Research on Energy and Economics of Self-Made Catalyst-Coated Membrane for Fuel Cell under Different Oxidants
Abstract
:1. Introduction
2. Materials and Methods
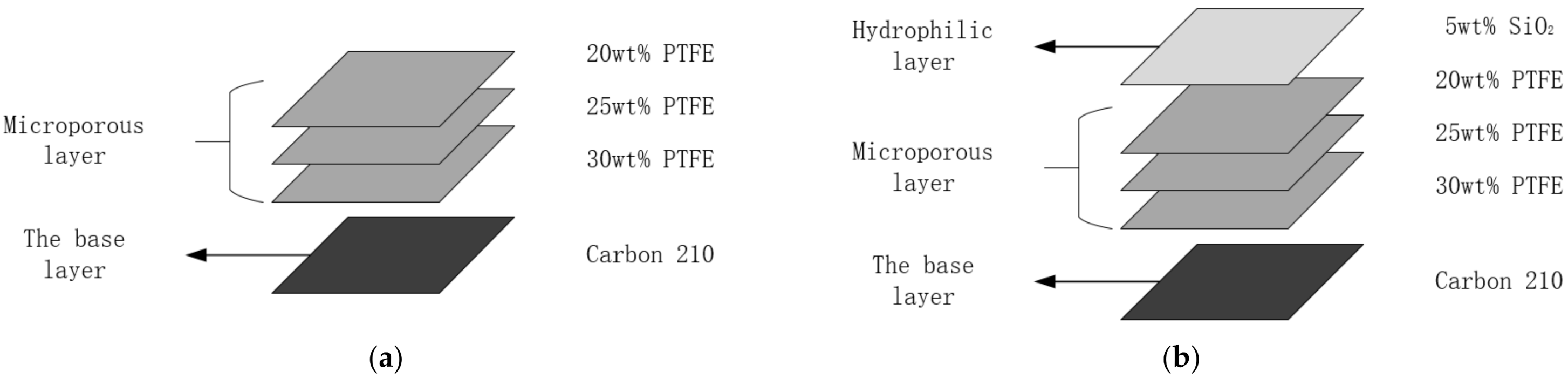
2.1. Material
- (1)
- Mix the catalyst with 5 wt% Nafion solution, then add a mixed solution of isopropanol solvent and deionized water, wherein the content ratio of catalyst to Nafion is 2:1, and place it into the ultrasonic oscillator for two hours to fully mix the solvent;
- (2)
- Clean the pipeline of the automatic spraying machine with isopropanol, open the air pressure bottle, and set the temperature of the heating plate to 80 °C;
- (3)
- After reaching the specified temperature, place the solution in (1) in the pipeline of the automatic spraying machine and conduct positioning test to determine whether the solution spraying can be carried out normally;
- (4)
- Set spraying parameters and conduct path test to determine whether the spraying position is correct. After confirmation, membrane spraying can be carried out;
- (5)
- Place the sprayed membrane in the oven and bake it at 60 °C for 8 h, then use shell protection paper for hot pressing protection (1000 psi-130 °C-3 min), and finally complete the production of the CCM.
- (1)
- Use a high-precision balance to measure the quality of carbon paper;
- (2)
- Mix carbon powder with isopropanol and place it into the ultrasonic oscillator for two hours to fully mix them;
- (3)
- In order to improve the hydrophobicity of the cathode, during the shaking process, PTFE (Teflon) is slowly added and the process is to use the mixed solution of carbon powder and PTFE as the hydrophobic layer material;
- (4)
- In order to improve the hydrophilicity of anode end, during the shaking process, SiO2 powder is slowly added and the process is to use the mixed solution of carbon powder and SiO2 as the hydrophilic layer material;
- (5)
- Spraying the solution prepared in (3) and (4) onto the surface of carbon paper with a spraying machine;
- (6)
- The sprayed GDL is placed in a high-temperature furnace for sintering, and the temperature is set as: (120 °C, 30 min), (280 °C, 30 min), (390 °C, 30 min).
- (1)
- Wipe the bipolar plate and graphite plate with industrial alcohol and check the surface flatness;
- (2)
- Stack the end plate with the red copper gold-plated collector plate, put the bolts into the fixing holes in sequence, and then sleeve the Teflon tube on the outer surface of the bolt, so as to fix the bolt position and prevent the anode and cathode of the PEMFC from conducting with each other;
- (3)
- Place the runner graphite plate on the bipolar plate, and then sleeve the MEA into the bolt;
- (4)
- Install the other side of the PEMFC according to the sequence in (1–3);
- (5)
- Tighten the PEMFC with a torque wrench in a diagonal locking manner. In order to avoid deformation of the MEA caused by stress concentration in the PEMFC, apply a force of 5 kg each time and gradually increase the locking force to 25 kg.
2.2. Method
- (1)
- Install the PEMFC on testing platform and complete the parameter setting;
- (2)
- Supply oxidant and reductant and maintain the open circuit voltage for 10 min. The next step can be carried out after the state is stable;
- (3)
- Set the working voltage of single PEMFC to 0.6 V and maintain for 30 min, then adjust the voltage to 0.2 V and maintain 30 min, cycle 12 times, a total of 12 h;
- (4)
- Observe whether the polarization curve of the PEMFC in (3) is stable and whether the performance meets the standard, and then the next test can be carried out.
2.3. The Stoichiometry of Oxidant and Reductant
3. Results
3.1. Working Principle of PEMFC
Cathode: 1/2O2 + 2H+ +2e− → H2O
Overall: H2 + 1/2O2 → H2O + heat
3.2. Differences in PEMFC Performance at Different Temperatures—Air
3.3. Differences in PEMFC Performance at Different Temperatures—Oxygen
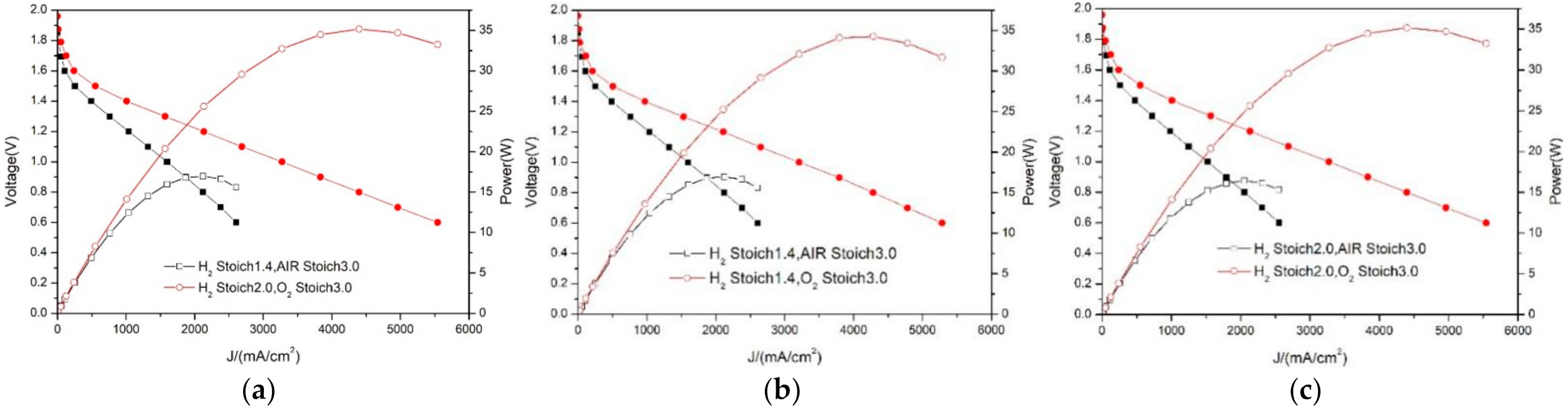
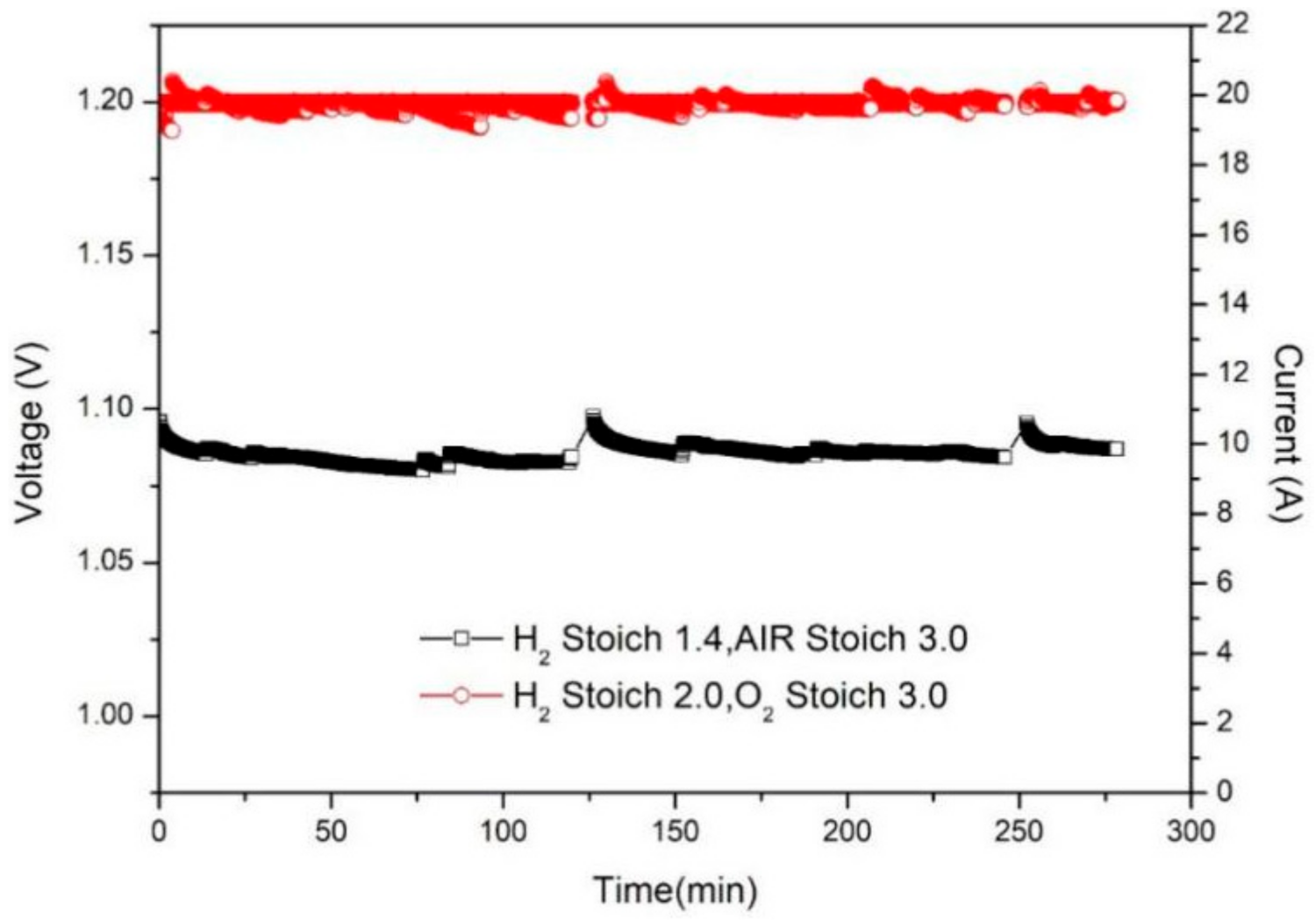
3.4. Comparison of Oxygen and Air Performance
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lu, W.; Li, X.; Zhang, H. The next generation vanadium flow batteries with high power density—A perspective. Phys. Chem. Chem. Phys. 2017, 20, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Sun, C. Cost-effective iron-based aqueous redox flow batteries for large-scale energy storage application: A review. J. Power Sources 2021, 493, 229445. [Google Scholar] [CrossRef]
- El-Eskandarany, M.; Ali, N.; Banyan, M.; Al-Ajmi, F. Cold Gas-Dynamic Spray for Catalyzation of Plastically Deformed Mg-Strips with Ni Powder. Nanomaterials 2021, 11, 1169. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhu, C.; Zhu, D.; Jia, X.; Zhang, Y.; Yu, J.; Li, X.; Yang, M. High Performance Low-Temperature Solid Oxide Fuel Cells Based on Nanostructured Ceria-Based Electrolyte. Nanomaterials 2021, 11, 2231. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Mi, Y.; Li, J.; Qi, F.; Yan, S.; Dong, W. Recent Progress in Semiconductor-Ionic Conductor Nanomaterial as a Membrane for Low-Temperature Solid Oxide Fuel Cells. Nanomaterials 2021, 11, 2290. [Google Scholar] [CrossRef]
- Zhu, S.; Ge, J.; Liu, C.; Xing, W. Atomic-level dispersed catalysts for PEMFCs: Progress and future prospects. EnergyChem 2019, 1, 100018. [Google Scholar] [CrossRef]
- Wang, M.; Chen, M.; Yang, Z.; Liu, G.; Lee, J.K.; Yang, W.; Wang, X. High-performance and durable cathode catalyst layer with hydrophobic C@PTFE particles for low-Pt loading membrane assembly electrode of PEMFC. Energy Convers. Manag. 2019, 191, 132–140. [Google Scholar] [CrossRef]
- Okonkwo, P.C.; Ige, O.O.; Barhoumi, E.M.; Uzoma, P.C.; Emori, W.; Benamor, A.; Abdullah, A.M. Platinum degradation mechanisms in proton exchange membrane fuel cell (PEMFC) system: A review. Int. J. Hydrog. Energy 2021, 46, 15850–15865. [Google Scholar] [CrossRef]
- Roshanravan, B.; Younesi, H.; Abdollahi, M.; Rahimnejad, M.; Pyo, S.-H. Application of proton-conducting sulfonated polysulfone incorporated MIL-100(Fe) composite materials for polymer-electrolyte membrane microbial fuel cells. J. Clean. Prod. 2021, 300, 126963. [Google Scholar] [CrossRef]
- Foglia, F.; Sakai, V.G.; Lyonnard, S.; McMillan, P.F. Decoupling polymer, water and ion transport dynamics in ion-selective membranes for fuel cell applications. J. Non-Cryst. Solids X 2022, 13, 100073. [Google Scholar] [CrossRef]
- Wang, Z.; Ren, J.; Sun, Y.; Wang, L.; Fan, Y.; Zheng, J.; Qian, H.; Li, S.; Xu, J.; Zhang, S. Fluorinated strategy of node structure of Zr-based MOF for construction of high-performance composite polymer electrolyte membranes. J. Membr. Sci. 2022, 645, 120193. [Google Scholar] [CrossRef]
- Lim, I.S.; Kang, B.; Park, J.Y.; Kim, M.S. Performance improvement of polymer electrolyte membrane fuel cell by gas diffusion layer with atomic-layer-deposited HfO2 on microporous layer. Energy Convers. Manag. 2021, 236, 114070. [Google Scholar] [CrossRef]
- Rajabi, Z.; Javanbakht, M.; Hooshyari, K.; Adibi, M.; Badiei, A. Phosphoric acid doped polybenzimidazole based polymer electrolyte membrane and functionalized SBA-15 mesoporous for elevated temperature fuel cell. Int. J. Hydrog. Energy 2021, 46, 33241–33259. [Google Scholar] [CrossRef]
- Liang, Y.; Zhang, H.; Zhang, J.; Cheng, X.; Zhu, Y.; Luo, L.; Lu, S.; Wei, J.; Wang, H. Porous 2D carbon nanosheets synthesized via organic groups triggered polymer particles exfoliation: An effective cathode catalyst for polymer electrolyte membrane fuel cells. Electrochim. Acta 2020, 332, 135397. [Google Scholar] [CrossRef]
- Kanani, H.; Shams, M.; Hasheminasab, M.; Bozorgnezhad, A. Model development and optimization of operating conditions to maximize PEMFC performance by response surface methodology. Energy Convers. Manag. 2015, 93, 9–22. [Google Scholar] [CrossRef]
- Deng, M.; Zhang, Q.; Huang, Y.; Zhang, X. Integration and optimization for a PEMFC and PSA oxygen production combined system. Energy Convers. Manag. 2021, 236, 114062. [Google Scholar] [CrossRef]
- Yin, L.; Li, Q.; Chen, W.; Wang, T.; Liu, H. Experimental analysis of optimal performance for a 5 kW PEMFC system. Int. J. Hydrog. Energy 2019, 44, 5499–5506. [Google Scholar] [CrossRef]
- Umap, V.M.; Ugwekar, R.P. Performance analysis of gas diffusion electrode with varying platinum loading under different oxidant condition. Renew. Energy 2020, 155, 1339–1346. [Google Scholar] [CrossRef]
- Chaurasia, P.B.L.; Panja, N.; Kendall, K. Performance study of power density in PEMFC for power generation from solar energy. Renew. Energy 2011, 36, 3305–3312. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, H.; Yu, Z.; Li, W.; Tang, J.; Xu, C.; Ding, J.; Wan, Z. Thermodynamic and economic assessment of a PEMFC-based micro-CCHP system integrated with geothermal-assisted methanol reforming. Int. J. Hydrog. Energy 2020, 45, 958–971. [Google Scholar] [CrossRef]
- Calise, F.; Figaj, R.D.; Massarotti, N.; Mauro, A.; Vanoli, L. Polygeneration system based on PEMFC, CPVT and electrolyzer: Dynamic simulation and energetic and economic analysis. Appl. Energy 2017, 192, 530–542. [Google Scholar] [CrossRef]
- Wu, Z.; Zhu, P.; Yao, J.; Tan, P.; Xu, H.; Chen, B.; Yang, F.; Zhang, Z.; Ni, M. Thermo-economic modeling and analysis of an NG-fueled SOFC-WGS-TSA-PEMFC hybrid energy conversion system for stationary electricity power generation. Energy 2020, 192, 116613. [Google Scholar] [CrossRef]
- Chen, M.; Zhao, C.; Sun, F.; Fan, J.; Li, H.; Wang, H. Research progress of catalyst layer and interlayer interface structures in membrane electrode assembly (MEA) for proton exchange membrane fuel cell (PEMFC) system. eTransportation 2020, 5, 100075. [Google Scholar] [CrossRef]
- Huang, B.; Chatillon, Y.; Bonnet, C.; Lapicque, F.; Leclerc, S.; Hinaje, M.; Raël, S. Experimental investigation of pinhole effect on MEA/cell aging in PEMFC. Int. J. Hydrog. Energy 2013, 38, 543–550. [Google Scholar] [CrossRef]
- Lin, R.; Zhong, D.; Lan, S.; Guo, R.; Ma, Y.; Cai, X. Experimental validation for enhancement of PEMFC cold start performance: Based on the optimization of micro porous layer. Appl. Energy 2021, 300, 117306. [Google Scholar] [CrossRef]
- Wang, H.; Lin, R.; Cai, X.; Liu, S.; Zhong, D.; Hao, Z. Transition metal dissolution control in Pt-alloy catalyst layers for low Pt-loaded PEMFCs for improving mass transfer. Int. J. Heat Mass Transf. 2021, 178, 121615. [Google Scholar] [CrossRef]
- Sun, C.; Złotorowicz, A.; Nawn, G.; Negro, E.; Bertasi, F.; Pagot, G.; Vezzù, K.; Pace, G.; Guarnieri, M.; Di Noto, V. [Nafion/(WO3)x] hybrid membranes for vanadium redox flow batteries. Solid State Ionics 2018, 319, 110–116. [Google Scholar] [CrossRef]
- Parnian, M.J.; Rowshanzamir, S.; Prasad, A.K.; Advani, S.G. High durability sulfonated poly (ether etherketone)-ceria nanocomposite membranes for proton exchange membrane fuel cell applications. J. Membr. Sci. 2018, 556, 12–22. [Google Scholar] [CrossRef]
- Shahgaldi, S.; Alaefour, I.; Li, X. Impact of manufacturing processes on proton exchange membrane fuel cell performance. Appl. Energy 2018, 225, 1022–1032. [Google Scholar] [CrossRef]
- Fofana, D.; Natarajan, S.K.; Hamelin, J.; Benard, P. Low platinum, high limiting current density of the PEMFC (proton exchange membrane fuel cell) based on multilayer cathode catalyst approach. Energy 2014, 64, 398–403. [Google Scholar] [CrossRef]
- Chugh, S.; Chaudhari, C.; Sonkar, K.; Sharma, A.; Kapur, G.; Ramakumar, S. Experimental and modelling studies of low temperature PEMFC performance. Int. J. Hydrog. Energy 2020, 45, 8866–8874. [Google Scholar] [CrossRef]
- Kahveci, E.E.; Taymaz, I. Hydrogen PEMFC stack performance analysis through experimental study of operating parameters by using response surface methodology (RSM). Int. J. Hydrog. Energy 2021, in press. [Google Scholar] [CrossRef]
- Xia, S.; Lin, R.; Cui, X.; Shan, J. The application of orthogonal test method in the parameters optimization of PEMFC under steady working condition. Int. J. Hydrog. Energy 2016, 41, 11380–11390. [Google Scholar] [CrossRef]
- Hu, D.; Wang, Y.; Li, J.; Yang, Q.; Wang, J. Investigation of optimal operating temperature for the PEMFC and its tracking control for energy saving in vehicle applications. Energy Convers. Manag. 2021, 249, 114842. [Google Scholar] [CrossRef]
- Ryu, S.K.; Vinothkannan, M.; Kim, A.R.; Yoo, D.J. Effect of type and stoichiometry of fuels on performance of polybenzimidazole-based proton exchange membrane fuel cells operating at the temperature range of 120–160 °C. Energy 2022, 238, 121791. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, Z.; Yang, L. Enhancement effects of the obstacle arrangement and gradient height distribution in serpentine flow-field on the performances of a PEMFC. Energy Convers. Manag. 2022, 252, 115077. [Google Scholar] [CrossRef]
- Chu, T.; Xie, M.; Yu, Y.; Wang, B.; Yang, D.; Li, B.; Ming, P.; Zhang, C. Experimental study of the influence of dynamic load cycle and operating parameters on the durability of PEMFC. Energy 2022, 239, 122356. [Google Scholar] [CrossRef]
- Kim, B.; Cha, D.; Kim, Y. The effects of air stoichiometry and air excess ratio on the transient response of a PEMFC under load change conditions. Appl. Energy 2015, 138, 143–149. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Q.; Xia, L.; Huang, D.; Fu, X.; Zhang, R.; Hu, S.; Zhao, F.; Li, X.; Bao, X. Poly(2,5-benzimidazole)/sulfonated sepiolite composite membranes with low phosphoric acid doping levels for PEMFC applications in a wide temperature range. J. Membr. Sci. 2019, 574, 282–298. [Google Scholar] [CrossRef] [Green Version]



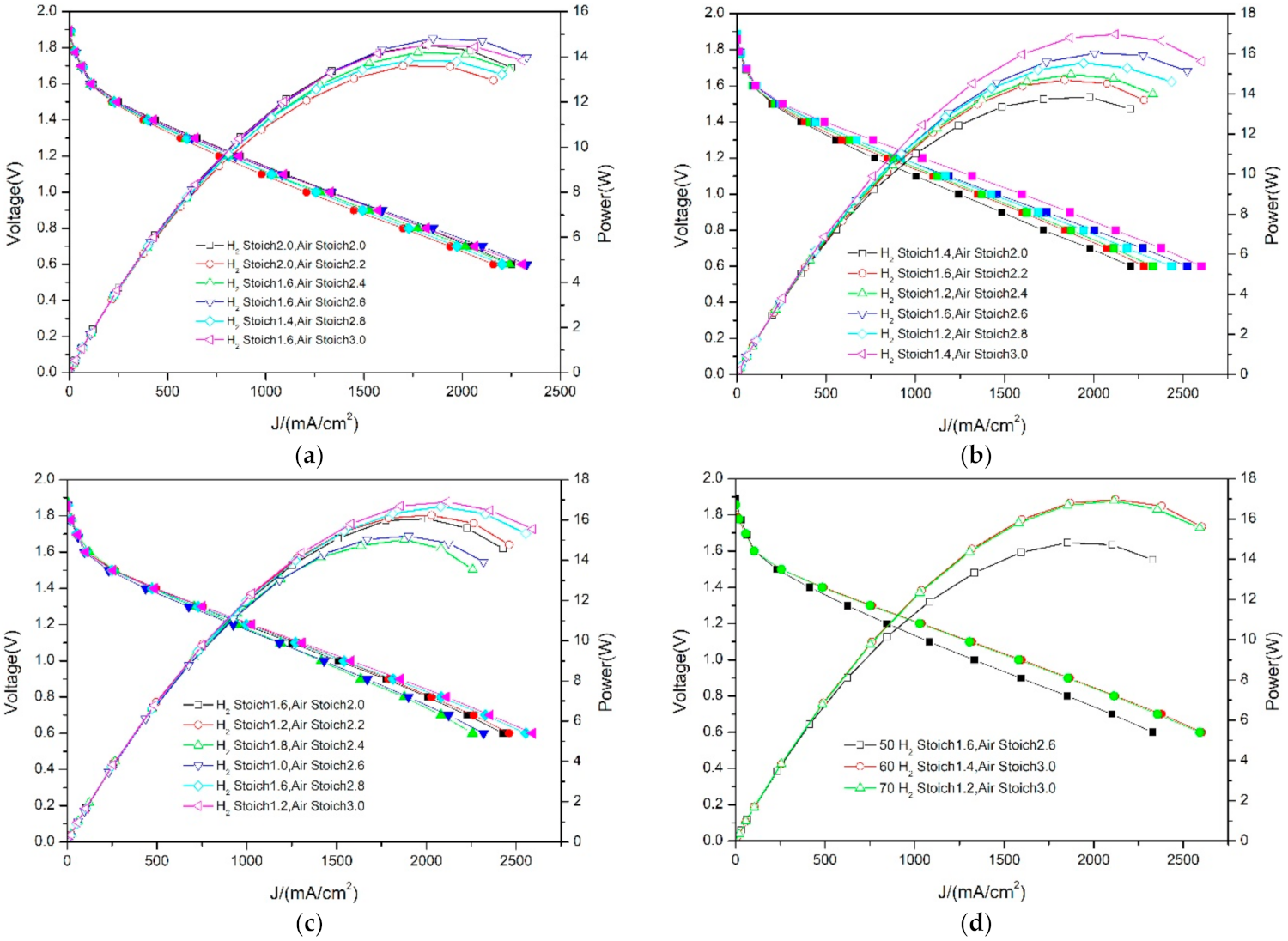
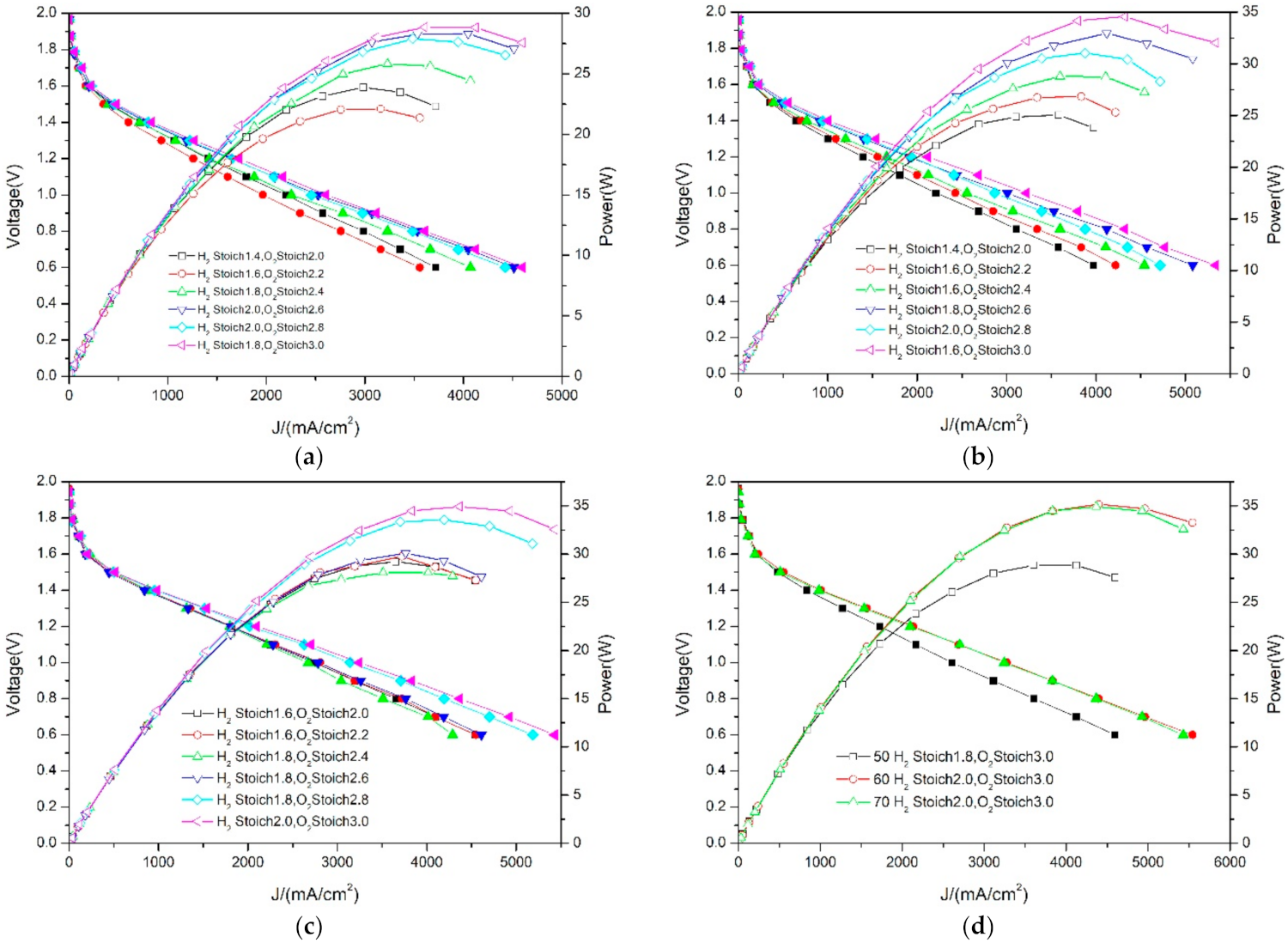
| air | 2.0(50–60–70) | 2.2(50–60–70) | 2.4(50–60–70) | 2.6(50–60–70) | 2.8(50–60–70) | 3.0(50–60–70) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H2 | |||||||||||||||||||
| 1.0 | 790 | 757 | 916 | 655 | 818 | 993 | 790 | 847 | 915 | 833 | 872 | 924 | 791 | 934 | 963 | 844 | 978 | 1015 | |
| 1.2 | 827 | 764 | 927 | 698 | 835 | 1022 | 793 | 871 | 926 | 836 | 897 | 912 | 798 | 920 | 993 | 849 | 1006 | 1029 | |
| 1.4 | 840 | 770 | 932 | 726 | 831 | 922 | 798 | 865 | 914 | 838 | 872 | 904 | 809 | 881 | 921 | 850 | 1038 | 1009 | |
| 1.6 | 731 | 779 | 989 | 741 | 843 | 1007 | 807 | 850 | 914 | 846 | 919 | 924 | 817 | 891 | 999 | 864 | 1009 | 1014 | |
| 1.8 | 754 | 778 | 998 | 756 | 845 | 988 | 813 | 849 | 947 | 839 | 961 | 917 | 826 | 881 | 1015 | 863 | 977 | 997 | |
| 2.0 | 870 | 820 | 988 | 764 | 831 | 952 | 817 | 872 | 953 | 849 | 974 | 948 | 828 | 921 | 1002 | 872 | 983 | 1003 | |
| O2 | 2.0(50–60–70) | 2.2(50–60–70) | 2.4(50–60–70) | 2.6(50–60–70) | 2.8(50–60–70) | 3.0(50–60–70) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H2 | |||||||||||||||||||
| 1.0 | 1304 | 1362 | 1594 | 1067 | 1455 | 1788 | 1342 | 1565 | 1637 | 1491 | 1658 | 1756 | 1439 | 1803 | 1878 | 1544 | 1926 | 2029 | |
| 1.2 | 1324 | 1344 | 1650 | 1152 | 1469 | 1861 | 1372 | 1612 | 1685 | 1529 | 1731 | 1760 | 1492 | 1786 | 1956 | 1579 | 2012 | 2078 | |
| 1.4 | 1411 | 1395 | 1696 | 1218 | 1521 | 1816 | 1397 | 1651 | 1674 | 1551 | 1727 | 1754 | 1538 | 1779 | 1953 | 1631 | 2107 | 2068 | |
| 1.6 | 1228 | 1387 | 1820 | 1260 | 1559 | 1863 | 1437 | 1658 | 1737 | 1565 | 1837 | 1802 | 1576 | 1835 | 1999 | 1702 | 2118 | 2109 | |
| 1.8 | 1236 | 1384 | 1757 | 1247 | 1546 | 1808 | 1422 | 1656 | 1799 | 1586 | 1911 | 1806 | 1612 | 1842 | 2020 | 1726 | 2100 | 2095 | |
| 2.0 | 1410 | 1460 | 1739 | 1245 | 1505 | 1675 | 1430 | 1682 | 1753 | 1639 | 1958 | 1867 | 1649 | 1935 | 2054 | 1718 | 2134 | 2097 | |
| Index | Cryogenic Method | Adsorption Separation Method | Membrane Separation Method | |
|---|---|---|---|---|
| High-Purity | Low-Purity | |||
| Yield (m3/h) | 50–100,000 | 1000–100,000 | 50–4000 | 25–15,000 |
| Purity (%) | 99.6 | 95.0 | 93.0 | 40.0 |
| Pressure (MPa) | 0.02–0.50 | 0.02–0.50 | 0.01 | 0.01 |
| Energy consumption (KWh.m−3) | 0.45–0.80 | 0.40–0.60 | 0.32–0.37 | <0.30 |
| Run-up time (h) | 30 | 28 | 0.2 | 0.1 |
| Product costs | middle | Slightly lower | Low | Low |
| Equipment investment | High | Slightly lower | Slightly lower | Low |
| Operability | Complex | Complex | Easy | Easy |
| Floor space | Big | Big | Middle | Small |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, Q.; Hsieh, C.; Li, S. Research on Energy and Economics of Self-Made Catalyst-Coated Membrane for Fuel Cell under Different Oxidants. Membranes 2022, 12, 128. https://doi.org/10.3390/membranes12020128
Bai Q, Hsieh C, Li S. Research on Energy and Economics of Self-Made Catalyst-Coated Membrane for Fuel Cell under Different Oxidants. Membranes. 2022; 12(2):128. https://doi.org/10.3390/membranes12020128
Chicago/Turabian StyleBai, Qiang, Chuangyu Hsieh, and Shaobo Li. 2022. "Research on Energy and Economics of Self-Made Catalyst-Coated Membrane for Fuel Cell under Different Oxidants" Membranes 12, no. 2: 128. https://doi.org/10.3390/membranes12020128
APA StyleBai, Q., Hsieh, C., & Li, S. (2022). Research on Energy and Economics of Self-Made Catalyst-Coated Membrane for Fuel Cell under Different Oxidants. Membranes, 12(2), 128. https://doi.org/10.3390/membranes12020128






