Evaluation of Nanofiltration Membranes for Pure Lactic Acid Permeability
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of the Lactic Acid Solution
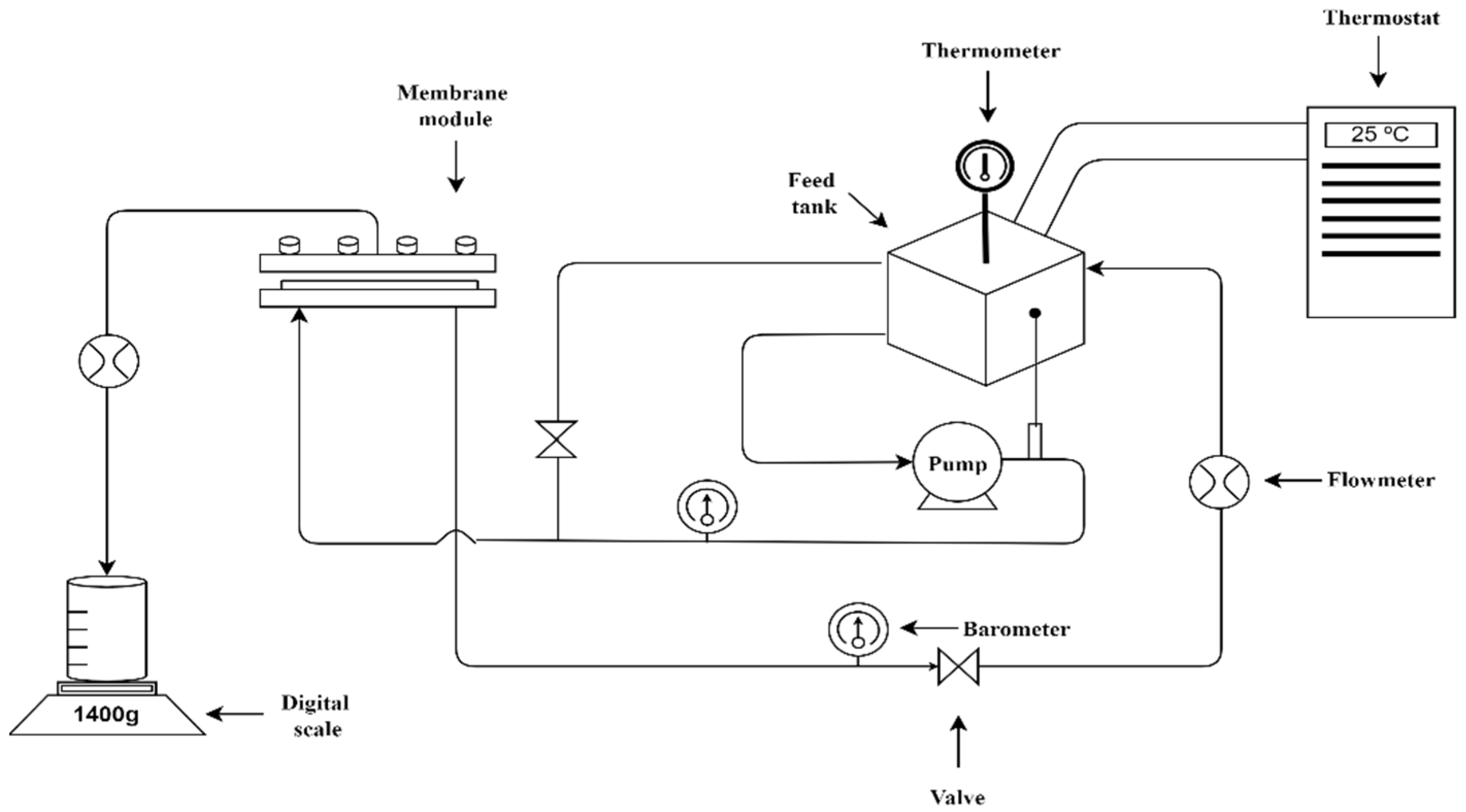
2.2. Experimental Set-Up and Nanofiltration Membranes
2.3. Operating Conditions
2.4. Lactic Acid Quantification
3. Results and Discussion
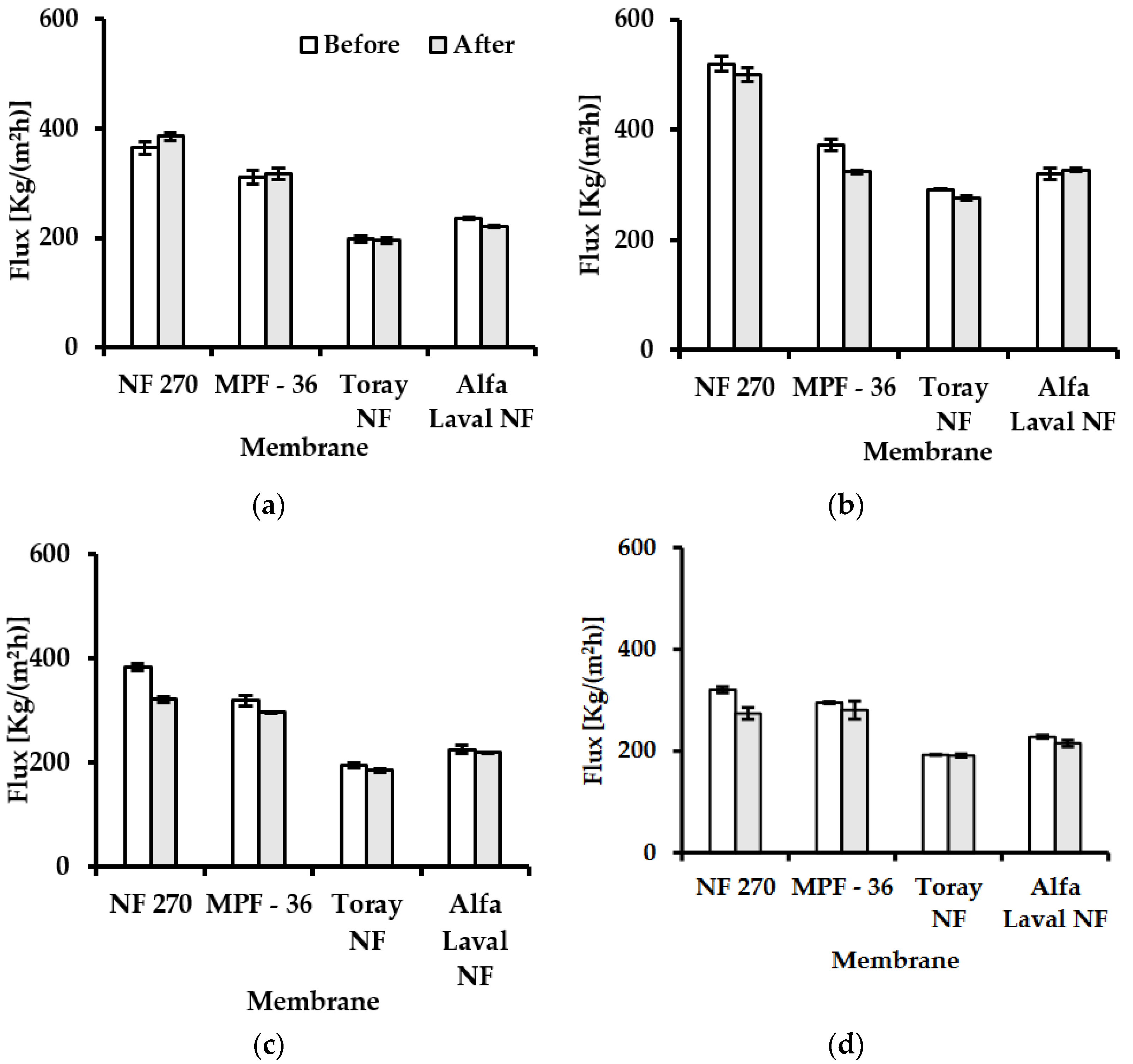
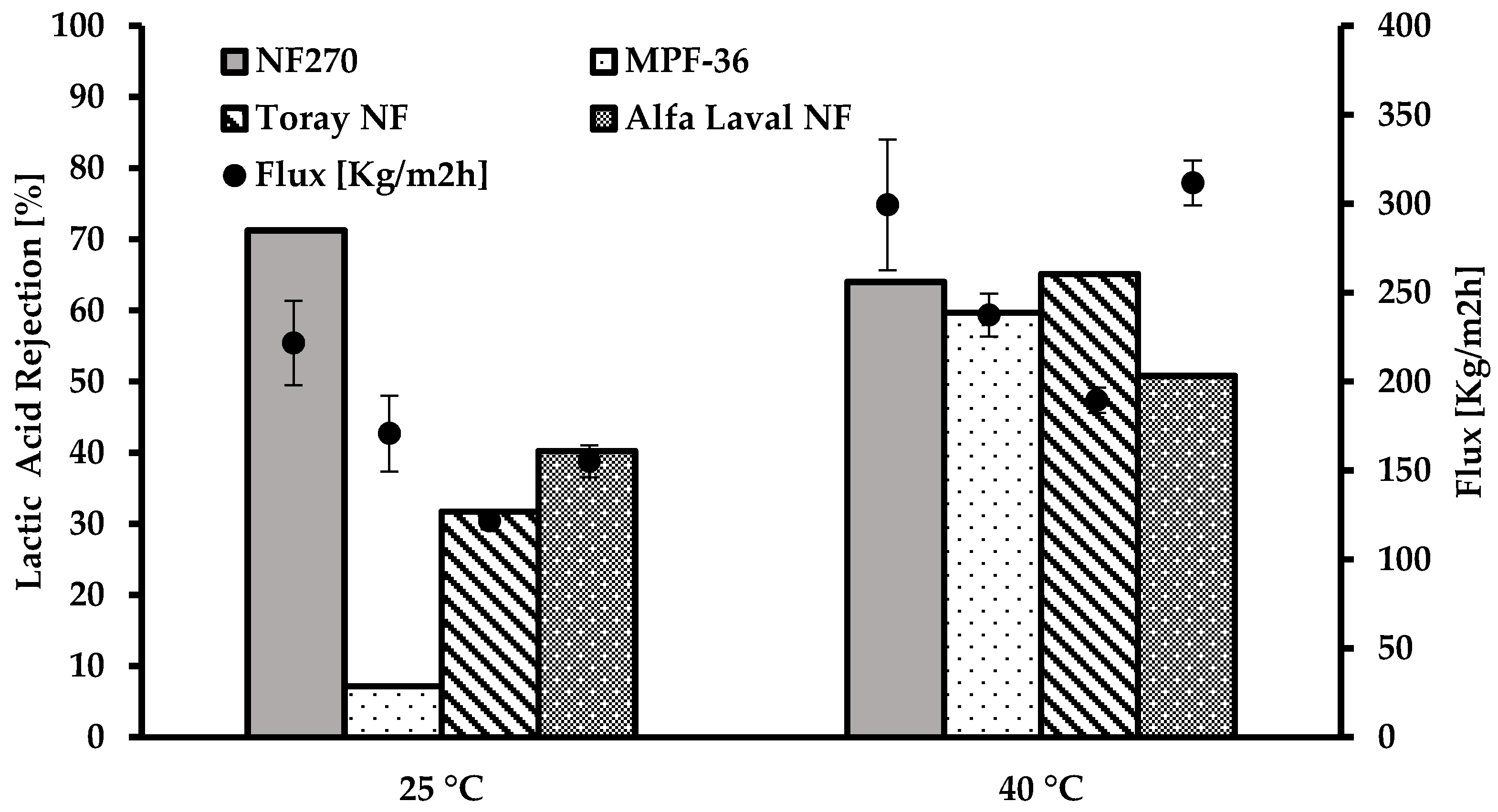
3.1. Water Flux and Flux Reduction
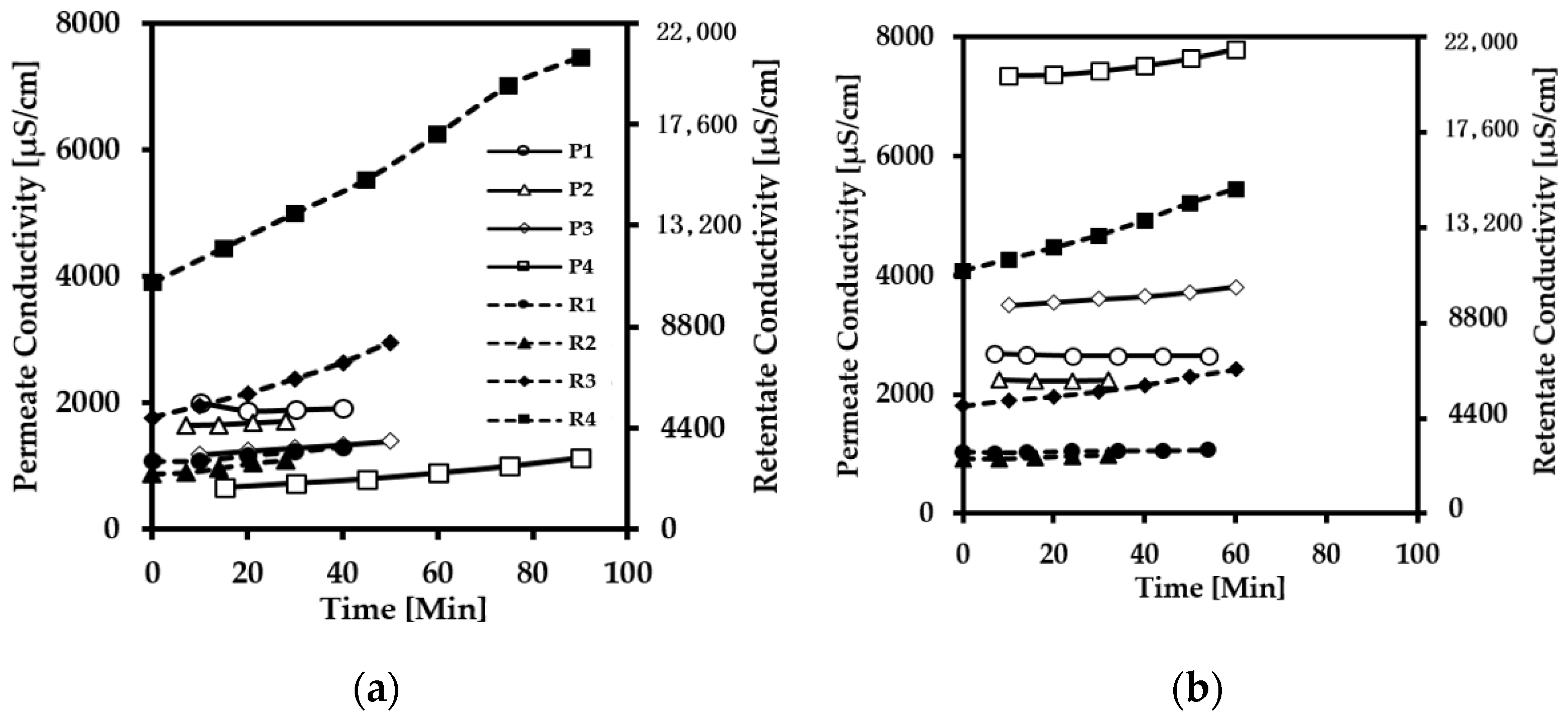
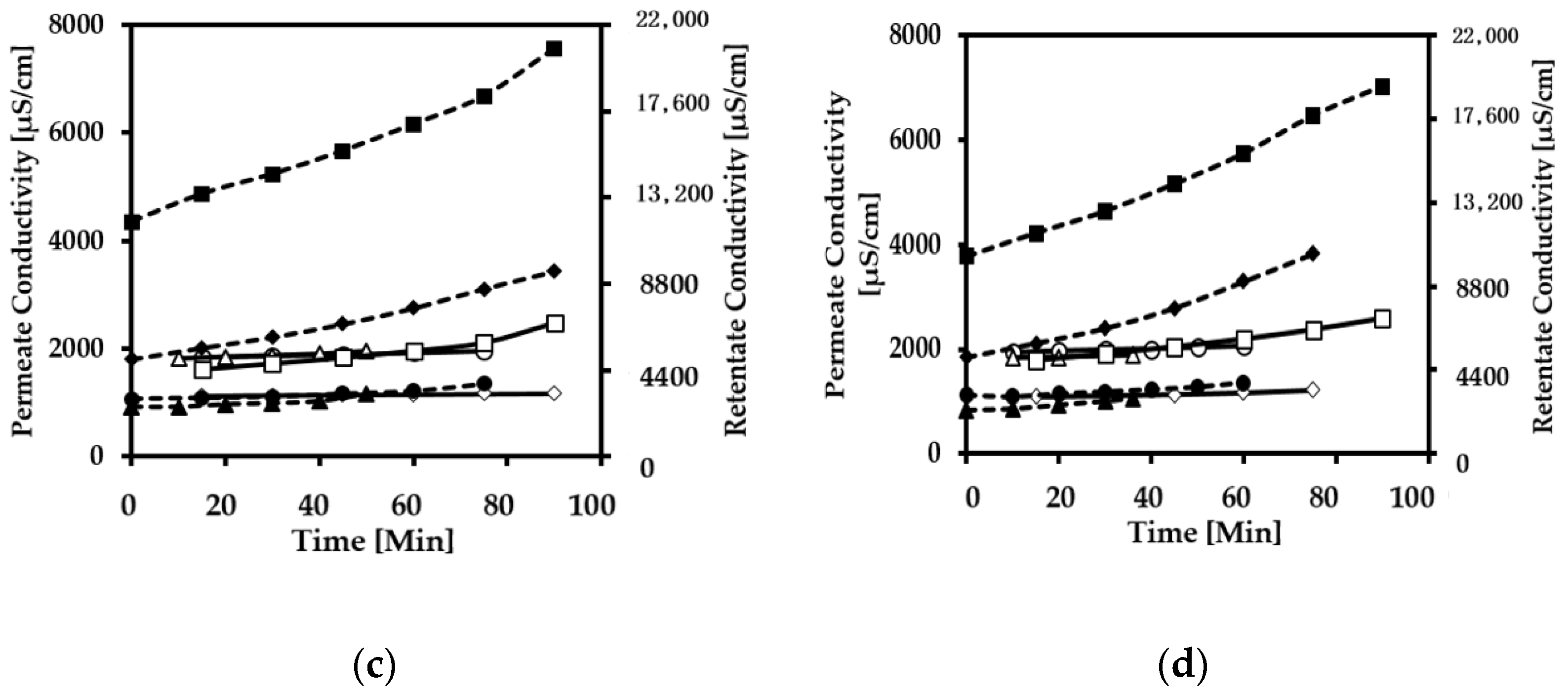
3.2. Conductivity
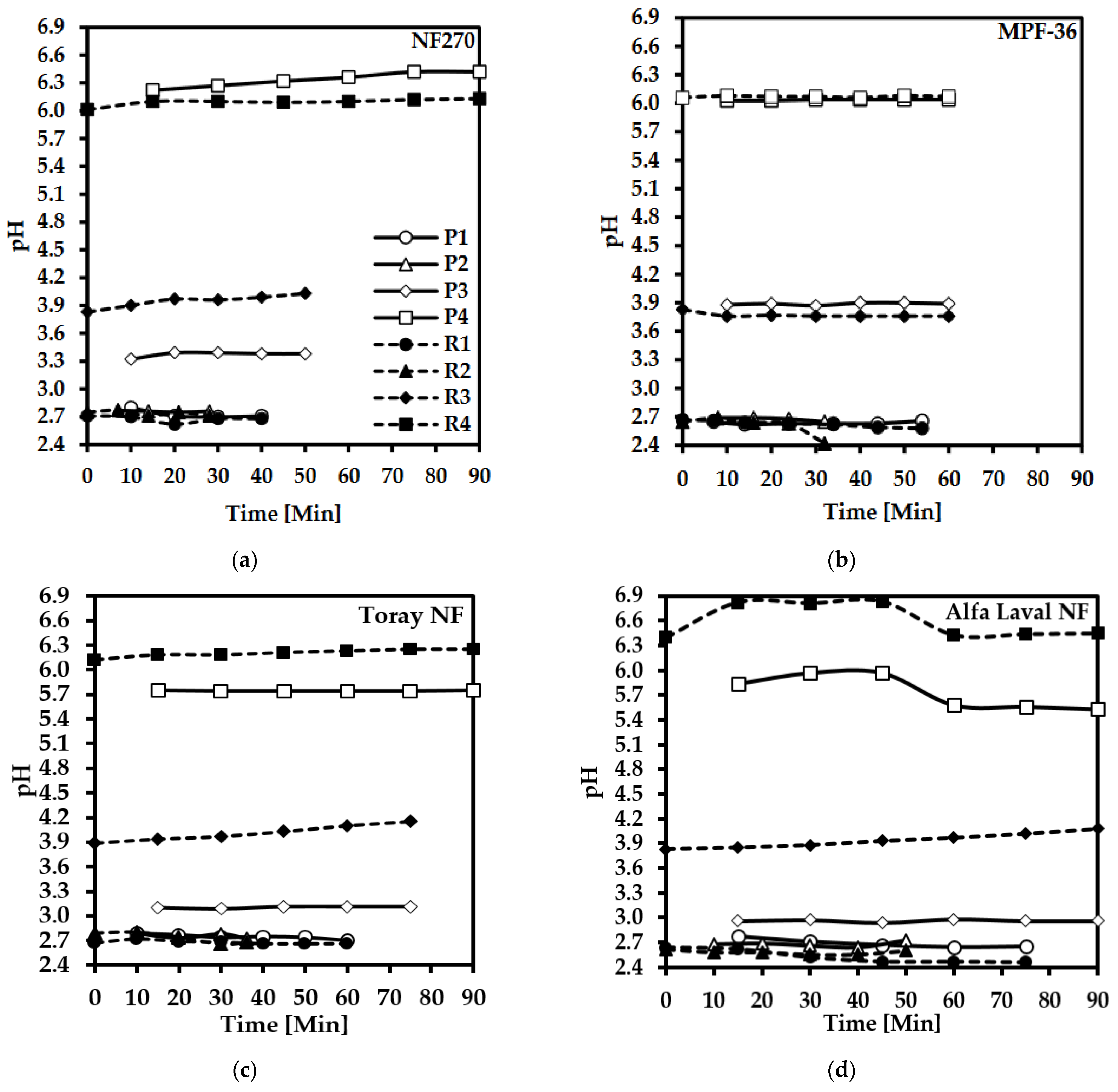
3.3. pH Variation in the Permeate and Retentate
3.4. Lactic Acid Permeability
3.4.1. Effect of the pH
3.4.2. Effect of Temperature
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Camesasca, L.; de Mattos, J.A.; Vila, E.; Cebreiros, F.; Lareo, C. Lactic acid production by Carnobacterium sp. isolated from a maritime Antarctic lake using eucalyptus enzymatic hydrolysate. Biotechnol. Rep. 2021, 31, e00643. [Google Scholar] [CrossRef] [PubMed]
- Agrefine. AgRefine Project: European Union’s Horizon 2020 Research and Innovation Programme under the Marie Skłodowska-Curie Grant Agreement No 860477. Available online: https://agrefine.eu/about-the-project/ (accessed on 26 January 2022).
- Karnaouri, A.; Asimakopoulou, G.; Kalogiannis, K.G.; Lappas, A.; Topakas, E. Efficient d-lactic acid production by Lactobacillus delbrueckii subsp. bulgaricus through conversion of organosolv pretreated lignocellulosic biomass. Biomass Bioenergy 2020, 140, 105672. [Google Scholar] [CrossRef]
- Alexandri, M.; Schneider, R.; Venus, J. Membrane Technologies for Lactic Acid Separation from Fermentation Broths Derived from Renewable Resources. Membranes 2018, 8, 94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Viesser, J.A.; de Melo Pereira, G.V.; de Carvalho Neto, D.P.; Rogez, H.; Góes-Neto, A.; Azevedo, V.; Brenig, B.; Aburjaile, F.; Soccol, C.R. Co-culturing fructophilic lactic acid bacteria and yeast enhanced sugar metabolism and aroma formation during cocoa beans fermentation. Int. J. Food Microbiol. 2021, 339, 109015. [Google Scholar] [CrossRef]
- Kumar, S.; Yadav, N.; Nain, L.; Khare, S.K. A simple downstream processing protocol for the recovery of lactic acid from the fermentation broth. Bioresour. Technol. 2020, 318, 124260. [Google Scholar] [CrossRef]
- Joglekar, H.G.; Rahman, I.; Babu, S.; Kulkarni, B.D.; Joshi, A. Comparative assessment of downstream processing options for lactic acid. Sep. Purif. Technol. 2006, 52, 1–17. [Google Scholar] [CrossRef]
- Komesu, A.; Wolf Maciel, M.R.; Filho, M. Separation and purifications technologies for lactic acid—A Brief Review. BioResources 2017, 12, 6885–6901. [Google Scholar] [CrossRef]
- Taleghani, H.G.; Ghoreyshi, A.A.; Najafpour, G.D. Thin film composite nanofiltration membrane for lactic acid production in membrane bioreactor. Biochem. Eng. J. 2018, 132, 152–160. [Google Scholar] [CrossRef]
- Phanthumchinda, N.; Thitiprasert, S.; Tanasupawat, S.; Assabumrungrat, S.; Thongchul, N. Process and cost modeling of lactic acid recovery from fermentation broths by membrane-based process. Process Biochem. 2018, 68, 205–213. [Google Scholar] [CrossRef]
- Desiriani, R.; Kresnowati, M.T.A.P.; Wenten, I.G. Membrane-Based Downstream Processing of Microbial Xylitol Production. Int. J. Technol. 2017, 8, 1393–1401. [Google Scholar] [CrossRef]
- Komesu, A.; Wolf Maciel, M.R.; Rocha de Oliveira, J.A.; Da Silva Martins, L.H.; Maciel Filho, R. Purification of Lactic Acid Produced by Fermentation: Focus on Non-traditional Distillation Processes. Sep. Purif. Rev. 2017, 46, 241–254. [Google Scholar] [CrossRef]
- Sabaté, J.; Pujolà, M.; Labanda, J.; Llorens, J. Influence of pH and operation variables on biogenic amines nanofiltration. Sep. Purif. Technol. 2008, 58, 424–428. [Google Scholar] [CrossRef]
- Chandrapala, J.; Chen, G.Q.; Kezia, K.; Bowman, E.G.; Vasiljevic, T.; Kentish, S.E. Removal of lactate from acid whey using nanofiltration. J. Food Eng. 2016, 177, 59–64. [Google Scholar] [CrossRef]
- Oatley-Radcliffe, D.L.; Walters, M.; Ainscough, T.J.; Williams, P.M.; Mohammad, A.W.; Hilal, N. Nanofiltration membranes and processes: A review of research trends over the past decade. J. Water Process Eng. 2017, 19, 164–171. [Google Scholar] [CrossRef] [Green Version]
- Pal, P.; Sikder, J.; Roy, S.; Giorno, L. Process intensification in lactic acid production: A review of membrane based processes. Chem. Eng. Process. Process Intensif. 2009, 48, 1549–1559. [Google Scholar] [CrossRef]
- Oberherr, R.; Tassinary, R.F.; Vognach, L.; Stulp, S. Application of ultrafiltration and electrodialysis techniques in lactic acid removal from whey solutions. Eclet. Quim. J. 2019, 44, 39–45. [Google Scholar] [CrossRef] [Green Version]
- Lech, M.; Trusek, A. Batch Electrodialysis of Lactic Acid Obtained from Lab Fermentation. Pol. J. Chem. Technol. 2018, 20, 81–86. [Google Scholar] [CrossRef] [Green Version]
- Dey, P.; Linnanen, L.; Pal, P. Separation of lactic acid from fermentation broth by cross flow nanofiltration: Membrane characterization and transport modelling. Desalination 2012, 288, 47–57. [Google Scholar] [CrossRef]
- Diaz, P.A.B.; Araujo Kronemberger, F.; Habert, A.C. Predictive study of succinic acid transport via nanofiltration membranes using a Maxwell–Stefan approach. J. Chem. Technol. Biotechnol. 2021, 96, 785–800. [Google Scholar] [CrossRef]
- Khanlari, S.; Tofighy, M.A.; Mohammadi, T. Transport phenomena through nanocomposite membranes. In Nanocomposite Membranes for Water and Gas Separation; Elsevier: Amsterdam, The Netherlands, 2020; pp. 91–112. ISBN 9780128167106. [Google Scholar]
- Cao, X.; Zhou, M.; Jia, S.; Yuan, X.; Yu, K.-T. Maxwell–Stefan diffusion coefficient model derived from entropy generation minimization principle for binary liquid mixtures. Chem. Eng. Sci. 2019, 207, 30–38. [Google Scholar] [CrossRef]
- Ebneyamini, A.; Azimi, H.; Thibault, J.; Tezel, F.H. Description of butanol aqueous solution transport through commercial PDMS pervaporation membrane using extended Maxwell–Stefan model. Sep. Sci. Technol. 2018, 53, 1611–1627. [Google Scholar] [CrossRef]
- Ecker, J.; Raab, T.; Harasek, M. Nanofiltration as key technology for the separation of LA and AA. J. Membr. Sci. 2012, 389, 389–398. [Google Scholar] [CrossRef]
- Ribeiro, A.C.F.; Lobo, V.M.M.; Leaist, D.G.; Natividade, J.J.S.; Veríssimo, L.P.; Barros, M.C.F.; Cabral, A.M.T.D.P.V. Binary Diffusion Coefficients for Aqueous Solutions of Lactic Acid. J. Solut. Chem. 2005, 34, 1009–1016. [Google Scholar] [CrossRef] [Green Version]
- 2Semião, A.J.; Schäfer, A.I. Estrogenic micropollutant adsorption dynamics onto nanofiltration membranes. J. Membr. Sci. 2011, 381, 132–141. [Google Scholar] [CrossRef]
- Thamaraiselvan, C.; Michael, N.; Yoram, O. Selective Separation of Dyes and Brine Recovery from Textile Wastewater by Nanofiltration Membranes. Chem. Eng. Technol. 2018, 2, 285–293. [Google Scholar] [CrossRef]
- Ahmad, A.; Banat, F.; Taher, H. A review on the lactic acid fermentation from low-cost renewable materials: Recent developments and challenges. Environ. Technol. Innov. 2020, 20, 101138. [Google Scholar] [CrossRef]
- Zhou, F.; Wang, C.; Wei, J. Separation of acetic acid from monosaccharides by NF and RO membranes: Performance comparison. J. Membr. Sci. 2013, 429, 243–251. [Google Scholar] [CrossRef]
- Merino-Garcia, I.; Velizarov, S. New insights into the definition of membrane cleaning strategies to diminish the fouling impact in ion exchange membrane separation processes. Sep. Purif. Technol. 2021, 277, 119445. [Google Scholar] [CrossRef]
- Mänttäri, M.; Pekuri, T.; Nyström, M. NF270, a new membrane having promising characteristics and being suitable for treatment of dilute effluents from the paper industry. J. Membr. Sci. 2004, 242, 107–116. [Google Scholar] [CrossRef]
- Liikanen, R.; Kiuru, H.; Tuhkanen, T.; Nyström, M. Nanofiltration membrane fouling by conventionally treated surface water. Water Sci. Technol. Water Supply 2003, 3, 183–190. [Google Scholar] [CrossRef]
- Ding, J.; Zhao, H.; Xu, B.; Yu, H. Biomimetic Sustainable Graphene Ultrafast-Selective Nanofiltration Membranes. ACS Sustain. Chem. Eng. 2020, 8, 8986–8993. [Google Scholar] [CrossRef]
- Ortiz-Albo, P.; Ibañez, R.; Urtiaga, A.; Ortiz, I. Phenomenological prediction of desalination brines nanofiltration through the indirect determination of zeta potential. Sep. Purif. Technol. 2019, 210, 746–753. [Google Scholar] [CrossRef]
- Zhang, L.; Hamelers, H.; Biesheuvel, P.M. Modeling permeate pH in RO membranes by the extended Donnan steric partitioning pore model. J. Membr. Sci. 2020, 613, 118511. [Google Scholar] [CrossRef]
- Ang, M.B.M.Y.; Tang, C.-L.; de Guzman, M.R.; Maganto, H.L.C.; Caparanga, A.R.; Huang, S.-H.; Tsai, H.-A.; Hu, C.-C.; Lee, K.-R.; Lai, J.-Y. Improved performance of thin-film nanofiltration membranes fabricated with the intervention of surfactants having different structures for water treatment. Desalination 2020, 481, 114352. [Google Scholar] [CrossRef]
- González, M.I.; Alvarez, S.; Riera, F.A.; Álvarez, R. Lactic acid recovery from whey ultrafiltrate fermentation broths and artificial solutions by nanofiltration. Desalination 2008, 228, 84–96. [Google Scholar] [CrossRef]
- Novalin, S.; Zweckmair, T. Renewable resources-green biorefinery: Separation of valuable substances from fluid-fractions by means of membrane technology. Biofuels Bioprod. Bioref. 2009, 3, 20–27. [Google Scholar] [CrossRef]


| Property | Value |
|---|---|
| Molecular structure | 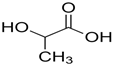 |
| Molecular formula | C3H6O3 |
| Molecular weight g mol−1 | 90.08 |
| Dissociation constant (pKa) at 25 °C | 3.86 |
| Dissociation constant (pKa) at 40 °C | 3.67 |
| Diffusion coefficient at 30 °C [25] | 11.2 × 10−10 m2 s−1 |
| Parameter | NF270 | SelRO® MPF-36 | Toray NF | Alfa Laval NF |
|---|---|---|---|---|
| Manufacture | FilmTec™ | Koch | Toray | Alfa Laval |
| Material | Polypiperazine | Polysulfone | Polypiperazineamide | Polyamide |
| MWCO (g mol−1) | 200 | 1000 | 200 | 300 |
| Maximum operating temperature (°C) | 45 | 60 | 50 | 50 |
| Operating pH range | 3–10 | 3–10 | 3.5–10.5 | 3–10 |
| Max operating pressure (bar) | 41 | 35 | 55.2 | 55 |
| Isoelectric point (pH) | 3.6 [26] | 5–6.5 [27] | 4.0 | 4.0 |
| Experiment | Pressure (bar) | Temperature (°C) | pH | Lactic Acid (g L−1) |
|---|---|---|---|---|
| 1 | 32 | 25 | 2.8 | 25 |
| 2 | 32 | 40 | 2.8 | 25 |
| 3 | 32 | 25 | 3.9 | 25 |
| 4 | 32 | 25 | 6.0 | 25 |
| Experiment | Value |
|---|---|
| Equipment | Shimadzu UFLC |
| Flow (mL min−1) | 0.6 |
| Injection volume (µL) | 10 |
| Mobile phase of H2SO4 (mM) | 5 |
| Gradient | Isocratic |
| Oven temperature (°C) | 50 |
| Refractive index detector | RID-10A |
| column | Shodex SH1011 (8 × 300 mm) |
| Guard column | SH-G Sugar |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cabrera-González, M.; Ahmed, A.; Maamo, K.; Salem, M.; Jordan, C.; Harasek, M. Evaluation of Nanofiltration Membranes for Pure Lactic Acid Permeability. Membranes 2022, 12, 302. https://doi.org/10.3390/membranes12030302
Cabrera-González M, Ahmed A, Maamo K, Salem M, Jordan C, Harasek M. Evaluation of Nanofiltration Membranes for Pure Lactic Acid Permeability. Membranes. 2022; 12(3):302. https://doi.org/10.3390/membranes12030302
Chicago/Turabian StyleCabrera-González, Mayuki, Amal Ahmed, Khaled Maamo, Mohammad Salem, Christian Jordan, and Michael Harasek. 2022. "Evaluation of Nanofiltration Membranes for Pure Lactic Acid Permeability" Membranes 12, no. 3: 302. https://doi.org/10.3390/membranes12030302
APA StyleCabrera-González, M., Ahmed, A., Maamo, K., Salem, M., Jordan, C., & Harasek, M. (2022). Evaluation of Nanofiltration Membranes for Pure Lactic Acid Permeability. Membranes, 12(3), 302. https://doi.org/10.3390/membranes12030302








