Abstract
Environmentalists are prioritizing reuse, recycling, and recovery systems to meet rising water demand. Diving into produced water treatment to enable compliance by the petroleum industry to meet discharge limits has increased research into advanced treatment technologies. The integration of biological degradation of pollutants and membrane separation has been recognized as a versatile technology in dealing with produced water with strength of salts, minerals, and oils being produced during crude refining operation. This review article presents highlights on produced water, fundamental principles of membrane bioreactors (MBRs), advantages of MBRs over conventional technologies, and research progress in the application of MBRs in treating produced water. Having limited literature that specifically addresses MBRs for PW treatment, this review also attempts to elucidate the treatment efficiency of MBRs PW treatment, integrated MBR systems, general fouling, and fouling mitigation strategies.
1. Introduction
The petroleum refinery industry plays a major role in providing energy to meet the world’s energy demand and industrial activities; its exploration comes with huge environmental risks. Despite the rate of renewable energy growth due to fossil fuel depletion, the oil and gas industry is anticipated to contribute 40–50% of the total global energy mix between 2040–2050 [1,2]. The value chain of crude oil and gas can be categorized into the upstream (exploration, drilling, and extraction), midstream (processing, transportation, and storage) and downstream (conversion, refinery, marketing and distribution) [3,4], where the transportation in the midstream is mostly via pipeline, rail, and shipments.
Consequentially, refining crude oil into useful products demands huge amounts of water for processes such as distillation, cracking, polymerization, alkylation, hydrotreating, desalting, treatment, and finishing of petroleum products [3,5,6]. In this case, it is estimated that one barrel of refined oil produces nearly 10 barrels of wastewater [5]. This wastewater is highly complex, containing high concentrations of residual free and emulsified oils, hydrocarbons (representing the majority of the organic load), dissolved salts (halides, phosphates, sulfates, and sulfides), and carcinogenic and mutagenic substances that require rigorous intervention to be eliminated [5,6,7,8]. During oil and gas processes, about 80% liquid waste, often at temperatures >50 °C is commonly referred to as produced water (PW) [7]. The ratio of PW to oil extracted from the reservoirs are estimated at 3:1 [6,7]. Basically, the total water consists of water in the cavities of the subsurface formations and injected water which has a dual purpose of enhancing the recovery of oil and gas and maintaining pressure in the reservoir [9,10,11]. For gas production wells, PW also consists of condensed water [12,13]. Inclusive of water, PW is also a complex mixture of soluble and insoluble high molecular weight hydrocarbons (aromatic and saturated), heavy metals, anions, and other impurities [14]. Their varying quantity, constituents, and characteristics are dependent on the geographic location of the field, geo-structure of the well, age of well, reservoir drive mechanism, mechanical integrity, well drilling and production technology, refinery technology, and the type of hydrocarbon products [15,16].
Water demand and issues of scarcity cannot be overlooked as water is essential to life and the functioning of ecosystems on earth. The demand and withdrawal concerns does not pertain to only volume, but rather, to volume and quality [17]. A fundamental proceeding in the protection of quality water involves investigating and developing efficient technologies for the treatment and conversion of these complex toxic compounds into harmless ones. A viable route to water sustainability and fresh water scarcity is the recycling and reuse of reclaimed wastewater for non-potable and potable use [18,19]. This environmentally and financially sustainable approach also involves the recovery of resources such as nitrogen, organic matter, phosphorus, heavy metals, and value addition of sludge through its beneficial usage as fertilizers, green energy, and biosolids [20].
For the beneficial reuse of PW for agricultural irrigation, a much-supported application that is aimed at transforming waste streams into a valuable resource requires a level of treatment before using to avoid operational challenges [21,22]. In the treatment of PW, the general objectives are to achieve the removal of both free and dispersed oil (de-oiling), disinfection, the removal of suspended particles, removal of various dissolved gases, desalination, and demineralization, softening and deionization, and sodicity level adjustment. Comparing membrane bioreactors (MBRs) to Physico-chemical processes, trickling bed, and activated sludge, MBRs have shown quite high treatment performances in removing both organic and inorganic contaminants [23,24,25]. While most of the reviews available consider generalized performances of membrane bioreactors on generalized wastewater, less attention has been paid to MBRs for PW treatment. This review, based on a systematic literature survey approach, considers the application of MBRs specifically to PW treatment. Sections of this succinct, easy to refer review expounds on PWs, MBRs, and the output of the investigations on MBRs in PW treatment, which includes the system configuration, integration of MBR, and other processes to treat PWs and modeling of MBR in PW treatment. The remaining phase is an overview of fouling in MBRs in PW treatment and fouling control in MBRs with its adaptability in PW treatment. Finally, the authors provide their perspective on the future of MBR research in PW treatment, which is expected to provide research gaps to scientists and engineers engaged in this field.
2. Produced Water
2.1. Production and Mangement of PW
Globally, PW generation is estimated at 250 million barrels per day with a foreseeable increase that is aligned to the increased oil and gas production and ageing of wells [16]. PW from oil and gas exploration fields contribute to 60% of annual generation. About 30% of the global oil production is contributed by offshore production [26]. In the United States of America (USA) alone, an estimated 21.6 billion barrels of PW is generated every year with onshore production contributing to about 97% and the remainder attributed to offshore sites [27]. PW from offshore exploration is mostly discharged to the immediate aquatic environment. Figure 1 shows the water life cycle of PW. However due to the hazardous constituents of PW, its management has been met with stricter oil and gas policies and legislation to avoid interference with environmental sustainability. These regulatory policies and standards vary from country to country and non-compliance could result in civil penalties, large fines, international criminal prosecution, and lost or deferred production [28]. One major concern of relevance that has led to these legal considerations is the biological effect of PW. Adopted management practices include the reuse of PW for drilling and operational purposes and deep well injection with no intent of accessibility [29]. The most adopted energy demanding, carbon-intensive, and expensive injection technique costs between $0.3–10 USD per barrel with associated environmental effects such as underground water contamination and induced seismicity [30,31,32].
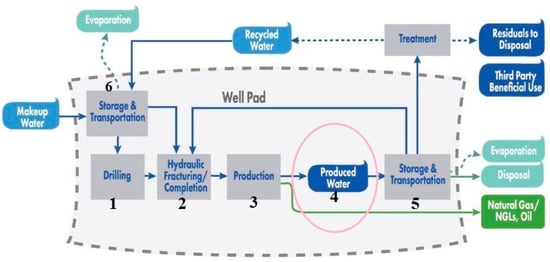
Figure 1.
Water life cycle of PW in an unconventional oil and gas production [28].
2.2. Characteristics of Produced Water
PW is hypersaline in nature and it is, therefore, denser than seawater. Compared to the 30 g/L of total dissolved solids (TDS) of sea water, as high as 300–400 g/L has been recorded for PW from some oil and gas fields [33,34,35]. Hypersaline concentration up to 800 g/L has previously been reported [36]. The dissolved Na+ and Cl− contributes more to salinity than the chloride salts of calcium, magnesium, and potassium. Findings show that the higher the temperature of the reservoir, the higher the TDS concentration in PW [37]. Aside chloride ions, other anions present in PW includes sulfate, carbonate, and biocarbonate. As such, much of this environmental concern arises when the PW is discharged into land surface and fresh water rather than the ocean [38,39].
The range of chemical additives used in drilling exploitation and production are not limited to corrosion and scaling inhibitors, emulsion breakers, fracturing fluids, clarifiers, solvents, coagulants, surfactants, biocides, and flocculants [40,41,42]. These treatment chemicals at a lower concentration of 0.1 ppm are considered highly toxic. This array forms part of the molecular composition of the PW. During the oil and gas extraction process, technologically enhanced naturally occurring radioactive materials (TERNOMS) or naturally occurring radioactive materials (NORM) are found in drill cuttings, flowback water, pipe scale, sludges, sediments, and filters captured as liquid or gases [43].
A quick comparison on the bases of extraction fields reveal that PW from gas fields is lower in volume but has high acidity and higher concentration of volatile components. The pH of PW from oil fields fall on a wide scale of 4.3–10 while that of gas fields range between pH 3.10–4.4. Additionally, benzene, toluene, ethyl benzene, xylene (BTEX), and naphthalene are found in higher concentrations in PW from gas fields than PW from the oil fields [44]. Straight chain alkanes (C10–C30) are the most dominant hydrocarbons, approximated at 90% in detection of which 25% is higher molecular weight n-alkanes ranging from C21 to C34. Table 1 shows a list of other constituents in PW. With petroleum compounds making up PW constituents, total organic carbon (TOC) of PW is also expected to be high.

Table 1.
Produced water composition.
2.3. PW Treatment Technologies
In general, wastewater treatment technologies can be categorized into preliminary, primary, secondary, and tertiary treatments. Preliminary treatment involves separating of entrained coarse solids such as sticks, grits, rags, and other floatable suspended solids. The primary process uses filtration and sedimentation to remove portions of suspended and organic matter, thereby accomplishing about 50–70% suspended solids and 35–40% biochemical oxygen demand [65,66,67]. Secondary treatment incorporates biological processes such as activated sludge and trickling filtration together with chemical precipitation in achieving high effluent. An estimated 85–95% biological oxygen demand (BOD) and suspended solids removal can be accomplished for a well operated and designed secondary treatment plant [68,69]. Also referred to as the polishing stage, tertiary treatment is required as an add-up when effluent characteristics from the secondary treatment process does not meet regulatory requirement. An estimated 99% removal is achieved in this process and it may involve physico-chemical techniques [70,71].
Various physico-chemical and biological treatments widely known in wastewater treatment have been applied to produce water are alkaline chemical precipitation, adsorption, ion exchange, membrane separation, and coagulation-flocculation [72,73]. Technologies—such as hydrocyclone, gas flotation, and gravity-based separators have also been used in the oil and gas sector for water purification and the reduction of oil–water concentration [74]. Table 2 accounts for the advantages and disadvantages of some available PW treatment technologies.

Table 2.
Advantages and disadvantages of selected PW treatment technologies [57,75,76,77,78,79,80,81].
3. Membrane Bioreactors (MBRs)
MBRs, a combination of selective membrane process such as microfiltration or ultrafiltration and a biological process in a simplified single unit has become an alternative technology for wastewater treatment. Being an alternative to the conventional activated sludge (CAS), MBRs have attracted much attention as a cost competitive and effective alternative. Whilst the biological process of the MBR degrades organic pollutants by adapted microorganisms, the membrane establishes a physical barrier that separates the biomass from the treated wastewater [82], hence, the possibility of recycling the activated sludge as recycled activated sludge (RAS).
The introduction of membrane bioreactors (MBRs) as a treatment technology has given rise to reduced plant foot print through the elimination of processes such as secondary clarification, tertiary filtration and UV disinfection and works with low feed to microorganism ratio [83,84]. Other advantages of MBRs include good disinfection capability, high quality effluent, good organic and inorganic separation ability, high organic loading resisting capability, absolute control for a longer sludge retention time (SRT) and shorter hydraulic retention time (HRT), and low sludge production rate due to negligible settleability [85,86,87]. Having a longer SRT allows slow-growing microorganisms responsible for the degradation of most nitrogen-based compounds to develop. Over the years, MBRs have been used to effectively treat industrial and municipal wastewater at mixed liquor suspended solids (MLSS) levels up to 12 mg/L, a level that CAS can only handle a minor fraction [88]. Despite treatment reliability of MBRs, they are associated with high operational and capital costs, membrane fouling phenomena, and high energy demand [89]. A visual comparison of CAS and MBR is shown in Figure 2.
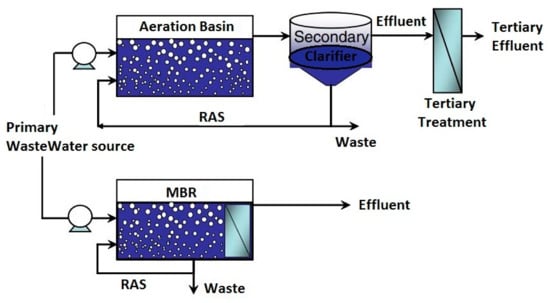
Figure 2.
Difference between CAS and MBR. Adapted from [87].
Over 50 years from the first CAS patent [90], history has it that, Smith et al. [91] in 1969, under the Dorr-Oliver research program introduced the MBR technology. In place of a sedimentation tank used in a typical CAS system, Smith et al. [91] installed an ultrafiltration membrane outside the bioreactor. Despite high-quality effluent produced out of the treatment of sewage, energy consumption from the recirculating pump, membrane fouling, and specific applicability restricted its wide usage in North America. However, a configurational improvement and innovation from the pioneering work of Yamamoto et al. in 1989 included the placement of membranes into the bioreactor unit, installation of pressured pumps to circulate mixed liquor and application of suction pressure into the unit [92]. These earlier designed configurations now exist as first generation side-stream MBRs where the activated sludge flows at high velocities through a tubular or flat sheet membrane module in a typical cross flow filtration mode and the second generation MBRs where the membranes are submerged in the aerated tank in a more dead end filtration mode [93]. The energy consumption in the side stream is therefore usually higher due to the recycle flow velocity. Figure 3 shows the two configurations for (a) side stream and (b) submerged configurations. Over the years, modifications have resulted in the airlift external circulation (AEC) MBR with a combined advantage of the side-stream and submerged MBRs. Additionally, air sparging control for hollow tube membranes used in side streams and a solid retention control (SRC) system has been developed as improvement to the conventional MBRs.
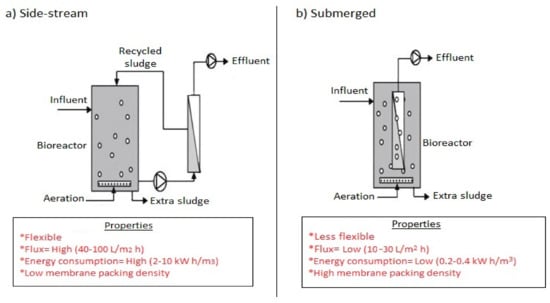
Figure 3.
Schematic diagram of (a) side stream MBR and (b) submerged MBR.
In MBRs, the microfiltration (MF) and ultrafiltration (UF) membranes are primarily used with UF being the most effective for oily wastewater treatment when compared to the low efficiency and high operational cost of conventional methods [94]. The MF and UF membranes utilize a separation mechanism to retain micron and macromolecular or particles specifically in the range of 0.1–10 μm for MFs and 5 to 100 nm for UFs. Therefore, considering the functionality of the membrane in the MBR system, the general mass balance for solute separation in the process can simply be presented as
where Q = flowrate, C = solute concentration, and subscripts f, p, and c denote the feed stream, permeate stream, and concentrate stream, respectively.
The materials used in making these membranes can be grouped into ceramic, polymeric, and composite or modified membranes. Although ceramic membranes have excellent fouling resistance, chemical and mechanical stability, and low operating costs, their high manufacturing and brittle nature makes polymeric membranes a popular choice for MBRs [95,96]. However, due to the hydrophobic nature, polymeric membranes are easily fouled. Polymeric membrane materials include polytetrafluoroethylene (PTFE), polyvinylidene fluoride (PVDF), polysulfone (PSO), polyacrylonitrile (PAN), polyethylene (PE), polypropylene (PF), polyvinyl butyral (PVB), and polyethersulfone (PESO) [66]. A composite or modified membrane is a combination of different materials; one as active surface and the other as a layer support to provide a synergic effect. Typical to surface modification is using plasma treatment and incorporation of photocatalytic nanomaterials.
Based on the purpose of the membrane usage, MBRs are divided into four categories namely, (1) biomass separation membrane bioreactor (BSMBRs), (2) membrane aeration bioreactors (MABRs), (3) extractive membrane bioreactor (EMBR) and (4) ion exchange membrane bioreactors (IEMBRs) [97]. The BSMBRs serve as biomass separators in wastewater settings. In MABRs, the membrane cavity is supplied with pressurized oxygen or air which diffuses though the membrane pores [98]. The biofilm on the membrane side receives the oxygen, creating a nutrient rich profile for better removal of the pollutants as the counter diffusion of the bubbleless aerating oxygen and substrate occurs [99,100]. On the side of EMBRs, hydrophobic-organophilic membranes are used to provide selective transport of specific toxic recalcitrant organic compounds such as dichloroaniline, chloronitrobenzene, phenol, nitrates, and many more through the solution-diffusion mechanism into biofilms for biodegradation as the wastewater passes through the membrane lumen [101,102,103,104]. The IEMBR was patented two decades ago and it incorporates Donnan dialysis, a concentration gradient driven counter transport process where the feed stream with targeted ionic pollutants pass through a non-porous anion exchange membrane into a receiving bio-compartment, which bio reduces under anoxic conditions [105,106,107,108]. This includes the electrodialysis ion exchange membrane bioreactor (EDIMB) and the innovative osmotic membrane bioreactor (OsMBR) which has also been investigated independently on a laboratory scale [109,110]. Schematic diagrams of an EMBR, EDIMB, and OsMBR are presented in Figure 4.
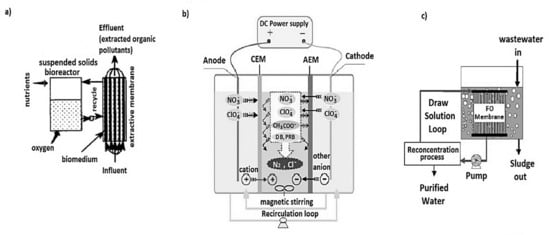
Figure 4.
(a) Extractive membrane reactor; (b) Electro-dialysis ion exchange membrane bioreactor; (c) Osmotic bioreactor adapted from [109,110].
3.1. MBR in PW Treatment
This subsection tabulates the various experimental works performed using MBRs in PW treatment which takes into account the MBR performances. A summary of the mechanism of action is shown in Figure 5 while the treatment performance of MBRs, mostly dominated by the submerged MBR is shown in Table 3. From Table 3, it is established that MBRs are efficient in treating PW pollutants having observed an 80 to >90% oil and grease removal, COD (>90%), TOC (>90%), and 30 to > 60% for phosphate.
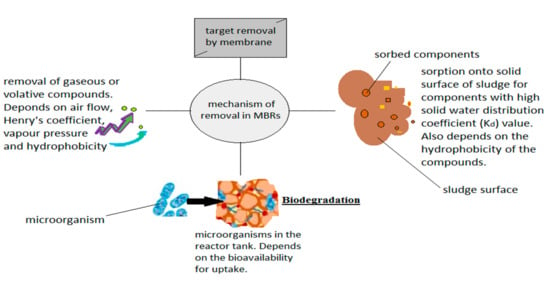
Figure 5.
Treatment mechanism of an MBR.

Table 3.
Performance of MBR in produced water treatment.
3.1.1. Integrated Treatment Processes
Integrated treatment processes in the wastewater treatment industry have been widely practiced to obtain treatment wastewater that meets the legislated quality standards. This involves the combination of conventional and hybrid systems which can be categorized as the fusion of physical, biological and chemical processes. The synergic goals are not limited to overcoming the limitations in a stand-alone process. Notable among integrated processes for treating PW are connected process streams of sedimentation, hydrocyclone, electrocoagulation, reverse osmosis [117], aeration skimmer, and activated sludge-filtration [44], a PW zero discharge system consisting of coagulation, flocculation, filtration (sand), adsorption (granulated activated carbon), RO and crystallization [118], Ti/IrO2–Ta2O5, and BDD electrodes for a flotation and photo-Fenton technique [119], an integrated biochemical and capacitive deionization system called microbial capacitive desalination cell (MCDC) [120], gravity separation-hydrocyclone-sand filtration for non-H2S PW and gravity separation-induced gas flotation-nutsell filtration for PW containing H2S [121] and an infused pretreatment process consisting of gravity driven ultrafiltration, solar aeration, and GAC adsorption [122]. The gravity separation-hydrocyclone-nutshell filtration-mechanical vapor compression-storage chain process was also considered for the treatment of PW for internal reuse. Additionally, integrated pressure systems for desalination that combines reverse osmosis and chemical or slurry precipitation has been used in the treatment of produced water due to the salt concentration range [123].
Although there is limited literature on integrated systems involving MBRs, the organic removal and total membrane resistance of an SMBR and an integrated system consisting of ozonation and a moving bed biofilm SMBR has recently been compared. Lui et al. [124] reported that the total membrane resistance was 40.1% lesser in the integrated system with removal rates of DOC and total nitrogen reported as 3.9% and 18.4% higher, respectively [124].
3.1.2. Modeling MBR Systems
The simplicity of the complex process in modeling and simulation is an answer to ‘what ifs’ and this leads to the consideration and varying of factors for optimum response, troubleshooting, and data collection for designing new systems using virtual applications [125]. In terms of simulating and predicting the PW concentration, discharge, dispersion, and environmental risks, four mathematical modeling techniques which are empirical and analytical solutions aimed to develop expressions of the plume parameters; numerical methods for directly solving the advection-diffusion equation on fluxes of pollutants; random walk particle tracking (RWPT) model for tracking individual particle transport; and jet-type integral methods based on the mass, momentum, and concentration and buoyancy conservation are widely used [11,126]. Others include the integration of the Princeton hydrodynamic ocean model and random walk model [127]. Statistical modeling has also been used to achieve similar objectives by using an analytical technique in assessing the PW contaminant levels and its ecological impact [128,129].
Modeling and simulation techniques have also been used in PW treatment, specifically the treatment of PW using MBRs. The Box–Behnken statistical experimental design was used to study the effect of HRT (16–32 h), SRT (60–120 days) and Temperature (22–38 °C) on COD, TOC and oxygen uptake rate (OUR) [130]. Using a hollow fiber submerged UF membrane continuous MBR, Janson et al. [130] reported that the average COD, TOC, and OUR removals were 60%, 59%, and 0.13 mg with high removals occurring at low HRT. Furthermore, no specific trend was observed as it would have been expected that the mixed liquor volatile suspended solids (MLVSS) would increase with increasing HRT.
A one-way ANOVA acclimatization of the aerobic non-halophilic bacteria of two MBR systems with MLSS adjusted at 5834 ± 877 mg/L and 6655 ± 643 mg/L, respectively was conducted at a varied C/N/P ratio of 100/10/1 and 100/2/1. The reduction in nitrogen in the C/N/P ratio from 10 to 2 inhibited the growth and metabolism of bacteria, thereby causing the reduction in average MLSS for each system. Steady state conditions with statistical significance (p-value less than 0.05) was achieved after 21 days [116].
Optimum range of values of the functional dimensions and designed parameters of a submerged CSTR MBR was obtained by simulating the MBR’s performance on COD, TSS, TOC, TDS, and oil and grease removal [131]. The performance equations based on the law of conservation of mass were developed with assumptions such as constant flowrate, no concentration gradient, no contaminant diffusion/dispersion and operation under isothermal, isobaric, and steady state conditions. From Dagde et al.’s [131] model, different volume, height, and hydraulic retention time are required to obtain 95% and 99% conversion with an SRT of 82.7 days. The fundamental fact therefore, still remains that the MBR plant is more complicated in both design and operation and there is greater risk of failure [132].
4. Fouling and Fouling Controls in MBRs
The performance and life span of membranes are affected by membrane fouling, which is a major setback in the application of membrane-based technologies in wastewater treatment. Massive global attention has been given to fouling in membrane integrated systems attributable to the deposit of foulants such as colloids, hydrophilic dissolved organic matter, salts, sludge flocs, and suspended particulates. This results in reduced permeate flux, increased feed stream pressure, an increase in operational and maintenance costs, and an increased system downtime especially during the treatment of high strength organic matter [133]. The tendency, behavior, and extent of fouling varying is highly dependent on the nature of foulants, mode of operation, and the physico-chemical interaction occurring between the foulants and the properties of the membrane material. Membrane fouling is inevitable in MBR application in PW treatment due to the heterogeneous nature of PWs. As such, it is possible to have all the classification of fouling with respect to the type of foulants—namely, organic, inorganic, or scaling, particulate and biofouling, occurring in MBRs during PW treatment. During treatment, the significant challenges occurs for high bacteria population of 42 × 106 colony-forming units (cfu)/L [134]. The characteristics of the various fouling types are presented in Table 4 [135].

Table 4.
Different foulants and their characteristics [135].
Generally, fouling mechanism in MBRs involve adsorption and accumulative deposition of foulants on the surface of the membrane, and precipitation in pore blocking. The surface adsorption, mechanism and accumulative deposition results in reversible fouling while irreversible fouling is attributed to the deposition in the membrane micro pores. Clogging of the pores is dependent on the size and shape of the particle, and the pore and pore size distribution of the membrane. Figure 6 is a schematic view of the different pore blocking mechanisms. The blocking index (n) of intermediate blocking, standard blocking, complete blocking, and cake formation are 1, 1.5, 2, and 0, respectively. The rate of fouling is higher in anaerobic MBRs than under aerobic conditions.
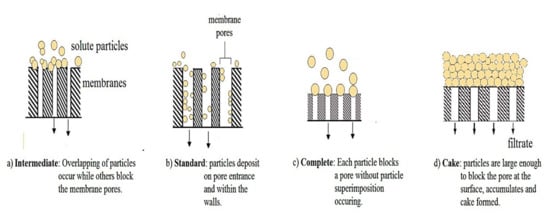
Figure 6.
Schematic view of the different pore blocking mechanisms.
4.1. Monitoring Fouling
Fouling over the years has been monitored in membrane-based processes through transmembrane pressure (TMP) and change in flux. The change in flux is directly proportional to the change in TMP. In effect, membrane fouling and damaging is reflected through the decline in flux and TMP.
The propensity of fouling by PW was determined by the flux step method and long-term operation. For PW with an MLSS concentration of 6 g/L, the critical flux was found to be 6 LMH with a corresponding TMP of 12 mbar [114]. Permeability was found to decrease by 50–65% for each 3 LMH flux step between 3–15 LMH and a further decreasing of permeability at an imposed flux of 18 LMH. Similarly, the average flux and TMP of PW with an MLSS of 9.1 g/L increased from 9 LMH and 0.4 kPa to 15 LMH and 2.7 kPa, respectively after 45 days was observed. This prompted the need for a 24 h fouling treatment [111].
The transitional flux for PW with biomass concentration of 6–18 g/L was reportedly between 8–14 LMH. The 6 g/L increments in biomass concentration did not have a direct effect on fouling rates as the fouling rate for high biomass concentration was 36% less than PW with lower concentration [112]. Complexity and variability in the biomass component would be the reason behind the observation, however, PW with high EPS may contribute to severe flux decline due to their large molecular size over membrane pore size [136]. Kose et al. [115] identified the sustainable TMP as 80 kPa and attributed the fouling to physical reversible cake layer and chemical reversible fouling.
4.2. General Mitigation Strategies
Strategies to control membrane fouling includes the pretreatment of the feed, optimization of operational conditions, activated sludge modification, membrane design and surface modification, and membrane cleaning. Generally, the antifouling and cleaning activities undertaken to recover the initial permeate flux is determined by the fouling and membrane type used. These strategies are equally major energy enhancing strategies as attempts are made to reduce the overall energy consumption in MBRs. The cost of these activities is also inevitable as membrane permeability maintenance has the most significant impact on operational expenditure [132]. As such, one antifouling technique is the introduction of air flow directly to the membrane surface through additional diffusers below the membrane module. Aeration as a control strategy is a main energy consumer of MBR systems with a percentage of 36–68% [137]. An overview of existing and innovative antifouling strategies for MBRs are illustrated in Figure 7.
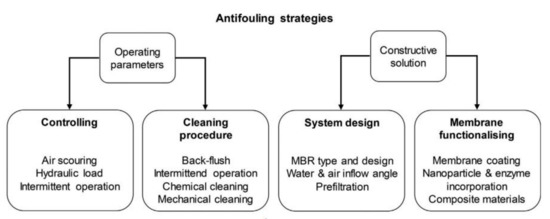
Figure 7.
Antifouling strategies adapted from [136].
Cleaning by ultrasonication has been effective in breaking down thick foulant layers from the membrane surface, however, its effect on anaerobic bacterial activity remains a concern [138,139]. Although research on MBR fouling management in PW treatment is sparse, generic parts such as membrane fouling during MBR wastewater treatment and reclamation [140], membrane fouling from a process control viewpoint [73], and membrane fouling mechanism in anaerobic MBRs [140] exist. Mechanism and limitation of new physical and chemical biofouling control in MBRs are provided in Table 5 [141].

Table 5.
Mechanism and limitation of selection physical and chemical biofouling control processes.
In the area of system design in fouling control, the EMBR, IEMBR, EDIMB, and OsMBR novel systems provide low fouling, high treatment performance, and energy saving characteristics. A number of MBR systems use rotation, vibration (longitudinally, transversely, torsionally, magnetically, or the combination) movement to increase shear-enhanced filtration. To improve cleaning efficiency, eliminate membrane fouling, and stabilize transmembrane pressure, the membrane cassette can also be reciprocated [142,143,144]. Alternative designs have employed the usage of baffles to divide the bioreactor compartment into zones in creating an anoxic–aerobic condition in the reactor vessel for efficient nitrogen removal through simultaneous nitrification and denitrification [145,146].
Current practices in improving membrane functionality, high flux and mitigating membrane fouling in MBRs is by embedding nanomaterials into the polymer support structure or deposition on the surface of the membrane to achieve such characteristics such as hydrophilicity, hydraulic stability, antimicrobial ability (inhibition of metabolism), thermal stability, and photocatalytic self-cleaning. The different nanomaterial membrane bioreactors (NMsMBRs) therefore classified by the nanomaterials as nanofibers membrane bioreactor (NFs-MBR), nanotubes membrane bioreactor (NTs-MBR), nano particle membrane bioreactor (NPs-MBR), nanosheets membrane bioreactor (NSs-MBR), nanowires membrane bioreactor (NWs-MBR) and nanocrystals membrane bioreactor (NCs-MBR) [147,148] as illustrated in Figure 8.
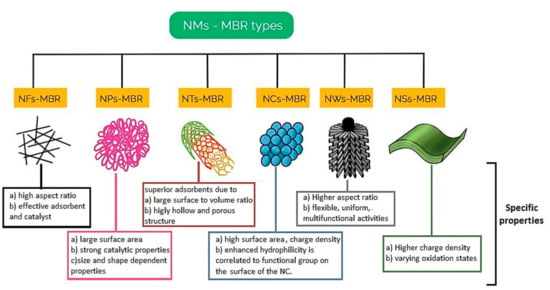
Figure 8.
Types of nanomaterials of hybrid NMs-MBR systems adapted and modified from [146,147].
A thorough search on the use of NMs-MBR systems for PW treatment revealed recent work by Fonouni et al. [149] and Etemadi et al. [150] by comparing a commercial PP membrane and TiO2/PP. While achieving a nanocomposite membrane with porosity and tensile strength of 19.26% and 0.6 MPa higher than commercial PP, respectively. The nanocomposite NM-MBR had higher flux recovery ratio (FRR) and lower irreversible fouling ratio (IFR) to demonstrate its better flux recovery and total fouling control over commercial PP membranes. The analysis of the impact of aeration on fouling, using the Hermia’s model, predicted that at a lower aeration rate, the fouling mechanism was by cake formation [150].
These NMs-MBRs have shown various membrane functional limitations and a few generalized to specific drawbacks are listed below:
- (1)
- NFs, such as Ag/polyamine, decay as a result of irreversible fouling [151,152].
- (2)
- Recovery of NP to encourage reusability concerns, accumulation of NP in MBR and the leaching of NPs. Additionally, the stability of TiO2 on PES, PVDF, and PAN in ensuring fouling mitigation is affected by the immobilization technique [153,154].
- (3)
- Be it single walled carbon nanotubes (SWCNT) or multiwalled carbon nanotubes (MWCNT), graphene oxide or reduced graphene oxide application in NTs-MBR, the functionality and properties are specific to the surface modification technique. This mostly contributes to specific affinity and long term stability is not assured due to poor dispersion over time [155,156].
- (4)
- Pristine cellulose NC-MBR and modified cellulose NCs have limited membrane lifetime due to biodegradability of cellulose [157].
5. Future Perspective
In spite of the progress in the usage of MBRs in treating different wastewater and the continuous evolution of membrane technology, there are limited applications of MBR technology in PW treatment. There are challenges to be addressed from a laboratory scale before an acceleration into large-commercial scale application.
- Very useful data is available from peer-reviewed literature on the treatment of PW using biological and membrane technology. However, the use of MBR systems (including hybrid structures) and its integration with other treatment systems such as RO, NMs (NPs-MBR, NTs-MBR, NCs-MBR, NWs-MBR, and NSs-MBR) and AOP is limited, and much focus must be channeled to establish the independent process efficiency and synergic output.
- With PW being comprised of more different components than just oil–water emulsion, the individual interactive influence of PW components on properties and parameters of a conventional MBR and modified system can be studied systematically to give new insights. For instance, the degradation chemistry of initial pollutants should be understood. Additionally, dynamic models could be developed which should focus on individual characteristic treatment and hydrodynamic flow behavior of synthetic and real feeds in the reactor.
- The impact of chemical, physical, biological fouling, and constructive control strategies on the performance of MBRs on laboratory and pilot scale must be conducted in relation to duration, dosage, metabolic activity, process stability, membrane improvement, behavior in active layer transport of membrane and sustainability, effectiveness, and environmental safety. The development of modified multi-functional low-cost membranes with superior antifouling characteristics can be pursued.
- The high salinity and hydrocarbon content of PW makes PW treatment very energy demanding. Owing to on-site MBR systems, another research direction focusing on powering MBR systems with renewable energies coupled with intelligent process monitoring control systems in achieving an autonomous MBR system should be carried out.
6. Conclusions
The implementation of ecofriendly wastewater technologies is important in achieving management policies of meeting discharge limits and increasing reusability of treated wastewater as a source water. MBR technology offers process advantages over conventional activated sludge processes, adsorption technology, hydrocyclones, gravity settling, precipitation, and many more. This article reviews the treatment of PW using membrane bioreactors which included the treatment schemes, models, and integrated processes. Other aspects addressed includes general fouling and fouling control associated with MBRs for PW treatment. The use of MBRs have demonstrated good performances in the removal of pollutants; however, there are several research gaps to be filled in that area. The scopes should include the MBR configuration and hybrid systems for improved treatment. The cost of large-scale manufacturing of membranes, a key component of MBR and the chemistry of degradation of pollutants in conventional and control of fouling using modified membranes should also be considered. The continuous advancements, especially in membrane design and technology, is key in achieving a process and energy efficient MBR for PW treatment.
Author Contributions
Conceptualization, D.A.-S. and E.K.T.; funding acquisition, E.K.T. and S.R.; writing—original draft, D.A.-S.; writing—reviewing and editing, E.K.T., S.R. and E.K.A.; supervision, S.R.; project administration, E.K.T. and D.A.-S. All authors have read and agreed to the published version of the manuscript.
Funding
National Research Foundation for the innovation grant number 138046.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors wish to thank the Durban University of Technology, Green Engineering and Sustainability Research Group. The authors also wish to thank the National Research Foundation for innovation gran no. 138046.
Conflicts of Interest
The authors declare no conflict of interest and the funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Lu, H.; Guo, L.; Zhang, Y. Oil and gas companies’ low-carbon emission transition to integrated energy companies. Sci. Total Environ. 2019, 686, 1202–1209. [Google Scholar] [CrossRef] [PubMed]
- DNV GL. Oil and Gas Forecast-Energy Transition Outlook 2017; DNV: Oslo, Norway, 2017. [Google Scholar]
- Abudu, H.; Sai, R. Examining prospects and challenges of Ghana’s petroleum industry: A systematic review. Energy Rep. 2020, 6, 841–858. [Google Scholar] [CrossRef]
- Blake, U. The unconventional oil and gas process, and an introduction to exposure pathways. In Environmental and Health Issues in Unconventional Oil and Gas Development; Elsevier: Amsterdam, The Netherlands, 2016; pp. 1–12. ISBN 9780128041253. [Google Scholar]
- Hedar, Y. Budiyono pollution impact and alternative treatment for produced water. In Proceedings of the E3S Web of Conferences, Semarang, Indonesia, 15–16 August 2017; EDP Sciences: Les Ulis, France, 2018; Volume 31, pp. 1–12. [Google Scholar]
- Igunnu, E.T.; Chen, G.Z. Produced water treatment technologies. Int. J. Low-Carbon Technol. 2014, 9, 157–177. [Google Scholar] [CrossRef]
- Al-ghouti, M.A.; Al-kaabi, M.A.; Ashfaq, M.Y.; Adel, D. Journal of Water Process Engineering Produced water characteristics, treatment and reuse: A review. J. Water Process Eng. 2019, 28, 222–239. [Google Scholar] [CrossRef]
- Khader, E.H.; Mohammed, T.H.J.; Mirghaffari, N. Use of Natural Coagulants for Removal of COD, Oil and Turbidity from Produced Waters in the Petroleum Industry. J. Pet. Environ. Biotechnol. 2018, 9, 3. [Google Scholar]
- Abbas, A.J.; Gzar, H.A.; Rahi, M.N. Oilfield-produced water characteristics and treatment technologies: A mini review. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1058, 012063. [Google Scholar] [CrossRef]
- Izadmehr, M.; Daryasafar, A.; Bakhshi, P. Determining influence of different factors on production optimization by developing production scenarios. J. Pet. Explor. Prod. Technol. 2018, 8, 505–520. [Google Scholar] [CrossRef]
- Niu, H.; Lee, K.; Robinson, B.; Cobanli, S.; Li, P. Monitoring and modeling the dispersion of produced water on the Scotian Shelf. Environ. Syst. Res. 2016, 5, 19. [Google Scholar] [CrossRef]
- Xiao, Q.; Wang, Z.; Liao, Z.; Ma, Y.; Huang, X.; Yu, X.; Ren, X. Amass balance method formeasuring condensed water content in gas reservoirs. J. Geophys. Eng. 2020, 17, 517–524. [Google Scholar] [CrossRef]
- Chikwe, T.; Okwa, F. Evaluation of the physico-chemical properties of produced water from oil producing well in the Niger Delta Area, Nigeria. J. Appl. Sci. Environ. Manag. 2017, 20, 1113. [Google Scholar] [CrossRef][Green Version]
- Jin, Y.; Davarpanah, A. Using Photo-Fenton and Floatation Techniques for the Sustainable Management of Flow-Back Produced Water Reuse in Shale Reservoirs Exploration. Water Air Soil Pollut. 2020, 231, 441. [Google Scholar] [CrossRef]
- Isehunwa, S.G.U.S.O.; Oguamah, N.U.O.I.U. Treatment of produced water from Niger Delta oil fields using simultaneous mixture of local materials. J. Pet. Explor. Prod. Technol. 2021, 11, 289–302. [Google Scholar]
- Yousef, R.; Qiblawey, H.; El-Naas, M.H. Adsorption as a Process for Produced Water Treatment: A Review. Processes 2020, 8, 1657. [Google Scholar] [CrossRef]
- Asante-Sackey, D.; Rathilal, S.; Pillay, L.V.; Kweinor Tetteh, E. Ion Exchange Dialysis for Aluminium Transport through a Face-Centred Central Composite Design Approach. Processes 2020, 8, 160. [Google Scholar] [CrossRef]
- Tetteh, E.K.; Rathilal, S.; Asante-Sackey, D.; Chollom, M.N. Prospects of Synthesized Magnetic TiO2-Based Membranes for Wastewater Treatment: A Review. Materials 2021, 14, 3524. [Google Scholar] [CrossRef] [PubMed]
- Asante-Sackey, D.; Rathilal, S.; Pillay, L.; Tetteh, E.K. Effect of ion exchange dialysis process variables on aluminium permeation using response surface methodology. Environ. Eng. Res. 2019, 25, 714–721. [Google Scholar] [CrossRef]
- Tetteh, E.K.; Asante-Sackey, D.; Armah, E.K.; Rathilal, S. Tapping wastewater resource: Why and how? In Handbook of Biofuels; Academic Press, Elsevier: Amsterdam, The Netherlands, 2022; pp. 125–146. [Google Scholar]
- Miller, H.; Dias, K.; Hare, H.; Borton, M.A.; Blotevogel, J.; Danforth, C.; Wrighton, K.C.; Ippolito, J.A.; Borch, T. Reusing oil and gas produced water for agricultural irrigation: Effects on soil health and the soil microbiome. Sci. Total Environ. 2020, 722, 137888. [Google Scholar] [CrossRef]
- Szép, A.; Kohlheb, R. Water treatment technology for produced water. Water Sci. Technol. 2010, 62, 2372–2380. [Google Scholar] [CrossRef]
- Tam, L.S.; Tang, T.W.; Lau, G.N.; Sharma, K.R.; Chen, G.H. A pilot study for wastewater reclamation and reuse with MBR/RO and MF/RO systems. Desalination 2007, 202, 106–113. [Google Scholar] [CrossRef]
- Attiogbe, F. Comparison of membrane bioreactor technology and conventional activated sludge system for treating bleached kraft mill effluent. Afr. J. Environ. Sci. Technol. Full 2013, 7, 292–306. [Google Scholar]
- Kitanou, S.; Tahri, M.; Bachiri, B.; Mahi, M.; Hafsi, M.; Taky, M.; Elmidaoui, A. Comparative study of membrane bioreactor (MBR) and activated sludge processes in the treatment of Moroccan domestic wastewater. Water Sci. Technol. 2018, 78, 1129–1136. [Google Scholar] [CrossRef] [PubMed]
- Yacovitch, T.I.; Daube, C.; Herndon, S.C. Methane Emissions from Offshore Oil and Gas Platforms in the Gulf of Mexico. Environ. Sci. Technol. 2020, 54, 3530–3538. [Google Scholar] [CrossRef] [PubMed]
- Oetjen, K.; Giddings, C.G.S.; McLaughlin, M.; Nell, M.; Blotevogel, J.; Helbling, D.E.; Mueller, D.; Higgins, C.P. Emerging analytical methods for the characterization and quantification of organic contaminants in flowback and produced water. Trends Environ. Anal. Chem. 2017, 15, 12–23. [Google Scholar] [CrossRef]
- GWPC. Produced Water Report: Regulations, Current Practices, and Research Needs; Ground Water Protection Council: Oklahoma City, OK, USA, 2019. [Google Scholar]
- EPA. Summary of Input on Oil and Gas Extraction Wastewater Management Practices Under the Clean Water Act; U.S. Environmental Protection Agency: Washington, DC, USA, 2020. [Google Scholar]
- Echchelh, A.; Hess, T.; Sakrabani, R.; Prigent, S.; Stefanakis, A.I. Towards agro-environmentally sustainable irrigation with treated produced water in hyper-arid environments. Agric. Water Manag. 2021, 243, 106449. [Google Scholar] [CrossRef]
- Berbellini, A.; Zaccarelli, L.; Faenza, L.; Garcia, A.; Improta, L.; De Gori, P.; Morelli, A. Effect of Groundwater on Noise-Based Monitoring of Crustal Velocity Changes Near a Produced Water Injection Well in Val d’Agri (Italy). Front. Earth Sci. 2021, 9, 1–13. [Google Scholar] [CrossRef]
- Dvory, N.Z.; Zoback, M.D. Prior oil and gas production can limit the occurrence of injection-induced seismicity: A case study in the Delaware Basin of western Texas and southeastern New Mexico, USA. Geology 2021, 49, 1193–1203. [Google Scholar] [CrossRef]
- Li, L.; Al-Muntasheri, G.A.; Liang, F. A review of crosslinked fracturing fluids prepared with produced water. Petroleum 2016, 2, 313–323. [Google Scholar] [CrossRef]
- Tetteh, E.K.; Ezugbe, E.O.; Rathilal, S.; Asante-Sackey, D. Removal of COD and SO42-from oil refinery wastewater using a photo-catalytic system-comparing TiO2 and zeolite efficiencies. Water 2020, 12, 214. [Google Scholar] [CrossRef]
- Tetteh, E.K.; Rathilal, S.; Asante-sackey, D. Assessment of Forward Osmosis in PRO Mode during Desalination of a Local Oil Refinery Effluent. Membranes 2021, 11, 801. [Google Scholar] [CrossRef]
- Menzie, A.C. The environmental implications of offshore oil and gas activities: An overview of the effects associated with routine discharges based on the American experience. Environ. Sci. Technol. 1982, 16, 1267–1275. [Google Scholar] [CrossRef]
- Joel, O.; Amajuoyi, C.; Nwokoye, C. Characterization of Formation Water Constituents and the Effect of Fresh Water Dilution from Land Rig Location of the Niger Delta, Nigeria. J. Appl. Sci. Environ. Manag. 2010, 14. [Google Scholar] [CrossRef][Green Version]
- Varonka, M.S.; Gallegos, T.J.; Bates, A.L.; Doolan, C.; Orem, W.H. Organic compounds in produced waters from the Bakken Formation and Three Forks Formation in the Williston Basin, North Dakota. Heliyon 2020, 6, e03590. [Google Scholar] [CrossRef] [PubMed]
- Neff, J.; Lee, K.; DeBlois, E.M. Produced water: Overview of composition, fates, and effects. In Produced Water; Springer: New York, NY, USA, 2011; pp. 3–54. ISBN 9781461400462. [Google Scholar]
- Hansen, B.H.; Sørensen, L.; Størseth, T.R.; Altin, D.; Gonzalez, S.V.; Skancke, J.; Rønsberg, M.U.; Nordtug, T. The use of PAH, metabolite and lipid profiling to assess exposure and effects of produced water discharges on pelagic copepods. Sci. Total Environ. 2020, 714, 136674. [Google Scholar] [CrossRef] [PubMed]
- Chapman, E.C.; Capo, R.C.; Stewart, B.W.; Kirby, C.S.; Hammack, R.W.; Schroeder, K.T.; Edenborn, H.M. Geochemical and strontium isotope characterization of produced waters from marcellus shale natural gas extraction. Environ. Sci. Technol. 2012, 46, 3545–3553. [Google Scholar] [CrossRef] [PubMed]
- WEF. Fundamentals of Produced Water Treatment in the Oil and Gas industry; Webinar Report Handout; Water Environment Federation: Alexandria, VA, USA, 2019. [Google Scholar]
- Babatunde, B.B.; Sikoki, F.D.; Avwiri, G.O.; Chad-umoreh, Y.E. Review of the status of radioactivity pro fi le in the oil and gas producing areas of the Niger delta region of Nigeria. J. Environ. Radioact. 2019, 202, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Tellez, G.T.; Nirmalakhandan, N.; Gardea-Torresdey, J.L. Kinetic evaluation of a field-scale activated sludge system for removing petroleum hydrocarbons from oilfield-produced water. Environ. Prog. 2005, 24, 96–104. [Google Scholar] [CrossRef]
- Batarseh, S.I.; Harith, A.; Othman, H.; Advanced, E. Efficient low maintenance natural ceramic technology to treat sea and produced water. In Proceedings of the SPE Middle East Oil & Gas Show and Conference, Manama, Bahrain, 4–9 March 2017. [Google Scholar]
- Liu, Z.; Li, Q.; Wu, Q.; Kuo, D.T.F.; Chen, S.; Hu, X.; Deng, M.; Zhang, H.; Luo, M. Removal Efficiency and Risk Assessment of Polycyclic Aromatic Hydrocarbons in a Typical Municipal Wastewater Treatment Facility in Guangzhou, China. Int. J. Environ. Res. Public Health 2017, 14, 861. [Google Scholar] [CrossRef]
- Faksness, L. Partitioning of semi-soluble organic compounds between the water phase and oil droplets in produced water. Mar. Pollut. Bull. 2004, 48, 731–742. [Google Scholar] [CrossRef]
- Operations, D. Chemical Analysis of Wastewater from Unconventional Drilling Operations. Water 2015, 7, 1568–1579. [Google Scholar] [CrossRef]
- Shores, A.; Laituri, M.; Butters, G. Produced Water Surface Spills and the Risk for BTEX and Naphthalene Groundwater Contamination. Water Air Soil Pollut. 2017, 228, 435. [Google Scholar] [CrossRef]
- Schneider, J.; Grosser, R.; Jayasimhulu, K.; Xue, W.; Warshawsky, D. Degradation of Pyrene, Benz[a]anthracene, and Benzo[a]pyrene by Mycobacterium sp. Strain RJGII-135, Isolated from a Former Coal Gasification Site. Appl. Environ. Microbiol. 1996, 62, 1491. [Google Scholar] [CrossRef] [PubMed]
- Ranck, J.M.; Bowman, R.S.; Weeber, J.L.; Katz, L.E.; Sullivan, E.J. BTEX Removal from Produced Water Using Surfactant-Modified Zeolite. J. Environ. Eng. 2005, 131, 434–442. [Google Scholar] [CrossRef]
- Ofosu, F.; Fosu-Mensah, B.Y.; Nukpezah, D.; Mensah, M. Concentration of heavy metals in two fish species (Cynoscion regalis and Pomatomus saltatrix) from an oil drilling area in western coast of ghana and public health risk assessment. J. Appl. Nat. Sci. 2021, 13, 520–529. [Google Scholar] [CrossRef]
- Hardi, M.; Siregar, Y.I.; Anita, S.; Ilza, M. Determination of heavy metals concentration in produced water of oil field exploration in siak regency. In Proceedings of the Journal of Physics: Conference Series; IOP Publishing: Bristol, UK, 2019; Volume 1156. [Google Scholar]
- Shpiner, R.; Vathi, S.; Stuckey, D.C. Treatment of oil well “produced water” by waste stabilization ponds: Removal of heavy metals. Water Res. 2009, 43, 4258–4268. [Google Scholar] [CrossRef] [PubMed]
- Akhbarizadeh, R. Improved waste-sourced biocomposite for simultaneous removal of crude oil and heavy metals from synthetic and real oilfield-produced water. Environ. Sci. Pollut. Res. 2018, 25, 31407–31420. [Google Scholar] [CrossRef]
- Lipus, D.; Roy, D.; Khan, E.; Ross, D.; Vikram, A.; Gulliver, D.; Hammack, R.; Bibby, K. Microbial communities in Bakken region produced water. FEMS Microbiol. Lett. 2018, 365, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Duraisamy, R.T.; Heydari, A.B.; Henni, A. State of the art treatment of produced water. In Water Treatment; InTech: London, UK, 2013. [Google Scholar]
- Booker, A.E.; Borton, M.A.; Daly, R.A.; Welch, S.A.; Nicora, C.D.; Hoyt, D.W.; Wilson, T.; Purvine, S.O.; Wolfe, R.A.; Sharma, S.; et al. Sulfide Generation by Dominant Halanaerobium Microorganisms in Hydraulically Fractured Shales. mSphere 2017, 2, e00257-17. [Google Scholar] [CrossRef]
- Ali, M.M.M.; Zhao, H.; Li, Z.; Ayoub, A.A.T. A review about radioactivity in TENORMs of produced water waste from petroleum industry and its environmental and health effects. IOP Conf. Ser. Earth Environ. Sci. 2020, 467, 012120. [Google Scholar] [CrossRef]
- Pillay, A.E.; Salih, F.M.; Maleek, M.I. Radioactivity in oily sludge and produced waste water from oil: Environmental concerns and potential remedial measures. Sustainability 2010, 2, 890–901. [Google Scholar] [CrossRef]
- Chad-Umoren, Y.E.; Briggs-Kamara, M.A. Environmental ionizing radiation distribution in rivers state, Nigeria. J. Environ. Eng. Landsc. Manag. 2010, 18, 154–161. [Google Scholar] [CrossRef]
- Haluszczak, L.O.; Rose, A.W.; Kump, L.R. Applied Geochemistry Geochemical evaluation of flowback brine from Marcellus gas wells in. Appl. Geochem. 2013, 28, 55–61. [Google Scholar] [CrossRef]
- Rodriguez, A.Z.; Wang, H.; Hu, L.; Zhang, Y.; Xu, P. Treatment of produced water in the permian basin for hydraulic fracturing: Comparison of different coagulation processes and innovative filter media. Water 2020, 12, 770. [Google Scholar] [CrossRef]
- Liu, Q.; Jin, Z.; Liu, W.; Wu, X.; Gao, B.; Zhang, D.; Hu, A.; Yang, C. Origin of marine sour natural gas and gas-filling model in the Puguang giant gas field, Sichuan Basin, China. Energy Explor. Exploit. 2014, 32, 113–138. [Google Scholar] [CrossRef]
- Jasim, N.A. The design for wastewater treatment plant (WWTP) with GPS X modelling. Cogent Eng. 2020, 7, 1723782. [Google Scholar] [CrossRef]
- Tetteh, E.K.; Rathilal, S.; Chetty, M.; Kwaku Armah, E.; Asante-Sackey, D. Treatment of water and wastewater for reuse and energy generation-emerging technologies. In Water and Wastewater Treatment; IntechOpen: London, UK, 2019. [Google Scholar]
- Jiménez, B.; Mara, D.; Carr, R.; Brissaud, F. Wastewater Irrigation and Health; Bahri, A., Drechsel, P., Raschid-Sally, L., Redwood, M., Eds.; Routledge: Oxfordshire, UK, 2009; ISBN 9781136544477. [Google Scholar]
- Barber, W.P.F. Influence of wastewater treatment on sludge production and processing. Water Environ. J. 2014, 28, 1–10. [Google Scholar] [CrossRef]
- Englande, A.J.; Krenkel, P.; Shamas, J. Wastewater treatment & water reclamation. In Reference Module in Earth Systems and Environmental Sciences; Elsevier: Amsterdam, The Netherlands, 2015; ISBN 9780124095489. [Google Scholar]
- Moran, S. Dirty water unit operation design. In An Applied Guide to Water and Effluent Treatment Plant Design; Elsevier: Amsterdam, The Netherlands, 2018; pp. 171–202. ISBN 9780128113097. [Google Scholar]
- Ranade, V.V.; Bhandari, V.M. Industrial Wastewater Treatment, Recycling, and Reuse: An Overview; Elsevier Ltd.: Amsterdam, The Netherlands, 2014; ISBN 9780444634030. [Google Scholar]
- Ibrahim, M.H.; Moussa, D.T.; El-naas, M.H.; Nasser, M.S. Journal of Water Process Engineering A perforated electrode design for passivation reduction during the electrochemical treatment of produced water. J. Water Process Eng. 2020, 33, 101091. [Google Scholar] [CrossRef]
- Jepsen, K.; Bram, M.; Pedersen, S.; Yang, Z. Membrane Fouling for Produced Water Treatment: A Review Study from a Process Control Perspective. Water 2018, 10, 847. [Google Scholar] [CrossRef]
- Coca-Prados, J.; Gutiérrez-Cervelló, G. Water Purification and Management; Springer: Dordrecht, The Netherlands, 2010; ISBN 978-90-481-9775-0. [Google Scholar]
- Souza, J.S.; Paiva, M.K.N.; Farias, F.P.M.; Farias Neto, S.R.; Lima, A.G.B. Hydrocyclone applications in produced water: A steady-state numerical analysis. Braz. J. Pet. Gas 2012, 6, 133–143. [Google Scholar] [CrossRef]
- Walsh, J.M. Produced-Water-Treating Systems: Comparison of North Sea and Deepwater Gulf of Mexico. Oil Gas Facil. 2015, 4, 073–086. [Google Scholar] [CrossRef]
- Stewart, M.; Arnold, K. Produced Water Treating Systems. In Emulsions and Oil Treating Equipment; Elsevier: Amsterdam, The Netherlands, 2009; pp. 107–211. [Google Scholar]
- Jiménez, S.; Andreozzi, M.; Micó, M.M.; Álvarez, M.G.; Contreras, S. Produced water treatment by advanced oxidation processes. Sci. Total Environ. 2019, 666, 12–21. [Google Scholar] [CrossRef]
- Arthur, J.D.; Langhus, B.G.; Patel, C. Technical Summary of Oil & Gas Produced Water Treatment Technologies; ALL Consulting, LLC: Tulsa, OK, USA, 2005. [Google Scholar]
- Saththasivam, J.; Loganathan, K.; Sarp, S. Chemosphere An overview of oil e water separation using gas flotation systems. Chemosphere 2016, 144, 671–680. [Google Scholar] [CrossRef] [PubMed]
- Judd, S.; Qiblawey, H.; Al-Marri, M.; Clarkin, C.; Watson, S.; Ahmed, A.; Bach, S. The size and performance of offshore produced water oil-removal technologies for reinjection. Sep. Purif. Technol. 2014, 134, 241–246. [Google Scholar] [CrossRef]
- Visvanathan, C.; Abeynayaka, A. Developments and future potentials of anaerobic membrane bioreactors (AnMBRs). Membr. Water Treat. 2012, 3, 1–23. [Google Scholar] [CrossRef]
- Lazarova, V.; Martin Ruel, S.; Barillon, B.; Dauthuille, P. The role of MBR technology for the improvement of environmental footprint of wastewater treatment. Water Sci. Technol. 2012, 66, 2056–2064. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hai, F.I.; Riley, T.; Shawkat, S.; Magram, S.F.; Yamamoto, K. Removal of pathogens by membrane bioreactors: A review of the mechanisms, influencing factors and reduction in chemical disinfectant dosing. Water 2014, 6, 3603–3630. [Google Scholar] [CrossRef]
- Chang, J.-S.; Chang, C.-Y.; Chen, A.-C.; Erdei, L.; Vigneswaran, S. Long-term operation of submerged membrane bioreactor for the treatment of high strength acrylonitrile-butadiene-styrene (ABS) wastewater: Effect of hydraulic retention time. Desalination 2006, 191, 45–51. [Google Scholar] [CrossRef]
- Skouteris, G.; Saroj, D.; Melidis, P.; Hai, F.I.; Ouki, S. The effect of activated carbon addition on membrane bioreactor processes for wastewater treatment and reclamation–A critical review. Bioresour. Technol. 2015, 185, 399–410. [Google Scholar] [CrossRef]
- Le-Clech, P. Membrane bioreactors and their uses in wastewater treatments. Appl. Microbiol. Biotechnol. 2010, 88, 1253–1260. [Google Scholar] [CrossRef]
- Bernal, R.; von Gottberg, A.; Mack, B. Using membrane bioreactors for wastewater treatment for small communities. Proc. Water Environ. Fed. 2012, 2002, 515–524. [Google Scholar] [CrossRef]
- Judd, S. The status of membrane bioreactor technology. Trends Biotechnol. 2008, 26, 109–116. [Google Scholar] [CrossRef]
- Lofrano, G.; Brown, J. Wastewater management through the ages: A history of mankind. Sci. Total Environ. 2010, 408, 5254–5264. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.; Di Gregorio, D.; Talcott, R. The use of ultrafiltration membrane for activated sludge separation. In Proceedings of the 24th Annual Purdue Industrial Waste Conference, West Lafayette, IN, USA, 6–8 May 1969; pp. 1300–1310. [Google Scholar]
- Yamamoto, K.; Hiasa, M.; Mahmood, T.; Matsuo, T. Direct solid-liquid separation using hollow fiber membrane in an activated sludge. Water Sci. Technol. 1989, 21, 43–54. [Google Scholar] [CrossRef]
- Ben Aim, R.M.; Semmens, M.J. Membrane bioreactors for wastewater treatment and reuse: A success story. Water Sci. Technol. 2003, 47, 1–5. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Jiang, Z.-W. Technology review: Treating oilfield wastewater. Filtr. Sep. 2008, 45, 14–16. [Google Scholar] [CrossRef]
- Hofs, B.; Ogier, J.; Vries, D.; Beerendonk, E.F.; Cornelissen, E.R. Comparison of ceramic and polymeric membrane permeability and fouling using surface water. Sep. Purif. Technol. 2011, 79, 365–374. [Google Scholar] [CrossRef]
- Mutamim, N.S.A.; Noor, Z.Z.; Hassan, M.A.A.; Olsson, G. Application of membrane bioreactor technology in treating high strength industrial wastewater: A performance review. Desalination 2012, 305, 1–11. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, Z.; Mai, S.; Yang, C.; Wang, X.; An, Y.; Zhou, Z. Research and applications of membrane bioreactors in China: Progress and prospect. Sep. Purif. Technol. 2008, 62, 249–263. [Google Scholar] [CrossRef]
- Li, T.; Liu, J. Factors affecting performance and functional stratification of membrane-aerated biofilms with a counter-diffusion configuration. RSC Adv. 2019, 9, 29337–29346. [Google Scholar] [CrossRef]
- Kinh, C.T.; Riya, S.; Hosomi, M.; Terada, A. Identification of hotspots for NO and N2O production and consumption in counter- and co-diffusion biofilms for simultaneous nitrification and denitrification. Bioresour. Technol. 2017, 245, 318–324. [Google Scholar] [CrossRef]
- Gong, W.; Fan, A.; Zhang, H.; Luo, L.; Liang, H. Cow manure anaerobic fermentation effluent treatment by oxygen-based membrane aerated biofilm reactor. Chem. Eng. J. 2020, 395, 125116. [Google Scholar] [CrossRef]
- Gede Wenten, I.; Friatnasary, D.L.; Khoiruddin, K.; Setiadi, T.; Boopathy, R. Extractive membrane bioreactor (EMBR): Recent advances and applications. Bioresour. Technol. 2020, 297, 122424. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Chen, R.; Zhang, X.; Shao, J.; He, Y. Bioresource Technology Phenol biodegradation and microbial community dynamics in extractive membrane bioreactor (EMBR) for phenol-laden saline wastewater. Bioresour. Technol. 2017, 244, 1121–1128. [Google Scholar] [CrossRef] [PubMed]
- Casey, E.; Glennon, B.; Hamer, G. Review of membrane aerated biofilm reactors. Resour. Conserv. Recycl. 1999, 27, 203–215. [Google Scholar] [CrossRef]
- Ren, L.; Hao, H.; Bu, C.; Ge, C.; Ni, S.; Shao, J. Novel external extractive membrane bioreactor (EMBR) using electrospun polydimethylsiloxane/polymethyl methacrylate membrane for phenol-laden saline wastewater. Chem. Eng. J. 2019, 383, 123179. [Google Scholar] [CrossRef]
- Fox, S.; Oren, Y.; Ronen, Z.; Gilron, J. Ion exchange membrane bioreactor for treating groundwater contaminated with high perchlorate concentrations. J. Hazard. Mater. 2014, 264, 552–559. [Google Scholar] [CrossRef] [PubMed]
- Ricardo, A.R.; Carvalho, G.; Velizarov, S.; Crespo, J.G.; Reis, M.A.M. Kinetics of nitrate and perchlorate removal and biofilm stratification in an ion exchange membrane bioreactor. Water Res. 2012, 46, 4556–4568. [Google Scholar] [CrossRef]
- Velizarov, S.; Reis, M.A.; Crespo, J.G. The ion exchange membrane bioreactor developments and perspectives in drinking water treatment. In NATO Science for Peace and Security Series C: Environmental Security; Coca-Prados, J., Gutiérrez-Cervelló, G., Eds.; NATO Science for Peace and Security Series C: Environmental Security; Springer: Dordrecht, The Netherlands, 2011; Volume 101, pp. 1–27. ISBN 978-90-481-9774-3. [Google Scholar]
- Asante-Sackey, D.; Rathilal, S.; Kweinor Tetteh, E.; Ezugbe, E.O.; Pillay, L.V. Donnan membrane process for the selective recovery and removal of target metal ions—A mini review. Membranes 2021, 11, 358. [Google Scholar] [CrossRef]
- Achilli, A.; Cath, T.Y.; Marchand, E.A.; Childress, A.E. The forward osmosis membrane bioreactor: A low fouling alternative to MBR processes. Desalination 2009, 239, 10–21. [Google Scholar] [CrossRef]
- Chen, J.; Gu, M.; Zhou, Y.; Wan, D.; He, Q.; Shi, Y.; Liu, Y. Efficient nitrate and perchlorateremoval from aqueous solution via a novel electro-dialysis ion-exchange membrane bioreactor. Chem. Eng. J. 2022, 430, 132952. [Google Scholar] [CrossRef]
- Qin, J.J.; Oo, M.H.; Tao, G.; Kekre, K.A. Feasibility study on petrochemical wastewater treatment and reuse using submerged MBR. J. Membr. Sci. 2007, 293, 161–166. [Google Scholar] [CrossRef]
- Brookes, A. Immersed Membrane Bioreactor for Produced Water Treatment. Ph.D. Thesis, Cranfield University, Bedford, UK, 2005. [Google Scholar]
- Li, L.; Goel, R. Biodegradation of naphthalene, benzene, toluene, ethyl benzene, and xylene in batch and membrane bioreactors. Environ. Eng. Sci. 2012, 29, 42–51. [Google Scholar] [CrossRef]
- Brookes, A.; Jefferson, B.; Le-Clech, P.; Judd, S. Fouling of membrane bioreactors during treatment of produced water. In Proceedings of the International Membrane Science and Technology (IMSTEC), Sydney, NSW, Australia, 11–13 November 2003. [Google Scholar]
- Kose, B.; Ozgun, H.; Ersahin, M.E.; Dizge, N.; Koseoglu-Imer, D.Y.; Atay, B.; Kaya, R.; Altinbas, M.; Sayili, S.; Hoshan, P.; et al. Performance evaluation of a submerged membrane bioreactor for the treatment of brackish oil and natural gas field produced water. Desalination 2012, 285, 295–300. [Google Scholar] [CrossRef]
- Fulazzaky, M.; Setiadi, T.; Fulazzaky, M.A. An evaluation of the oilfield-produced water treatment by the membrane bioreactor. J. Environ. Chem. Eng. 2020, 8, 104417. [Google Scholar] [CrossRef]
- Rahman, I.U. Produced water treatment through an integrated system: A case study. In Proceedings of the 5th Online International Conference on Sustainability in Process Industry (SPI-2020), Peshawar, Pakistan, 15–16 December 2021. [Google Scholar]
- Dastgheib, S.A. An Integrated Supercritical System for Efficient Produced Water Treatment and Power Generation; The Board of Trustees of the University of Illinois Office of Sponsored Programs & Research Administration: Campaign, IL, USA, 2018. [Google Scholar]
- da Silva, A.J.C.; dos Santos, E.V.; de Oliveira Morais, C.C.; Martínez-Huitle, C.A.; Castro, S.S.L. Electrochemical treatment of fresh, brine and saline produced water generated by petrochemical industry using Ti/IrO2-Ta2O5 and BDD in flow reactor. Chem. Eng. J. 2013, 233, 47–55. [Google Scholar] [CrossRef]
- Stoll, Z.A.; Forrestal, C.; Ren, Z.J.; Xu, P. Shale gas produced water treatment using innovative microbial capacitive desalination cell. J. Hazard. Mater. 2015, 283, 847–855. [Google Scholar] [CrossRef]
- Bagheri, M.; Roshandel, R.; Shayegan, J. Optimal selection of an integrated produced water treatment system in the upstream of oil industry. Process Saf. Environ. Prot. 2018, 117, 67–81. [Google Scholar] [CrossRef]
- Tang, P.; Li, J.; Li, T.; Tian, L.; Sun, Y.; Xie, W.; He, Q. Efficient integrated module of gravity driven membrane filtration, solar aeration and GAC adsorption for pretreatment of shale gas wastewater. J. Hazard. Mater. 2020, 405, 124166. [Google Scholar] [CrossRef]
- Xu, P.; Cath, T.; Drewes, J.E. Novel and emerging technologies for produced water treatment. In Proceedings of the US EPA Technical Workshops for the Hydraulic Fracturing, Arlington, VA, USA, 29–30 March 2011. [Google Scholar]
- Liu, X.; Tang, P.; Liu, Y.; Xie, W.; Chen, C.; Li, T.; He, Q.; Bao, J.; Tiraferri, A.; Liu, B. Efficient removal of organic compounds from shale gas wastewater by coupled ozonation and moving-bed-biofilm submerged membrane bioreactor. Bioresour. Technol. 2021, 344, 126191. [Google Scholar] [CrossRef]
- Bafleur, M.; Caignet, F.; Nolhier, N. Modeling and simulation methods. In ESD Protection Methodologies; Elsevier: Amsterdam, The Netherlands, 2017; pp. 111–175. [Google Scholar]
- Zhao, L.; Chen, Z.; Lee, K. Modelling the dispersion of wastewater discharges from offshore outfalls: A review. Environ. Rev. 2011, 19, 107–120. [Google Scholar] [CrossRef]
- Chen, Z.; Zhao, L.; Lee, K.; Hannath, C. Modeling and assessment of the produced water discharges from offshore petroleum platforms. Water Qual. Res. J. Can. 2007, 42, 303–310. [Google Scholar] [CrossRef]
- Ganat, T.A.; Hrairi, M.; Mohyaldinn, M.E. Experimental study to evaluate the environmental impacts of disposed produced water on the surrounding ecosystems. Int. J. Environ. Sci. Technol. 2020, 17, 1439–1454. [Google Scholar] [CrossRef]
- Ribeiro, F.A.L.; do Rosário, F.F.; Bezerra, M.C.M.; Bastos, A.L.M.; de Melo, V.L.A.; Poppi, R.J. Assessment of the chemical composition of waters associated with oil production using PARAFAC. Chemom. Intell. Lab. Syst. 2012, 115, 18–24. [Google Scholar] [CrossRef]
- Janson, A.F.; Santos, A.; Hussain, A.; Minier-Matar, J.; Judd, S.; Adham, S. Application of membrane bioreactor technology for produced water treatment. In Proceedings of the 4th International Gas Processing Symposium; Elsevier: Amsterdam, The Netherlands, 2015; pp. 293–300. [Google Scholar]
- Dagde, K.K.; Nwidadaa, N.J. Computer-Aided Design and Simulation of a Membrane Bioreactor for Produced Water Treatment. Adv. Chem. Eng. Sci. 2018, 8, 144–160. [Google Scholar] [CrossRef][Green Version]
- Hill, C.H.M. Design, operation and maintenance. In The MBR Book; Elsevier: Amsterdam, The Netherlands, 2011; pp. 209–288. [Google Scholar]
- Wu, B.; Fane, A.G. Microbial Relevant Fouling in Membrane Bioreactors: Influencing Factors, Characterization, and Fouling Control. Membranes 2012, 2, 565–584. [Google Scholar] [CrossRef] [PubMed]
- Mondal, S. Polymeric membranes for produced water treatment: An overview of fouling behavior and its control. Rev. Chem. Eng. 2016, 32, 611–628. [Google Scholar] [CrossRef]
- Sadr, S.M.K.; Saroj, D.P. Membrane technologies for municipal wastewater treatment. In Advances in Membrane Technologies for Water Treatment; Elsevier: Amsterdam, The Netherlands, 2015; pp. 443–463. ISBN 9781782421269. [Google Scholar]
- Dizge, N.; Soydemir, G.; Karagunduz, A.; Keskinler, B. Influence of type and pore size of membranes on cross flow microfiltration of biological suspension. J. Membr. Sci. 2011, 366, 278–285. [Google Scholar] [CrossRef]
- Ho, J.; Smith, S.; Patamasank, J.; Tontcheva, P.; Kim, G.D.; Roh, H.K. Pilot Demonstration of Energy-Efficient Membrane Bioreactor (MBR) Using Reciprocating Submerged Membrane. Water Environ. Res. 2015, 87, 266–273. [Google Scholar] [CrossRef]
- Xu, M.; Wen, X.; Huang, X.; Li, Y. Membrane fouling control in an anaerobic membrane bioreactor coupled with online ultrasound equipment for digestion of waste activated sludge. Sep. Sci. Technol. 2010, 45, 941–947. [Google Scholar] [CrossRef]
- Wen, X.; Sui, P.; Huang, X. Exerting ultrasound to control the membrane fouling in filtration of anaerobic activated sludge–Mechanism and membrane damage. Water Sci. Technol. 2008, 57, 773–779. [Google Scholar] [CrossRef]
- Iorhemen, O.T.; Hamza, R.A.; Tay, J.H. Membrane bioreactor (Mbr) technology for wastewater treatment and reclamation: Membrane fouling. Membranes 2016, 6, 33. [Google Scholar] [CrossRef]
- Cui, Y.; Gao, H.; Yu, R.; Gao, L.; Zhan, M. Biological-based control strategies for MBR membrane biofouling: A review. Water Sci. Technol. 2021, 83, 2597–2614. [Google Scholar] [CrossRef] [PubMed]
- Komesli, O.T.; Gökçay, C.F. Investigation of sludge viscosity and its effects on the performance of a vacuum rotation membrane bioreactor. Environ. Technol. 2014, 35, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Rector, T.J.; Garland, J.L.; Starr, S.O. Dispersion characteristics of a rotating hollow fiber membrane bioreactor: Effects of module packing density and rotational frequency. J. Membr. Sci. 2006, 278, 144–150. [Google Scholar] [CrossRef]
- Paul, P.; Anderson Jones, F. Development of a Comprehensive Fouling Model for a Rotating Membrane Bioreactor System Treating Wastewater. Water 2015, 7, 377–397. [Google Scholar] [CrossRef]
- Kimura, K.; Watanabe, Y. Baffled membrane bioreactor (BMBR) for advanced wastewater treatment: Easy modification of existing MBRs for efficient nutrient removal. Water Sci. Technol. J. Int. Assoc. Water Pollut. Res. 2005, 52, 427–434. [Google Scholar] [CrossRef]
- Kimura, K.; Nishisako, R.; Miyoshi, T.; Shimada, R.; Watanabe, Y. Baffled Membrane Bioreactor (BMBR) for Efficient Nutrient Removal from Municipal Wastewater. Water Res. 2008, 42, 625–632. [Google Scholar] [CrossRef]
- Pervez, M.N.; Balakrishnan, M.; Hasan, S.W.; Choo, K.-H.; Zhao, Y.; Cai, Y.; Zarra, T.; Belgiorno, V.; Naddeo, V. A critical review on nanomaterials membrane bioreactor (NMs-MBR) for wastewater treatment. NPJ Clean Water 2020, 3, 43. [Google Scholar] [CrossRef]
- Qin, L.; Zhang, Y.; Xu, Z.; Zhang, G. Advanced membrane bioreactors systems: New materials and hybrid process design. Bioresour. Technol. 2018, 269, 476–488. [Google Scholar] [CrossRef]
- Fonouni, M.; Etemadi, H.; Yegani, R.; Zarin, S. Fouling characterization of TiO2 nanoparticle embedded polypropylene membrane in oil refinery wastewater treatment using membrane bioreactor (MBR). Desalin. Water Treat. 2017, 90, 99–109. [Google Scholar] [CrossRef]
- Etemadi, H.; Fonouni, M.; Yegani, R. Investigation of antifouling properties of polypropylene/TiO2 nanocomposite membrane under different aeration rate in membrane bioreactor system. Biotechnol. Rep. 2020, 25, e00414. [Google Scholar] [CrossRef]
- Daels, N.; De Vrieze, S.; Decostere, B.; Dejans, P.; Dumoulin, A.; De Clerck, K.; Westbroek, P.; Van Hulle, S.W.H. The use of electrospun flat sheet nanofibre membranes in MBR applications. Desalination 2010, 257, 170–176. [Google Scholar] [CrossRef]
- Bjorge, D.; Daels, N.; De Vrieze, S.; Dejans, P.; Van Camp, T.; Audenaert, W.; Hogie, J.; Westbroek, P.; De Clerck, K.; Van Hulle, S.W.H. Performance assessment of electrospun nanofibers for filter applications. Desalination 2009, 249, 942–948. [Google Scholar] [CrossRef]
- Bae, T.H.; Tak, T.M. Preparation of TiO2 self-assembled polymeric nanocomposite membranes and examination of their fouling mitigation effects in a membrane bioreactor system. J. Membr. Sci. 2005, 266, 1–5. [Google Scholar] [CrossRef]
- Bae, T.H.; Tak, T.M. Effect of TiO2 nanoparticles on fouling mitigation of ultrafiltration membranes for activated sludge filtration. J. Membr. Sci. 2005, 249, 1–8. [Google Scholar] [CrossRef]
- Rashed, A.O.; Merenda, A.; Kondo, T.; Lima, M.; Razal, J.; Kong, L.; Huynh, C.; Dumée, L.F. Carbon nanotube membranes–strategies and challenges towards scalable manufacturing and practical separation applications. Sep. Purif. Technol. 2020, 257, 117929. [Google Scholar] [CrossRef]
- Jun, L.Y.; Mubarak, N.M.; Yee, M.J.; Yon, L.S.; Bing, C.H.; Khalid, M.; Abdullah, E.C. An overview of functionalised carbon nanomaterial for organic pollutant removal. J. Ind. Eng. Chem. 2018, 67, 175–186. [Google Scholar] [CrossRef]
- Reshmy, R.; Thomas, D.; Philip, E.; Paul, S.A.; Madhavan, A.; Sindhu, R.; Binod, P.; Sirohi, R.; Tarafdar, A.; Pandey, A.; et al. Potential of nanocellulose for wastewater treatment. Chemosphere 2021, 281, 130738. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).