Functional Properties of Casein and Caseinate Produced by Electrodialysis with Bipolar Membrane Coupled to an Ultrafiltration Module
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Protocol
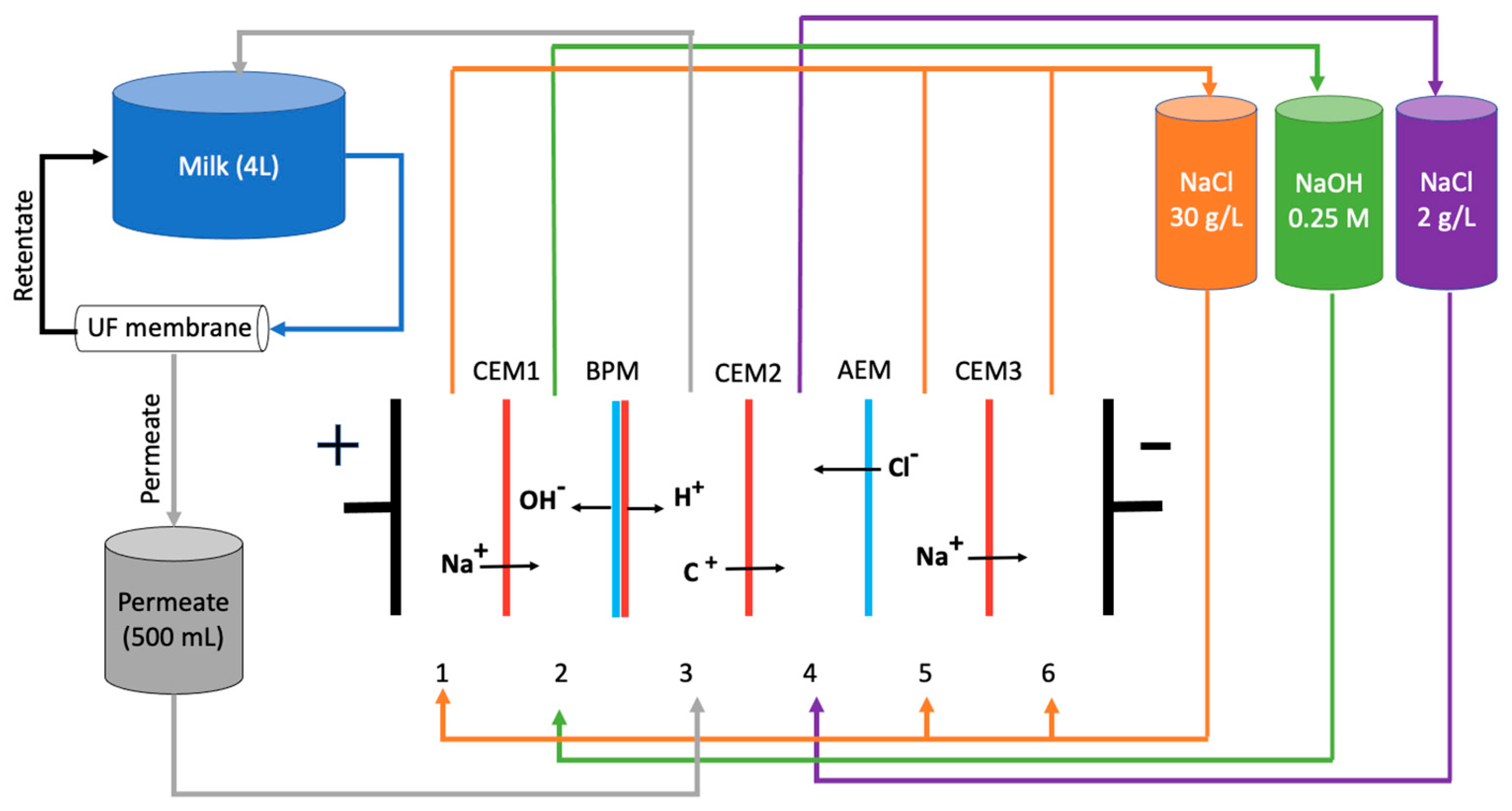
2.2.1. Production of Casein and Caseinate by EDBM-UF
2.2.2. Chemical Production of Casein and Caseinate
2.3. Analyses
2.3.1. Milk Composition
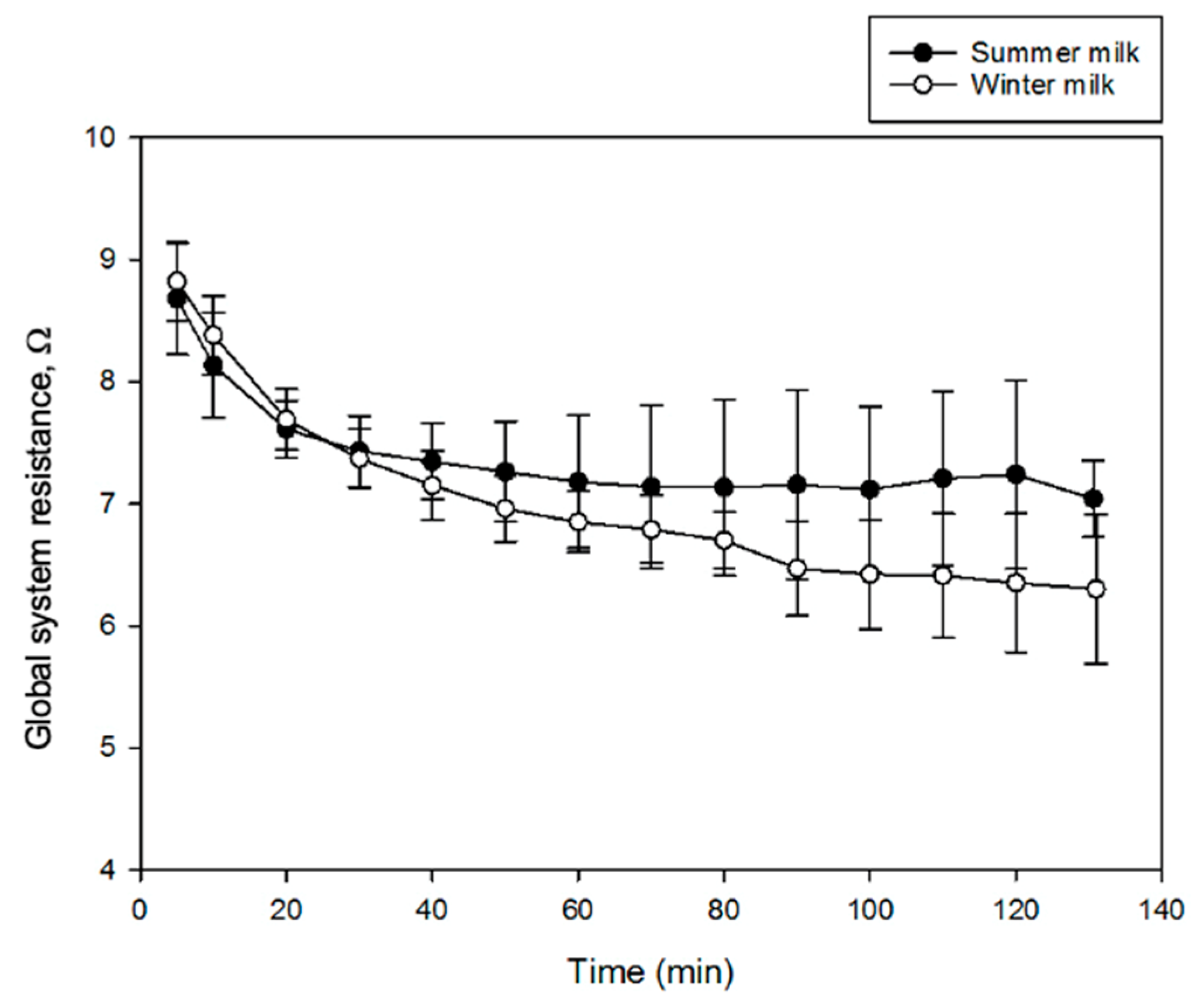
2.3.2. Global System Resistance
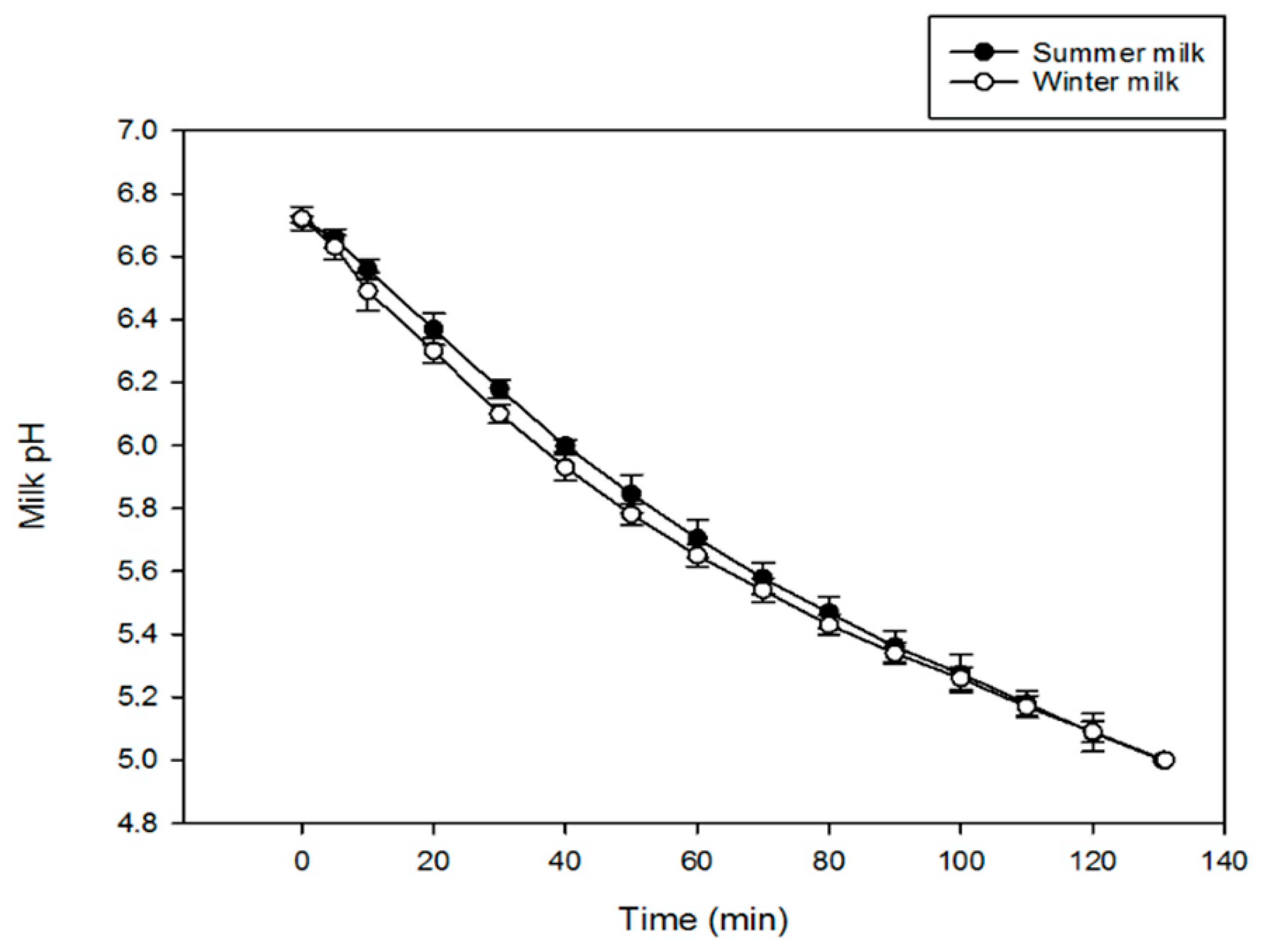
2.3.3. Milk pH
2.3.4. Membranes Characterization
2.3.5. Casein and Caseinate Composition
Moisture Content
Lactose Content
Protein Content
Ash Content
2.3.6. Functional Properties of Casein and Caseinate
Solubility
Hygroscopicity
Water-Holding Capacity
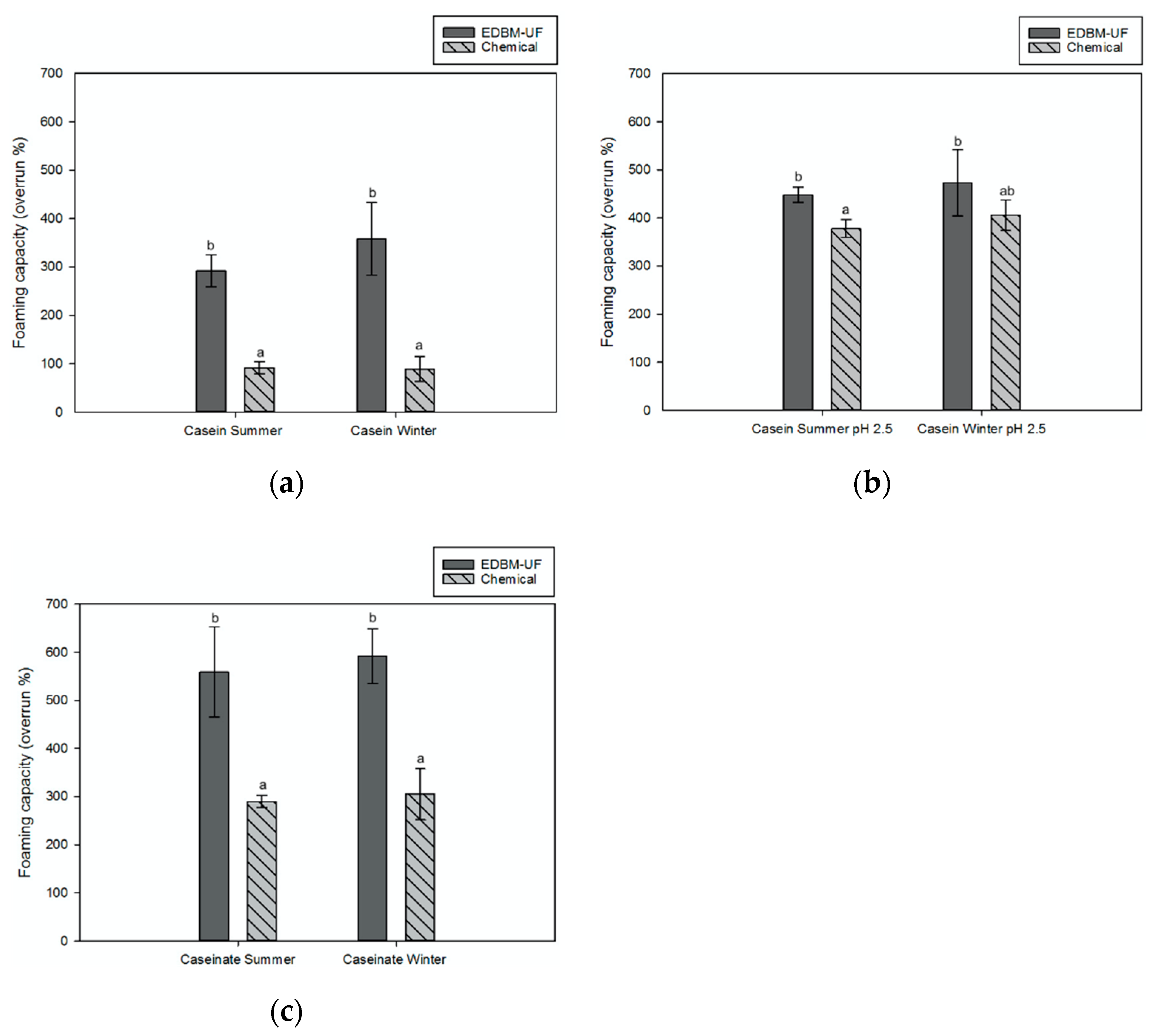
Foaming Capacity
Foam Stability
Emulsifying Activity
Emulsifying Capacity
Emulsion Stability
Gelling Capacity
2.3.7. Statistical Analyses
3. Results and Discussion
3.1. Proximal Composition of Milks
3.2. EDBM Parameters
3.2.1. pH Evolution during Milk Electroacidification
3.2.2. Global System Resistance
3.2.3. Membranes Characterization
3.3. Analyses
3.3.1. Casein and Caseinate Composition
3.3.2. Casein and Caseinate Functional Properties
Solubility
Hygroscopicity
Water-Holding Capacity
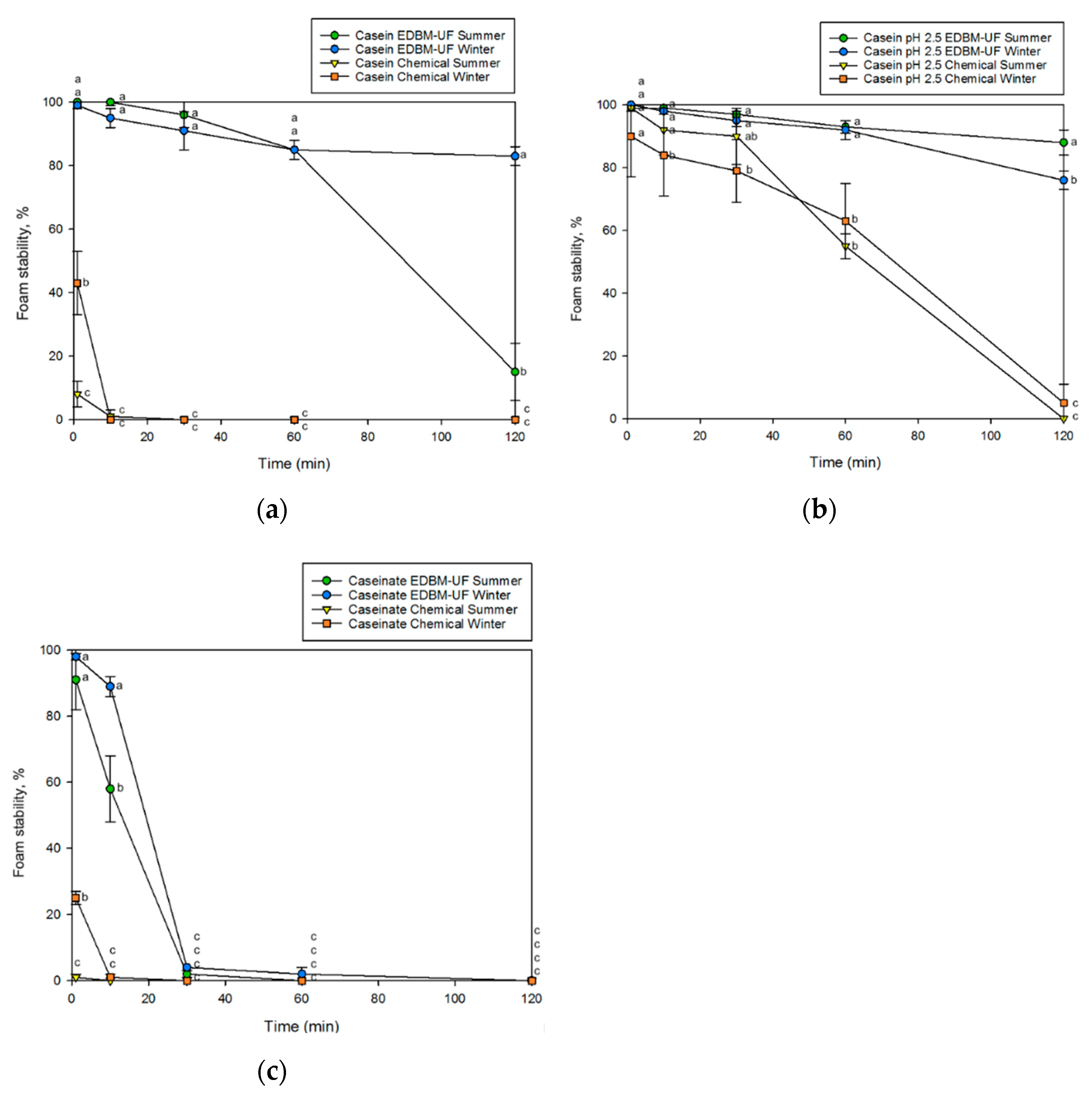
Foaming Properties
Emulsifying Properties
Gelling Capacity
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wong, D.W.S.; Camirand, W.M.; Pavlath, A.E.; Parris, D.N.; Friedman, D.M. Structures and functionalities of milk proteins, Critical Reviews in Food Science & Nutrition. Crit. Rev. Food Sci. Nutr. 1996, 36, 807–844. [Google Scholar] [CrossRef] [PubMed]
- Chandan, R.C.; Kilara, A. Dairy Ingredients for Food Processing; Blackwell Publishing: Hoboken, NJ, USA, 2011. [Google Scholar] [CrossRef]
- Gaudichon, C. Les Protéines Laitières: Intérêts Technologiques et Nutritionnels; Tech & Doc: Paris, France, 2001. [Google Scholar]
- Paul, M. Formation of Bioactive Peptides from Dairy Products; Gene-Tech Books: Chicago, IL, USA, 2007. [Google Scholar]
- Sarode, A.; Sawale, P.; Khedkar, C.; Kalyankar, S. Casein and Caseinate: Methods of Manufacture. In The Encyclopedia of Food and Health; Babalerro, B., Finglas, P., Toldra, F., Eds.; Oxfard: London, UK, 2016; pp. 676–682. [Google Scholar]
- Huppertz, T.; Fox, P.F.; Kelly, A.L. The caseins: Structure, stability, and functionality. In Proteins in Food Processing; Yada, R.Y., Ed.; Springer: Berlin, Germany, 2018; pp. 49–92. [Google Scholar]
- Brule, G.; Fauquant, J.; Maubois, J.-L. Preparation of “Native” Phosphocaseinate by Combining Membrane Ultrafiltration and Ultracentrifugation. J. Dairy Sci. 1979, 62, 869–875. [Google Scholar] [CrossRef]
- Mikhaylin, S.; Patouillard, L.; Margni, M.; Bazinet, L. Milk protein production by a more environmentally sustainable process: Bipolar membrane electrodialysis coupled with ultrafiltration. Green Chem. 2018, 20, 449–456. [Google Scholar] [CrossRef]
- Masson, F.-A.; Mikhaylin, S.; Bazinet, L. Production of calcium- and magnesium-enriched caseins and caseinates by an ecofriendly technology. J. Dairy Sci. 2018, 101, 7002–7012. [Google Scholar] [CrossRef] [PubMed]
- Mikhaylin, S.; Sion, A.-V. Improvement of a sustainable hybrid technology for caseins isoelectric precipitation (electrodialysis with bipolar membrane/ultrafiltration) by mitigation of scaling on cation-exchange membrane. Innov. Food Sci. Emerg. Technol. 2016, 33, 571–579. [Google Scholar] [CrossRef]
- Carr, A.; Golding, M. Functional Milk Proteins Production and Utilization: Casein-Based Ingredients. Adv. Dairy Chem. 2016, 1B, 35–66. [Google Scholar] [CrossRef]
- AOAC. Method 972.16 Fat, Lactose, Protein, and Solids in Milk. In Official Methods of Analysis of AOAC International, 17th ed.; AOAC: Gaithersburg, MD, USA, 1995; Volume 2. [Google Scholar]
- Lemay, N.; Mikhaylin, S.; Bazinet, L. Voltage spike and electroconvective vortices generation during electrodialysis under pulsed electric field: Impact on demineralization process efficiency and energy consumption. Innov. Food Sci. Emerg. Technol. 2019, 52, 221–231. [Google Scholar] [CrossRef]
- ISO 22662; Milk and Milk Products-Determining of Lactose Content by High-Performance Liquid Chromatography (Reference Method). International Organization for Standardization: Geneva, Switzerland, 2007.
- FAO. Nitrogen and Protein Content Measurement and Nitrogen to Protein Conversion Factors for Dairy and Soy Protein-Based Foods: A Systematic Review and Modelling Analysis. Available online: https://apps.who.int/iris/bitstream/handle/10665/331206/9789241516983-eng.pdf (accessed on 23 February 2021).
- AOAC. Method 945.46: Ash in milk. In Official Methods of Analysis of AOAC International, 17th ed.; AOAC: Gaithersburg, MD, USA, 1995; Volume 2. [Google Scholar]
- Haque, E.; Whittaker, A.K.; Gidley, M.J.; Deeth, H.C.; Fibrianto, K.; Bhandari, B.R. Kinetics of enthalpy relaxation of milk protein concentrate powder upon ageing and its effect on solubility. Food Chem. 2012, 134, 1368–1373. [Google Scholar] [CrossRef]
- Anema, S.G.; Pinder, D.N.; Hunter, R.J.; Hemar, Y. Effects of storage temperature on the solubility of milk protein concentrate (MPC85). Food Hydrocoll. 2006, 20, 386–393. [Google Scholar] [CrossRef]
- Ma, J.-J.; Mao, X.-Y.; Wang, Q.; Yang, S.; Zhang, D.; Chen, S.-W.; Li, Y.-H. Effect of spray drying and freeze drying on the immunomodulatory activity, bitter taste and hygroscopicity of hydrolysate derived from whey protein concentrate. Food Sci. Technol. 2014, 56, 296–302. [Google Scholar] [CrossRef]
- Mirmoghtadaie, L.; Kadivar, M.; Shahedi, M. Effects of succinylation and deamidation on functional properties of oat protein isolate. Food Chem. 2009, 114, 127–131. [Google Scholar] [CrossRef]
- Lin, M.J.Y.; Humbert, E.S.; Sosulski, F.W. Certain functional properties of sunflower meal products. J. Food Sci. 1974, 39, 368–370. [Google Scholar] [CrossRef]
- Kato, A.; Takahashi, A.; Matsudomi, N.; Kobayashi, K. Determination of Foaming Properties of Proteins by Conductivity Measurements. J. Food Sci. 1983, 48, 62–65. [Google Scholar] [CrossRef]
- Neto, V.Q.; Narain, N.; Silva, J.B.; Bora, P.S. Functional properties of raw and heat processed cashew nut (Anacardium occidentale, L.) kernel protein isolates. Mol. Nutr. 2001, 45, 258–262. [Google Scholar] [CrossRef]
- Mohanty, B.; Mulvihill, D.M.; Fox, P.F. Emulsifying and Foaming Properties of Acidic Caseins and Sodium Caseinate. Food Chem. 1988, 28, 17–30. [Google Scholar] [CrossRef]
- Stone, A.K.; Nickerson, M.T. Formation and functionality of whey protein isolate-(kappa-, iota-, and lambda-type) carrageenan electrostatic complexes. Food Hydrocoll. 2012, 27, 271–277. [Google Scholar] [CrossRef]
- Myllarinen, P.; Buchert, J.; Autio, K. Effect of transglutaminase on rheological properties and microstructure of chemically acidified sodium caseinate gels. Int. Dairy J. 2005, 17, 800–807. [Google Scholar] [CrossRef]
- Bernabucci, U.; Basiricò, L.; Morera, P.; Dipasquale, D.; Vitali, A.; Cappelli, F.P.; Calamar, L. Effect of summer season on milk protein fractions in Holstein cows. J. Dairy Sci. 2015, 98, 1815–1827. [Google Scholar] [CrossRef]
- Godden, S.M.; Lissemore, K.D.; Kelton, D.F.; Lesli, K.E.; Walton, J.S.; Lumsden, J.H. Factors Associated with Milk Urea Concentrations in Ontario Dairy Cows. J. Dairy Sci. 2001, 84, 107–114. [Google Scholar] [CrossRef]
- Salaün, F.; Mietton, B.; Gaucheron, F. Buffering capacity of dairy products. Int. Dairy J. 2005, 15, 95–109. [Google Scholar] [CrossRef]
- Bazinet, L.; Lamarche, F.; Ippersiel, D.; Gendron, C.; Mahdavi, B.; Amiot, J. Comparaison of Electrochemical and Chemical Acidification of skim milk. J. Food Sci. 2000, 65, 1303–1307. [Google Scholar] [CrossRef]
- Bazinet, L.; Lamarche, F.; Ippersiel, D.; Amiot, J. Bipolar Membrane Electroacidification To Produce Bovine Milk Casein Isolate. J. Agric. Food Chem. 1999, 47, 5291–5296. [Google Scholar] [CrossRef] [PubMed]
- Bazinet, L.; Ippersiel, D.; Gendron, C.; Beaudry, J.; Mahdavi, B.; Amiot, J.; Lamarche, F. Cationic balance in skim milk during bipolar membrane electroacidification. J. Membr. Sci. 2000, 173, 201–209. [Google Scholar] [CrossRef]
- Mikhaylin, S.; Nikonenko, V.; Pourcelly, G.; Bazinet, L. Hybrid bipolar membrane electrodialysis/ultrafiltration technology assisted by a pulsed electric field for casein production. Green Chem. 2016, 18, 307–314. [Google Scholar] [CrossRef]
- Bazinet, L.; Ippersiel, D.; Gendron, C.; Tétreault, C.; René-Paradis, J.; Beaudry, J.; Britten, M.; Mahdavi, B.; Amiot, J.; Lamarche, F. Comparison between reconstituted and fresh skim milk chemical and electrochemical acidifications. J. Sci. Food Agric. 2002, 82, 1356–1364. [Google Scholar] [CrossRef]
- Li, S.; Ye, A.; Singh, H. Seasonal variations in composition, properties, and heat-induced changes in bovine milk in a seasonal calving system. J. Dairy Sci. 2019, 102, 7747–7759. [Google Scholar] [CrossRef]
- Mier, M.P.; Ibañez, R.; Ortiz, I. Influence of process variables on the production of bovine milk casein by electrodialysis with bipolar membranes. Biophys. Eng. J. 2008, 40, 304–311. [Google Scholar] [CrossRef]
- Bazinet, L.; Gendron, C.; Ippersiel, D.; René-Paradis, J.; Tétreault, C.; Beaudry, J.; Britten, M.; Mahdavi, B.; Amiot, J.; Lamarche, F. Effects of Type of Added Salt and Ionic Strength on Physicochemical and Functional Properties of Casein Isolates Produced by Electroacidification. J. Agric. Food Chem. 2002, 50, 6875–6881. [Google Scholar] [CrossRef]
- Zayas, J.F. Functionality of Proteins in Food; Springer: Berlin/Heidelberg, Germany, 1997; p. 373. [Google Scholar] [CrossRef]
- Le Graet, Y.; Brulé, G. Les équilibres minéraux du lait: Influence du pH et de la force ionique. Lait 1993, 73, 51–60. [Google Scholar] [CrossRef][Green Version]
- Kinsella, J.E.; Morr, C.V. Milk proteins: Physicochemical and functional properties. Crit. Rev. Food Sci. Nutr. 1984, 21, 197–253. [Google Scholar] [CrossRef]
- Kneifel, W.; Seiler, A. Water-holding Properties of Milk Protein Products—A Review. Food Struct. 1993, 13, 3. [Google Scholar]
- Teo, C.T.; Munro, P.A.; Singh, H.; Hudson, R.C. Effects of pH and temperature on the water-holding capacity of casein curds and whey protein gels. J. Dairy Res. 2009, 63, 83–95. [Google Scholar] [CrossRef]
- Cayot, P.; Lorient, D. Structures et Technofonctions des Protéines de Lait; Tec & Doc—Lavoisier: Paris, France, 1998; p. 384. [Google Scholar]
- Lorient, D.; Closs, B.; Courthaudon, J.-L. Surface properties of the bovine casein components: Relationships between structure and foaming properties. J. Dairy Sci. 1989, 56, 495–502. [Google Scholar] [CrossRef]
- Sarkar, A.; Singh, H. Emulsions and Foams Stabilized by Milk Proteins. In Volume 1B: Proteins: Applied Aspects; McSweeney, P., O’Mahony, J., Eds.; Springer: New York, NY, USA, 2016; pp. 133–154. [Google Scholar]
- Marinova, K.G.; Basheva, E.S.; Nenova, B.; Temelska, M.; Mirarefi, A.Y.; Campbell, B.; Ivanov, I.B. Physico-chemical factors controlling the foamability and foam stability of milk proteins: Sodium caseinate and whey protein concentrates. Food Hydrocoll. 2009, 23, 1864–1876. [Google Scholar] [CrossRef]
- Kinsella, J.E. Functional properties of proteins: Possible relationships between structure and function in foams. Food Chem. 1981, 7, 273–288. [Google Scholar] [CrossRef]
- Leman, J.; Kinsella, J.E.; Kilara, A. Surface activity, film formation, and emulsifying properties of milk proteins. Crit. Rev. Food Sci. Nutr. 1989, 28, 115–138. [Google Scholar] [CrossRef]
- Singh, H.; Ye, A. Interactions and functionality of milk proteins in food emulsions. In Milk Proteins from Expression to Food; Thompson, A., Boland, M., Singh, H., Eds.; Elsevier: Amsterdam, The Netherlands, 2009; pp. 321–340. [Google Scholar]
- Lucey, J.A. Formation, Structural Properties, and Rheology of Acid-Coagulated Milk Gels. In Cheese, Chemistry, Physics and Microbiology, 3rd ed.; Fox, P.F., Sweeney, P.L.H.M., Cogan, T.M., Guinee, T.P., Eds.; Elsevier Academic: London, UK, 2004; Volume 1, pp. 105–122. [Google Scholar]

| Compound | Summer Milk | Winter Milk |
|---|---|---|
| Fat (% m/m) | 0.07 ± 0.01 a* | 0.07 ± 0.01 a |
| Protein (% m/m) | 3.29 ± 0.01 a | 3.42 ± 0.03 b |
| Lactose (% m/m) | 4.74 ± 0.01 a | 4.74 ± 0.02 a |
| Solids (% m/m) | 9.02 ± 0.01 a | 9.16 ± 0.03 b |
| Nonfat solids (% m/m) | 8.26 ± 0.01 a | 8.40 ± 0.04 b |
| Casein (g/L) | 26.37 ± 0.05 a | 27.47 ± 0.19 b |
| NPN/CU * (mg/100 g) | 13.20 ± 0.80 b | 11.50 ± 0.80 a |
| Membrane | Thickness (mm) | Conductivity (mS/cm) | ||
|---|---|---|---|---|
| Before | After | Before | After | |
| AEM | 0.142 ± 0.004 a* | 0.142 ± 0.005 a | 5.4 ± 0.4 a | 5.3 ± 0.2 a |
| CEM1 | 0.151 ± 0.002 a | 0.151 ± 0.001 a | 8.8 ± 0.6 a | 7.9 ± 0.7 a |
| CEM2 | 0.152 ± 0.001 a | 0.153 ± 0.001 a | 8.6 ± 0.3 a | 7.9 ± 0.5 a |
| CEM3 | 0.152 ± 0.001 a | 0.153 ± 0.002 a | 8.9 ± 0.2 a | 8.6 ± 0.6 a |
| BPM | 0.240 ± 0.003 a | 0.241 ± 0.003 a | - | - |
| Compound | Casein | Caseinate | ||||||
|---|---|---|---|---|---|---|---|---|
| EDBM-UF | Chemical | EDBM-UF | Chemical | |||||
| Summer | Winter | Summer | Winter | Summer | Winter | Summer | Winter | |
| Lactose (% dry weight) | 0.037 ± 0.003 a* | 0.038 ± 0.005 a | 0.078 ± 0.016 b | 0.089 ± 0.010 b | 0.028 ± 0.006 a | 0.038 ± 0.008 a | 0.068 ± 0.006 b | 0.087 ± 0.010 b |
| Ash (% dry weight) | 3.18 ± 0.13 b | 2.91 ± 0.23 ab | 3.20 ± 0.10 b | 2.81 ± 0.02 a | 5.07 ± 0.50 a | 5.77 ± 0.03 b | 5.42 ± 0.32 ab | 5.72 ± 0.05 b |
| Protein (% dry weight) | 92 ± 2 b | 93 ± 1 b | 85 ± 4 a | 87 ± 2 a | 88 ± 1 b | 89 ± 1 b | 79 ± 5 a | 83 ± 3 a |
| Functional Property | Casein | Caseinate | ||||||
|---|---|---|---|---|---|---|---|---|
| EDBM-UF | Chemical | EDBM-UF | Chemical | |||||
| Summer | Winter | Summer | Winter | Summer | Winter | Summer | Winter | |
| Solubility (%) | - | - | - | - | 98.3 ± 0.7 a* | 98.1 ± 0.5 a | 98.2 ± 0.9 a | 98.8 ± 1.3 a |
| Water-holding capacity (g of water/g of protein) | 2.01 ± 0.05 a | 3.09 ± 0.14 b | 2.10 ± 0.21 a | 3.33 ± 0.43 b | - | - | - | - |
| Hygroscopicity (g of water/100 g of dry solids) | 24.9 ± 0.7 b | 22.7 ± 0.8 a | 30.0 ± 0.7 c | 24.4 ± 0.1 b | 41.1 ± 3.4 b | 32.1 ± 2.1 a | 44.6 ± 3.6 b | 32.2 ± 1.9 a |
| Emulsifying activity (%) | 48 ± 2 a | 48 ± 1 a | 47 ± 2 a | 49 ± 1 a | 46 ± 2 a | 49 ± 1 a | 47 ± 2 a | 47 ± 3 a |
| Emulsion stability (%) | 48 ± 2 a | 49 ± 5 a | 47 ± 1 a | 43 ± 3 a | 36 ± 2 a | 37 ± 4 a | 37 ± 2 a | 37 ± 3 a |
| Emulsifying capacity (g of oil/100 g of dry proteins) | 33 ± 3 a | 32 ± 2 a | 34 ± 2 a | 33 ± 2 a | 34 ± 1 a | 38 ± 4 a | 35 ± 2 a | 37 ± 2 a |
| Gel firmness (G) | - | - | - | - | 22 ± 5 a | 26 ± 4 a | 22 ± 1 a | 24 ± 1 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deschênes Gagnon, R.; Bazinet, L.; Mikhaylin, S. Functional Properties of Casein and Caseinate Produced by Electrodialysis with Bipolar Membrane Coupled to an Ultrafiltration Module. Membranes 2022, 12, 270. https://doi.org/10.3390/membranes12030270
Deschênes Gagnon R, Bazinet L, Mikhaylin S. Functional Properties of Casein and Caseinate Produced by Electrodialysis with Bipolar Membrane Coupled to an Ultrafiltration Module. Membranes. 2022; 12(3):270. https://doi.org/10.3390/membranes12030270
Chicago/Turabian StyleDeschênes Gagnon, Rosie, Laurent Bazinet, and Sergey Mikhaylin. 2022. "Functional Properties of Casein and Caseinate Produced by Electrodialysis with Bipolar Membrane Coupled to an Ultrafiltration Module" Membranes 12, no. 3: 270. https://doi.org/10.3390/membranes12030270
APA StyleDeschênes Gagnon, R., Bazinet, L., & Mikhaylin, S. (2022). Functional Properties of Casein and Caseinate Produced by Electrodialysis with Bipolar Membrane Coupled to an Ultrafiltration Module. Membranes, 12(3), 270. https://doi.org/10.3390/membranes12030270







