Effect of Co-Existing Ions on Salinity Gradient Power Generation by Reverse Electrodialysis Using Different Ion Exchange Membrane Pairs
Abstract
:1. Introduction
2. Materials and Methods
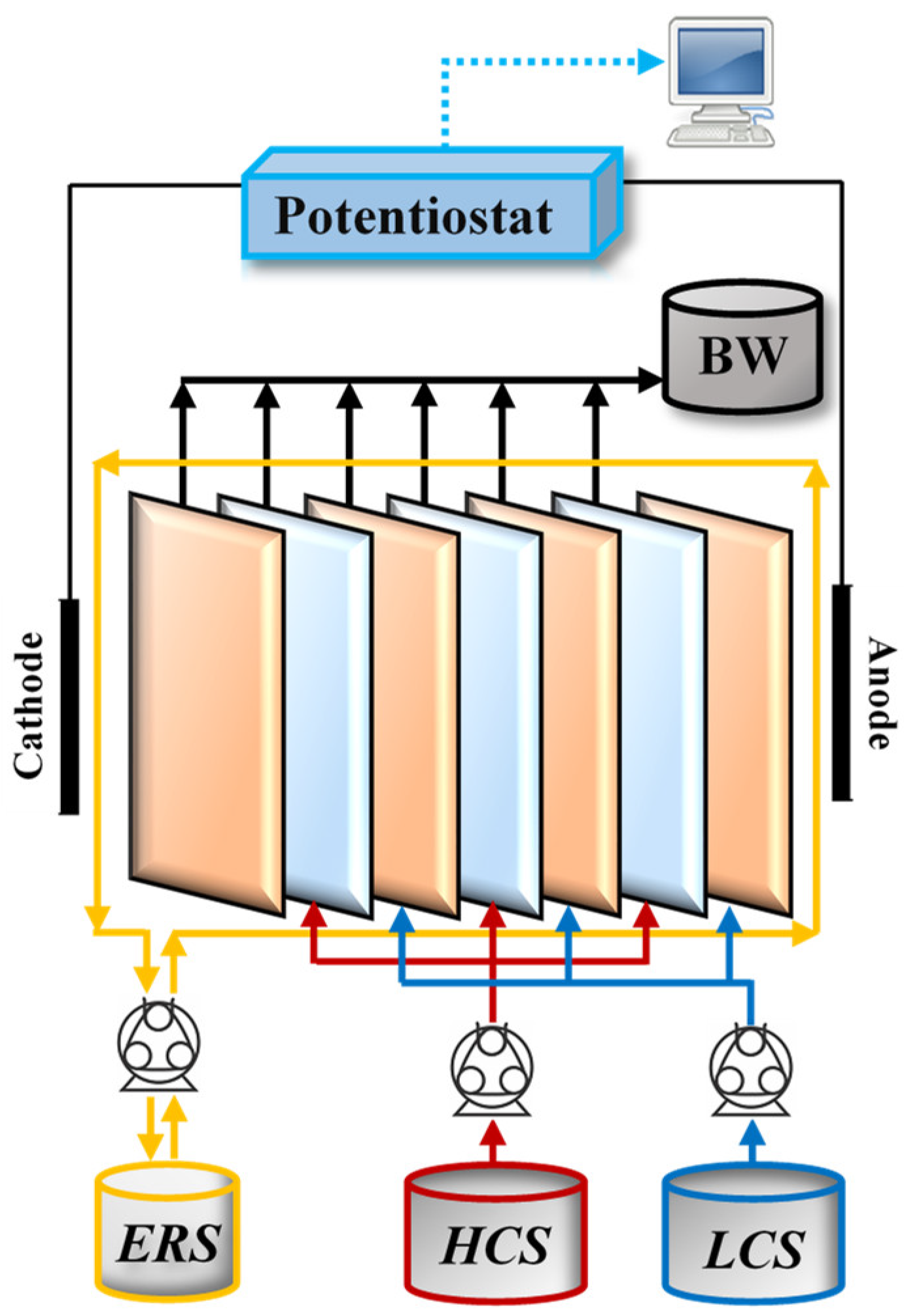
2.1. RED Tests
2.2. Ion Exchange Membranes (IEMs)
2.3. Feed Solutions
2.4. RED Tests Performed for Investigation of Co-Existing Ion Effects
2.5. Performance Analysis of the RED System
3. Results and Discussion
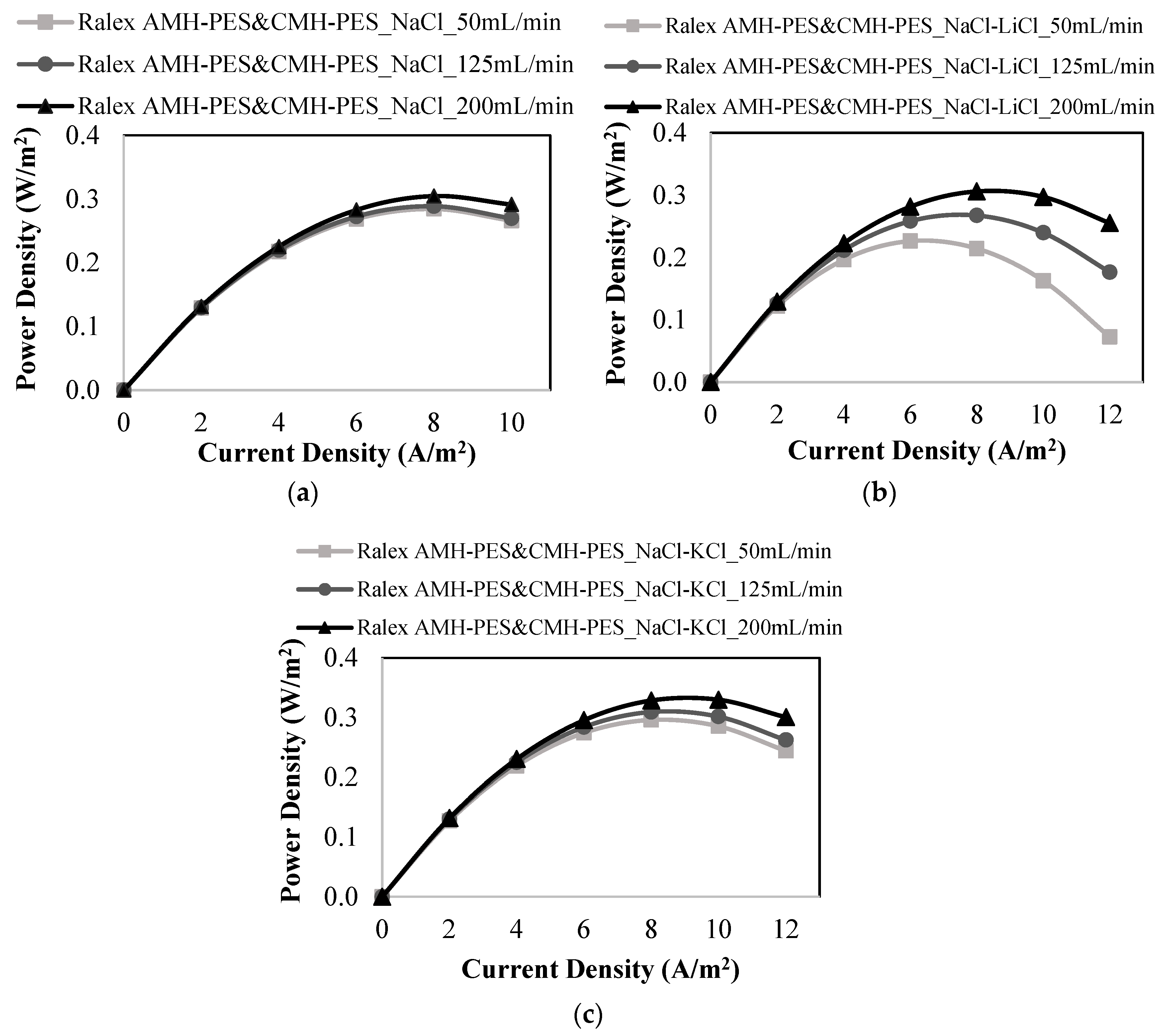
3.1. RED Studies Performed with Ralex Membranes
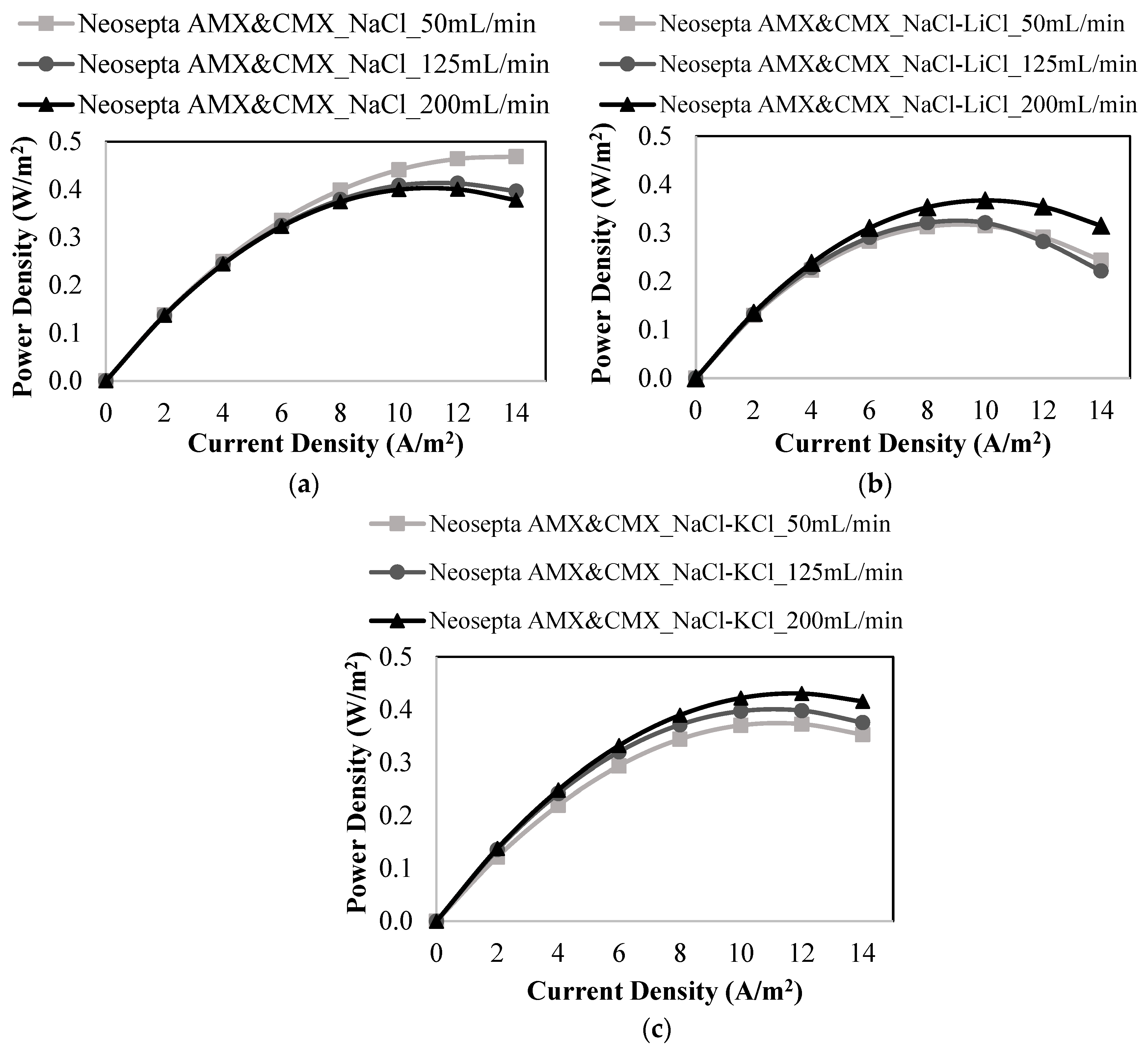
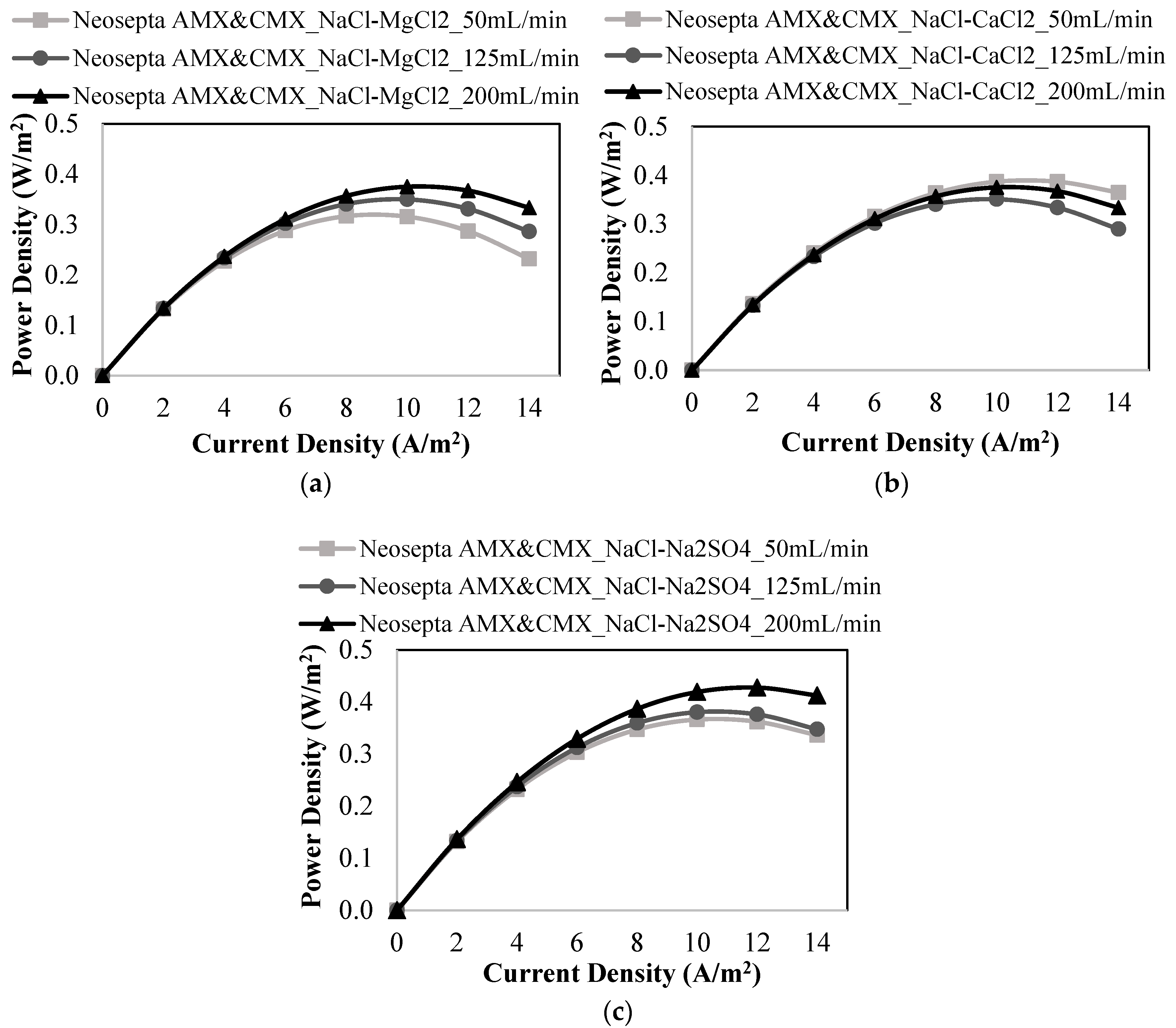
3.2. RED Studies Performed with Neosepta Membranes
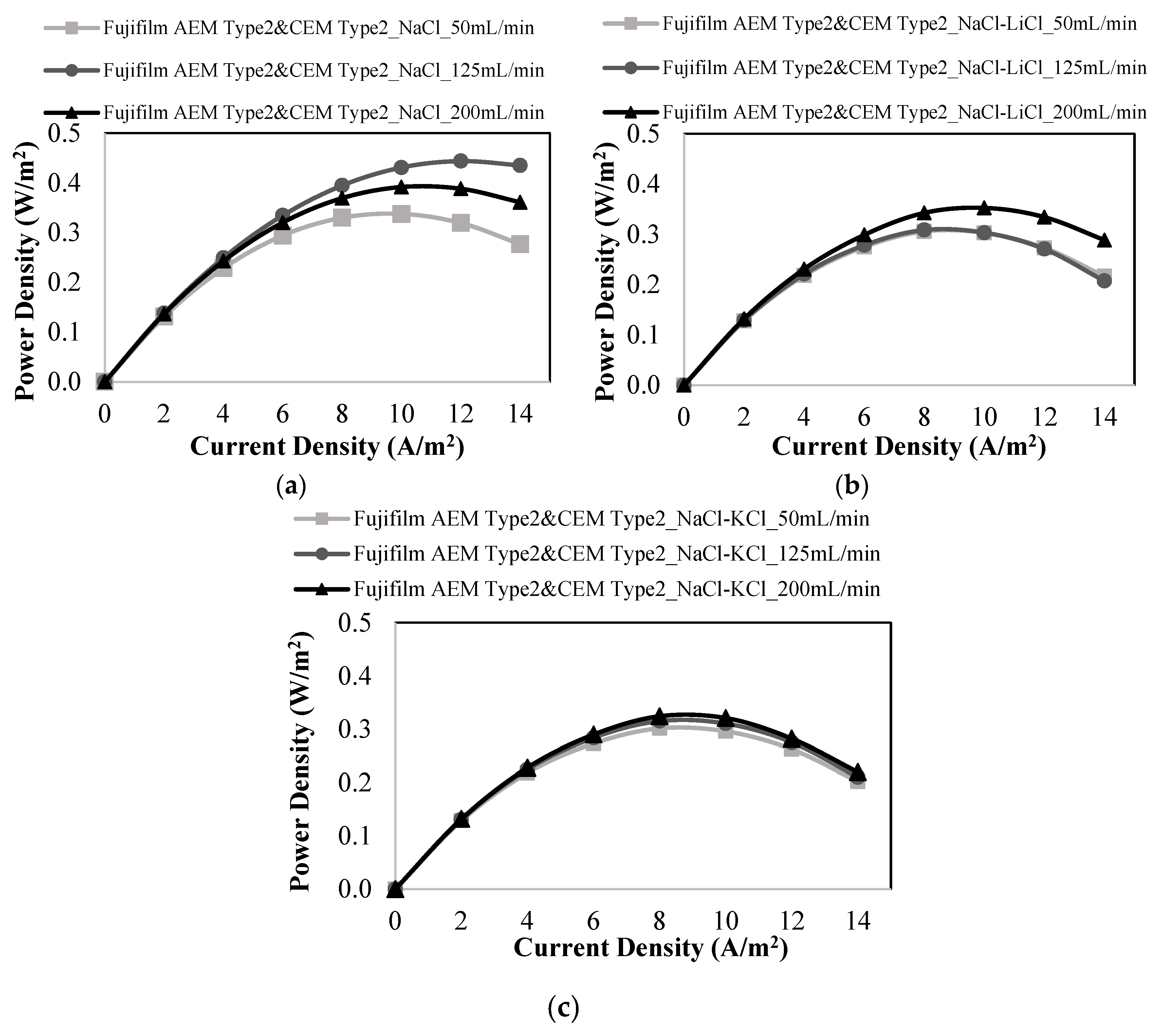
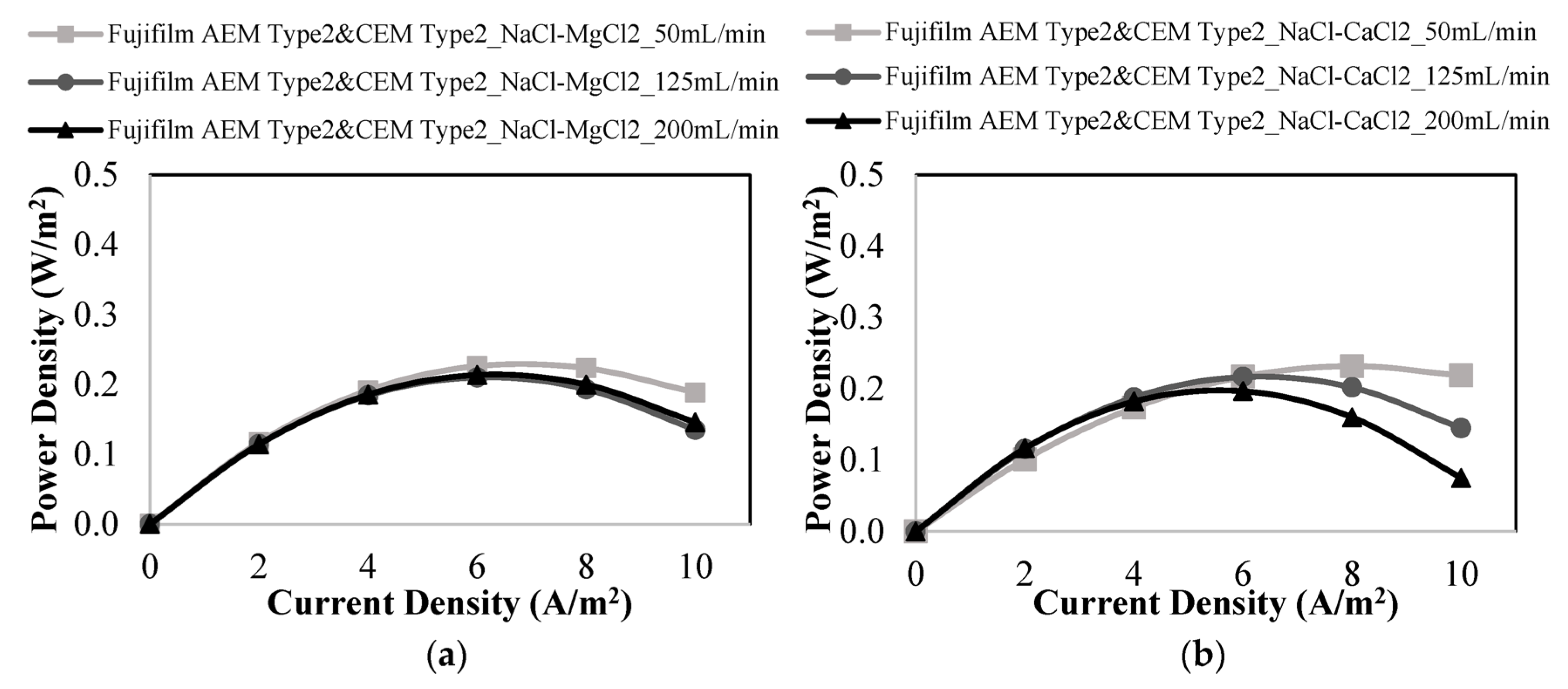
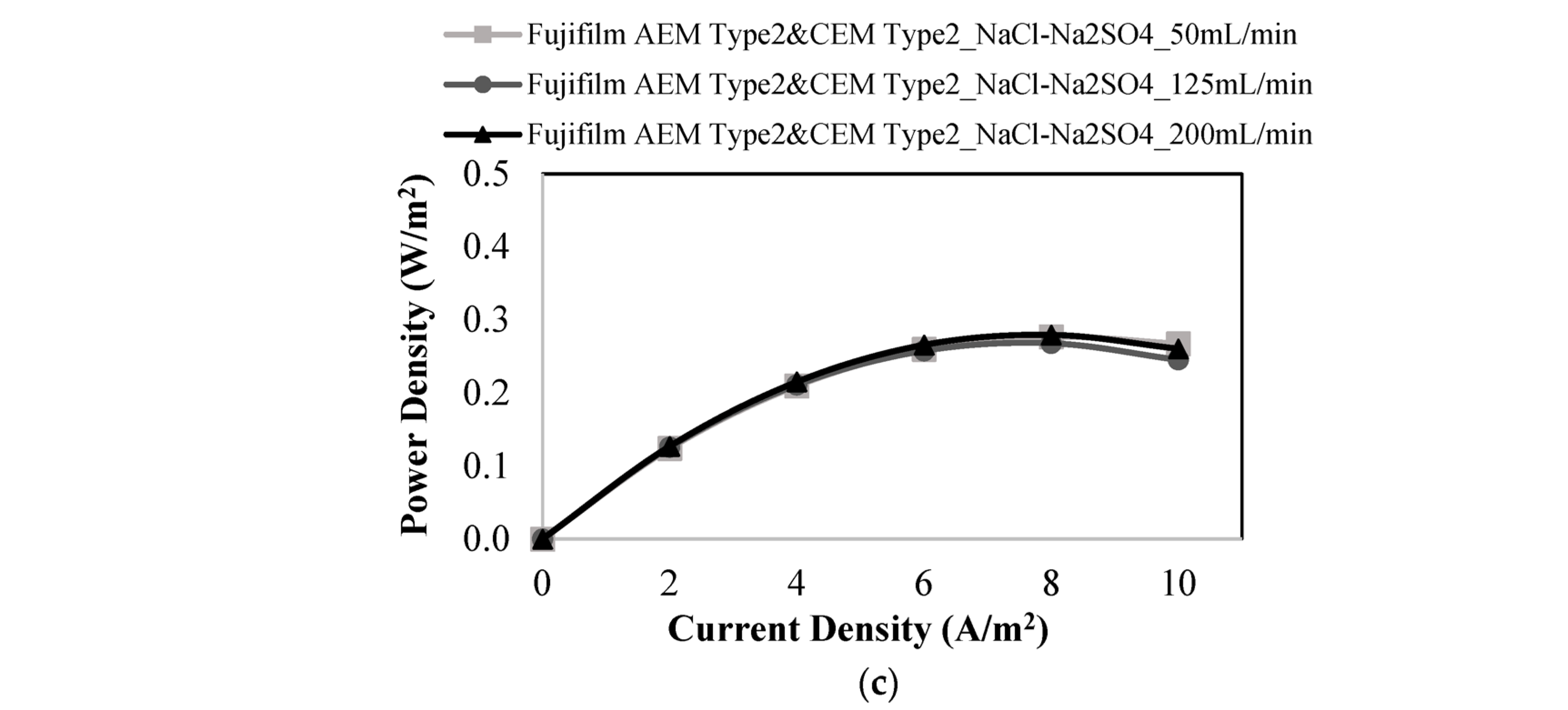
3.3. RED Studies Performed with Fujifilm Membranes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nijmeijer, K.; Metz, S. Chapter 5: Salinity Gradient Energy. Sustain. Sci. Eng. 2010, 2, 95–139. [Google Scholar] [CrossRef]
- Archer, C.L.; Jacobson, M.Z. Evaluation of global wind power. J. Geophys. Res. D Atmos. 2005, 110, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Moya, D.; Aldás, C.; Kaparaju, P. Geothermal energy: Power plant technology and direct heat applications. Renew. Sustain. Energy Rev. 2018, 94, 889–901. [Google Scholar] [CrossRef]
- Roberts, A.; Thomas, B.; Sewell, P.; Khan, Z.; Balmain, S.; Gillman, J. Current tidal power technologies and their suitability for applications in coastal and marine areas. J. Ocean Eng. Mar. Energy 2016, 2, 227–245. [Google Scholar] [CrossRef] [Green Version]
- Shahbaz, M.; Rasool, G.; Ahmed, K.; Mahalik, M.K. Considering the effect of biomass energy consumption on economic growth: Fresh evidence from BRICS region. Renew. Sustain. Energy Rev. 2016, 60, 1442–1450. [Google Scholar] [CrossRef] [Green Version]
- Jang, J.; Kang, Y.; Han, J.H.; Jang, K.; Kim, C.M.; Kim, I.S. Developments and future prospects of reverse electrodialysis for salinity gradient power generation: Influence of ion exchange membranes and electrodes. Desalination 2020, 491, 114540. [Google Scholar] [CrossRef]
- Pattle, R. Production of Electric Power by mixing Fresh and Salt Water in the Hydroelectric Pile. Nature 1954, 174, 660. [Google Scholar] [CrossRef]
- Logan, B.E.; Elimelech, M. Membrane-based processes for sustainable power generation using water. Nature 2012, 488, 313–319. [Google Scholar] [CrossRef]
- Wick, G.L. Power from salinity gradients. Energy 1978, 3, 95–100. [Google Scholar] [CrossRef]
- Post, J.W. Blue Energy: Electricity Production from Salinity Gradients by Reverse Electrodialysis; Wageningen University: Wageningen, The Netherlands, 2009. [Google Scholar]
- Yang, S.T.; Huang, H.; Tay, A.; Qin, W.; De Guzman, L.; Nicolas, E.C.S. Extractive Fermentation for the Production of Carboxylic Acids. Bioprocess. Value-Added Prod. Renew. Resour. New Technol. Appl. 2007, 421–446. [Google Scholar] [CrossRef]
- Altıok, E.; Kaya, T.Z.; Güler, E.; Kabay, N.; Bryjak, M. Performance of reverse electrodialysis system for salinity gradient energy generation by using a commercial ion exchange membrane pair with homogeneous bulk structure. Water 2021, 13, 814. [Google Scholar] [CrossRef]
- Pawlowski, S.; Crespo, J.; Velizarov, S. Sustainable power generation from salinity gradient energy by reverse electrodialysis. Electrokinet. Across Discip. Cont. New Strateg. Sustain. Dev. 2015, 57–80. [Google Scholar] [CrossRef]
- Kaya, T.Z. Evaluation of Reverse Electrodialysis (RED) System Performance with Various Compositions of Salt Solutions for Production of Salinity Gradient Energy (SGE); Ege University Graduate School of Applied and Natural Sciences: Izmir, Turkey, 2021. [Google Scholar]
- Guler, E. Anion Exchange Membrane Design for Reverse Electrodialysis. Ph.D. Thesis, Universiteit Twente, Enschede, The Netherlands, 2014. [Google Scholar]
- Guo, Z.Y.; Ji, Z.Y.; Zhang, Y.G.; Yang, F.J.; Liu, J.; Zhao, Y.Y.; Yuan, J.S. Effect of ions (K+, Mg2+, Ca2+ and SO42−) and temperature on energy generation performance of reverse electrodialysis stack. Electrochim. Acta 2018, 290, 282–290. [Google Scholar] [CrossRef]
- Yip, N.Y.; Vermaas, D.A.; Nijmeijer, K.; Elimelech, M. Thermodynamic, energy efficiency, and power density analysis of reverse electrodialysis power generation with natural salinity gradients. Environ. Sci. Technol. 2014, 48, 4925–4936. [Google Scholar] [CrossRef]
- Vermaas, D.A.; Veerman, J.; Saakes, M.; Nijmeijer, K. Influence of multivalent ions on renewable energy generation in reverse electrodialysis. Energy Environ. Sci. 2014, 7, 1434–1445. [Google Scholar] [CrossRef] [Green Version]
- Post, J.W.; Hamelers, H.V.M.; Buisman, C.J.N. Influence of multivalent ions on power production from mixing salt and fresh water with a reverse electrodialysis system. J. Memb. Sci. 2009, 330, 65–72. [Google Scholar] [CrossRef]
- Geise, G.M.; Paul, D.R.; Freeman, B.D. Fundamental water and salt transport properties of polymeric materials. Prog. Polym. Sci. 2014, 39, 1–42. [Google Scholar] [CrossRef]
- Samson, E.; Marchand, J.; Snyder, K.A. Calculation of ionic diffusion coefficients on the basis of migration test results. Mater. Struct. Constr. 2003, 36, 156–165. [Google Scholar] [CrossRef]
- CRC. Handbook of Chemistry and Physics, 1st ed.; Weast, R.C., Ed.; CRC Press, Inc.: Boca Raton, FL, USA; Wolfe Medical Publications: London, UK, 1988; ISBN 0-8493-0740-6. [Google Scholar] [CrossRef]
- Pintossi, D.; Simões, C.; Saakes, M.; Borneman, Z.; Nijmeijer, K. Predicting reverse electrodialysis performance in the presence of divalent ions for renewable energy generation. Energy Convers. Manag. 2021, 243, 9. [Google Scholar] [CrossRef]
- Hong, J.G.; Zhang, W.; Luo, J.; Chen, Y. Modeling of power generation from the mixing of simulated saline and freshwater with a reverse electrodialysis system: The effect of monovalent and multivalent ions. Appl. Energy 2013, 110, 244–251. [Google Scholar] [CrossRef]
- Długołecki, P.; Nymeijer, K.; Metz, S.; Wessling, M. Current status of ion exchange membranes for power generation from salinity gradients. J. Memb. Sci. 2008, 319, 214–222. [Google Scholar] [CrossRef]
- Astom Corporation. Products Catalogue: Ion Exchange Membranes. Available online: http://www.astom-corp.jp/en/catalog/pdf/Astom_Products_Catalogue.pdf (accessed on 21 September 2022).
- Fujifilm.com. Ion Exchange Membranes. Available online: https://www.fujifilm.com/nl/en/business/manufacturing-process/ion-exchange-membranes/overview (accessed on 27 October 2022).
- Pintossi, D. Fouling in Reverse Electrodialysis: Monitoring, Modeling, and Control. Ph.D. Thesis, Technische Universiteit Eindhoven, Eindhoven, The Netherlands, 2021. [Google Scholar]
- Avci, A.H.; Sarkar, P.; Tufa, R.A.; Messana, D.; Argurio, P.; Fontananova, E.; di Profio, G.; Curcio, E. Effect of Mg2+ ions on energy generation by Reverse Electrodialysis. J. Memb. Sci. 2016, 520, 499–506. [Google Scholar] [CrossRef]
- Moreno, J.; Díez, V.; Saakes, M.; Nijmeijer, K. Mitigation of the effects of multivalent ion transport in reverse electrodialysis. J. Memb. Sci. 2018, 550, 155–162. [Google Scholar] [CrossRef]
- Rijnaarts, T.; Huerta, E.; van Baak, W.; Nijmeijer, K. Effect of Divalent Cations on RED Performance and Cation Exchange Membrane Selection to Enhance Power Densities. Environ. Sci. Technol. 2017, 51, 13028–13035. [Google Scholar] [CrossRef]
- Oh, Y.; Jeong, Y.; Han, S.J.; Kim, C.S.; Kim, H.; Han, J.H.; Hwang, K.S.; Jeong, N.; Park, J.S.; Chae, S. Effects of Divalent Cations on Electrical Membrane Resistance in Reverse Electrodialysis for Salinity Power Generation. Ind. Eng. Chem. Res. 2018, 57, 15803–15810. [Google Scholar] [CrossRef]


| Ion | Hydrated Radius (×10−10 m) [20] | Diffusion Coefficient (×10−9 m2/s) [21,22] |
|---|---|---|
| Na+ | 3.58 | 1.334 |
| Li+ | 3.82 | 1.029 |
| K+ | 3.31 | 1.957 |
| Ca2+ | 4.12 | 0.792 |
| Mg2+ | 4.28 | 0.706 |
| SO42− | 3.79 | 1.065 |
| Cl− | 3.32 | 2.032 |
| Parameter | Specification | |
|---|---|---|
| Effective area of membrane/electrode (cm2) | 10 × 10 | |
| Electrodes (anode and cathode) | Mesh type and alloyed with Ti/Ru-Ir (mesh 1.0, area: 10 × 10 cm) | |
| Thickness of spacer (µm) | 465 | |
| Volumetric flow rate of electrode solution (mL/min) | 300 | |
| Composition of electrode solution | Mixture of 0.05 M K4Fe(CN)6 and 0.05 M K3Fe(CN)6 in 0.25 M NaCl | |
| Salinity of feed solutions (g NaCl/L) | Low saline: 1 | High saline: 30 |
| Flow rates of feed solutions (mL/min) | 50, 125, and 200 | |
| Specification | Ralex [15] AMH-PES | Ralex [15] CMH-PES | Neosepta [26] AMX | Neosepta [26] CMX | Fujifilm [27] AEM Type 2 | Fujifilm [27] CEM Type 2 |
|---|---|---|---|---|---|---|
| Type | Heterogeneous | Homogeneous | ||||
| Functionality | Anion exchange (Cl− form) | Cation exchange (Na+ form) | Anion exchange (Cl− form | Cation exchange (Na+ form) | Anion exchange (Cl− form) | Cation exchange(Na+ form) |
| δ (μm) | 714 | 700 | 140 | 170 | 210 | 190 |
| IEC (mmol·g−1) | 1.97 | 2.34 | 1.25 | 1.62 | 1.08 ± 0.05 | 1.35 ± 0.05 |
| R (Ω·cm2) | 7.66 | 11.33 | 2.0–3.5 | 2.0–3.5 | 5 | 8 |
| α (%) | 89.3 | 94.7 | 91.0 ± 0.4 | 92.5 ± 0.6 | 95 | 96 |
| SD (%) | 56.0 | 31.0 | 16.4 ± 0.5 | 21.5 ± 0.2 | - | - |
| CD (meq·g−1 H2O) | 3.5 | 7.6 | 5.4 | 9 | - | - |
| BS (kg.cm−2) | - | - | 4.5–5.5 | 3.5–6.0 | 5.0 | 4.7 |
| Salt Pairs Used in Feed Solutions | LCC 1 (M) | HCC 2 (M) | |
|---|---|---|---|
| NaCl | 0.0171 | 0.5128 | |
| NaCl-LiCl | NaCl | 0.0154 | 0.4615 |
| LiCl | 0.0024 | 0.0708 | |
| NaCl-KCl | NaCl | 0.0154 | 0.4615 |
| KCl | 0.0013 | 0.0402 | |
| NaCl-CaCl2 | NaCl | 0.0154 | 0.4615 |
| CaCl2 | 0.0009 | 0.0270 | |
| NaCl-MgCl2 | NaCl | 0.0154 | 0.4615 |
| MgCl2 | 0.0011 | 0.0315 | |
| NaCl-Na2SO4 | NaCl | 0.0154 | 0.4615 |
| Na2SO4 | 0.0007 | 0.0211 | |
| Membranes | Binary Salt Mixtures Used in Feed Solutions |
|---|---|
| Ralex AMH-PES & CMH-PES | NaCl |
| NaCl + LiCl | |
| Neosepta AMX & CMX | NaCl + KCl |
| NaCl + MgCl2 | |
| Fujifilm AEM Type 2 & CEM Type 2 | NaCl + CaCl2 |
| NaCl + Na2SO4 |
| Membranes | Flow Rates of Feed Solutions (mL/min) | Salt Mixtures in Feed Solutions | Power Density (W/m2) | OCV (V) |
|---|---|---|---|---|
| Ralex AMH-PES & Ralex CMH-PES | 50 | NaCl | 0.284 | 0.742 |
| 125 | 0.289 | 0.743 | ||
| 200 | 0.305 | 0.749 | ||
| 50 | NaCl-LiCl | 0.226 | 0.727 | |
| 125 | 0.267 | 0.731 | ||
| 200 | 0.306 | 0.736 | ||
| 50 | NaCl-KCl | 0.296 | 0.722 | |
| 125 | 0.309 | 0.742 | ||
| 200 | 0.330 | 0.743 | ||
| 50 | NaCl-MgCl2 | 0.243 | 0.694 | |
| 125 | 0.242 | 0.733 | ||
| 200 | 0.291 | 0.732 | ||
| 50 | NaCl-CaCl2 | 0.225 | 0.717 | |
| 125 | 0.264 | 0.733 | ||
| 200 | 0.289 | 0.726 | ||
| 50 | NaCl-Na2SO4 | 0.219 | 0.718 | |
| 125 | 0.244 | 0.723 | ||
| 200 | 0.279 | 0.728 |
| Membranes | Flow Rates of Feed Solutions (mL/min) | Salt Mixtures in Feed Solutions | Power Density (W/m2) | OCV (V) |
|---|---|---|---|---|
| Neosepta AMX & Neosepta CMX | 50 | NaCl | 0.469 | 0.755 |
| 125 | 0.413 | 0.756 | ||
| 200 | 0.400 | 0.759 | ||
| 50 | NaCl-LiCl | 0.315 | 0.735 | |
| 125 | 0.321 | 0.746 | ||
| 200 | 0.367 | 0.753 | ||
| 50 | NaCl-KCl | 0.373 | 0.666 | |
| 125 | 0.398 | 0.752 | ||
| 200 | 0.431 | 0.759 | ||
| 50 | NaCl-MgCl2 | 0.317 | 0.744 | |
| 125 | 0.350 | 0.750 | ||
| 200 | 0.375 | 0.743 | ||
| 50 | NaCl-CaCl2 | 0.387 | 0.757 | |
| 125 | 0.351 | 0.748 | ||
| 200 | 0.375 | 0.743 | ||
| 50 | NaCl-Na2SO4 | 0.366 | 0.738 | |
| 125 | 0.380 | 0.748 | ||
| 200 | 0.427 | 0.752 |
| Membranes | Flow Rates of Feed Solutions (mL/min) | Salt Mixtures in Feed Solutions | Power Density (W/m2) | OCV (V) |
|---|---|---|---|---|
| Fujifilm AEM Type 2 & Fujifilm CEM Type 2 | 50 | NaCl | 0.338 | 0.735 |
| 125 | 0.444 | 0.757 | ||
| 200 | 0.392 | 0.756 | ||
| 50 | NaCl-LiCl | 0.306 | 0.732 | |
| 125 | 0.309 | 0.738 | ||
| 200 | 0.353 | 0.741 | ||
| 50 | NaCl-KCl | 0.302 | 0.743 | |
| 125 | 0.315 | 0.749 | ||
| 200 | 0.324 | 0.750 | ||
| 50 | NaCl-MgCl2 | 0.226 | 0.692 | |
| 125 | 0.210 | 0.685 | ||
| 200 | 0.213 | 0.677 | ||
| 50 | NaCl-CaCl2 | 0.232 | 0.572 | |
| 125 | 0.217 | 0.608 | ||
| 200 | 0.197 | 0.705 | ||
| 50 | NaCl-Na2SO4 | 0.277 | 0.714 | |
| 125 | 0.268 | 0.731 | ||
| 200 | 0.280 | 0.733 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaya, T.Z.; Altıok, E.; Güler, E.; Kabay, N. Effect of Co-Existing Ions on Salinity Gradient Power Generation by Reverse Electrodialysis Using Different Ion Exchange Membrane Pairs. Membranes 2022, 12, 1240. https://doi.org/10.3390/membranes12121240
Kaya TZ, Altıok E, Güler E, Kabay N. Effect of Co-Existing Ions on Salinity Gradient Power Generation by Reverse Electrodialysis Using Different Ion Exchange Membrane Pairs. Membranes. 2022; 12(12):1240. https://doi.org/10.3390/membranes12121240
Chicago/Turabian StyleKaya, Tuğçe Zeynep, Esra Altıok, Enver Güler, and Nalan Kabay. 2022. "Effect of Co-Existing Ions on Salinity Gradient Power Generation by Reverse Electrodialysis Using Different Ion Exchange Membrane Pairs" Membranes 12, no. 12: 1240. https://doi.org/10.3390/membranes12121240
APA StyleKaya, T. Z., Altıok, E., Güler, E., & Kabay, N. (2022). Effect of Co-Existing Ions on Salinity Gradient Power Generation by Reverse Electrodialysis Using Different Ion Exchange Membrane Pairs. Membranes, 12(12), 1240. https://doi.org/10.3390/membranes12121240









