Compact Carbon-Based Membrane Reactors for the Intensified Anaerobic Decolorization of Dye Effluents
Abstract
1. Introduction
2. Materials and Methods
2.1. Fabrication of Ceramic-Supported Carbon Membrane
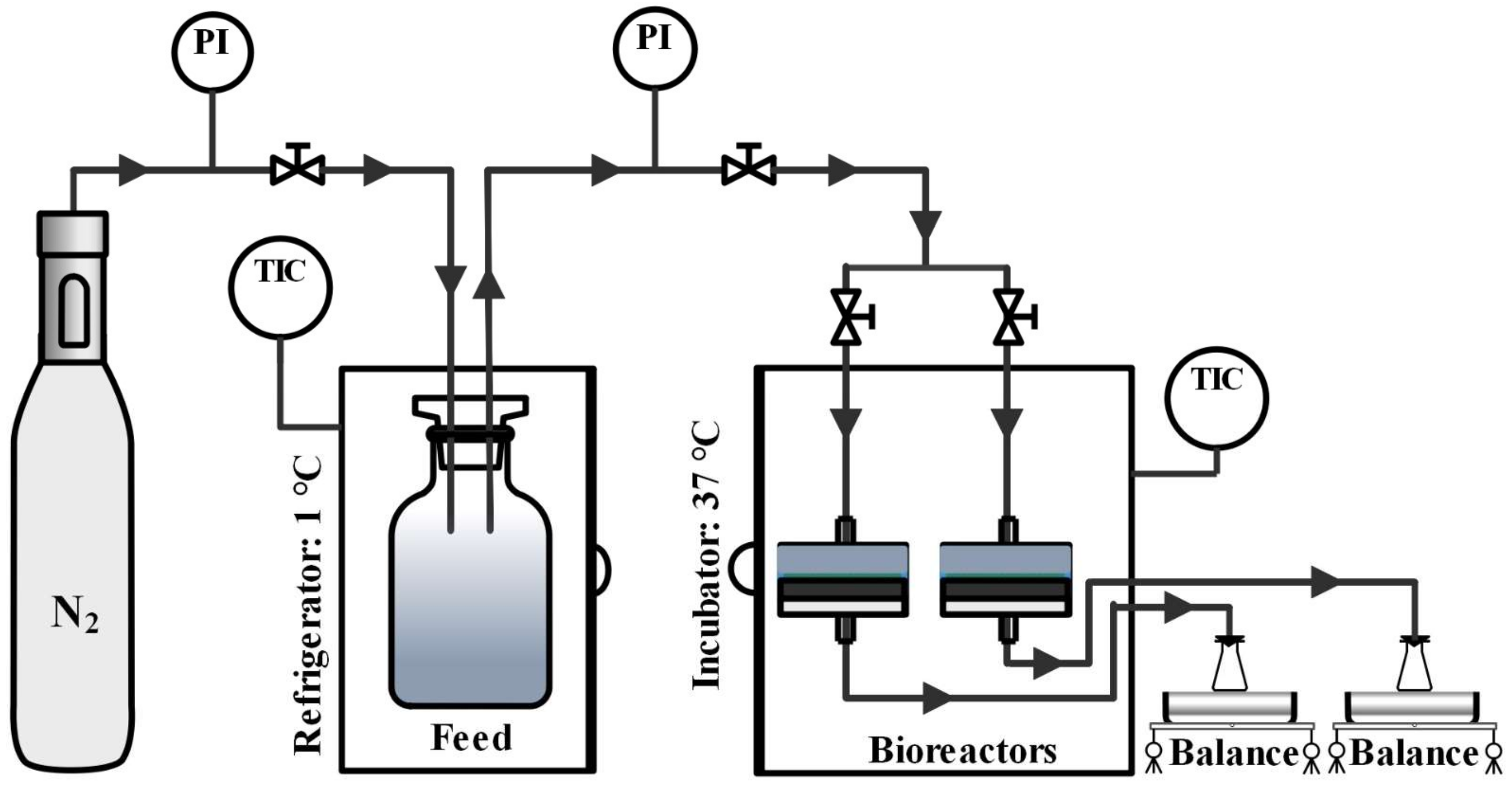
2.2. Experimental Set-Up for Anaerobic Biodegradation
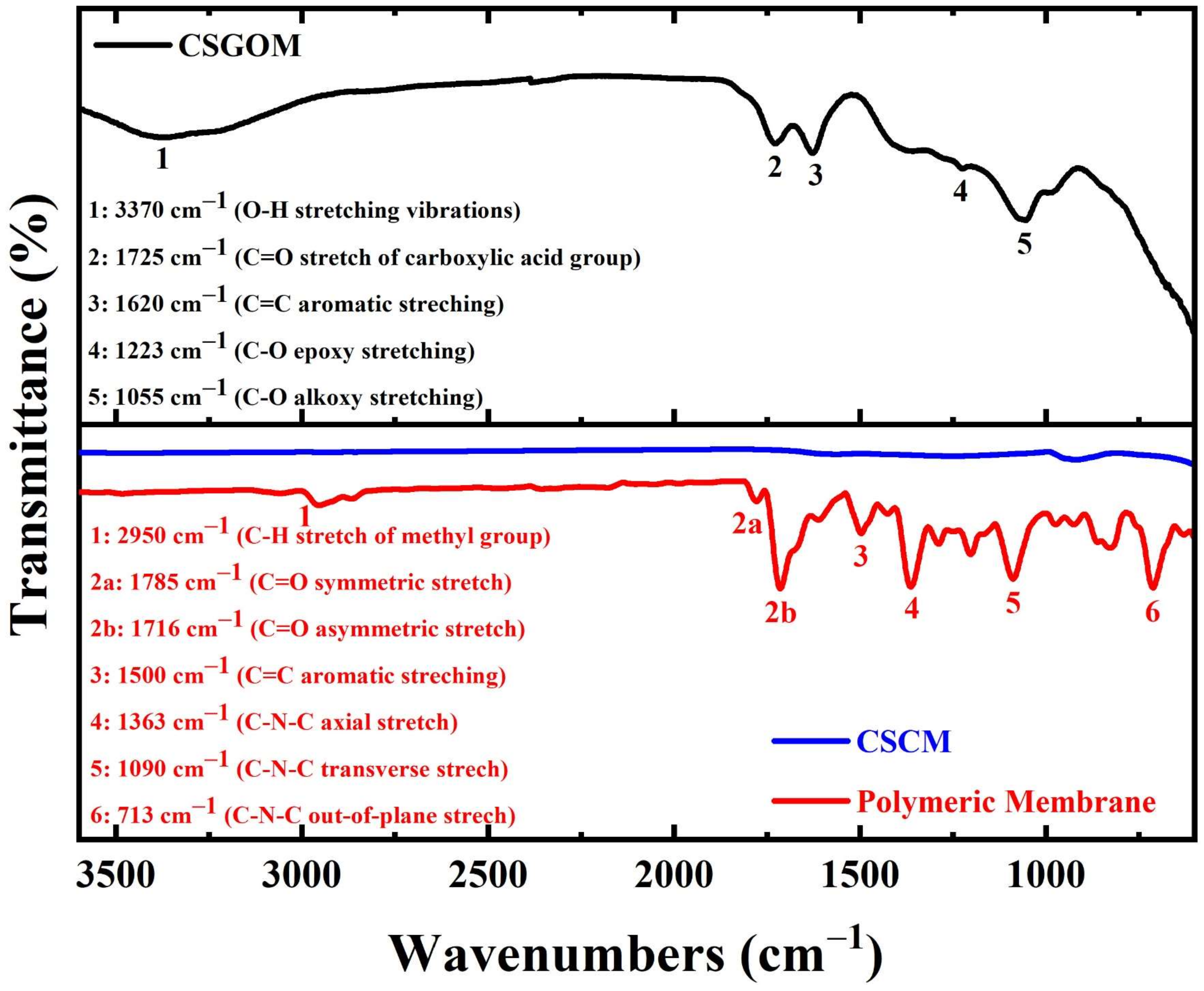
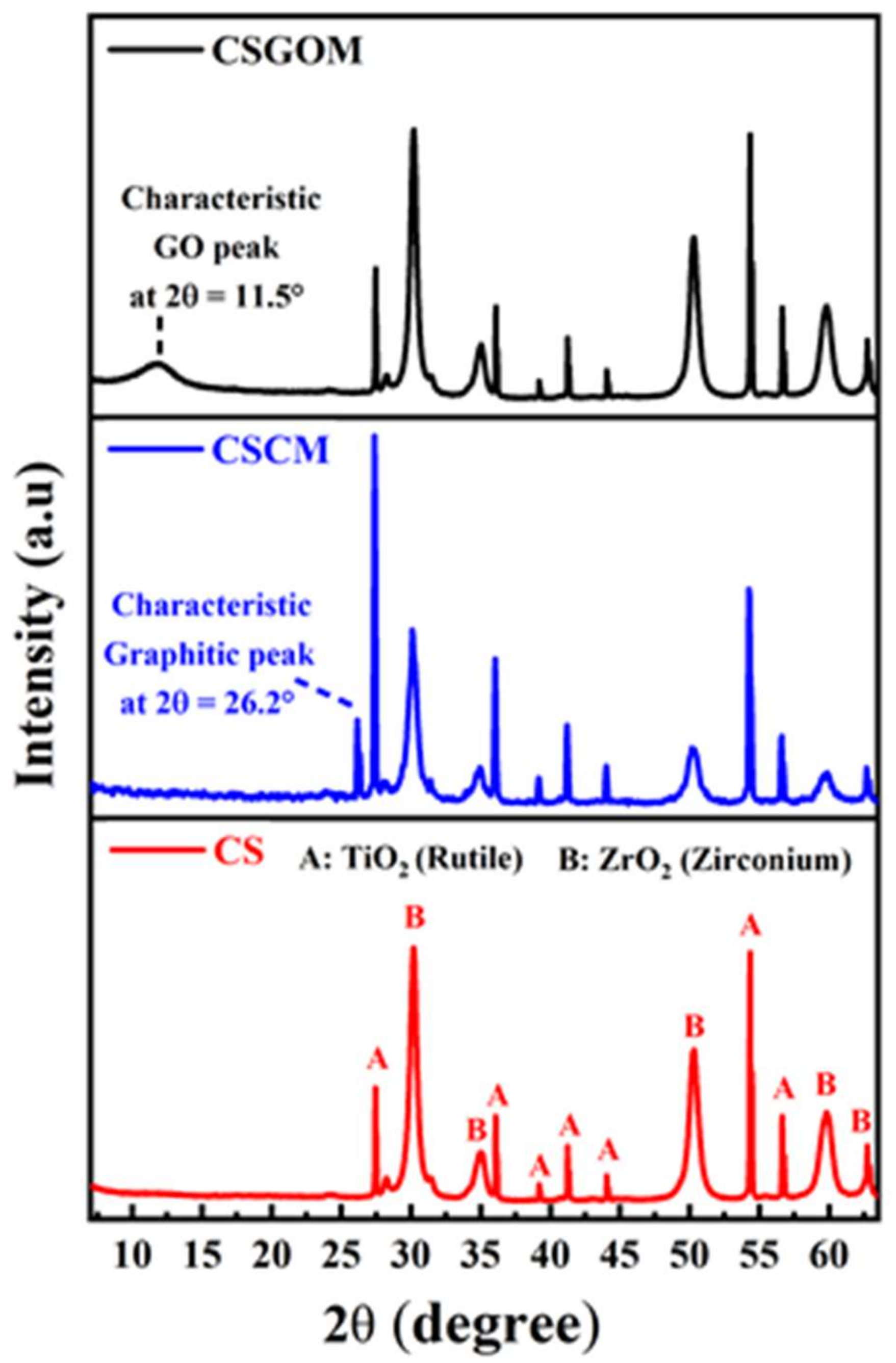
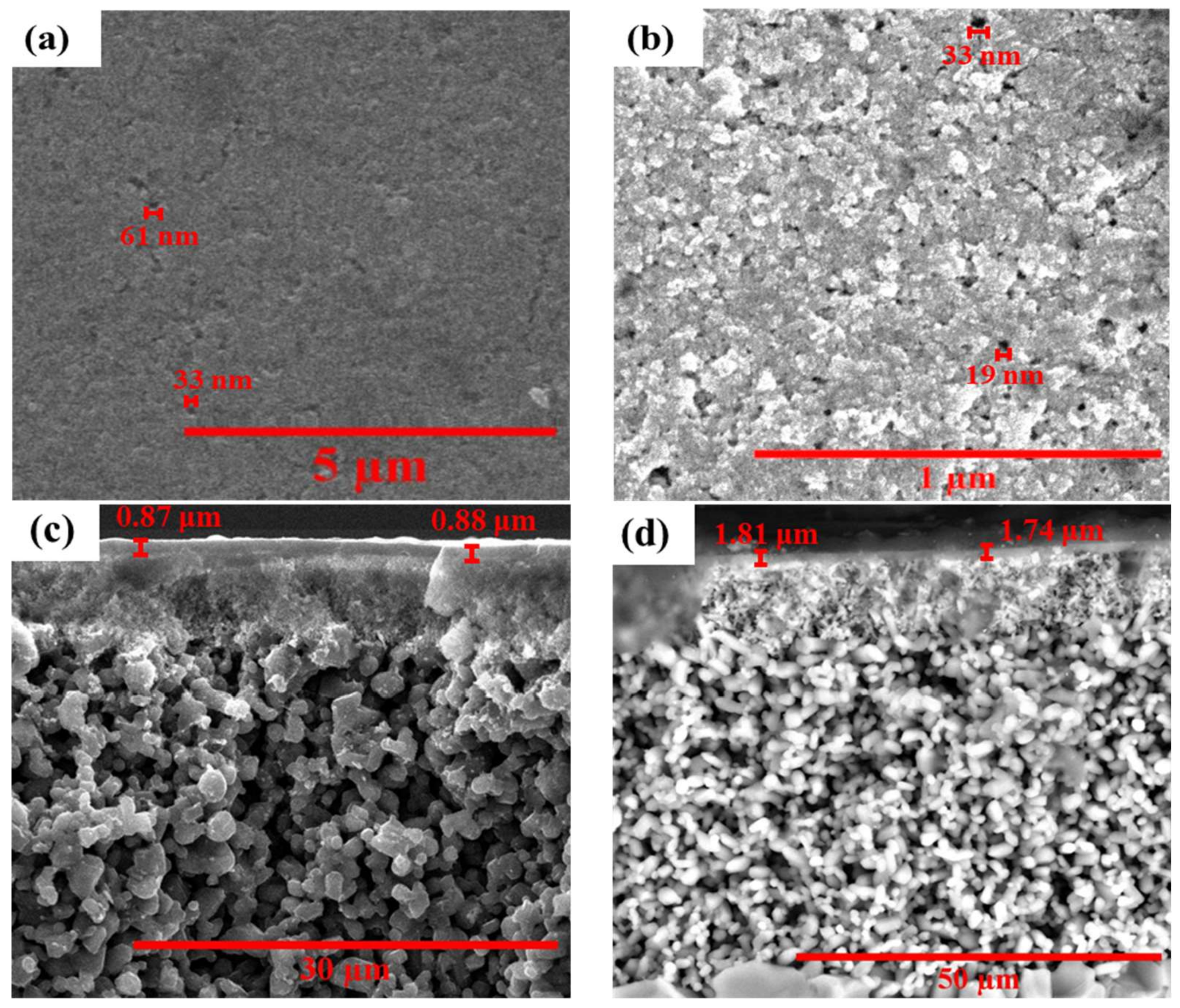
2.3. Membrane Characterization
3. Result and Discussion
3.1. Structural and Chemical Characterization of Ceramic-Supported Carbon Membranes
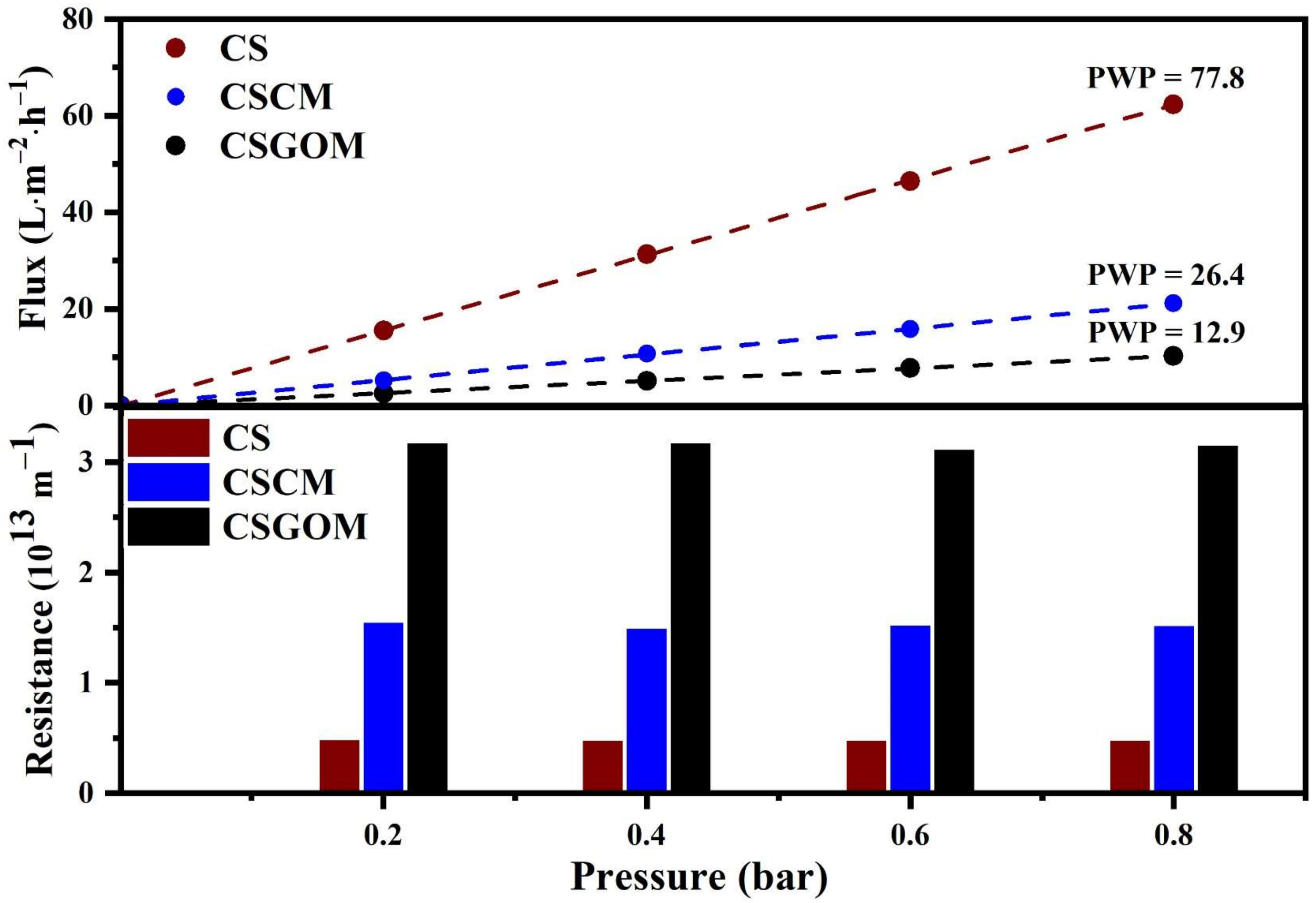
3.2. Impact of the Carbonaceous Layer on Flux and Resistance
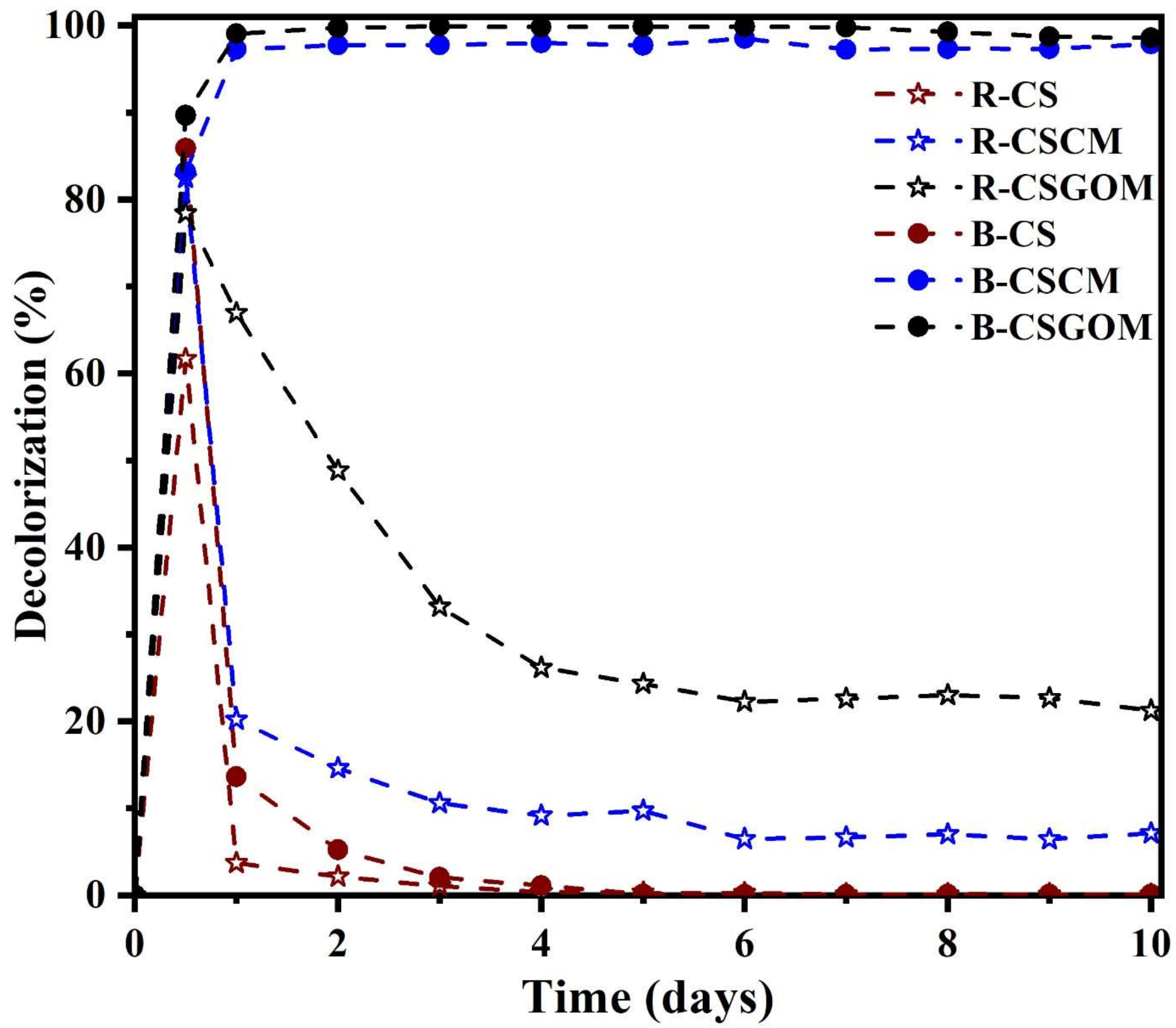
3.3. Role of the Membrane Precursors on Anaerobic Decolorization of Dye Molecules
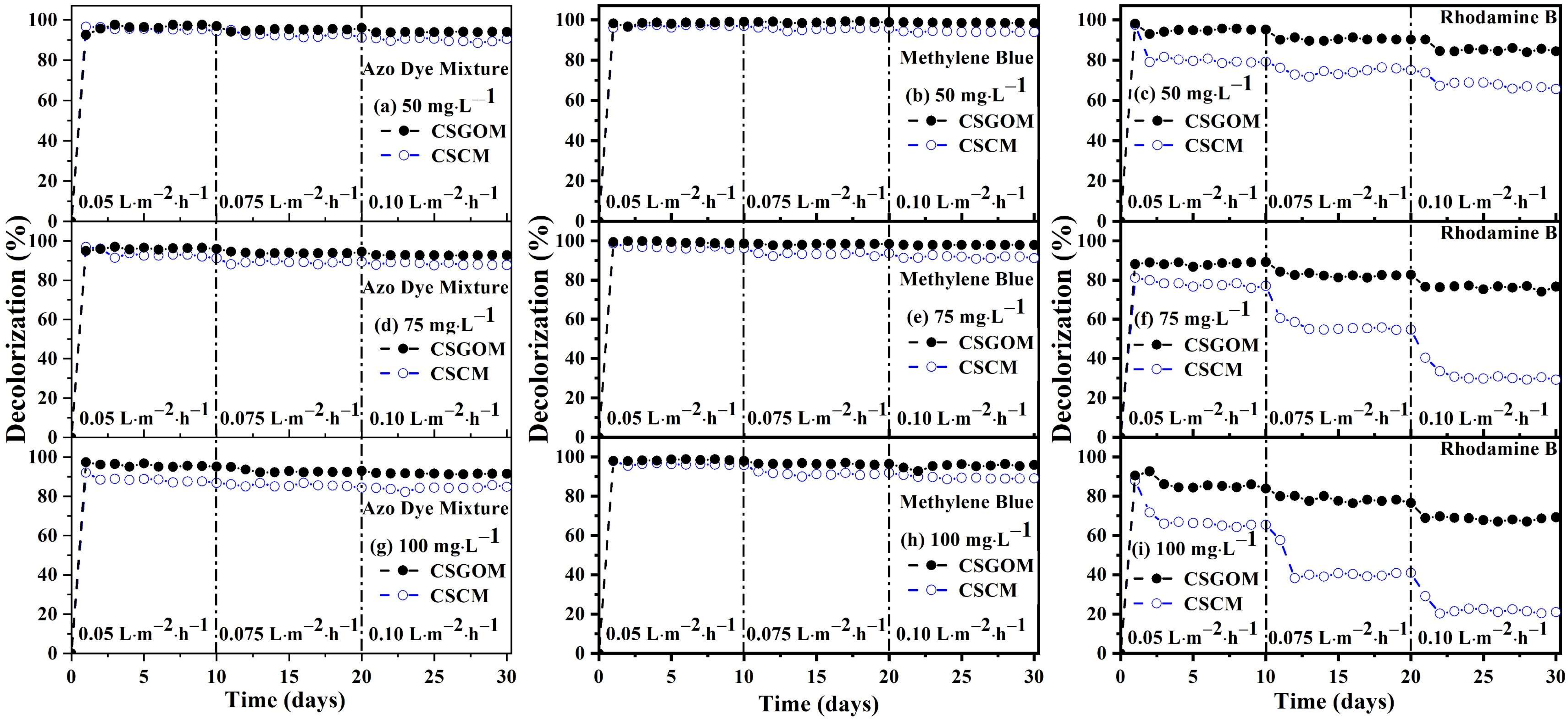
3.4. Biodecolorization Performance of CSCM and CSGOM
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Adeel, S.; Saeed, M.; Shahzad, M.; Haq, A.; Muneer, M.; Younas, M. Decolorization of Rhodamine B Dye in Aqueous Medium. Chiang Mai J. Sci. 2015, 42, 730–744. [Google Scholar]
- Gupta, V.K.; Khamparia, S.; Tyagi, I.; Jaspal, D.; Malviya, A. Decolorization of mixture of dyes: A critical review. Glob. J. Environ. Sci. Manag. 2015, 1, 71–94. [Google Scholar] [CrossRef]
- Holkar, C.R.; Jadhav, A.J.; Pinjari, D.V.; Mahamuni, N.M.; Pandit, A.B. A critical review on textile wastewater treatments: Possible approaches. J. Environ. Manag. 2016, 182, 351–366. [Google Scholar] [CrossRef]
- Elshahawy, M.F.; Mahmoud, G.A.; Raafat, A.I.; Ali, A.E.-H.; Soliman, E.S.A. Fabrication of TiO2 Reduced Graphene Oxide Based Nanocomposites for Effective of Photocatalytic Decolorization of Dye Effluent. J. Inorg. Organomet. Polym. Mater. 2020, 30, 2720–2735. [Google Scholar] [CrossRef]
- Pavithra, K.G.; Jaikumar, V. Removal of colorants from wastewater: A review on sources and treatment strategies. J. Ind. Eng. Chem. 2019, 75, 1–19. [Google Scholar] [CrossRef]
- Ledakowicz, S.; Paździor, K. Recent Achievements in Dyes Removal Focused on Advanced Oxidation Processes Integrated with Biological Methods. Molecules 2021, 26, 870. [Google Scholar] [CrossRef]
- Wang, Y.; Pan, J.; Li, Y.; Zhang, P.; Li, M.; Zheng, H.; Zhang, X.; Li, H.; Du, Q. Methylene blue adsorption by activated carbon, nickel alginate/activated carbon aerogel, and nickel alginate/graphene oxide aerogel: A comparison study. J. Mater. Res. Technol. 2020, 9, 12443–12460. [Google Scholar] [CrossRef]
- Uddin, M.J.; Islam, M.A.; Haque, S.A.; Hasan, S.; Amin, M.S.A.; Rahman, M.M. Preparation of nanostructured TiO2-based photocatalyst by controlling the calcining temperature and pH. Int. Nano Lett. 2012, 2, 19. [Google Scholar] [CrossRef]
- Lellis, B.; Fávaro-Polonio, C.Z.; Pamphile, J.A.; Polonio, J.C. Effects of textile dyes on health and the environment and bioremediation potential of living organisms. Biotechnol. Res. Innov. 2019, 3, 275–290. [Google Scholar] [CrossRef]
- Hassan, M.M.; Carr, C.M. A critical review on recent advancements of the removal of reactive dyes from dyehouse effluent by ion-exchange adsorbents. Chemosphere 2018, 209, 201–219. [Google Scholar] [CrossRef] [PubMed]
- Caprarescu, S.; Miron, A.R.; Purcar, V.; Radu, A.-L.; Sarbu, A.; Ion-Ebrasu, D.; Atanase, L.-I.; Ghiurea, M. Efficient removal of Indigo Carmine dye by a separation process. Water Sci. Technol. 2016, 74, 2462–2473. [Google Scholar] [CrossRef]
- Modrogan, C.; Cǎprǎrescu, S.; Dǎncilǎ, A.M.; Orbuleț, O.D.; Grumezescu, A.M.; Purcar, V.; Radițoiu, V.; Fierascu, R.C. Modified Composite Based on Magnetite and Polyvinyl Alcohol: Synthesis, Characterization, and Degradation Studies of the Methyl Orange Dye from Synthetic Wastewater. Polymers 2021, 13, 3911. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.A.; Amin, M.S.A.; Hoinkis, J. Optimal design of an activated sludge plant: Theoretical analysis. Appl. Water Sci. 2013, 3, 375–386. [Google Scholar] [CrossRef][Green Version]
- Obotey Ezugbe, E.; Rathilal, S. Membrane Technologies in Wastewater Treatment: A Review. Membranes 2020, 10, 89. [Google Scholar] [CrossRef]
- García-Martínez, Y.; Chirinos, J.; Bengoa, C.; Stüber, F.; Font, J.; Fortuny, A.; Fabregat, A. Fast Aqueous Biodegradation of Highly-Volatile Organic Compounds in a Novel Anaerobic Reaction Setup. Environments 2018, 5, 115. [Google Scholar] [CrossRef]
- Tao, P.; Xu, Y.; Song, C.; Yin, Y.; Yang, Z.; Wen, S.; Wang, S.; Liu, H.; Li, S.; Li, C.; et al. A novel strategy for the removal of rhodamine B (RhB) dye from wastewater by coal-based carbon membranes coupled with the electric field. Sep. Purif. Technol. 2017, 179, 175–183. [Google Scholar] [CrossRef]
- Xiao, X.; Li, T.-T.; Lu, X.-R.; Feng, X.-L.; Han, X.; Li, W.-W.; Li, Q.; Yu, H.-Q. A simple method for assaying anaerobic biodegradation of dyes. Bioresour. Technol. 2018, 251, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Ion-Ebrasu, D.; Pollet, B.G.; Spinu-Zaulet, A.; Soare, A.; Carcadea, E.; Varlam, M.; Caprarescu, S. Graphene modified fluorinated cation-exchange membranes for proton exchange membrane water electrolysis. Int. J. Hydrog. Energy 2019, 44, 10190–10196. [Google Scholar] [CrossRef]
- Ion-Ebrasu, D.; Pollet, B.G.; Caprarescu, S.; Chitu, A.; Trusca, R.; Niculescu, V.; Gabor, R.; Carcadea, E.; Varlam, M.; Vasile, B.S. Graphene inclusion effect on anion-exchange membranes properties for alkaline water electrolyzers. Int. J. Hydrog. Energy 2020, 45, 17057–17066. [Google Scholar] [CrossRef]
- Pan, Z.; Song, C.; Li, L.; Wang, H.; Pan, Y.; Wang, C.; Li, J.; Wang, T.; Feng, X. Membrane technology coupled with electrochemical advanced oxidation processes for organic wastewater treatment: Recent advances and future prospects. Chem. Eng. J. 2019, 376, 120909. [Google Scholar] [CrossRef]
- Amin, M.S.A.; Stüber, F.; Giralt, J.; Fortuny, A.; Fabregat, A.; Font, J. Comparative Anaerobic Decolorization of Azo Dyes by Carbon-Based Membrane Bioreactor. Water 2021, 13, 1060. [Google Scholar] [CrossRef]
- Khraisheh, M.; Elhenawy, S.; AlMomani, F.; Al-Ghouti, M.; Hassan, M.K.; Hameed, B.H. Recent Progress on Nanomaterial-Based Membranes for Water Treatment. Membranes 2021, 11, 995. [Google Scholar] [CrossRef] [PubMed]
- Isaeva, V.I.; Vedenyapina, M.D.; Kurmysheva, A.Y.; Weichgrebe, D.; Nair, R.R.; Nguyen, N.P.; Kustov, L.M. Modern Carbon–Based Materials for Adsorptive Removal of Organic and Inorganic Pollutants from Water and Wastewater. Molecules 2021, 26, 6628. [Google Scholar] [CrossRef]
- Manawi, Y.; Kochkodan, V.; Hussein, M.A.; Khaleel, M.A.; Khraisheh, M.; Hilal, N. Can carbon-based nanomaterials revolutionize membrane fabrication for water treatment and desalination? Desalination 2016, 391, 69–88. [Google Scholar] [CrossRef]
- Perreault, F.; Fonseca de Faria, A.; Elimelech, M. Environmental applications of graphene-based nanomaterials. Chem. Soc. Rev. 2015, 44, 5861–5896. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.S.A.; Stüber, F.; Giralt, J.; Fortuny, A.; Fabregat, A.; Font, J. Ceramic-supported graphene oxide membrane bioreactor for the anaerobic decolorization of azo dyes. J. Water Process Eng. 2022, 45, 102499. [Google Scholar] [CrossRef]
- Giménez-Pérez, A.; Bikkarolla, S.K.; Benson, J.; Bengoa, C.; Stüber, F.; Fortuny, A.; Fabregat, A.; Font, J.; Papakonstantinou, P. Synthesis of N-doped and non-doped partially oxidised graphene membranes supported over ceramic materials. J. Mater. Sci. 2016, 51, 8346–8360. [Google Scholar] [CrossRef]
- Chen, A.; Yang, B.; Zhou, Y.; Sun, Y.; Ding, C. Effects of azo dye on simultaneous biological removal of azo dye and nutrients in wastewater. R. Soc. Open Sci. 2018, 5, 180795. [Google Scholar] [CrossRef] [PubMed]
- El Bouraie, M.; El Din, W.S. Biodegradation of Reactive Black 5 by Aeromonas hydrophila strain isolated from dye-contaminated textile wastewater. Sustain. Environ. Res. 2016, 26, 209–216. [Google Scholar] [CrossRef]
- Sazali, N.; Salleh, W.N.W.; Md Nordin, N.A.H.; Harun, Z.; Ismail, A.F. Matrimid-based carbon tubular membranes: The effect of the polymer composition. J. Appl. Polym. Sci. 2015, 132, 42394. [Google Scholar] [CrossRef]
- Dohade, M. Incorporation of carbon nanofibers into a Matrimid polymer matrix: Effects on the gas permeability and selectivity properties. J. Appl. Polym. Sci. 2018, 135, 46019. [Google Scholar] [CrossRef]
- Sazali, N.; Salleh, W.N.W.; Nordin, N.A.H.M.; Ismail, A.F. Matrimid-based carbon tubular membrane: Effect of carbonization environment. J. Ind. Eng. Chem. 2015, 32, 167–171. [Google Scholar] [CrossRef]
- Rattana; Chaiyakun, S.; Witit-anun, N.; Nuntawong, N.; Chindaudom, P.; Oaew, S.; Kedkeaw, C.; Limsuwan, P. Preparation and characterization of graphene oxide nanosheets. Procedia Eng. 2012, 32, 759–764. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, P.; Liang, B.; Liu, Y.; Xu, T.; Wang, L.; Cao, B.; Pan, K. Graphene Oxide as an Effective Barrier on a Porous Nanofibrous Membrane for Water Treatment. ACS Appl. Mater. Interfaces 2016, 8, 6211–6218. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Cai, Q.; Li, X.; Pan, Z.; Li, Y.; Chen, X.; Yan, Q. Preparation and characterization of ZrO2/TiO2 composite photocatalytic film by micro-arc oxidation. Trans. Nonferrous Met. Soc. China 2013, 23, 2945–2950. [Google Scholar] [CrossRef]
- Ruppert, A.M.; Agulhon, P.; Grams, J.; Wąchała, M.; Wojciechowska, J.; Świerczyński, D.; Cacciaguerra, T.; Tanchoux, N.; Quignard, F. Synthesis of TiO2–ZrO2 Mixed Oxides via the Alginate Route: Application in the Ru Catalytic Hydrogenation of Levulinic Acid to Gamma-Valerolactone. Energies 2019, 12, 4706. [Google Scholar] [CrossRef]
- Ismail, N.H.; Salleh, W.N.W.; Sazali, N.; Ismail, A.F. Development and characterization of disk supported carbon membrane prepared by one-step coating-carbonization cycle. J. Ind. Eng. Chem. 2018, 57, 313–321. [Google Scholar] [CrossRef]
- Johra, F.T.; Lee, J.-W.; Jung, W.-G. Facile and safe graphene preparation on solution based platform. J. Ind. Eng. Chem. 2014, 20, 2883–2887. [Google Scholar] [CrossRef]
- Bikkarolla, S.K.; Cumpson, P.; Joseph, P.; Papakonstantinou, P. Oxygen reduction reaction by electrochemically reduced graphene oxide. Faraday Discuss. 2014, 173, 415–428. [Google Scholar] [CrossRef]
- Thema, F.T.; Moloto, M.J.; Dikio, E.D.; Nyangiwe, N.N.; Kotsedi, L.; Maaza, M.; Khenfouch, M. Synthesis and Characterization of Graphene Thin Films by Chemical Reduction of Exfoliated and Intercalated Graphite Oxide. J. Chem. 2013, 2013, 150536. [Google Scholar] [CrossRef]
- Ismail, N.H.; Salleh, W.N.W.; Sazali, N.; Ismail, A.F.; Yusof, N.; Aziz, F. Disk supported carbon membrane via spray coating method: Effect of carbonization temperature and atmosphere. Sep. Purif. Technol. 2018, 195, 295–304. [Google Scholar] [CrossRef]
- Wu, S.; Zhang, B.; Liu, Y.; Suo, X.; Li, H. Influence of surface topography on bacterial adhesion: A Review. Biointerphases 2018, 13, 060801. [Google Scholar] [CrossRef] [PubMed]
- Oder, M.; Arlič, M.; Bohinc, K.; Fink, R. Escherichia coli biofilm formation and dispersion under hydrodynamic conditions on metal surfaces. Int. J. Environ. Health Res. 2018, 28, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Niu, F.; Huang, M.; Cai, T.; Meng, L. Effect of Membrane Thickness on Properties of FO Membranes with Nanofibrous Substrate. IOP Conf. Ser. Earth Environ. Sci. 2018, 170, 052005. [Google Scholar] [CrossRef]
- Ismail, N.H.; Salleh, W.N.W.; Sazali, N.; Ismail, A.F. Effect of intermediate layer on gas separation performance of disk supported carbon membrane. Sep. Sci. Technol. 2017, 52, 2137–2149. [Google Scholar] [CrossRef]
- Yang, M.; Zhao, C.; Zhang, S.; Li, P.; Hou, D. Preparation of graphene oxide modified poly(m-phenylene isophthalamide) nanofiltration membrane with improved water flux and antifouling property. Appl. Surf. Sci. 2016, 394, 149–159. [Google Scholar] [CrossRef]
- Xu, K.; Feng, B.; Zhou, C.; Huang, A. Synthesis of highly stable graphene oxide membranes on polydopamine functionalized supports for seawater desalination. Chem. Eng. Sci. 2016, 146, 159–165. [Google Scholar] [CrossRef]
- Lou, Y.; Liu, G.; Liu, S.; Shen, J.; Jin, W. A facile way to prepare ceramic-supported graphene oxide composite membrane via silane-graft modification. Appl. Surf. Sci. 2014, 307, 631–637. [Google Scholar] [CrossRef]
- Yaseen, D.A.; Scholz, M. Textile dye wastewater characteristics and constituents of synthetic effluents: A critical review. Int. J. Environ. Sci. Technol. 2019, 16, 1193–1226. [Google Scholar] [CrossRef]
- Xu, N.; Guo, D.; Xiao, C. Fe/Mn oxide decorated polyacrylonitrile hollow fiber membrane as heterogeneous Fenton reactor for methylene blue decolorization. J. Appl. Polym. Sci. 2019, 136, 48217. [Google Scholar] [CrossRef]
- Ming, J.; Sun, D.; Wei, J.; Chen, X.; Zheng, N. Adhesion of Bacteria to a Graphene Oxide Film. ACS Appl. Biol. Mater. 2020, 3, 704–712. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Gao, D.; Wang, B.; Zhao, R.; Guan, M.; Zheng, L.-; Zhou, X.; Chai, Z.; Feng, W. Graphene oxide as an anaerobic membrane scaffold for the enhancement of B. adolescentis proliferation and antagonistic effects against pathogens E. coli and S. aureus. Nanotechnology 2014, 25, 165101. [Google Scholar] [CrossRef]
- Mezohegyi, G.; Bengoa, C.; Stuber, F.; Font, J.; Fabregat, A.; Fortuny, A. Novel bioreactor design for decolourisation of azo dye effluents. Chem. Eng. J. 2008, 143, 293–298. [Google Scholar] [CrossRef]
- Hashemi Salehi, M.; Yousefi, M.; Hekmati, M.; Balali, E. Application of palladium nanoparticle-decorated Artemisia abrotanum extract-modified graphene oxide for highly active catalytic reduction of methylene blue, methyl orange and rhodamine B. Appl. Organomet. Chem. 2019, 33, e5123. [Google Scholar] [CrossRef]
- Dhale, A.D.; Mahajani, V.V. Studies in treatment of disperse dye waste: Membrane–wet oxidation process. Waste Manag. 2000, 20, 85–92. [Google Scholar] [CrossRef]
- Zhang, X.; Hu, Y.; Yang, W.; Feng, M. Ag-loaded and Pd-loaded ZnO nanofiber membranes: Preparation via electrospinning and application in photocatalytic antibacterial and dye degradation. Appl. Nanosci. 2021, in press. [Google Scholar] [CrossRef]
- Ismail, A.M.; Menazea, A.A.; Ali, H. Selective adsorption of cationic azo dyes onto zeolite nanorod-based membranes prepared via laser ablation. J. Mater. Sci. Mater. Electron. 2021, 32, 19352–19367. [Google Scholar] [CrossRef]
- Ravadelli, M.; da Costa, R.E.; Lobo-Recio, M.A.; Akaboci, T.R.V.; Bassin, J.P.; Lapolli, F.R.; Belli, T.J. Anoxic/oxic membrane bioreactor assisted by electrocoagulation for the treatment of azo-dye containing wastewater. J. Environ. Chem. Eng. 2021, 9, 105286. [Google Scholar] [CrossRef]
- Chakraborty, S.; Purkait, M.K.; DasGupta, S.; De, S.; Basu, J.K. Nanofiltration of textile plant effluent for color removal and reduction in COD. Sep. Purif. Technol. 2003, 31, 141–151. [Google Scholar] [CrossRef]
- Ye, Q.; Li, J.; Han, Q.; Xu, M.; Jiang, L.; Yan, B. Preparation of Activated Carbon-PVDF Blend Membrane and Its Effect on the Decolorization of Azo Dyes. IOP Conf. Ser. Earth Environ. Sci. 2021, 793, 012012. [Google Scholar] [CrossRef]
- Rahimi, M.; Aghel, B.; Sadeghi, M.; Ahmadi, M. Using Y-shaped microreactor for continuous decolorization of an Azo dye. Desalin. Water Treat. 2014, 52, 5513–5519. [Google Scholar] [CrossRef]
- Ewida, A.Y.I.; El-Sesy, M.E.; Abou Zeid, A. Complete degradation of azo dye acid red 337 by Bacillus megaterium KY848339.1 isolated from textile wastewater. Water Sci. 2019, 33, 154–161. [Google Scholar] [CrossRef]
- Li, Q.; Tang, X.; Sun, Y.; Wang, Y.; Long, Y.; Jiang, J.; Xu, H. Removal of Rhodamine B from wastewater by modified Volvariella volvacea: Batch and column study. RSC Adv. 2015, 5, 25337–25347. [Google Scholar] [CrossRef]
- Popli, S.; Patel, U.D. Destruction of azo dyes by anaerobic–aerobic sequential biological treatment: A review. Int. J. Environ. Sci. Technol. 2015, 12, 405–420. [Google Scholar] [CrossRef]
- Khan, R.; Bhawana, P.; Fulekar, M.H. Microbial decolorization and degradation of synthetic dyes: A review. Rev. Environ. Sci. Bio Technol. 2013, 12, 75–97. [Google Scholar] [CrossRef]
- Solís, M.; Solís, A.; Pérez, H.I.; Manjarrez, N.; Flores, M. Microbial decolouration of azo dyes: A review. Process Biochem. 2012, 47, 1723–1748. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amin, M.S.A.; Stüber, F.; Giralt, J.; Fortuny, A.; Fabregat, A.; Font, J. Compact Carbon-Based Membrane Reactors for the Intensified Anaerobic Decolorization of Dye Effluents. Membranes 2022, 12, 174. https://doi.org/10.3390/membranes12020174
Amin MSA, Stüber F, Giralt J, Fortuny A, Fabregat A, Font J. Compact Carbon-Based Membrane Reactors for the Intensified Anaerobic Decolorization of Dye Effluents. Membranes. 2022; 12(2):174. https://doi.org/10.3390/membranes12020174
Chicago/Turabian StyleAmin, Mohammad Shaiful Alam, Frank Stüber, Jaume Giralt, Agustin Fortuny, Azael Fabregat, and Josep Font. 2022. "Compact Carbon-Based Membrane Reactors for the Intensified Anaerobic Decolorization of Dye Effluents" Membranes 12, no. 2: 174. https://doi.org/10.3390/membranes12020174
APA StyleAmin, M. S. A., Stüber, F., Giralt, J., Fortuny, A., Fabregat, A., & Font, J. (2022). Compact Carbon-Based Membrane Reactors for the Intensified Anaerobic Decolorization of Dye Effluents. Membranes, 12(2), 174. https://doi.org/10.3390/membranes12020174






