Electrophoretic Deposition and Characterization of the Doped BaCeO3 Barrier Layers on a Supporting Ce0.8Sm0.2O1.9 Solid-State Electrolyte
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis and Characterization of the Electrolytes
2.2. Preparation and Characterization of BCSCuO Suspensions for EPD
2.3. Electrophoretic Deposition and Characterization of BCSCuO Films
2.4. Electrochemical Characterization of SDC Substrates with Deposited BCSCuO Films under Open-Circuit Conditions
3. Results
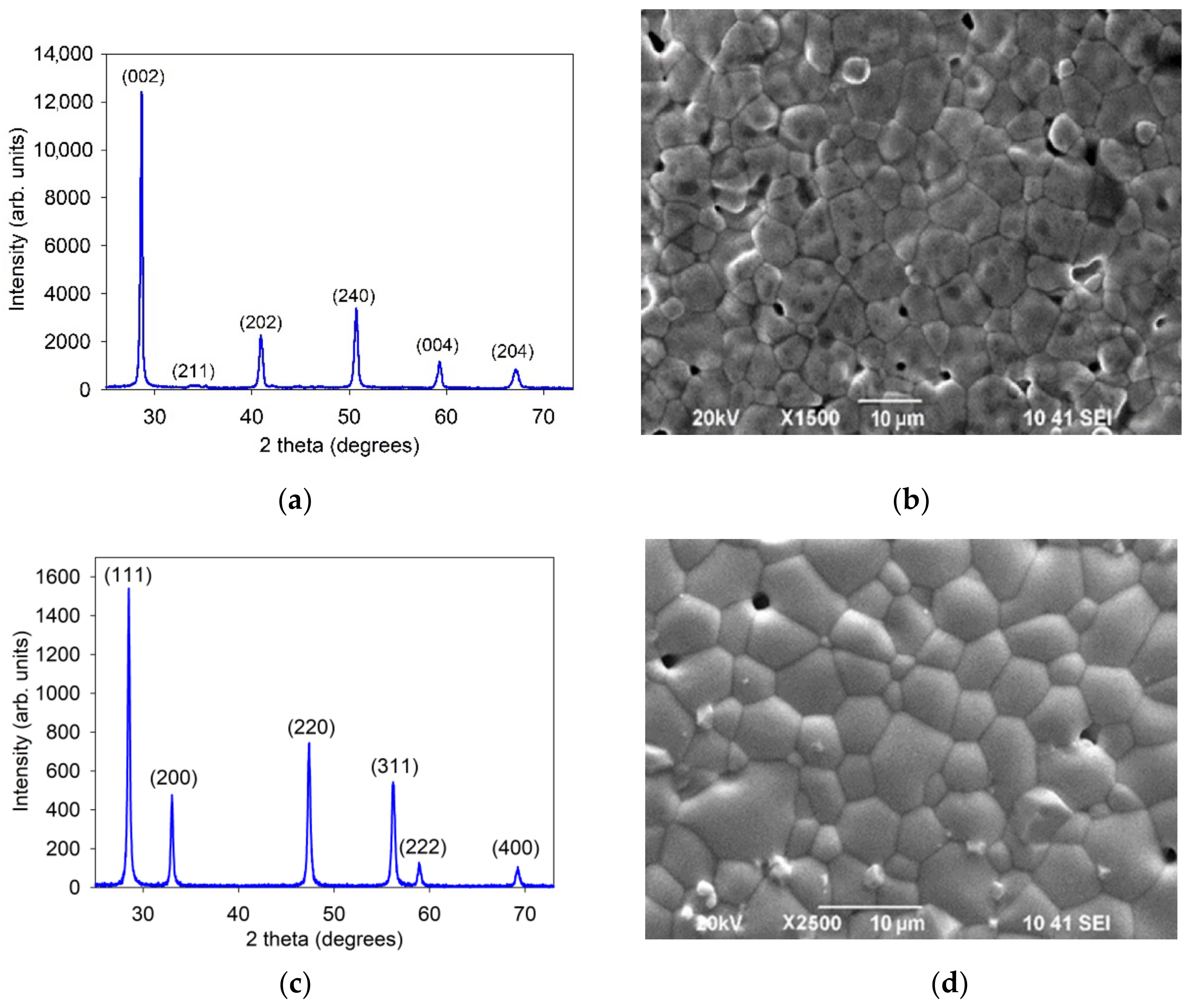
3.1. Characteristics of the SDC and BCSCu Electrolyte Materials
3.2. Preparation and Study of the Fractional Composition of the Base Suspension of the BCSCuO Electrolyte Powder for EPD
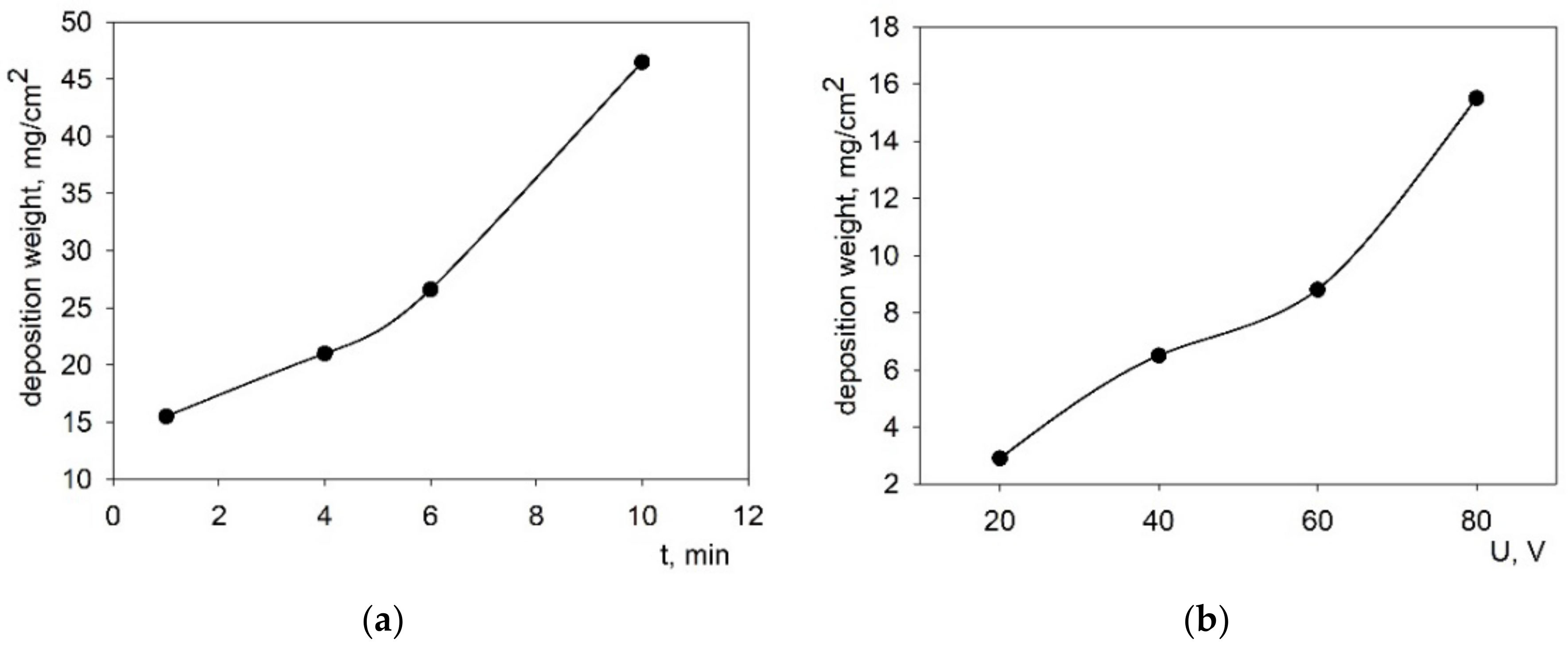
3.3. EPD from the Suspension of BCSCuO Powder on a Model Substrate (Ni-Foil): Determination of Optimal Deposition Modes
3.4. EPD of the BCSCuO Layer on the SDC Substrate with a Predeposited Sublayer of Finely Dispersed Platinum
3.5. Formation of a Conductive Polypyrrole Sublayer on the Surface of the Dense SDC Electrolyte Substrates
3.6. EPD of BCSCuO Barrier Layers on Dense SDC Supporting Substrates with Predeposited PPy Sublayers
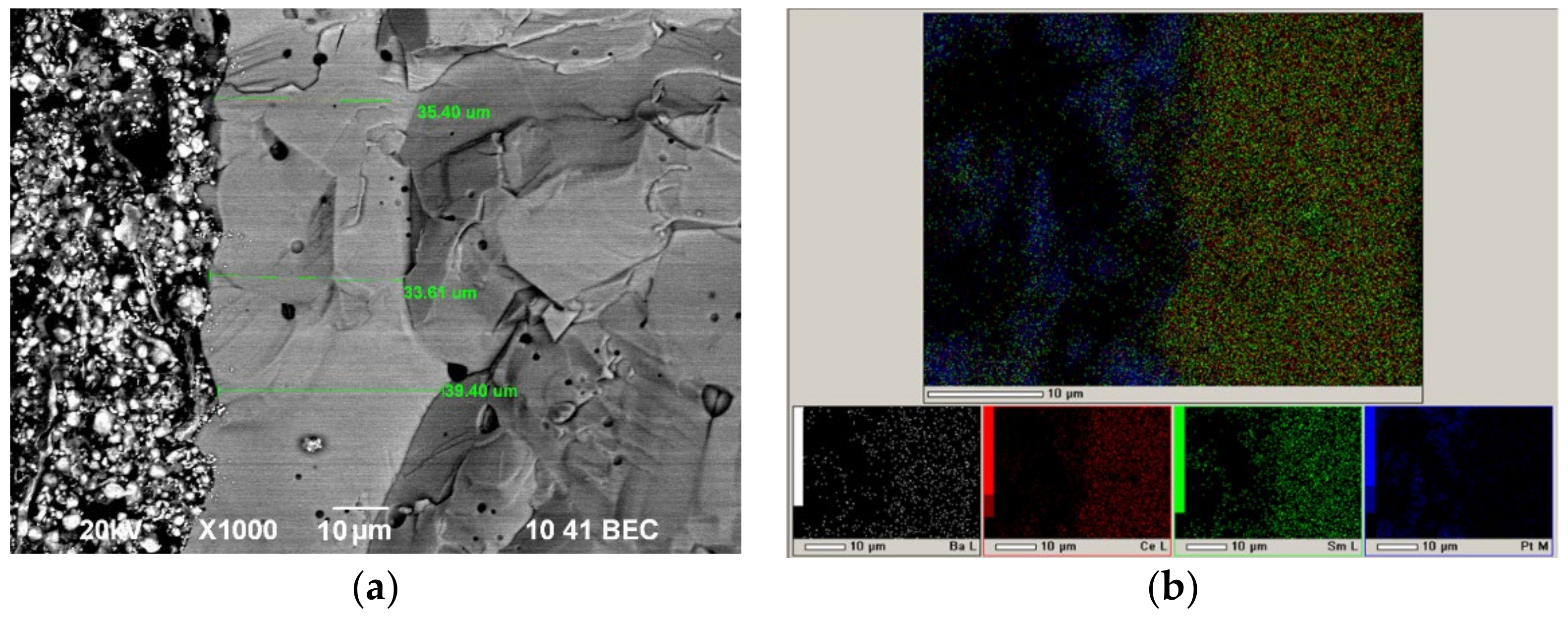
3.7. Electrochemical Characterization and Microstructure of the Deposited Films
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khan, M.Z.; Song, R.-H.; Mehran, M.T.; Lee, S.-B.; Lim, T.-H. Controlling cation migration and inter-diffusion across cathode/interlayer/electrolyte interfaces of solid oxide fuel cells: A review. Ceram. Int. 2021, 47, 5839–5869. [Google Scholar] [CrossRef]
- Pikalova, E.Y.; Kalinina, E.G. Solid oxide fuel cells based on ceramic membranes with mixed conductivity: Improving efficiency. Russ. Chem. Rev. 2021, 90, 703. [Google Scholar] [CrossRef]
- Lowrance, Y.N.; Azham Azmi, M.; Rahman, H.A.; Zakaria, H.; Hassan, S. A review on the solid oxide fuel cells (SOFCs) interconnect coating quality on electrophoretic deposition (EPD) processing parameters. AIP Conf. Proc. 2021, 2339, 020182. [Google Scholar] [CrossRef]
- Franco, T.; Ruckdäschel, R.; Lang, M.; Schiller, G.; Szabo, P. Diffusion and Protecting Barrier Layers in a Substrate Supported SOFC Concept. In Proceedings of the 7th International Fuel Solid Oxide Cell Forum, Luzern, Switzerland, 3–7 July 2006; Available online: https://elib.dlr.de/46236/ (accessed on 8 February 2022).
- Sazali, N.; Wan Salleh, W.N.; Jamaludin, A.S.; Mhd Razali, M.N. New Perspectives on Fuel Cell Technology: A Brief Review. Membranes 2020, 10, 99. [Google Scholar] [CrossRef] [PubMed]
- Shri Prakash, B.; Pavitra, R.; Senthil Kumar, S.; Aruna, S.T. Electrolyte bi-layering strategy to improve the performance of an intermediate temperature solid oxide fuel cell: A review. J. Power Sources 2018, 381, 136–155. [Google Scholar] [CrossRef]
- Klyndyuk, A.I.; Chizhova, E.A.; Kharytonau, D.S.; Medvedev, D.A. Layered oxygen-deficient double perovskites as promising cathode materials for solid oxide fuel cells. Materials 2022, 15, 141. [Google Scholar] [CrossRef]
- Tarutin, A.P.; Lyagaeva, J.G.; Medvedev, D.A.; Bi, L.; Yaremchenko, A.A. Recent advances in layered Ln2NiO4+δ nickelates: Fundamentals and prospects of their applications in protonic ceramic fuel and electrolysis cells. J. Mater. Chem. A 2021, 9, 154–195. [Google Scholar] [CrossRef]
- Coppola, N.; Polverino, P.; Carapella, G.; Ciancio, R.; Rajak, P.; Dario, M.; Martinelli, F.; Maritato, L.; Pianese, C. Large Area Deposition by Radio Frequency Sputtering of Gd0.1Ce0.9O1.95 Buffer Layers in Solid Oxide Fuel Cells: Structural, Morphological and Electrochemical Investigation. Materials 2021, 14, 5826. [Google Scholar] [CrossRef]
- Morales, M.; Pesce, A.; Slodczyk, A.; Torrell, M.; Piccardo, P.; Montinaro, D.; Tarancón, A.; Morata, A. Enhanced Performance of Gadolinia-Doped Ceria Diffusion Barrier Layers Fabricated by Pulsed Laser Deposition for Large-Area Solid Oxide Fuel Cells. ACS Appl. Energy Mater. 2018, 1, 1955–1964. [Google Scholar] [CrossRef]
- Solovyev, A.A.; Shipilova, A.V.; Ionov, I.V.; Kovalchuk, A.N.; Rabotkin, S.V.; Oskirko, V.O. Magnetron-sputtered YSZ and CGO electrolytes for SOFC. J. Electron. Mater. 2016, 45, 3921–3928. [Google Scholar] [CrossRef]
- Liu, J.; Zhu, C.; Zhu, D.; Jia, X.; Zhang, Y.; Yu, J.; Li, X.; Yang, M. High Performance Low-Temperature Solid Oxide Fuel Cells Based on Nanostructured Ceria-Based Electrolyte. Nanomaterials 2021, 11, 2231. [Google Scholar] [CrossRef] [PubMed]
- Mendonça, C.; Ferreira, A.; Santos, D.M.F. Towards the Commercialization of Solid Oxide Fuel Cells: Recent Advances in Materials and Integration Strategies. Fuels 2021, 2, 393–419. [Google Scholar] [CrossRef]
- Lyu, Y.; Xie, J.; Wang, D.; Wang, J. Review of cell performance in solid oxide fuel cells. J. Mater. Sci. 2020, 55, 7184–7207. [Google Scholar] [CrossRef]
- Løken, A.; Ricote, S.; Wachowski, S. Thermal and Chemical Expansion in Proton Ceramic Electrolytes and Compatible Electrodes. Crystals 2018, 8, 365. [Google Scholar] [CrossRef] [Green Version]
- Medvedev, D.; Murashkina, A.; Pikalova, E.; Demin, A.; Podias, A.; Tsiakaras, P. BaCeO3: Materials development, properties and application. Progr. Mater. Sci. 2014, 60, 72–129. [Google Scholar] [CrossRef]
- Medvedev, D.; Maragou, V.; Pikalova, E.; Demin, A.; Tsiakaras, P. Novel composite solid state electrolytes on the base of BaCeO3 and CeO2 for intermediate temperature electrochemical devices. J. Power Sources 2013, 221, 217–227. [Google Scholar] [CrossRef]
- Medvedev, D.; Pikalova, E.; Demin, A.; Podias, A.; Korzun, I.; Antonov, B.; Tsiakaras, P. Structural, thermomechanical and electrical properties of new (1 − x)Ce0.8Nd0.2O2−δ–xBaCe0.8Nd0.2O3−δ composites. J. Power Sources 2014, 267, 269–279. [Google Scholar] [CrossRef]
- Riess, I. The possible use of mixed ionic electronic conductors instead of electrolytes in fuel cells. Solid State Ion. 1992, 52, 127–134. [Google Scholar] [CrossRef]
- Wang, X.X.; Zhang, T.; Kang, J.H.; Zhao, L.; Guo, L.T.; Feng, P.Z.; Zhou, F.B.; Ling, Y.H. Numerical modeling of ceria-based SOFCs with bi-layer electrolyte free from internal short circuit: Comparison of two cell configurations. Electrochim. Acta 2017, 248, 356–367. [Google Scholar] [CrossRef]
- Menzler, N.H.; Tietz, F.; Uhlenbruck, S.; Buchkremer, H.P.; Stöver, D. Materials and manufacturing technologies for solid oxide fuel cells. J. Mater. Sci. 2010, 45, 3109–3135. [Google Scholar] [CrossRef]
- Pikalova, E.Y.; Kalinina, E.G. Place of Electrophoretic Deposition Among Thin-Film Methods Adapted to the Solid Oxide Fuel Cell Technology: A Short Review. Int. J. Energy Prod. Manag. 2019, 4, 1–27. [Google Scholar] [CrossRef] [Green Version]
- Song, J.-H.; Jung, M.G.; Park, H.W.; Lim, H.-T. The Effect of Fabrica-tion Conditions for GDC Buffer Layer on Electrochemical Performance of Solid Oxide Fuel Cells. Nano-Micro Lett. 2013, 5, 151–158. [Google Scholar] [CrossRef]
- Kim, J.; Kim, J.; Yoon, K.J.; Son, J.-W.; Lee, J.-H.; Lee, J.-H.; Lee, H.-W.; Ji, H.-I. Solid oxide fuel cells with zirconia/ceria bilayer electrolytes via roll calendering process. J. Alloys Compd. 2020, 846, 156318. [Google Scholar] [CrossRef]
- Medvedev, D.; Lyagaeva, J.; Vdovin, G.; Beresnev, S.; Demin, A.; Tsiakaras, P. A tape calendering method as an effective way for the preparation of proton ceramic fuel cells with enhanced performance. Electrochim. Acta 2016, 210, 681–688. [Google Scholar] [CrossRef]
- Bouleau, L.; Coton, N.; Coquoz, P.; Ihringer, R.; Billard, A.; Briois, P. GDC Buffer Layer Synthesized by Reactive Magnetron Sputtering: Effect of Total Pressure and Thickness on SOFC Performances. Crystals 2020, 10, 759. [Google Scholar] [CrossRef]
- Solovyev, A.A.; Lebedynskiy, A.M.; Shipilova, A.V.; Ionov, I.V.; Smolyanskiy, E.A.; Lauk, A.L.; Remnev, G.E.; Maslov, A.S. Scale-up of Solid Oxide Fuel Cells with Magnetron Sputtered Electrolyte. Fuel Cells 2017, 17, 378–382. [Google Scholar] [CrossRef]
- Rabo, J.R.; Takayanagi, M.; Tsuchiya, T.; Nakajima, H.; Terabe, K.; Cervera, R.B.M. Effects of Oxygen Partial Pressure and Substrate Temperature on the Structure and Morphology of Sc and Y Co-Doped ZrO2 Solid Electrolyte Thin Films Prepared via Pulsed Laser Deposition. Materials 2022, 15, 410. [Google Scholar] [CrossRef]
- Wang, Y.; Jia, C.; Lyu, Z.; Han, M.; Wu, J.; Sun, Z.; Iguchi, F.; Yashiro, K.; Kawada, T. Performance and stability analysis of SOFC containing thin and dense gadolinium-doped ceria interlayer sintered at low temperature. J. Mater. 2022, 8, 347–357. [Google Scholar] [CrossRef]
- Schüller, E.; Vaßen, R.; Stöver, D. Thin Electrolyte Layers for SOFC via Wet Powder Spraying (WPS). Adv. Mater. 2002, 4, 659–662. [Google Scholar] [CrossRef]
- Erilin, I.S.; Burmistrov, I.N.; Agarkov, D.A.; Pukha, V.E.; Yalovenko, D.V.; Lyskov, N.V.; Levin, M.N.; Bredikhin, S.I. Aerosol Deposition of Thin-Film Single- and Bi-layered Solid Electrolytes for Intermediate Temperature Planar Solid Oxide Fuel Cells. ECS Trans. 2021, 103, 1695–1703. [Google Scholar] [CrossRef]
- Hu, S.; Li, W.; Yao, M.; Li, T.; Liu, X. Electrophoretic Deposition of Gadolinium-doped Ceria as a Barrier Layer on Yttrium-stabilized Zirconia Electrolyte for Solid Oxide Fuel Cells. Fuel Cells 2017, 17, 869–874. [Google Scholar] [CrossRef]
- Hu, S. Scalable and Cost-Effective Barrier Layer Coating to Improve Stability and Performance of SOFC Cathode; Graduate Theses Dissertations, and Problem Reports; West Virginia University: Morgantown, WV, USA, 2019; Volume 7465, Available online: https://researchrepository.wvu.edu/etd/7465 (accessed on 7 February 2022).
- Kovrova, A.I.; Gorelov, V.P.; Kuzmin, A.V.; Tropin, E.S.; Osinkin, D.A. Influence of Ce0.8R0.2O2–a (R = Y, Sm, Tb) submicron barrier layers at the La2NiO4+δ/YSZ boundary on the electrochemical performance of a cathode. J. Solid State Electrochem. 2021, 25, 1789–1796. [Google Scholar] [CrossRef]
- Hierso, J.; Boy, P.; Vallé, K.; Vulliet, J.; Blein, F.; Laberty-Robert, C.; Sanchez, C. Ceria based thin films (≤1 μm) As cathode/electrolyte interfaces. J. Solid State Chem. 2013, 197, 113–119. [Google Scholar] [CrossRef]
- Oh, E.-O.; Whang, C.-M.; Lee, Y.-R.; Park, S.-Y.; Prasad, D.H.; Yoon, K.J.; Son, J.-W.; Lee, J.-H.; Lee, H.-W. Extremely Thin Bilayer Electrolyte for Solid Oxide Fuel Cells (SOFCs) Fabricated by Chemical Solution Deposition (CSD). Adv. Mater. 2012, 24, 3373–3377. [Google Scholar] [CrossRef]
- Kalinina, E.G.; Pikalova, E.Y. New trends in the development of electrophoretic deposition method in the solid oxide fuel cell technology: Theoretical approaches, experimental solutions and development prospects. Russ. Chem. Rev. 2019, 88, 1179–1219. [Google Scholar] [CrossRef]
- Sakka, Y.; Uchikoshi, T. Forming and microstructure control of ceramics by electrophoretic deposition (EPD). KONA Powder Part. J. 2010, 28, 74–90. [Google Scholar] [CrossRef]
- Kalinina, E.G.; Pikalova, E.Y.; Kolchugin, A.A.; Pikalova, N.S.; Farlenkov, A.S. Comparative Study of Electrophoretic Deposition of Doped BaCeO3-Based Films on La2NiO4+δ and La1.7Ba0.3NiO4+δ Cathode Substrates. Materials 2019, 12, 2545. [Google Scholar] [CrossRef] [Green Version]
- Kalinina, E.; Kolchugin, A.; Shubin, K.; Farlenkov, A.; Pikalova, E. Features of electrophoretic deposition of a Ba-containing thin-film proton-conducting electrolyte on a porous cathode substrate. Appl. Sci. 2020, 10, 6535. [Google Scholar] [CrossRef]
- Besra, L.; Compson, C.; Liu, M. Electrophoretic deposition on non-conducting substrates: The case of YSZ film on NiO–YSZ composite substrates for solid oxide fuel cell application. J. Power Sources 2007, 173, 130–136. [Google Scholar] [CrossRef]
- Lankin, M.K.; Karan, K. Effect of Processing Conditions on Curvature of Anode/Electrolyte SOFC Half-Cells Fabricated by Electrophoretic Deposition. J. Fuel Cell Sci. Technol. 2009, 6, 021001. [Google Scholar] [CrossRef]
- Pikalova, E.; Kalinina, E. Electrophoretic deposition in the solid oxide fuel cell technology: Fundamentals and recent advances. Renew. Sust. Energy Rev. 2019, 116, 109440. [Google Scholar] [CrossRef]
- Park, I.; Kim, J.; Choi, J.; Lee, H.; Park, J.; Shin, D. Enhanced sintering behavior mechanism of nanocrystalline BaCe0.8Sm0.2O3−δ by Cu doping. Int. J. Hydrogen Energy 2013, 38, 7423–7429. [Google Scholar] [CrossRef]
- Pikalova, E.; Bogdanovich, N.; Kolchugin, A.; Shubin, K.; Ermakova, L.; Eremeev, N.; Farlenkov, A.; Khrustov, A.; Filonova, E.; Sadykov, V. Development of composite LaNi0.6Fe0.4O3-δ-based air electrodes for solid oxide fuel cells with a thin-film bilayer electrolyte. Int. J. Hydrogen Energy 2021, 46, 16947–16964. [Google Scholar] [CrossRef]
- Gorbova, E.; Maragou, V.; Medvedev, D.; Demin, A.; Tsiakaras, P. Investigation of the protonic conduction in Sm doped BaCeO3. J. Power Sources 2008, 181, 207–213. [Google Scholar] [CrossRef]
- Rehman, S.U.; Shaur, A.; Kim, H.; Joh, D.W.; Song, R.; Lim, T.; Hong, J.; Parl, S.; Lee, S. Effect of transition metal doping on the sintering and electrochemical properties of GDC buffer layer in SOFCs. Int. J. Appl. Ceram. Technol. 2021, 18, 511–524. [Google Scholar] [CrossRef]
- Nicollet, C.; Waxin, J.; Dupeyron, T.; Flura, A.; Jean-Marc Heintz, J.-M.; Ouweltjes, P.; Piccardo, P.; Rougier, A.; Grenier, J.-C.; Bassat, J.-M. Gadolinium doped ceria interlayers for Solid Oxide Fuel Cells cathodes: Enhanced reactivity with sintering aids (Li, Cu, Zn), and improved densification by infiltration. J. Power Sources 2017, 372, 157–165. [Google Scholar] [CrossRef]
- Ananyev, M.; Medvedev, D.; Gavrilyuk, A.; Mitri, S.; Demin, A.; Malkov, V.; Tsiakaras, P. Cu and Gd co-doped BaCeO3 proton conductors: Experimental vs. SEM image algorithmic-segmentation results. Electrochim. Acta 2014, 125, 371–379. [Google Scholar] [CrossRef]
- Koettgen, J.; Martin, M. The ionic conductivity of Sm-doped ceria. J. Americ. Ceram. Soc. 2020, 103, 3776–3787. [Google Scholar] [CrossRef] [Green Version]
- Pikalova, E.Y.; Maragou, V.I.; Demina, A.N.; Demin, A.K. The effect of co-dopant addition on the properties of Ln0.2Ce0.8O2−δ (Ln = Gd, Sm, La) solid-state electrolyte. J. Power Sources 2008, 181, 199–206. [Google Scholar] [CrossRef]
- Gorelov, V.P.; Balakireva, V.B.; Yaroslavtsev, I.Y.; Kazantsev, V.A.; Vaganova, E.G. Conductivity and thermal expansion of the Ce0.8Gd0.2O1.9 solid electrolyte in the oxidizing and reducing atmospheres. Russ. J. Electrochem. 2007, 43, 888–893. [Google Scholar] [CrossRef]
- Hirabayashi, D.; Tomita, A.; Teranishi, S.; Hibino, T.; Sano, M. Improvement of a reduction-resistant Ce0.8Sm0.2O1.9 electrolyte by optimizing a thin BaCe1-xSmxO3-δ layer for intermediate-temperature SOFCs. Solid State Ion. 2005, 176, 881–887. [Google Scholar] [CrossRef]
- Dusoulier, L.; Cloots, R.; Vertruyen, B.; Moreno, R.; Burgos-Montes, O.; Ferrari, B. YBa2Cu3O7−x dispersion in iodine acetone for electrophoretic deposition: Surface charging mechanism in a halogenated organic media. J. Eur. Ceram. Soc. 2011, 31, 1075–1086. [Google Scholar] [CrossRef] [Green Version]
- Nazani, N.; Aghajani, H. Suspension chemistry and electrophoretic deposition of YSZ-NiO nano-composite films on an iron-nickel based superalloy. J. Dispers. Sci. Technol. 2020, 41, 1754–1767. [Google Scholar] [CrossRef]
- Kalinina, E.G.; Pikalova, E.Y. Modifying Suspensions for the Electrophoretic Deposition of BaCe0.5Zr0.3Y0.1Yb0.1O3–δ Solid Electrolyte. Russ. J. Phys. Chem. 2021, 95, 1942–1947. [Google Scholar] [CrossRef]
- Kalinina, E.; Pikalova, E.; Ermakova, L.; Bogdanovich, N. Challenges of Formation of Thin-Film Solid Electrolyte Layers on Non-Conductive Substrates by Electrophoretic Deposition. Coatings 2021, 11, 805. [Google Scholar] [CrossRef]
- Vernitskaya, T.V.; Efimov, O.N. Polypyrrole: A conducting polymer; its synthesis, properties and applications. Russ. Chem. Rev. 1997, 66, 443–457. [Google Scholar] [CrossRef]
- Sakthivel, S.; Boopathi, A. Synthesis and Characterization of Polypyrrole (PPY) Thin Film by Spin Coating Technique. J. Chem. Cheml. Sci. 2014, 4, 150–155. Available online: http://chemistry-journal.org/dnload/S-Sakthivel-and-A-Boopathi/CHEMISTRY-JOURNAL-CHJV04I03P0150.pdf (accessed on 7 February 2022).
- Suzuki, H.T.; Uchikoshi, T.; Kobayashi, K.; Suzuki, T.S.; Sugiyama, T.; Furuya, K.; Matsuda, M.; Sakka, Y.; Munakata, F. Fabrication of GDC/LSGM/GDC tri-layers on polypyrrole-coated NiO-YSZ by electrophoretic deposition for anode-supported SOFC. J. Ceram. Soc. Jpn. 2009, 117, 1246–1248. [Google Scholar] [CrossRef] [Green Version]
- Shen, S.; Wang, Z.; Liu, Y.; Zhang, Q.; Zheng, K. A new experimental method to estimate the leakage current in the solid oxide fuel cell with a mixed ionic and electronic conducting electrolyte. J. Power Sources 2018, 406, 88–95. [Google Scholar] [CrossRef]
- Pikalova, E.; Medvedev, D. Effect of anode gas mixture humidification on the electrochemical performance of the BaCeO3-based protonic ceramic fuel cell. Int. J. Hydrogen Energy 2016, 41, 4016–4025. [Google Scholar] [CrossRef]
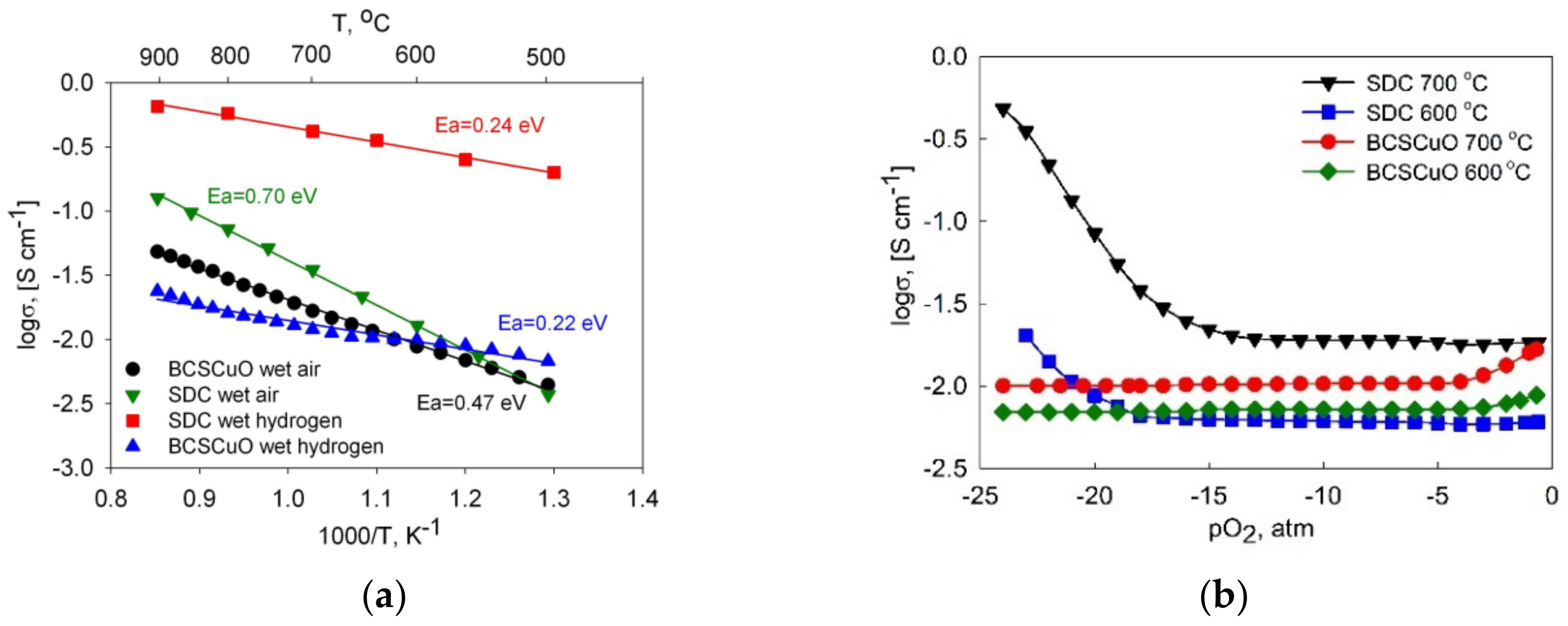

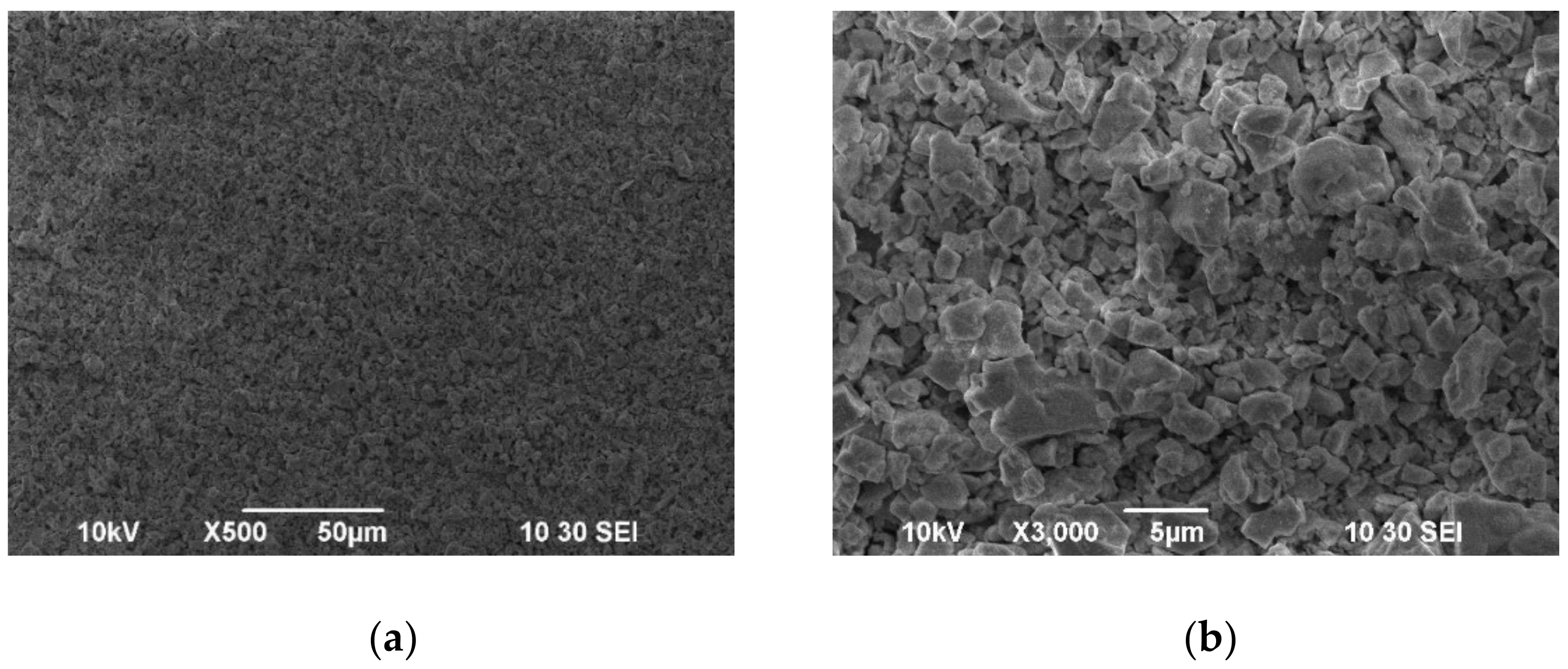
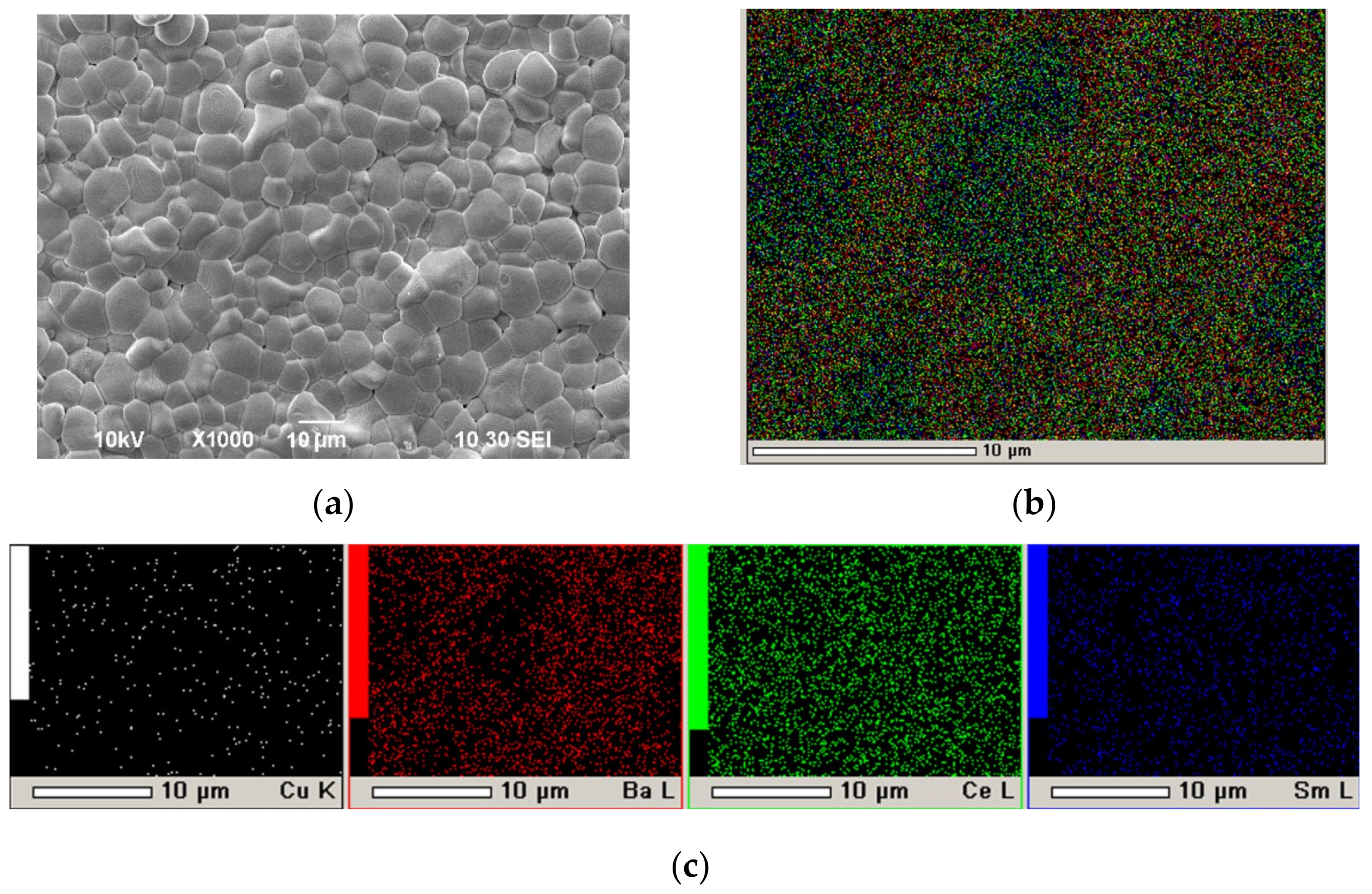
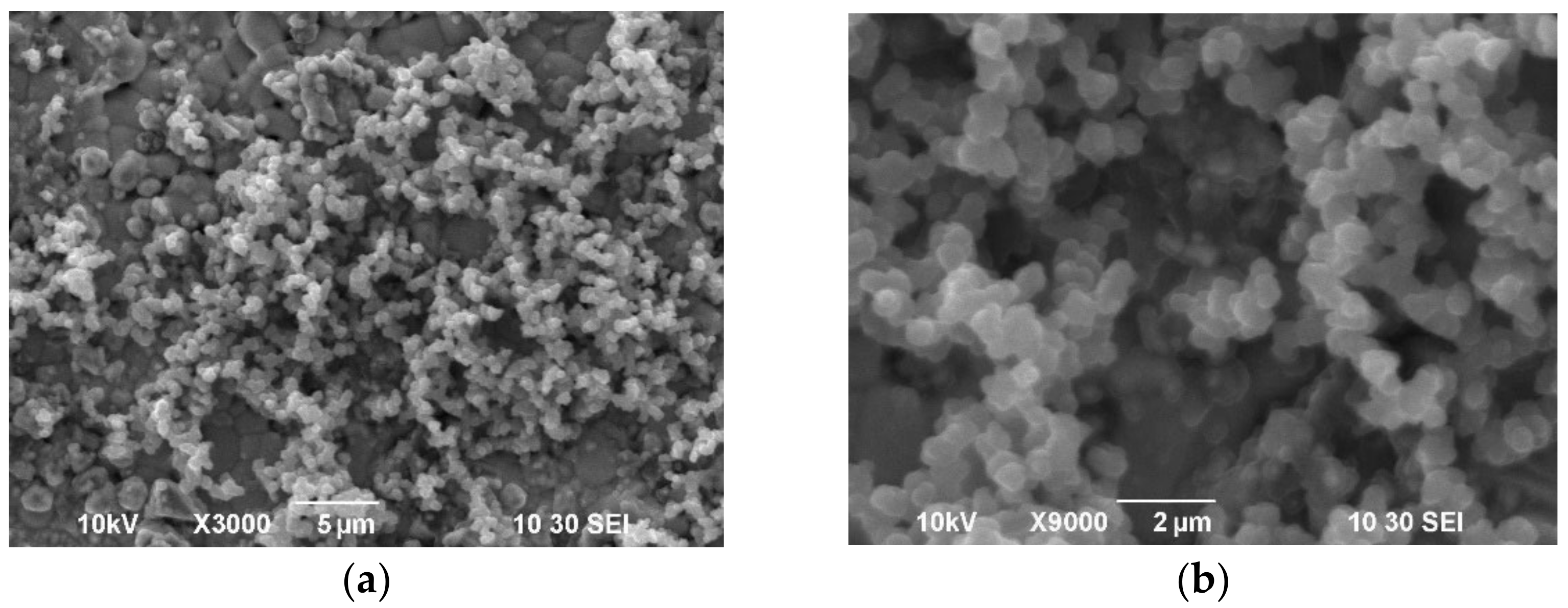

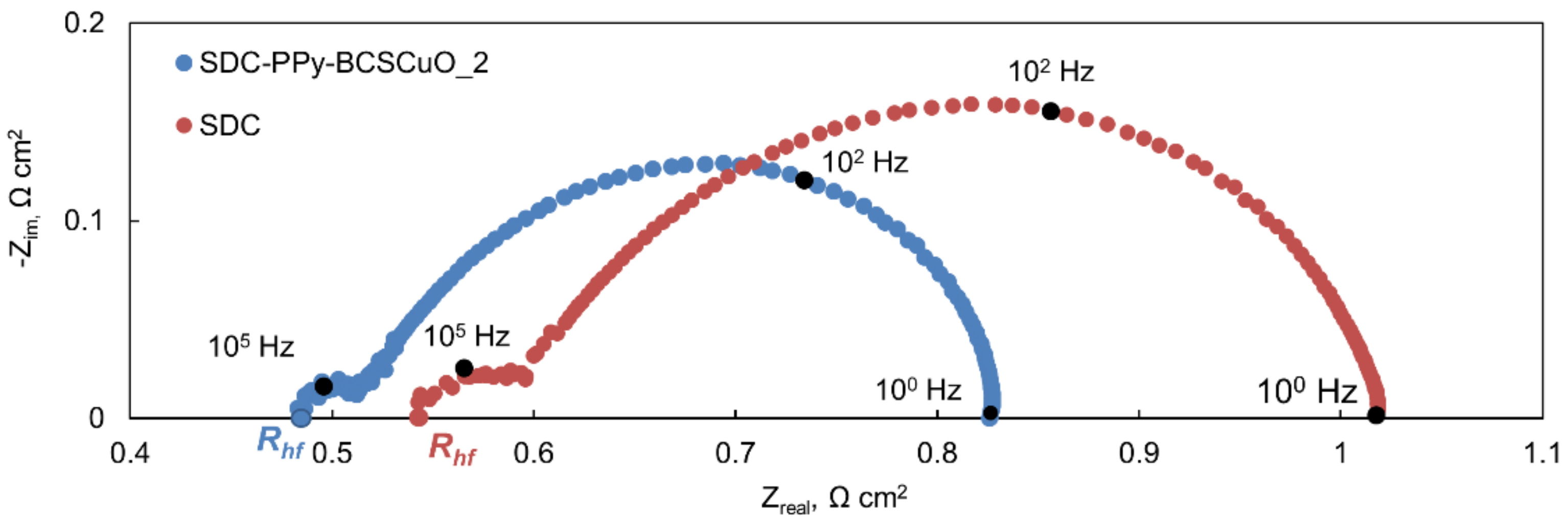



| Samples | 600 °C | 700 °C | ||||
|---|---|---|---|---|---|---|
| σe, mS/cm | σi, mS/cm | σtot, mS/cm | σe, mS/cm | σi, mS/cm | σtot, mS/cm | |
| 0.21 atm | ||||||
| BCSCuO | 1.6 | 7.2 | 8.8 | 6.4 | 10.3 | 16.7 |
| SDC | ~0.01 | 6.1 | 6.1 | ~0.01 | 18.4 | 18.4 |
| 10−22 atm | ||||||
| BCSCuO | 0.07 | 6.9 | 7.0 | 0.07 | 9.6 | 9.7 |
| SDC | 8.1 | 6.1 | 14.09 | 201.9 | 18.4 | 220.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalinina, E.; Shubin, K.; Pikalova, E. Electrophoretic Deposition and Characterization of the Doped BaCeO3 Barrier Layers on a Supporting Ce0.8Sm0.2O1.9 Solid-State Electrolyte. Membranes 2022, 12, 308. https://doi.org/10.3390/membranes12030308
Kalinina E, Shubin K, Pikalova E. Electrophoretic Deposition and Characterization of the Doped BaCeO3 Barrier Layers on a Supporting Ce0.8Sm0.2O1.9 Solid-State Electrolyte. Membranes. 2022; 12(3):308. https://doi.org/10.3390/membranes12030308
Chicago/Turabian StyleKalinina, Elena, Kirill Shubin, and Elena Pikalova. 2022. "Electrophoretic Deposition and Characterization of the Doped BaCeO3 Barrier Layers on a Supporting Ce0.8Sm0.2O1.9 Solid-State Electrolyte" Membranes 12, no. 3: 308. https://doi.org/10.3390/membranes12030308
APA StyleKalinina, E., Shubin, K., & Pikalova, E. (2022). Electrophoretic Deposition and Characterization of the Doped BaCeO3 Barrier Layers on a Supporting Ce0.8Sm0.2O1.9 Solid-State Electrolyte. Membranes, 12(3), 308. https://doi.org/10.3390/membranes12030308