Novel Magnetic Mixed Cellulose Acetate Matrix Membranes with Oxygen-Enrichment Potential
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
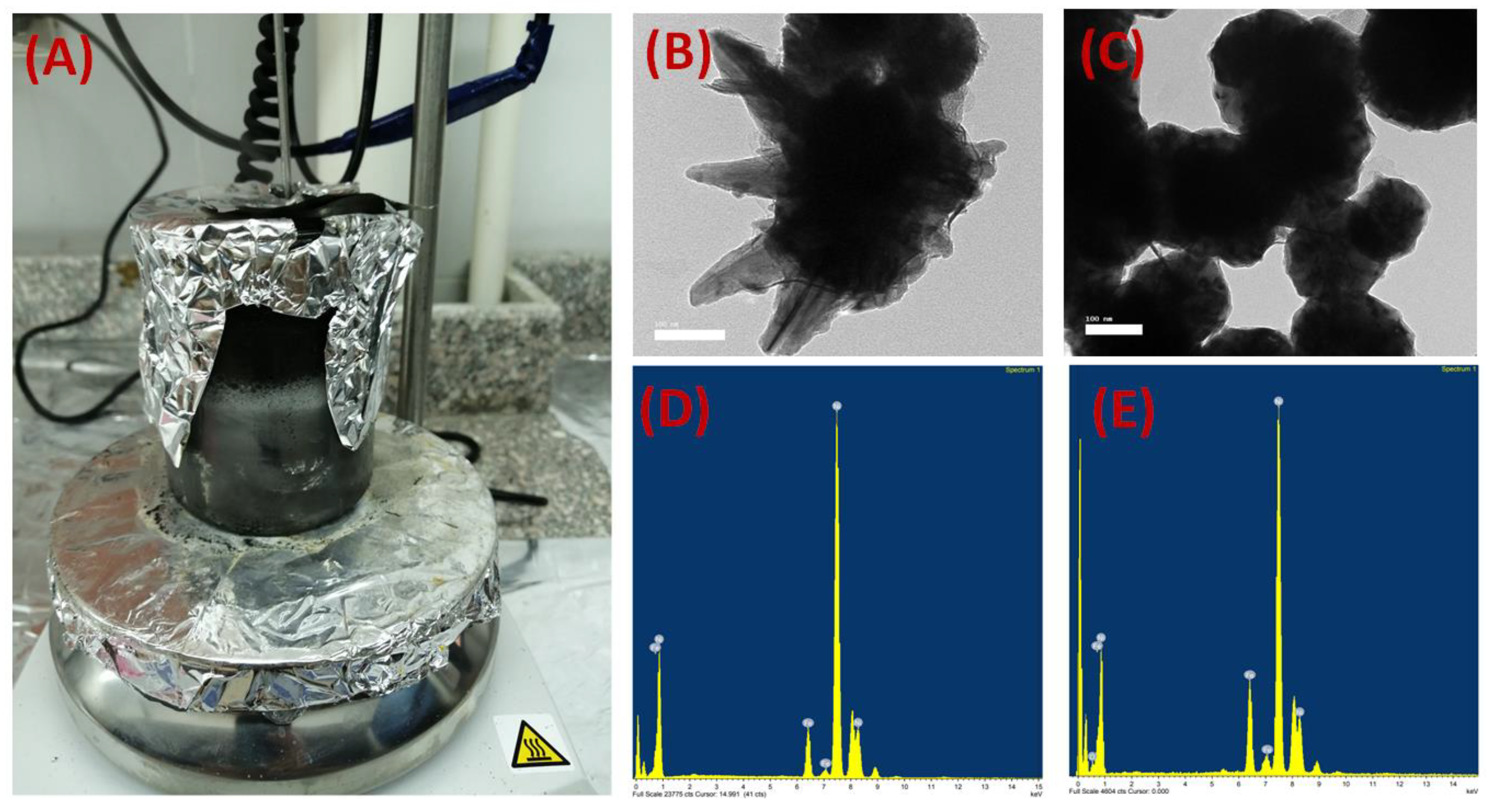
2.2.1. Preparation of the Iron–Nickel Magnetic Alloys/Fillers
2.2.2. Preparation of Cellulose Acetate (CA) Polymeric Dope
2.2.3. Preparation of Magnetic Mixed Cellulose Acetate Matrix Membranes
2.2.4. Iron–Nickel Alloys and Membrane Characterization
3. Results
3.1. Iron–Nickel Alloys/Fillers
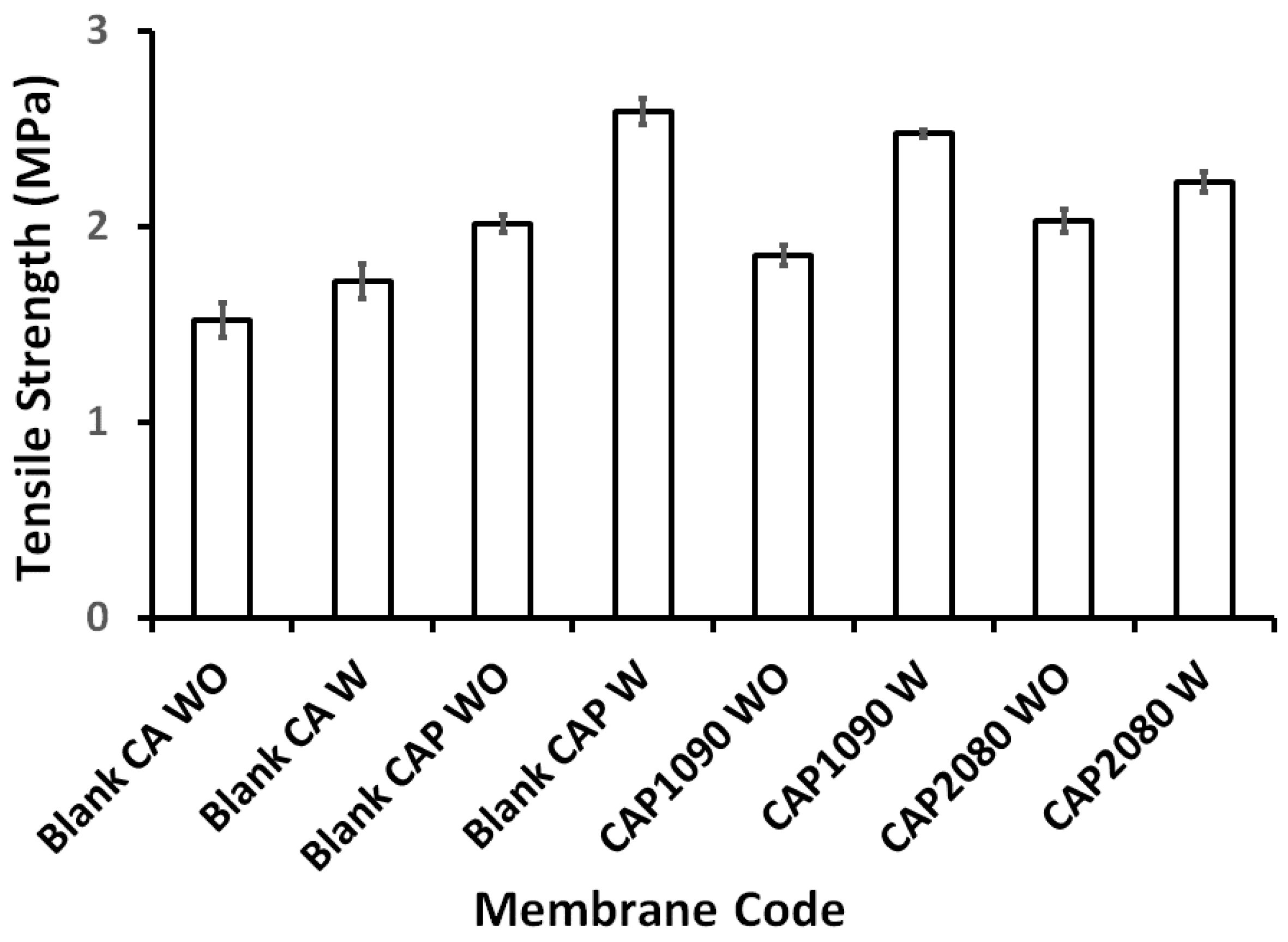
3.2. Magnetic Mixed Cellulose Acetate (CA) Matrix Membranes
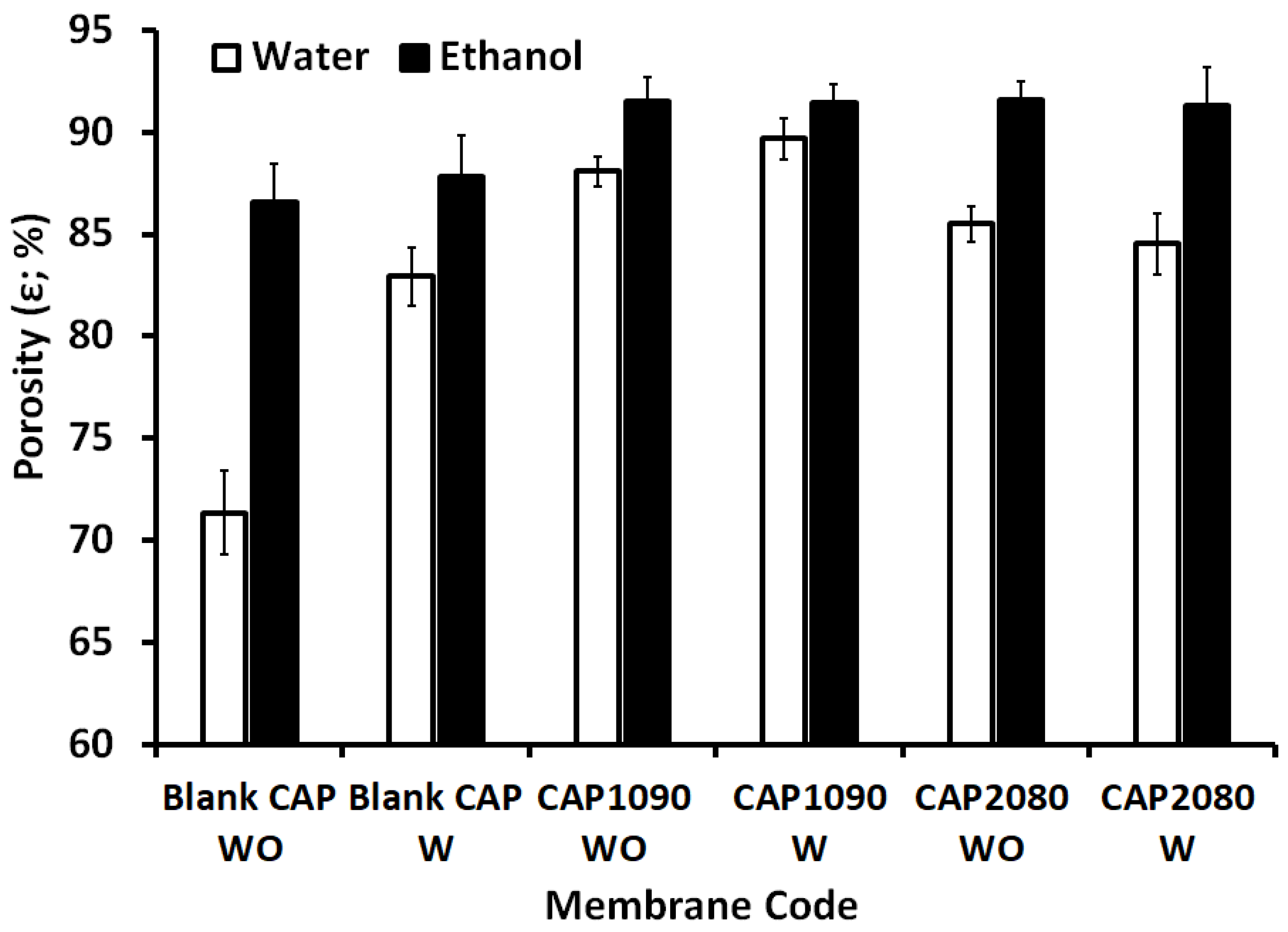
3.3. Membrane Porosity and Thickness
3.4. Membrane Microstructure
3.5. The Elemental Composition
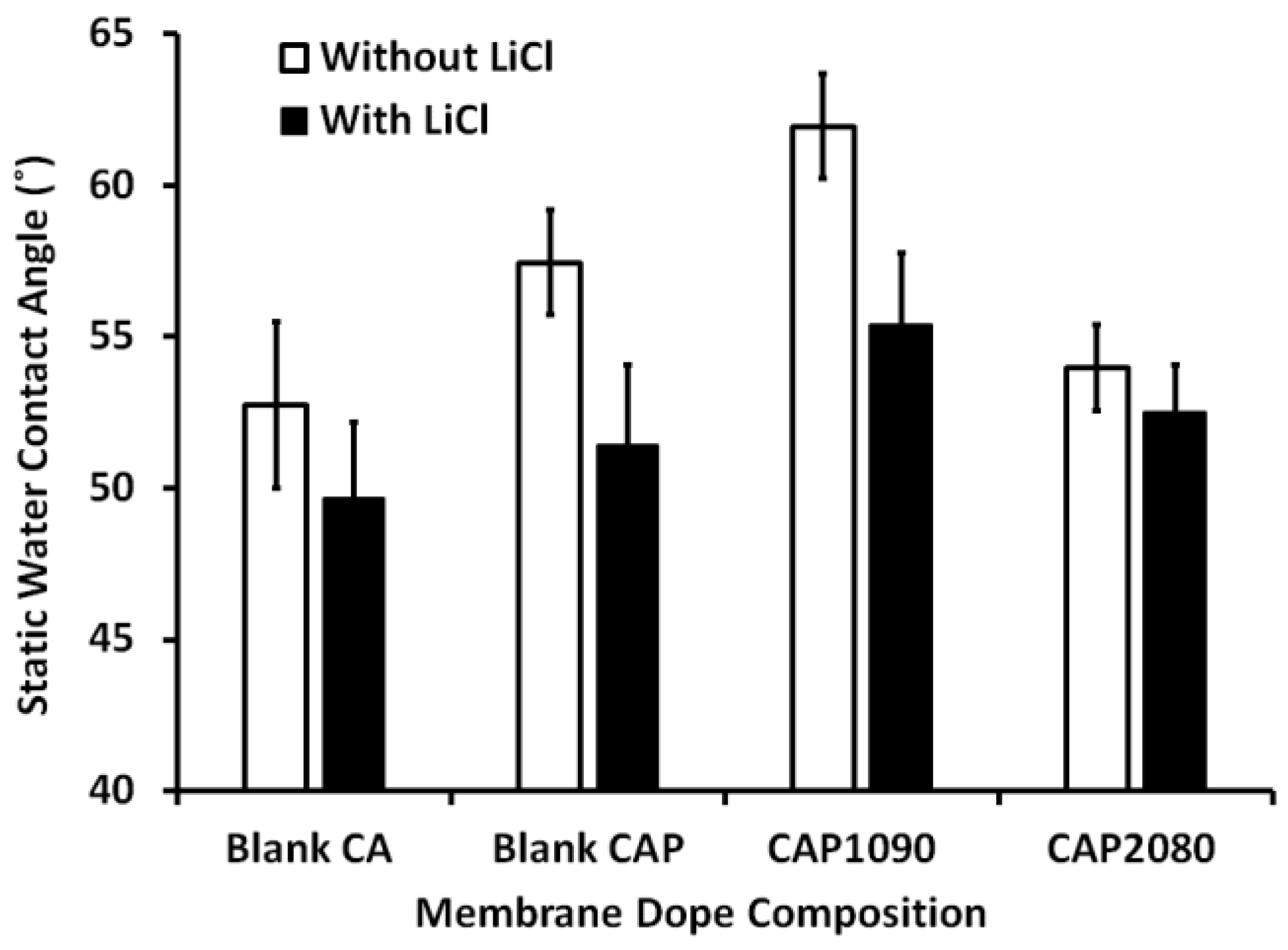
3.6. Membrane Hydrophilicity
3.7. X-ray Diffraction (XRD)
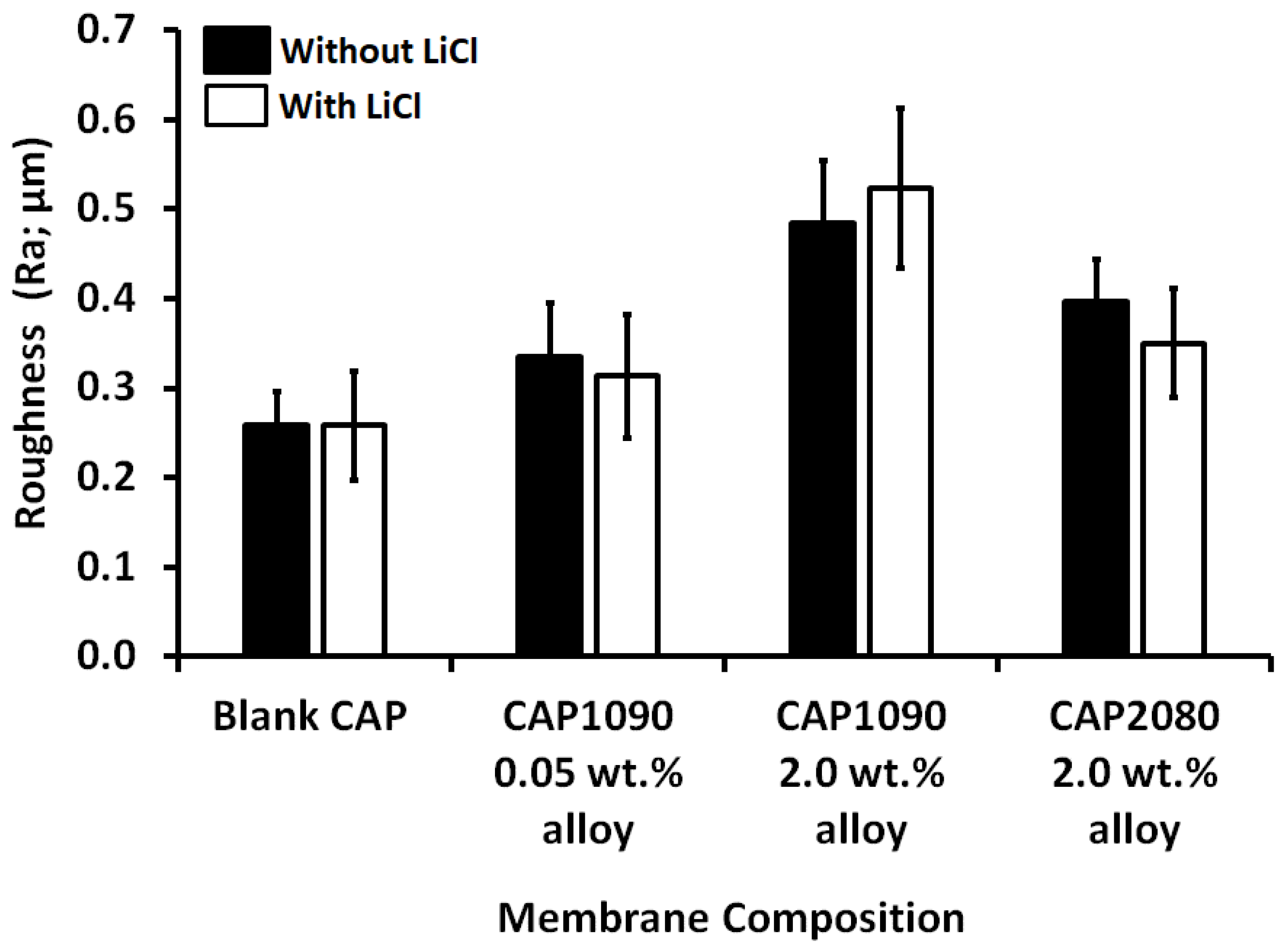
3.8. Membrane Roughness
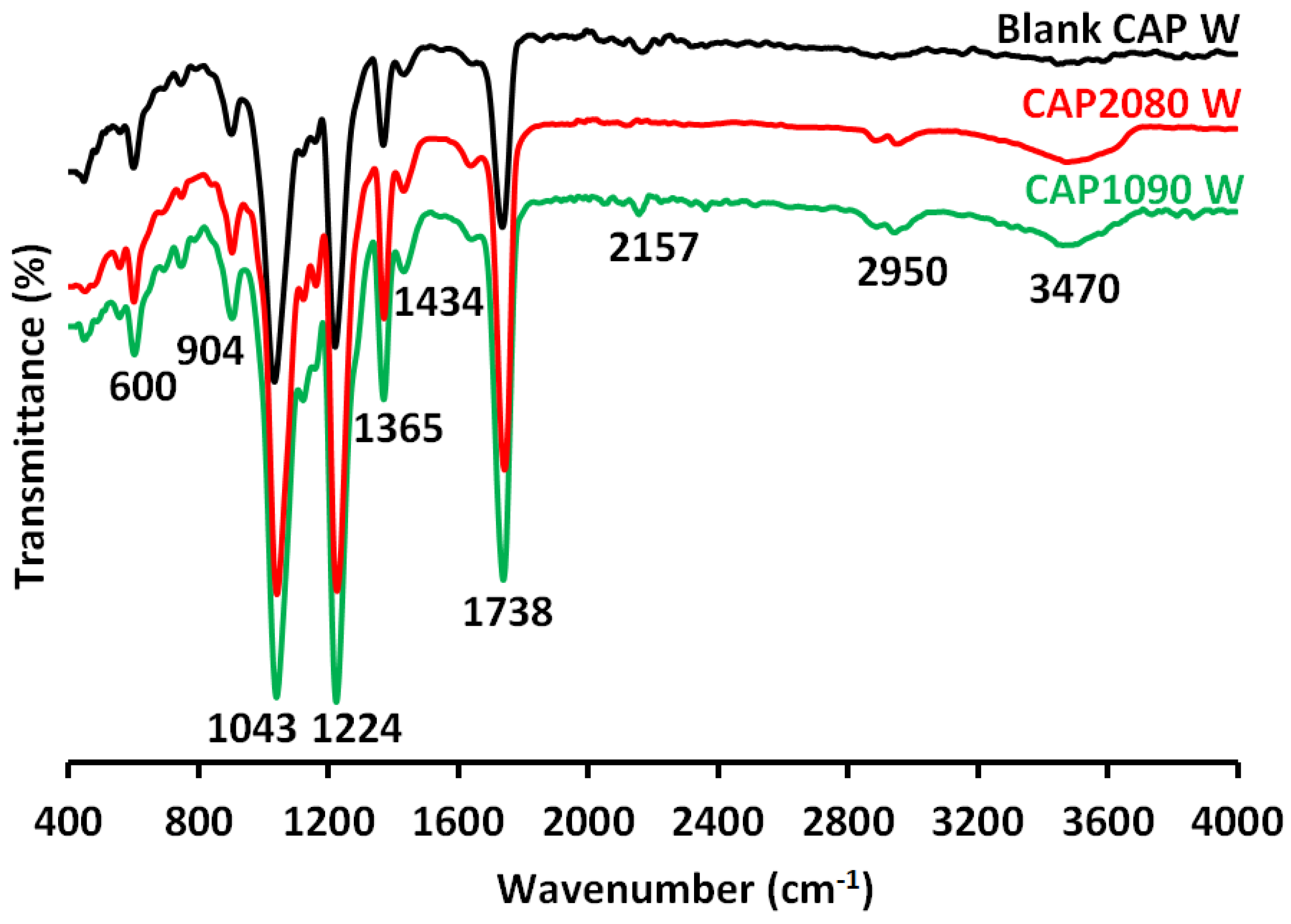
3.9. Fourier-Transform Infrared Spectroscopy-Attenuated Total Reflectance (FTIR-ATR) Analysis
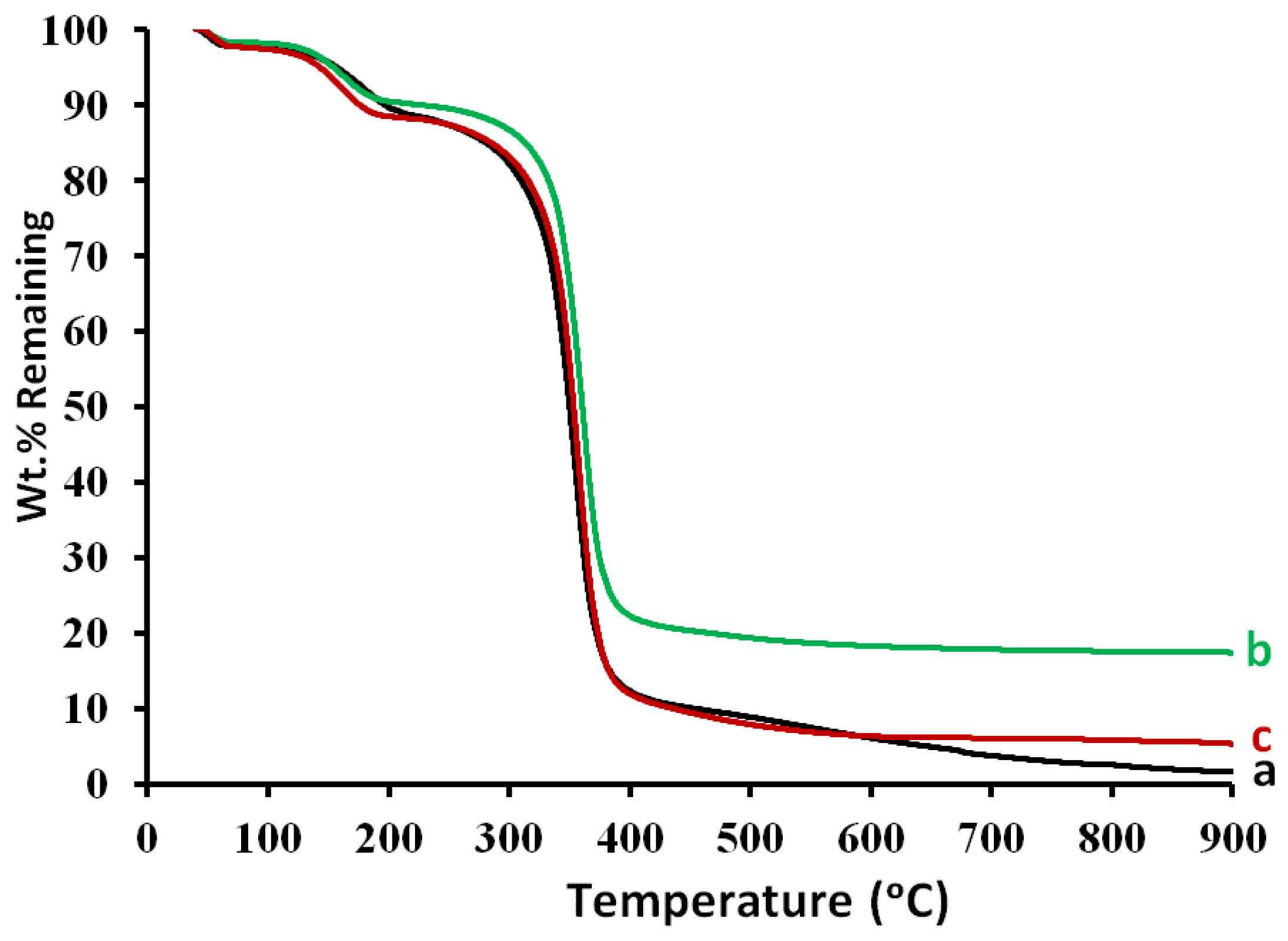
3.10. Thermogravimetric Analysis (TGA)
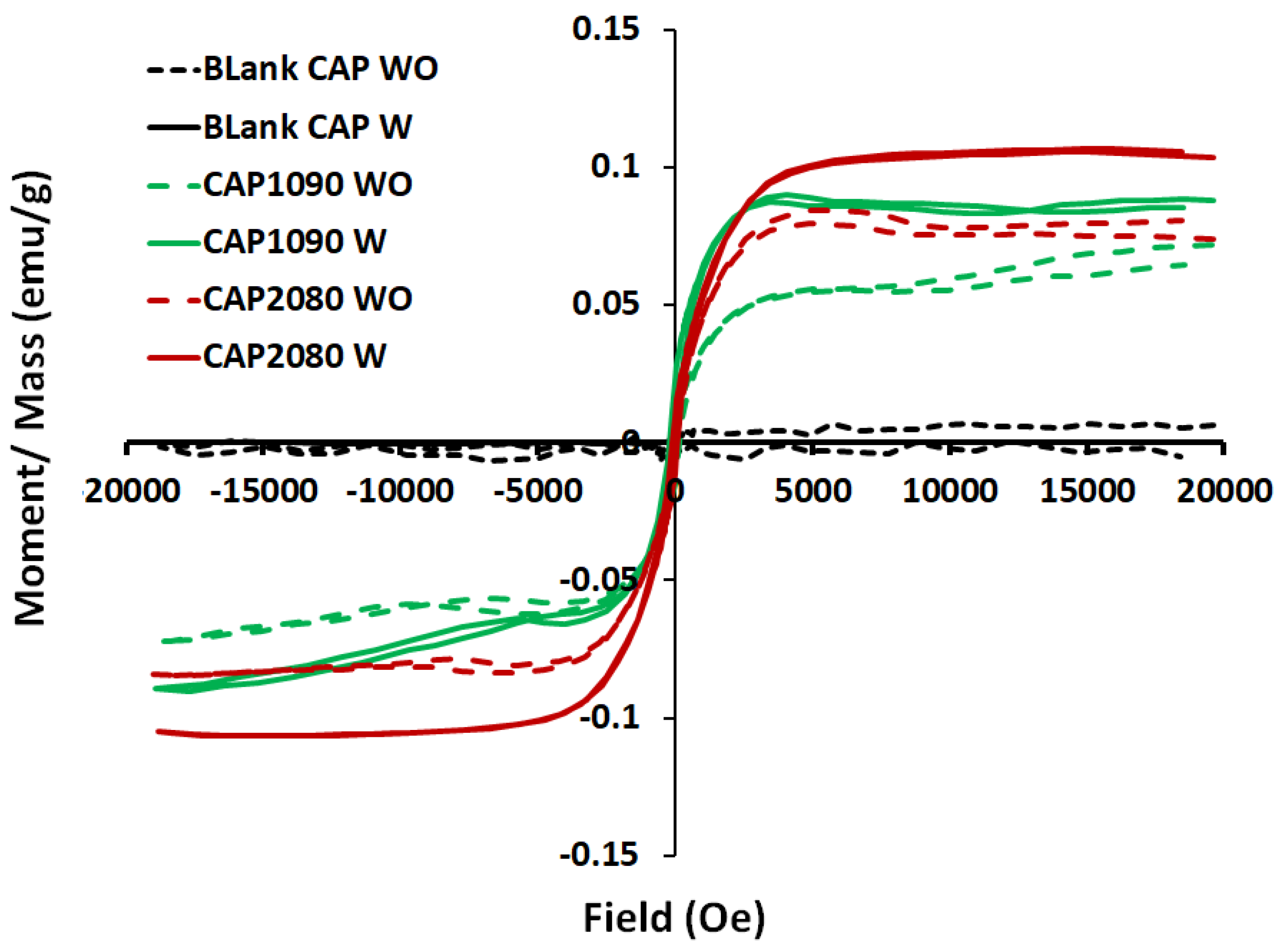
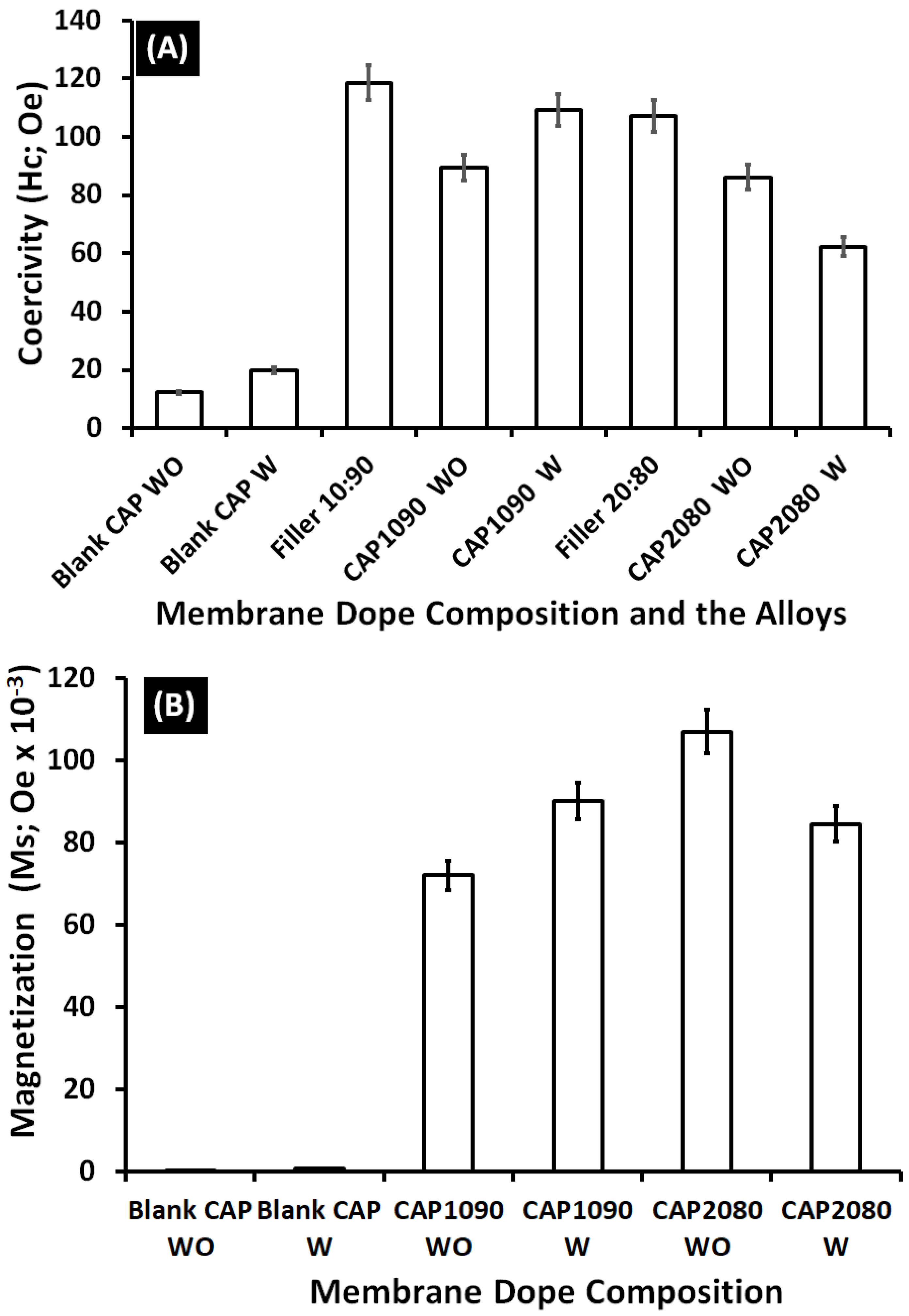
3.11. Vibrating Sample Magnetometer (VSM) Analysis
3.12. Oxygen Transmission Rate (OTR)
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Aroon, M.A.; Ismail, A.F.; Matsuura, T.; Montazer-Rahmati, M.M. Performance studies of mixed matrix membranes for gas separation: A review. Sep. Purif. Technol. 2010, 75, 229–242. [Google Scholar] [CrossRef]
- Qadir, D.; Mukhtar, H.; Keong, L.K. Mixed matrix membranes for water purification applications. Sep. Purif. Rev. 2017, 46, 62–80. [Google Scholar] [CrossRef]
- Tavolaro, P.; Martino, G.; Andò, S.; Tavolaro, A. Fabrication and evaluation of novel zeolite membranes to control the neoplastic activity and anti-tumoral drug treatments in human breast cancer cells. Part 1: Synthesis and characterization of pure zeolite membranes and mixed matrix membranes for adhesion and growth of cancer cells. Mater. Sci. Eng. C 2016, 69, 894–904. [Google Scholar]
- Lin, L.; Wang, A.; Zhang, L.; Dong, M.; Zhang, Y. Novel mixed matrix membranes for sulfur removal and for fuel cell applications. J. Power Sources 2012, 220, 138–146. [Google Scholar] [CrossRef]
- Jia, Z.; Wu, G. Metal-organic frameworks based mixed matrix membranes for pervaporation. Microporous Mesoporous Mater. 2016, 235, 151–159. [Google Scholar] [CrossRef]
- Himma, N.F.; Wardani, A.K.; Prasetya, N.; Aryanti PT, P.; Wenten, I.G. Recent progress and challenges in membrane-based O2/N2 separation. Rev. Chem. Eng. 2019, 35, 591–625. [Google Scholar] [CrossRef]
- Shakeel, I.; Hussain, A.; Farrukh, S. Effect analysis of nickel ferrite (NiFe2O4) and titanium dioxide (TiO2) nanoparticles on CH4/CO2 gas permeation properties of cellulose acetate based mixed matrix membranes. J. Polym. Environ. 2019, 27, 1449–1464. [Google Scholar] [CrossRef]
- De Mukherjee, R.S. Adsorptive removal of phenolic compounds using cellulose acetate phthalate–alumina nanoparticle mixed matrix membrane. J. Hazard. Mater. 2014, 265, 8–19. [Google Scholar] [CrossRef]
- Nasir, R.; Mukhtar, H.; Man, Z.; Mohshim, D.F. Material Advancements in Fabrication of Mixed-Matrix Membranes. Chem. Eng. Technol. 2013, 36, 717–727. [Google Scholar] [CrossRef]
- Dudek, G.; Turczyn, R.; Strzelewicz, A.; Rybak, A.; Krasowska, M.; Grzywna, Z.J. Preparation and characterization of iron oxides–polymer composite membranes. Sep. Sci. Technol. 2012, 47, 1390–1394. [Google Scholar] [CrossRef]
- Chong, K.; Lai, S.; Thiam, H.; Teoh, H.; Heng, S. Recent progress of oxygen/nitrogen separation using membrane technology. J. Eng. Sci. Technol. 2016, 11, 1016–1030. [Google Scholar]
- Moghadassi, A.R.; Rajabi, Z.; Hosseini, S.M.; Mohammadi, M. Fabrication and modification of cellulose acetate based mixed matrix membrane: Gas separation and physical properties. J. Ind. Eng. Chem. 2014, 20, 1050–1060. [Google Scholar] [CrossRef]
- Nouri, M.; Marjani, A. Surface modification of a cellulose acetate membrane using a nanocomposite suspension based on magnetic particles. Cellulose 2019, 26, 7995–8006. [Google Scholar] [CrossRef]
- Sanaeepur, H.; Nasernejad, B.; Kargari, A. Cellulose acetate/nano-porous zeolite mixed matrix membrane for CO2 separation. Greenh. Gases Sci. Technol. 2015, 5, 291–304. [Google Scholar] [CrossRef]
- Ahmad, A.L.; Jawad, Z.A.; Low, S.C.; Zein SH, S. A cellulose acetate/multi-walled carbon nanotube mixed matrix membrane for CO2/N2 separation. J. Membr. Sci. 2014, 451, 55–66. [Google Scholar] [CrossRef]
- Mubashir, M.; Yeong, Y.F.; Lau, K.K.; Chew, T.L.; Norwahyu, J. Efficient CO2/N2 and CO2/CH4 separation using NH2-MIL-53 (Al)/cellulose acetate (CA) mixed matrix membranes. Sep. Purif. Technol. 2018, 199, 140–151. [Google Scholar] [CrossRef]
- Sahraei, R.; Shahalizade, T.; Ghaemy, M.; Mahdavi, H. Fabrication of cellulose acetate/Fe3O4@GO-APTS-poly(AMPS-co-MA) mixed matrix membrane and its evaluation on anionic dyes removal. Cellulose 2018, 25, 3519–3532. [Google Scholar] [CrossRef]
- Chaudhary, M.; Maiti, A. Fe–Al–Mn@ chitosan based metal oxides blended cellulose acetate mixed matrix membrane for fluoride decontamination from water: Removal mechanisms and antibacterial behavior. J. Membr. Sci. 2020, 611, 118372. [Google Scholar] [CrossRef]
- De Guzman, M.R.; Andra CK, A.; Ang MB, M.Y.; Dizon GV, C.; Caparanga, A.R.; Huang, S.-H.; Lee, K. Increased performance and antifouling of mixed-matrix membranes of cellulose acetate with hydrophilic nanoparticles of polydopamine-sulfobetaine methacrylate for oil-water separation. J. Membr. Sci. 2021, 620, 118881. [Google Scholar] [CrossRef]
- Abdel-Karim, A.; El-Naggar, M.E.; Radwan, E.K.; Mohamed, I.M.; Azaam, M.; Kenawy, E.-R. High-performance mixed-matrix membranes enabled by organically/inorganic modified montmorillonite for the treatment of hazardous textile wastewater. Chem. Eng. J. 2021, 405, 126964. [Google Scholar] [CrossRef]
- Ali AS, M.; Soliman, M.M.; Kandil, S.H.; Khalil MM, A. Emerging mixed matrix membranes based on zeolite nanoparticles and cellulose acetate for water desalination. Cellulose 2021, 28, 6417–6426. [Google Scholar] [CrossRef]
- Andrade, M.C.; Pereira, J.C.; de Almeida, N.; Marques, P.; Faria, M.; Gonçalves, M.C. Improving hydraulic permeability, mechanical properties, and chemical functionality of cellulose acetate-based membranes by co-polymerization with tetraethyl orthosilicate and 3-(aminopropyl)triethoxysilane. Carbohydr. Polym. 2021, 261, 117813. [Google Scholar] [CrossRef] [PubMed]
- Nady, N.; Salem, N.; Mohamed, M.A.; Kandil, S.H. Iron-Nickel Alloy with Starfish-like Shape and Its Unique Magnetic Properties: Effect of Reaction Volume and Metal Concentration on the Synthesized Alloy. Nanomaterials 2021, 11, 3034. [Google Scholar] [CrossRef] [PubMed]
- Nady, N.; Salem, N.; Kandil, S.H. Novel magnetic iron–nickel/poly(ethersulfone) mixed matrix membranes for oxygen separation potential without applying an external magnetic field. Sci. Rep. 2022, 12, 13675. [Google Scholar] [CrossRef]
- Abdel-Karim, A.; Leaper, S.; Alberto, M.; Vijayaraghavan, A.; Fan, X.; Holmes, S.M.; Souaya, E.R.; Badawy, M.I.; Gorgojo, P. High flux and fouling resistant flat sheet polyethersulfone membranes incorporated with graphene oxide for ultrafiltration applications. Chem. Eng. J. 2018, 334, 789–799. [Google Scholar] [CrossRef]
- Frommer, M.A.; Messalen, R.M. Mechanism of membrane formation, VI. Convective flows and large voids formation during membrane preparation. Ind. Eng. Chem. Prod. Res. Dev. 1973, 12, 328. [Google Scholar]
- Cheng, Y.-T.; Rodak, D.E.; Angelopoulos, A.; Gacek, T. Microscopic observations of condensation of water on lotus leaves. Appl. Phys. Lett. 2005, 87, 194112. [Google Scholar] [CrossRef]
- Endoh, R.; Tanaka, T.; Kurihara, M.; Ikeda, K. New Polymeric materials for reverse osmosis membranes. Desalination 1977, 21, 35. [Google Scholar] [CrossRef]
- Mohd Shafie ZM, H.; Ahmad, A.L.; Low, S.C.; Rode, S.; Belaissaoui, B. Lithium chloride (LiCl)-modified polyethersulfone (PES) substrate surface pore architectures on thin poly(dimethylsiloxane) (PDMS) dense layer formation and the composite membrane’s performance in gas separation. RSC Adv. 2020, 10, 9500–9511. [Google Scholar] [CrossRef]
- Ali, U.; Karim, K.J.B.A.; Buang, N.A.A. review of the properties and applications of poly(methyl methacrylate)(PMMA). Polym. Rev. 2015, 55, 678–705. [Google Scholar] [CrossRef]
- Ito, A.; Shin, A.; Nitta, K. Additive effects of lithium halides on the tensile and rheological properties of poly(methyl methacrylate). Polym. J. 2022, 54, 1279–1285. [Google Scholar] [CrossRef]
- Sudiarti, T.; Wahyuningrum, D.; Bundjali, B.; Made Arcana, I. Mechanical strength and ionic conductivity of polymer electrolyte membranes prepared from cellulose acetate-lithium perchlorate. IOP Conf. Ser. Mater. Sci. Eng. 2017, 223, 012052. [Google Scholar] [CrossRef]





| No. | Membrane Code | Filler Concen. (wt.%) | Filler Shape | LiCl Concen. (wt.%) | PMMA Concen. (wt.%) |
|---|---|---|---|---|---|
| 1 | Blank CA WO | 0 | No filler | 0.0 | 0.0 |
| 2 | Blank CAP WO | 0 | No filler | 0.0 | 0.8 |
| 3 | Blank CA W | 0 | No filler | 0.1 | 0.0 |
| 4 | Blank CAP W | 0 | No filler | 0.1 | 0.8 |
| 5 | CA1090 WO | 2 | Starfish-like | 0.0 | 0.0 |
| 6 | CAP1090 WO | 2 | Starfish-like | 0.0 | 0.8 |
| 7 | CA1090 W | 2 | Starfish-like | 0.1 | 0.0 |
| 8 | CAP1090 W | 2 | Starfish-like | 0.1 | 0.8 |
| 9 | CA2080 WO | 2 | Necklace-like | 0.0 | 0.0 |
| 10 | CAP2080 WO | 2 | Necklace-like | 0.0 | 0.8 |
| 11 | CA2080 W | 2 | Necklace-like | 0.1 | 0.0 |
| 12 | CAP2080 W | 2 | Necklace-like | 0.1 | 0.8 |
| Alloy Code | Adsorbed Amount (Vm) | Surface Area (as) | Total Pore Volume | Average Pore Diameter |
|---|---|---|---|---|
| cm3 (STP) g−1 | m2·g−1 | cm3·g−1 | nm | |
| Fe10Ni90—starfish-like | 1.0852 | 4.72 | 0.05 | 43.40 |
| Fe20Ni80—necklace-like | 1.7209 | 7.49 | 0.08 | 40.32 |
| Membrane Code | Atomic % | Mass % | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Fe | Ni | O | C | Cl | Fe | Ni | O | C | Cl | |
| Blank CAP WO | 0.00 | 0.00 | 52.87 | 47.13 | 0.00 | 0.00 | 0.00 | 59.91 | 40.09 | 0.00 |
| Blank CA W | 0.00 | 0.00 | 51.12 | 48.07 | 0.81 | 0.00 | 0.00 | 57.43 | 40.54 | 2.03 |
| CAP1090 WO | 0.16 | 0.75 | 39.42 | 59.67 | 0.00 | 0.64 | 3.15 | 45.04 | 51.17 | 0.00 |
| CAP1090 W | 0.35 | 0.64 | 50.22 | 48.74 | 0.05 | 1.35 | 2.59 | 55.50 | 40.44 | 0.12 |
| CAP2080 WO | 0.22 | 0.07 | 40.11 | 59.60 | 0.00 | 0.74 | 0.15 | 44.04 | 55.07 | 0.00 |
| CAP2080 W | 1.25 | 22.49 | 26.59 | 48.25 | 1.42 | 2.87 | 53.99 | 17.40 | 23.69 | 2.05 |
| Membrane Code | Atomic % | Mass % | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Fe | Ni | O | C | Cl | Fe | Ni | O | C | Cl | |
| Blank CAP W | 0.00 | 0.00 | 2.87 | 97.56 | 0.0 | 0.00 | 0.00 | 3.81 | 97.33 | 0.00 |
| CAP1090 W | 0.78 | 2.49 | 1.44 | 95.09 | 0.20 | 3.20 | 10.75 | 1.69 | 83.84 | 0.53 |
| CAP2080 W | 1.96 | 0.01 | 14.66 | 83.11 | 0.28 | 8.10 | 0.03 | 17.35 | 73.84 | 0.73 |
| No. | Membrane Code | Filler Concen. (wt.%) | Filler Shape | Coercivity (Hc) (emu/g) | Total Porosity (%) | Large-Size Porosity (%) | OTR cm3/(m2·s) × 10−5 |
|---|---|---|---|---|---|---|---|
| 1 | Blank PES W | 0 | No filler | 79.33 | 89 ± 1.0 | 70 ± 6 | 0.00 |
| 2 | Blank CAP W | 0 | No filler | 19.89 | 87 ± 2.0 | 82 ± 2 | 0.01 |
| 3 | PES1090 W | 2 | Starfish-like | 103.40 | 93 ± 0.7 | 88 ± 3 | 3.61 |
| 4 | CAP1090 W | 2 | Starfish-like | 109.26 | 91 ± 0.8 | 89 ± 1 | 1.27 |
| 5 | PES2080 W | 2 | Necklace-like | 73.67 | 92 ± 0.7 | 86 ± 2 | 1.36 |
| 6 | CAP2080 W | 2 | Necklace-like | 62.28 | 91 ± 2.0 | 84 ± 2 | 0.57 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nady, N.; Salem, N.; Elmarghany, M.R.; Salem, M.S.; Kandil, S.H. Novel Magnetic Mixed Cellulose Acetate Matrix Membranes with Oxygen-Enrichment Potential. Membranes 2022, 12, 1259. https://doi.org/10.3390/membranes12121259
Nady N, Salem N, Elmarghany MR, Salem MS, Kandil SH. Novel Magnetic Mixed Cellulose Acetate Matrix Membranes with Oxygen-Enrichment Potential. Membranes. 2022; 12(12):1259. https://doi.org/10.3390/membranes12121259
Chicago/Turabian StyleNady, Norhan, Noha Salem, Mohamed R. Elmarghany, Mohamed S. Salem, and Sherif H. Kandil. 2022. "Novel Magnetic Mixed Cellulose Acetate Matrix Membranes with Oxygen-Enrichment Potential" Membranes 12, no. 12: 1259. https://doi.org/10.3390/membranes12121259
APA StyleNady, N., Salem, N., Elmarghany, M. R., Salem, M. S., & Kandil, S. H. (2022). Novel Magnetic Mixed Cellulose Acetate Matrix Membranes with Oxygen-Enrichment Potential. Membranes, 12(12), 1259. https://doi.org/10.3390/membranes12121259






