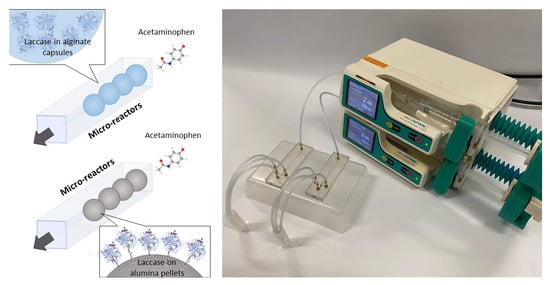
Comparison of Acetaminophen Degradation by Laccases Immobilized by Two Different Methods via a Continuous Flow Microreactor Process Scheme
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Laccase Immobilization on Silanized Al2O3 Pellets
2.3. Laccase Encapsulation into Alginate Microcapsules via Microfluidics
2.4. Laccase Encapsulation/Immobilization Efficiencies
2.5. Quantification of Laccase
2.6. SEM Characterization
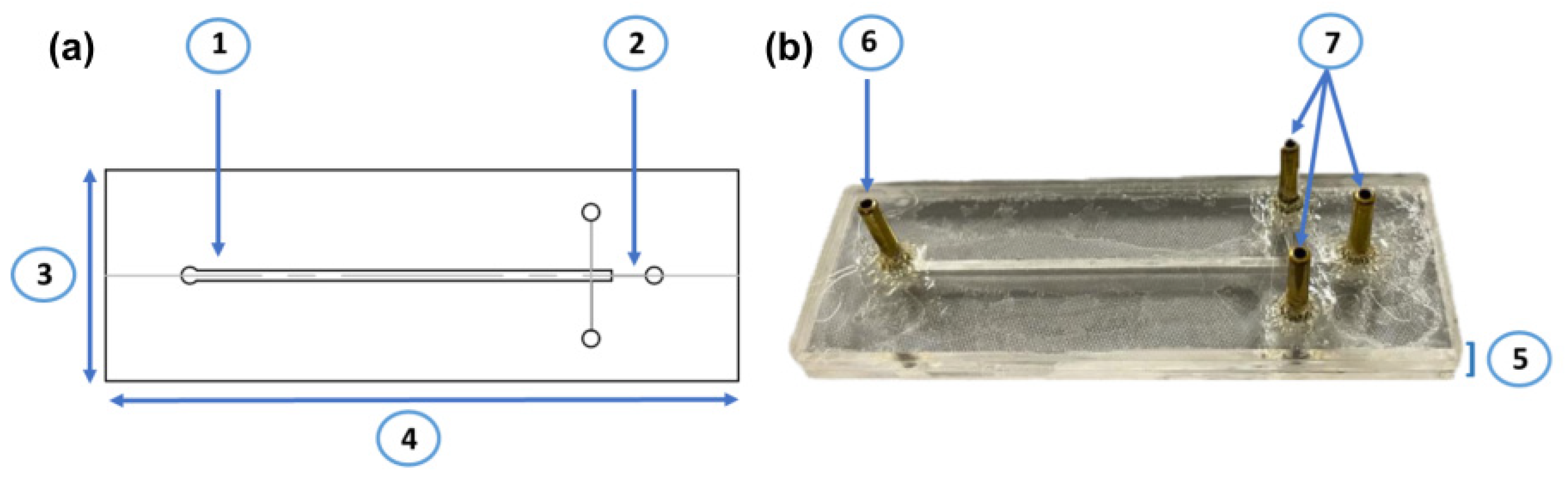
2.7. Design of the Continuous Flow Microreactor for Acetaminophen Treatment
2.8. Acetaminophen Treatment in Microreactors with Immobilized Laccase
2.9. Phytotoxicity Test
2.10. Statistical Analysis
3. Results
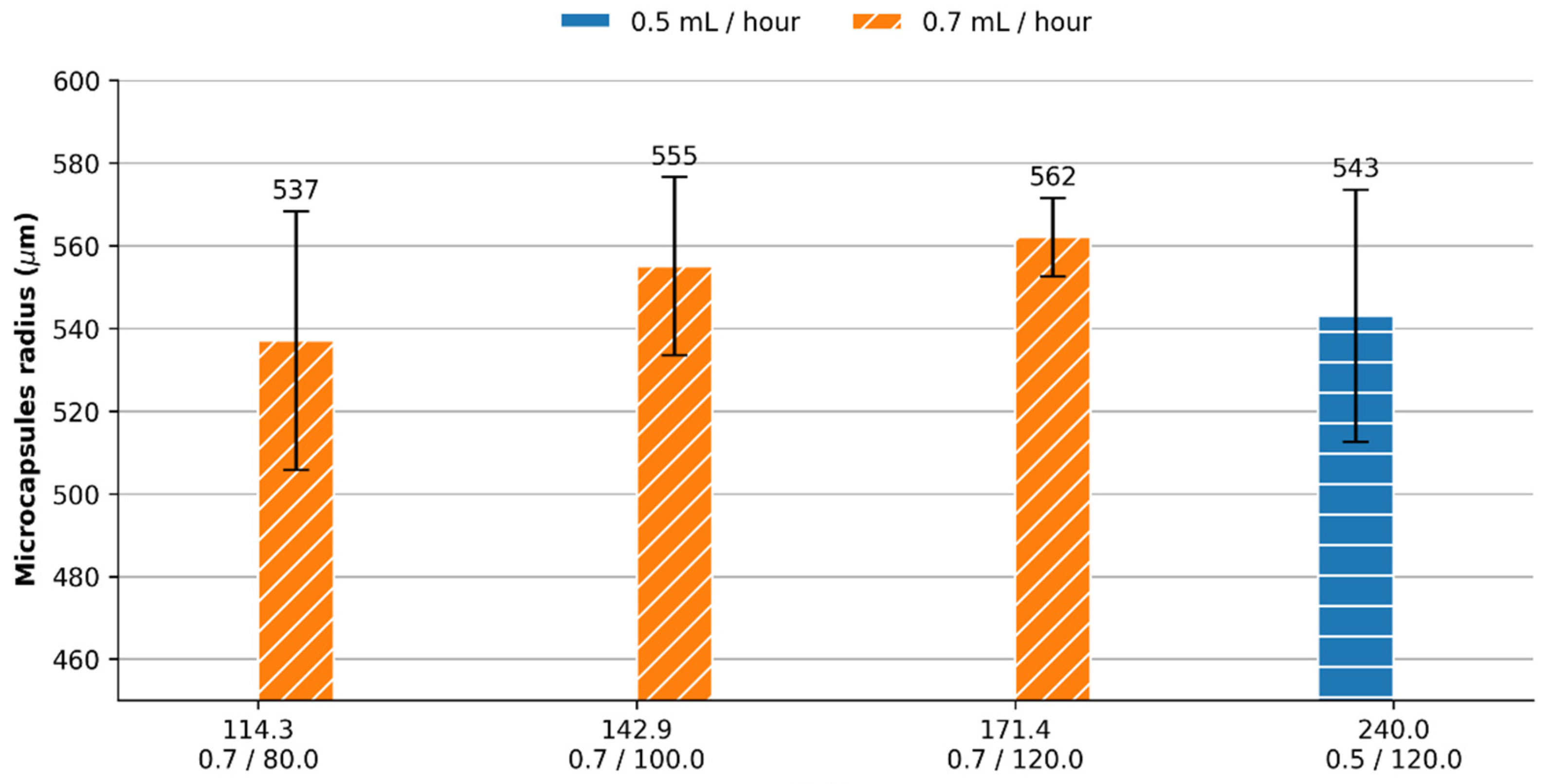
3.1. Laccase Encapsulation into Alginate Microcapsules via Microfluidics
3.2. Laccase Immobilization Efficiencies
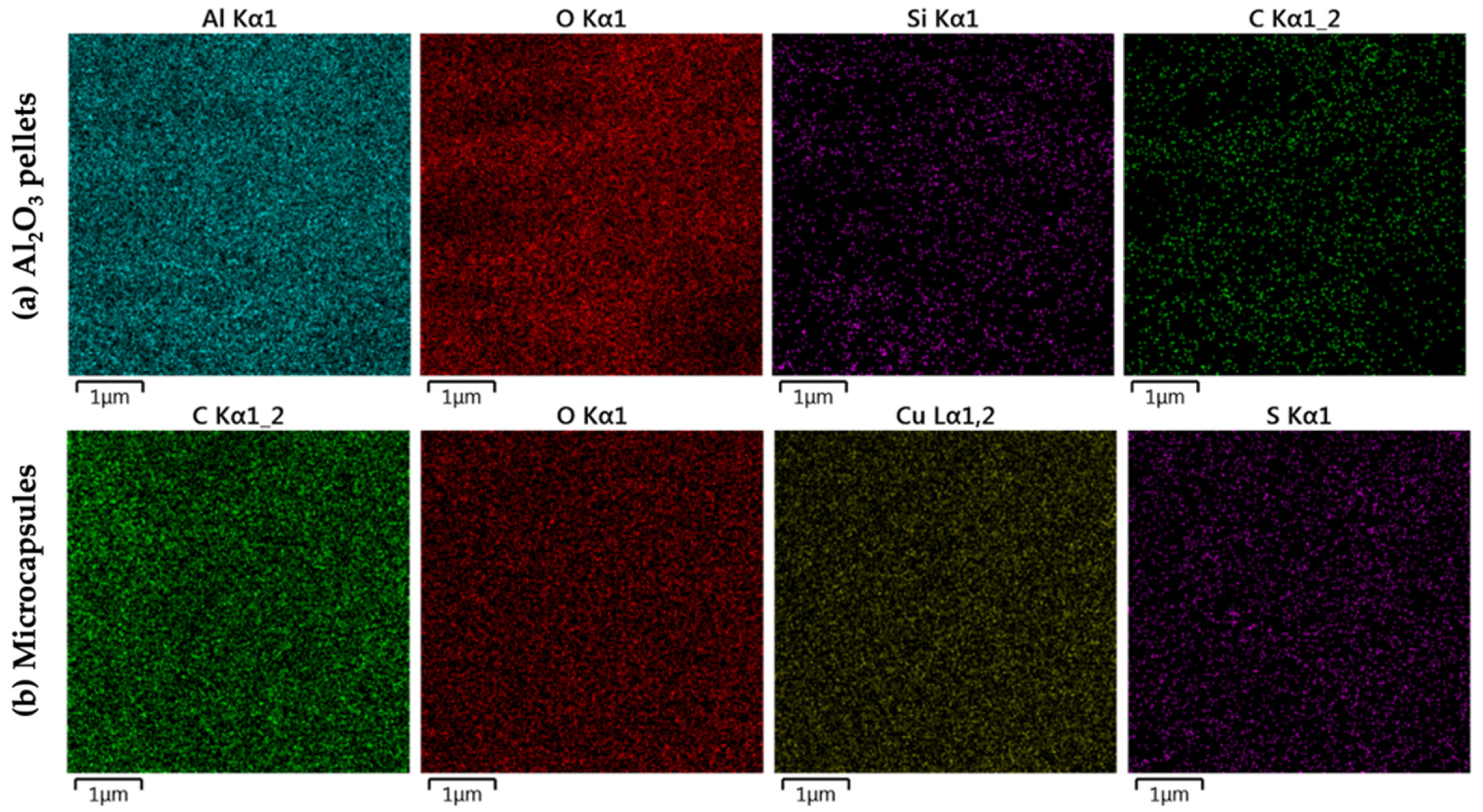
3.3. Microscopy Characterization of Immobilized Pellets and Microcapsules
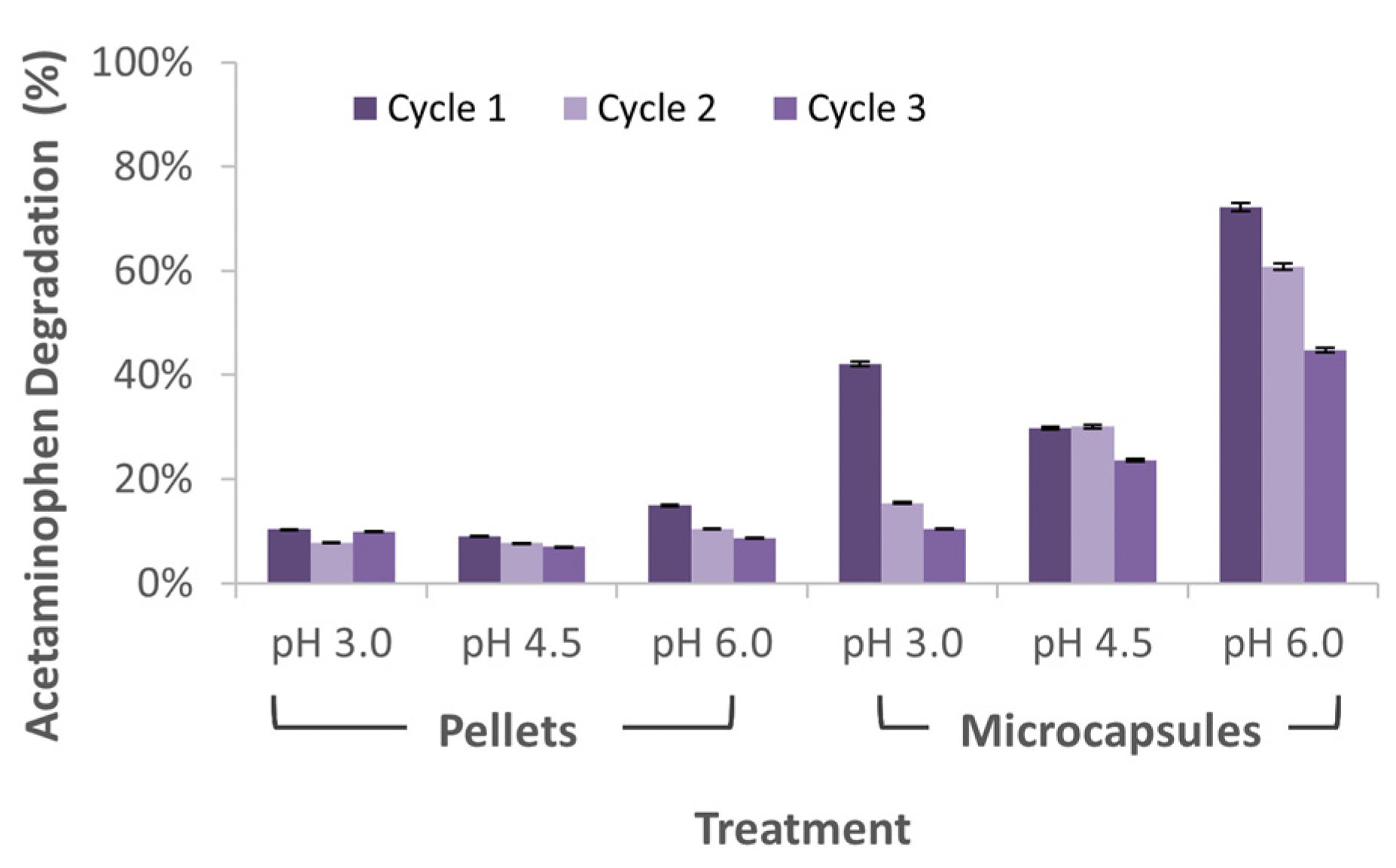
3.4. Acetaminophen Treatment in Microreactors Packed with Immobilized Laccase Preparations
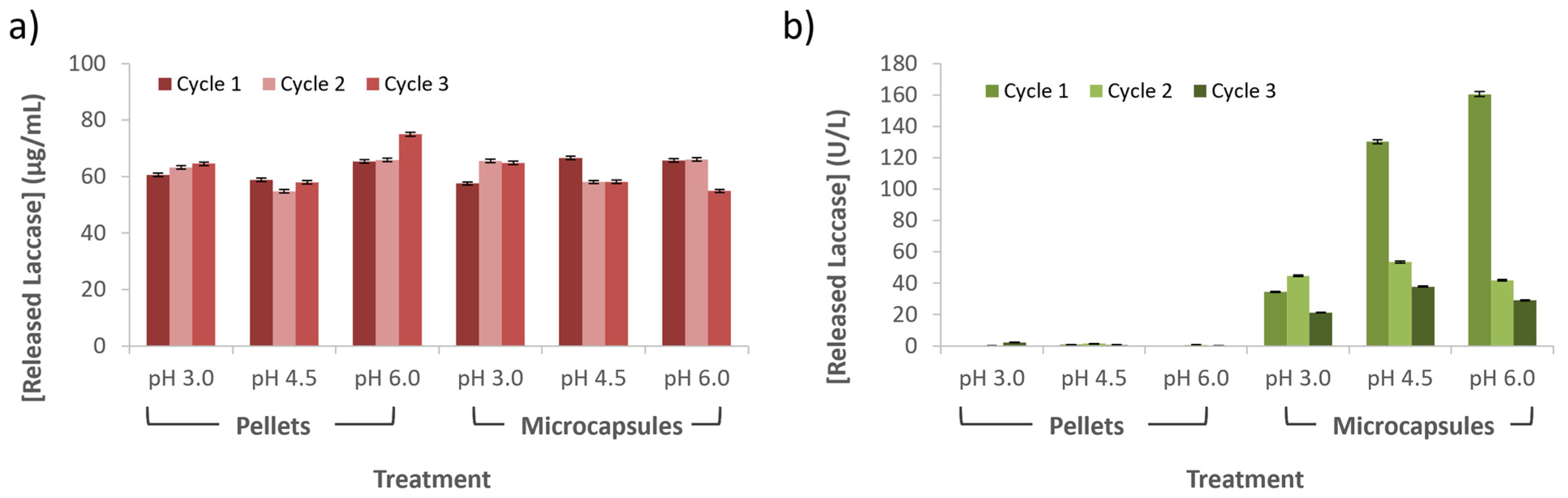
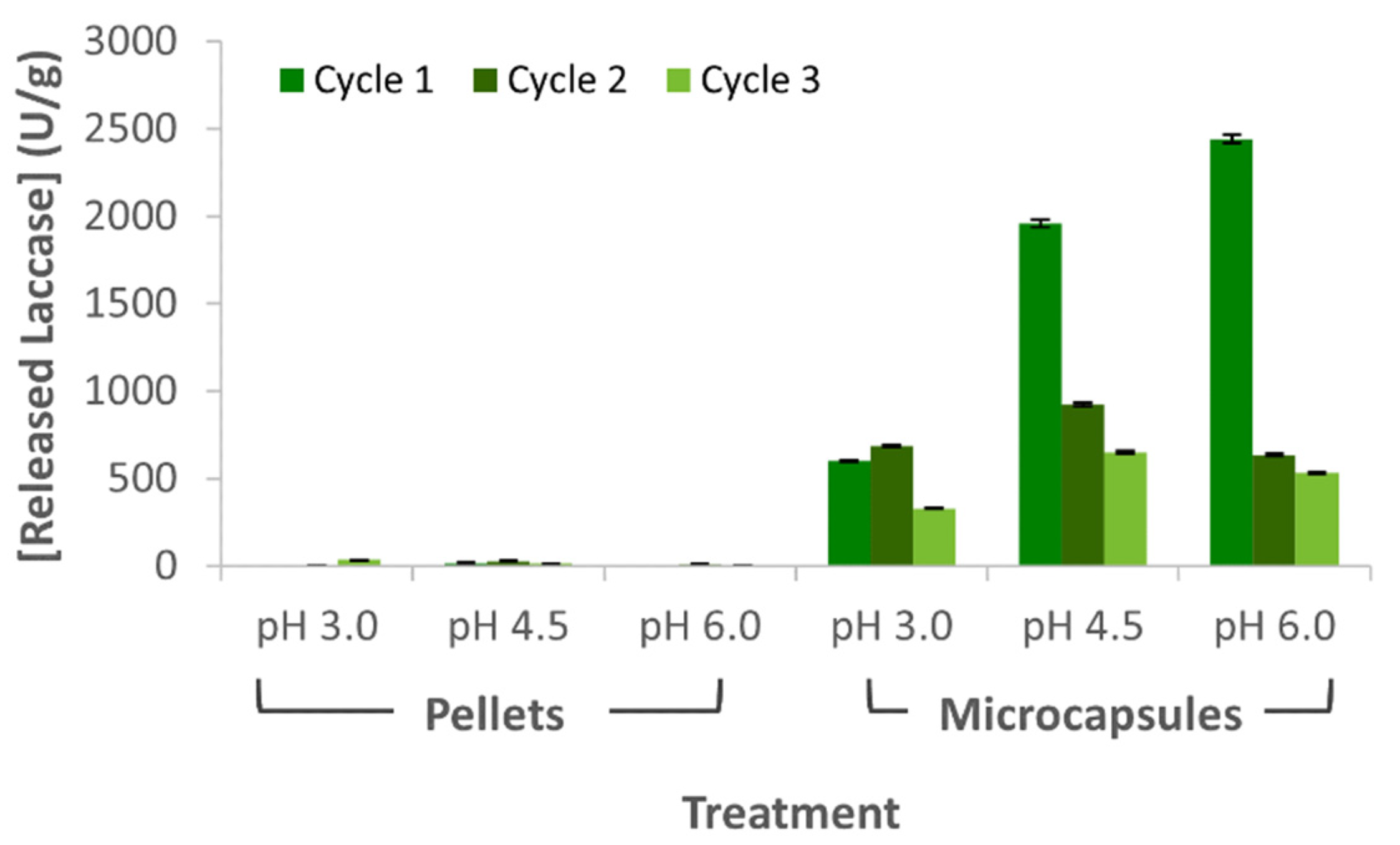
3.5. Released and Laccase Losses during Wastewater Treatment
3.6. Phytotoxicity Test
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tilley, E. Compendium of Sanitation Systems and Technologies; Swiss Federal Institute of Aquatic Science and Technology (Eawag): Dübendorf, Switzerland, 2014. [Google Scholar]
- Tripathi, V.K.; Rajput, T.B.S.; Patel, N. Performance of different filter combinations with surface and subsurface drip irrigation systems for utilizing municipal wastewater. Irrig. Sci. 2014, 32, 379–391. [Google Scholar] [CrossRef]
- UNESCO. The United Nations World Water Development Report 2017. Wastewater: The Untapped Resource; The United Nations Educational, Scientific and Cultural Organization: Paris, France, 2017. [Google Scholar]
- Margot, J.; Rossi, L.; Barry, D.; Holliger, C. A review of the fate of micropollutants in wastewater treatment plants. WIREs Water 2015, 2, 457–487. [Google Scholar] [CrossRef] [Green Version]
- Kubiňáková, E.; Fašková, L.; Králiková, E.; Híveša, J.; Mackuľak, T. Micropollutants in wastewater and their degradation by ferrates (VI). Acta Chim. Slovaca 2018, 11, 43–50. [Google Scholar] [CrossRef] [Green Version]
- Paul, P.E.V.; Sangeetha, V.; Deepika, R.G. Emerging Trends in the Industrial Production of Chemical Products by Microorganisms. In Recent Developments in Applied Microbiology and Biochemistry; Academic Press: Cambridge, MA, USA, 2018; pp. 107–125. [Google Scholar] [CrossRef]
- Ferreira, D.P.; Cruz, J.; Fangueiro, R. Surface modification of natural fibers in polymer composites. In Green Composites for Automotive Applications; Woodhead Publishing: Sawston, UK, 2018; pp. 3–41. [Google Scholar] [CrossRef]
- Berg, J.; Tymoczko, J.; Stryer, L. Biochemistry, 5th ed.; Freeman Publishing: New York, NY, USA, 2002. [Google Scholar]
- Jegannathan, K.R.; Nielsen, P.H. Environmental assessment of enzyme use in industrial production—A literature review. J. Clean. Prod. 2013, 42, 228–240. [Google Scholar] [CrossRef] [Green Version]
- Chapman, J.; Ismail, A.E.; Dinu, C.Z. Industrial Applications of Enzymes: Recent Advances, Techniques, and Outlooks. Catalysts 2018, 8, 238. [Google Scholar] [CrossRef] [Green Version]
- Langbehn, R.K.; Michels, C.; Soares, H.M. Antibiotics in wastewater: From its occurrence to the biological removal by environmentally conscious technologies. Environ. Pollut. 2021, 275, 116603. [Google Scholar] [CrossRef]
- Naghdi, M.; Taheran, M.; Brar, S.K.; Kermanshahi-Pour, A.; Verma, M.; Surampalli, R. Removal of pharmaceutical compounds in water and wastewater using fungal oxidoreductase enzymes. Environ. Pollut. 2018, 234, 190–213. [Google Scholar] [CrossRef]
- Osma, J.F.; Toca-Herrera, J.L.; Rodríguez-Couto, S. Uses of Laccases in the Food Industry. Enzym. Res. 2010, 2010, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Chauhan, A.K.; Choudhury, B. Synthetic dyes degradation using lignolytic enzymes produced from Halopiger aswanensis strain ABC_IITR by Solid State Fermentation. Chemosphere 2021, 273, 129671. [Google Scholar] [CrossRef]
- Liu, Z.; Smith, S.R. Enzyme Recovery from Biological Wastewater Treatment. Waste Biomass Valorization 2021, 12, 4185–4211. [Google Scholar] [CrossRef]
- Yuan, Z.; Chen, L. The Role of Endophytic Fungal Individuals and Communities in the Decomposition of Pinus massoniana Needle Litter. PLoS ONE 2014, 9, e105911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barber, E.A.; Liu, Z.; Smith, S.R. Organic Contaminant Biodegradation by Oxidoreductase Enzymes in Wastewater Treatment. Microorganisms 2020, 8, 122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mashayekh-Salehi, A.; Moussavi, G.; Yaghmaeian, K. Preparation, characterization and catalytic activity of a novel mesoporous nanocrystalline MgO nanoparticle for ozonation of acetaminophen as an emerging water contaminant. Chem. Eng. J. 2017, 310, 157–169. [Google Scholar] [CrossRef]
- Vo, H.N.P.; Le, G.K.; Nguyen, T.M.H.; Bui, X.-T.; Nguyen, K.H.; Rene, E.R.; Vo, T.D.H.; Cao, N.-D.T.; Mohan, R. Acetaminophen micropollutant: Historical and current occurrences, toxicity, removal strategies and transformation pathways in different environments. Chemosphere 2019, 236, 124391. [Google Scholar] [CrossRef]
- Maryšková, M.; Schaabová, M.; Tománková, H.; Novotný, V.; Rysová, M. Wastewater Treatment by Novel Polyamide/Polyethylenimine Nanofibers with Immobilized Laccase. Water 2020, 12, 588. [Google Scholar] [CrossRef] [Green Version]
- Morsi, R.; Bilal, M.; Iqbal, H.M.; Ashraf, S.S. Laccases and peroxidases: The smart, greener and futuristic biocatalytic tools to mitigate recalcitrant emerging pollutants. Sci. Total Environ. 2020, 714, 136572. [Google Scholar] [CrossRef]
- Arya, P.S.; Yagnik, S.M.; Rajput, K.N.; Panchal, R.R.; Raval, V.H. Understanding the Basis of Occurrence, Biosynthesis, and Implications of Thermostable Alkaline Proteases. Appl. Biochem. Biotechnol. 2021, 193, 4113–4150. [Google Scholar] [CrossRef]
- Basu, A.K.; Basu, A.; Bhattacharya, S. Study of pH induced conformational change of papain using polymeric nano-cantilever. AIP Conf. Proc. 2019, 2083, 030001. [Google Scholar] [CrossRef]
- Federsel, H.-J.; Moody, T.; Taylor, S. Recent Trends in Enzyme Immobilization—Concepts for Expanding the Biocatalysis Toolbox. Molecules 2021, 26, 2822. [Google Scholar] [CrossRef]
- Rangel-Muñoz, N.; González-Barrios, A.; Pradilla, D.; Osma, J.; Cruz, J. Novel Bionanocompounds: Outer Membrane Protein A and Lacasse Co-Immobilized on Magnetite Nanoparticles for Produced Water Treatment. Nanomaterials 2020, 10, 2278. [Google Scholar] [CrossRef]
- Lopez-Barbosa, N.; Florez, S.L.; Cruz, J.C.; Ornelas-Soto, N.; Osma, J.F. Congo Red Decolorization Using Textile Filters and Laccase-Based Nanocomposites in Continuous Flow Bioreactors. Nanomaterials 2020, 10, 1227. [Google Scholar] [CrossRef] [PubMed]
- García-Morales, R.; Garcia, A.; Orona-Navar, C.; Osma, J.F.; Nigam, K.; Ornelas-Soto, N. Biotransformation of emerging pollutants in groundwater by laccase from P. sanguineus CS43 immobilized onto titania nanoparticles. J. Environ. Chem. Eng. 2018, 6, 710–717. [Google Scholar] [CrossRef]
- Singh, M.; Hemant, K.; Ram, M.; Shivakumar, H. Microencapsulation: A promising technique for controlled drug delivery. Res. Pharm. Sci. 2010, 5, 65–77. [Google Scholar] [PubMed]
- Campaña, A.L.; Sotelo, D.C.; Oliva, H.A.; Aranguren, A.; Ornelas-Soto, N.; Cruz, J.C.; Osma, J.F. Fabrication and Characterization of a Low-Cost Microfluidic System for the Manufacture of Alginate–Lacasse Microcapsules. Polymers 2020, 12, 1158. [Google Scholar] [CrossRef]
- Ozkan, G.; Franco, P.; De Marco, I.; Xiao, J.; Capanoglu, E. A review of microencapsulation methods for food antioxidants: Principles, advantages, drawbacks and applications. Food Chem. 2019, 272, 494–506. [Google Scholar] [CrossRef]
- van der Helm, M.P.; Bracco, P.; Busch, H.; Szymańska, K.; Jarzębski, A.B.; Hanefeld, U. Hydroxynitrile lyases covalently immobilized in continuous flow microreactors. Catal. Sci. Technol. 2019, 9, 1189–1200. [Google Scholar] [CrossRef] [Green Version]
- Ramírez-Cavazos, L.I.; Junghanns, C.; Ornelas-Soto, N.; Cárdenas-Chávez, D.L.; Luna, C.E.H.; Demarche, P.; Enaud, E.; García-Morales, R.; Agathos, S.; Parra, R. Purification and characterization of two thermostable laccases from Pycnoporus sanguineus and potential role in degradation of endocrine disrupting chemicals. J. Mol. Catal. B Enzym. 2014, 108, 32–42. [Google Scholar] [CrossRef] [Green Version]
- Lopez-Barbosa, N.; Suárez-Arnedo, A.; Cifuentes, J.; Barrios, A.F.G.; Batista, C.A.S.; Osma, J.F.; Muñoz-Camargo, C.; Cruz, J.C. Magnetite–OmpA Nanobioconjugates as Cell-Penetrating Vehicles with Endosomal Escape Abilities. ACS Biomater. Sci. Eng. 2020, 6, 415–424. [Google Scholar] [CrossRef]
- Niku-Paavola, M.; Raaska, L.; Itävaara, M. Detection of white-rot fungi by a non-toxic stain. Mycol. Res. 1990, 94, 27–31. [Google Scholar] [CrossRef]
- Peretz, S.; Anghel, D.F.; Vasilescu, E.; Florea-Spiroiu, M.; Stoian, C.; Zgherea, G. Synthesis, characterization and adsorption properties of alginate porous beads. Polym. Bull. 2015, 72, 3169–3182. [Google Scholar] [CrossRef]
- Banerjee, S.; Dubey, S.; Gautam, R.K.; Chattopadhyaya, M.; Sharma, Y.C. Adsorption characteristics of alumina nanoparticles for the removal of hazardous dye, Orange G from aqueous solutions. Arab. J. Chem. 2019, 12, 5339–5354. [Google Scholar] [CrossRef]
- Chen, T.S.; Huang, K.L. Electrochemical detection and degradation of acetaminophen in aqueous solutions. Int. J. Electrochem. Sci. 2012, 7, 6877–6892. [Google Scholar]
- Zdarta, J.; Jesionowski, T.; Pinelo, M.; Meyer, A.S.; Iqbal, H.M.; Bilal, M.; Nguyen, L.N.; Nghiem, L.D. Free and immobilized biocatalysts for removing micropollutants from water and wastewater: Recent progress and challenges. Bioresour. Technol. 2022, 344, 126201. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Chen, Y.; Wei, N. Biocatalytic properties of cell surface display laccase for degradation of emerging contaminant acetaminophen in water reclamation. Biotechnol. Bioeng. 2020, 117, 342–353. [Google Scholar] [CrossRef] [Green Version]
- Ratanapongleka, K.; Punbut, S. Removal of acetaminophen in water by laccase immobilized in barium alginate. Environ. Technol. 2018, 39, 336–345. [Google Scholar] [CrossRef]
- Brás, E.J.S.; Chu, V.; Conde, J.P.; Fernandes, P. Recent developments in microreactor technology for biocatalysis applications. React. Chem. Eng. 2021, 6, 815–827. [Google Scholar] [CrossRef]
- Kazan, A.; Hu, X.; Stahl, A.; Frerichs, H.; Smirnova, I.; Yesil-Celiktas, O. An enzyme immobilized microreactor for continuous-flow biocatalysis of ginsenoside Rb1. J. Chem. Technol. Biotechnol. 2021, 96, 3349–3357. [Google Scholar] [CrossRef]
- Bermudez, J.F.; Saldarriaga, J.F.; Osma, J.F. Portable and Low-Cost Respirometric Microsystem for the Static and Dynamic Respirometry Monitoring of Compost. Sensors 2019, 19, 4132. [Google Scholar] [CrossRef] [Green Version]
- Fuentes, O.P.; Noguera, M.J.; Peñaranda, P.A.; Flores, S.L.; Cruz, J.C.; Osma, J.F. Micromixers for Wastewater Treatment and Their Life Cycle Assessment (LCA). In Advances in Microfluidics and Nanofluids; IntechOpen: London, UK, 2021. [Google Scholar] [CrossRef]
- Ghanbari, F.; Giannakis, S.; Lin, K.-Y.A.; Wu, J.; Madihi-Bidgoli, S. Acetaminophen degradation by a synergistic peracetic acid/UVC-LED/Fe(II) advanced oxidation process: Kinetic assessment, process feasibility and mechanistic considerations. Chemosphere 2021, 263, 128119. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sotelo, L.D.; Sotelo, D.C.; Ornelas-Soto, N.; Cruz, J.C.; Osma, J.F. Comparison of Acetaminophen Degradation by Laccases Immobilized by Two Different Methods via a Continuous Flow Microreactor Process Scheme. Membranes 2022, 12, 298. https://doi.org/10.3390/membranes12030298
Sotelo LD, Sotelo DC, Ornelas-Soto N, Cruz JC, Osma JF. Comparison of Acetaminophen Degradation by Laccases Immobilized by Two Different Methods via a Continuous Flow Microreactor Process Scheme. Membranes. 2022; 12(3):298. https://doi.org/10.3390/membranes12030298
Chicago/Turabian StyleSotelo, Laura D., Diana C. Sotelo, Nancy Ornelas-Soto, Juan C. Cruz, and Johann F. Osma. 2022. "Comparison of Acetaminophen Degradation by Laccases Immobilized by Two Different Methods via a Continuous Flow Microreactor Process Scheme" Membranes 12, no. 3: 298. https://doi.org/10.3390/membranes12030298
APA StyleSotelo, L. D., Sotelo, D. C., Ornelas-Soto, N., Cruz, J. C., & Osma, J. F. (2022). Comparison of Acetaminophen Degradation by Laccases Immobilized by Two Different Methods via a Continuous Flow Microreactor Process Scheme. Membranes, 12(3), 298. https://doi.org/10.3390/membranes12030298