Low-Molecular-Weight Lignin Recovery with Nanofiltration in the Kraft Pulping Process
Abstract
:1. Introduction
2. Materials and Methods
2.1. Feed Solution
2.2. Membranes
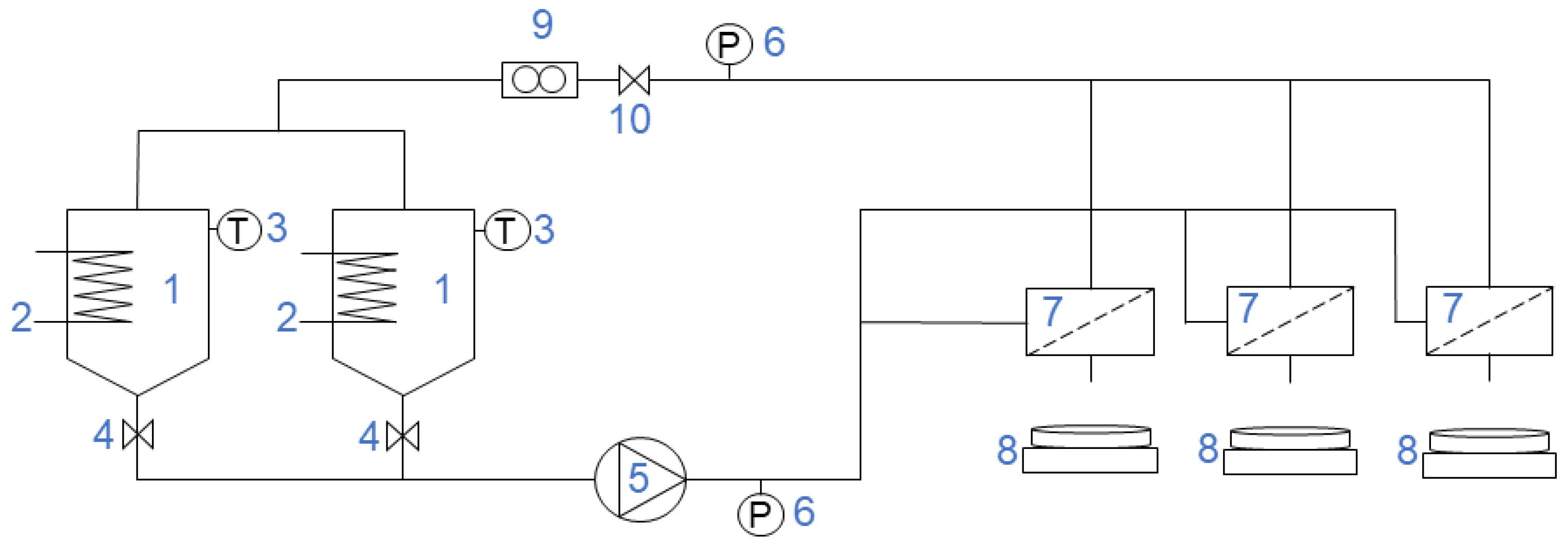
2.3. Experimental Setup
2.4. Experimental Procedure
2.4.1. Cleaning and PWF Measurements
2.4.2. Sodium Hydroxide Conditioning
2.4.3. Parametric Studies
2.4.4. Concentration Studies
2.4.5. Analysis
3. Results and Discussion
3.1. Parametric Studies
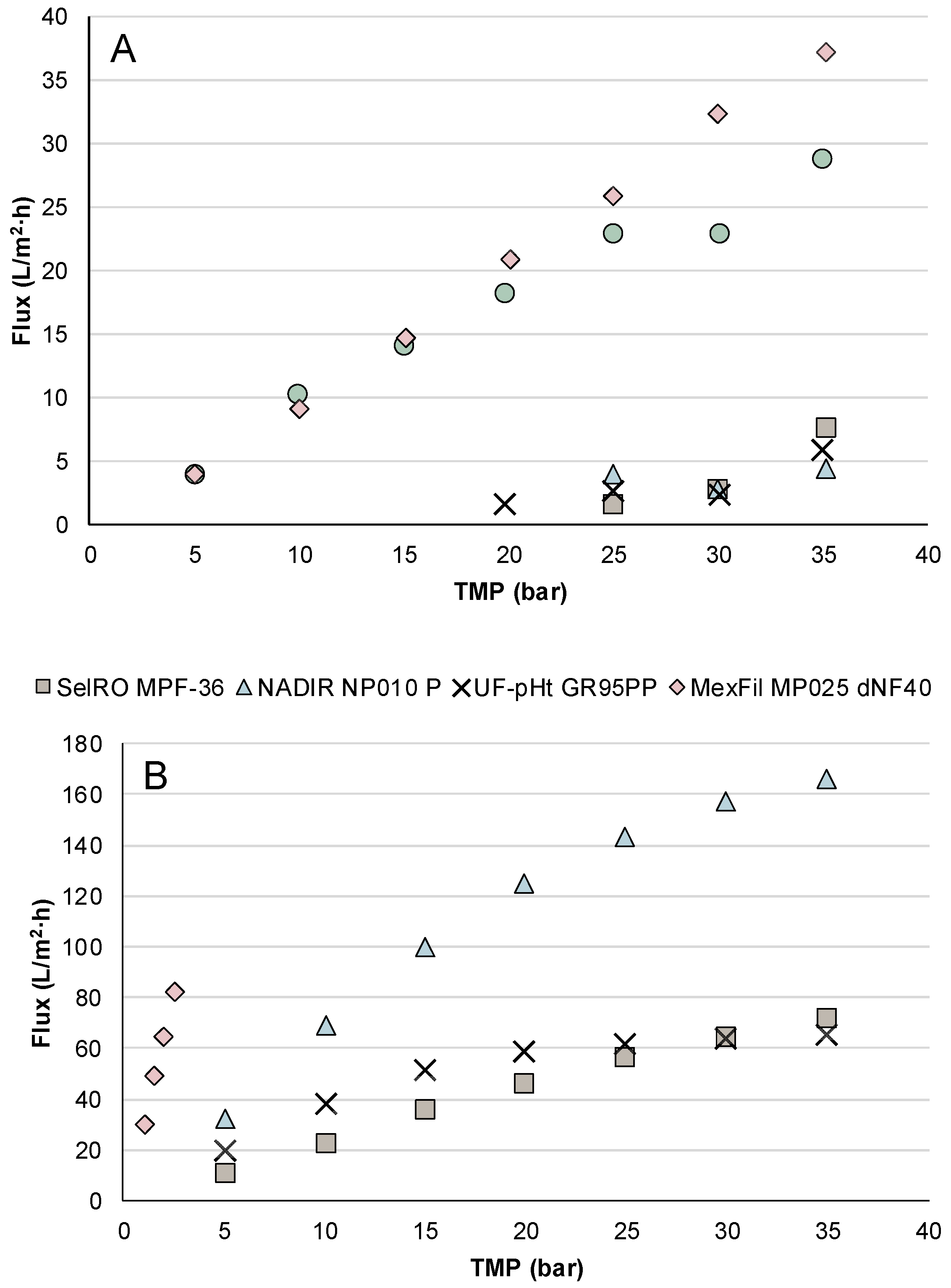
3.1.1. Influence of TMP on the Flux
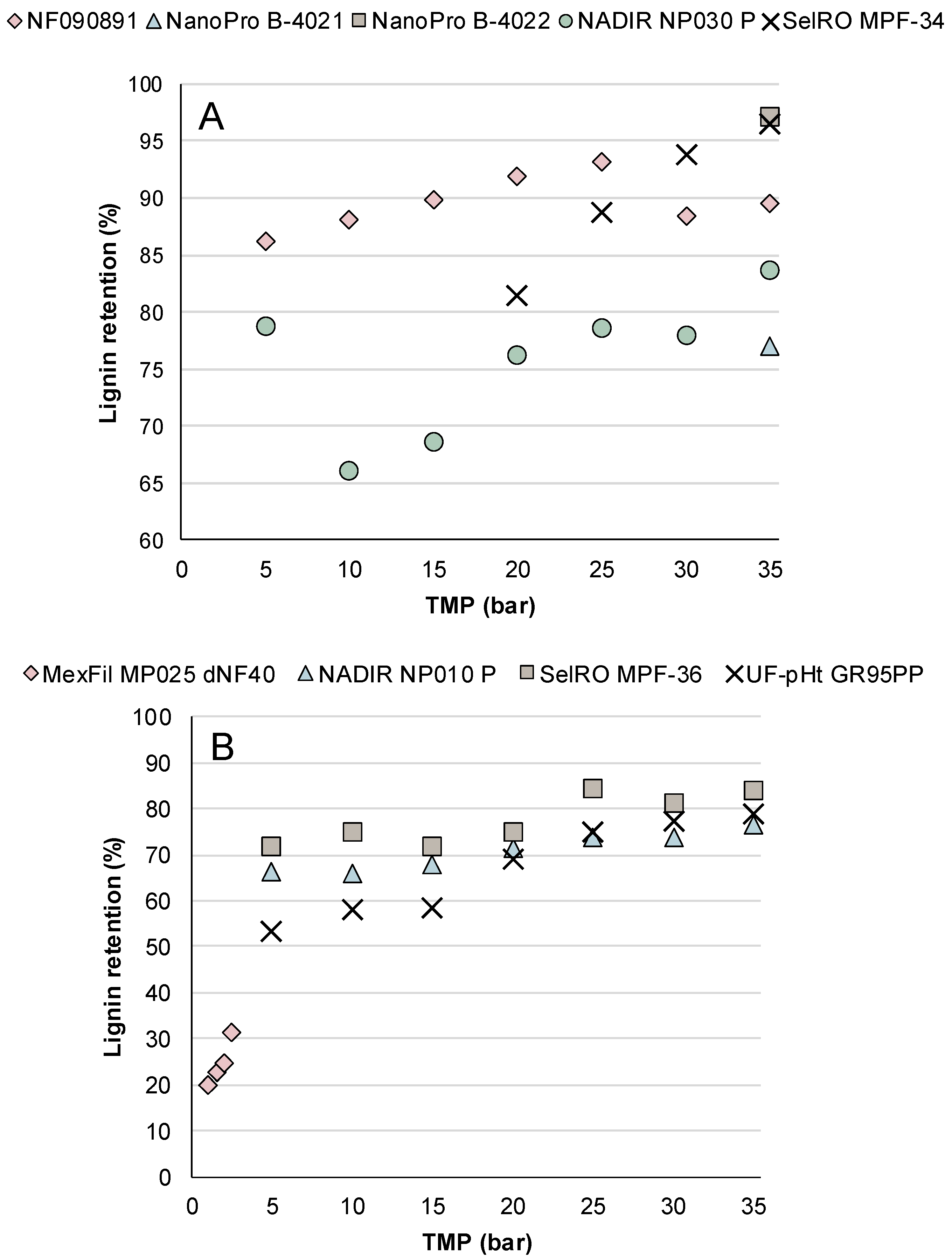
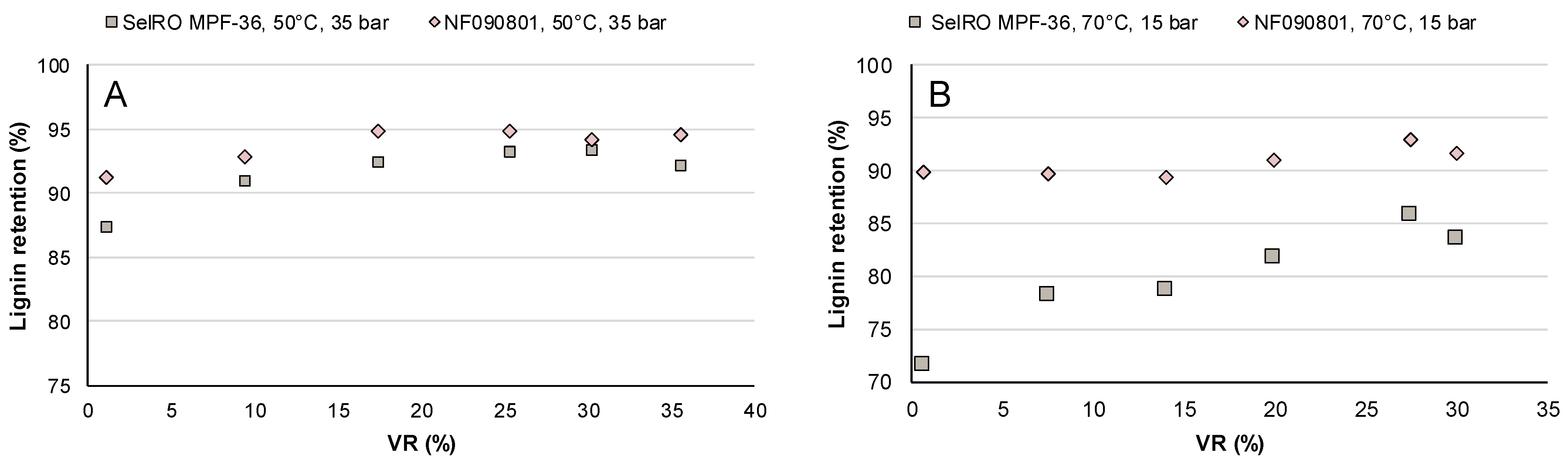
3.1.2. Lignin Retention
3.2. Concentration Studies
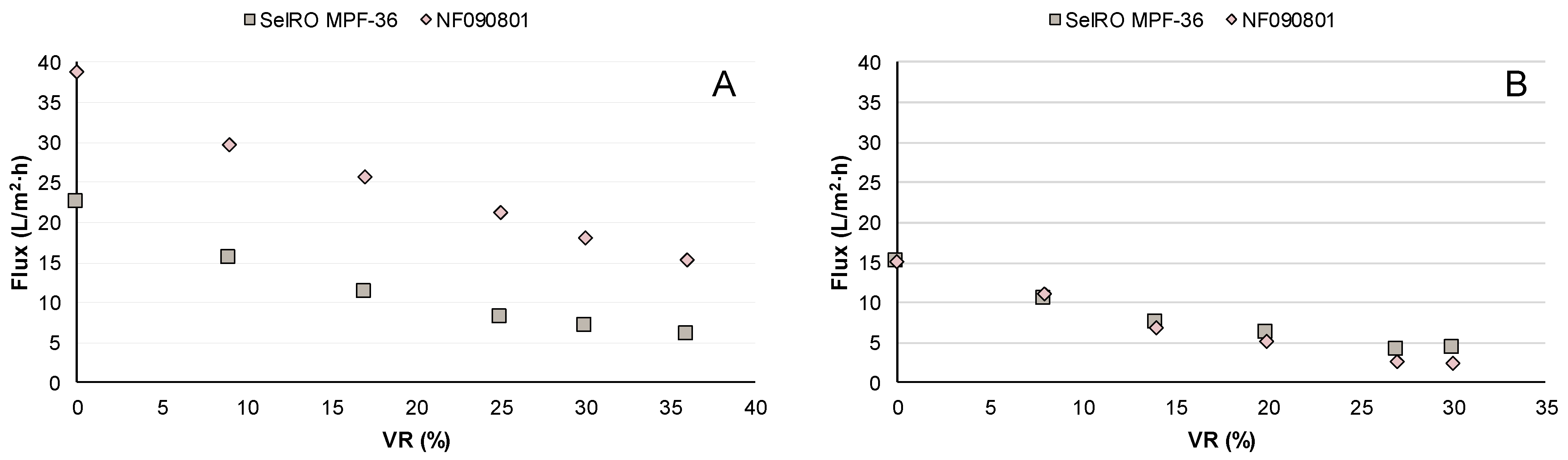
3.2.1. Lignin Retention
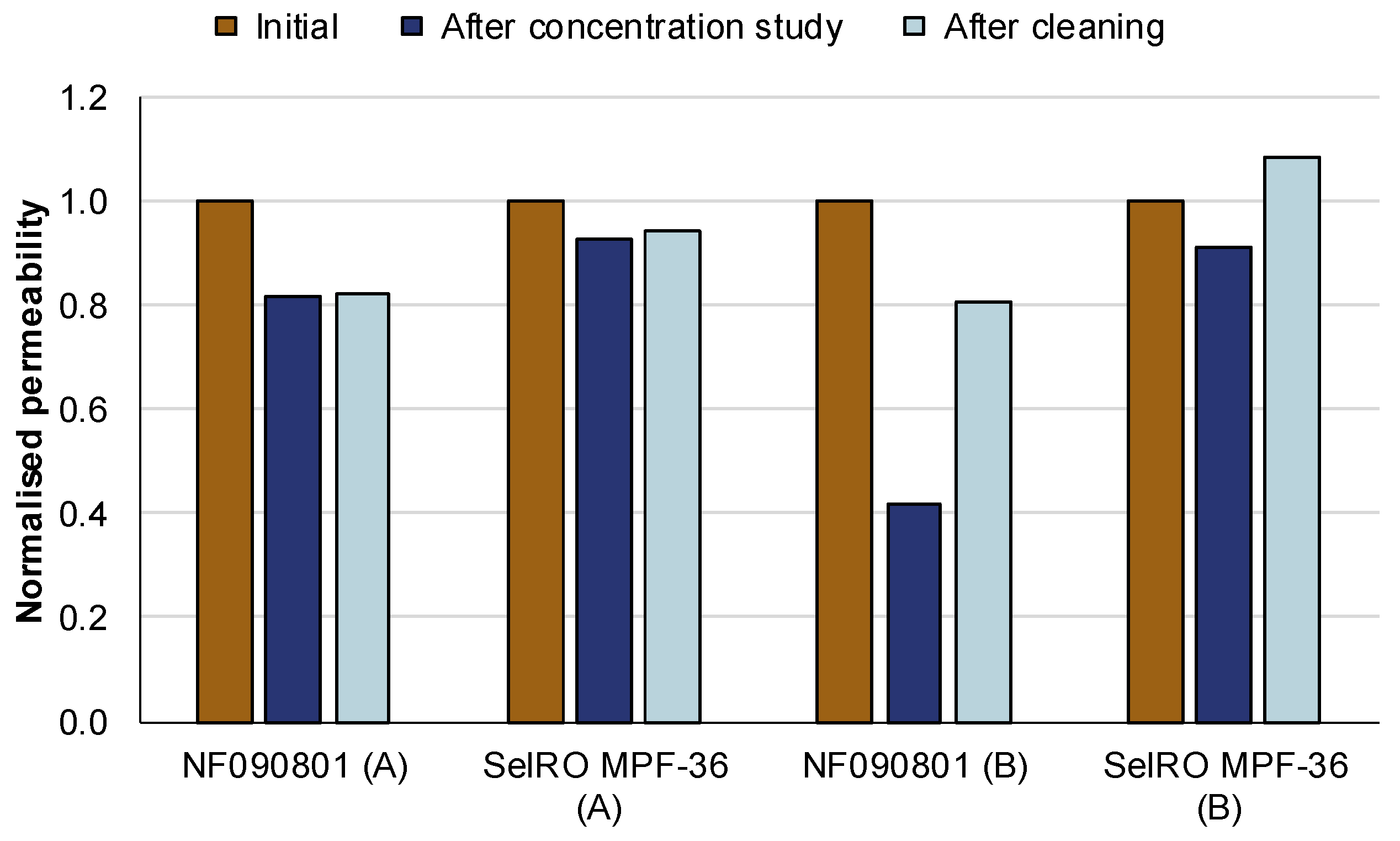
3.2.2. Membrane Permeability and Fouling
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Glasser, W.G. About Making Lignin Great Again—Some Lessons from the Past. Front. Chem. 2019, 7, 565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.; Kazzaz, A.E.; Alipoormazandarani, N.; Feizi, Z.H.; Fatehi, P. Production of Flocculants, Adsorbents, and Dispersants from Lignin. Molecules 2018, 23, 868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dieste, A.; Clavijo, L.; Torres, A.I.; Barbe, S.; Oyarbide, I.; Bruno, L.; Cassella, F. Lignin from Eucalyptus spp. Kraft Black Liquor as Biofuel. Energy Fuels 2016, 30, 10494–10498. [Google Scholar] [CrossRef]
- Tran, H.; Vakkilainnen, E.K. The Kraft Chemical Recovery Process. Tappi Kraft Pulping Short Course 2008, 1–8. Available online: https://www.tappi.org/content/events/08kros/manuscripts/1-1.pdf (accessed on 9 December 2021).
- Berlin, A.; Balakshin, M. Industrial Lignins: Analysis, Properties, and Applications. In Bioenergy Research: Advances and Applications; Elsevier: Amsterdam, The Netherlands, 2014; pp. 315–336. [Google Scholar] [CrossRef]
- Axegård, P. Possibilities for Improving the Pulping Process. 2003, pp. 1–6. Available online: https://www.eucalyptus.com.br/icep01/peter_axegard.pdf (accessed on 10 December 2021).
- Jönsson, A.-S. Membranes for lignin and hemicellulose recovery in pulp mills. In Membrane Technologies for Biorefining; Elsevier: Amsterdam, The Netherlands, 2016; pp. 105–133. [Google Scholar]
- Loufti, H.; Blackwell, B.; Uloth, V. Lignin recovery from kraft black liquor: Preliminary process design. Tappi J. 1991, 74, 203. [Google Scholar]
- Zhu, W. Precipitation of Kraft Lignin Yield and Equilibrium, Chalmers University of Technology. 2015. Available online: http://publications.lib.chalmers.se/records/fulltext/216246/216246.pdf (accessed on 10 December 2021).
- Uloth, V.; Wearing, J.T. Kraft Lignin Recovery: Acid Precipitation Versus Ultrafiltration, Part I: Laboratory Test Results. Pulp Pap. Can. 1989, 90, 67–71. [Google Scholar]
- Pye, E.K. Industrial Lignin Production and Applications; Wiley: Hoboken, NJ, USA, 2005; pp. 165–200. [Google Scholar] [CrossRef]
- Öhman, F.; Wallmo, H.; Theliander, H. An improved method for washing lignin precipitated from kraft black liquor—The key to a new biofuel. Filtration 2007, 7, 309–315. [Google Scholar]
- Tomani, P. The LignoBoost Process. Cellul. Chem. Technol. 2010, 44, 53–58. [Google Scholar]
- Wallberg, O.; Jönsson, A.-S. Separation of lignin in kraft cooking liquor from a continuous digester by ultrafiltration at temperatures above 100 °C. Desalination 2006, 195, 187–200. [Google Scholar] [CrossRef]
- Toledano, A.; García, A.; Mondragon, I.; Labidi, J. Lignin separation and fractionation by ultrafiltration. Sep. Purif. Technol. 2010, 71, 38–43. [Google Scholar] [CrossRef]
- Kirkman, A.G.; Gratzl, J.S.; Edwards, L.L. Kraft Lignin Recovery by Ultrafiltration: Economic Feasibility and Impact on the Kraft Recovery System. 1986. Available online: https://www.osti.gov/biblio/6429790 (accessed on 15 November 2021).
- Wallberg, O.; Jönsson, A.-S. Influence of the Membrane Cut-off during Ultrafiltration of Kraft Black Liquor with Ceramic Membranes. Chem. Eng. Res. Des. 2003, 81, 1379–1384. [Google Scholar] [CrossRef]
- Holmqvist, A.; Wallberg, O.; Jönsson, A.-S. Ultrafiltration of Kraft Black Liquor from Two Swedish Pulp Mills. Chem. Eng. Res. Des. 2005, 83, 994–999. [Google Scholar] [CrossRef]
- Liu, G.; Liu, Y.; Ni, J.; Shi, H.; Qian, Y. Treatability of kraft spent liquor by microfiltration and andultrafiltration. Desalination 2004, 160, 131–141. [Google Scholar] [CrossRef]
- Keyoumu, A.; Sjödahl, R.; Henriksson, G.; Ek, M.; Gellerstedt, G.; Lindström, M.E. Continuous nano- and ultra-filtration of kraft pulping black liquor with ceramic filters: A method for lowering the load on the recovery boiler while generating valuable side-products. Ind. Crops Prod. 2004, 20, 143–150. [Google Scholar] [CrossRef]
- Arkell, A.; Olsson, J.; Wallberg, O. Process performance in lignin separation from softwood black liquor by membrane filtration. Chem. Eng. Res. Des. 2014, 92, 1792–1800. [Google Scholar] [CrossRef]
- Dafinov, A.; Font, J.; Garcia-Valls, R. Processing of black liquors by UF/NF ceramic membranes. Desalination 2005, 173, 83–90. [Google Scholar] [CrossRef]
- Hulteberg, C.; Ekelund, L.; Kollberg, J.; Sundin, M.; Arkell, A. SunCarbon: Creating a New Value Chain from Forest to Re-Fineries Using Homogeneous and Heterogeneous Catalysis; EuropaCat: Aachen, Germany, 2019. [Google Scholar]
- Valderrama, O.J.; Zedda, K.L.; Velizarov, S. Membrane Filtration Opportunities for the Treatment of Black Liquor in the Paper and Pulp Industry. Water 2021, 13, 2270. [Google Scholar] [CrossRef]
- Wang, Z.; Ma, C.; Sinquefield, S.A.; Shofner, M.L.; Nair, S. High-Performance Graphene Oxide Nanofiltration Membranes for Black Liquor Concentration. ACS Sustain. Chem. Eng. 2019, 7, 14915–14923. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of Structural Carbohydrates and Lignin in Biomass, Golden, Colorado. 2012. Available online: http://www.nrel.gov/docs/gen/fy13/42618.pdf (accessed on 22 September 2021).
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of Ash in Biomass, Golden, Colorado, 2005. Available online: https://www.nrel.gov/docs/gen/fy08/42622.pdf (accessed on 22 September 2021).
- Jönsson, A.-S.; Nordin, A.-K.; Wallberg, O. Concentration and purification of lignin in hardwood kraft pulping liquor by ultrafiltration and nanofiltration. Chem. Eng. Res. Des. 2008, 86, 1271–1280. [Google Scholar] [CrossRef]
- Schlackl, K.; Herchl, R.; Almhofer, L.; Bischof, R.; Fackler, K.; Samhaber, W. Intermolecular Interactions in the Membrane Filtration of Highly Alkaline Steeping Lye. Membranes 2021, 11, 88. [Google Scholar] [CrossRef] [PubMed]
| Data | KBL UF Permeate |
|---|---|
| pH | 13.13 ± 0.08 |
| TDS (g/L) | 199.14 ± 2.44 |
| Ash (g/L) | 73.59 ± 1.38 |
| Total hemicelluloses (g/L) | 2.01 |
| Arabinose (g/L) | 0.44 ± 0.05 |
| Galactose (g/L) | 1.19 ± 0.05 |
| Glucose (g/L) | 0.11 ± 0.03 |
| Xylose (g/L) | 0.27 ± 0.05 |
| Total lignin (g/L) | 29.72 ± 0.81 |
| Klason lignin (g/L) | 22.78 ± 0.32 |
| Name | Manufacturer | Type | MWCO (Da) | Material |
|---|---|---|---|---|
| MexFil MP025 dNF40 | NXFiltration, Enschede, The Netherlands | Hollow-fiber | 400 | Modified PES |
| UF-pHt GR95PP | Alfa Laval, Nakskov, Denmark | Flat-sheet | 2000 | PES |
| NADIR NP030 P | MANN+HUMMEL Water & Fluid Solutions, Wiesbaden, Germany | Flat-sheet | 500–600 | PES |
| NADIR NP010 P | MANN+HUMMEL Water & Fluid Solutions, Wiesbaden, Germany | Flat-sheet | 1000–2000 | PES |
| SelRO MPF-36 | Koch Separation Solutions, Wilmington, MA, USA | Flat-sheet | 1000 | Not specified |
| SelRO MPF-34 | Koch Separation Solutions, Wilmington, MA, USA | Flat-sheet | 200 | Not specified |
| NF090801 | SolSep BV, Apeldoorn, The Netherlands | Flat-sheet | 350 | PES |
| NanoPro B-4021 | AMS Technologies, Or Yehuda, Israel | Flat-sheet | 100 | Not specified |
| NanoPro B-4022 | AMS Technologies, Or Yehuda, Israel | Flat-sheet | 150 | Not specified |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Battestini Vives, M.; Thuvander, J.; Arkell, A.; Lipnizki, F. Low-Molecular-Weight Lignin Recovery with Nanofiltration in the Kraft Pulping Process. Membranes 2022, 12, 310. https://doi.org/10.3390/membranes12030310
Battestini Vives M, Thuvander J, Arkell A, Lipnizki F. Low-Molecular-Weight Lignin Recovery with Nanofiltration in the Kraft Pulping Process. Membranes. 2022; 12(3):310. https://doi.org/10.3390/membranes12030310
Chicago/Turabian StyleBattestini Vives, Mariona, Johan Thuvander, Anders Arkell, and Frank Lipnizki. 2022. "Low-Molecular-Weight Lignin Recovery with Nanofiltration in the Kraft Pulping Process" Membranes 12, no. 3: 310. https://doi.org/10.3390/membranes12030310
APA StyleBattestini Vives, M., Thuvander, J., Arkell, A., & Lipnizki, F. (2022). Low-Molecular-Weight Lignin Recovery with Nanofiltration in the Kraft Pulping Process. Membranes, 12(3), 310. https://doi.org/10.3390/membranes12030310









