A Review on the Morphology and Material Properties of the Gas Separation Membrane: Molecular Simulation
Abstract
:1. Introduction
2. The Transport Mechanism of Gas Molecules
2.1. The Porous Membrane
- (1)
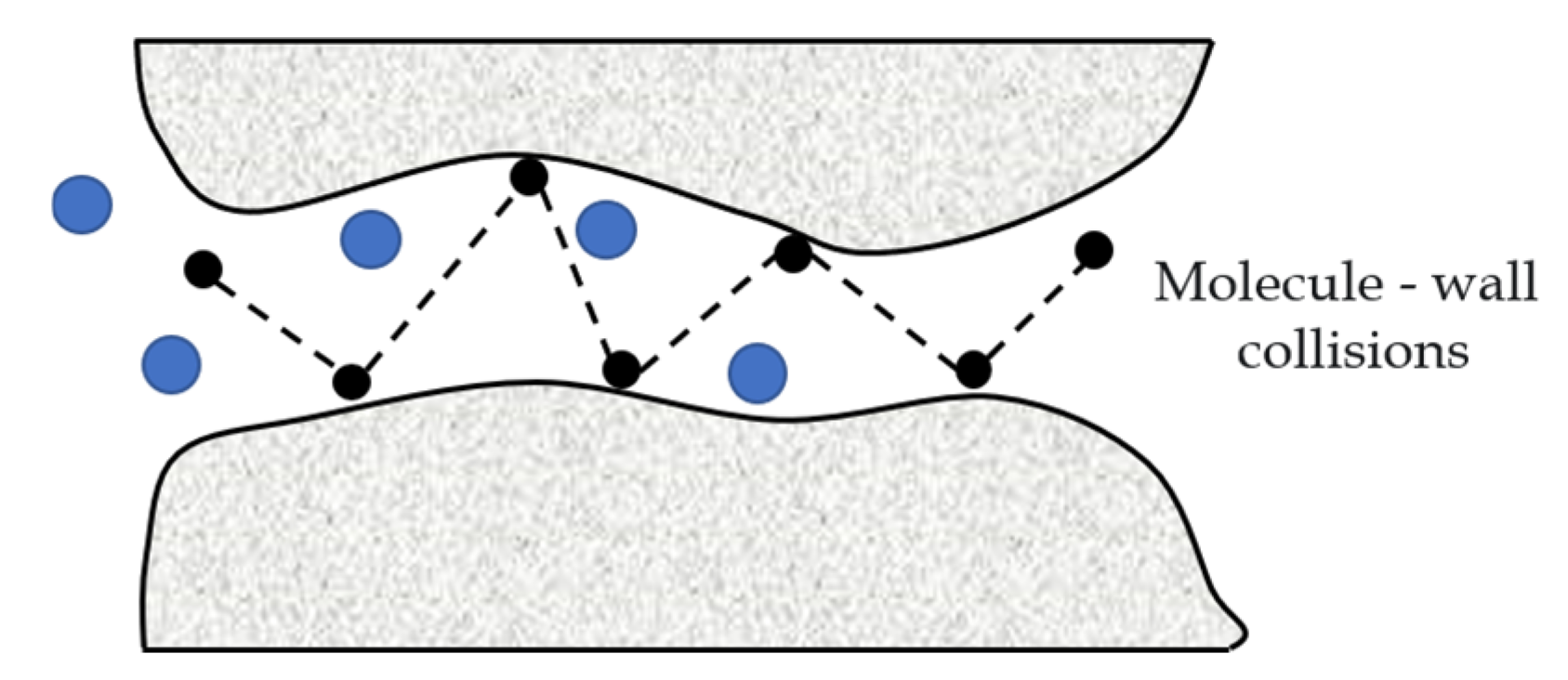
- Knudsen diffusion.
- (2)
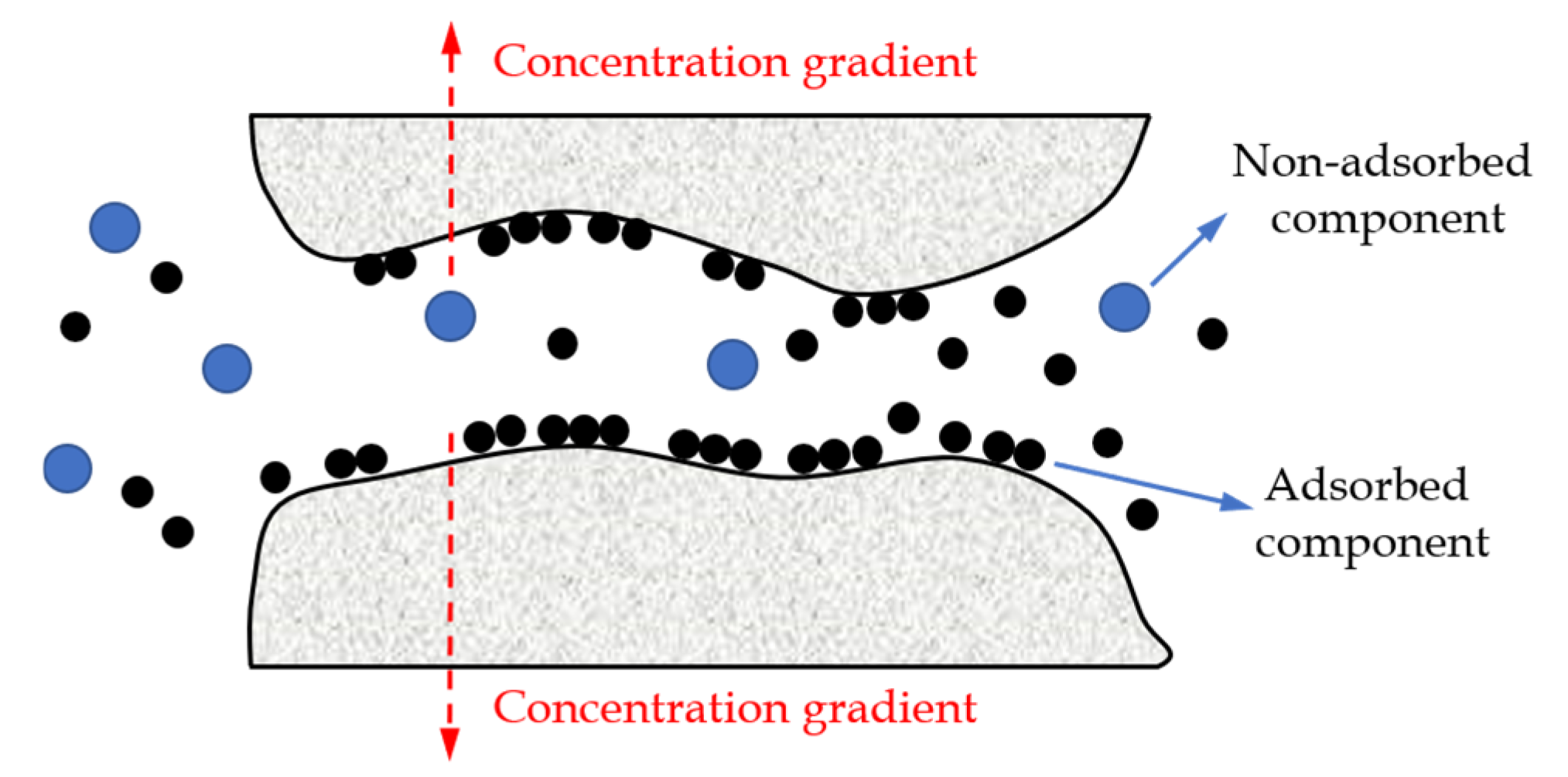
- Surface diffusion.
- (3)
- Molecular sieving.
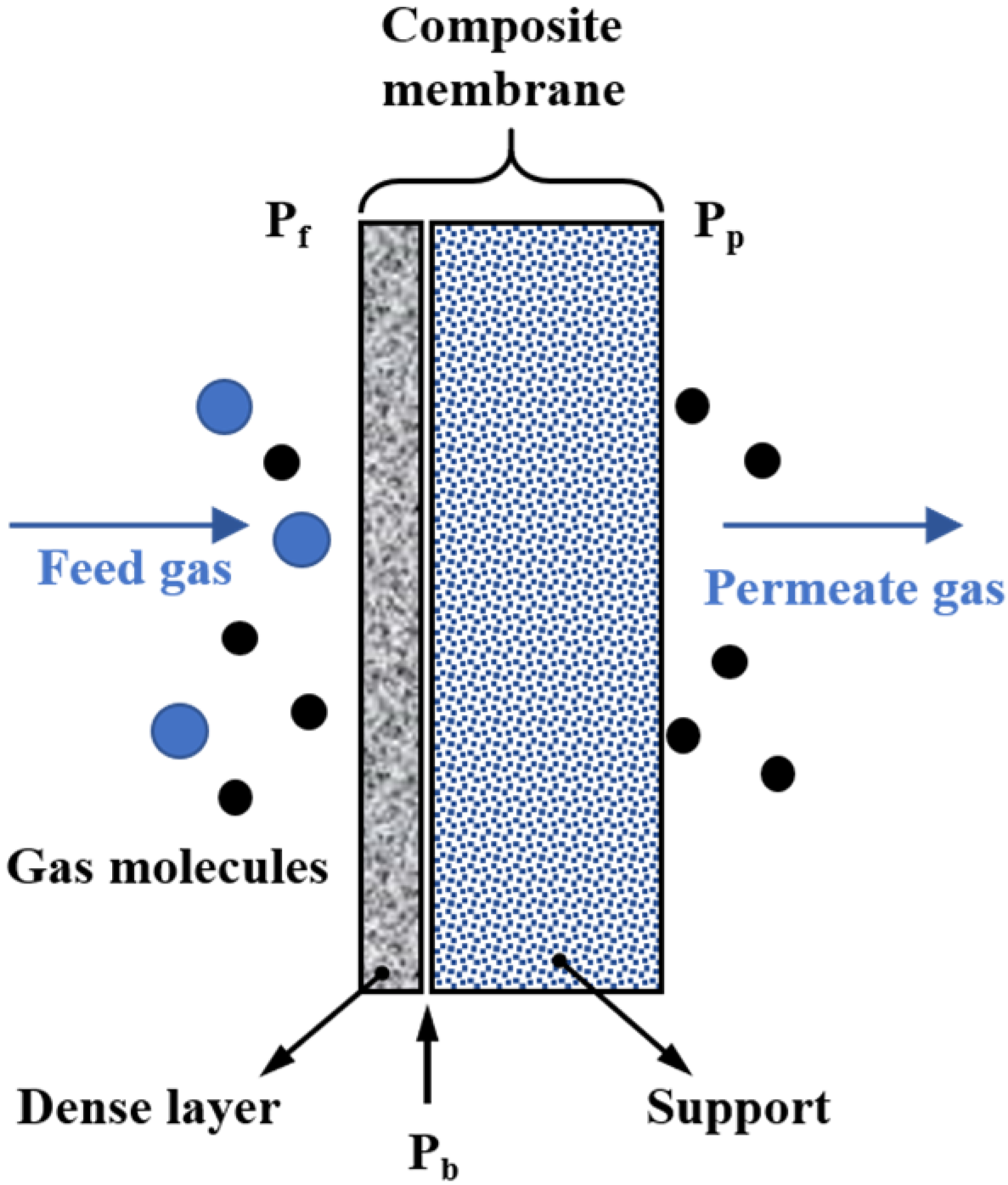
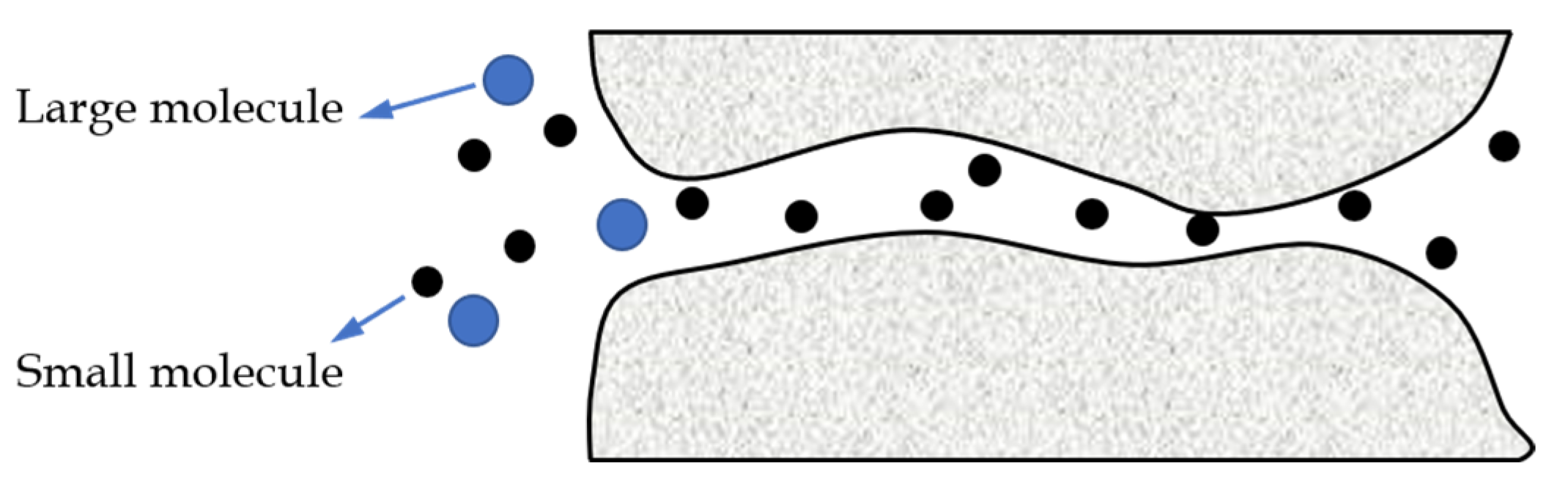
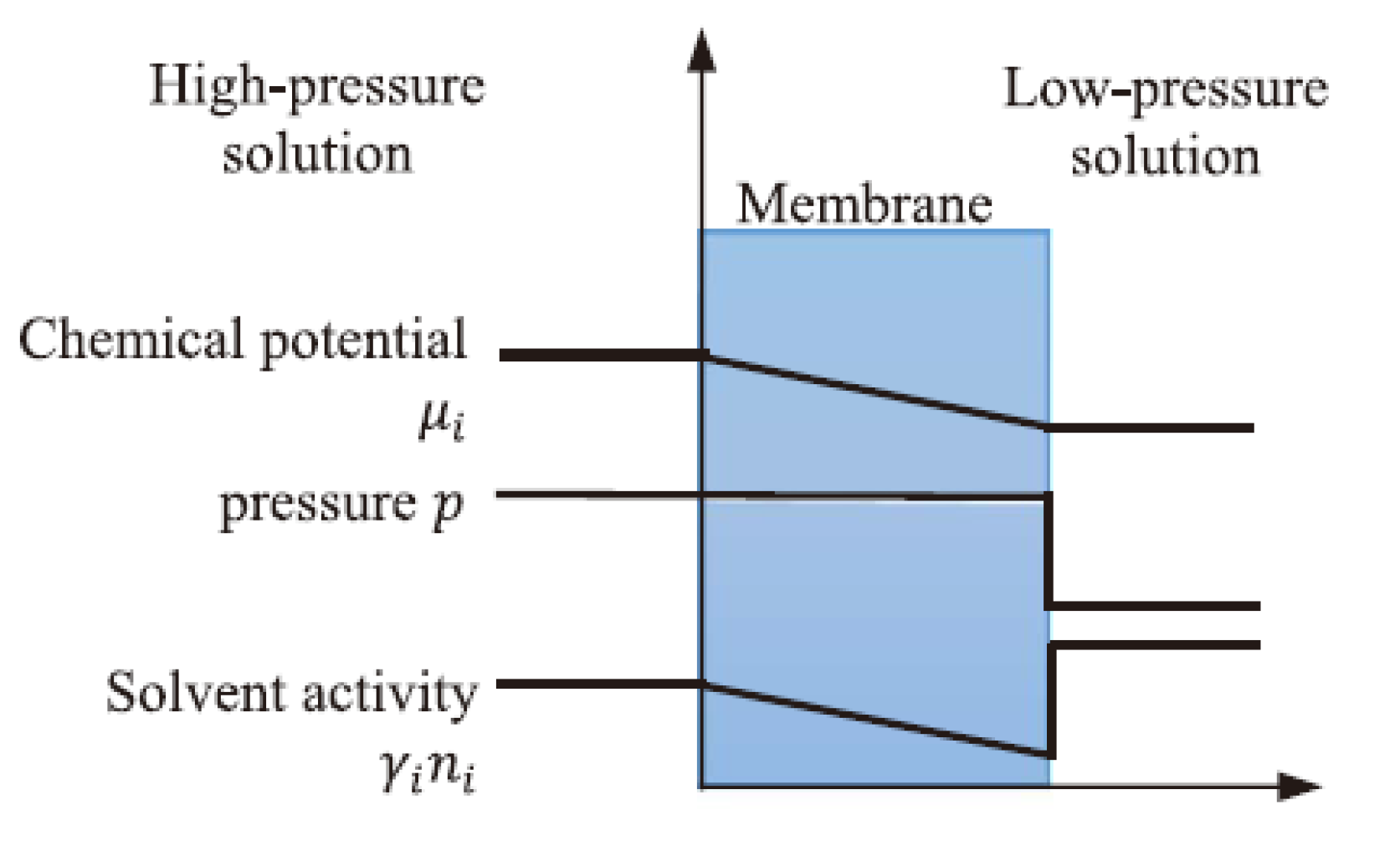
2.2. The Non-Porous Membrane
3. The Relationship between Membrane Properties and Membrane Morphology
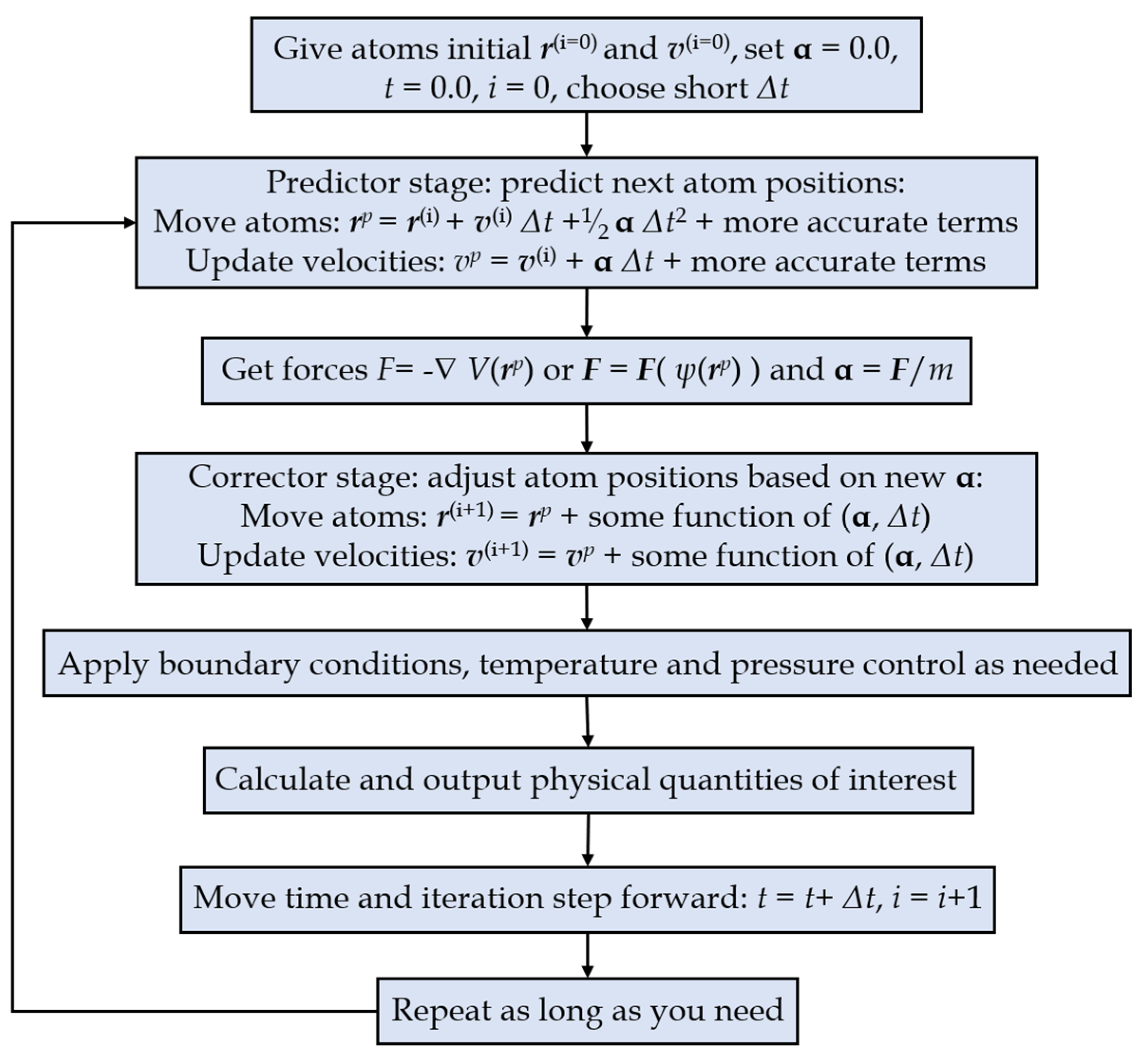
3.1. The Molecular Dynamics Method
3.2. Physical and Chemical Properties
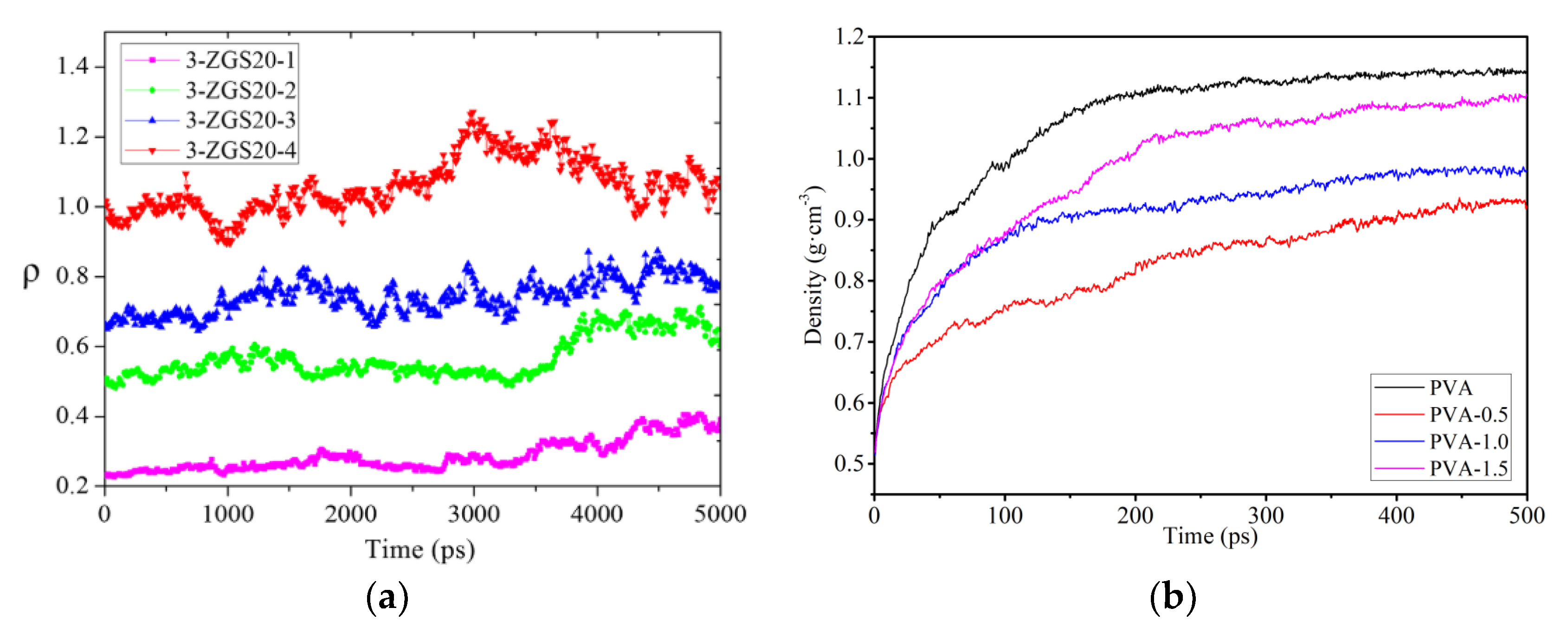
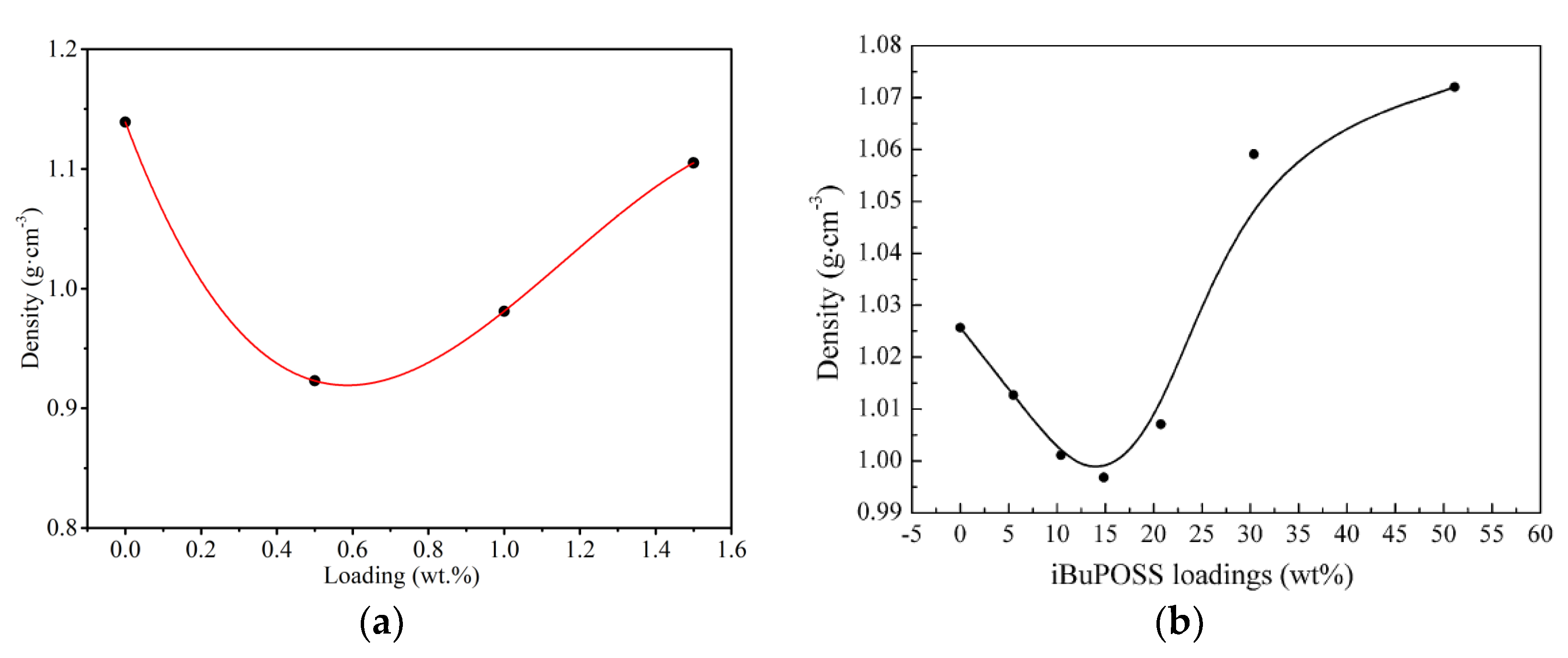
3.2.1. Densities
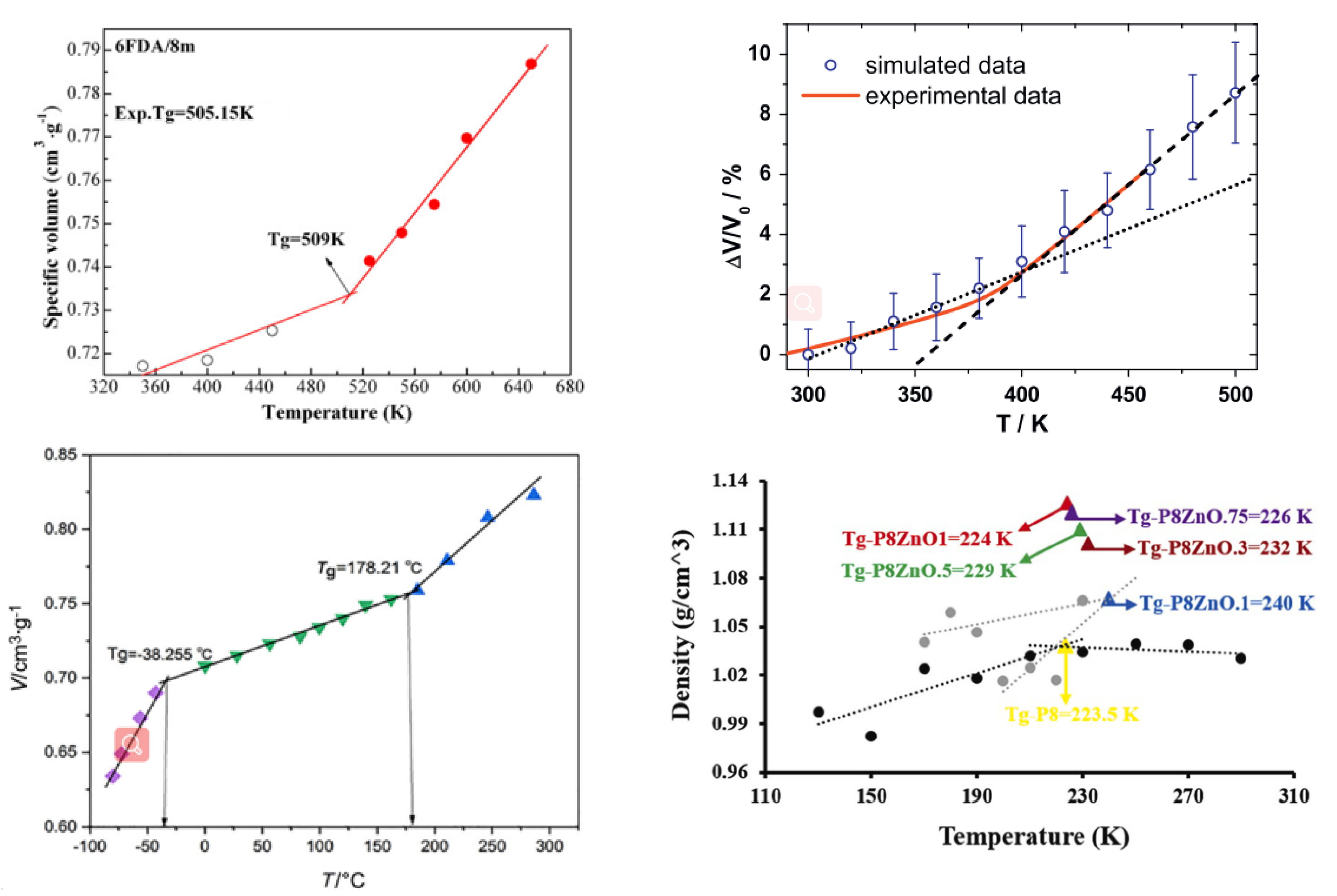
3.2.2. The Glass Transition Temperature (Tg)
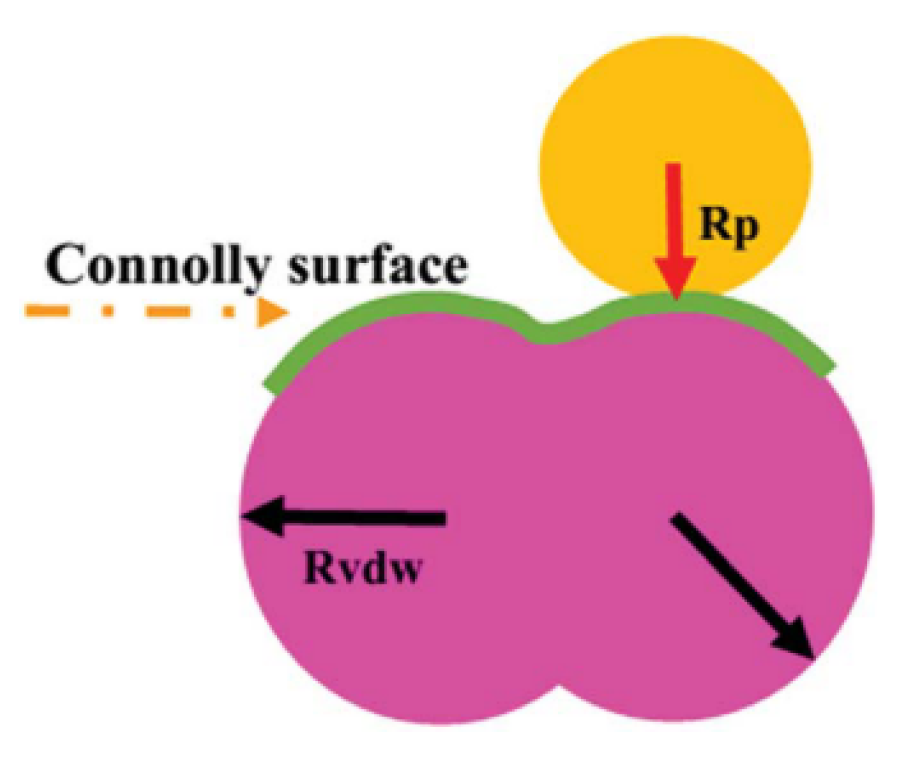
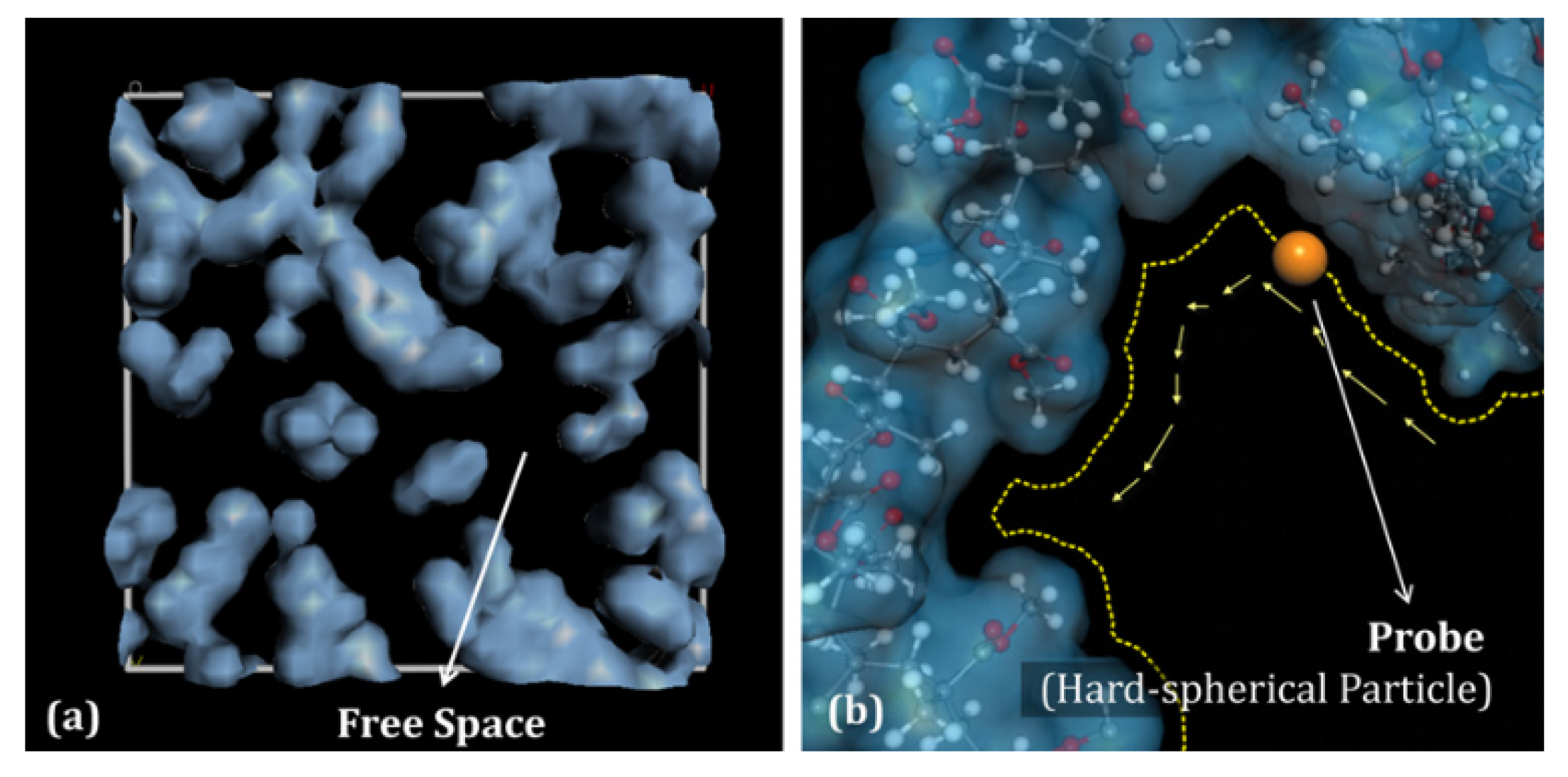
3.2.3. Fractional Free Volume (FFV)
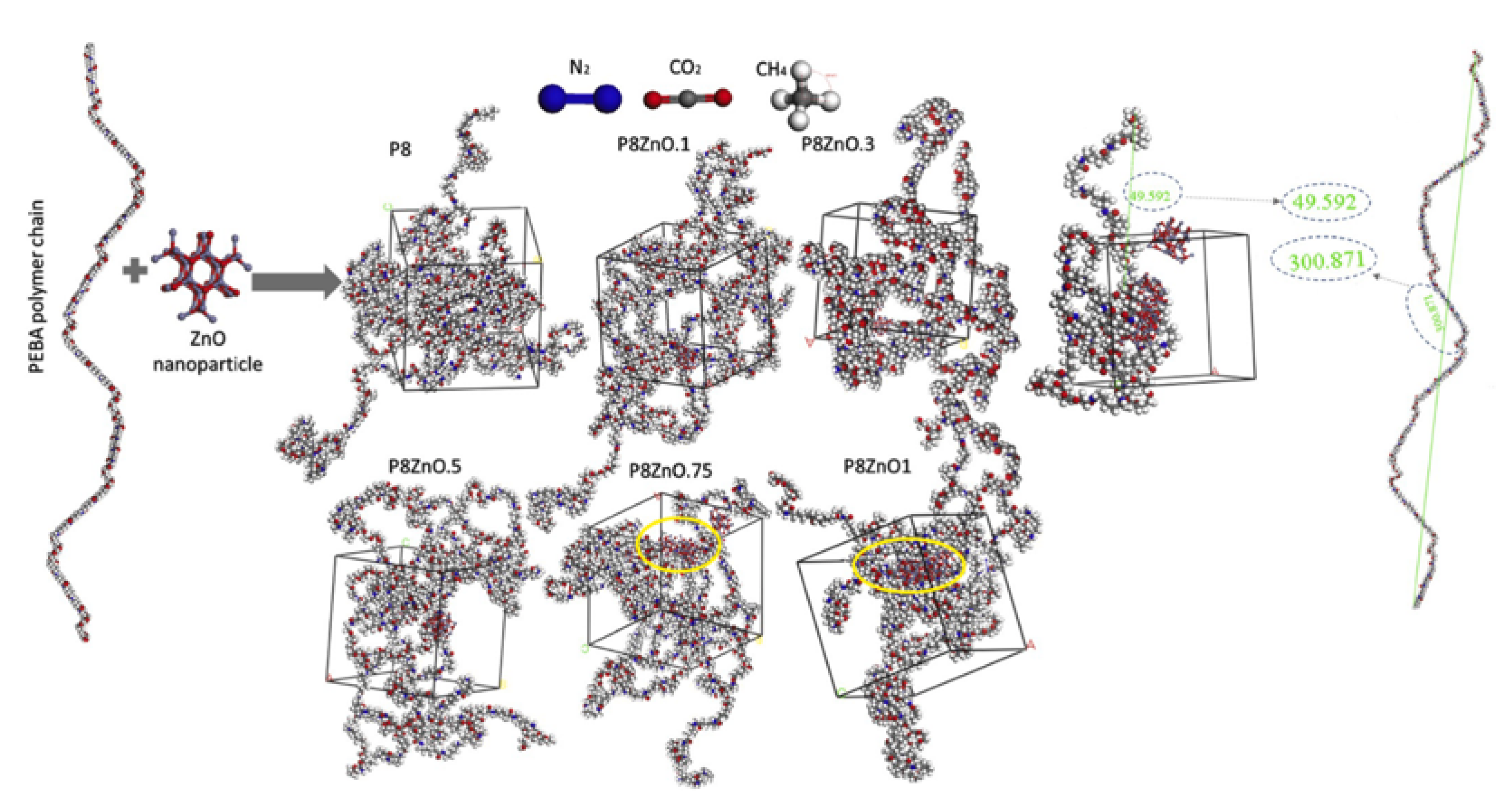
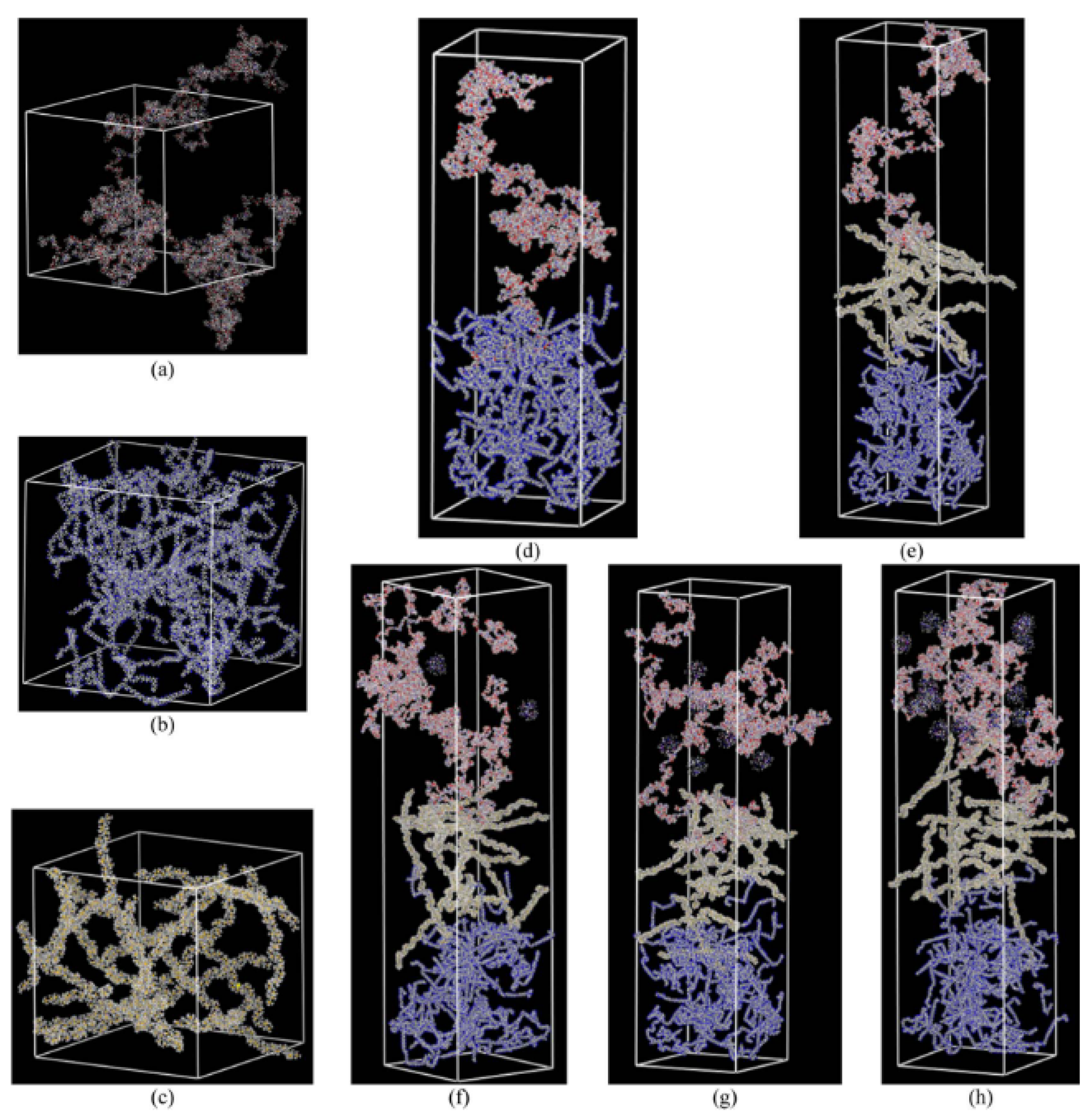
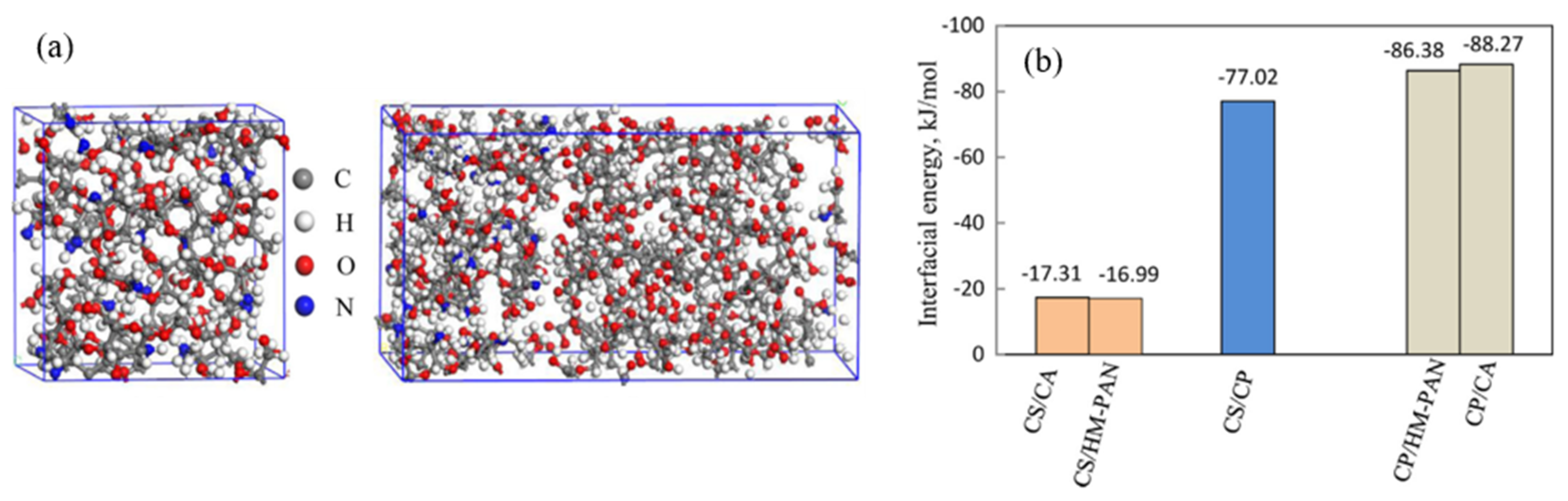
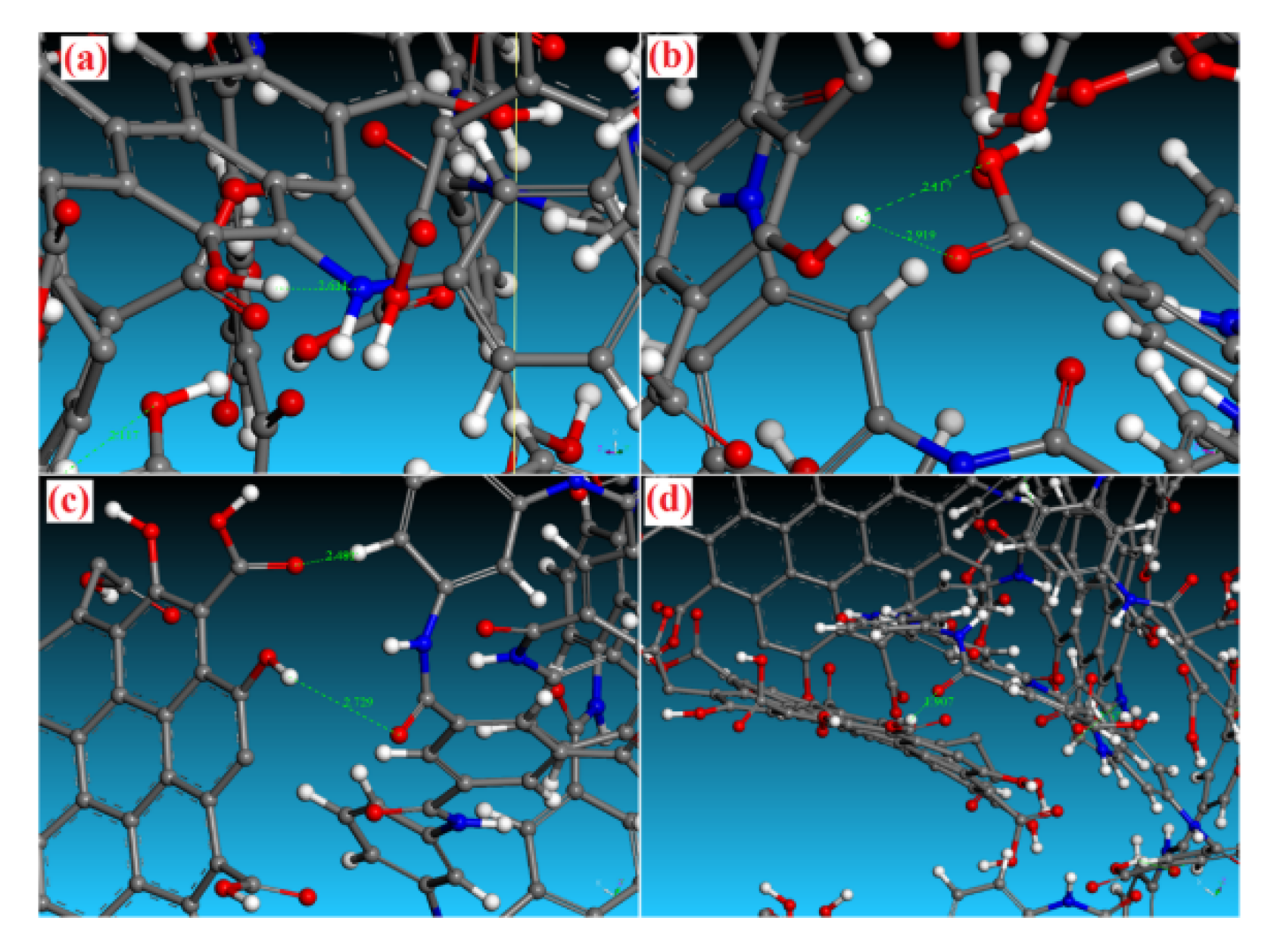
3.3. Interfacial Interactions
4. Transport Characteristics
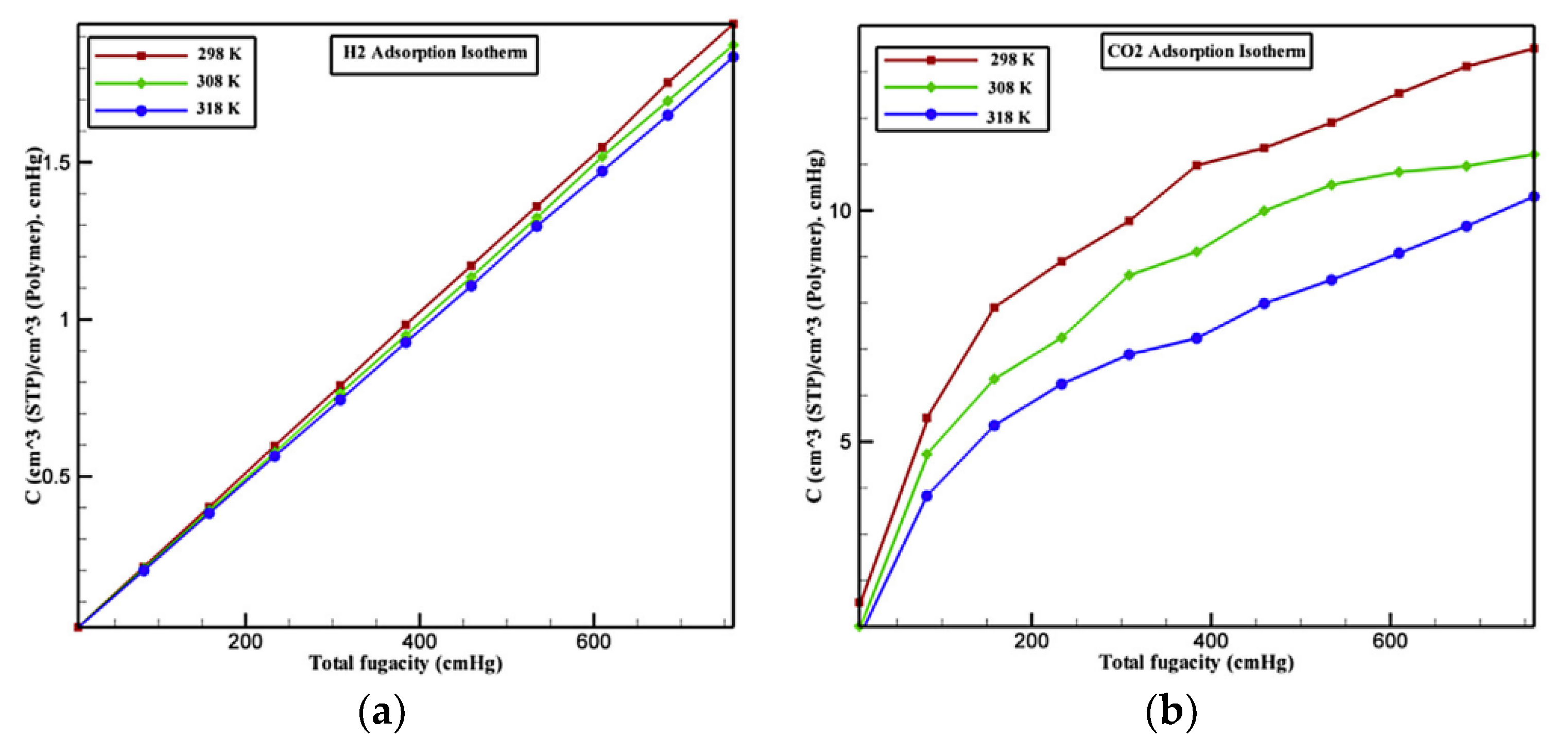
4.1. Adsorption
4.2. Diffusion
4.3. Permeability and Selectivity
5. Conclusions and Perspectives
- (1)
- A large number of studies focus on the solubility and diffusion coefficients of polymer membranes, and few studies report the relationship between membrane morphology, properties and membrane permeability and selectivity;
- (2)
- In most calculations, gas molecules are simplified as rigid molecules. The potential energy equations of gas–gas and gas–membrane interactions generally only consider L-J potential energy and coulomb force. The collision between molecules and walls is considered as elastic collision. Based on the above assumptions, the molecular simulation is still far from an accurate quantitative analysis of the actual membrane separation process;
- (3)
- The existing empirical potential energy parameters cannot meet the needs of some simulations. The necessary potential energy parameters and suitable force field equations are lacking. Considerable work should be undertaken in parameter selection and molecular model building;
- (4)
- At present, there are few commercial products of composite polymer membranes used for gas separation. The parameters required for modeling (the material properties of the separation layer, crystal type and microscopic shape of membrane pore, etc.) and the experimental data for comparing simulation results are scarce.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Nomenclature
| c | Concentration (mol/m3) | P | The permeability coefficient (Barrer) |
| D | Diffusion coefficient (cm2/s) | p | The pressure (Pa) |
| Ds | Surface diffusion coefficient (cm2/s) | R | The gas constant (J/mol K) |
| d1/d2 | The membrane thickness changes due to the surface adsorption | rp | The membrane pore radius (m) |
| Eint | The interfacial energies of the composite membrane (kcal/mol) | S | The solution coefficient (cm3 (STP)/(cm3 kPa)) |
| Elayer,1-2 | The total energies of the composite membrane (kcal/mol) | T | The temperature (K) |
| Elayer,1 | The energies of separation layer (kcal/mol) | Vs | The reciprocal density |
| Elayer,2 | The energies of support layer (kcal/mol) | VVdW | The van der Waals volume |
| FFV | Fractional free volume (%) | Greek | |
| fk | The gas flux due to Knudsen diffusion (kg/(m2 s)) | ρ | The density (kg/m3) |
| fs | The gas flux due to surface diffusion (kg/(m2 s)) | ε | The porosity of porous media |
| M | The gas molecular weight (kg/mol) | μs | The shape factor |
| Nα | The atoms number | α | The selectivity |
References
- Stookey, D.J.; Patton, C.J.; Malcolm, G.L. Membrane Separation Technology; American Institute of Chemical Engineers: New York, NY, USA, 2003. [Google Scholar]
- Chong, K.; Lai, S.; Thiam, H.; Teoh, H.; Heng, S. Recent progress of oxygennitrogen separation using membrane technology. J. Eng. Sci. Technol. 2016, 11, 1016–1030. [Google Scholar]
- Haggin, J. Ceramic, metallic devices extend membrane separation technology. Chem. Eng. News 1989, 67, 34–35. [Google Scholar] [CrossRef]
- Shi, J.; Gong, L.; Zhang, T.; Sun, S. Study of the Seawater Desalination Performance by Electrodialysis. Membranes 2022, 12, 767. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Li, C.; Sun, S. Effect of Temperature on Oil-Water Separations Using Membranes in Horizontal Separators. Membranes 2022, 12, 232. [Google Scholar] [CrossRef] [PubMed]
- Echakouri, M.; Henni, A.; Salama, A. High-Frequency Pulsatile Parameterization Study for the Titania Ceramic Membrane Fouling Mitigation in Oily Wastewater Systems Using the Box–Behnken Response Surface Methodology. Membranes 2022, 12, 1198. [Google Scholar] [CrossRef]
- Kikkinides, E.S.; Yang, R.T.; Cho, S.H. Concentration and recovery of carbon dioxide from flue gas by pressure swing adsorption. Ind. Eng. Chem. Res. 1993, 32, 2714–2720. [Google Scholar] [CrossRef]
- Iizuka, A.; Hashimoto, K.; Nagasawa, H.; Kumagai, K.; Yanagisawa, Y.; Yamasaki, A. Carbon dioxide recovery from carbonate solutions using bipolar membrane electrodialysis. Sep. Purif. Technol. 2012, 101, 49–59. [Google Scholar] [CrossRef]
- Uddin, M.W.; Hgg, M.B. Natural gas sweetening—The effect on CO2–CH4 separation after exposing a facilitated transport membrane to hydrogen sulfide and higher hydrocarbons. J. Membr. Sci. 2012, 423–424, 143–149. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, Z.; Qiu, W.; Liu, G.; Eddaoudi, M.; Koros, W.J. Penetrant competition and plasticization in membranes: How negatives can be positives in natural gas sweetening. J. Membr. Sci. 2021, 627, 119201. [Google Scholar] [CrossRef]
- Bhide, B.D.; Stern, S.A. A new evaluation of membrane processes for the oxygen-enrichment of air. ii. effects of economic parameters and membrane propertie. J. Membr. Sci. 1991, 62, 37–58. [Google Scholar] [CrossRef]
- Micari, M.; Agrawal, K.V. Oxygen enrichment of air: Performance guidelines for membranes based on techno-economic assessment. J. Membr. Sci. 2022, 641, 119883. [Google Scholar] [CrossRef]
- Ding, Y. Volatile Organic Compound Liquid Recovery by the Dead End Gas Separation Membrane Process: Theory and Process Simulation. Ind. Eng. Chem. Res. 2019, 58, 5008–5017. [Google Scholar] [CrossRef]
- Zhang, X.R.; Zhang, L.Z.; Pei, L.X. Sorption, permeation and selective transport of moisture/VOCs through a CA membrane for total heat recovery. Int. J. Low-Carbon Technol. 2012, 8, 64–69. [Google Scholar] [CrossRef]
- Liu, Y.; Wei, Y.; Su, J.; Zhang, L.; Jin, L. Surface-modified PVA/PVDF hollow fiber composite membrane for air dehumidification. J. Mater. Sci. 2020, 55, 5415–5430. [Google Scholar] [CrossRef]
- Bui, D.T.; Vivekh, P.; Islam, M.R.; Chua, K.J. Studying the characteristics and energy performance of a composite hollow membrane for air dehumidification. Appl. Energy 2022, 306, 118161. [Google Scholar] [CrossRef]
- Ziaka, Z.; Vasileiadis, S. Pretreated Landfill Gas Conversion Process via a Catalytic Membrane Reactor for Renewable Combined Fuel Cell-Power Generation. J. Renew. Energy 2013, 2013, 1–8. [Google Scholar] [CrossRef]
- Hao, J.; Rice, P.A.; Stern, S.A. Upgrading low-quality natural gas with H2S- and CO2-selective polymer membranes Part I. Process design and economics of membrane stages without recycle streams. J. Membr. Sci. 2002, 209, 177–206. [Google Scholar] [CrossRef]
- Ma, X.; Li, K.; Zhu, Z.; Dong, H.; Lv, J.; Wang, Y.; Pinnau, I.; Li, J.; Chen, B.; Han, Y. High-performance polymer molecular sieve membranes prepared by direct fluorination for efficient helium enrichment. J. Mater. Chem. A 2021, 9, 18313–18322. [Google Scholar] [CrossRef]
- Quader, M.A.; Rufford, T.E.; Smart, S. Modeling and cost analysis of helium recovery using combined-membrane process configurations. Sep. Purif. Technol. 2020, 236, 116269. [Google Scholar] [CrossRef]
- Locatelli, D.P.; Girgenti, P.; Moncini, L.; Limonta, L. Semifield study on the effect of membrane-based nitrogen production for the control of the eggs of Sitophilus oryzaeand and Tribolium confusum. J. Entomol. Acarol. Res. 2020, 52. [Google Scholar] [CrossRef]
- Bozorg, M.; Addis, B.; Piccialli, V.; Ramírez-Santos, Á.A.; Castel, C.; Pinnau, I.; Favre, E. Polymeric membrane materials for nitrogen production from air: A process synthesis study. Chem. Eng. Sci. 2019, 207, 1196–1213. [Google Scholar] [CrossRef]
- Itoh, M.; Saito, M.; Tajima, N.; Machida, K. Ammonia synthesis using atomic hydrogen supplied from silver-palladium alloy membrane. Mater. Sci. Forum 2007, 561–565, 1597–1600. [Google Scholar] [CrossRef]
- Zhang, X.; Li, C.; He, Z.; Han, T. Integration of Ammonia Synthesis Gas Production and N2O Decomposition into a Membrane Reactor. Ind. Eng. Chem. Res. 2020, 60, 3066–3072. [Google Scholar] [CrossRef]
- Tüzün, F.N.; Arçevik, E. Pore Modification in Porous Ceramic Membranes with Sol-Gel Process and Determination of Gas Permeability and Selectivity. Macromol. Symp. 2010, 287, 135–142. [Google Scholar] [CrossRef]
- Cui, Z.F.; Chang, S.; Fane, A.G. The use of gas bubbling to enhance membrane processes. J. Membr. Sci. 2003, 221, 1–35. [Google Scholar] [CrossRef]
- Stranick, S.J.; Parikh, A.N.; Allara, D.L.; Weiss, P.S. A New Mechanism for Surface Diffusion: Motion of a Substrate-Adsorbate Complex. J. Phys. Chem. B 1994, 98, 11136–11142. [Google Scholar] [CrossRef]
- Wang, Y.; Han, Q.; Zhou, Q.; Du, X.; Xue, L. Molecular sieving effect of zeolites on the properties of PVA based composite membranes for total heat recovery in ventilation systems. RSC Adv. 2016, 6, 66767–66773. [Google Scholar] [CrossRef]
- Li, H.; Song, Z.; Zhang, X.; Huang, Y.; Li, S.; Mao, Y.; Ploehn, H.J.; Bao, Y.; Yu, M. Ultrathin Molecular-Sieving Graphene Oxide Membranes for Selective Hydrogen Separation. Science 2013, 342, 95–98. [Google Scholar] [CrossRef]
- Golchoobi, A.; Tasharrofi, S.; Taghdisian, H. Functionalized nanoporous graphene membrane for water desalination; Effect of feed salinity on permeability and salt rejection, a molecular dynamics study. Comput. Mater. Sci. 2020, 172, 109399. [Google Scholar] [CrossRef]
- Golzar, K.; Modarress, H.; Amjad-Iranagh, S. Separation of gases by using pristine, composite and nanocomposite polymeric membranes: A molecular dynamics simulation study. J. Membr. Sci. 2017, 539, 238–256. [Google Scholar] [CrossRef]
- Karatasos, K.; Kritikos, G. Characterization of a graphene oxide/poly(acrylic acid) nanocomposite by means of molecular dynamics simulations. RSC Adv. 2016, 6, 109267–109277. [Google Scholar] [CrossRef]
- Khosravi, V.; Mahmood, S.M.; Zivar, D.; Sharifigaliuk, H. Investigating the Applicability of Molecular Dynamics Simulation for Estimating the Wettability of Sandstone Hydrocarbon Formations. ACS Omega 2020, 5, 22852–22860. [Google Scholar] [CrossRef]
- Zalc, J.M.; Reyes, S.C.; Iglesia, E. Monte-Carlo simulations of surface and gas phase diffusion in complex porous structures. Chem. Eng. Sci. 2003, 58, 4605–4617. [Google Scholar] [CrossRef]
- Salestan, S.K.; Seyedpour, S.F.; Rahimpour, A.; Shamsabadi, A.A.; Tiraferri, A.; Soroush, M. Molecular Dynamics Insights into the Structural and Water Transport Properties of a Forward Osmosis Polyamide Thin-Film Nanocomposite Membrane Modified with Graphene Quantum Dots. Ind. Eng. Chem. Res. 2020, 59, 14447–14457. [Google Scholar] [CrossRef]
- Szutkowski, K.; Sikorska, E.; Bakanovych, I.; Choudhury, A.R.; Perdih, A.; Jurga, S.; Novic, M.; Zhukov, I. Structural Analysis and Dynamic Processes of the Transmembrane Segment Inside Different Micellar Environments-Implications for the TM4 Fragment of the Bilitranslocase Protein. Int. J. Mol. Sci. 2019, 20, 4172. [Google Scholar] [CrossRef] [Green Version]
- Shen, A. Effects of Gas Concentration Difference on Permeation Separation. J. Yuzhou Univ. 1990, 23, 209–233. [Google Scholar] [CrossRef]
- Backhaus, C.; Werneke, H. Method for Separating a Membrane and Device for Separating a Membrane for Enriching at Least One Gas Component in a Gas Flow. U.S. Patent 20050229778A1, 20 October 2005. [Google Scholar]
- Zheng, Q. Efficient CO2 Delivery from Flue Gas to Microalgae Ponds through a Novel Membrane System. Ph.D. Thesis, University of Melbourne, Melbourne, Australia, 2017. [Google Scholar]
- Dong, L.I.; Hao, J. Temperature-pressure-permeability equation of gas separation in inorganic membrane and its application on adsorption. Membr. Sci. Technol. 2018, 38, 127–131. [Google Scholar]
- Wijmans, J.G.; Baker, R.W. The solution-diffusion model a review. J. Membr. Sci. 1995, 107, 1–21. [Google Scholar] [CrossRef]
- Lee, S.; Mattice, W.L. A “phantom bubble” model for the distribution of free volume in polymers. Comput. Theor. Polym. Sci. 1999, 9, 57–61. [Google Scholar] [CrossRef]
- Velioğlu, S.; Ahunbay, M.G.; Tantekin-Ersolmaz, S.B. Investigation of CO2-induced plasticization in fluorinated polyimide membranes via molecular simulation. J. Membr. Sci. 2012, 417–418, 217–227. [Google Scholar] [CrossRef]
- Ahmadi, M.; Tas, E.; Kilic, A.; Kumbaraci, V.; Talinli, N.; Ahunbay, M.G.; Tantekin-Ersolmaz, S.B. Highly CO(2) Selective Microporous Metal-Imidazolate Framework-Based Mixed Matrix Membranes. ACS Appl. Mater. Interfaces 2017, 9, 35936–35946. [Google Scholar] [CrossRef] [PubMed]
- Kılıç, A.; Atalay-Oral, Ç.; Sirkecioğlu, A.; Tantekin-Ersolmaz, Ş.B.; Ahunbay, M.G. Sod-ZMOF/Matrimid (R) mixed matrix membranes for CO2 separation. J. Membr. Sci. 2015, 489, 81–89. [Google Scholar] [CrossRef]
- Liu, Y.; Cui, X.; Yan, W.; Wang, J.; Su, J.; Jin, L. A molecular level based parametric study of transport behavior in different polymer composite membranes for water vapor separation. Appl. Energ. 2022, 326, 120007. [Google Scholar] [CrossRef]
- Yu, Y.; Zhao, C.; Tao, Y.; Chen, X.; He, Y.L. Superior thermal energy storage performance of NaCl-SWCNT composite phase change materials: A molecular dynamics approach. Appl. Energ. 2021, 290, 116799. [Google Scholar] [CrossRef]
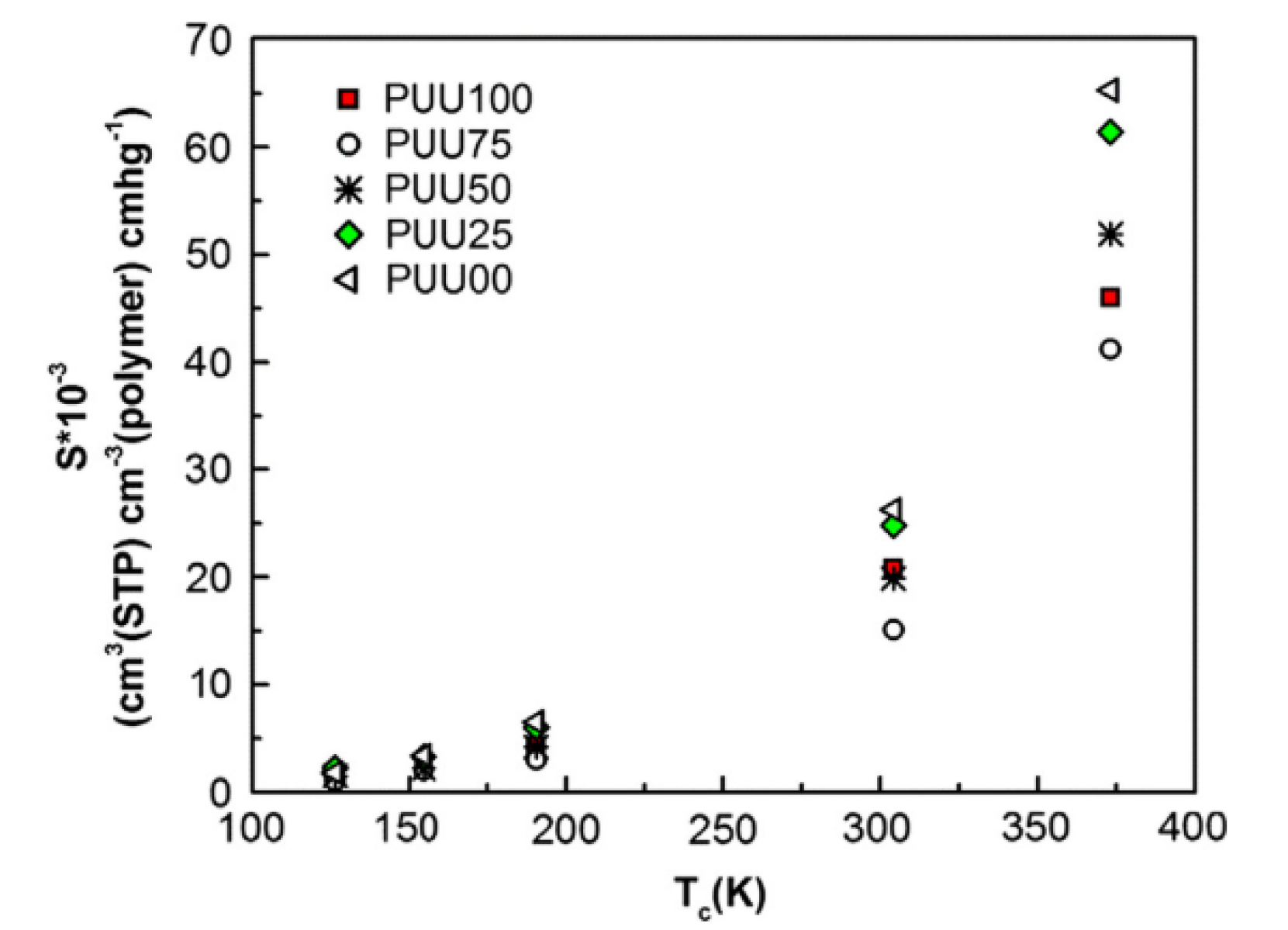
- Amani, M.; Amjad-Iranagh, S.; Golzar, K.; Sadeghi, G.M.M.; Modarress, H. Study of nanostructure characterizations and gas separation properties of poly(urethane–urea)s membranes by molecular dynamics simulation. J. Membr. Sci. 2014, 462, 28–41. [Google Scholar] [CrossRef]
- Azizi, M.; Mousavi, S.A. CO2/H2 separation using a highly permeable polyurethane membrane: Molecular dynamics simulation. J. Mol. Struct. 2015, 1100, 401–414. [Google Scholar] [CrossRef]
- Chang, K.-S.; Yoshioka, T.; Kanezashi, M.; Tsuru, T.; Tung, K.-L. Molecular simulation of micro-structures and gas diffusion behavior of organic–inorganic hybrid amorphous silica membranes. J. Membr. Sci. 2011, 381, 90–101. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Q.L.; Zhu, A.M.; Zhang, Q.G.; Wu, J.Y. Molecular simulation of CO2/CH4 permeabilities in polyamide–imide isomers. J. Membr. Sci. 2010, 348, 204–212. [Google Scholar] [CrossRef]
- Hölck, O.; Dermitzaki, E.; Wunderle, B.; Bauer, J.; Michel, B. Basic thermo-mechanical property estimation of a 3D-crosslinked epoxy/SiO2 interface using molecular modelling. MiRe 2011, 51, 1027–1034. [Google Scholar] [CrossRef]
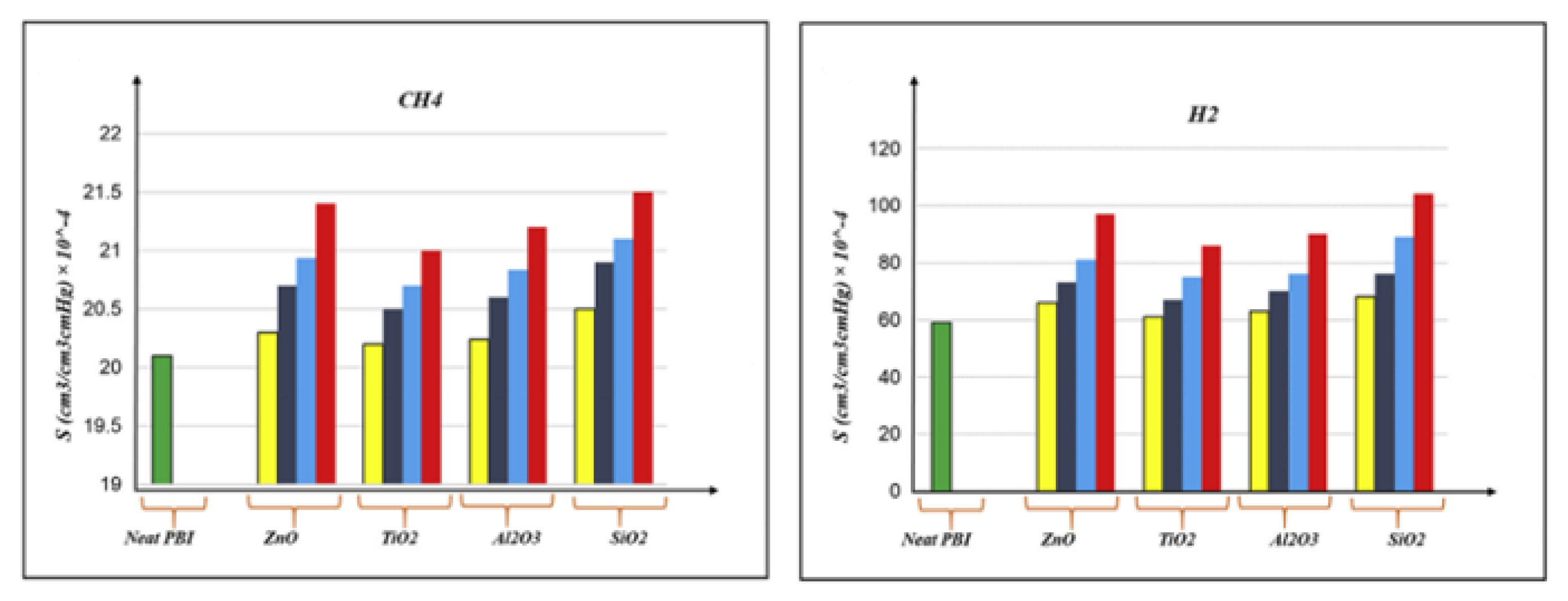
- Khosravanian, A.; Dehghani, M.; Pazirofteh, M.; Asghari, M.; Mohammadi, A.H.; Shahsavari, D. Grand canonical Monte Carlo and molecular dynamics simulations of the structural properties, diffusion and adsorption of hydrogen molecules through poly(benzimidazoles)/nanoparticle oxides composites. Int. J. Hydrogen Energy 2018, 43, 2803–2816. [Google Scholar] [CrossRef]
- Liu, S.; Cao, X.; Wang, H.; Yuan, S.; Hou, S. Zwitterionic polymers functionalised nanoporous graphene for water desalination: A molecular dynamics study. Mol. Simul. 2017, 44, 349–357. [Google Scholar] [CrossRef]
- Liu, Y.; Su, J.; Duan, F.; Cui, X.; Yan, W.; Jin, L. Molecular simulation of enhanced separation of humid air components using GO-PVA nanocomposite membranes under differential pressures. Phys. Chem. Chem. Phys. 2022, 24, 16442–16452. [Google Scholar] [CrossRef]
- Amirkhani, F.; Harami, H.R.; Asghari, M. CO2/CH4 mixed gas separation using poly(ether-b-amide)-ZnO nanocomposite membranes: Experimental and molecular dynamics study. Polym. Test. 2020, 86, 106464. [Google Scholar] [CrossRef]
- Zhang, Q.G.; Liu, Q.L.; Wu, J.Y.; Chen, Y.; Zhu, A.M. Structure-related diffusion in poly(methyl methacrylate)/polyhedral oligomeric silsesquioxanes composites: A molecular dynamics simulation study. J. Membr. Sci. 2009, 342, 105–112. [Google Scholar] [CrossRef]
- Golzar, K.; Amjad-Iranagh, S.; Amani, M.; Modarress, H. Molecular simulation study of penetrant gas transport properties into the pure and nanosized silica particles filled polysulfone membranes. J. Membr. Sci. 2014, 451, 117–134. [Google Scholar] [CrossRef]
- Dashti, A.; Asghari, M.; Dehghani, M.; Rezakazemi, M.; Mohammadi, A.H.; Bhatia, S.K. Molecular dynamics, grand canonical Monte Carlo and expert simulations and modeling of water–acetic acid pervaporation using polyvinyl alcohol/tetraethyl orthosilicates membrane. J. Mol. Liq. 2018, 265, 53–68. [Google Scholar] [CrossRef]
- Liang, T.; Yang, X.; Zhang, X. Prediction of polyimide materials with high glass-transition temperatures. J. Polym. Sci. Part B Polym. Phys. 2001, 39, 2243–2251. [Google Scholar] [CrossRef]
- Mark, J.E.; Ngai, K.; Graessley, W.W.; Mandelkern, L.; Wignall, G. Physical Properties of Polymers. In Physical Properties of Polymers; Cambridge University Press: Cambridge, UK, 2004. [Google Scholar]
- Hatami, A.; Salahshoori, I.; Rashidi, N.; Nasirian, D. The effect of ZIF-90 particle in Pebax/Psf composite membrane on the transport properties of CO2, CH4 and N2 gases by Molecular Dynamics Simulation method. Chin. J. Chem. Eng. 2020, 28, 2267–2284. [Google Scholar] [CrossRef]
- Cheng, X.; Cai, W.; Chen, X.; Shi, Z.; Li, J. Preparation of graphene oxide/poly(vinyl alcohol) composite membrane and pervaporation performance for ethanol dehydration. RSC Adv. 2019, 9, 15457–15465. [Google Scholar] [CrossRef] [Green Version]
- Huang, K.; Liu, G.; Jin, W. Vapor transport in graphene oxide laminates and their application in pervaporation. Curr. Opin. Chem. Eng. 2017, 16, 56–64. [Google Scholar] [CrossRef]
- Connolly, M.L. Computation of molecular volume. J. Am. Chem. Soc. 1985, 107, 1118–1124. [Google Scholar] [CrossRef]
- Park, J.Y.; Paul, D.R. Correlation and prediction of gas permeability in glassy polymer membrane materials via a modified free volume based group contribution method. J. Membr. Sci. 1997, 125, 23–39. [Google Scholar] [CrossRef]
- Dong, X.; Liu, Q.; Cui, L.; Yu, Y.; Zhang, M. Molecular simulation and experimental study on propylene dehumidification through a PVA–PAA blend membrane. J. Mater. Chem. A 2014, 2, 16687–16696. [Google Scholar] [CrossRef]
- Pan, F.; Jia, H.; Jiang, Z.; Zheng, X. Enhanced dehumidification performance of PVA membranes by tuning the water state through incorporating organophosphorus acid. J. Membr. Sci. 2008, 325, 727–734. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, H.; Liu, H.; Hu, J.; Tan, S.; Wu, T. Using diethylamine as crosslinking agent for getting polyepichlorohydrin-based composite membrane with high tensile strength and good chemical stability. Polym. Bull. 2016, 74, 625–639. [Google Scholar] [CrossRef]
- Huang, R.Y.M.; Pal, R.; Moon, G.Y. Crosslinked chitosan composite membrane for the pervaporation dehydration of alcohol mixtures and enhancement of structural stability of chitosanpolysulfone composite membranes. J. Membr. Sci. 1990, 160, 17–30. [Google Scholar] [CrossRef]
- Aburabie, J.; Peinemann, K.-V. Crosslinked poly(ether block amide) composite membranes for organic solvent nanofiltration applications. J. Membr. Sci. 2017, 523, 264–272. [Google Scholar] [CrossRef]
- Zhao, C.; Wu, H.; Li, X.; Pan, F.; Li, Y.; Zhao, J.; Jiang, Z.; Zhang, P.; Cao, X.; Wang, B. High performance composite membranes with a polycarbophil calcium transition layer for pervaporation dehydration of ethanol. J. Membr. Sci. 2013, 429, 409–417. [Google Scholar] [CrossRef]
- Shao, P.; Ji, G.; Chen, P. Gold nanotube membranes: Preparation, characterization and application for enantioseparation. J. Membr. Sci. 2005, 255, 1–11. [Google Scholar] [CrossRef]
- Sun, J.; Huang, L.; Wei, Y.; Wang, Y.; Chen, D.; Du, W.; Wu, H.; Liu, C. Prevalence of emerging porcine parvoviruses and their co-infections with porcine circovirus type 2 in China. Arch. Virol. 2015, 160, 1339–1344. [Google Scholar] [CrossRef]
- Adoor, S.G.; Prathab, B.; Manjeshwar, L.S.; Aminabhavi, T.M. Mixed matrix membranes of sodium alginate and poly(vinyl alcohol) for pervaporation dehydration of isopropanol at different temperatures. Polymer 2007, 48, 5417–5430. [Google Scholar] [CrossRef]
- Bhowmik, R.; Katti, K.S.; Katti, D. Molecular dynamics simulation of hydroxyapatite–polyacrylic acid interfaces. Polymer 2007, 48, 664–674. [Google Scholar] [CrossRef]
- Wang, B.; Nie, Y.; Ma, J. The effect of bioadhesive on the interfacial compatibility and pervaporation performance of composite membranes by MD and GCMC simulation. J. Mol. Graph. Model. 2018, 80, 113–120. [Google Scholar] [CrossRef]
- Wu, H.; Zhang, X.; Xu, D.; Li, B.; Jiang, Z. Enhancing the interfacial stability and solvent-resistant property of PDMS/PES composite membrane by introducing a bifunctional aminosilane. J. Membr. Sci. 2009, 337, 61–69. [Google Scholar] [CrossRef]
- Truhlar, D.G. Topics in Physical Chemistry; Oxford University Press: New York, NY, USA, 1997. [Google Scholar]
- Zhao, J.; Ma, J.; Chen, J.; Pan, F.; Jiang, Z. Experimental and molecular simulation investigations on interfacial characteristics of gelatin/polyacrylonitrile composite pervaporation membrane. Chem. Eng. J. 2011, 178, 1–7. [Google Scholar] [CrossRef]
- Ma, Y.Q.; Velioğlu, S.; Tanis-Kanbur, M.B.; Wang, R.; Jia, W.C. Mechanistic understanding of the adsorption of natural organic matter by heated aluminum oxide particles (HAOPs) via molecular dynamics simulation. J. Membr. Sci. 2020, 598, 117651. [Google Scholar] [CrossRef]
- Ozkan, A.; Sitharam, M.; Flores-Canales, J.C.; Prabhu, R.; Kurnikova, M. Baseline Comparisons of Complementary Sampling Methods for Assembly Driven by Short-Ranged Pair Potentials toward Fast and Flexible Hybridization. J. Chem. Theory Comput. 2021, 17, 1967–1987. [Google Scholar] [CrossRef]
- Saha, B.B.; El-Sharkawy, I.I.; Shahzad, M.W.; Thu, K.; Ang, L.; Ng, K.C. Fundamental and application aspects of adsorption cooling and desalination. Appl. Therm. Eng. 2016, 97, 68–76. [Google Scholar] [CrossRef] [Green Version]
- Sun, S.-Y.; Nie, X.-Y.; Huang, J.; Yu, J.-G. Molecular simulation of diffusion behavior of counterions within polyelectrolyte membranes used in electrodialysis. J. Membr. Sci. 2020, 595, 117528. [Google Scholar] [CrossRef]
- Roy, P.K.; Kumar, K.; Thakkar, F.M.; Pathak, A.D.; Ayappa, K.G.; Maiti, P.K. Investigations on 6FDA/BPDA-DAM polymer melt properties and CO2 adsorption using molecular dynamics simulations. J. Membr. Sci. 2020, 613, 118377. [Google Scholar] [CrossRef]
- Cabrales-Navarro, F.A.; Gómez-Ballesteros, J.L.; Balbuena, P.B. Molecular dynamics simulations of metal-organic frameworks as membranes for gas mixtures separation. J. Membr. Sci. 2013, 428, 241–250. [Google Scholar] [CrossRef]
- Liu, C.; Ding, C.; Hao, X.; Li, C.; An, X.; Wang, P.; Zhang, B.; Gao, F.; Peng, C.; Guan, G. Molecular dynamics simulation and experimental investigation of furfural separation from aqueous solutions via PEBA-2533 membranes. Sep. Purif. Technol. 2018, 207, 42–50. [Google Scholar] [CrossRef]
- Asghari, M.; Mosadegh, M.; Harami, H.R. Supported PEBA-zeolite 13X nano-composite membranes for gas separation: Preparation, characterization and molecular dynamics simulation. Chem. Eng. Sci. 2018, 187, 67–78. [Google Scholar] [CrossRef]
- Lim, S.Y.; Tsotsis, T.T.; Sahimi, M. Molecular simulation of diffusion and sorption of gases in an amorphous polymer. J. Chem. Phys. 2003, 119, 496–504. [Google Scholar] [CrossRef]
- Ni, F.; Wang, G.; Zhao, H. Molecular and condition parameters dependent diffusion coefficient of water in poly(vinyl alcohol): A molecular dynamics simulation study. Colloid. Polym. Sci. 2017, 295, 859–868. [Google Scholar] [CrossRef]
- Metz, S.; Vandeven, W.; Potreck, J.; Mulder, M.; Wessling, M. Transport of water vapor and inert gas mixtures through highly selective and highly permeable polymer membranes. J. Membr. Sci. 2005, 251, 29–41. [Google Scholar] [CrossRef]
- Comesaña-Gándara, B.; Chen, J.; Bezzu, C.G.; Carta, M.; Rose, I.; Ferrari, M.-C.; Esposito, E.; Fuoco, A.; Jansen, J.C.; McKeown, N.B. Redefining the Robeson upper bounds for CO2/CH4 and CO2/N2 separations using a series of ultrapermeable benzotriptycene-based polymers of intrinsic microporosity. Energy Environ. Sci. 2019, 12, 2733–2740. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.; Guo, W.; Mahurin, S.M.; Wang, S.; Chen, H.; Cheng, L.; Jie, K.; Meyer, H.M.; Jiang, D.-e.; Liu, G.; et al. Surpassing Robeson Upper Limit for CO2/N2 Separation with Fluorinated Carbon Molecular Sieve Membranes. Chem 2020, 6, 631–645. [Google Scholar] [CrossRef]
- Akhtar, F.H.; Vovushua, H.; Villalobos, L.F.; Shevate, R.; Kumar, M.; Nunes, S.P.; Schwingenschlögl, U.; Peinemann, K.-V. Highways for water molecules: Interplay between nanostructure and water vapor transport in block copolymer membranes. J. Membr. Sci. 2019, 572, 641–649. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Li, N.; Cui, X.; Yan, W.; Su, J.; Jin, L. A Review on the Morphology and Material Properties of the Gas Separation Membrane: Molecular Simulation. Membranes 2022, 12, 1274. https://doi.org/10.3390/membranes12121274
Liu Y, Li N, Cui X, Yan W, Su J, Jin L. A Review on the Morphology and Material Properties of the Gas Separation Membrane: Molecular Simulation. Membranes. 2022; 12(12):1274. https://doi.org/10.3390/membranes12121274
Chicago/Turabian StyleLiu, Yilin, Na Li, Xin Cui, Weichao Yan, Jincai Su, and Liwen Jin. 2022. "A Review on the Morphology and Material Properties of the Gas Separation Membrane: Molecular Simulation" Membranes 12, no. 12: 1274. https://doi.org/10.3390/membranes12121274
APA StyleLiu, Y., Li, N., Cui, X., Yan, W., Su, J., & Jin, L. (2022). A Review on the Morphology and Material Properties of the Gas Separation Membrane: Molecular Simulation. Membranes, 12(12), 1274. https://doi.org/10.3390/membranes12121274