Hybrid Membrane Technology for Acid Recovery from Wastewater in Coated Steel Wire Production: A Pilot Scale Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Membranes
2.2. Waste Characteristics
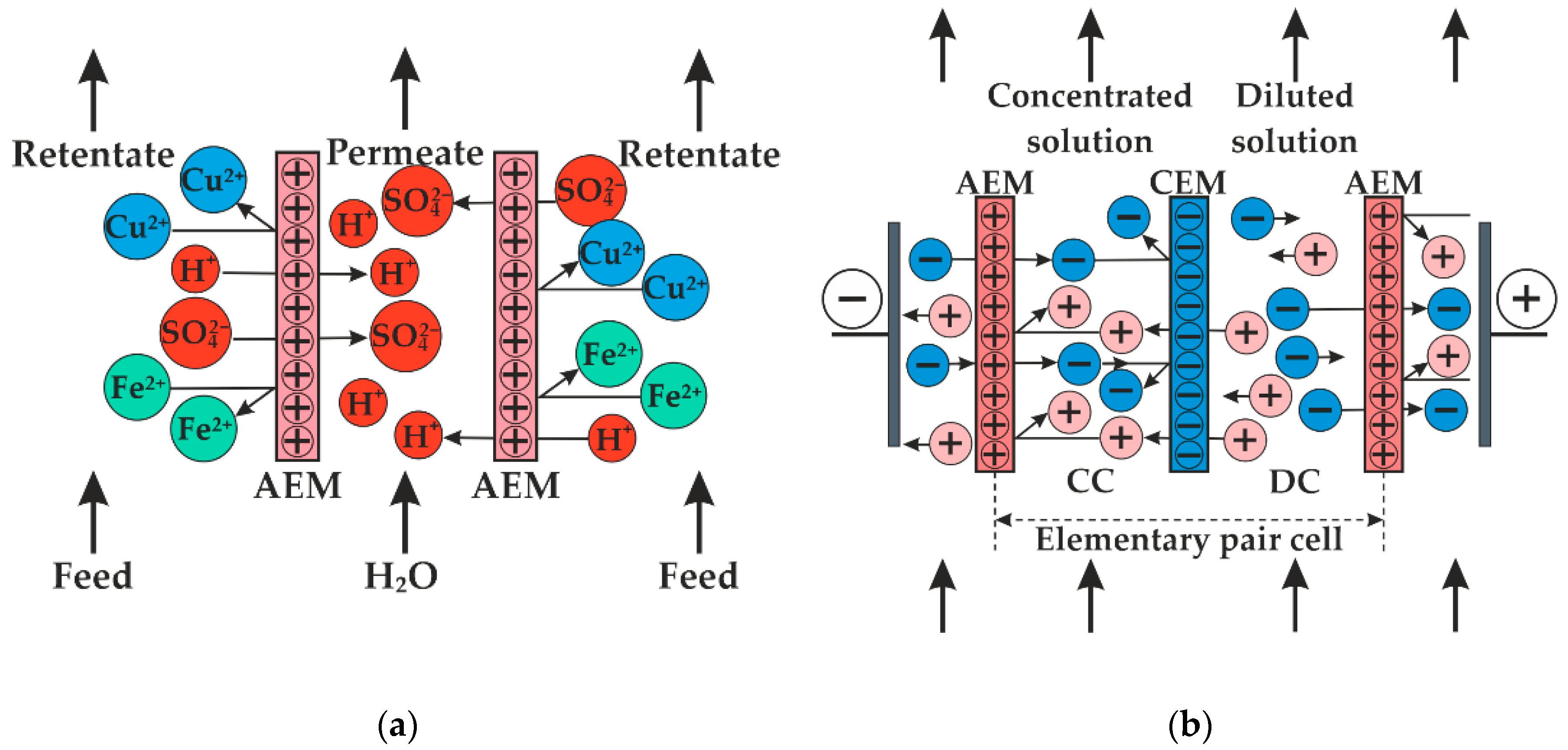
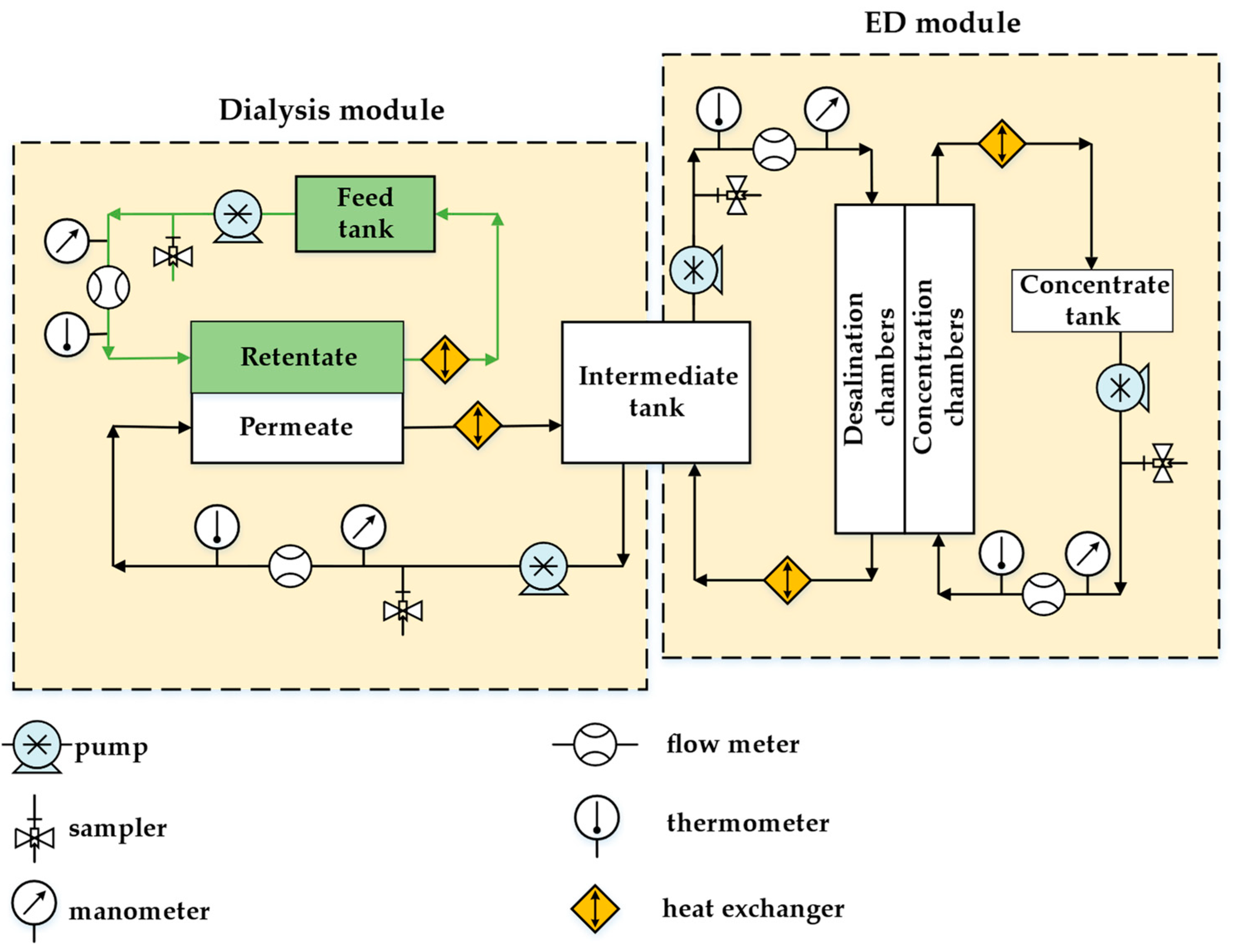
2.3. Pilot Scale Hybrid Installation
2.4. Method of Determining the Composition of Solutions Containing Acid and Metal Salts
3. Results
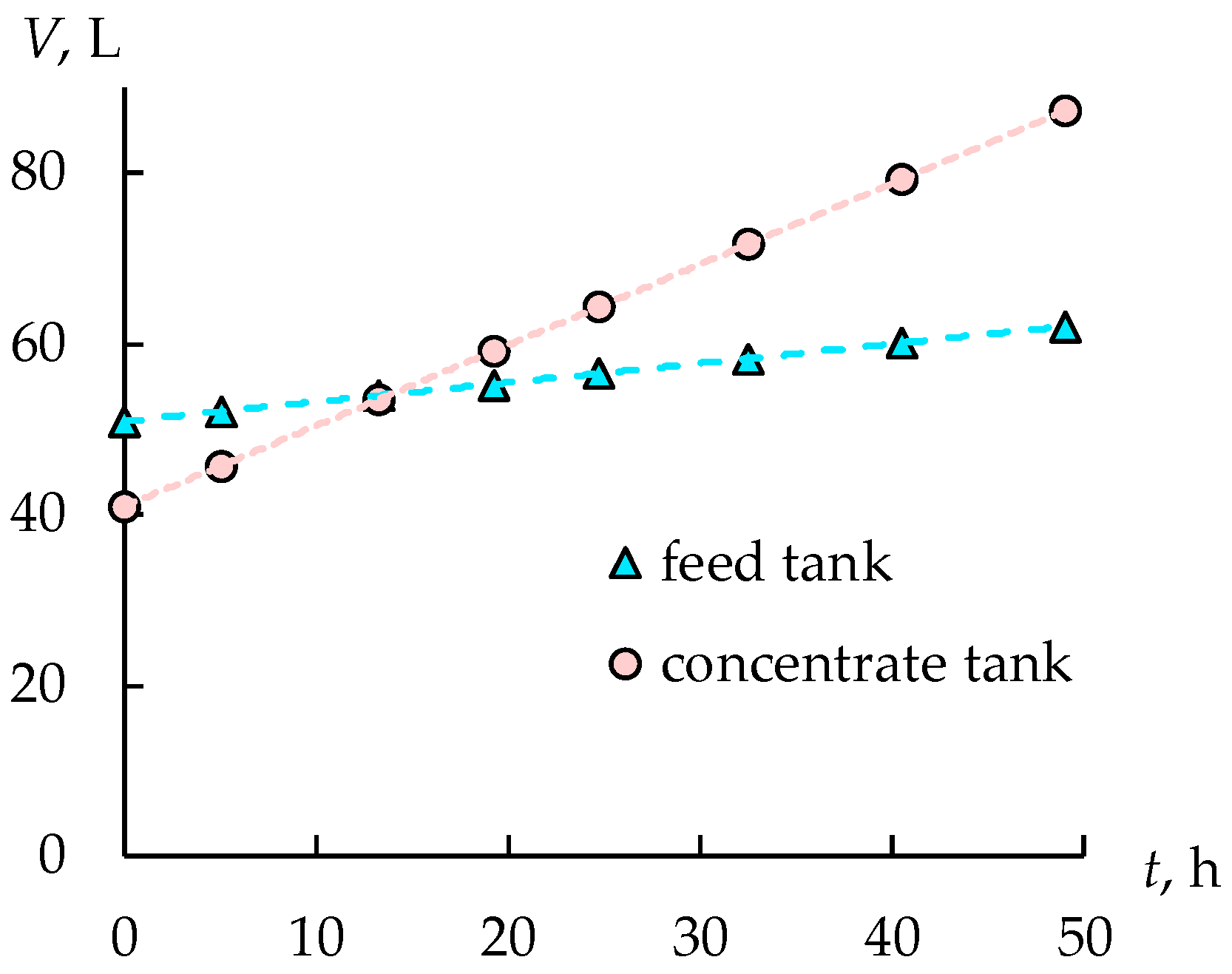
3.1. Acid Recovery from Wastewater of the Coated Steel Wire Production. Stage 1
3.2. Acid Recovery from Wastewater of the Coated Steel Wire Production. Stage 2
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Appendix A


References
- Agrawal, A.; Sahu, K.K. An overview of the recovery of acid from spent acidic solutions from steel and electroplating industries. J. Hazard. Mater. 2009, 171, 61–75. [Google Scholar] [CrossRef]
- Chen, Q.; Yao, Y.; Li, X.; Lu, J.; Zhou, J.; Huang, Z. Comparison of heavy metal removals from aqueous solutions by chemical precipitation and characteristics of precipitates. J. Water Process Eng. 2018, 26, 289–300. [Google Scholar] [CrossRef]
- Malviya, R.; Chaudhary, R. Leaching behavior and immobilization of heavy metals in solidified/stabilized products. J. Hazard. Mater. 2006, 137, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.A.R.; Mater, L.; Souza-Sierra, M.M.; Corrêa, A.X.R.; Sperb, R.; Radetski, C.M. Small hazardous waste generators in developing countries: Use of stabilization/solidification process as an economic tool for metal wastewater treatment and appropriate sludge disposal. J. Hazard. Mater. 2007, 147, 986–990. [Google Scholar] [CrossRef] [PubMed]
- Panagopoulos, A.; Haralambous, K.J.; Loizidou, M. Desalination brine disposal methods and treatment technologies—A review. Sci. Total Environ. 2019, 693, 133545. [Google Scholar] [CrossRef]
- Yaqub, M.; Lee, W. Zero-liquid discharge (ZLD) technology for resource recovery from wastewater: A review. Sci. Total Environ. 2019, 681, 551–563. [Google Scholar] [CrossRef]
- An, W.; Zhao, J.; Lu, J.; Han, Y.; Li, D. Zero-liquid discharge technologies for desulfurization wastewater: A review. J. Environ. Manag. 2022, 321, 115953. [Google Scholar] [CrossRef]
- Du, J.; Waite, T.D.; Biesheuvel, P.M.; Tang, W. Recent advances and prospects in electrochemical coupling technologies for metal recovery from water. J. Hazard. Mater. 2023, 442, 130023. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Wu, C.; Xu, T.; Wu, Y. Diffusion dialysis-concept, principle and applications. J. Memb. Sci. 2011, 366, 1–16. [Google Scholar] [CrossRef]
- Yaroslavtsev, A.B.; Nikonenko, V.V. Ion-exchange membrane materials: Properties, modification, and practical application. Nanotechnologies Russ. 2009, 4, 137–159. [Google Scholar] [CrossRef]
- Arana Juve, J.M.; Christensen, F.M.S.; Wang, Y.; Wei, Z. Electrodialysis for metal removal and recovery: A review. Chem. Eng. J. 2022, 435, 134857. [Google Scholar] [CrossRef]
- Melnikov, S.; Sheldeshov, N.; Zabolotsky, V.; Loza, S.; Achoh, A. Pilot scale complex electrodialysis technology for processing a solution of lithium chloride containing organic solvents. Sep. Purif. Technol. 2017, 189, 74–81. [Google Scholar] [CrossRef]
- Sata, T.; Sata, T.; Yang, W. Studies on cation-exchange membranes having permselectivity between cations in electrodialysis. J. Memb. Sci. 2002, 206, 31–60. [Google Scholar] [CrossRef]
- Ge, L.; Wu, L.; Wu, B.; Wang, G.; Xu, T. Preparation of monovalent cation selective membranes through annealing treatment. J. Memb. Sci. 2014, 459, 217–222. [Google Scholar] [CrossRef]
- Zhang, Y.; Paepen, S.; Pinoy, L.; Meesschaert, B.; Van Der Bruggen, B. Selectrodialysis: Fractionation of divalent ions from monovalent ions in a novel electrodialysis stack. Sep. Purif. Technol. 2012, 88, 191–201. [Google Scholar] [CrossRef]
- Wang, W.; Liu, R.; Tan, M.; Sun, H.; Niu, Q.J.; Xu, T.; Nikonenko, V.; Zhang, Y. Evaluation of the ideal selectivity and the performance of selectrodialysis by using TFC ion exchange membranes. J. Memb. Sci. 2019, 582, 236–245. [Google Scholar] [CrossRef]
- Zabolotsky, V.I.; Achoh, A.R.; Lebedev, K.A.; Melnikov, S.S. Permselectivity of bilayered ion-exchange membranes in ternary electrolyte. J. Memb. Sci. 2020, 608, 118152. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, W.; Wang, Y. Diffusion dialysis for acid recovery from acidic waste solutions: Anion exchange membranes and technology integration. Membranes 2020, 10, 169. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhang, Y.; Huang, J.; Zhu, X.; Wang, Y. Separation and recovery of sulfuric acid from acidic vanadium leaching solution by diffusion dialysis. Sep. Purif. Technol. 2012, 96, 44–49. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, Y.; Huang, J.; Liu, T.; Wang, J. Recovery of sulfuric acid from a stone coal acid leaching solution by diffusion dialysis. Hydrometallurgy 2017, 173, 9–14. [Google Scholar] [CrossRef]
- Yan, J.; Wang, H.; Fu, R.; Fu, R.; Li, R.; Chen, B.; Jiang, C.; Ge, L.; Liu, Z.; Wang, Y.; et al. Ion exchange membranes for acid recovery: Diffusion Dialysis (DD) or Selective Electrodialysis (SED)? Desalination 2022, 531, 115690. [Google Scholar] [CrossRef]
- Zhang, X.; Li, C.; Wang, X.; Wang, Y.; Xu, T. Recovery of hydrochloric acid from simulated chemosynthesis aluminum foils wastewater: An integration of diffusion dialysis and conventional electrodialysis. J. Memb. Sci. 2012, 409–410, 257–263. [Google Scholar] [CrossRef]
- Khan, M.I.; Khraisheh, M.; Almomani, F. Fabrication and characterization of pyridinium functionalized anion exchange membranes for acid recovery. Sci. Total Environ. 2019, 686, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Aguirre, A.; Lopez, J.; Gueccia, R.; Randazzo, S.; Cipollina, A.; Cortina, J.L.; Micale, G. Diffusion dialysis for the treatment of H2SO4-CuSO4 solutions from electroplating plants: Ions membrane transport characterization and modelling. Sep. Purif. Technol. 2021, 266, 118215. [Google Scholar] [CrossRef]
- Merkel, A.; Čopák, L.; Dvořák, L.; Golubenko, D.; Šeda, L. Recovery of spent sulphuric acid by diffusion dialysis using a spiral wound module. Int. J. Mol. Sci. 2021, 22, 11819. [Google Scholar] [CrossRef] [PubMed]
- Merkel, A.; Čopák, L.; Golubenko, D.; Dvořák, L.; Vavro, M.; Yaroslavtsev, A.; Šeda, L. Recovery of Hydrochloric Acid from Industrial Wastewater by Diffusion Dialysis Using a Spiral-Wound Module. Int. J. Mol. Sci. 2022, 23., 6212. [Google Scholar] [CrossRef]
- Koros, W.J.; Ma, Y.H.; Shimidzu, T. Terminology for membranes and membrane processes (IUPAC Recommendations 1996). Pure Appl. Chem. 1996, 68, 1479–1489. [Google Scholar] [CrossRef]
- Kavitha, E.; Poonguzhali, E.; Nanditha, D.; Kapoor, A.; Arthanareeswaran, G.; Prabhakar, S. Current status and future prospects of membrane separation processes for value recovery from wastewater. Chemosphere 2022, 291, 132690. [Google Scholar] [CrossRef]
- López, J.; Gibert, O.; Cortina, J.L. Integration of membrane technologies to enhance the sustainability in the treatment of metal-containing acidic liquid wastes. An overview. Sep. Purif. Technol. 2021, 265, 118485. [Google Scholar] [CrossRef]
- Al-Jubainawi, A.; Ma, Z.; Guo, Y.; Nghiem, L.D.; Cooper, P.; Li, W. Factors governing mass transfer during membrane electrodialysis regeneration of LiCl solution for liquid desiccant dehumidification systems. Sustain. Cities Soc. 2017, 28, 30–41. [Google Scholar] [CrossRef] [Green Version]
- Zabolotskii, V.I.; Demin, A.V.; Demina, O.A. Ion and water transport during lithium chloride concentration from aqueous organic solutions by electrodialysis. Russ. J. Electrochem. 2011, 47, 327–335. [Google Scholar] [CrossRef]
- Melnikov, S.S.; Mugtamov, O.A.; Zabolotsky, V.I. Study of electrodialysis concentration process of inorganic acids and salts for the two-stage conversion of salts into acids utilizing bipolar electrodialysis. Sep. Purif. Technol. 2020, 235, 116198. [Google Scholar] [CrossRef]
- Loza, S.A.; Smyshlyaev, N.A.; Korzhov, A.N.; Romanyuk, N.A. Electrodialysis concentration of sulfuric acid. Chim. Techno Acta 2021, 8, 20218106. [Google Scholar] [CrossRef]
- Lorrain, Y.; Pourcelly, G.; Gavach, C. Transport mechanism of sulfuric acid through an anion exchange membrane. Desalination 1997, 109, 231–239. [Google Scholar] [CrossRef]
- Zhu, M.; Tian, B.; Luo, S.; Chi, Y.; Aishajiang, D.; Zhang, Y.; Yang, M. High-value conversion of waste Na2SO4 by a bipolar membrane electrodialysis metathesis system. Resour. Conserv. Recycl. 2022, 186, 106556. [Google Scholar] [CrossRef]
- Kuldeep; Kauranen, P.; Pajari, H.; Pajarre, R.; Murtomäki, L. Electrodiffusion of ions in ion exchange membranes: Finite element simulations and experiments. Chem. Eng. J. Adv. 2021, 8, 100169. [Google Scholar] [CrossRef]







| Membrane | l, μm | Q, mmol/gwet |
|---|---|---|
| Ralex CMHPES | 540 | 1.16 |
| Ralex AMHPES | 550 | 0.86 |
| Component | C, mol/L |
|---|---|
| H2SO4 | 1.45 |
| CuSO4 | 0.176 |
| FeSO4 | 0.67 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Loza, S.; Loza, N.; Korzhov, A.; Romanyuk, N.; Kovalchuk, N.; Melnikov, S. Hybrid Membrane Technology for Acid Recovery from Wastewater in Coated Steel Wire Production: A Pilot Scale Study. Membranes 2022, 12, 1196. https://doi.org/10.3390/membranes12121196
Loza S, Loza N, Korzhov A, Romanyuk N, Kovalchuk N, Melnikov S. Hybrid Membrane Technology for Acid Recovery from Wastewater in Coated Steel Wire Production: A Pilot Scale Study. Membranes. 2022; 12(12):1196. https://doi.org/10.3390/membranes12121196
Chicago/Turabian StyleLoza, Sergey, Natalia Loza, Alexander Korzhov, Nazar Romanyuk, Nikita Kovalchuk, and Stanislav Melnikov. 2022. "Hybrid Membrane Technology for Acid Recovery from Wastewater in Coated Steel Wire Production: A Pilot Scale Study" Membranes 12, no. 12: 1196. https://doi.org/10.3390/membranes12121196
APA StyleLoza, S., Loza, N., Korzhov, A., Romanyuk, N., Kovalchuk, N., & Melnikov, S. (2022). Hybrid Membrane Technology for Acid Recovery from Wastewater in Coated Steel Wire Production: A Pilot Scale Study. Membranes, 12(12), 1196. https://doi.org/10.3390/membranes12121196






