Breaking the Saturated Vapor Layer with a Thin Porous Membrane
Abstract
1. Introduction
2. Experimental Section
2.1. Membranes
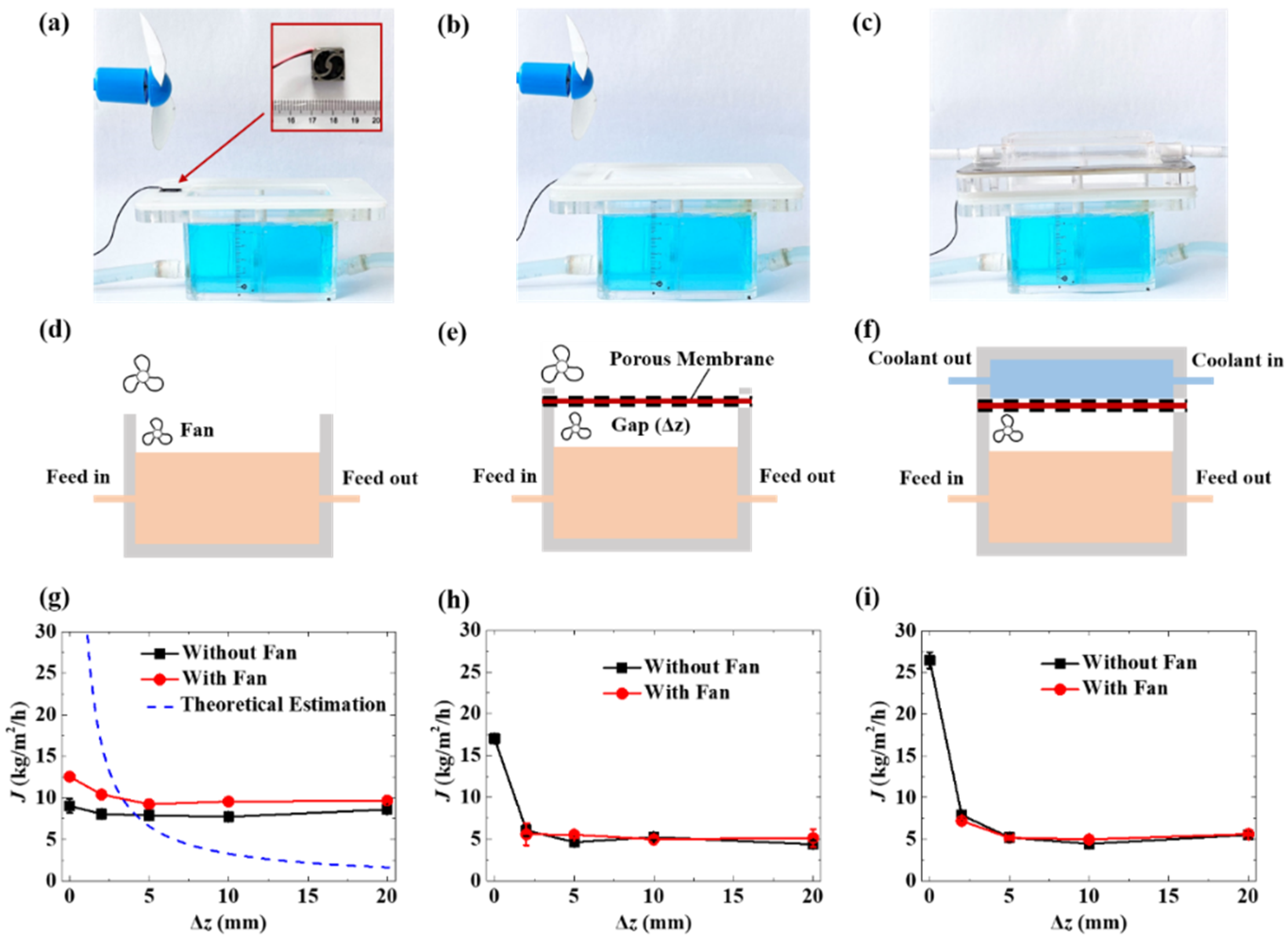
2.2. Evaporation Test System
2.3. Free-Surface Evaporation
2.4. Membrane-Covered Evaporation
2.5. Membrane Distillation
3. Results and Discussion
3.1. Free-Surface Evaporation and Transmembrane Evaporation
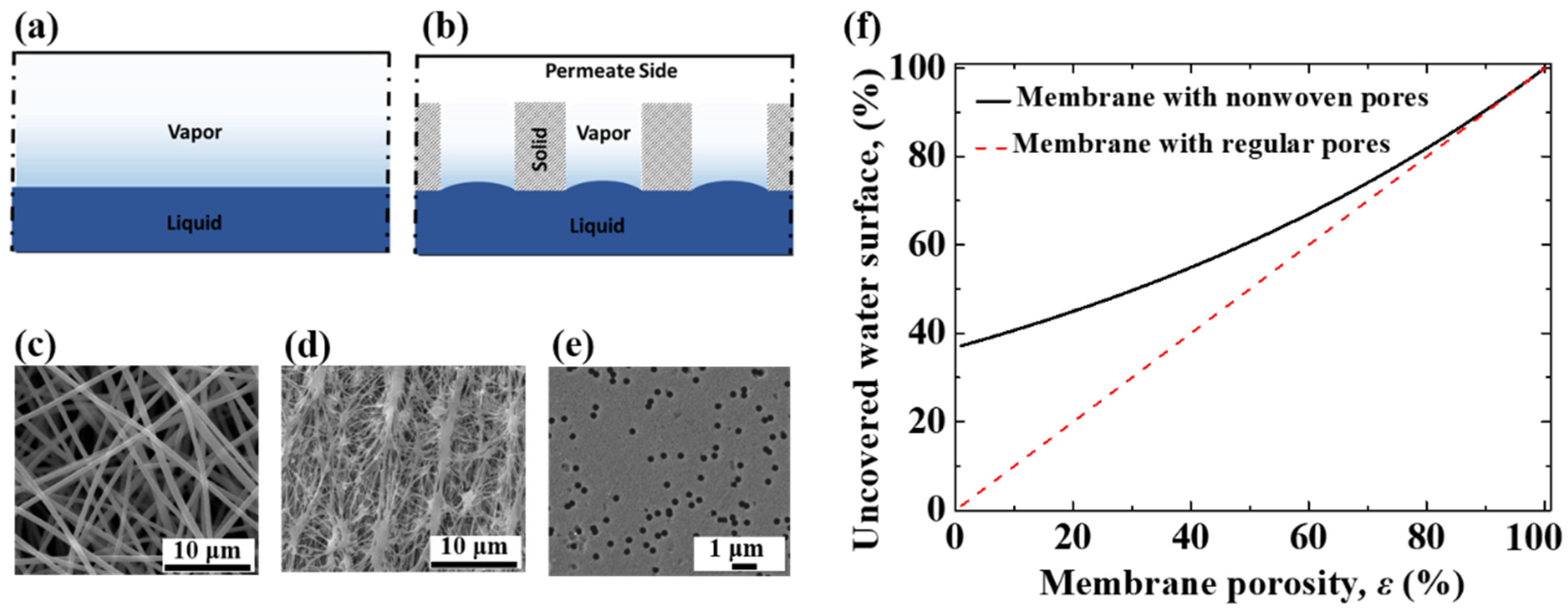
3.2. Diffusion Capacity through a Porous Membrane
3.3. Dialectical Effect of Porous Membrane on Evaporation
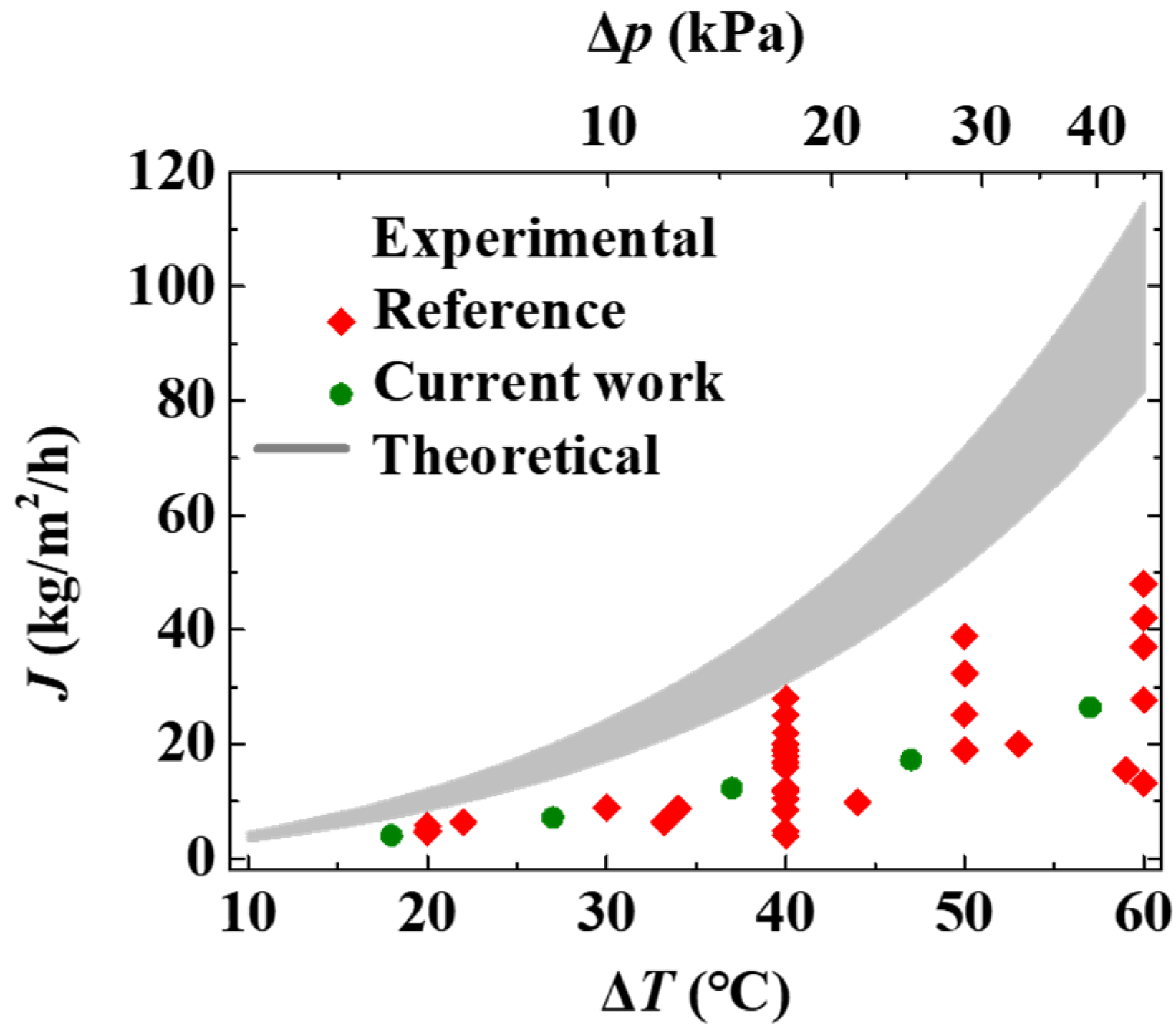
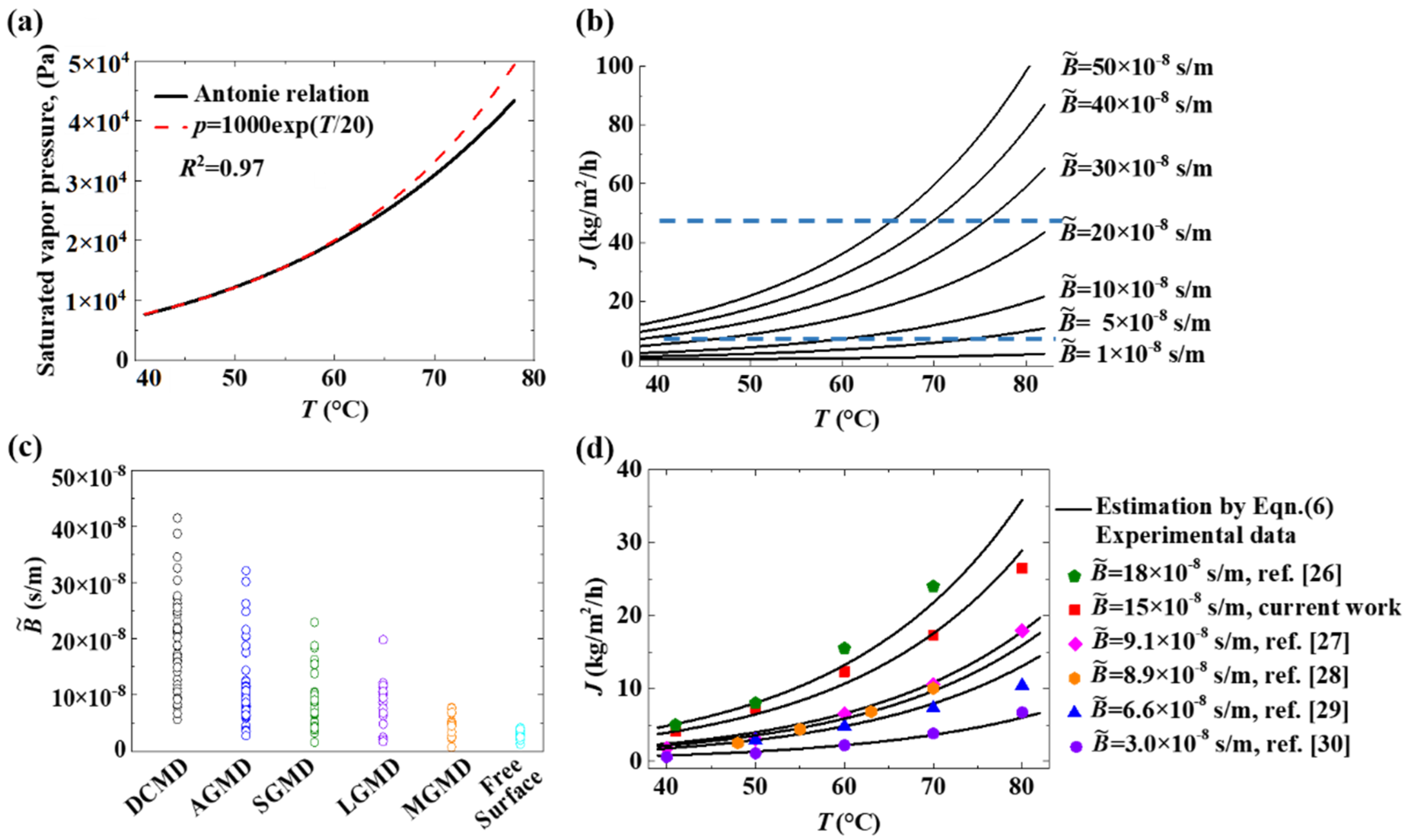
3.4. Transmembrane Flux Capacity of MD
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tao, P.; Ni, G.; Song, C.; Shang, W.; Wu, J.; Zhu, J.; Chen, G.; Deng, T. Solar-driven interfacial evaporation. Nat. Energy 2018, 3, 1031–1041. [Google Scholar] [CrossRef]
- Jodat, A.; Moghiman, M.; Shirkhani, G. An experimental investigation on the effects of surface gravity waves on the water evaporation rate in different air flow regimes. Heat Mass Transf. 2013, 49, 1823–1830. [Google Scholar] [CrossRef]
- Bower, S.; Saylor, J. A study of the Sherwood–Rayleigh relation for water undergoing natural convection-driven evaporation. Int. J. Heat Mass Transf. 2009, 52, 3055–3063. [Google Scholar] [CrossRef]
- Wu, L.; Dong, Z.; Cai, Z.; Ganapathy, T.; Fang, N.X.; Li, C.; Yu, C.; Zhang, Y.; Song, Y. Highly efficient three-dimensional solar evaporator for high salinity desalination by localized crystallization. Nat. Commun. 2020, 11, 521. [Google Scholar] [CrossRef]
- Curcio, E.; Drioli, E. Membrane Distillation and Related Operations—A Review. Sep. Purif. Rev. 2005, 34, 35–86. [Google Scholar] [CrossRef]
- Lawson, K.W.; Lloyd, D.R. Membrane distillation. J. Membr. Sci. 1997, 124, 1–25. [Google Scholar] [CrossRef]
- Elsaid, K.; Kamil, M.; Sayed, E.T.; Abdelkareem, M.A.; Wilberforce, T.; Olabi, A. Environmental impact of desalination technologies: A review. Sci. Total. Environ. 2020, 748, 141528. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Liang, H.; Xu, Z.; Wang, Z. Harnessing Solar-Driven Photothermal Effect toward the Water-Energy Nexus. Adv. Sci. 2019, 6, 1900883. [Google Scholar] [CrossRef]
- Deshmukh, A.; Boo, C.; Karanikola, V.; Lin, S.; Straub, A.P.; Tong, T.; Warsinger, D.M.; Elimelech, M. Membrane distillation at the water-energy nexus: Limits, opportunities, and challenges. Energy Environ. Sci. 2018, 11, 1177–1196. [Google Scholar] [CrossRef]
- Alklaibi, A.M.; Lior, N. Membrane-distillation desalination: Status and potential. Desalination 2005, 171, 111–131. [Google Scholar] [CrossRef]
- Yao, M.; Tijing, L.D.; Naidu, G.; Kim, S.-H.; Matsuyama, H.; Fane, A.G.; Shon, H.K. A review of membrane wettability for the treatment of saline water deploying membrane distillation. Desalination 2020, 479, 114312. [Google Scholar] [CrossRef]
- Rezaei, M.; Warsinger, D.M.; Duke, M.C.; Matsuura, T.; Samhaber, W.M. Wetting phenomena in membrane distillation: Mechanisms, reversal, and prevention. Water Res. 2018, 139, 329–352. [Google Scholar] [CrossRef]
- Castro-Muñoz, R.; Boczkaj, G.; Gontarek, E.; Cassano, A.; Fíla, V. Membrane technologies assisting plant-based and agro-food by-products processing: A comprehensive review. Trends Food Sci. Technol. 2019, 95, 219–232. [Google Scholar] [CrossRef]
- Xiao, Z.; Zheng, R.; Liu, Y.; He, H.; Yuan, X.; Ji, Y.; Li, D.; Yin, H.; Zhang, Y.; Li, X.-M.; et al. Slippery for scaling resistance in membrane distillation: A novel porous micropillared superhydrophobic surface. Water Res. 2019, 155, 152–161. [Google Scholar] [CrossRef]
- Xie, M.; Shon, H.K.; Gray, S.R.; Elimelech, M. Membrane-based processes for wastewater nutrient recovery: Technology, challenges, and future direction. Water Res. 2016, 89, 210–221. [Google Scholar] [CrossRef]
- Incropera, F.P.; DeWitt, D.P.; Bergman, T.L.; Lavine, A.S. Fundamentals of Heat and Mass Transfer; John Wiley & Sons: New York, NY, USA, 2006. [Google Scholar]
- Cai, J.; Liu, X.; Zhao, Y.; Guo, F. Membrane desalination using surface fluorination treated electrospun polyacrylonitrile membranes with nonwoven structure and quasi-parallel fibrous structure. Desalination 2018, 429, 70–75. [Google Scholar] [CrossRef]
- Al-Shammiri, M. Evaporation rate as a function of water salinity. Desalination 2002, 150, 189–203. [Google Scholar] [CrossRef]
- Menon, A.K.; Haechler, I.; Kaur, S.; Lubner, S.; Prasher, R.S. Enhanced solar evaporation using a photo-thermal umbrella for wastewater management. Nat. Sustain. 2020, 3, 144–151. [Google Scholar] [CrossRef]
- Blázquez, J.L.F.; Maestre, I.R.; Gallero, F.J.G.; Gómez, P. Experimental test for the estimation of the evaporation rate in indoor swimming pools: Validation of a new CFD-based simulation methodology. Build. Environ. 2018, 138, 293–299. [Google Scholar] [CrossRef]
- Raimundo, A.M.; Gaspar, A.R.; Oliveira, A.V.M.; Quintela, D.A. Wind tunnel measurements and numerical simulations of water evaporation in forced convection airflow. Int. J. Therm. Sci. 2014, 86, 28–40. [Google Scholar] [CrossRef]
- Asdrubali, F. A scale model to evaluate water evaporation from indoor swimming pools. Energy Build. 2009, 41, 311–319. [Google Scholar] [CrossRef]
- Korolkov, I.V.; Yeszhanov, A.B.; Zdorovets, M.V.; Gorin, Y.G.; Güven, O.; Dosmagambetova, S.S.; Khlebnikov, N.A.; Serkov, K.V.; Krasnopyorova, M.V.; Milts, O.S.; et al. Modification of PET ion track membranes for membrane distillation of low-level liquid radioactive wastes and salt solutions. Sep. Purif. Technol. 2019, 227, 115694. [Google Scholar] [CrossRef]
- Guo, F.; Silverberg, G.; Bowers, S.; Kim, S.-P.; Datta, D.; Shenoy, V.; Hurt, R.H. Graphene-Based Environmental Barriers. Environ. Sci. Technol. 2012, 46, 7717–7724. [Google Scholar] [CrossRef]
- Zhu, X.; Liu, Y.; Du, F.; Han, J.; Hao, G.; Li, L.; Ma, Q. Geothermal direct contact membrane distillation system for purifying brackish water. Desalination 2020, 500, 114887. [Google Scholar] [CrossRef]
- Thakur, A.K.; Hsieh, I.-M.; Islam, M.R.; Lin, B.; Chen, C.-C.; Malmali, M. Performance of sweeping gas membrane distillation for treating produced water: Modeling and experiments. Desalination 2020, 492, 114597. [Google Scholar] [CrossRef]
- Guo, F.; Servi, A.; Liu, A.; Gleason, K.K.; Rutledge, G.C. Desalination by Membrane Distillation using Electrospun Polyamide Fiber Membranes with Surface Fluorination by Chemical Vapor Deposition. ACS Appl. Mater. Interfaces 2015, 7, 8225–8232. [Google Scholar] [CrossRef]
- Tian, M.; Yuan, S.; Decaesstecker, F.; Zhu, J.; Volodine, A.; Van der Bruggen, B. One-step fabrication of isotropic poly(vinylidene fluoride) membranes for direct contact membrane distillation (DCMD). Desalination 2019, 477, 114265. [Google Scholar] [CrossRef]
- Alsaadi, A.; Ghaffour, N.; Li, J.-D.; Gray, S.; Francis, L.; Maab, H.; Amy, G. Modeling of air-gap membrane distillation process: A theoretical and experimental study. J. Membr. Sci. 2013, 445, 53–65. [Google Scholar] [CrossRef]
- Yao, M.; Woo, Y.C.; Tijing, L.D.; Shim, W.-G.; Choi, J.-S.; Kim, S.-H.; Shon, H.K. Effect of heat-press conditions on electrospun membranes for desalination by direct contact membrane distillation. Desalination 2016, 378, 80–91. [Google Scholar] [CrossRef]
- Tow, E.W.; Warsinger, D.M.; Trueworthy, A.M.; Swaminathan, J.; Thiel, G.P.; Zubair, S.M.; Myerson, A.S.; Lienhard, V.J.H. Comparison of fouling propensity between reverse osmosis, forward osmosis, and membrane distillation. J. Membr. Sci. 2018, 556, 352–364. [Google Scholar] [CrossRef]
- González, D.; Amigo, J.; Suárez, F. Membrane distillation: Perspectives for sustainable and improved desalination. Renew. Sustain. Energy Rev. 2017, 80, 238–259. [Google Scholar] [CrossRef]
- Singh, D.; Sirkar, K.K. Performance of PVDF flat membranes and hollow fibers in desalination by direct contact membrane distillation at high temperatures. Sep. Purif. Technol. 2017, 187, 264–273. [Google Scholar] [CrossRef]
- Leitch, M.E.; Li, C.; Ikkala, O.; Mauter, M.S.; Lowry, G.V. Bacterial Nanocellulose Aerogel Membranes: Novel High-Porosity Materials for Membrane Distillation. Environ. Sci. Technol. Lett. 2016, 3, 85–91. [Google Scholar] [CrossRef]
- Wang, W.; Du, X.; Vahabi, H.; Zhao, S.; Yin, Y.; Kota, A.K.; Tong, T. Trade-off in membrane distillation with monolithic omniphobic membranes. Nat. Commun. 2019, 10, 3220. [Google Scholar] [CrossRef] [PubMed]
- Boo, C.; Lee, J.; Elimelech, M. Engineering Surface Energy and Nanostructure of Microporous Films for Expanded Membrane Distillation Applications. Environ. Sci. Technol. 2016, 50, 8112–8119. [Google Scholar] [CrossRef]
- Abdelrazeq, H.; Khraisheh, M.; Al Momani, F.; McLeskey, J.T.; Hassan, M.K.; Gad-El-Hak, M.; Tafreshi, H.V. Performance of electrospun polystyrene membranes in synthetic produced industrial water using direct-contact membrane distillation. Desalination 2020, 493, 114663. [Google Scholar] [CrossRef]
- Khayet, M. Membranes and theoretical modeling of membrane distillation: A review. Adv. Colloid Interface Sci. 2011, 164, 56–88. [Google Scholar] [CrossRef]
- Deshpande, J.; Nithyanandam, K.; Pitchumani, R. Analysis and design of direct contact membrane distillation. J. Membr. Sci. 2017, 523, 301–316. [Google Scholar] [CrossRef]
- Tijing, L.D.; Woo, Y.C.; Shim, W.-G.; He, T.; Choi, J.-S.; Kim, S.-H.; Shon, H.K. Superhydrophobic nanofiber membrane containing carbon nanotubes for high-performance direct contact membrane distillation. J. Membr. Sci. 2016, 502, 158–170. [Google Scholar] [CrossRef]
- Kim, S.; Kim, S.; Ahmed, Z.; Cha, D.K.; Cho, J. Flux model for the membrane distillation process to treat wastewater: Effect of solids concentration. J. Membr. Sci. 2018, 566, 396–405. [Google Scholar] [CrossRef]
- Anari, Z.; Sengupta, A.; Sardari, K.; Wickramasinghe, S.R. Surface modification of PVDF membranes for treating produced waters by direct contact membrane distillation. Sep. Purif. Technol. 2019, 224, 388–396. [Google Scholar] [CrossRef]
- Ke, H.; Feldman, E.; Guzman, P.; Cole, J.; Wei, Q.; Chu, B.; Alkhudhiri, A.; Alrasheed, R.; Hsiao, B.S. Electrospun polystyrene nanofibrous membranes for direct contact membrane distillation. J. Membr. Sci. 2016, 515, 86–97. [Google Scholar] [CrossRef]
- Muster-Slawitsch, B.; Dow, N.; Desai, D.; Pinches, D.; Brunner, C.; Duke, M. Membrane distillation for concentration of protein-rich waste water from meat processing. J. Water Process Eng. 2021, 44, 102285. [Google Scholar] [CrossRef]
- Olatunji, S.O.; Camacho, L.M. Heat and Mass Transport in Modeling Membrane Distillation Configurations: A Review. Front. Energy Res. 2018, 6. [Google Scholar] [CrossRef]
- Camacho, L.M.; Pinion, T.A.; Olatunji, S.O. Behavior of mixed-matrix graphene oxide—Polysulfone membranes in the process of direct contact membrane distillation. Sep. Purif. Technol. 2020, 240, 116645. [Google Scholar] [CrossRef]
- Chen, Y.; Zheng, R.; Wang, J.; Liu, Y.; Wang, Y.; Li, X.-M.; He, T. Laminated PTFE membranes to enhance the performance in direct contact membrane distillation for high salinity solution. Desalination 2017, 424, 140–148. [Google Scholar] [CrossRef]
- Singh, D.; Sirkar, K.K. Desalination by air gap membrane distillation using a two hollow-fiber-set membrane module. J. Membr. Sci. 2012, 421–422, 172–179. [Google Scholar] [CrossRef]
- Warsinger, D.E.; Swaminathan, J.; Maswadeh, L.A. Superhydrophobic condenser surfaces for air gap membrane distillation. J. Membr. Sci. 2015, 492, 578–587. [Google Scholar] [CrossRef]
- Ray, S.S.; Chen, S.-S.; Sangeetha, D.; Chang, H.-M.; Thanh, C.N.D.; Le, Q.H.; Ku, H.-M. Developments in forward osmosis and membrane distillation for desalination of waters. Environ. Chem. Lett. 2018, 16, 1247–1265. [Google Scholar] [CrossRef]
- Warsinger, D.M.; Swaminathan, J.; Morales, L.L. Comprehensive condensation flow regimes in air gap membrane distillation: Visualization and energy efficiency. J. Membr. Sci. 2018, 555, 517–528. [Google Scholar] [CrossRef]
- Amaya-Vías, D.; López-Ramírez, J.A.; Gray, S.; Zhang, J.; Duke, M. Diffusion behavior of humic acid during desalination with air gap and water gap membrane distillation. Water Res. 2019, 158, 182–192. [Google Scholar] [CrossRef] [PubMed]
- Thomas, N.; Swaminathan, J.; Zaragoza, G.; Abu Al-Rub, R.K.; Lienhard, V.J.H.; Arafat, H.A. Comparative assessment of the effects of 3D printed feed spacers on process performance in MD systems. Desalination 2021, 503, 114940. [Google Scholar] [CrossRef]
- Khayet, M.; Matsuura, T. Membrane Distillation: Principles and Applications, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Khayet, M.; Cojocaru, C.; Baroudi, A. Modeling and optimization of sweeping gas membrane distillation. Desalination 2012, 287, 159–166. [Google Scholar] [CrossRef]
- Khayet, M.; Cojocaru, C. Artificial neural network model for desalination by sweeping gas membrane distillation. Desalination 2013, 308, 102–110. [Google Scholar] [CrossRef]
- Wang, F.; Li, J.; Zhu, H.; Zhang, H.; Tang, H.; Chen, J.; Guo, Y. Physical modification of polytetrafluoroethylene flat membrane by a simple heat setting process and membrane wetting remission in SGMD for desalination. Desalination 2014, 354, 143–152. [Google Scholar] [CrossRef]
- Zhao, S.; Feron, P.H.; Xie, Z.; Zhang, J.; Hoang, M. Condensation studies in membrane evaporation and sweeping gas membrane distillation. J. Membr. Sci. 2014, 462, 9–16. [Google Scholar] [CrossRef]
- Perfilov, V.; Fila, V.; Marcano, J.S. A general predictive model for sweeping gas membrane distillation. Desalination 2018, 443, 285–306. [Google Scholar] [CrossRef]
- Francis, L.; Ghaffour, N.; Alsaadi, A.A.; Amy, G.L. Material gap membrane distillation: A new design for water vapor flux enhancement. J. Membr. Sci. 2013, 448, 240–247. [Google Scholar] [CrossRef]
- Essalhi, M.; Khayet, M. Application of a porous composite hydrophobic/hydrophilic membrane in desalination by air gap and liquid gap membrane distillation: A comparative study. Sep. Purif. Technol. 2014, 133, 176–186. [Google Scholar] [CrossRef]
- Swaminathan, J.; Chung, H.W.; Warsinger, D.M.; AlMarzooqi, F.A.; Arafat, H.A.; Lienhard, J.H.V. Energy efficiency of permeate gap and novel conductive gap membrane distillation. J. Membr. Sci. 2016, 502, 171–178. [Google Scholar] [CrossRef]
- Cheng, L.; Zhao, Y.; Li, P.; Li, W.; Wang, F. Comparative study of air gap and permeate gap membrane distillation using internal heat recovery hollow fiber membrane module. Desalination 2018, 426, 42–49. [Google Scholar] [CrossRef]
- Mahmoudi, F.; Date, A.; Akbarzadeh, A. Further investigation of simultaneous fresh water production and power generation concept by permeate gap membrane distillation system. J. Membr. Sci. 2018, 572, 230–245. [Google Scholar] [CrossRef]
- Birgi, P.Y.; Ali, M.H.; Swaminathan, J.; Lienhard, J.; Arafat, H.A. Computational fluid dynamics modeling for performance assessment of permeate gap membrane distillation. J. Membr. Sci. 2018, 568, 55–66. [Google Scholar] [CrossRef]
- Cai, J.; Yin, H.; Guo, F. Transport analysis of material gap membrane distillation desalination processes. Desalination 2020, 481, 114361. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Guo, F. Breaking the Saturated Vapor Layer with a Thin Porous Membrane. Membranes 2022, 12, 1231. https://doi.org/10.3390/membranes12121231
Zhang Y, Guo F. Breaking the Saturated Vapor Layer with a Thin Porous Membrane. Membranes. 2022; 12(12):1231. https://doi.org/10.3390/membranes12121231
Chicago/Turabian StyleZhang, Yaoling, and Fei Guo. 2022. "Breaking the Saturated Vapor Layer with a Thin Porous Membrane" Membranes 12, no. 12: 1231. https://doi.org/10.3390/membranes12121231
APA StyleZhang, Y., & Guo, F. (2022). Breaking the Saturated Vapor Layer with a Thin Porous Membrane. Membranes, 12(12), 1231. https://doi.org/10.3390/membranes12121231







