The Role of Extracellular Vesicles in Osteoporosis: A Scoping Review
Abstract
:1. Introduction
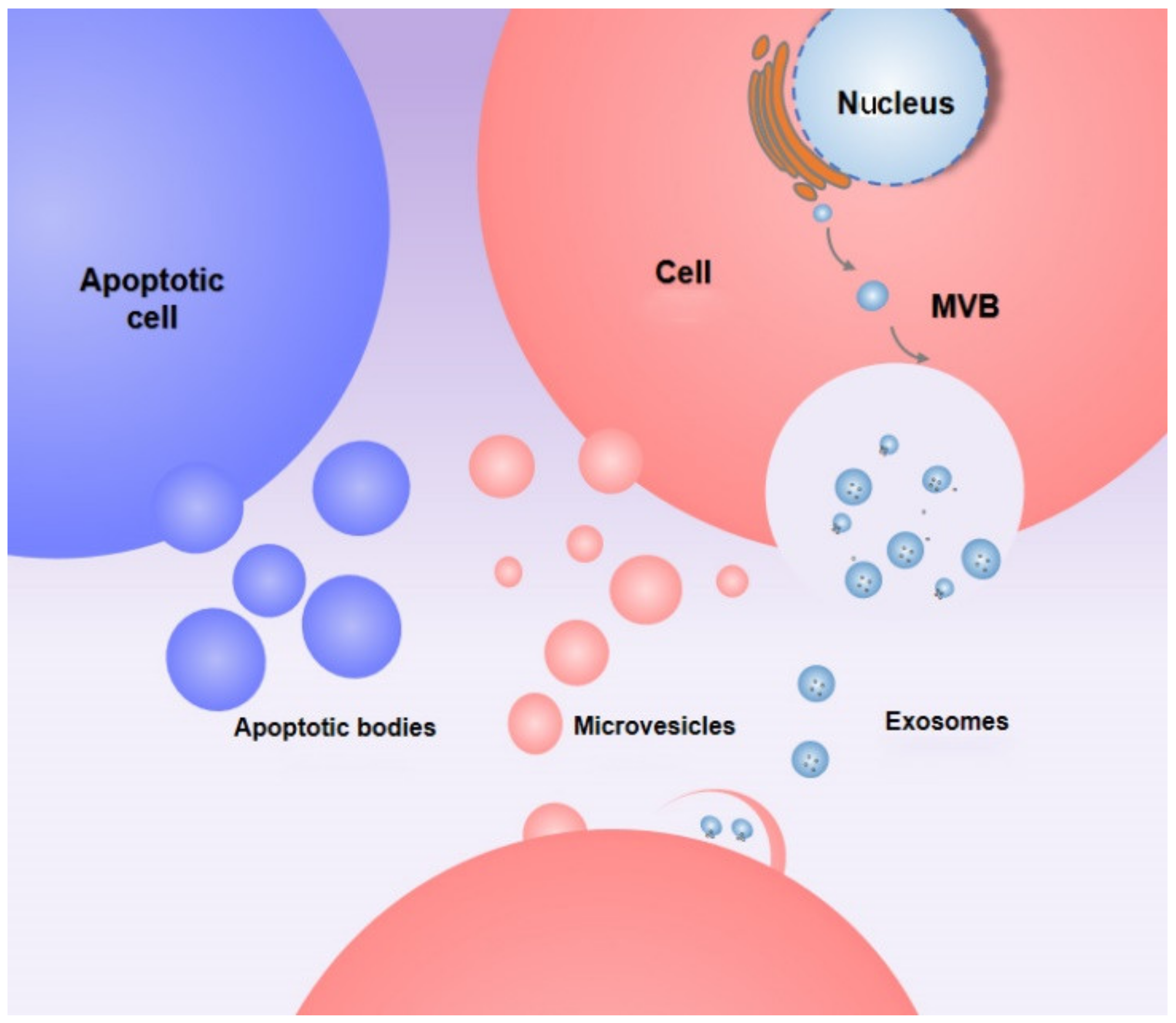
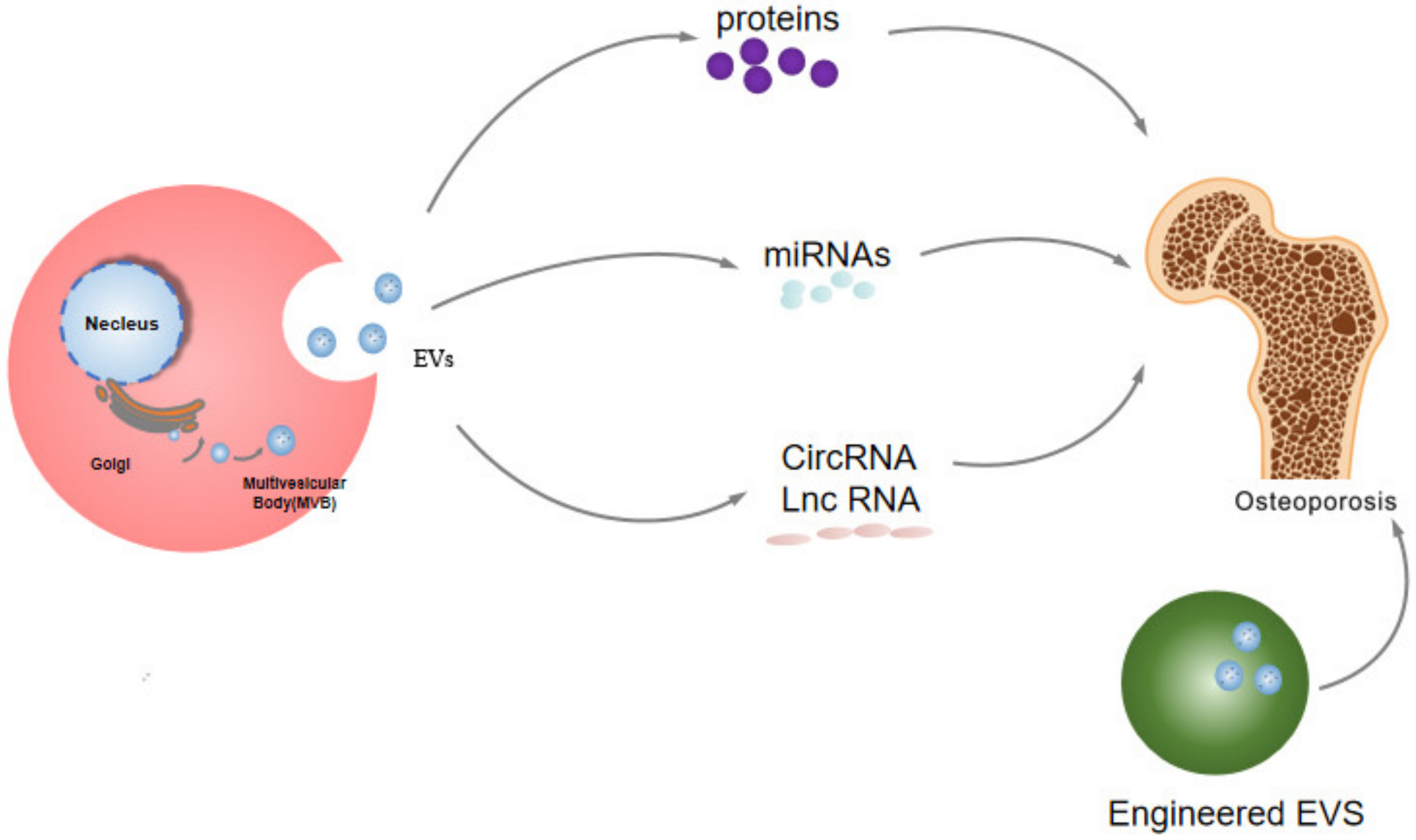
2. Production and Release of EVs
3. The Potential Role of EVs in the Etiology of Osteoporosis
4. EVs as Potential Diagnostic Tool in Osteoporosis
| Moleculars | Regulated | Samples (Experimental Group-Control Group) | p Value | Reference |
|---|---|---|---|---|
| Vinculin et al. | upregulated | 28-28 | <0.05 | [32] |
| PSMB9 et al. | upregulated | 60-60 | <0.001 | [33] |
| miR-4746-3p et al. | down-regulated | 12-6 | 0.000487 | [34] |
| tRF-25 et al. | upregulated | 40-40 | <0.05 | [35] |
5. The Potential Therapeutic Effects of EVs in Osteoporosis
5.1. The Potential Therapeutic Effects of Proteins of EVs in Osteoporosis
| Proteins | Source | Regulated | Functions | Reference |
|---|---|---|---|---|
| CTHRC1/OPG | EVs | upregulated | inhibit osteoporosis | [9] |
| RNF146 | apoptotic body | upregulated | inhibit osteoporosis | [21] |
| CLEC11A | EVs | upregulated | inhibit osteoporosis | [62] |
| NLRP3 | exosomes | upregulated | inhibit osteoporosis | [63] |
| WNT1/WNT5A/WNT7A/WNT9A | EVs | down | inhibit osteoporosis | [64] |
| OPG | EVs | upregulated | inhibit osteoporosis | [65] |
5.2. MiRNA
| Gene | Source | Regulated | Functions | Reference |
|---|---|---|---|---|
| miR-31 | microvesicles | upregulated | promote osteoporosis | [27] |
| miR-21 | exosomes | upregulated | promote osteoporosis | [66] |
| miR-328-3P | apoptotic bodies | upregulated | inhibit osteoporosis | [21] |
| miR-155 | exosomes | upregulated | inhibit osteoporosis | [67] |
| miR-3960 | EVs | upregulated | inhibit osteoporosis | [20] |
| miR-22-3p | EVs | down | promote osteoporosis | [68] |
| miR-214-3p | exosomes | down | promote osteoporosis | [69] |
| miR-186 | exosomes | upregulated | inhibit osteoporosis | [70] |
| miR-29b-3p | EVs | down | inhibit osteoporosis | [71] |
| miR-143/145 | EVs | upregulated | promote osteoporosis | [72] |
| miR-139-5p | exosomes | upregulated | promote osteoporosis | [73] |
| miR-935 | exosomes | upregulated | inhibit osteoporosis | [74] |
| miR-424-5p | exosomes | upregulated | promote osteoporosis | [75] |
| miRNA-19b-3p | exosomes | upregulated | inhibit osteoporosis | [76] |
| miR-27a-5p | EVs | upregulated | inhibit osteoporosis | [77] |
| miR-27a | EVs | upregulated | inhibit osteoporosis | [78] |
5.3. lncRNA and circRNA
5.4. The Role of Engineered EVs in the Treatment of Osteoporosis
| Material | Source | Assembly Method | Functions | Reference |
|---|---|---|---|---|
| Magnetic hydroxyapatite | exosomes | MHA stimulation | inhibit osteoporosis | [97] |
| alendronate | EVs | Assembly of drugs into EVs | inhibit osteoporosis | [98] |
| GPNMB-EVs | EVs | Lentiviral transfected cells | inhibit osteoporosis | [99] |
| sEV-20a | EVs | Transfection into EVs | inhibit osteoporosis | [100] |
| T cell-depleting nanoparticles | EVs | Extract EVs | inhibit osteoporosis | [101] |
| alendronic acid | EVs | biomimicking polymer vesicle | inhibit osteoporosis | [102] |
6. Clinical Progress and Future Prospects of EVs in Osteoporosis
7. Limitations and Coping Strategies of EVs
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Compston, J.E.; McClung, M.R.; Leslie, W.D. Osteoporosis. Lancet 2019, 393, 364–376. [Google Scholar] [CrossRef]
- Zhao, J.G.; Zeng, X.T.; Wang, J.; Liu, L. Association between Calcium or Vitamin D Supplementation and Fracture Incidence in Community-Dwelling Older Adults: A Systematic Review and Meta-analysis. JAMA 2017, 318, 2466–2482. [Google Scholar] [CrossRef] [PubMed]
- Cauley, J.A. Screening for Osteoporosis. JAMA 2018, 319, 2483–2485. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wei, Q.; An, R.; Zhang, H.M.; Shen, J.Y.; Qin, X.Y.; Han, X.L.; Li, J.; Li, X.W.; Gao, X.M.; et al. Anti-osteoporosis effect of Semen Cuscutae in ovariectomized mice through inhibition of bone resorption by osteoclasts. J. Ethnopharmacol. 2022, 285, 114834. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Yang, P.; Liu, P.; Wang, H.; Ke, E.; Li, K.; Yan, H. Targeting Filamin A alleviates ovariectomy-induced bone loss in mice via the WNT/β-catenin signaling pathway. Cell. Signal. 2022, 90, 110191. [Google Scholar] [CrossRef]
- Liu, Z.; Lee, H.L.; Suh, J.S.; Deng, P.; Lee, C.R.; Bezouglaia, O.; Mirnia, M.; Chen, V.; Zhou, M.; Cui, Z.K.; et al. The ERα/KDM6B regulatory axis modulates osteogenic differentiation in human mesenchymal stem cells. Bone Res. 2022, 10, 3. [Google Scholar] [CrossRef]
- Peng, S.; Gao, Y.; Shi, S.; Zhao, D.; Cao, H.; Fu, T.; Cai, X.; Xiao, J. LncRNA-AK137033 inhibits the osteogenic potential of adipose-derived stem cells in diabetic osteoporosis by regulating Wnt signaling pathway via DNA methylation. Cell Prolif. 2021, 55, e13174. [Google Scholar] [CrossRef]
- Li, C.H.; Lü, Z.R.; Zhao, Z.D.; Wang, X.Y.; Leng, H.J.; Niu, Y.; Wang, M.P. Nitazoxanide, an Antiprotozoal Drug, Reduces Bone Loss in Ovariectomized Mice by Inhibition of RANKL-Induced Osteoclastogenesis. Front. Pharmacol. 2021, 12, 781640. [Google Scholar] [CrossRef]
- Hayes, K.N.; Baschant, U.; Hauser, B.; Burden, A.M.; Winter, E.M. When to Start and Stop Bone-Protecting Medication for Preventing Glucocorticoid-Induced Osteoporosis. Front. Endocrinol. 2021, 12, 782118. [Google Scholar] [CrossRef]
- van der Burgh, A.C.; de Keyser, C.E.; Zillikens, M.C.; Stricker, B.H. The Effects of Osteoporotic and Non-osteoporotic Medications on Fracture Risk and Bone Mineral Density. Drugs 2021, 81, 1831–1858. [Google Scholar] [CrossRef]
- de Roij van Zuijdewijn, C.; van Dorp, W.; Florquin, S.; Roelofs, J.; Verburgh, K. Bisphosphonate nephropathy: A case series and review of the literature. Br. J. Clin. Pharmacol. 2021, 87, 3485–3491. [Google Scholar] [CrossRef]
- Villatoro-Villar, M.; Kwoh, C.K. Bisphosphonates, Bone and Joint Pain. Curr. Osteoporos. Rep. 2021, 19, 417–428. [Google Scholar] [CrossRef]
- Tkach, M.; Théry, C. Communication by Extracellular Vesicles: Where We Are and Where We Need to Go. Cell 2016, 164, 1226–1232. [Google Scholar] [CrossRef] [Green Version]
- Shao, H.; Im, H.; Castro, C.M.; Breakefield, X.; Weissleder, R.; Lee, H. New Technologies for Analysis of Extracellular Vesicles. Chem. Rev. 2018, 118, 1917–1950. [Google Scholar] [CrossRef]
- Théry, C. Cancer: Diagnosis by extracellular vesicles. Nature 2015, 523, 161–162. [Google Scholar] [CrossRef]
- Pavlyukov, M.S.; Yu, H.; Bastola, S.; Minata, M.; Shender, V.O.; Lee, Y.; Zhang, S.; Wang, J.; Komarova, S.; Wang, J.; et al. Apoptotic Cell-Derived Extracellular Vesicles Promote Malignancy of Glioblastoma via Intercellular Transfer of Splicing Factors. Cancer Cell 2018, 34, 119–135.e10. [Google Scholar] [CrossRef] [Green Version]
- Schneider, E.; Winzer, R.; Rissiek, A.; Ricklefs, I.; Meyer-Schwesinger, C.; Ricklefs, F.L.; Bauche, A.; Behrends, J.; Reimer, R.; Brenna, S.; et al. CD73-mediated adenosine production by CD8 T cell-derived extracellular vesicles constitutes an intrinsic mechanism of immune suppression. Nat. Commun. 2021, 12, 5911. [Google Scholar] [CrossRef]
- Chen, C.Y.; Rao, S.S.; Tan, Y.J.; Luo, M.J.; Hu, X.K.; Yin, H.; Huang, J.; Hu, Y.; Luo, Z.W.; Liu, Z.Z.; et al. Extracellular vesicles from human urine-derived stem cells prevent osteoporosis by transferring CTHRC1 and OPG. Bone Res. 2019, 7, 18. [Google Scholar] [CrossRef] [Green Version]
- Lin, X.; Wang, Q.; Gu, C.; Li, M.; Chen, K.; Chen, P.; Tang, Z.; Liu, X.; Pan, H.; Liu, Z.; et al. Smart Nanosacrificial Layer on the Bone Surface Prevents Osteoporosis through Acid-Base Neutralization Regulated Biocascade Effects. J. Am. Chem. Soc. 2020, 142, 17543–17556. [Google Scholar] [CrossRef]
- Hu, Y.; Xu, R.; Chen, C.Y.; Rao, S.S.; Xia, K.; Huang, J.; Yin, H.; Wang, Z.X.; Cao, J.; Liu, Z.Z.; et al. Extracellular vesicles from human umbilical cord blood ameliorate bone loss in senile osteoporotic mice. Metab. Clin. Exp. 2019, 95, 93–101. [Google Scholar] [CrossRef]
- Liu, D.; Kou, X.; Chen, C.; Liu, S.; Liu, Y.; Yu, W.; Yu, T.; Yang, R.; Wang, R.; Zhou, Y.; et al. Circulating apoptotic bodies maintain mesenchymal stem cell homeostasis and ameliorate osteopenia via transferring multiple cellular factors. Cell Res. 2018, 28, 918–933. [Google Scholar] [CrossRef] [Green Version]
- Murphy, C.; Withrow, J.; Hunter, M.; Liu, Y.; Tang, Y.L.; Fulzele, S.; Hamrick, M.W. Emerging role of extracellular vesicles in musculoskeletal diseases. Mol. Asp. Med. 2018, 60, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Vietri, M.; Radulovic, M.; Stenmark, H. The many functions of ESCRTs. Nat. Rev. Mol. Cell Biol. 2020, 21, 25–42. [Google Scholar] [CrossRef] [PubMed]
- Elsherbini, A.; Bieberich, E. Ceramide and Exosomes: A Novel Target in Cancer Biology and Therapy. Adv. Cancer Res. 2018, 140, 121–154. [Google Scholar]
- Szempruch, A.J.; Sykes, S.E.; Kieft, R.; Dennison, L.; Becker, A.C.; Gartrell, A.; Martin, W.J.; Nakayasu, E.S.; Almeida, I.C.; Hajduk, S.L.; et al. Extracellular Vesicles from Trypanosoma brucei Mediate Virulence Factor Transfer and Cause Host Anemia. Cell 2016, 164, 246–257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, R.; Shen, X.; Si, Y.; Fu, Y.; Zhu, W.; Xiao, T.; Fu, Z.; Zhang, P.; Cheng, J.; Jiang, H. MicroRNA-31a-5p from aging BMSCs links bone formation and resorption in the aged bone marrow microenvironment. Aging Cell 2018, 17, e12794. [Google Scholar] [CrossRef] [PubMed]
- Weilner, S.; Schraml, E.; Wieser, M.; Messner, P.; Schneider, K.; Wassermann, K.; Micutkova, L.; Fortschegger, K.; Maier, A.B.; Westendorp, R.; et al. Secreted microvesicular miR-31 inhibits osteogenic differentiation of mesenchymal stem cells. Aging Cell 2016, 15, 744–754. [Google Scholar] [CrossRef] [Green Version]
- Martin, P.J.; Haren, N.; Ghali, O.; Clabaut, A.; Chauveau, C.; Hardouin, P.; Broux, O. Adipogenic RNAs are transferred in osteoblasts via bone marrow adipocytes-derived extracellular vesicles (EVs). BMC Cell Biol. 2015, 16, 10. [Google Scholar] [CrossRef] [Green Version]
- Liu, D.S.; Frampton, A.E. Plasma extracellular vesicles contain unannotated small RNA clusters suitable as biomarkers for detecting early hepatocellular carcinoma. Gut 2021, 2021, 325798. [Google Scholar] [CrossRef]
- Penders, J.; Nagelkerke, A.; Cunnane, E.M.; Pedersen, S.V.; Pence, I.J.; Coombes, R.C.; Stevens, M.M. Single Particle Automated Raman Trapping Analysis of Breast Cancer Cell-Derived Extracellular Vesicles as Cancer Biomarkers. ACS Nano 2021, 15, 18192–18205. [Google Scholar] [CrossRef]
- Fitz, N.F.; Wang, J.; Kamboh, M.I.; Koldamova, R.; Lefterov, I. Small nucleolar RNAs in plasma extracellular vesicles and their discriminatory power as diagnostic biomarkers of Alzheimer’s disease. Neurobiol. Dis. 2021, 159, 105481. [Google Scholar] [CrossRef]
- Huo, C.; Li, Y.; Qiao, Z.; Shang, Z.; Cao, C.; Hong, Y.; Xiao, H. Comparative proteomics analysis of microvesicles in human serum for the evaluation of osteoporosis. Electrophoresis 2019, 40, 1839–1847. [Google Scholar] [CrossRef]
- Chen, M.; Li, Y.; Lv, H.; Yin, P.; Zhang, L.; Tang, P. Quantitative proteomics and reverse engineer analysis identified plasma exosome derived protein markers related to osteoporosis. J. Proteom. 2020, 228, 103940. [Google Scholar] [CrossRef]
- Shao, J.L.; Li, H.; Zhang, X.R.; Zhang, X.; Li, Z.Z.; Jiao, G.L.; Sun, G.D. Identification of Serum Exosomal MicroRNA Expression Profiling in Menopausal Females with Osteoporosis by High-throughput Sequencing. Curr. Med. Sci. 2020, 40, 1161–1169. [Google Scholar] [CrossRef]
- Zhang, Y.; Cai, F.; Liu, J.; Chang, H.; Liu, L.; Yang, A.; Liu, X. Transfer RNA-derived fragments as potential exosome tRNA-derived fragment biomarkers for osteoporosis. Int. J. Rheum. Dis. 2018, 21, 1659–1669. [Google Scholar] [CrossRef]
- Tian, F.; Zhang, S.; Liu, C.; Han, Z.; Liu, Y.; Deng, J.; Li, Y.; Wu, X.; Cai, L.; Qin, L.; et al. Protein analysis of extracellular vesicles to monitor and predict therapeutic response in metastatic breast cancer. Nat. Commun. 2021, 12, 2536. [Google Scholar] [CrossRef]
- Wang, X.; Hu, S.; Li, J.; Zhu, D.; Wang, Z.; Cores, J.; Cheng, K.; Liu, G.; Huang, K. Extruded Mesenchymal Stem Cell Nanovesicles Are Equally Potent to Natural Extracellular Vesicles in Cardiac Repair. ACS Appl. Mater. Interfaces 2021, 13, 55767–55779. [Google Scholar] [CrossRef]
- Crunkhorn, S. Extracellular vesicles target neuronal AMPK. Nat. Rev. Drug Discov. 2021, 20, 898. [Google Scholar] [CrossRef]
- Schatz, D.; Schleyer, G.; Saltvedt, M.R.; Sandaa, R.A.; Feldmesser, E.; Vardi, A. Ecological significance of extracellular vesicles in modulating host-virus interactions during algal blooms. ISME J. 2021, 15, 3714–3721. [Google Scholar] [CrossRef]
- Zeng, T.; Yuan, P.; Liang, L.; Zhang, X.; Zhang, H.; Wu, W. Cartilaginous Extracellular Matrix Enriched with Human Gingival Mesenchymal Stem Cells Derived “Matrix Bound Extracellular Vesicles” Enabled Functional Reconstruction of Tracheal Defect. Adv. Sci. 2021, 9, e2102735. [Google Scholar] [CrossRef]
- Zuo, R.; Ye, L.F.; Huang, Y.; Song, Z.Q.; Wang, L.; Zhi, H.; Zhang, M.Y.; Li, J.Y.; Zhu, L.; Xiao, W.J.; et al. Hepatic small extracellular vesicles promote microvascular endothelial hyperpermeability during NAFLD via novel-miRNA-7. J. Nanobiotechnol. 2021, 19, 396. [Google Scholar] [CrossRef] [PubMed]
- Perluigi, M.; Picca, A.; Montanari, E.; Calvani, R.; Marini, F.; Matassa, R.; Tramutola, A.; Villani, A.; Familiari, G.; Domenico, F.D.; et al. Aberrant crosstalk between insulin signaling and mTOR in young Down syndrome individuals revealed by neuronal-derived extracellular vesicles. Alzheimer’s Dement. J. Alzheimer’s Assoc. 2021. [Google Scholar] [CrossRef] [PubMed]
- Ge, L.; Xun, C.; Li, W.; Jin, S.; Liu, Z.; Zhuo, Y.; Duan, D.; Hu, Z.; Chen, P.; Lu, M. Extracellular vesicles derived from hypoxia-preconditioned olfactory mucosa mesenchymal stem cells enhance angiogenesis via miR-612. J. Nanobiotechnol. 2021, 19, 380. [Google Scholar] [CrossRef] [PubMed]
- Casanova, M.R.; Osório, H.; Reis, R.L.; Martins, A.; Neves, N.M. Chondrogenic differentiation induced by extracellular vesicles bound to a nanofibrous substrate. NPJ Regen. Med. 2021, 6, 79. [Google Scholar] [CrossRef]
- Tan, S.H.S.; Wong, J.R.Y.; Sim, S.J.Y.; Tjio, C.K.E.; Wong, K.L.; Chew, J.R.J.; Hui, J.H.P.; Toh, W.S. Mesenchymal stem cell exosomes in bone regenerative strategies—A systematic review of preclinical studies. Mater. Today. Bio 2020, 7, 100067. [Google Scholar] [CrossRef]
- Seo, N.; Shirakura, Y.; Tahara, Y.; Momose, F.; Harada, N.; Ikeda, H.; Akiyoshi, K.; Shiku, H. Activated CD8(+) T cell extracellular vesicles prevent tumour progression by targeting of lesional mesenchymal cells. Nat. Commun. 2018, 9, 435. [Google Scholar] [CrossRef] [Green Version]
- Jiang, L.; Shen, Y.; Guo, D.; Yang, D.; Liu, J.; Fei, X.; Yang, Y.; Zhang, B.; Lin, Z.; Yang, F.; et al. EpCAM-dependent extracellular vesicles from intestinal epithelial cells maintain intestinal tract immune balance. Nat. Commun. 2016, 7, 13045. [Google Scholar] [CrossRef] [Green Version]
- Eichholz, K.F.; Woods, I.; Riffault, M.; Johnson, G.P.; Corrigan, M.; Lowry, M.C.; Shen, N.; Labour, M.N.; Wynne, K.; O’Driscoll, L.; et al. Human bone marrow stem/stromal cell osteogenesis is regulated via mechanically activated osteocyte-derived extracellular vesicles. Stem Cells Transl. Med. 2020, 9, 1431–1447. [Google Scholar] [CrossRef]
- Negri, S.; Wang, Y.; Sono, T.; Lee, S.; Hsu, G.C.; Xu, J.; Meyers, C.A.; Qin, Q.; Broderick, K.; Witwer, K.W.; et al. Human perivascular stem cells prevent bone graft resorption in osteoporotic contexts by inhibiting osteoclast formation. Stem Cells Transl. Med. 2020, 9, 1617–1630. [Google Scholar] [CrossRef]
- Gatti, M.; Beretti, F.; Zavatti, M.; Bertucci, E.; Ribeiro Luz, S.; Palumbo, C.; Maraldi, T. Amniotic Fluid Stem Cell-Derived Extracellular Vesicles Counteract Steroid-Induced Osteoporosis In Vitro. Int. J. Mol. Sci. 2020, 22, 38. [Google Scholar] [CrossRef]
- Niedermair, T.; Lukas, C.; Li, S.; Stöckl, S.; Craiovan, B.; Brochhausen, C.; Federlin, M.; Herrmann, M.; Grässel, S. Influence of Extracellular Vesicles Isolated from Osteoblasts of Patients with Cox-Arthrosis and/or Osteoporosis on Metabolism and Osteogenic Differentiation of BMSCs. Front. Bioeng. Biotechnol. 2020, 8, 615520. [Google Scholar] [CrossRef]
- Liu, J.H.; Chen, C.Y.; Liu, Z.Z.; Luo, Z.W.; Rao, S.S.; Jin, L.; Wan, T.F.; Yue, T.; Tan, Y.J.; Yin, H.; et al. Extracellular Vesicles from Child Gut Microbiota Enter into Bone to Preserve Bone Mass and Strength. Adv. Sci. 2021, 8, 2004831. [Google Scholar] [CrossRef]
- Bei, H.P.; Hung, P.M.; Yeung, H.L.; Wang, S.; Zhao, X. Bone-a-Petite: Engineering Exosomes towards Bone, Osteochondral, and Cartilage Repair. Small 2021, 17, e2101741. [Google Scholar] [CrossRef]
- Yang, R.Z.; Xu, W.N.; Zheng, H.L.; Zheng, X.F.; Li, B.; Jiang, L.S.; Jiang, S.D. Exosomes derived from vascular endothelial cells antagonize glucocorticoid-induced osteoporosis by inhibiting ferritinophagy with resultant limited ferroptosis of osteoblasts. J. Cell. Physiol. 2021, 236, 6691–6705. [Google Scholar] [CrossRef]
- Pinson, M.R.; Chung, D.D.; Adams, A.M.; Scopice, C.; Payne, E.A.; Sivakumar, M.; Miranda, R.C. Extracellular Vesicles in Premature Aging and Diseases in Adulthood Due to Developmental Exposures. Aging Dis. 2021, 12, 1516–1535. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, C.; Wang, J.; Liu, H.; Wang, M. Bone-Adipose Tissue Crosstalk: Role of Adipose Tissue Derived Extracellular Vesicles in Bone Diseases. J. Cell. Physiol. 2021, 236, 7874–7886. [Google Scholar] [CrossRef]
- Qi, X.; Zhang, J.; Yuan, H.; Xu, Z.; Li, Q.; Niu, X.; Hu, B.; Wang, Y.; Li, X. Exosomes Secreted by Human-Induced Pluripotent Stem Cell-Derived Mesenchymal Stem Cells Repair Critical-Sized Bone Defects through Enhanced Angiogenesis and Osteogenesis in Osteoporotic Rats. Int. J. Biol. Sci. 2016, 12, 836–849. [Google Scholar] [CrossRef] [Green Version]
- Deng, L.; Peng, Y.; Jiang, Y.; Wu, Y.; Ding, Y.; Wang, Y.; Xu, D.; Fu, Q. Imipramine Protects against Bone Loss by Inhibition of Osteoblast-Derived Microvesicles. Int. J. Mol. Sci. 2017, 18, 1013. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.; Sun, Y.; Zhang, Q. Emerging Role of Extracellular Vesicles in Bone Remodeling. J. Dent. Res. 2018, 97, 859–868. [Google Scholar] [CrossRef]
- Xie, Y.; Gao, Y.; Zhang, L.; Chen, Y.; Ge, W.; Tang, P. Involvement of serum-derived exosomes of elderly patients with bone loss in failure of bone remodeling via alteration of exosomal bone-related proteins. Aging Cell 2018, 17, e12758. [Google Scholar] [CrossRef]
- Wei, Y.; Tang, C.; Zhang, J.; Li, Z.; Zhang, X.; Miron, R.J.; Zhang, Y. Extracellular vesicles derived from the mid-to-late stage of osteoblast differentiation markedly enhance osteogenesis in vitro and in vivo. Biochem. Biophys. Res. Commun. 2019, 514, 252–258. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, Y.; Ni, C.Y.; Chen, C.Y.; Rao, S.S.; Yin, H.; Huang, J.; Tan, Y.J.; Wang, Z.X.; Cao, J.; et al. Human umbilical cord mesenchymal stromal cells-derived extracellular vesicles exert potent bone protective effects by CLEC11A-mediated regulation of bone metabolism. Theranostics 2020, 10, 2293–2308. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, Q.; Su, H.; Cheng, J. Exosomes from adipose derived mesenchymal stem cells alleviate diabetic osteoporosis in rats through suppressing NLRP3 inflammasome activation in osteoclasts. J. Biosci. Bioeng. 2021, 131, 671–678. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Chen, Y.; Yu, D.; Zhang, L.; Dou, X.; Wu, G.; Wang, Y.; Zhang, S. Evaluation of the cargo contents and potential role of extracellular vesicles in osteoporosis. Aging 2021, 13, 19282–19292. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.S.; Lee, J.; Kim, H.K.; Yeom, S.H.; Woo, C.H.; Jung, Y.J.; Yun, Y.E.; Park, S.Y.; Han, J.; Kim, E.; et al. Extracellular vesicles from adipose tissue-derived stem cells alleviate osteoporosis through osteoprotegerin and miR-21-5p. J. Extracell. Vesicles 2021, 10, e12152. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.B.; Tian, L.; Zhang, C.G. Bone marrow stem cells-derived exosomes extracted from osteoporosis patients inhibit osteogenesis via microRNA-21/SMAD7. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 6221–6229. [Google Scholar] [PubMed]
- Song, H.; Li, X.; Zhao, Z.; Qian, J.; Wang, Y.; Cui, J.; Weng, W.; Cao, L.; Chen, X.; Hu, Y.; et al. Reversal of Osteoporotic Activity by Endothelial Cell-Secreted Bone Targeting and Biocompatible Exosomes. Nano Lett. 2019, 19, 3040–3048. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Y.; Zhao, H.; Han, X.; Zhao, T.; Qu, P.; Li, G.; Wang, W. Extracellular vesicle-encapsulated miR-22-3p from bone marrow mesenchymal stem cell promotes osteogenic differentiation via FTO inhibition. Stem Cell Res. Ther. 2020, 11, 227. [Google Scholar] [CrossRef]
- Wang, X.; Li, X.; Li, J.; Zhai, L.; Liu, D.; Abdurahman, A.; Zhang, Y.; Yokota, H.; Zhang, P. Mechanical loading stimulates bone angiogenesis through enhancing type H vessel formation and downregulating exosomal miR-214-3p from bone marrow-derived mesenchymal stem cells. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2021, 35, e21150. [Google Scholar] [CrossRef]
- Li, L.; Zhou, X.; Zhang, J.T.; Liu, A.F.; Zhang, C.; Han, J.C.; Zhang, X.Q.; Wu, S.; Zhang, X.Y.; Lv, F.Q. Exosomal miR-186 derived from BMSCs promote osteogenesis through hippo signaling pathway in postmenopausal osteoporosis. J. Orthop. Surg. Res. 2021, 16, 23. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, W.; Wang, Y.; Zhao, H.; Han, X.; Zhao, T.; Qu, P. Extracellular Vesicle-Encapsulated miR-29b-3p Released from Bone Marrow-Derived Mesenchymal Stem Cells Underpins Osteogenic Differentiation. Front. Cell Dev. Biol. 2020, 8, 581545. [Google Scholar] [CrossRef]
- Xu, R.; Shen, X.; Xie, H.; Zhang, H.; Liu, D.; Chen, X.; Fu, Y.; Zhang, P.; Yang, Y.; Cheng, J.; et al. Identification of the canonical and noncanonical role of miR-143/145 in estrogen-deficient bone loss. Theranostics 2021, 11, 5491–5510. [Google Scholar] [CrossRef]
- Lu, Q.; Qin, H.; Tan, H.; Wei, C.; Yang, X.; He, J.; Liang, W.; Li, J. Senescence Osteoblast-Derived Exosome-Mediated miR-139-5p Regulates Endothelial Cell Functions. BioMed Res. Int. 2021, 2021, 5576023. [Google Scholar] [CrossRef]
- Zhang, Y.; Cao, X.; Li, P.; Fan, Y.; Zhang, L.; Ma, X.; Sun, R.; Liu, Y.; Li, W. microRNA-935-modified bone marrow mesenchymal stem cells-derived exosomes enhance osteoblast proliferation and differentiation in osteoporotic rats. Life Sci. 2021, 272, 119204. [Google Scholar] [CrossRef]
- Wei, Y.; Ma, H.; Zhou, H.; Yin, H.; Yang, J.; Song, Y.; Yang, B. miR-424-5p shuttled by bone marrow stem cells-derived exosomes attenuates osteogenesis via regulating WIF1-mediated Wnt/β-catenin axis. Aging 2021, 13, 17190–17201. [Google Scholar] [CrossRef]
- Xun, J.; Li, C.; Liu, M.; Mei, Y.; Zhou, Q.; Wu, B.; Xie, F.; Liu, Y.; Dai, R. Serum exosomes from young rats improve the reduced osteogenic differentiation of BMSCs in aged rats with osteoporosis after fatigue loading in vivo. Stem Cell Res. Ther. 2021, 12, 424. [Google Scholar] [CrossRef]
- Li, X.; Chen, R.; Li, Y.; Wang, P.; Cui, Y.; Yang, L.; Zhu, X.; Zhang, R. miR-27a-5p-Abundant Small Extracellular Vesicles Derived from Epimedium-Preconditioned Bone Mesenchymal Stem Cells Stimulate Osteogenesis by Targeting Atg4B-Mediated Autophagy. Front. Cell Dev. Biol. 2021, 9, 642646. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, X.; Wang, D. Mesenchymal Stem Cell-Derived Extracellular Vesicles Inhibit Osteoporosis via MicroRNA-27a-Induced Inhibition of DKK2-Mediated Wnt/β-Catenin Pathway. Inflammation 2021. [Google Scholar] [CrossRef]
- Tu, C.; He, J.; Chen, R.; Li, Z. The Emerging Role of Exosomal Non-coding RNAs in Musculoskeletal Diseases. Curr. Pharm. Des. 2019, 25, 4523–4535. [Google Scholar] [CrossRef]
- Cao, Q.; Guo, Z.; Yan, Y.; Wu, J.; Song, C. Exosomal long noncoding RNAs in aging and age-related diseases. IUBMB Life 2019, 71, 1846–1856. [Google Scholar] [CrossRef]
- Yang, X.; Yang, J.; Lei, P.; Wen, T. LncRNA MALAT1 shuttled by bone marrow-derived mesenchymal stem cells-secreted exosomes alleviates osteoporosis through mediating microRNA-34c/SATB2 axis. Aging 2019, 11, 8777–8791. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, R.; Liang, F.; Deng, C.; Seidi, F.; Xiao, H. Fluorescent paper-based analytical devices for ultra-sensitive dual-type RNA detections and accurate gastric cancer screening. Biosens. Bioelectron. 2022, 197, 113781. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Liu, J.; Deng, H.; Ma, R.; Liao, J.Y.; Liang, H.; Hu, J.; Li, J.; Guo, Z.; Cai, J.; et al. Targeting Mitochondria-Located circRNA SCAR Alleviates NASH via Reducing mROS Output. Cell 2020, 183, 76–93.e22. [Google Scholar] [CrossRef] [PubMed]
- Shan, S.K.; Lin, X.; Li, F.; Xu, F.; Zhong, J.Y.; Guo, B.; Wang, Y.; Zheng, M.H.; Wu, F.; Yuan, L.Q. Exosomes and Bone Disease. Curr. Pharm. Des. 2019, 25, 4536–4549. [Google Scholar] [CrossRef] [PubMed]
- Cao, G.; Meng, X.; Han, X.; Li, J. Exosomes derived from circRNA Rtn4-modified BMSCs attenuate TNF-α-induced cytotoxicity and apoptosis in murine MC3T3-E1 cells by sponging miR-146a. Biosci. Rep. 2020, 40, BSR20193436. [Google Scholar] [CrossRef]
- Zhan, J.; Wang, Y.; Ma, S.; Qin, Q.; Wang, L.; Cai, Y.; Yang, Z. Organelle-inspired supramolecular nanomedicine to precisely abolish liver tumor growth and metastasis. Bioact. Mater. 2022, 9, 120–133. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, L.; Zheng, M.; Zhu, C.; Wang, G.; Xia, Y.; Blumenthal, E.J.; Mao, W.; Wan, Y. Engineered extracellular vesicles for concurrent Anti-PDL1 immunotherapy and chemotherapy. Bioact. Mater. 2022, 9, 251–265. [Google Scholar] [CrossRef]
- Zhang, S.; Dong, Y.; Wang, Y.; Sun, W.; Wei, M.; Yuan, L.; Yang, G. Selective Encapsulation of Therapeutic mRNA in Engineered Extracellular Vesicles by DNA Aptamer. Nano Lett. 2021, 21, 8563–8570. [Google Scholar] [CrossRef]
- Zhai, X.; Chen, K.; Yang, H.; Li, B.; Zhou, T.; Wang, H.; Zhou, H.; Chen, S.; Zhou, X.; Wei, X.; et al. Extracellular vesicles derived from CD73 modified human umbilical cord mesenchymal stem cells ameliorate inflammation after spinal cord injury. J. Nanobiotechnol. 2021, 19, 274. [Google Scholar] [CrossRef]
- Silva, A.M.; Lázaro-Ibáñez, E.; Gunnarsson, A.; Dhande, A.; Daaboul, G.; Peacock, B.; Osteikoetxea, X.; Salmond, N.; Friis, K.P.; Shatnyeva, O.; et al. Quantification of protein cargo loading into engineered extracellular vesicles at single-vesicle and single-molecule resolution. J. Extracell. Vesicles 2021, 10, e12130. [Google Scholar] [CrossRef]
- Xing, Z.; Zhao, C.; Wu, S.; Yang, D.; Zhang, C.; Wei, X.; Wei, X.; Su, H.; Liu, H.; Fan, Y. Hydrogel Loaded with VEGF/TFEB-Engineered Extracellular Vesicles for Rescuing Critical Limb Ischemia by a Dual-Pathway Activation Strategy. Adv. Healthc. Mater. 2021, 11, e2100334. [Google Scholar] [CrossRef]
- Dooley, K.; McConnell, R.E.; Xu, K.; Lewis, N.D.; Haupt, S.; Youniss, M.R.; Martin, S.; Sia, C.L.; McCoy, C.; Moniz, R.J.; et al. A versatile platform for generating engineered extracellular vesicles with defined therapeutic properties. Mol. Ther. J. Am. Soc. Gene Ther. 2021, 29, 1729–1743. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, H.; Gu, J.; Zhang, J.; Shi, H.; Qian, H.; Wang, D.; Xu, W.; Pan, J.; Santos, H.A. Engineered Extracellular Vesicles for Cancer Therapy. Adv. Mater. 2021, 33, e2005709. [Google Scholar] [CrossRef]
- Yao, X.; Lyu, P.; Yoo, K.; Yadav, M.K.; Singh, R.; Atala, A.; Lu, B. Engineered extracellular vesicles as versatile ribonucleoprotein delivery vehicles for efficient and safe CRISPR genome editing. J. Extracell. Vesicles 2021, 10, e12076. [Google Scholar] [CrossRef]
- Sheller-Miller, S.; Radnaa, E.; Yoo, J.K.; Kim, E.; Choi, K.; Kim, Y.; Kim, Y.N.; Richardson, L.; Choi, C.; Menon, R. Exosomal delivery of NF-κB inhibitor delays LPS-induced preterm birth and modulates fetal immune cell profile in mouse models. Sci. Adv. 2021, 7, eabd3865. [Google Scholar] [CrossRef]
- Tang, S.; Salazar-Puerta, A.; Richards, J.; Khan, S.; Hoyland, J.A.; Gallego-Perez, D.; Walter, B.; Higuita-Castro, N.; Purmessur, D. Non-viral reprogramming of human nucleus pulposus cells with FOXF1 via extracellular vesicle delivery: An in vitro and in vivo study. Eur. Cells Mater. 2021, 41, 90–107. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, Z.; Zhang, Y.; Lan, F.; He, J.; Wu, Y. The essential role of osteoclast-derived exosomes in magnetic nanoparticle-infiltrated hydroxyapatite scaffold modulated osteoblast proliferation in an osteoporosis model. Nanoscale 2020, 12, 8720–8726. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yao, J.; Cai, L.; Liu, T.; Wang, X.; Zhang, Y.; Zhou, Z.; Li, T.; Liu, M.; Lai, R.; et al. Bone-Targeted Extracellular Vesicles from Mesenchymal Stem Cells for Osteoporosis Therapy. Int. J. Nanomed. 2020, 15, 7967–7977. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Su, Y.; Shen, E.; Song, M.; Liu, D.; Qi, H. Extracellular vesicles from GPNMB-modified bone marrow mesenchymal stem cells attenuate bone loss in an ovariectomized rat model. Life Sci. 2021, 272, 119208. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Huang, J.; Chen, F.; Xie, D.; Wang, L.; Ye, C.; Zhu, Q.; Li, X.; Li, X.; Yang, L. MSC-derived small extracellular vesicles overexpressing miR-20a promoted the osteointegration of porous titanium alloy by enhancing osteogenesis via targeting BAMBI. Stem Cell Res. Ther. 2021, 12, 348. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhou, F.; Yuan, P.; Dou, G.; Liu, X.; Liu, S.; Wang, X.; Jin, R.; Dong, Y.; Zhou, J.; et al. T cell-depleting nanoparticles ameliorate bone loss by reducing activated T cells and regulating the Treg/Th17 balance. Bioact. Mater. 2021, 6, 3150–3163. [Google Scholar] [CrossRef]
- Zhou, X.; Cornel, E.J.; Fan, Z.; He, S.; Du, J. Bone-Targeting Polymer Vesicles for Effective Therapy of Osteoporosis. Nano Lett. 2021, 21, 7998–8007. [Google Scholar] [CrossRef]
- Melnik, B.C.; Schmitz, G. Exosomes of pasteurized milk: Potential pathogens of Western diseases. J. Transl. Med. 2019, 17, 3. [Google Scholar] [CrossRef] [Green Version]
- Stickney, Z.; Losacco, J.; McDevitt, S.; Zhang, Z.; Lu, B. Development of exosome surface display technology in living human cells. Biochem. Biophys. Res. Commun. 2016, 472, 53–59. [Google Scholar] [CrossRef]
- Fuhrmann, G.; Serio, A.; Mazo, M.; Nair, R.; Stevens, M.M. Active loading into extracellular vesicles significantly improves the cellular uptake and photodynamic effect of porphyrins. J. Control. Release Off. J. Control. Release Soc. 2015, 205, 35–44. [Google Scholar] [CrossRef]
- Yuan, F.; Li, Y.M.; Wang, Z. Preserving extracellular vesicles for biomedical applications: Consideration of storage stability before and after isolation. Drug Deliv. 2021, 28, 1501–1509. [Google Scholar] [CrossRef]
- Abhange, K.; Makler, A.; Wen, Y.; Ramnauth, N.; Mao, W.; Asghar, W.; Wan, Y. Small extracellular vesicles in cancer. Bioact. Mater. 2021, 6, 3705–3743. [Google Scholar] [CrossRef]
- Cooper, J.M.; Wiklander, P.B.; Nordin, J.Z.; Al-Shawi, R.; Wood, M.J.; Vithlani, M.; Schapira, A.H.; Simons, J.P.; El-Andaloussi, S.; Alvarez-Erviti, L. Systemic exosomal siRNA delivery reduced alpha-synuclein aggregates in brains of transgenic mice. Mov. Disord. Off. J. Mov. Disord. Soc. 2014, 29, 1476–1485. [Google Scholar] [CrossRef] [Green Version]
- Cai, S.; Luo, B.; Jiang, P.; Zhou, X.; Lan, F.; Yi, Q.; Wu, Y. Immuno-modified superparamagnetic nanoparticles via host-guest interactions for high-purity capture and mild release of exosomes. Nanoscale 2018, 10, 14280–14289. [Google Scholar] [CrossRef]
- Jia, G.; Han, Y.; An, Y.; Ding, Y.; He, C.; Wang, X.; Tang, Q. NRP-1 targeted and cargo-loaded exosomes facilitate simultaneous imaging and therapy of glioma in vitro and in vivo. Biomaterials 2018, 178, 302–316. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; Huang, P.; Lin, J.; Zeng, H. The Role of Extracellular Vesicles in Osteoporosis: A Scoping Review. Membranes 2022, 12, 324. https://doi.org/10.3390/membranes12030324
Zhang W, Huang P, Lin J, Zeng H. The Role of Extracellular Vesicles in Osteoporosis: A Scoping Review. Membranes. 2022; 12(3):324. https://doi.org/10.3390/membranes12030324
Chicago/Turabian StyleZhang, Weifei, Pengzhou Huang, Jianjing Lin, and Hui Zeng. 2022. "The Role of Extracellular Vesicles in Osteoporosis: A Scoping Review" Membranes 12, no. 3: 324. https://doi.org/10.3390/membranes12030324
APA StyleZhang, W., Huang, P., Lin, J., & Zeng, H. (2022). The Role of Extracellular Vesicles in Osteoporosis: A Scoping Review. Membranes, 12(3), 324. https://doi.org/10.3390/membranes12030324






