Photoinduced Oxidation of Lipid Membranes in the Presence of the Nonsteroidal Anti-Inflammatory Drug Ketoprofen
Abstract
:1. Introduction
2. Materials and Methods
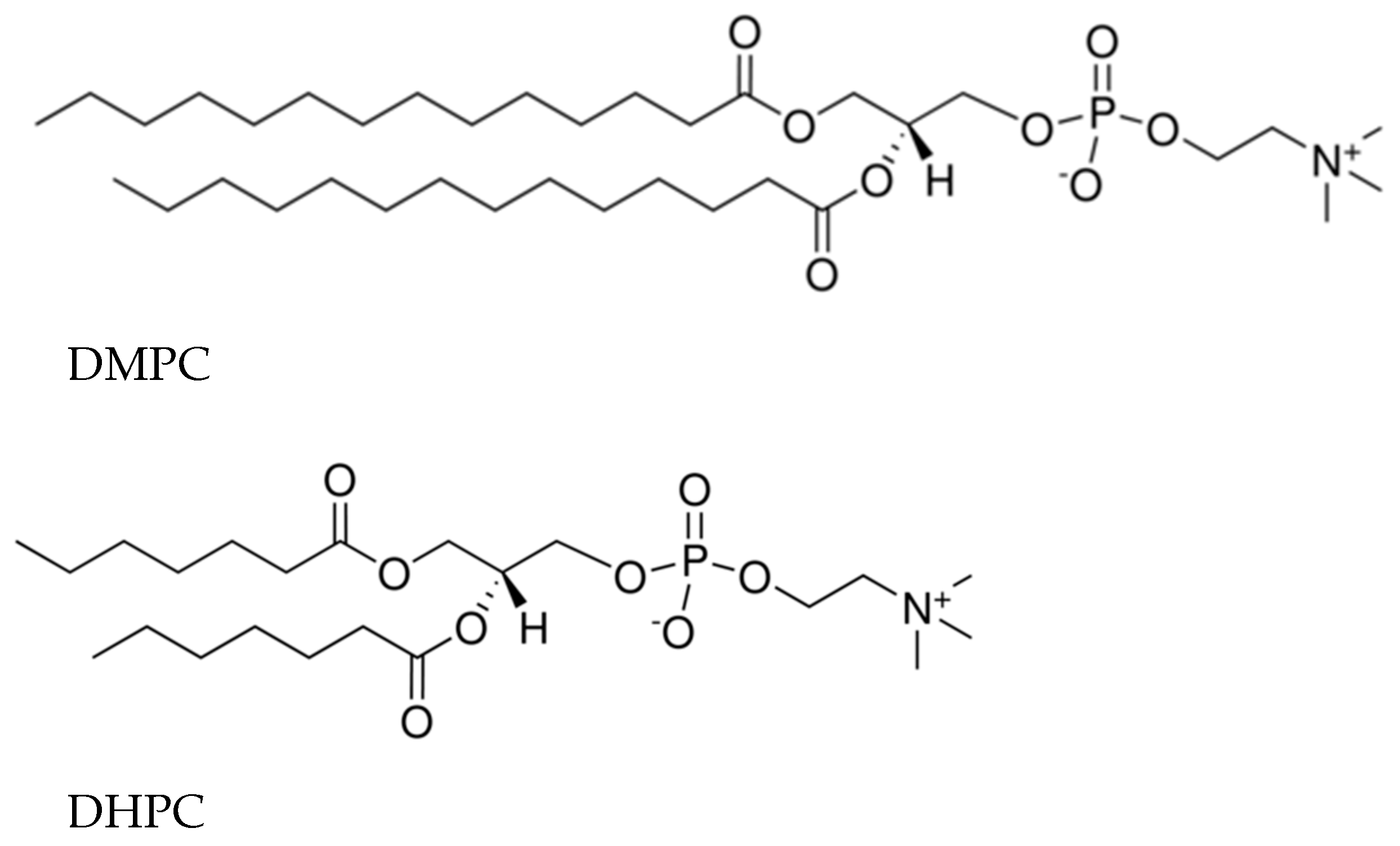
2.1. Materials
2.2. NMR and CIDNP Study
2.3. Molecular Dynamics Simulations
3. Results
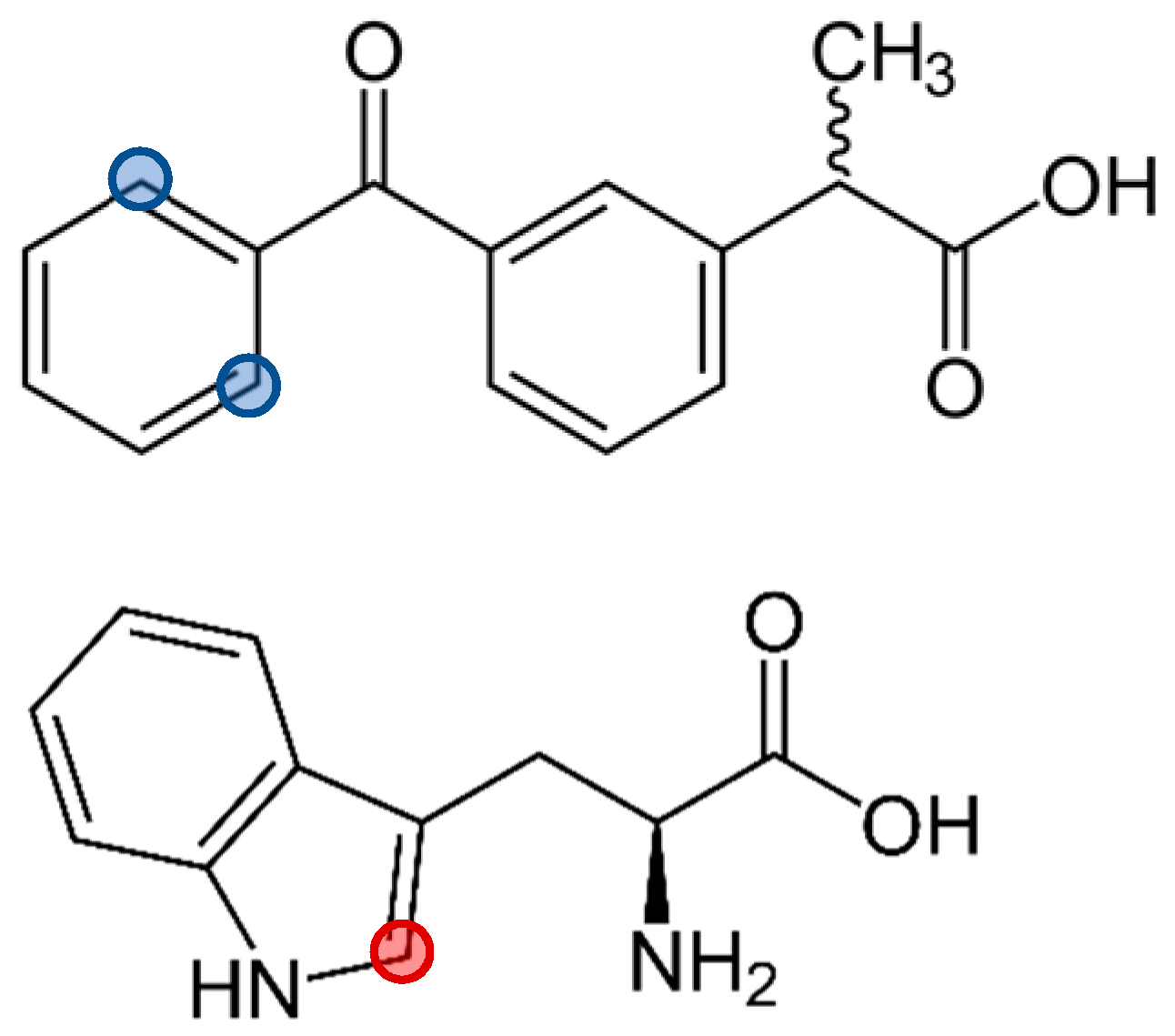
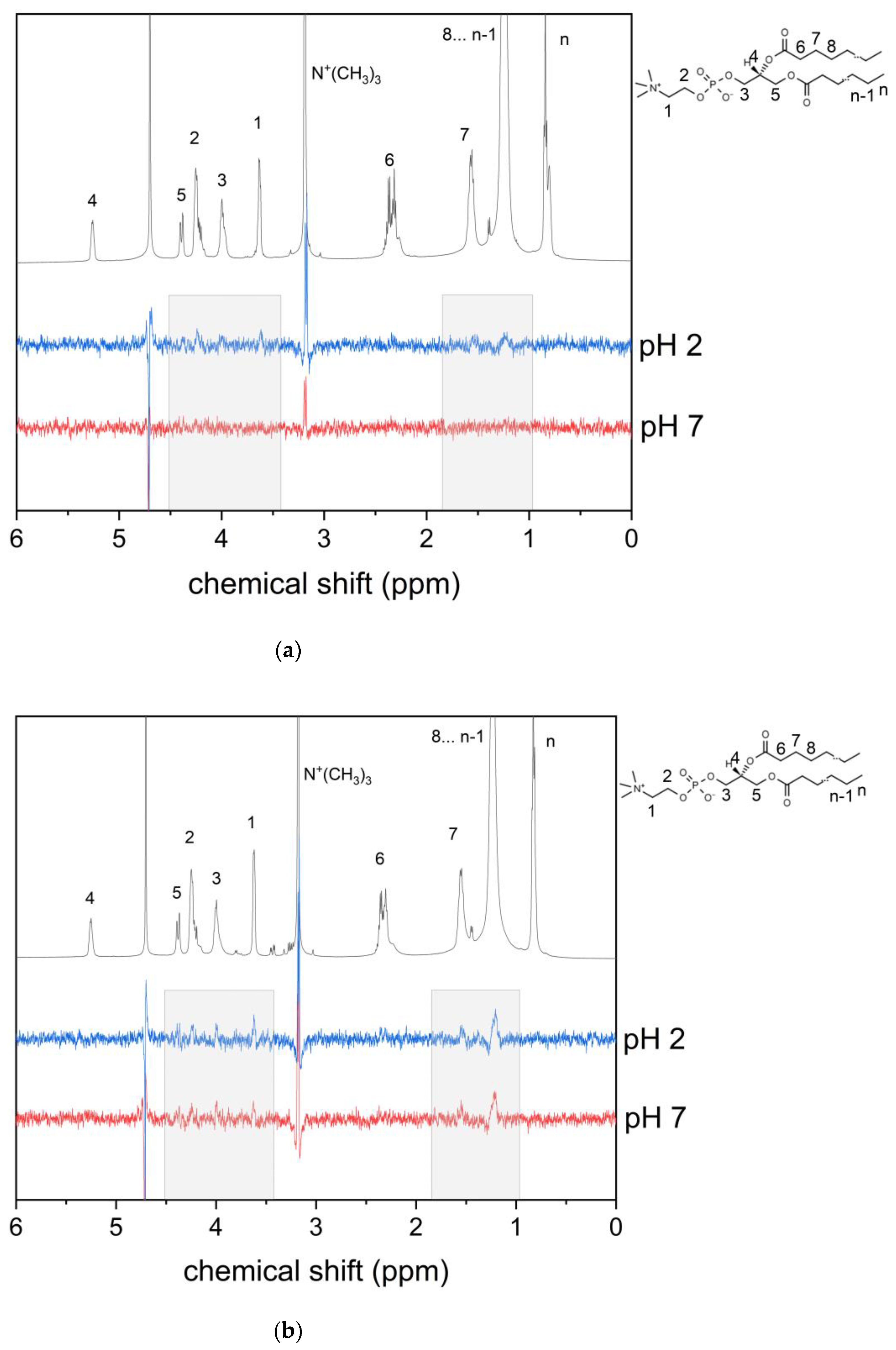
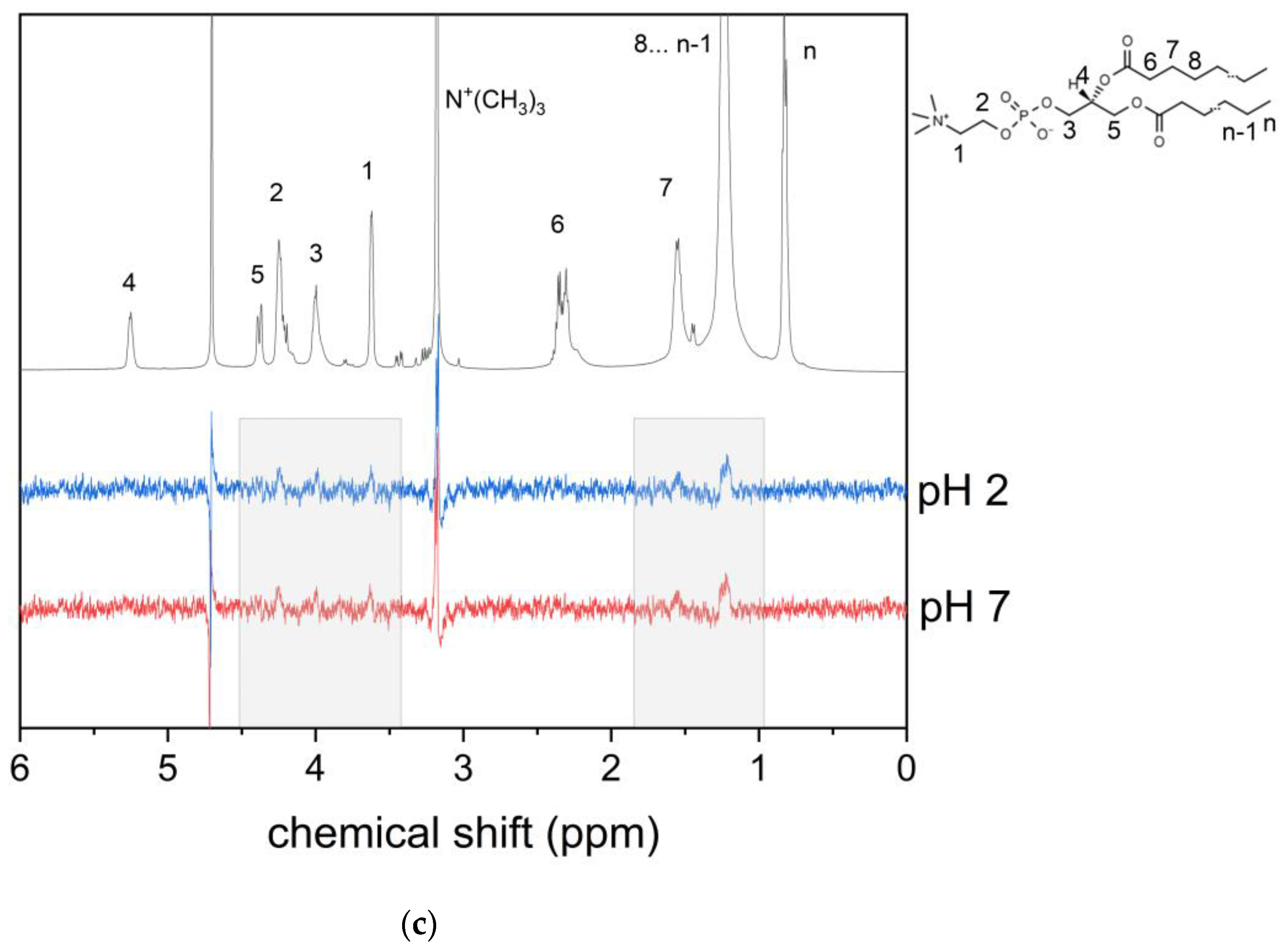
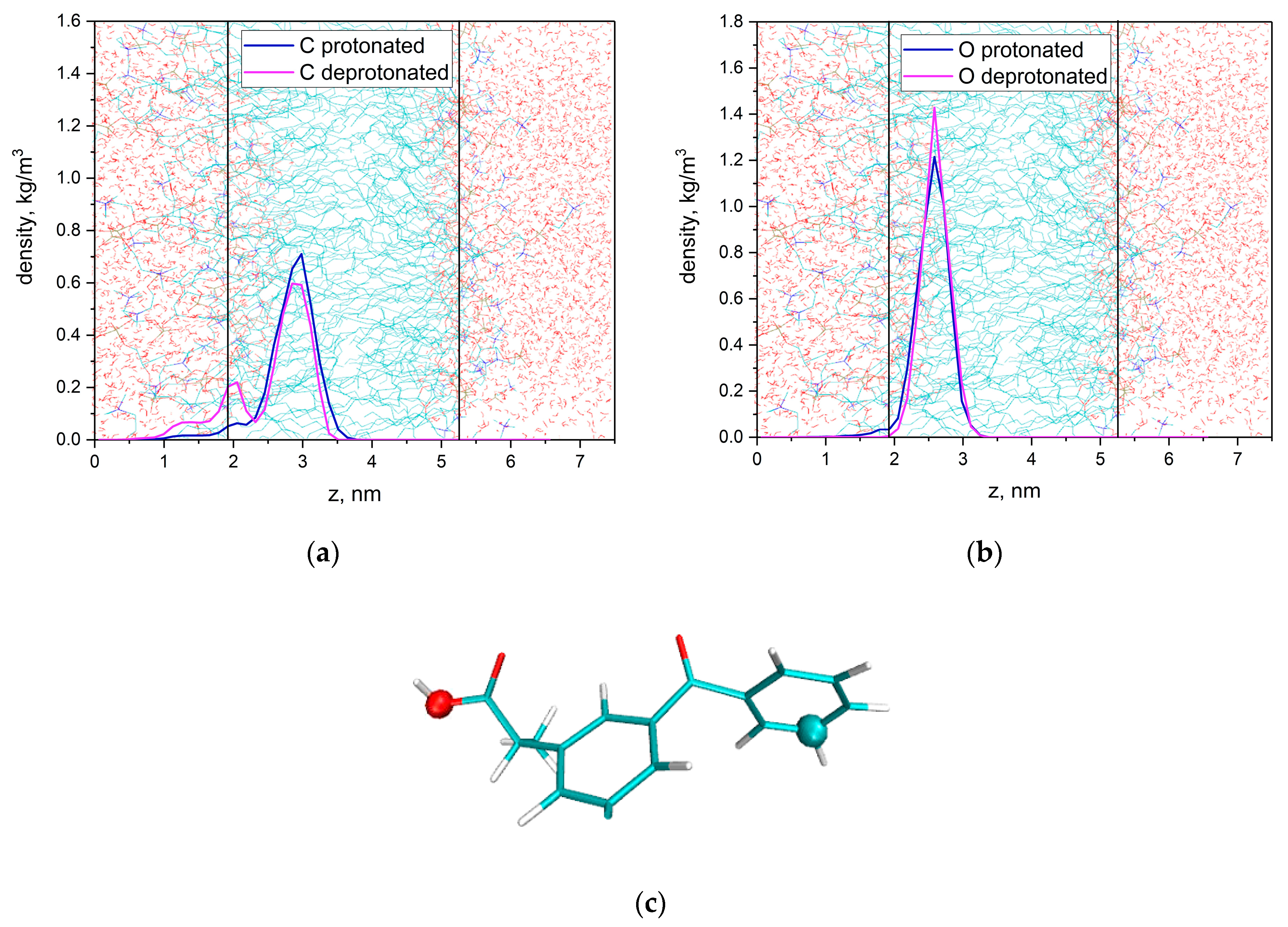
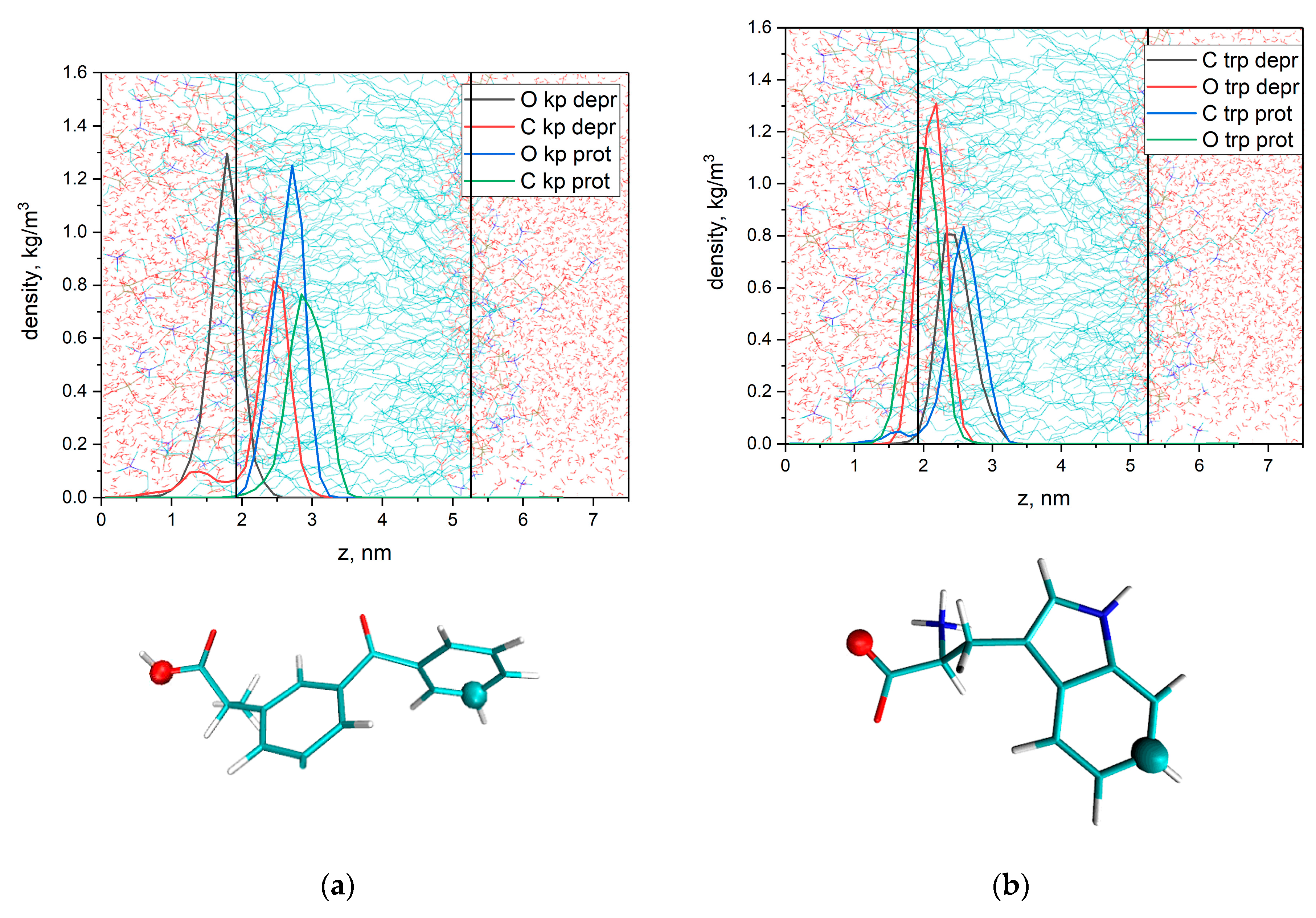
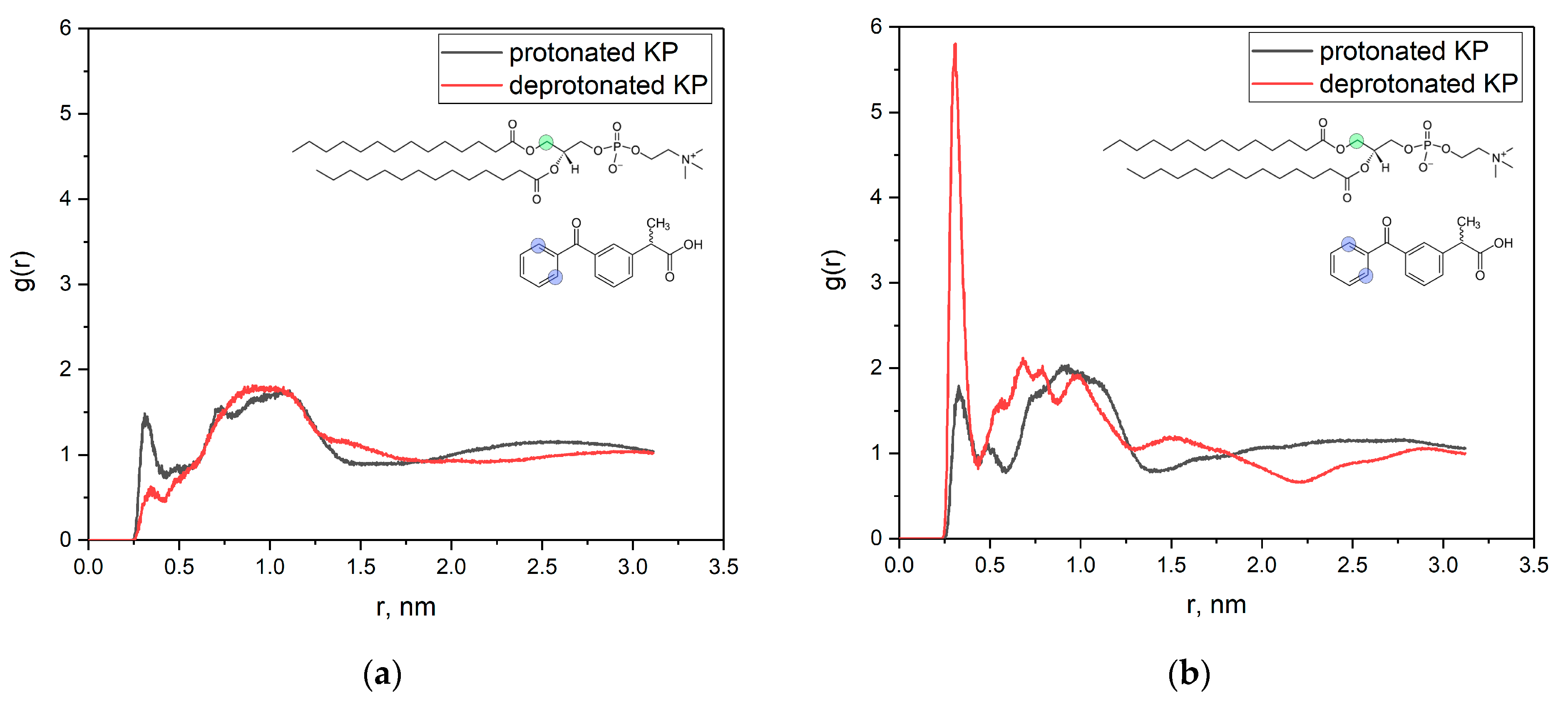
3.1. KP Interaction with Lipid Bilayer Studied by NMR Technique and Molecular Dynamic Simulation
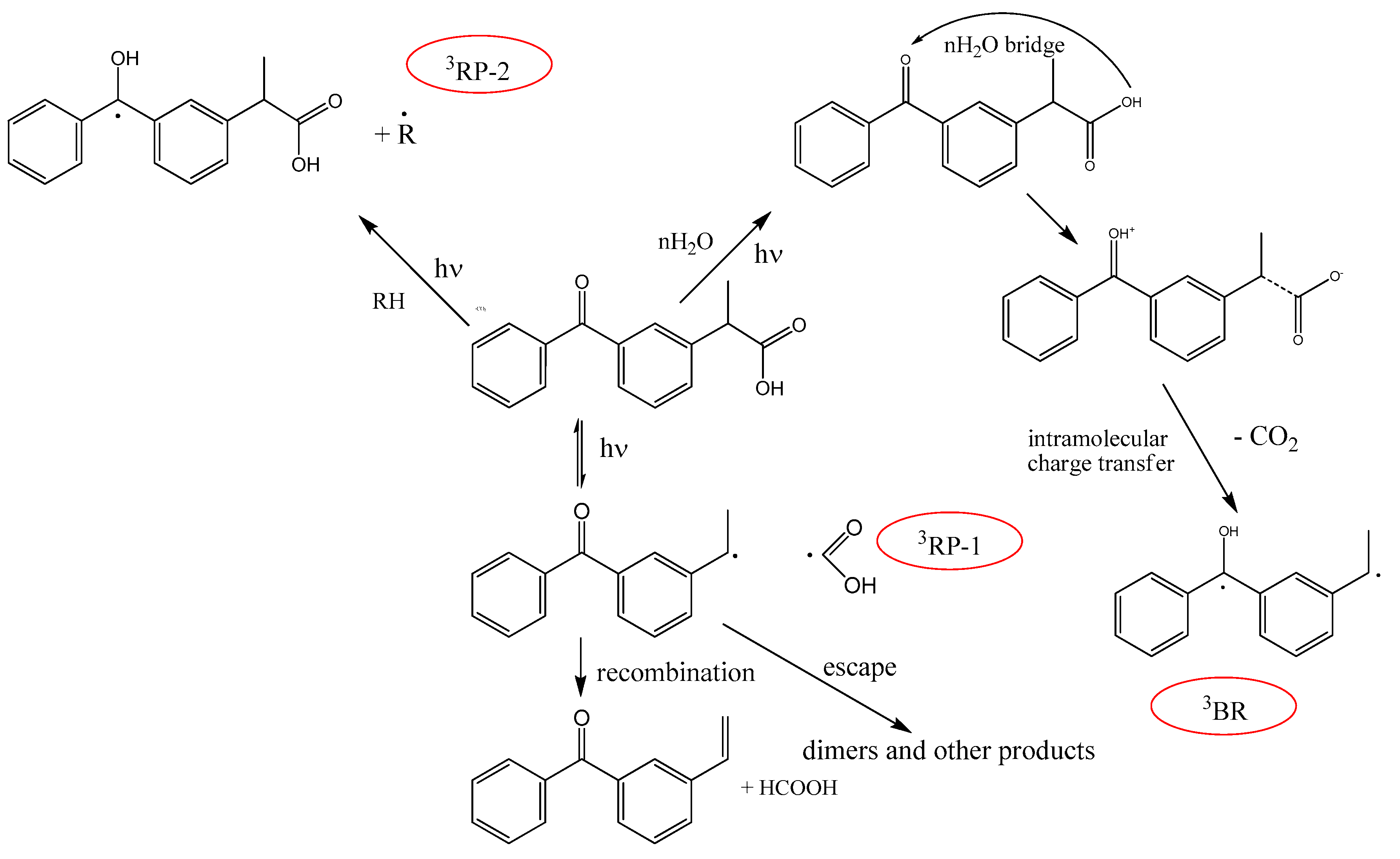
3.2. KP Photolysis in Phospholipid Bicelles
3.3. KP Photolysis in Phospholipid Bicelles in the Presence of Electron Donor Tryptophan
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CIDNP | chemically induced dynamic nuclear polarization |
| DHPC | 1,2-diheptanoyl-sn-glycero-3-phosphocholine |
| DMPC | 1,2-dimyristoyl-sn-glycero-3-phosphocholine |
| KP | ketoprofen |
| MD | molecular dynamics |
| NMR | nuclear magnetic resonance |
| NOESY | Nuclear Overhauser effect spectroscopy |
| PBS | phosphate buffered saline |
| PME | particle mesh Evald |
| SPC | single point charge |
| Trp | tryptophan |
| CIDNP | chemically induced dynamic nuclear polarization |
References
- Kelemen, H.I.; Hancu, G.; Kacsó, E.I.; Papp, L.-A. Photosensitivity Reactions Induced by Photochemical Degradation of Drugs. Adv. Pharm. Bull. 2021, 12, 77–85. [Google Scholar] [CrossRef]
- Kim, W.B.; Shelley, A.J.; Novice, K.; Joo, J.; Lim, H.W.; Glassman, S.J. Drug-induced phototoxicity: A systematic review. J. Am. Acad. Dermatol. 2018, 79, 1069–1075. [Google Scholar] [CrossRef] [PubMed]
- Lugović-Mihić, L.; Duvančić, T.; Fercek, I.; Vuković, P.; Japundžić, I.; Ćesić, D. Drug-Induced Photosensitivity—A Continuing Diagnostic Challenge. Acta Clin. Croat. 2017, 56, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, H.; Lhiaubet, V.; Montastruc, J.L.; Chouini-Lalanne, N. Photosensitivity to ketoprofen: Mechanisms and pharmacoepidemiological data. Drug Saf. 2000, 22, 339–349. [Google Scholar] [CrossRef]
- Liu, S.; Mizu, H.; Yamauchi, H. Molecular response to phototoxic stress of UVB-irradiated ketoprofen through arresting cell cycle in G2/M phase and inducing apoptosis. Biochem. Biophys. Res. Commun. 2007, 364, 650–655. [Google Scholar] [CrossRef]
- Boscá, F.; Miranda, M.A. New Trends in Photobiology (Invited Review) Photosensitizing drugs containing the benzophenone chromophore. J. Photochem. Photobiol. B Biol. 1998, 43, 1–26. [Google Scholar] [CrossRef]
- Costanzo, L.L.; De Guidi, G.; Condorelli, G.; Cambria, A.; Fama, M. Molecular mechanism of drug photosensitization—II. photohemolysis sensitized by ketoprofen. Photochem. Photobiol. 1989, 50, 359–365. [Google Scholar] [CrossRef]
- Ljunggren, B. Propionic acid-derived non-steroidal antiinflammatory drugs are phototoxic in vitro. Photodermatology 1985, 2, 3–9. [Google Scholar]
- Nihira, T.; Hagiwara, Y. Ketoprofen-induced photoallergic dermatitis. Pediatr. Int. 2019, 61, 610–611. [Google Scholar] [CrossRef]
- Loh, T.Y.; Cohen, P.R. Ketoprofen-induced photoallergic dermatitis. Indian J. Med. Res. 2016, 144, 803. [Google Scholar] [CrossRef]
- Borsarelli, C.D.; Braslavsky, S.E.; Sortino, S.; Marconi, G.; Monti, S. Photodecarboxylation of Ketoprofen in Aqueous Solution. A Time-resolved Laser-induced Optoacoustic Study. Photochem. Photobiol. 2000, 72, 163–171. [Google Scholar] [CrossRef]
- Monti, S.; Sortino, S.; De Guidi, G.; Marconi, G. Photochemistry of 2-(3-benzoylphenyl)propionic acid(ketoprofen) Part 1A picosecond and nanosecond time resolved study inaqueous solution. J. Chem. Soc. Faraday Trans. 1997, 93, 2269–2275. [Google Scholar] [CrossRef]
- Boscá, F.; Miranda, M.A.; Carganico, G.; Mauleon, D. Photochemical and photobiological properties of ketoprofen associated with the benzophenone chromophore. Photochem. Photobiol. 1994, 60, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Musa, K.A.K.; Matxain, J.M.; Eriksson, L.A. Mechanism of Photoinduced Decomposition of Ketoprofen. J. Med. Chem. 2007, 50, 1735–1743. [Google Scholar] [CrossRef]
- Okazaki, S.; Hirata, A.; Shogomori, Y.; Takemoto, M.; Nagata, T.; Hayashida, E.; Takeshita, K. Radical reactions induced by ketoprofen in phospholipid membranes under ultraviolet light irradiation. J. Photochem. Photobiol. B Biol. 2021, 214, 112090. [Google Scholar] [CrossRef]
- Babenko, S.V.; Kuznetsova, P.S.; Polyakov, N.E.; Kruppa, A.I.; Leshina, T.V. New insights into the nature of short-lived paramagnetic intermediates of ketoprofen. Photo-CIDNP study. J. Photochem. Photobiol. A Chem. 2020, 392, 112383. [Google Scholar] [CrossRef]
- Li, M.-D.; Yeung, C.S.; Guan, X.; Ma, J.; Li, W.; Ma, C.; Phillips, D.L. Water- and acid-mediated excited-state intramolecular proton transfer and decarboxylation reactions of ketoprofen in water-rich and acidic aqueous solutions. Chemistry 2011, 17, 10935–10950. [Google Scholar] [CrossRef]
- Suzuki, T.; Osanai, Y.; Isozaki, T. Effect of basic amino acids on photoreaction of ketoprofen in phosphate buffer solution. Photochem. Photobiol. 2012, 88, 884–888. [Google Scholar] [CrossRef]
- Suzuki, T.; Shinoda, M.; Osanai, Y.; Isozaki, T. Photochemical reaction of 2-(3-benzoylphenyl)propionic acid (ketoprofen) with basic amino acids and dipeptides. J. Phys. Chem. B 2013, 117, 9662–9668. [Google Scholar] [CrossRef]
- Shinoda, M.; Isozaki, T.; Suzuki, T. Photoreaction of Ketoprofen with Tryptophan and Tyrosine in Phosphate Buffer Solution. Photochem. Photobiol. 2014, 90, 92–98. [Google Scholar] [CrossRef]
- Kashihara, W.; Inoue, M.; Tanabe, S.; Miyata, S.; Sakai, K.; Isozaki, T.; Suzuki, T. Hydrogen Abstraction of Ketoprofen in the Excited Triplet State with Indole and Methylindoles. J. Phys. Chem. B 2019, 123, 9388–9394. [Google Scholar] [CrossRef]
- Boscá, F.; Carganico, G.; Castell, J.V.; Gómez-Lechón, M.J.; Hernandez, D.; Mauleón, D.; Martínez, L.A.; Miranda, M.A. Evaluation of ketoprofen (R, S and RS) phototoxicity by a battery of in vitro assays. J. Photochem. Photobiol. B Biol. 1995, 31, 133–138. [Google Scholar] [CrossRef]
- Stroet, M.; Caron, B.; Visscher, K.M.; Geerke, D.P.; Malde, A.K.; Mark, A.E. Automated Topology Builder Version 3.0: Prediction of Solvation Free Enthalpies in Water and Hexane. J. Chem. Theory Comput. 2018, 14, 5834–5845. [Google Scholar] [CrossRef] [PubMed]
- Poger, D.; Mark, A.E. On the validation of molecular dynamics simulations of saturated and cis-monounsaturated phosphatidylcholine lipid bilayers: A comparison with experiment. J. Chem. Theory Comput. 2010, 6, 325–336. [Google Scholar] [CrossRef] [PubMed]
- Parrinello, M.; Rahman, A. Polymorphic transitions in single crystals: A new molecular dynamics method. J. Appl. Phys. 1981, 52, 7182–7190. [Google Scholar] [CrossRef]
- Hoover, W.G. Canonical dynamics: Equilibrium phase-space distributions. Phys. Rev. A 1985, 31, 1695–1697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Essmann, U.; Perera, L.; Berkowitz, M.L.; Darden, T.; Lee, H.; Pedersen, L.G. A smooth particle mesh Ewald method. J. Chem. Phys. 1995, 103, 8577–8593. [Google Scholar] [CrossRef] [Green Version]
- Bocian, D.F.; Chan, S.I. NMR Studies of Membrane Structure and Dynamics. Annu. Rev. Phys. Chem. 2003, 29, 307–335. [Google Scholar] [CrossRef]
- Deese, A.J.; Dratz, E.A.; Hymel, L.; Fleischer, S. Proton NMR T1, T2, and T1 rho relaxation studies of native and reconstituted sarcoplasmic reticulum and phospholipid vesicles. Biophys. J. 1982, 37, 207–216. [Google Scholar] [CrossRef] [Green Version]
- Gabrielska, J.; Gagoś, M.; Gubernator, J.; Gruszecki, W.I. Binding of antibiotic amphotericin B to lipid membranes: A 1H NMR study. FEBS Lett. 2006, 580, 2677–2685. [Google Scholar] [CrossRef] [Green Version]
- Ellena, J.F.; Lepore, L.S.; Cafiso, D.S. Estimating lipid lateral diffusion in phospholipid vesicles from carbon-13 spin-spin relaxation. J. Phys. Chem. 1993, 97, 2952–2957. [Google Scholar] [CrossRef]
- Selyutina, O.Y.; Shelepova, E.A.; Paramonova, E.D.; Kichigina, L.A.; Khalikov, S.S.; Polyakov, N.E. Glycyrrhizin-induced changes in phospholipid dynamics studied by 1H NMR and MD simulation. Arch. Biochem. Biophys. 2020, 686, 108368. [Google Scholar] [CrossRef] [PubMed]
- Selyutina, O.Y.; Apanasenko, I.E.; Kim, A.V.; Shelepova, E.A.; Khalikov, S.S.; Polyakov, N.E. Spectroscopic and molecular dynamics characterization of glycyrrhizin membrane-modifying activity. Colloids Surf. B Biointerfaces 2016, 147, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Goez, M. Chapter 3 Photo-CIDNP Spectroscopy. Annu. Rep. NMR Spectrosc. 2009, 66, 77–147. [Google Scholar] [CrossRef]
- Morozova, O.B.; Ivanov, K.L.; Kiryutin, A.S.; Sagdeev, R.Z.; Köchling, T.; Vieth, H.M.; Yurkovskaya, A.V. Time-resolved CIDNP: An NMR way to determine the EPR parameters of elusive radicals. Phys. Chem. Chem. Phys. 2011, 13, 6619–6627. [Google Scholar] [CrossRef]
- Li, M.-D.; Du, Y.; Chuang, Y.P.; Xue, J.; Phillips, D.L. Water concentration dependent photochemistry of ketoprofen in aqueous solutions. Phys. Chem. Chem. Phys. 2010, 12, 4800–4808. [Google Scholar] [CrossRef] [PubMed]
- Ageeva, A.A.; Babenko, S.V.; Magin, I.M.; Plyusnin, V.F.; Kuznetsova, P.S.; Stepanov, A.A.; Vasilevsky, S.F.; Polyakov, N.E.; Doktorov, A.B.; Leshina, T.V. Stereoselectivity of Electron and Energy Transfer in the Quenching of (S/R)-Ketoprofen-(S)-Tryptophan Dyad Excited State. Int. J. Mol. Sci. 2020, 21, 5370. [Google Scholar] [CrossRef]
- Weller, A. Photoinduced electron transfer in solution: Exciplex and radical ion pair formation free enthalpies and their solvent dependence. Z. Phys. Chem. 1982, 133, 93–98. [Google Scholar] [CrossRef]
- Wang, X.Y.; Yang, D.; Gan, L.J.; Zhang, H.; Shin, J.A.; Lee, Y.H.; Jang, Y.S.; Lee, K.T. Effect of Positional Distribution of Linoleic Acid on Oxidative Stability of Triacylglycerol Molecules Determined by 1H NMR. J. Am. Oil Chem. Soc. 2015, 92, 157–165. [Google Scholar] [CrossRef]


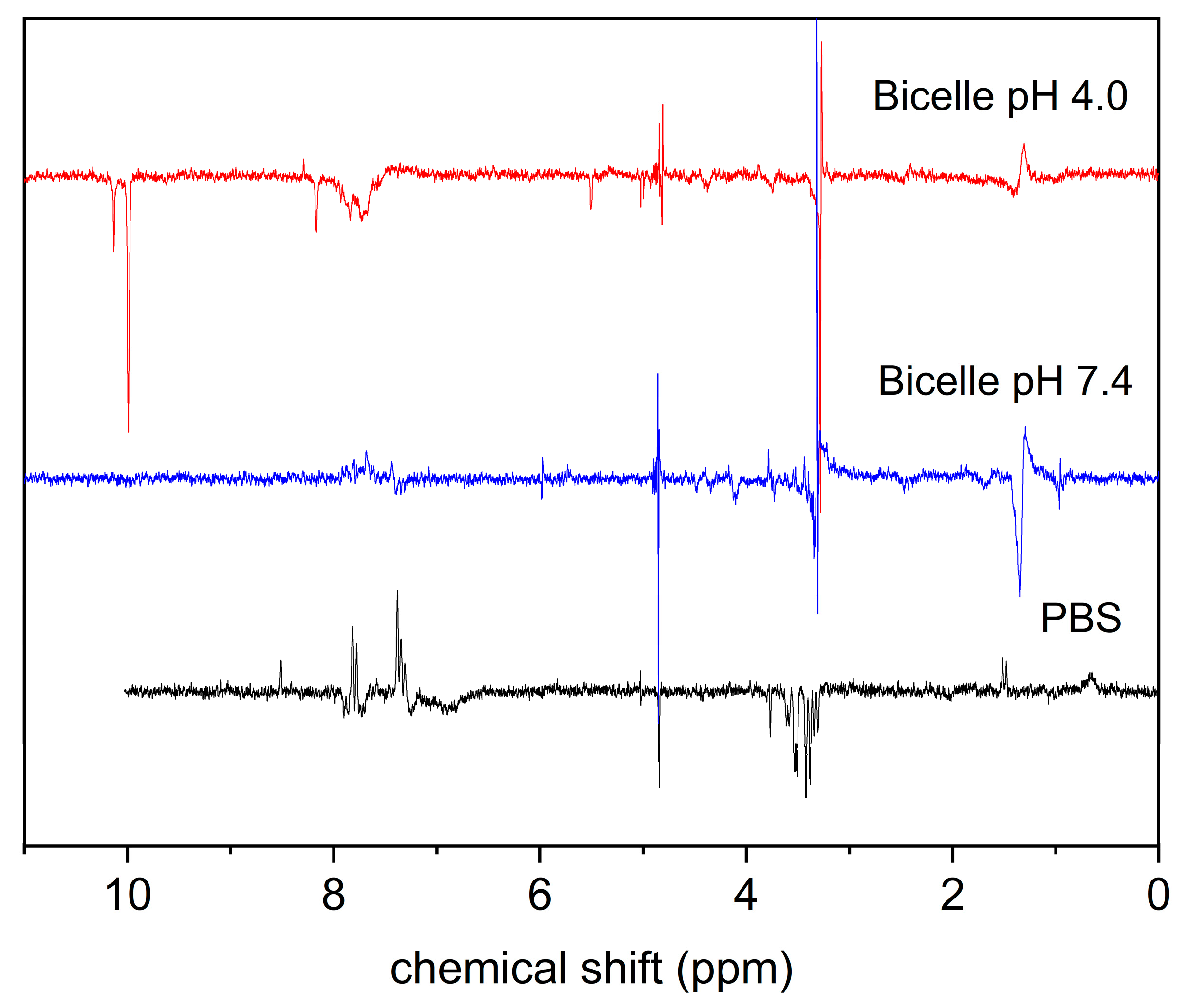
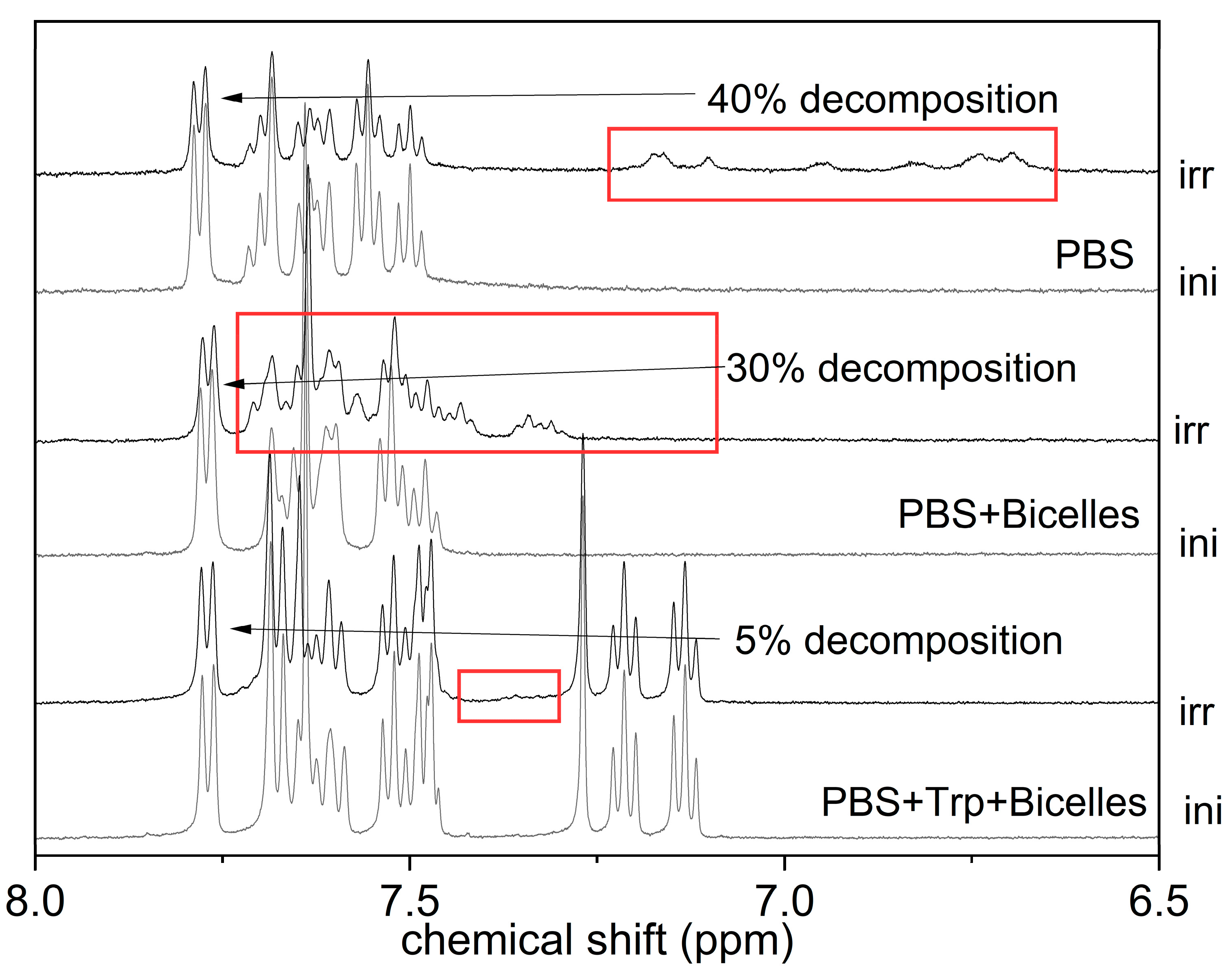
| Water, pH = 7 | Bicelles, pH = 7 | Bicelles, pH = 3.5 | |
|---|---|---|---|
| KP (7.8 ppm) without Trp | 2.5 ± 0.2 s | 1.2 ± 0.1 s | 1.1 ± 0.1 s |
| KP (7.8 ppm) with Trp | 3.1 ± 0.1 s | 3.0 ± 0.1 s | 1.0 ± 0.2 s |
| Trp (7.2 ppm) | 2.4 ± 0.2 s | 2.6 ± 0.1 s | 1.9 ± 0.2 s |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mastova, A.V.; Selyutina, O.Y.; Evseenko, V.I.; Polyakov, N.E. Photoinduced Oxidation of Lipid Membranes in the Presence of the Nonsteroidal Anti-Inflammatory Drug Ketoprofen. Membranes 2022, 12, 251. https://doi.org/10.3390/membranes12030251
Mastova AV, Selyutina OY, Evseenko VI, Polyakov NE. Photoinduced Oxidation of Lipid Membranes in the Presence of the Nonsteroidal Anti-Inflammatory Drug Ketoprofen. Membranes. 2022; 12(3):251. https://doi.org/10.3390/membranes12030251
Chicago/Turabian StyleMastova, Anna V., Olga Yu. Selyutina, Veronika I. Evseenko, and Nikolay E. Polyakov. 2022. "Photoinduced Oxidation of Lipid Membranes in the Presence of the Nonsteroidal Anti-Inflammatory Drug Ketoprofen" Membranes 12, no. 3: 251. https://doi.org/10.3390/membranes12030251
APA StyleMastova, A. V., Selyutina, O. Y., Evseenko, V. I., & Polyakov, N. E. (2022). Photoinduced Oxidation of Lipid Membranes in the Presence of the Nonsteroidal Anti-Inflammatory Drug Ketoprofen. Membranes, 12(3), 251. https://doi.org/10.3390/membranes12030251








