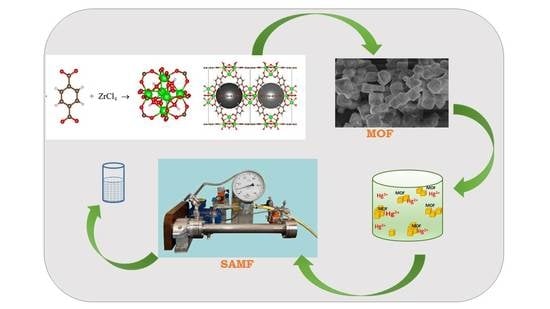
MOF-Based Sorbents Used for the Removal of Hg2+ from Aqueous Solutions via a Sorption-Assisted Microfiltration
Abstract
:1. Introduction
2. Experimental Methods
2.1. Chemicals
2.2. Analytical Methods
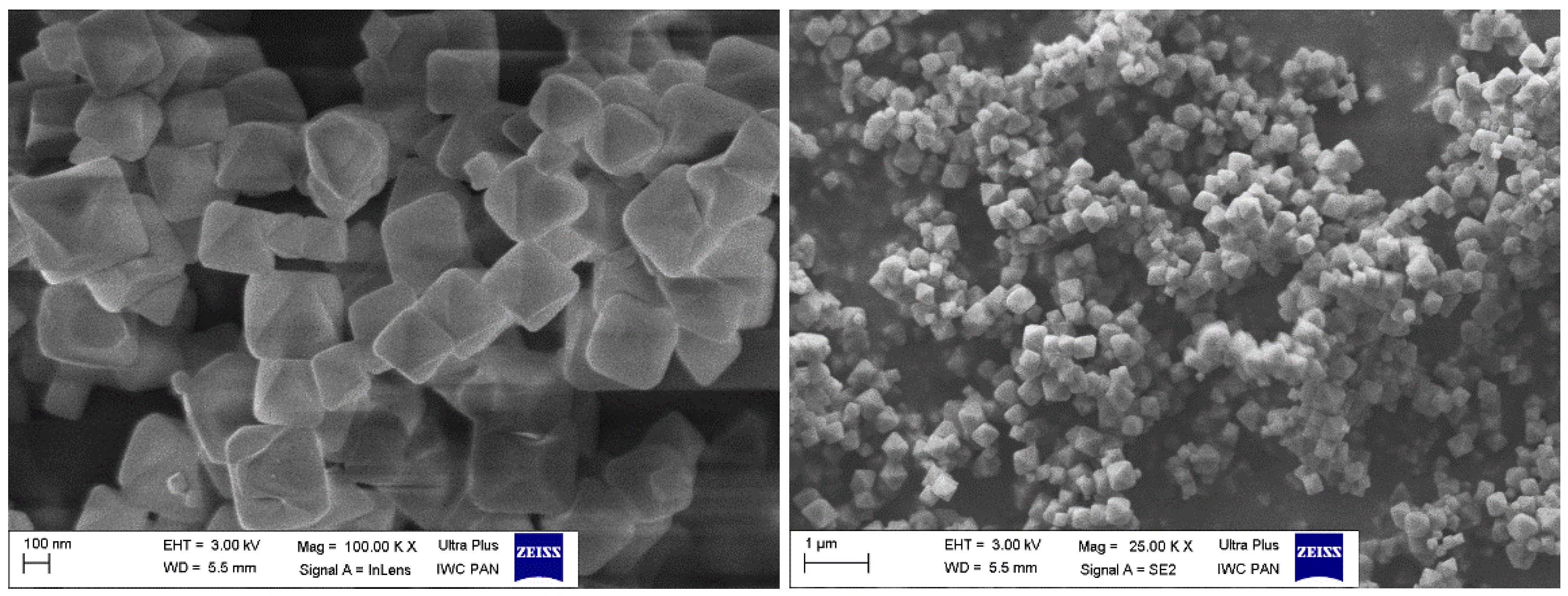
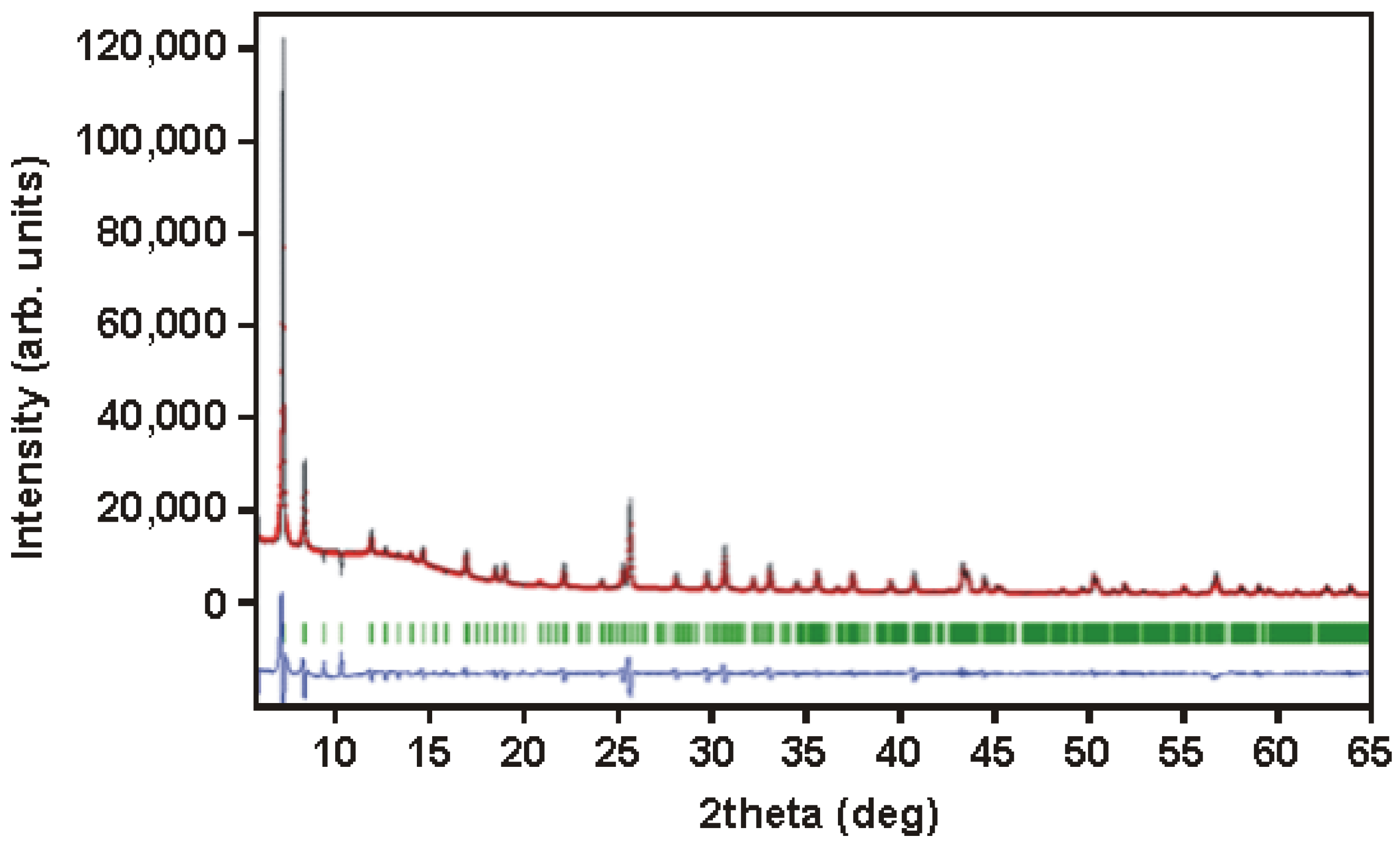
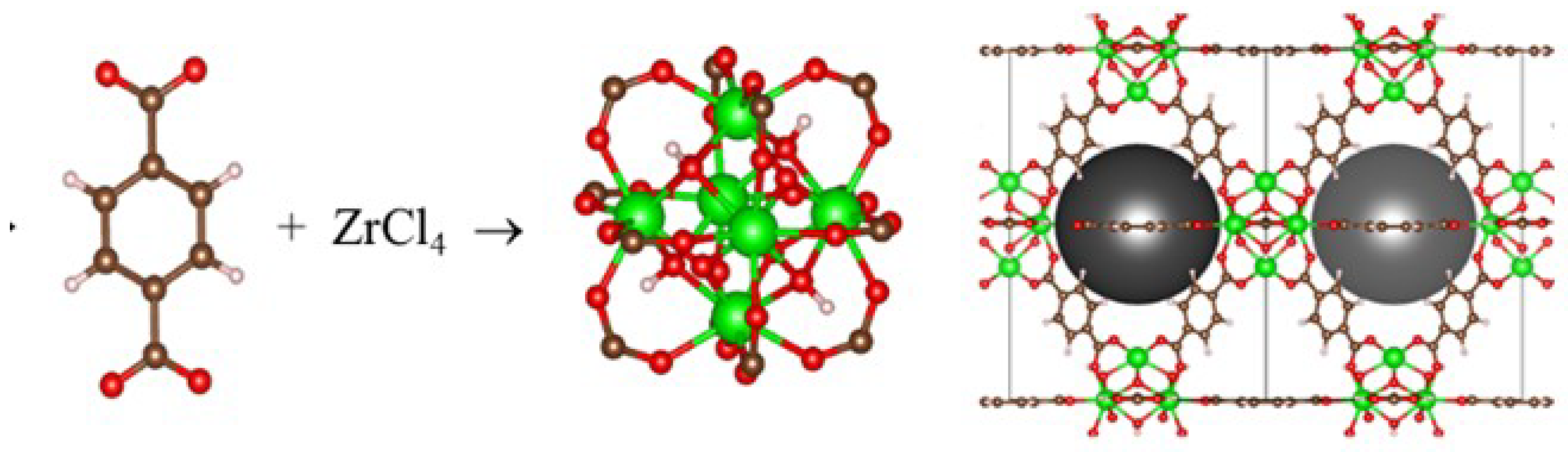
2.3. Synthesis and Characterisation of the MOF-Type Sorbents
2.3.1. Synthesis of MAA Sorbent
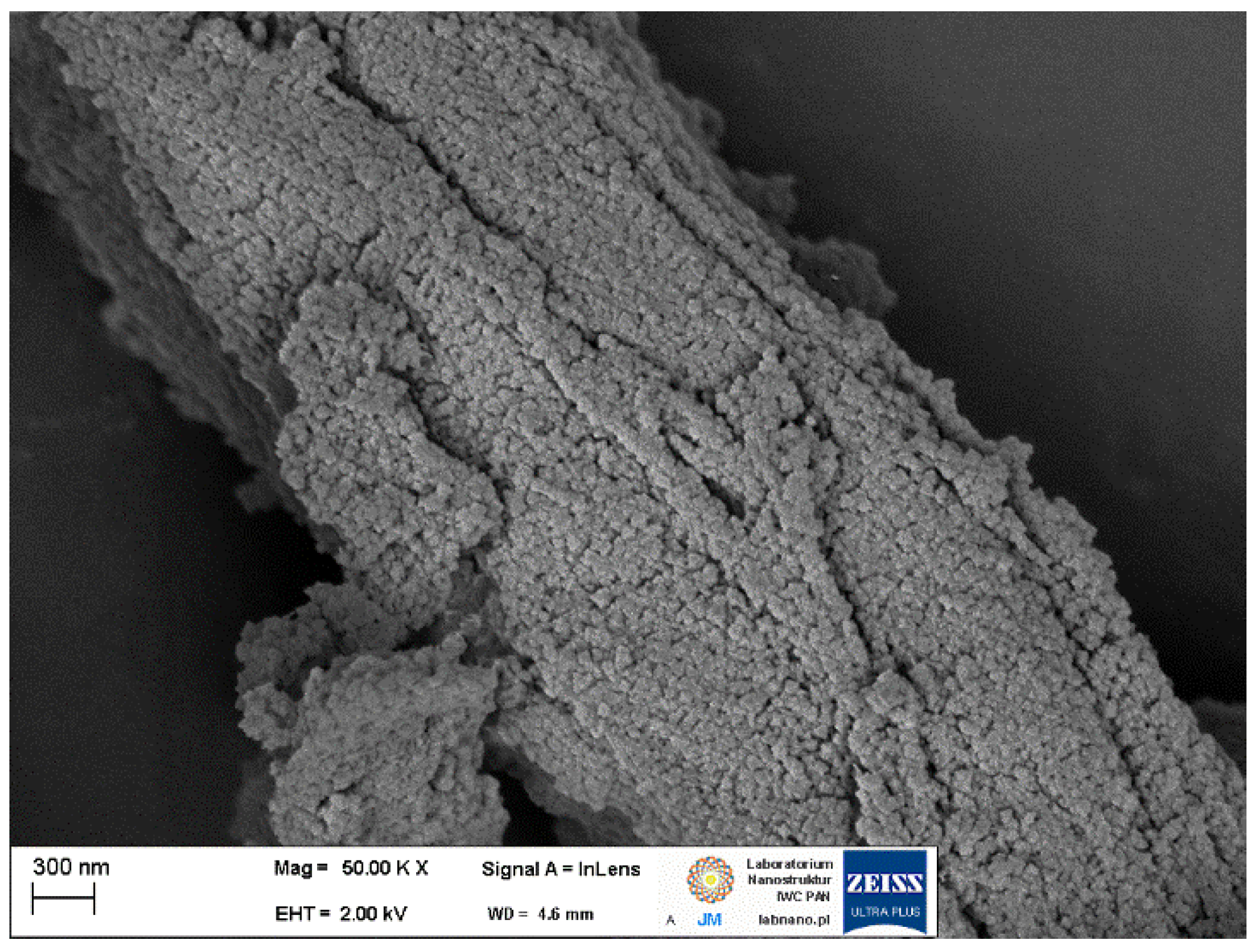
2.3.2. Synthesis of MOFs Composite Materials with Cellulose
2.4. Sorption of Mercury Ions in Batch System
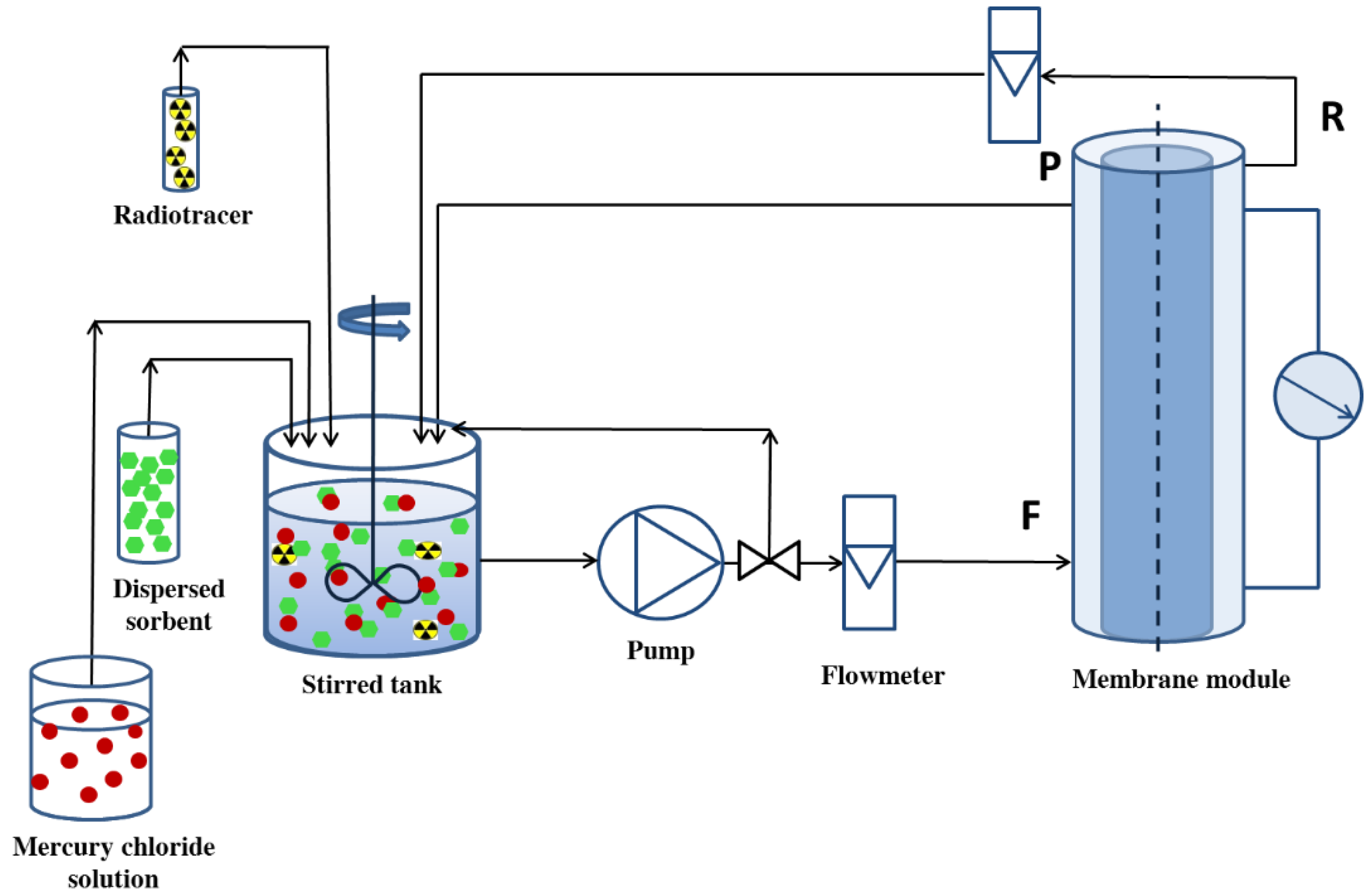
2.5. A Sorption-Assisted Microfiltration Process
3. Results and Discussion
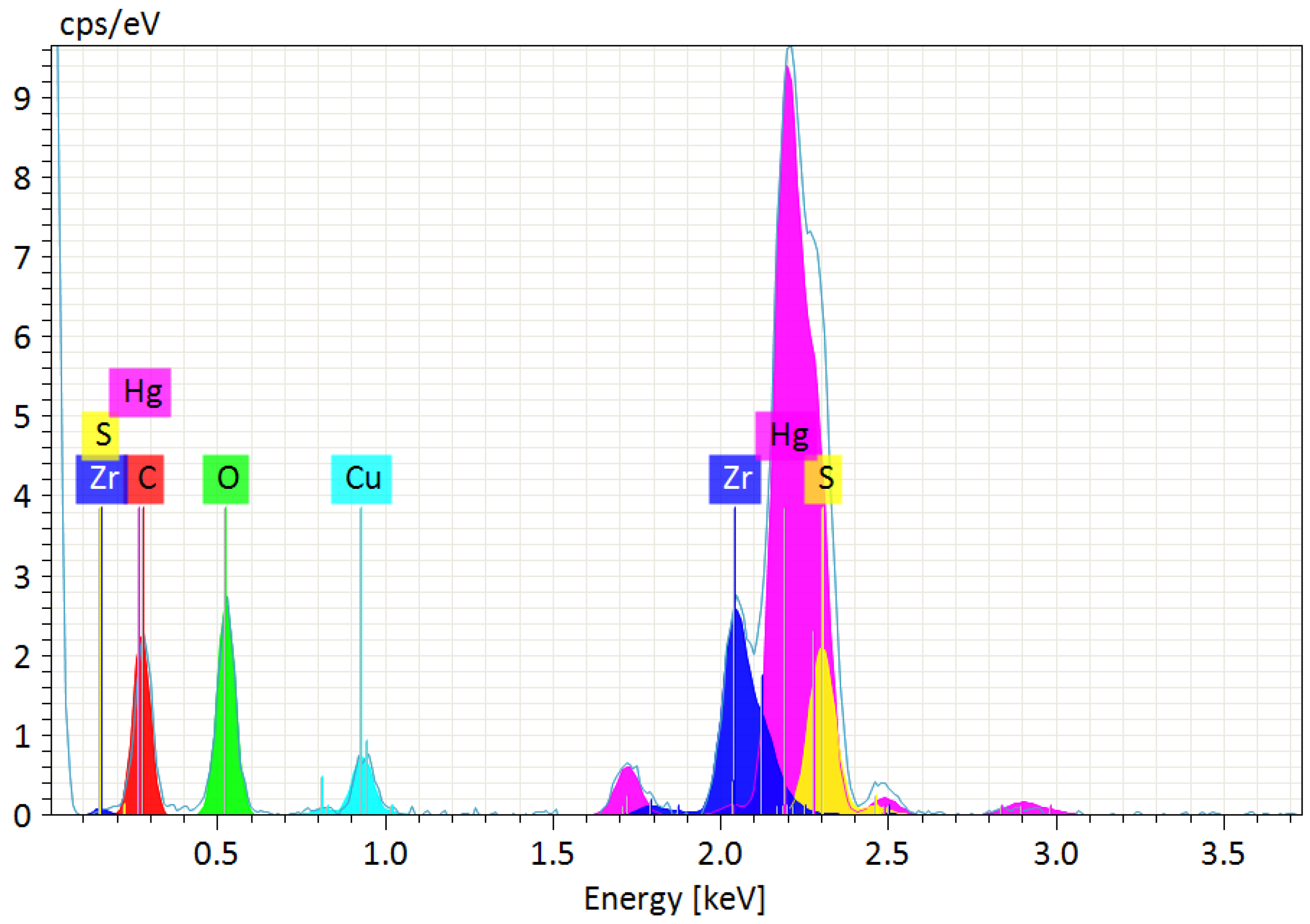
3.1. MOF as a Sorbent for Mercury Ions
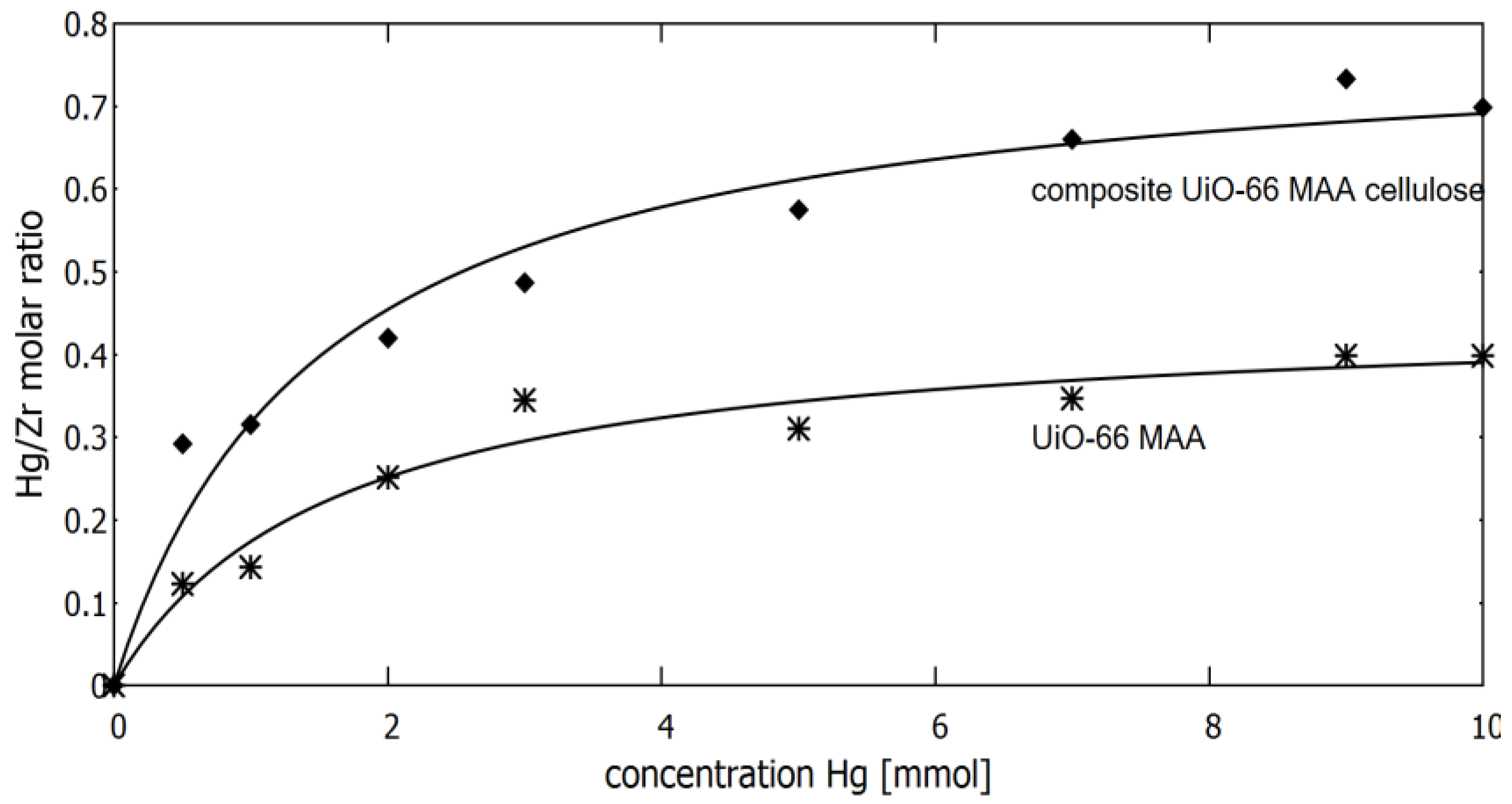
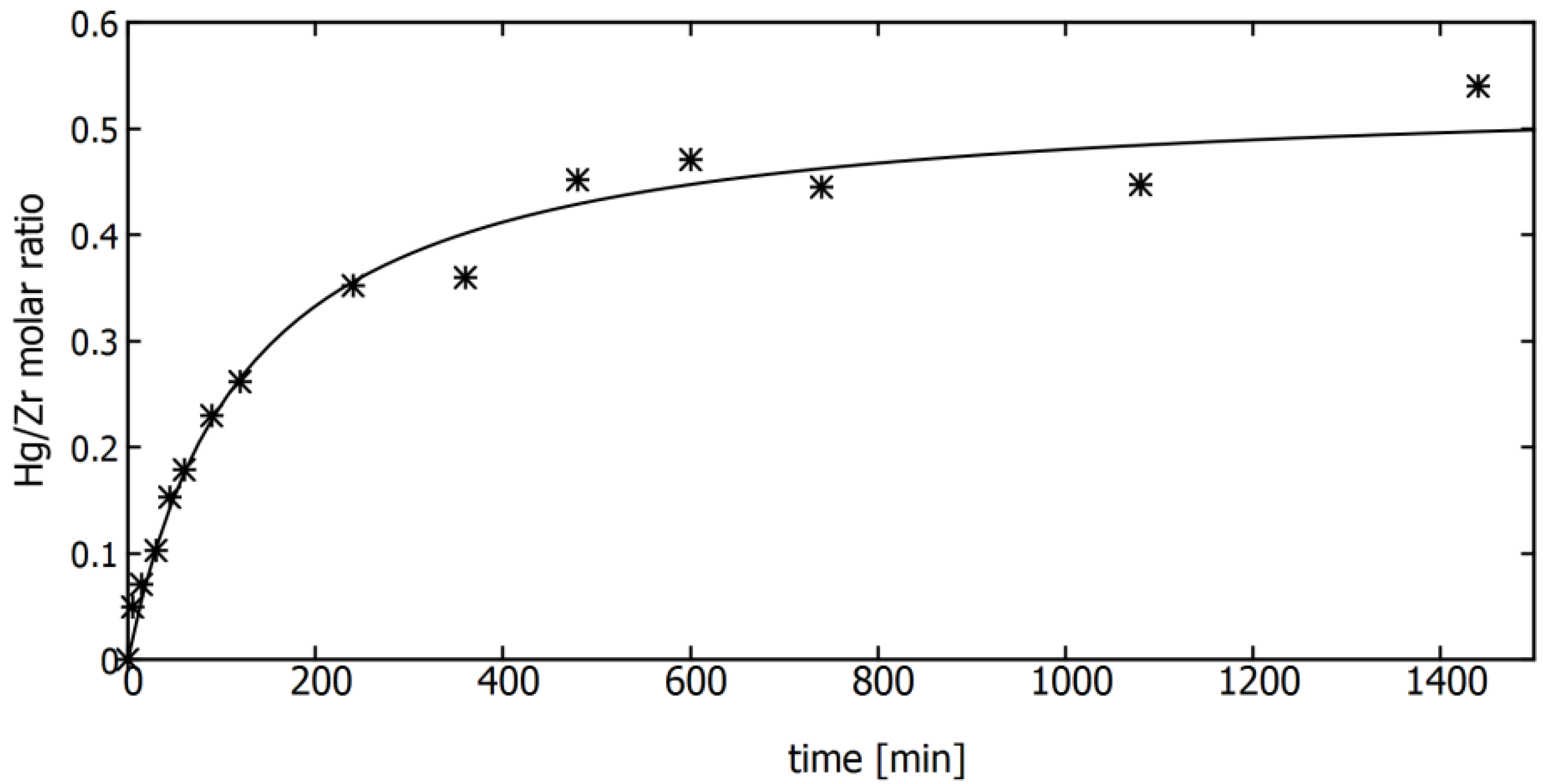
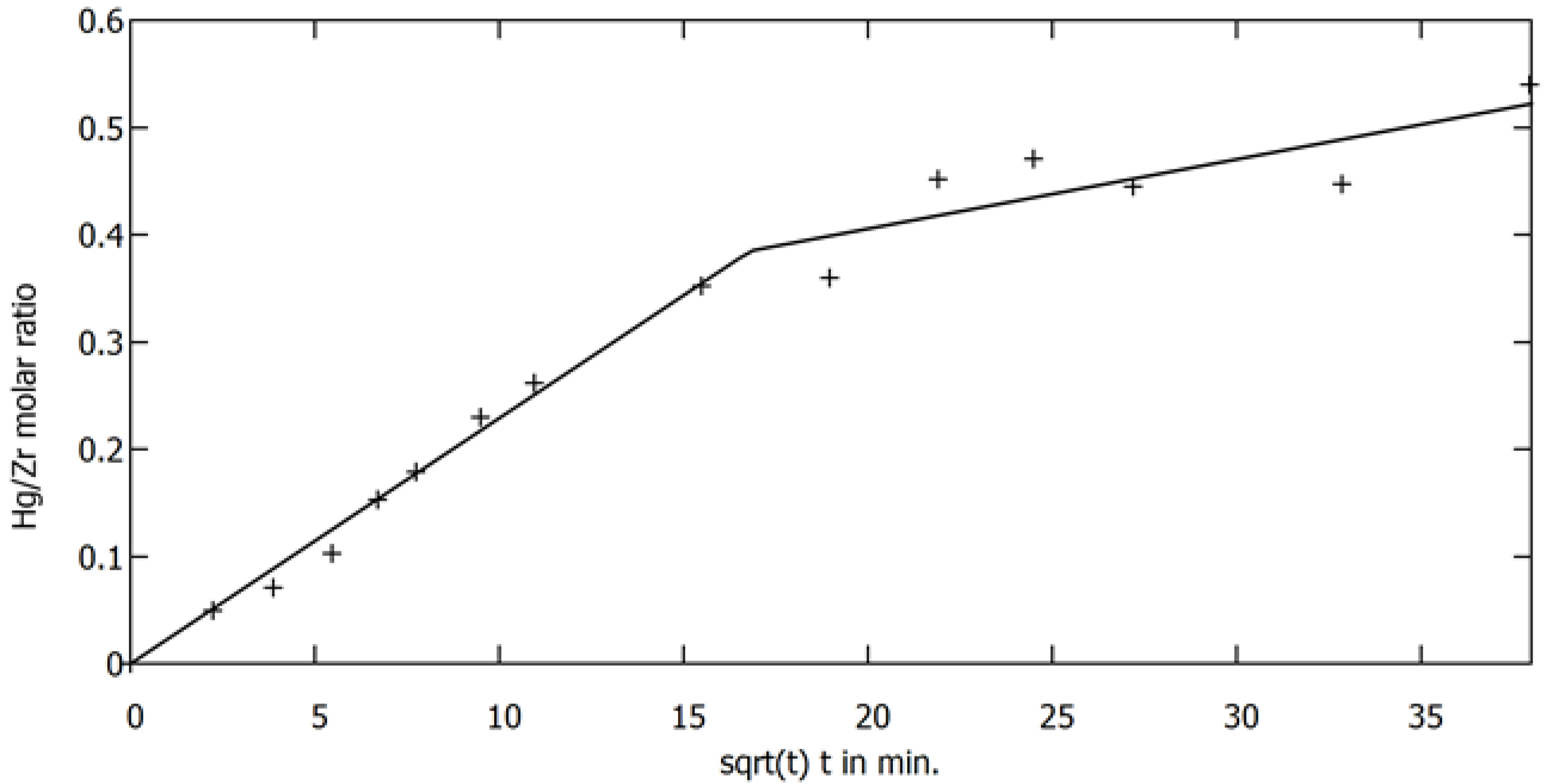
3.2. Sorption of Mercury Ions on MOF Material in Static Mode
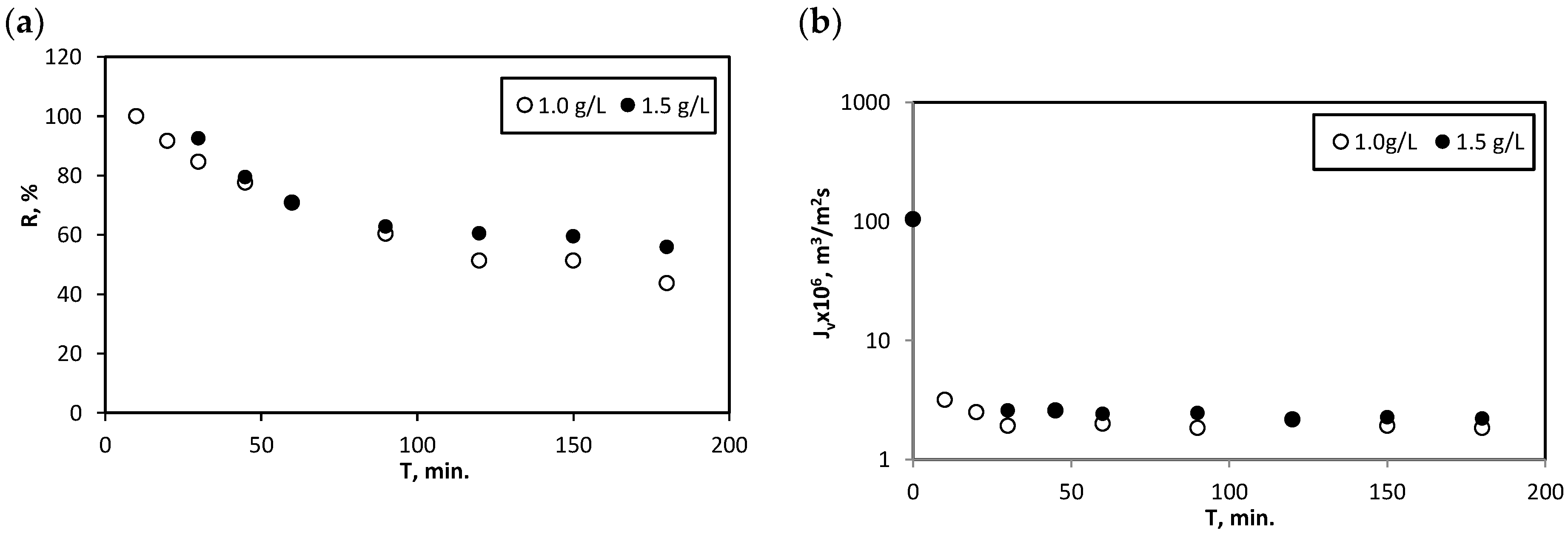
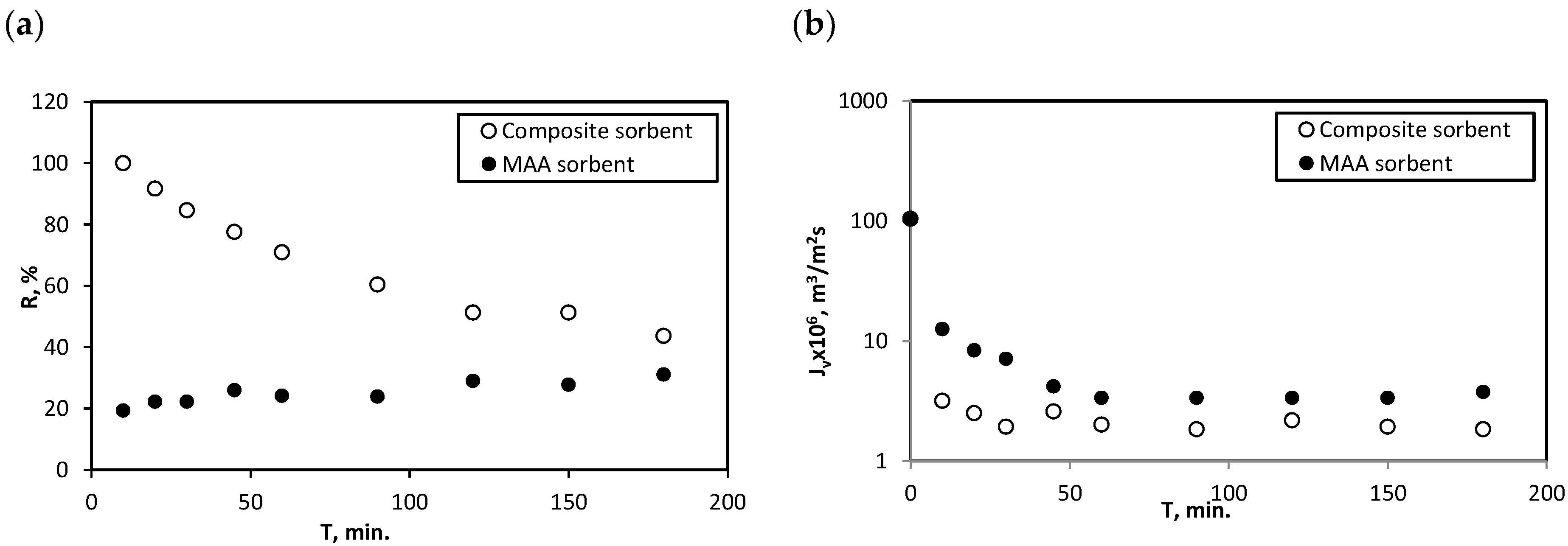
3.3. Sorption-Assisted Microfiltration for Mercury Ions Removal
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Qdais, H.A.; Moussa, H. Removal of heavy metals from wastewater by membrane processes: A comparative study. Desalination 2004, 164, 105–110. [Google Scholar] [CrossRef]
- Montaño-Medina, C.U.; Lopéz-Martínez, L.M.; Ochoa-Terán, A.; López-Maldonado, E.A.; Salazar-Gastelum, M.I.; Trujillo-Navarrete, B.; Pérez-Sicairos, S.; Cornejo-Bravo, J.M. New pyridyl and aniline-functionalized carbamoylcarboxylic acids for removal of metal ions from water by coagulation-flocculation process. Chem. Eng. J. 2023, 451, 138396. [Google Scholar] [CrossRef]
- El Samrani, A.G.; Lartiges, B.S.; Villie´ras, F. Chemical coagulation of combined sewer overflow: Heavy metal removal and treatment optimization. Wat. Res. 2008, 42, 951–960. [Google Scholar] [CrossRef] [PubMed]
- Milićević, S.; Milošević, V.; Povrenović, D.; Stojanović, J.; Martinović, S.; Babić, B. Removal of Heavy Metals from Aqueous Solution Using Natural and Fe(III) Oxyhydroxide Clinoptilolite. Clays Clay Miner. 2013, 61, 508–516. [Google Scholar] [CrossRef]
- Goyal, M.; Bhagat, M.; Dhawan, R. Removal of mercury from water by fixed bed activated carbon columns. J. Hazard. Mat. 2009, 171, 1009–1015. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.; Jiang, N.; Liu, Z.; Li, C.; Sun, H.; Guo, H.; Peng, B.; Li, J. Pulsed discharge plasma assisted with Z-scheme graphene-TiO2-MnFe2O4 for simultaneous removal of atrazine and Cr(VI): Performance and mechanism. Chem. Eng. J. 2023, 242, 139342. [Google Scholar] [CrossRef]
- Sun, H.; Wang, L.; Liu, Y.; Cheng, Z.; Zhao, Y.; Guo, H.; Qu, G.; Wang, T.; Yin, X. Photocatalytic reduction of Cr(VI) via surface modified g-C3N4 by acid-base regulation. J. Environ. Manag. 2022, 324, 116431. [Google Scholar] [CrossRef]
- Winarta, J.; Shan, B.; Mcintyre, S.M.; Ye, L.; Wang, C.; Liu, J.; Mu, B. A Decade of UiO-66 Research: A Historic Review of Dynamic Structure, Synthesis Mechanisms, and Characterization Techniques of an Archetypal Metal−Organic Framework. Cryst. Growth Des. 2020, 20, 1347–1362. [Google Scholar] [CrossRef]
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The Chemistry and Applications of Metal-Organic Frameworks. Science 2013, 341, 1230444. [Google Scholar] [CrossRef] [Green Version]
- Kaskel, S. The Chemistry of Metal-Organic Frameworks: Synthesis, Characterization, and Applications; Wiley-VCH Verlag GmbH & Co.KGaA: Weinheim, Germany, 2016. [Google Scholar]
- Ryu, U.; Jee, S.; Rao, P.C.; Shin, J.; Ko, C.; Yoon, M.; Park, K.S.; Choi, K.M. Recent advances in process engineering and upcoming applications of metal–organic frameworks. Coord. Chem. Rev. 2021, 426, 213544. [Google Scholar] [CrossRef]
- Ren, J.; Dyosiba, X.; Musyoka, N.M.; Langmi, H.W.; Mathe, M.; Liao, S. Review on the current practices and efforts towards pilot-scale production of metal-organic frameworks (MOFs). Coord. Chem. Rev. 2017, 352, 187–219. [Google Scholar] [CrossRef]
- Li, J.-R.; Kuppler, R.J.; Zhou, H.-C. Selective gas adsorption and separation in metal–organic frameworks. Chem. Soc. Rev. 2009, 38, 1477–1504. [Google Scholar] [CrossRef] [PubMed]
- Horcajada, P.; Chalati, T.; Serre, C.; Gillet, B.; Sebrie, C.; Baati, T.; Eubank, J.F.; Heurtaux, D.; Clayette, P.; Kreuz, C.; et al. Porous metal–organic-framework nanoscale carriers as a potential platform for drug delivery and imaging. Nat. Mat. 2010, 9, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Abdollahi, N.; Moussavi, G.; Giannakis, S. A review of heavy metals’ removal from aqueous matrices by Metal-Organic Frameworks (MOFs): State-of-the art and recent advances. J. Environ. Chem. Eng. 2022, 10, 107394. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, J.; Zhang, Y.; Ma, J.; Huang, L.; Yu, S.; Chen, L.; Song, G.; Qiu, M.; Wang, X. Applications of water-stable metal-organic frameworks in the removal of water pollutants: A review. Environ. Pollut. 2021, 291, 118076. [Google Scholar] [CrossRef]
- Manousi, N.; Giannakoudakis, D.A.; Rosenberg, E.; Zachariadis, G.A. Extraction of Metal Ions with Metal–Organic Frameworks. Molecules 2019, 24, 4605. [Google Scholar] [CrossRef] [Green Version]
- Jin, K.; Lee, B.; Park, J. Metal-organic frameworks as a versatile platform for radionuclide management. Coord. Chem. Rev. 2020, 427, 213473. [Google Scholar] [CrossRef]
- Chuhadiya, S.; Suthar, H.D.; Patel, S.L.; Dhaka, M.S. Metal organic frameworks as hybrid porous materials for energy storage and conversion devices: A review. Coord. Chem. Rev. 2021, 440, 214115. [Google Scholar] [CrossRef]
- Frameworks for commercial success. Nat. Chem. 2016, 8, 987. [CrossRef] [Green Version]
- Baticle, P.; Kiefer, C.; Lakhchaf, N.; Leclerc, O.; Persin, M.; Sarrazin, J. Treatment of nickel containing industrial effluents with a hybrid process comprising of polymer complexation–ultrafiltration–electrolysis. Sep. Purif. Technol. 2000, 18, 195–207. [Google Scholar] [CrossRef]
- Fan, X.; Tao, Y.; Wei, D.; Zhang, X.; Lei, Y.; Noguchi, H. Removal of organic matter and disinfection by-products precursors in a hybrid process combining ozonation with ceramic membrane ultrafiltration. Front. Environ. Sci. Eng. 2015, 9, 112–120. [Google Scholar] [CrossRef]
- Zeng, J.; Ye, H.; Hu, Z. Application of the hybrid complexation–ultrafiltration process for metal ion removal from aqueous solutions. J. Hazard. Mat. 2009, 161, 1491–1498. [Google Scholar] [CrossRef] [PubMed]
- Desai, K.R.; Murthy, Z.V.P. Removal of silver from aqueous solutions by complexation–ultrafiltration using anionic polyacrylamide. Chem. Eng. J. 2012, 185–186, 187–192. [Google Scholar] [CrossRef]
- Cojocaru, C.; Zakrzewska-Trznadel, G.; Miskiewicz, A. Removal of cobalt ions from aqueous solutions by polymer assisted ultrafiltration using experimental design approach Part 2: Optimization of hydrodynamic conditions for a crossflow ultrafiltration module with rotating part. J. Hazard. Mat. 2009, 169, 610–620. [Google Scholar] [CrossRef] [PubMed]
- Fuks, L.; Miśkiewicz, A.; Zakrzewska-Kołtuniewicz, G. Sorption-Assisted Ultrafiltration Hybrid Method for Treatment of the Radioactive Aqueous Solutions. Chemistry 2022, 4, 1076–1091. [Google Scholar] [CrossRef]
- Barron-Zambrano, J.; Laborie, S.; Viers, P.; Rakib, M.; Durand, G. Mercury removal from aqueous solutions by complexation–ultrafiltration. Desalination 2002, 144, 201–206. [Google Scholar] [CrossRef]
- Hafizovic Cavka, J.; Jakobsen, S.; Olsbye, U.; Guillou, N.; Lamberti, C.; Bordiga, S.; Lillerud, K.P. A New Zirconium Inorganic Building Brick Forming Metal Organic Frameworks with Exceptional Stability. J. Am. Chem. Soc. 2008, 130, 13850–13851. [Google Scholar] [CrossRef]
- Zakrzewska-Trznadel, G.; Harasimowicz, M.; Miskiewicz, A.; Jaworska, A.; Dłuska, E.; Wroński, S. Reducing fouling and boundary-layer by application of helical flow in ultrafiltration module employed for radioactive wastes processing. Desalination 2009, 240, 108–116. [Google Scholar] [CrossRef]
- Momma, K.; Izumi, F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- Schaate, A.; Roy, P.; Godt, A.; Lippke, J.; Waltz, F.; Wiebcke, M.; Behrens, P. Modulated Synthesis of Zr-Based Metal–Organic Frameworks: From Nano to Single Crystals. Chem. Eur. J. 2011, 17, 6643–6651. [Google Scholar] [CrossRef]
- Forgan, R. Modulated self-assembly of metal–organic frameworks. Chem. Sci. 2020, 11, 4546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cohen, S. Postsynthetic Methods for the Functionalization of Metal–Organic Frameworks. Chem. Rev. 2012, 112, 970–1000. [Google Scholar] [CrossRef]
- Ajsuvakova, O.P.; Tinkov, A.A.; Aschner, M.; Rocha, J.B.T.; Michalke, B.; Skalnaya, M.G.; Skalny, A.V.; Butnariu, M.; Dadar, M.; Sarac, I.; et al. Sulfhydryl groups as targets of mercury toxicity. Coord. Chem. Rev. 2020, 417, 213343. [Google Scholar] [CrossRef]
- Shellaiah, M.; Sun, K.-W. Progress in Metal-Organic Frameworks Facilitated Mercury Detection and Removal. Chemosensors 2021, 9, 101. [Google Scholar] [CrossRef]
- Yan, X.; Lia, P.; Song, X.; Li, J.; Ren, B.; Gao, S.; Cao, R. Recent progress in the removal of mercury ions from water-based MOFs materials. Coord. Chem. Rev. 2021, 443, 214034. [Google Scholar] [CrossRef]
- Wang, J.; Guo, X. Rethinking of the intraparticle adsorption kinetics model: Interpretations, solving methods and applications. Chemosphere 2022, 309, 136732. [Google Scholar] [CrossRef]
- Miśkiewicz, A.; Zakrzewska-Kołtuniewicz, G.; Starosta, W. MOF-assisted membrane process for removal of radionuclides and other hazardous elements from aqueous solutions. In Waste PET-MOF-Cleanwater: Waste PET-Derived Metal-Organic Framework (MOFs) as Cost-Effective Adsorbents for Removal of Hazardous Elements from Polluted Water; University of Johannesburg: Johannesburg, South Africa, 2022; pp. 45–56. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miśkiewicz, A.; Starosta, W.; Walczak, R.; Zakrzewska-Kołtuniewicz, G. MOF-Based Sorbents Used for the Removal of Hg2+ from Aqueous Solutions via a Sorption-Assisted Microfiltration. Membranes 2022, 12, 1280. https://doi.org/10.3390/membranes12121280
Miśkiewicz A, Starosta W, Walczak R, Zakrzewska-Kołtuniewicz G. MOF-Based Sorbents Used for the Removal of Hg2+ from Aqueous Solutions via a Sorption-Assisted Microfiltration. Membranes. 2022; 12(12):1280. https://doi.org/10.3390/membranes12121280
Chicago/Turabian StyleMiśkiewicz, Agnieszka, Wojciech Starosta, Rafał Walczak, and Grażyna Zakrzewska-Kołtuniewicz. 2022. "MOF-Based Sorbents Used for the Removal of Hg2+ from Aqueous Solutions via a Sorption-Assisted Microfiltration" Membranes 12, no. 12: 1280. https://doi.org/10.3390/membranes12121280
APA StyleMiśkiewicz, A., Starosta, W., Walczak, R., & Zakrzewska-Kołtuniewicz, G. (2022). MOF-Based Sorbents Used for the Removal of Hg2+ from Aqueous Solutions via a Sorption-Assisted Microfiltration. Membranes, 12(12), 1280. https://doi.org/10.3390/membranes12121280