Ionomers Based on Addition and Ring Opening Metathesis Polymerized 5-phenyl-2-norbornene as a Membrane Material for Ionic Actuators
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Polymers Characterization
2.3. Polymerization of 5-phenyl-2-norbornene (ROMP)
2.4. Synthesis of Hydrogenated Poly(5-phenyl-2-norbornene) HPPNB
2.5. Polymerization of 5-phenyl-2-norbornene (Addition Polymerization)
2.6. Copolymerization of 5-phenyl-2-norbornene and 5-docecyl-2-norbornene (Addition Polymerization)
2.7. Sulfonation Procedure
2.8. Synthesis of Polymer with Imidazolium and 1-methylimidazolium Cation
2.9. Degree of Sulfonation Measurement
2.10. Membrane Preparation
2.11. Membrane Characterization
2.12. Preparation of Actuators
2.13. Characterization of Actuators
3. Results
3.1. Polymer Synthesis and Characterization
3.2. Membrane Preparation and Characterization
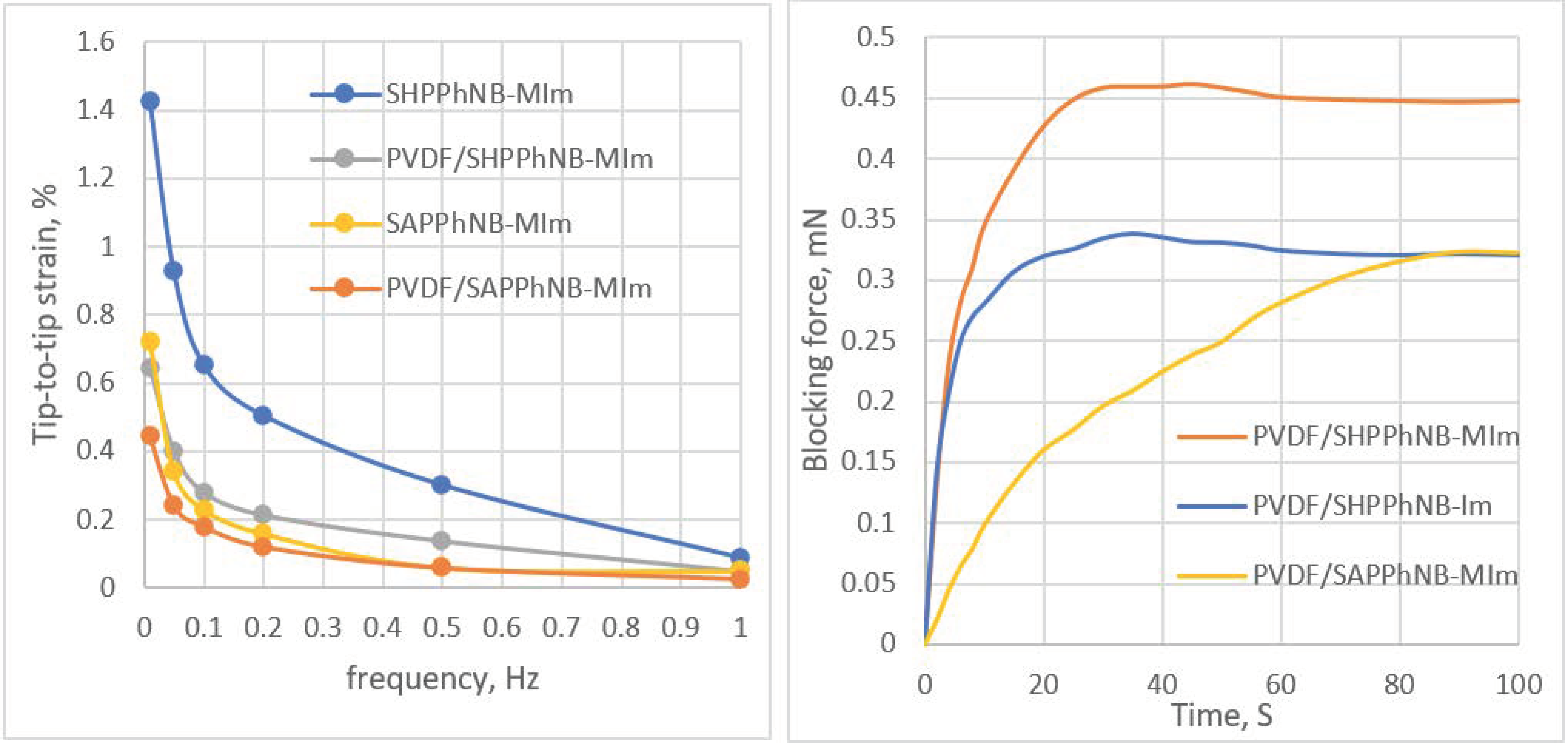
3.3. Bucky-Gel Actuators Fabrication and Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Asaka, K.; Okuzaki, H. Soft Actuators: Materials, Modeling, Applications, and Future Perspectives; Springer: Singapore, 2014; Volume 9784431547, ISBN 9784431547679. [Google Scholar]
- Oguro, K.; Kawami, Y.; Takenaka, H. Bending of an ion-conducting polymer filmelectrode composite by an electric stimulus at low voltage. J. Micromachine Soc. 1992, 5, 27. [Google Scholar]
- Asaka, K.; Oguro, K.; Nishimura, Y.; Mizuhata, M.; Takenaka, H. Bending of Polyelectrolyte Membrane-Platinum Composites by Electric Stimuli I. Response Characteristics to Various Waveforms. Polym. J. 1995, 27, 436–440. [Google Scholar] [CrossRef]
- Duncan, A.J.; Leo, D.J.; Long, T.E. Review Beyond Nafion: Charged Macromolecules Tailored for Performance as Ionic Polymer Transducers. Macromolecules 2008, 41, 7765–7775. [Google Scholar] [CrossRef]
- Jo, C.; Pugal, D.; Oh, I.-K.; Kim, K.J.; Asaka, K. Recent advances in ionic polymer–metal composite actuators and their modeling and applications. Prog. Polym. Sci. 2013, 38, 1037–1066. [Google Scholar] [CrossRef]
- Panwar, V.; Cha, K.; Park, J.-O.; Park, S. High actuation response of PVDF/PVP/PSSA based ionic polymer metal composites actuator. Sens. Actuators B Chem. 2012, 161, 460–470. [Google Scholar] [CrossRef]
- Jeon, J.H.; Kang, S.P.; Lee, S.; Oh, I.K. Novel biomimetic actuator based on SPEEK and PVDF. Sensors Actuators B Chem. 2009. [CrossRef]
- Wang, X.L.; Oh, I.K.; Cheng, T.H. Electro-active polymer actuators employing sulfonated poly(styrene-ran-ethylene) as ionic membranes. Polym. Int. 2010, 59, 305–312. [Google Scholar] [CrossRef]
- Wang, X.L.; Oh, I.K.; Lu, J.; Ju, J.; Lee, S. Biomimetic electro-active polymer based on sulfonated poly (styrene-b-ethylene-co-butylene-b-styrene). Mater. Lett. 2007, 61, 5117–5120. [Google Scholar] [CrossRef]
- Jung, J.-Y.; Oh, I.-K. Novel Nanocomposite Actuator Based on Sulfonated Poly(styrene-b-ethylene-co-butylene-b-styrene) Polymer. J. Nanosci. Nanotechnol. 2007, 7, 3740–3743. [Google Scholar] [CrossRef]
- Wang, X.-L.; Oh, I.-K.; Kim, J.-B. Enhanced electromechanical performance of carbon nano-fiber reinforced sulfonated poly(styrene-b-[ethylene/butylene]-b-styrene) actuator. Compos. Sci. Technol. 2009, 69, 2098–2101. [Google Scholar] [CrossRef]
- Rajagopalan, M.; Jeon, J.-H.H.; Oh, I.-K.K. Electric-stimuli-responsive bending actuator based on sulfonated polyetherimide. Sensors Actuators B Chem. 2010, 151, 198–204. [Google Scholar] [CrossRef]
- Song, J.; Jeon, J.-H.; Oh, I.-K.; Park, K.C. Electro-active Polymer Actuator Based on Sulfonated Polyimide with Highly Conductive Silver Electrodes Via Self-metallization. Macromol. Rapid Commun. 2011, 32, 1583–1587. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Hong, S.M.; Kim, J.; Koo, C.M. Novel sulfonated styrenic pentablock copolymer/silicate nanocomposite membranes with controlled ion channels and their IPMC transducers. Sens. Actuators B Chem. 2012, 162, 369–376. [Google Scholar] [CrossRef]
- Vargantwar, P.H.; Roskov, K.E.; Ghosh, T.K.; Spontak, R.J. Enhanced biomimetic performance of ionic polymer-metal composite actuators prepared with nanostructured block ionomers. Macromol. Rapid Commun. 2012, 33, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Yu, S.; Hong, S.M.; Koo, C.M. High-strain air-working soft transducers produced from nanostructured block copolymer ionomer/silicate/ionic liquid nanocomposite membranes. J. Mater. Chem. C 2013, 1, 3784–3793. [Google Scholar] [CrossRef]
- Tang, Y.; Chen, C.; Ye, Y.S.; Xue, Z.; Zhou, X.; Xie, X. The enhanced actuation response of an ionic polymer–metal composite actuator based on sulfonated polyphenylsulfone. Polym. Chem 2014, 5, 6097. [Google Scholar] [CrossRef]
- Khan, A.; Jain, R.K.; Ghosh, B.; Inamuddin, I.; Asiri, A.M. Novel ionic polymer-metal composite actuator based on sulfonated poly(1,4-phenylene ether-ether-sulfone) and polyvinylidene fluoride/sulfonated graphene oxide. RSC Adv. 2018, 8, 25423–25435. [Google Scholar] [CrossRef] [Green Version]
- Shahinpoor, M.; Bar-Cohen, Y.; Simpson, J.O.; Smith, J. Ionic polymer-metal composites (IPMCs) as biomimetic sensors, actuators and artificial muscles—A review. Smart Mater. Struct. 1998, 7, R15–R30. [Google Scholar] [CrossRef]
- Newbury, K.M.; Leo, D.J. Linear electromechanical model of ionic polymer transducers—Part I: Model development. J. Intell. Mater. Syst. Struct. 2003, 14, 333–342. [Google Scholar] [CrossRef]
- Jaakson, P.; Aabloo, A.; Tamm, T. Encapsulation of ionic electroactive polymers: Reducing the interaction with environment. In Proceedings of the Electroactive Polymer Actuators and Devices (EAPAD); Bar-Cohen, Y., Vidal, F., Eds.; SPIE: Bellingham, WA, USA, 2016; Volume 9798, p. 979825. [Google Scholar]
- Bennett, M.D.; Leo, D.J.; Wilkes, G.L.; Beyer, F.L.; Pechar, T.W. A model of charge transport and electromechanical transduction in ionic liquid-swollen Nafion membranes. Polymer 2006, 47, 6782–6796. [Google Scholar] [CrossRef]
- Ding, J.; Zhou, D.; Spinks, G.; Wallace, G.; Forsyth, S.; Forsyth, M.; MacFarlane, D. Use of ionic liquids as electrolytes in electromechanical actuator systems based on inherently conducting polymers. Chem. Mater. 2003, 15, 2392–2398. [Google Scholar] [CrossRef]
- Vidal, F.; Plesse, C.; Teyssié, D.; Chevrot, C. Long-life air working conducting semi-IPN/ionic liquid based actuator. Synth. Met. 2004, 142, 287–291. [Google Scholar] [CrossRef]
- Fukushima, T.; Asaka, K.; Kosaka, A.; Aida, T. Fully plastic actuator through layer-by-layer casting with ionic-liquid-based bucky gel. Angew. Chemie-Int. Ed. 2005, 44, 2410–2413. [Google Scholar] [CrossRef] [PubMed]
- Sahrash, R.; Siddiqa, A.; Razzaq, H.; Iqbal, T.; Qaisar, S. PVDF based ionogels: Applications towards electrochemical devices and membrane separation processes. Heliyon 2018, 4, e00847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mukai, K.; Asaka, K.; Kiyohara, K.; Sugino, T.; Takeuchi, I.; Fukushima, T.; Aida, T. High performance fully plastic actuator based on ionic-liquid-based bucky gel. Electrochim. Acta 2008, 53, 5555–5562. [Google Scholar] [CrossRef]
- Terasawa, N.; Asaka, K. Electrochemical and Electromechanical Properties of Activated Multi-walled Carbon Nanotube Polymer Actuator that Surpass the Performance of a Single-walled Carbon Nanotube Polymer Actuator. Mater. Today Proc. 2016, 3, S178–S183. [Google Scholar] [CrossRef]
- Terasawa, N. High-performance ionic and non-ionic fluoropolymer/ionic liquid gel hybrid actuators based on single-walled carbon nanotubes. RSC Adv. 2017, 7, 2443–2449. [Google Scholar] [CrossRef] [Green Version]
- Imaizumi, S.; Ohtsuki, Y.; Yasuda, T.; Kokubo, H.; Watanabe, M. Printable polymer actuators from ionic liquid, soluble polyimide, and ubiquitous carbon materials. ACS Appl. Mater. Interfaces 2013, 5, 6307–6315. [Google Scholar] [CrossRef]
- Kim, O.; Shin, T.J.; Park, M.J. Fast low-voltage electroactive actuators using nanostructured polymer electrolytes. Nat. Commun. 2013, 4, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Kim, O.; Kim, H.; Choi, U.H.; Park, M.J. One-volt-driven superfast polymer actuators based on single-ion conductors. Nat. Commun. 2016, 7, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Zhao, Y.; Feng, Z.; Xiang, X.; Wang, S.; Xie, X.; Ramani, V.K. Ring-opening metathesis polymerization for the preparation of polynorbornene-based proton exchange membranes with high proton conductivity. J. Memb. Sci. 2017, 528, 55–63. [Google Scholar] [CrossRef]
- Bermeshev, M.V.; Chapala, P.P. Addition polymerization of functionalized norbornenes as a powerful tool for assembling molecular moieties of new polymers with versatile properties. Prog. Polym. Sci. 2018, 84, 1–46. [Google Scholar] [CrossRef]
- Artemov, A.N.; Sazonova, E.V.; Revin, M.V.; Rybkin, K.V.; Lazarev, M.A.; Faerman, V.I. [2+4] Cycloaddition of η 6-(styrene)chromium tricarbonyl and conjugated dienes. Russ. Chem. Bull. 2011, 60, 2103–2106. [Google Scholar] [CrossRef]
- Burrell, A.K.; Del Sesto, R.E.; Baker, S.N.; McCleskey, T.M.; Baker, G.A. The large scale synthesis of pure imidazolium and pyrrolidinium ionic liquids. Green Chem. 2007, 9, 449–454. [Google Scholar] [CrossRef]
- Morozov, O.S.; Bulgakov, B.A.; Ivanchenko, A.V.; Shachneva, S.S.; Nechausov, S.S.; Bermeshev, M.V.; Kepman, A.V. Data on synthesis and characterization of sulfonated poly(phenylnorbornene) and polymer electrolyte membranes based on it. Data Br. 2019, 27, 104626. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, I.; Asaka, K.; Kiyohara, K.; Sugino, T.; Terasawa, N.; Mukai, K.; Fukushima, T.; Aida, T. Electromechanical behavior of fully plastic actuators based on bucky gel containing various internal ionic liquids. Electrochim. Acta 2009, 54, 1762–1768. [Google Scholar] [CrossRef]
- Tamagawa, H.; Yagasaki, K.; Nogata, F. Mechanical characteristics of ionic polymer-metal composite in the process of self-bending. J. Appl. Phys. 2002, 92, 7614–7618. [Google Scholar] [CrossRef]
- Li, S.; Burns, A.B.; Register, R.A.; Bell, A. Poly(phenylnorbornene) from ring-opening metathesis and its hydrogenated derivatives. Macromol. Chem. Phys. 2012, 213, 2027–2033. [Google Scholar] [CrossRef]
- Finkelshtein, E.S.; Bermeshev, M.V.; Gringolts, M.L.; Starannikova, L.E.; Yampolskii, Y.P. Substituted polynorbornenes as promising materials for gas separation membranes. Russ. Chem. Rev. 2011, 80, 341–361. [Google Scholar] [CrossRef]
- Janata, M.; Kůdela, V.; Gromadzki, D.; Štěpánek, P.; Nallet, F.; Diat, O.; Vlček, P.; Toman, L. Synthesis of highly sulfonated polystyrene-based block copolymers soluble in tetrahydrofuran. E-Polymers 2006, 6, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Bhutto, A.A.; Vesely, D.; Gabrys, B.J. Miscibility and interactions in polystyrene and sodium sulfonated polystyrene with poly(vinyl methyl ether) PVME blends. Part II. FTIR. Polymer 2003, 44, 6627–6631. [Google Scholar] [CrossRef]
- Brijmohan, S.B.; Swier, S.; Weiss, R.A.; Shaw, M.T. Synthesis and characterization of cross-linked sulfonated polystyrene nanoparticles. Ind. Eng. Chem. Res. 2005, 44, 8039–8045. [Google Scholar] [CrossRef]
- Vinhola, L.; Facci, T.; Dias, L.G.; De Azevedo, D.C.; Borissevitch, G.; Huguenin, F. Self-assembled films from chitosan and poly(vinyl sulfonic acid) on Nafion®® for direct methanol fuel cell. J. Braz. Chem. Soc. 2012, 23, 531–537. [Google Scholar] [CrossRef] [Green Version]
- Boroglu, M.S.; Celik, S.U.; Bozkurt, A.; Boz, I. The synthesis and characterization of anhydrous proton conducting membranes based on sulfonated poly(vinyl alcohol) and imidazole. J. Memb. Sci. 2011, 375, 157–164. [Google Scholar] [CrossRef]
- He, S.; Zhai, S.; Zhang, C.; Xue, Y.; Yang, W.; Lin, J. Effect of sulfonation degree and PVDF content on the structure and transport properties of SPEEK/PVDF blend membranes. Polymers 2019, 11, 676. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Xi, J.; Zhou, H.; Liu, L.; Wu, Z.; Qiu, X.; Chen, L. Preparation and characterization of sulfonated poly(ether ether ketone)/poly(vinylidene fluoride) blend membrane for vanadium redox flow battery application. J. Power Sources 2013, 237, 132–140. [Google Scholar] [CrossRef]
- Kim, O.; Kim, S.J.; Park, M.J. Low-voltage-driven soft actuators. Chem. Commun. 2018, 54, 4895–4904. [Google Scholar] [CrossRef] [Green Version]

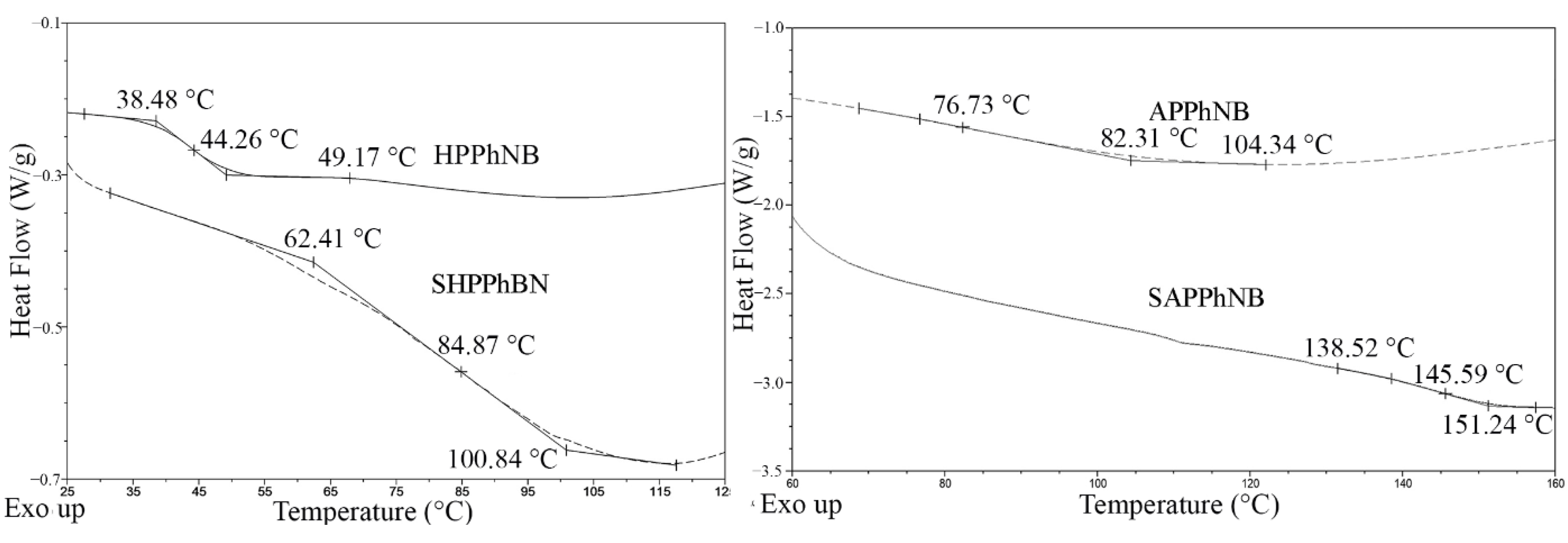
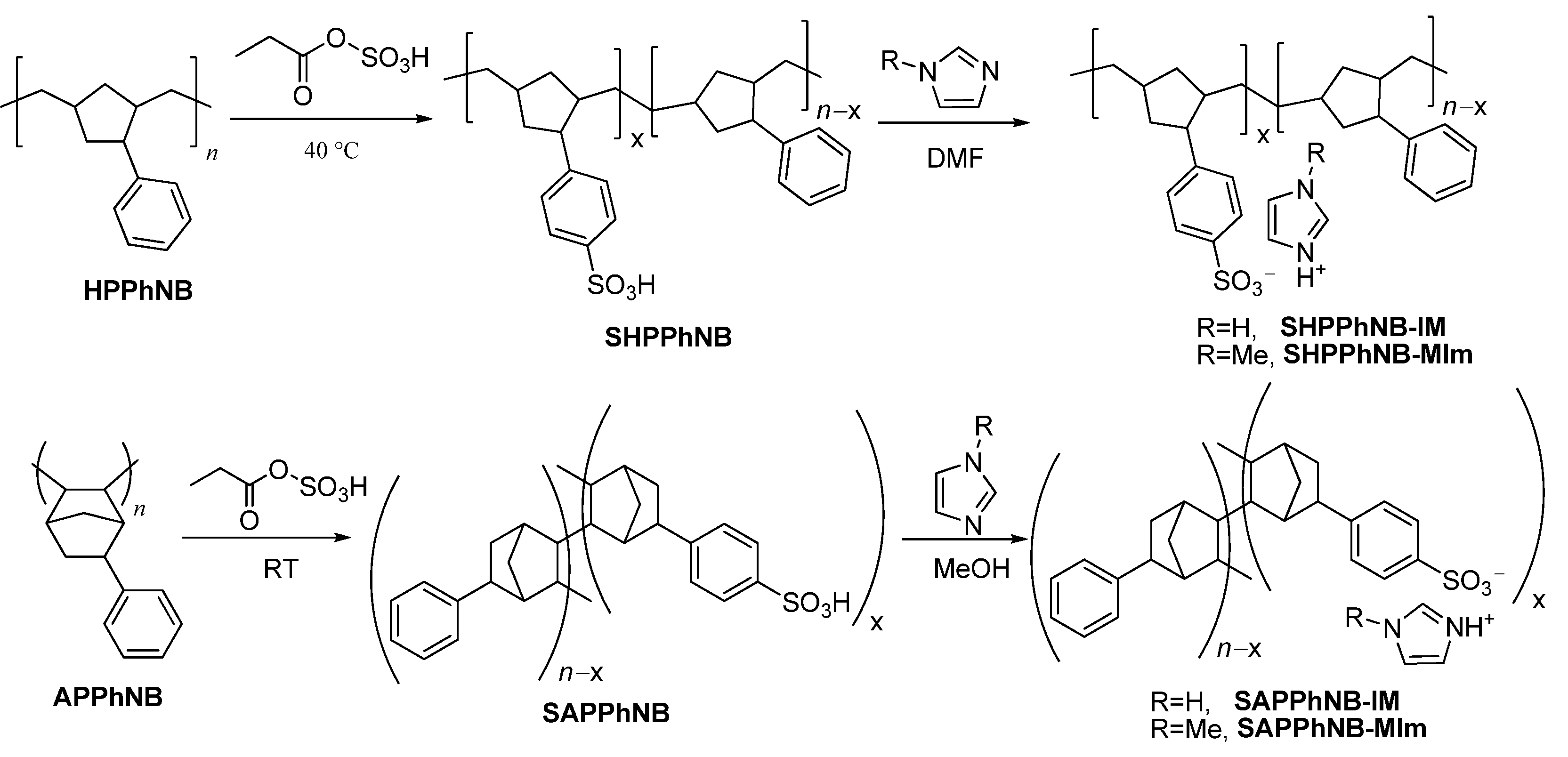

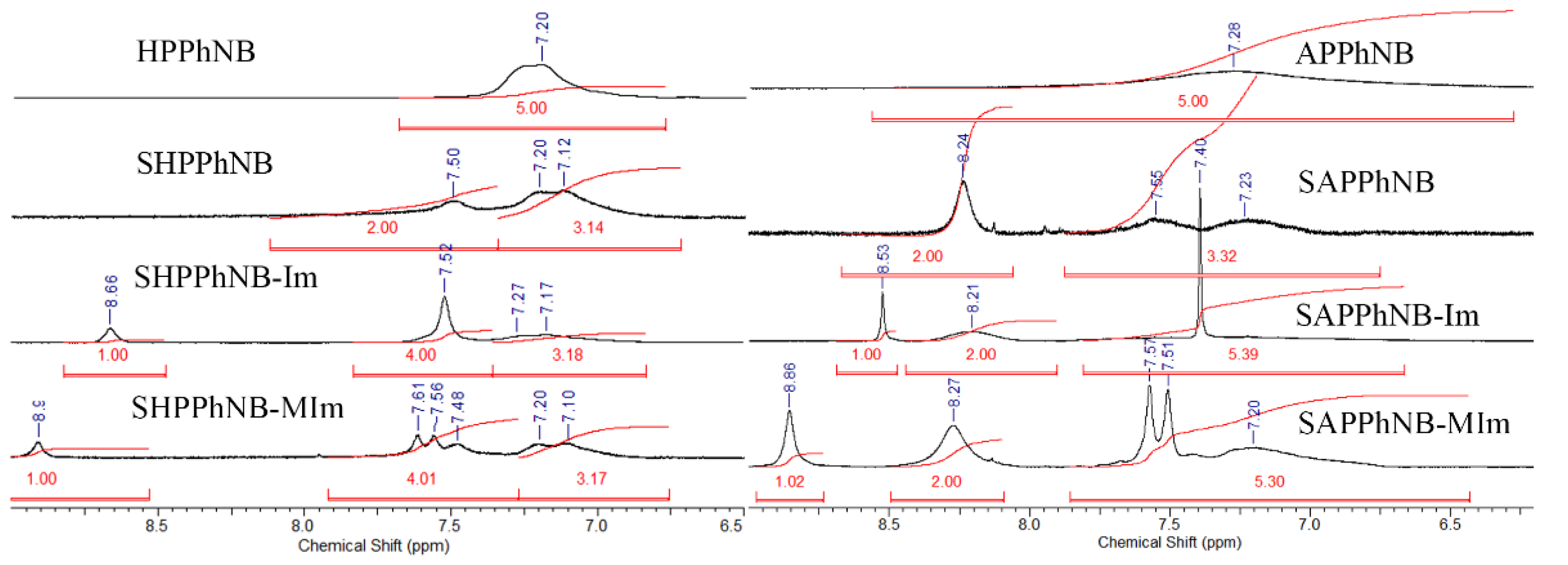

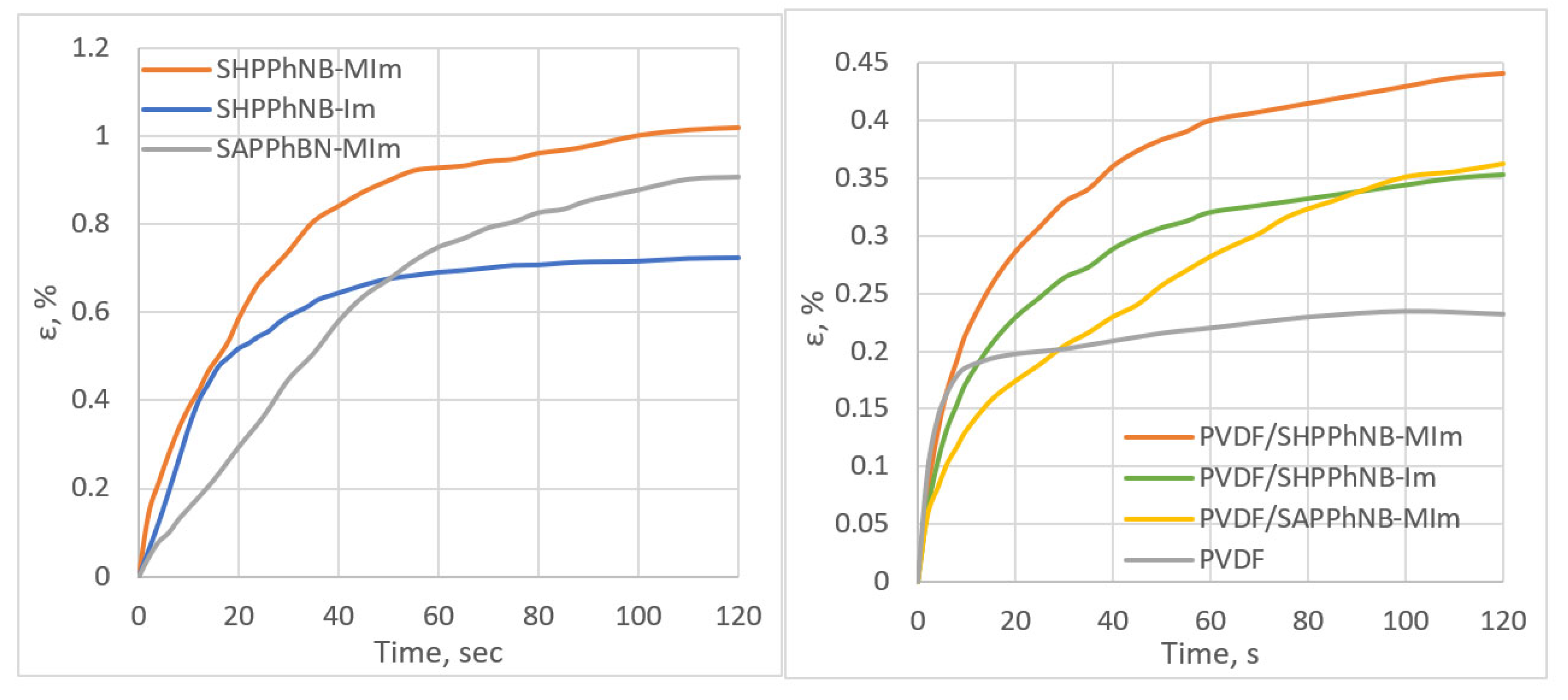
| Monomer/Catalyst Ratio | Cmonomer, mol/L | Yield, % | Mw·10−3 | Mw/Mn |
|---|---|---|---|---|
| 1000 | 0.19 | 86 | 1100 | 2.9 |
| 3000 * | 0.28 | 88 | 1200 | 3.5 |
| 4000 | 0.21 | 53 | 1200 | 2.9 |
| 6000 | 0.22 | 31 | 1600 | 2.7 |
| 6000 | 0.47 | 35 | 980 | 2.1 |
| 8000 | 0.49 | 25 | 1200 | 2.0 |
| Solvent | Gel Formation Time | Solubility | Sulfonation Degree | IEC, Meq/g |
|---|---|---|---|---|
| CH2Cl2 | 2 h | DMSO | 67% | 2.97 |
| CHCl3 | 2 h | DMF, NMP, DMSO | 53% | 2.47 |
| ClCH2CH2Cl | 4 h | CHCl3-MeOH, NMP, DMF, DMSO | 37% | 1.84 |
| CH2Cl2 * | 1.5 h | DMSO | 79% | 3.36 |
| Membrane | Composition, wt.% | IL/SO3X | σ, mS/cm |
|---|---|---|---|
| SHPPhNB-Im | 100 | 0.038 ± 0.0046 | |
| SHPPhNB-MIm | 100 | 0.11 ± 0.029 | |
| SHPPhNB/EMImBF4 | 50/50 | 1.19 | 1.39 ± 0.020 |
| SHPPhNB-Im/EMImBF4 | 50/50 | 1.52 | 3.45 ± 0.080 |
| SHPPhNB-MIm/EMImBF4 | 50/50 | 1.57 | 4.81 ± 0.15 |
| SAPPhNB-Im/EMImBF4 | 50/50 | 1.51 | 0.50 ± 0.051 |
| SAPPhNB-MIm/EMImBF4 | 50/50 | 1.54 | 1.02 ± 0.010 |
| Membrane | Thickness, µm | Modulus, MPa | Tensile Strength, MPa | Fracture Strain, % | σ, mS/cm |
|---|---|---|---|---|---|
| PVDF/SHPPhNB-Im | 93 ± 2 | 170 ± 0.6 | 5.1 | 15 | 0.41 ± 0.062 |
| PVDF/SHPPhNB-MIm | 102 ± 3 | 253 ± 2.0 | 12.1 | 34 | 1.24 ± 0.087 |
| PVDF/SAPPhNB-MIm | 95 ± 2 | 313 ± 1.2 | 10.8 | 24 | 0.33 ± 0.011 |
| Actuator | Applied Voltage | |
|---|---|---|
| 1 V | 2 V | |
| SHPPhNB-MIm | 0.27% | 1.14% |
| SHPPhNB-Im | 0.22% | 0.67% |
| PVDF/SHPPhNB-MIm | 0.10% | 0.48% |
| PVDF/SHPPhNB-Im | 0.08% | 0.35% |
| SAPPhNB-MIm | 0.25% | 0.95% |
| PVDF/SAPPhNB-MIm | 0.09% | 0.44% |
| PVDF | 0.02% | 0.23% |
| PVdF(HFP)/Nafion(1:3) [29] | 0.48% | |
| Actuator | σbeam, MPa | σHook, MPa |
|---|---|---|
| PVDF/SHPPhNB-MIm | 1.21 ± 0.02 | 1.18 |
| PVDF/SHPPhNB-Im | 0.87 ± 0.02 | 0.86 |
| PVDF/SAPPhNB-MIm | 1.19 ± 0.04 | 1.08 |
| PVDF/EMImBF4 | 0.69 ± 0.06 | 0.61 |
| PVdF(HFP)/Nafion(1:3)/EMImBF4 [29] | 0.84 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morozov, O.S.; Babkin, A.V.; Ivanchenko, A.V.; Shachneva, S.S.; Nechausov, S.S.; Alentiev, D.A.; Bermeshev, M.V.; Bulgakov, B.A.; Kepman, A.V. Ionomers Based on Addition and Ring Opening Metathesis Polymerized 5-phenyl-2-norbornene as a Membrane Material for Ionic Actuators. Membranes 2022, 12, 316. https://doi.org/10.3390/membranes12030316
Morozov OS, Babkin AV, Ivanchenko AV, Shachneva SS, Nechausov SS, Alentiev DA, Bermeshev MV, Bulgakov BA, Kepman AV. Ionomers Based on Addition and Ring Opening Metathesis Polymerized 5-phenyl-2-norbornene as a Membrane Material for Ionic Actuators. Membranes. 2022; 12(3):316. https://doi.org/10.3390/membranes12030316
Chicago/Turabian StyleMorozov, Oleg S., Alexander V. Babkin, Anna V. Ivanchenko, Svetlana S. Shachneva, Sergey S. Nechausov, Dmitry A. Alentiev, Maxim V. Bermeshev, Boris A. Bulgakov, and Alexey V. Kepman. 2022. "Ionomers Based on Addition and Ring Opening Metathesis Polymerized 5-phenyl-2-norbornene as a Membrane Material for Ionic Actuators" Membranes 12, no. 3: 316. https://doi.org/10.3390/membranes12030316
APA StyleMorozov, O. S., Babkin, A. V., Ivanchenko, A. V., Shachneva, S. S., Nechausov, S. S., Alentiev, D. A., Bermeshev, M. V., Bulgakov, B. A., & Kepman, A. V. (2022). Ionomers Based on Addition and Ring Opening Metathesis Polymerized 5-phenyl-2-norbornene as a Membrane Material for Ionic Actuators. Membranes, 12(3), 316. https://doi.org/10.3390/membranes12030316







