilot scale study. J. Clean. Prod. 2017, 147, 37–43. [Google Scholar] [CrossRef]
- He, L.P.; Sun, S.Y.; Song, X.F.; Yu, J.G. Leaching process for recovering valuable metals from the LiNi1/3Co1/3Mn1/3O2 cathode of lithium-ion batteries. Waste Manag. 2017, 64, 171–181. [Google Scholar] [CrossRef]
- Chen, M.; Ma, X.; Chen, B.; Arsenault, R.; Karlson, P.; Simon, N.; Wang, Y. Recycling End-of-Life Electric Vehicle Lithium-Ion Batteries. Joule 2019, 3, 2622–2646. [Google Scholar] [CrossRef]
- Li, L.; Ge, J.; Wu, F.; Chen, R.; Chen, S.; Wu, B. Recovery of cobalt and lithium from spent lithium ion batteries using organic citric acid as leachant. J. Hazard. Mater. 2010, 176, 288–293. [Google Scholar] [CrossRef]
- Zeng, X.; Li, J.; Shen, B. Novel approach to recover cobalt and lithium from spent lithium-ion battery using oxalic acid. J. Hazard. Mater. 2015, 295, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Zhang, X.; Zheng, X.; Lin, X.; Cao, H.; Zhang, Y.; Sun, Z. Lithium Carbonate Recovery from Cathode Scrap of Spent Lithium-Ion Battery: A Closed-Loop Process. Environ. Sci. Technol. 2017, 51, 1662–1669. [Google Scholar] [CrossRef] [PubMed]
- Boxall, N.J.; Cheng, K.Y.; Bruckard, W.; Kaksonen, A.H. Application of indirect non-contact bioleaching for extracting metals from waste lithium-ion batteries. J. Hazard. Mater. 2018, 360, 504–511. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Chen, Y.; Zhou, T.; Liu, D.; Hu, H.; Fan, S. Hydrometallurgical recovery of metal values from sulfuric acid leaching liquor of spent lithium-ion batteries. Waste Manag. 2015, 38, 349–356. [Google Scholar] [CrossRef]
- Meng, F.; McNeice, J.; Zadeh, S.S.; Ghahreman, A. Review of Lithium Production and Recovery from Minerals, Brines, and Lithium-Ion Batteries. Miner. Process. Extr. Metall. Rev. 2021, 42, 123–141. [Google Scholar] [CrossRef]
- Alkhadra, M.A.; Gao, T.; Conforti, K.M.; Tian, H.; Bazant, M.Z. Small-scale desalination of seawater by shock electrodialysis. Desalination 2020, 476, 114219. [Google Scholar] [CrossRef]
- Hwang, C.W.; Jeong, M.H.; Kim, Y.J.; Son, W.K.; Kang, K.S.; Lee, C.S.; Hwang, T.S. Process design for lithium recovery using bipolar membrane electrodialysis system. Sep. Purif. Technol. 2016, 166, 34–40. [Google Scholar] [CrossRef]
- Strathmann, H. Electrodialysis, a mature technology with a multitude of new applications. Desalination 2010, 264, 268–288. [Google Scholar] [CrossRef]
- Fernandez-Gonzalez, C.; Dominguez-Ramos, A.; Ibañez, R.; Irabien, A. Electrodialysis with Bipolar Membranes for Valorization of Brines. Sep. Purif. Rev. 2016, 45, 275–287. [Google Scholar] [CrossRef]
- Razmjou, A.; Asadnia, M.; Hosseini, E.; Habibnejad Korayem, A.; Chen, V. Design principles of ion selective nanostructured membranes for the extraction of lithium ions. Nat. Commun. 2019, 10, 5793. [Google Scholar] [CrossRef] [Green Version]
- Eisenman, G. Cation selective glass electrodes and their mode of operation. Biophys. J. 1962, 2, 259–323. [Google Scholar] [CrossRef] [Green Version]
- Horn, R.; Roux, B.; Åqvist, J. Permeation redux: Thermodynamics and kinetics of ion movement through potassium channels. Biophys. J. 2014, 106, 1859–1863. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, P.; Wang, M.; Liu, F.; Ding, S.; Wang, X.; Du, G.; Liu, J.; Apel, P.; Kluth, P.; Trautmann, C.; et al. Ultrafast ion sieving using nanoporous polymeric membranes. Nat. Commun. 2018, 9, 2225. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zaviska, F.; Liang, S.; Li, J.; He, L.; Yang, H.Y. A high charge efficiency electrode by self-assembling sulphonated reduced graphene oxide onto carbon fibre: Towards enhanced capacitive deionization. J. Mater. Chem. A 2014, 2, 3484–3491. [Google Scholar] [CrossRef]
- Razmjou, A.; Eshaghi, G.; Orooji, Y.; Hosseini, E.; Korayem, A.H.; Mohagheghian, F.; Boroumand, Y.; Noorbakhsh, A.; Asadnia, M.; Chen, V. Lithium ion-selective membrane with 2D subnanometer channels. Water Res. 2019, 159, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Wen, Q.; Yan, D.; Liu, F.; Wang, M.; Ling, Y.; Wang, P.; Kluth, P.; Schauries, D.; Trautmann, C.; Apel, P.; et al. Highly Selective Ionic Transport through Subnanometer Pores in Polymer Films. Adv. Funct. Mater. 2016, 26, 5796–5803. [Google Scholar] [CrossRef]
- Nie, X.Y.; Sun, S.Y.; Sun, Z.; Song, X.; Yu, J.G. Ion-fractionation of lithium ions from magnesium ions by electrodialysis using monovalent selective ion-exchange membranes. Desalination 2017, 403, 128–135. [Google Scholar] [CrossRef]
- Nie, X.Y.; Sun, S.Y.; Song, X.; Yu, J.G. Further investigation into lithium recovery from salt lake brines with different feed characteristics by electrodialysis. J. Memb. Sci. 2017, 530, 185–191. [Google Scholar] [CrossRef]
- Parsa, N.; Moheb, A.; Mehrabani-Zeinabad, A.; Masigol, M.A. Recovery of lithium ions from sodium-contaminated lithium bromide solution by using electrodialysis process. Chem. Eng. Res. Des. 2015, 98, 81–88. [Google Scholar] [CrossRef]
- Ji, Z.; Chen, Q.; Yuan, J.; Liu, J.; Zhao, Y.; Feng, W. Preliminary study on recovering lithium from high Mg2+/Li+ ratio brines by electrodialysis. Sep. Purif. Technol. 2017, 172, 168–177. [Google Scholar] [CrossRef]
- Hoshino, T. Development of technology for recovering lithium from seawater by electrodialysis using ionic liquid membrane. Fusion Eng. Des. 2013, 88, 2956–2959. [Google Scholar] [CrossRef]
- Hoshino, T. Innovative lithium recovery technique from seawater by using world-first dialysis with a lithium ionic superconductor. Desalination 2015, 359, 59–63. [Google Scholar] [CrossRef] [Green Version]
- Song, Y.; Zhao, Z. Recovery of lithium from spent lithium-ion batteries using precipitation and electrodialysis techniques. Sep. Purif. Technol. 2018, 206, 335–342. [Google Scholar] [CrossRef]
- Iizuka, A.; Yamashita, Y.; Nagasawa, H.; Yamasaki, A.; Yanagisawa, Y. Separation of lithium and cobalt from waste lithium-ion batteries via bipolar membrane electrodialysis coupled with chelation. Sep. Purif. Technol. 2013, 113, 33–41. [Google Scholar] [CrossRef]
- Afifah, D.N.; Ariyanto, T.; Supranto, S.; Prasetyo, I. Separation of lithium ion from lithium-cobalt mixture using electrodialysis monovalent selective ion exchange membrane. Eng. J. 2018, 22, 165–179. [Google Scholar] [CrossRef]
- Bunani, S.; Yoshizuka, K.; Nishihama, S.; Arda, M.; Kabay, N. Application of bipolar membrane electrodialysis (BMED) for simultaneous separation and recovery of boron and lithium from aqueous solutions. Desalination 2017, 424, 37–44. [Google Scholar] [CrossRef]
- Jiang, C.; Wang, Y.; Wang, Q.; Feng, H.; Xu, T. Production of lithium hydroxide from lake brines through electro-electrodialysis with bipolar membranes (EEDBM). Ind. Eng. Chem. Res. 2014, 53, 6103–6112. [Google Scholar] [CrossRef]
- Suss, M.E.; Porada, S.; Sun, X.; Biesheuvel, P.M.; Yoon, J.; Presser, V. Water desalination via capacitive deionization: What is it and what can we expect from it? Energy Environ. Sci. 2015, 8, 2296–2319. [Google Scholar] [CrossRef] [Green Version]
- Porada, S.; Zhao, R.; van der Wal, A.; Presser, V.; Biesheuvel, P.M. Review on the science and technology of water desalination by capacitive deionization. Prog. Mater. Sci. 2013, 58, 1388–1442. [Google Scholar] [CrossRef] [Green Version]
- Siekierka, A. Preparation of electrodes for hybrid capacitive deionization and its influence on the adsorption behaviour. Sep. Sci. Technol. 2019, 55, 2238–2249. [Google Scholar] [CrossRef]
- Kim, S.; Lee, J.; Kang, J.S.; Jo, K.; Kim, S.; Sung, Y.E.; Yoon, J. Lithium recovery from brine using a λ-MnO2/activated carbon hybrid supercapacitor system. Chemosphere 2015, 125, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Siekierka, A.; Bryjak, M. Hybrid capacitive deionization with anion-exchange membranes for lithium extraction. E3S Web Conf. 2017, 22, 00157. [Google Scholar] [CrossRef]
- Li, Q.; Zheng, Y.; Xiao, D.; Or, T.; Gao, R.; Li, Z.; Feng, M.; Shui, L.; Zhou, G.; Wang, X.; et al. Faradaic Electrodes Open a New Era for Capacitive Deionization. Adv. Sci. 2020, 7, 2002213. [Google Scholar] [CrossRef]
- Singh, K.; Bouwmeester, H.J.M.; De Smet, L.C.P.M.; Bazant, M.Z.; Biesheuvel, P.M. Theory of Water Desalination with Intercalation Materials. Phys. Rev. Appl. 2018, 9, 064036. [Google Scholar] [CrossRef] [Green Version]
- Feng, Q.; Kanoh, H.; Miyai, Y.; Ooi, K. Hydrothermal Synthesis of Lithium and Sodium Manganese Oxides and Their Metal. Ion Extraction/insertion Reactions. Chem. Mater. 1995, 7, 1226–1232. Available online: https://pubs.acs.org/sharingguidelines (accessed on 3 September 2019). [CrossRef]
- Siekierka, A.; Kujawa, J.; Kujawski, W.; Bryjak, M. Lithium dedicated adsorbent for the preparation of electrodes useful in the ion pumping method. Sep. Purif. Technol. 2018, 194, 231–238. [Google Scholar] [CrossRef]
- Pasta, M.; Wessells, C.D.; Cui, Y.; La Mantia, F. A Desalination Battery. Nano Lett. 2012, 12, 839–843. [Google Scholar] [CrossRef]
- Trócoli, R.; Battistel, A.; La Mantia, F. Nickel Hexacyanoferrate as Suitable Alternative to Ag for Electrochemical Lithium Recovery. ChemSusChem 2015, 8, 2514–2519. [Google Scholar] [CrossRef]
- Siekierka, A. Lithium iron manganese oxide as an adsorbent for capturing lithium ions in hybrid capacitive deionization with different electrical modes. Sep. Purif. Technol. 2019, 236, 116234. [Google Scholar] [CrossRef]
- Kim, C.; Srimuk, P.; Lee, J.; Aslan, M.; Presser, V. Semi-continuous capacitive deionization using multi-channel flow stream and ion exchange membranes. Desalination 2018, 425, 104–110. [Google Scholar] [CrossRef]
- Suss, M.E.; Baumann, T.F.; Bourcier, W.L.; Spadaccini, C.M.; Rose, K.A.; Santiago, J.G.; Stadermann, M. Capacitive desalination with flow-through electrodes. Energy Environ. Sci. 2012, 5, 9511. [Google Scholar] [CrossRef]
- Ryu, T.; Ryu, J.C.; Shin, J.; Lee, D.H.; Kim, Y.H.; Chung, K.S. Recovery of lithium by an electrostatic field-assisted desorption process. Ind. Eng. Chem. Res. 2013, 52, 13738–13742. [Google Scholar] [CrossRef]
- Ryu, T.; Lee, D.H.; Ryu, J.C.; Shin, J.; Chung, K.S.; Kim, Y.H. Lithium recovery system using electrostatic field assistance. Hydrometallurgy 2015, 151, 78–83. [Google Scholar] [CrossRef]
- Lee, D.H.; Ryu, T.; Shin, J.; Ryu, J.C.; Chung, K.S.; Kim, Y.H. Selective lithium recovery from aqueous solution using a modified membrane capacitive deionization system. Hydrometallurgy 2017, 173, 283–288. [Google Scholar] [CrossRef]
- Shi, W.; Liu, X.; Ye, C.; Cao, X.; Gao, C.; Shen, J. Efficient lithium extraction by membrane capacitive deionization incorporated with monovalent selective cation exchange membrane. Sep. Purif. Technol. 2019, 210, 885–890. [Google Scholar] [CrossRef]
- Siekierka, A.; Bryjak, M.; Wolska, J. The use of activated carbon modified with polypyrrole as a supporting electrode for lithium ions adsorption in capacitive deionization. Desalin. Water Treat. 2017, 64, 251–254. [Google Scholar] [CrossRef]
- Siekierka, A.; Wolska, J.; Bryjak, M.; Kujawski, W. Anion exchange membranes in lithium extraction by means of capacitive deionization system. Desalin. Water Treat. 2017, 75, 331–341. [Google Scholar] [CrossRef] [Green Version]
- Siekierka, A.; Bryjak, M. Novel anion exchange membrane for concentration of lithium salt in hybrid capacitive deionization. Desalination 2019, 452, 279–289. [Google Scholar] [CrossRef]
- Siekierka, A.; Kmiecik, E.; Tomaszewska, B.; Wator, K.; Bryjak, M. The evaluation of the effectiveness of lithium separation by hybrid capacitive deionization from geothermal water with the uncertainty measurement application. Desalin. Water Treat. 2018, 128, 259–264. [Google Scholar] [CrossRef]
- Zhao, X.; Feng, M.; Jiao, Y.; Zhang, Y.; Wang, Y.; Sha, Z. Lithium extraction from brine in an ionic selective desalination battery. Desalination 2020, 481, 114360. [Google Scholar] [CrossRef]
- Qiu, Y.; Ruan, H.; Tang, C.; Yao, L.; Shen, J.; Sotto, A. Study on recovering high-concentration lithium salt from lithium-containing wastewater using a hybrid reverse osmosis (RO)-electrodialysis (ED) process. ACS Sustain. Chem. Eng. 2019, 7, 13481–13490. [Google Scholar] [CrossRef]
- Recepoğlu, Y.K.; Kabay, N.; Yoshizuka, K.; Nishihama, S.; Yılmaz-Ipek, İ.; Arda, M.; Yüksel, M. Effect of Operational Conditions on Separation of Lithium from Geothermal Water by λ-MnO2 Using Ion Exchange-Membrane Filtration Hybrid Process. Solvent Extr. Ion Exch. 2018, 36, 499–512. [Google Scholar] [CrossRef]
- Umeno, A.; Miyai, Y.; Takagi, N.; Chitrakar, R.; Sakane, K.; Ooi, K. Preparation and adsorptive properties of membrane-type adsorbents for lithium recovery from seawater. Ind. Eng. Chem. Res. 2002, 41, 4281–4287. [Google Scholar] [CrossRef]
- Quist-Jensen, C.A.; Ali, A.; Mondal, S.; Macedonio, F.; Drioli, E. A study of membrane distillation and crystallization for lithium recovery from high-concentrated aqueous solutions. J. Memb. Sci. 2016, 505, 167–173. [Google Scholar] [CrossRef]
- Huang, Y.; Han, G.; Liu, J.; Chai, W.; Wang, W.; Yang, S.; Su, S. A stepwise recovery of metals from hybrid cathodes of spent Li-ion batteries with leaching-flotation-precipitation process. J. Power Sources 2016, 325, 555–564. [Google Scholar] [CrossRef]
- Torres, W.R.; Díaz Nieto, C.H.; Prévoteau, A.; Rabaey, K.; Flexer, V. Lithium carbonate recovery from brines using membrane electrolysis. J. Memb. Sci. 2020, 615, 118416. [Google Scholar] [CrossRef]
- Abdollahzadeh, M.; Chai, M.; Hosseini, E.; Zakertabrizi, M.; Mohammad, M.; Ahmadi, H.; Hou, J.; Lim, S.; Habibnejad Korayem, A.; Chen, V.; et al. Designing Angstrom-Scale Asymmetric MOF-on-MOF Cavities for High Monovalent Ion Selectivity. Adv. Mater. 2022, 34, 2107878. [Google Scholar] [CrossRef]
- Cha-umpong, W.; Li, Q.; Razmjou, A.; Chen, V. Concentrating brine for lithium recovery using GO composite pervaporation membranes. Desalination 2021, 500, 114894. [Google Scholar] [CrossRef]
- Johnston, T. Cost Structures for Lithium Carbonate Production—A World View Cost Structures for Lithium Carbonate Production—A World View; Hatch: Mississauga, ON, Canada; Available online: http://www.indmin.com/events/download.ashx/document/speaker/7125/a0ID000000X0jvsMAB/Presentation (accessed on 14 January 2022).
- Afonso, P.; Santana, A.; Afonso, P.; Zanin, A.; Wernke, R. Costing models for capacity optimization in Industry 4.0: Trade-off between used capacity and operational efficiency. Procedia Manuf. 2017, 13, 1183–1190. [Google Scholar] [CrossRef]
- Steward, D.; Mayyas, A.; Mann, M. Economics and challenges of Li-ion battery recycling from end-of-life vehicles. Procedia Manuf. 2019, 33, 272–279. [Google Scholar] [CrossRef]
- Kelleher Environmental. Research Study on Reuse and Recycling of Batteries Employed in Electric Vehicles: The Technical, Environmental, Economic, Energy and Cost Implications of Reusing and Recycling EV Batteries Project Report; Kelleher Environmental: East York, ON, Canada, 2019. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).