Fluidized Bed Membrane Reactor for the Direct Dehydrogenation of Propane: Proof of Concept
Abstract
:1. Introduction
2. Phenomenological Fluidized Bed (Membrane) Reactor Model Description
| (R1) | ||
| (R2) | ||
| (R3) | ||
| (R4) |
3. Materials and Methods
3.1. Fluidized Bed (Membrane) Reactor Setup
3.2. H2-Selective Membrane
3.3. Catalyst
4. Results and Discussion
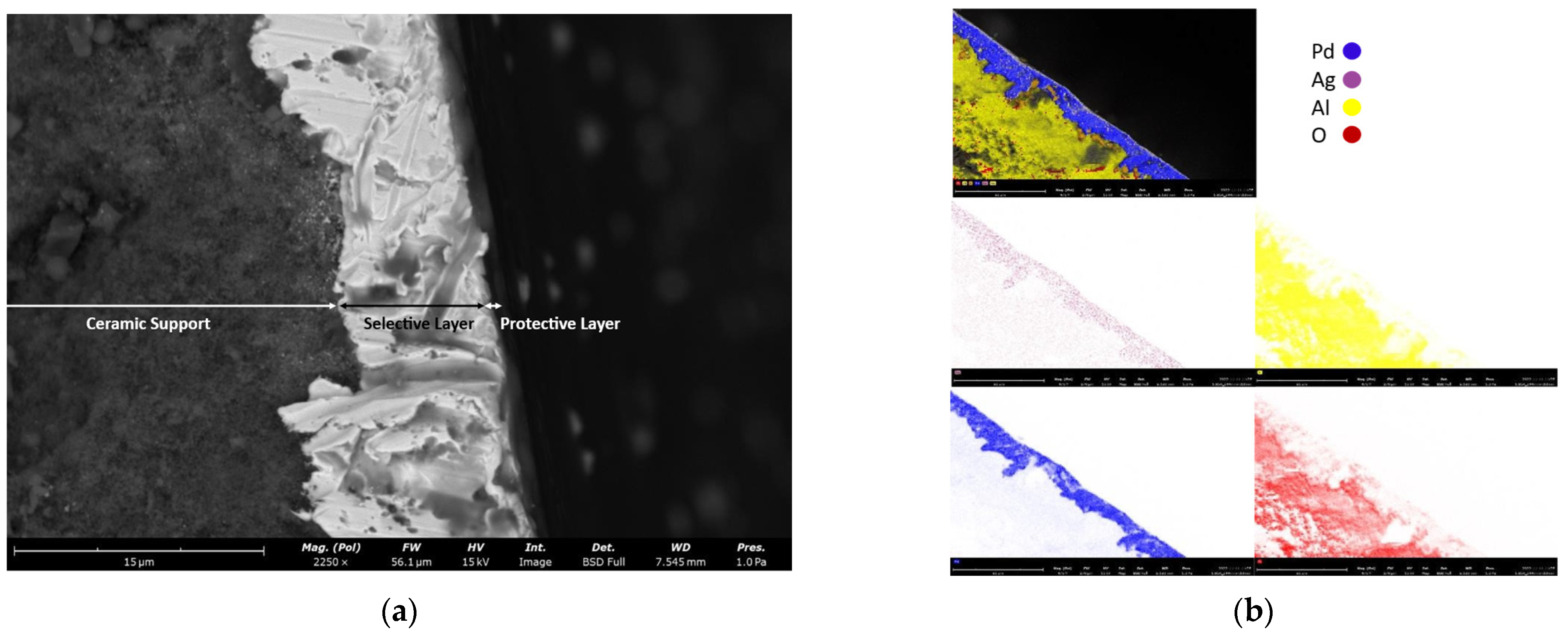
4.1. H2-Selective Membrane Characterization
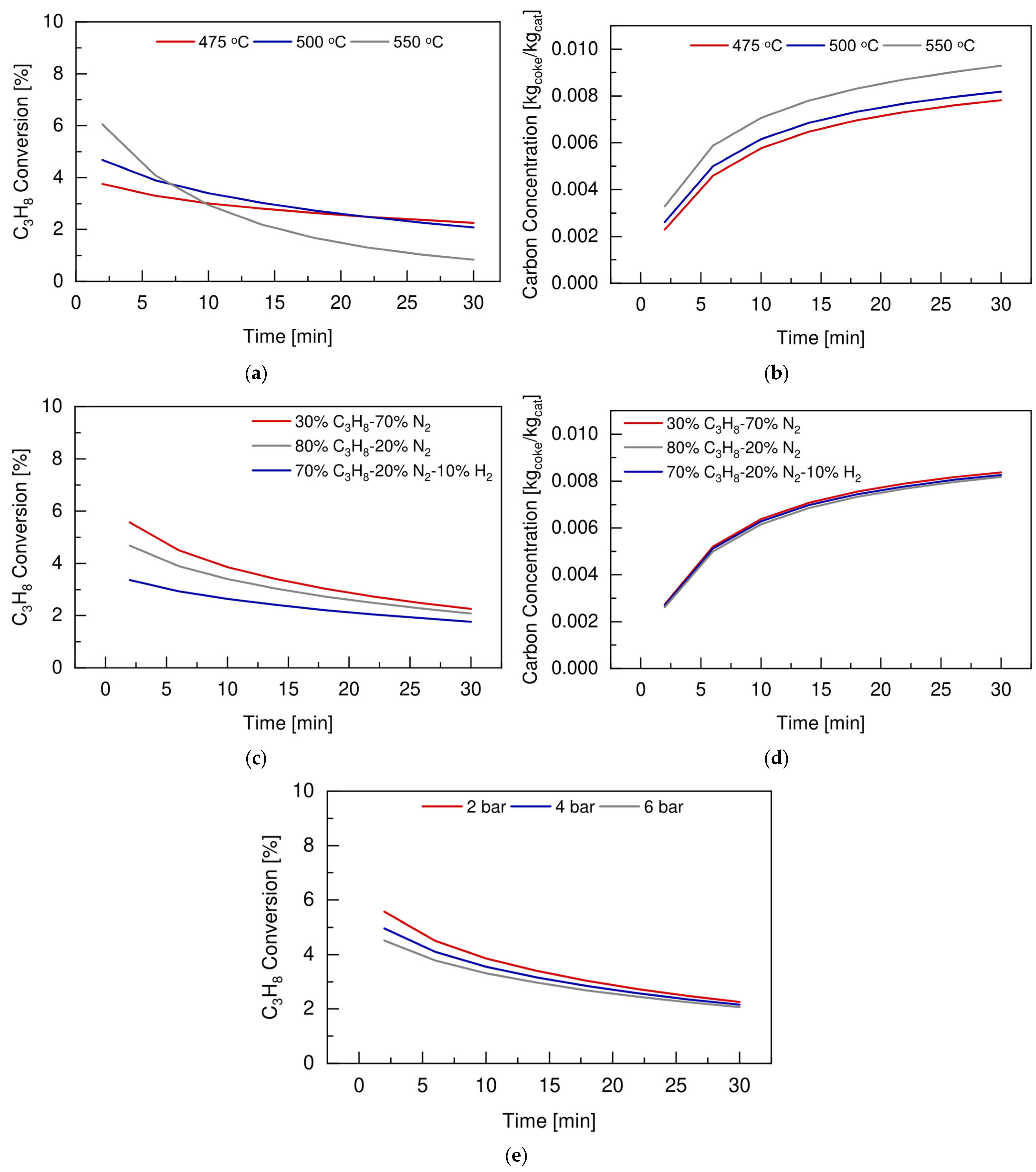
4.2. Preliminary Simulation Study to Identify Optimum Operative Conditions
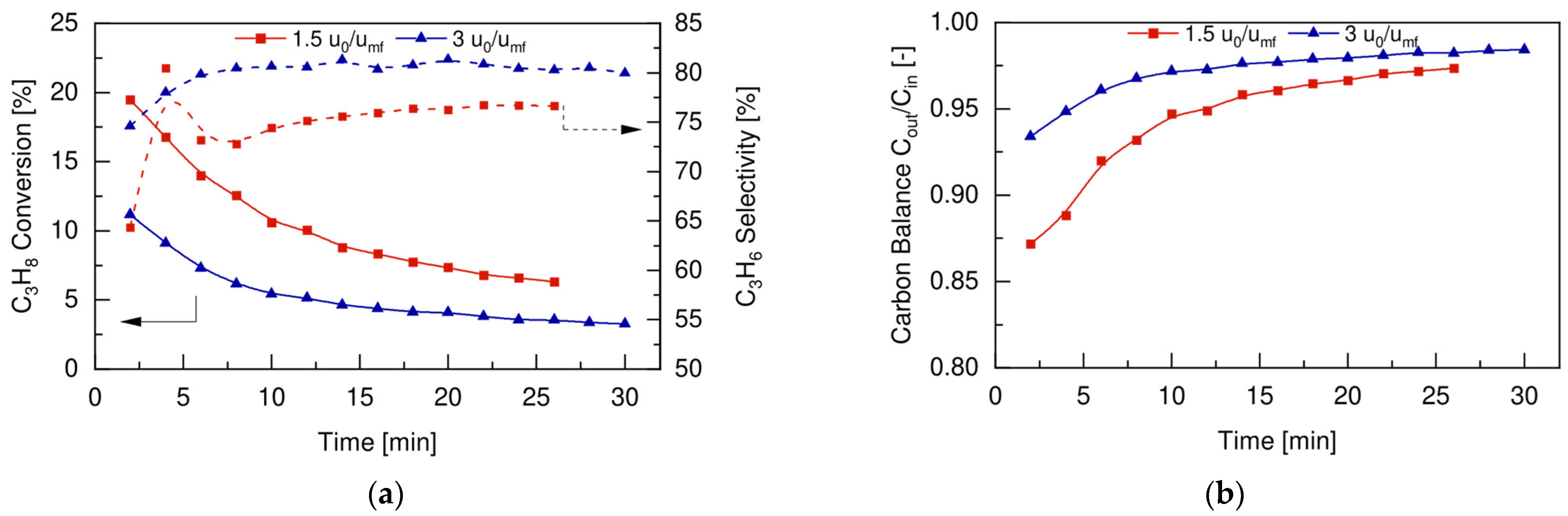
4.3. Conventional Fluidized Bed Reactor
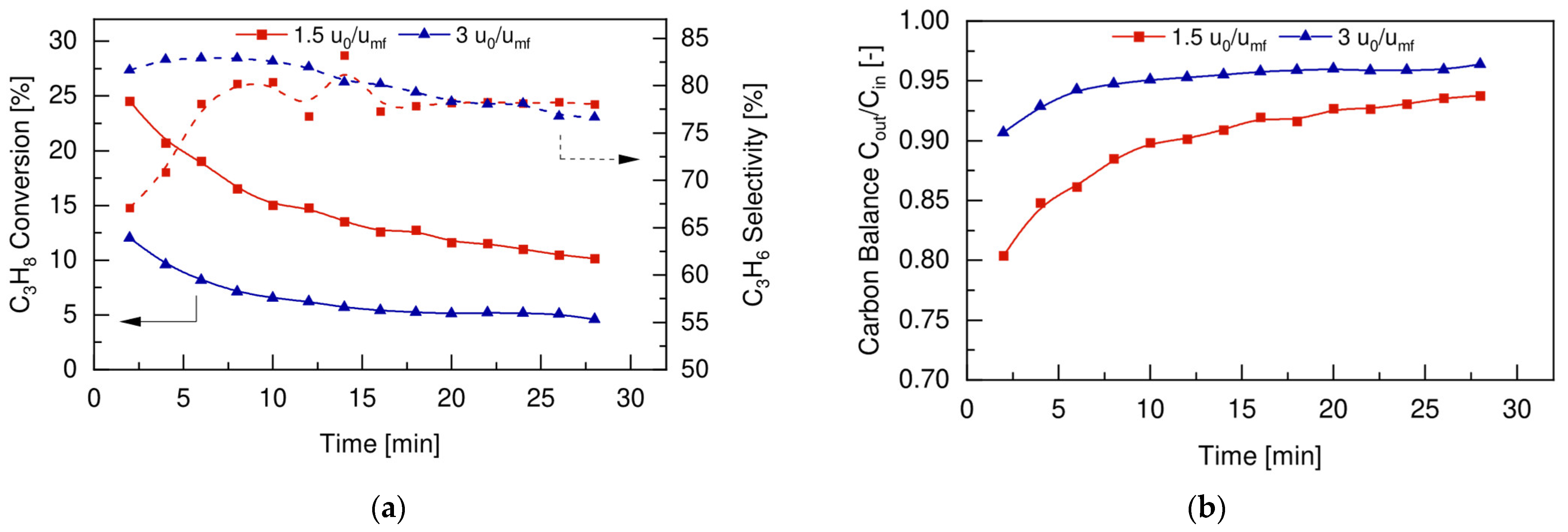
4.4. Fluidized Bed Membrane Reactor
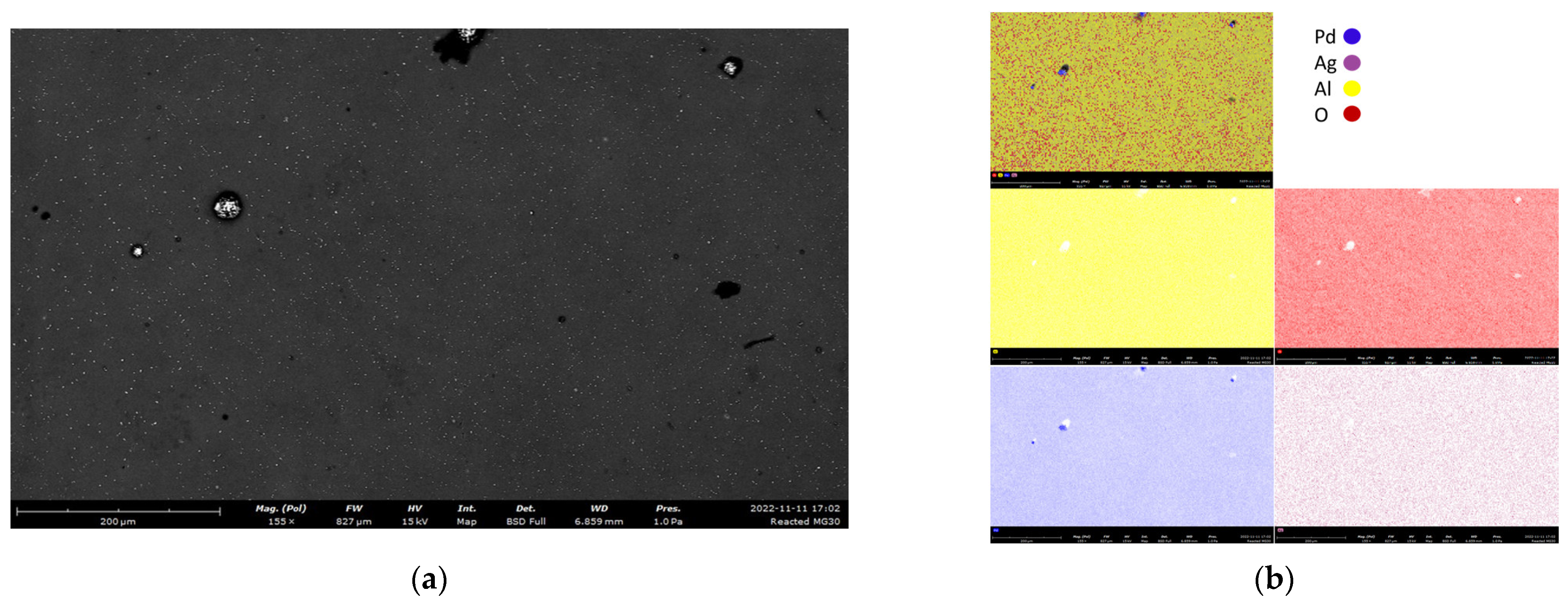
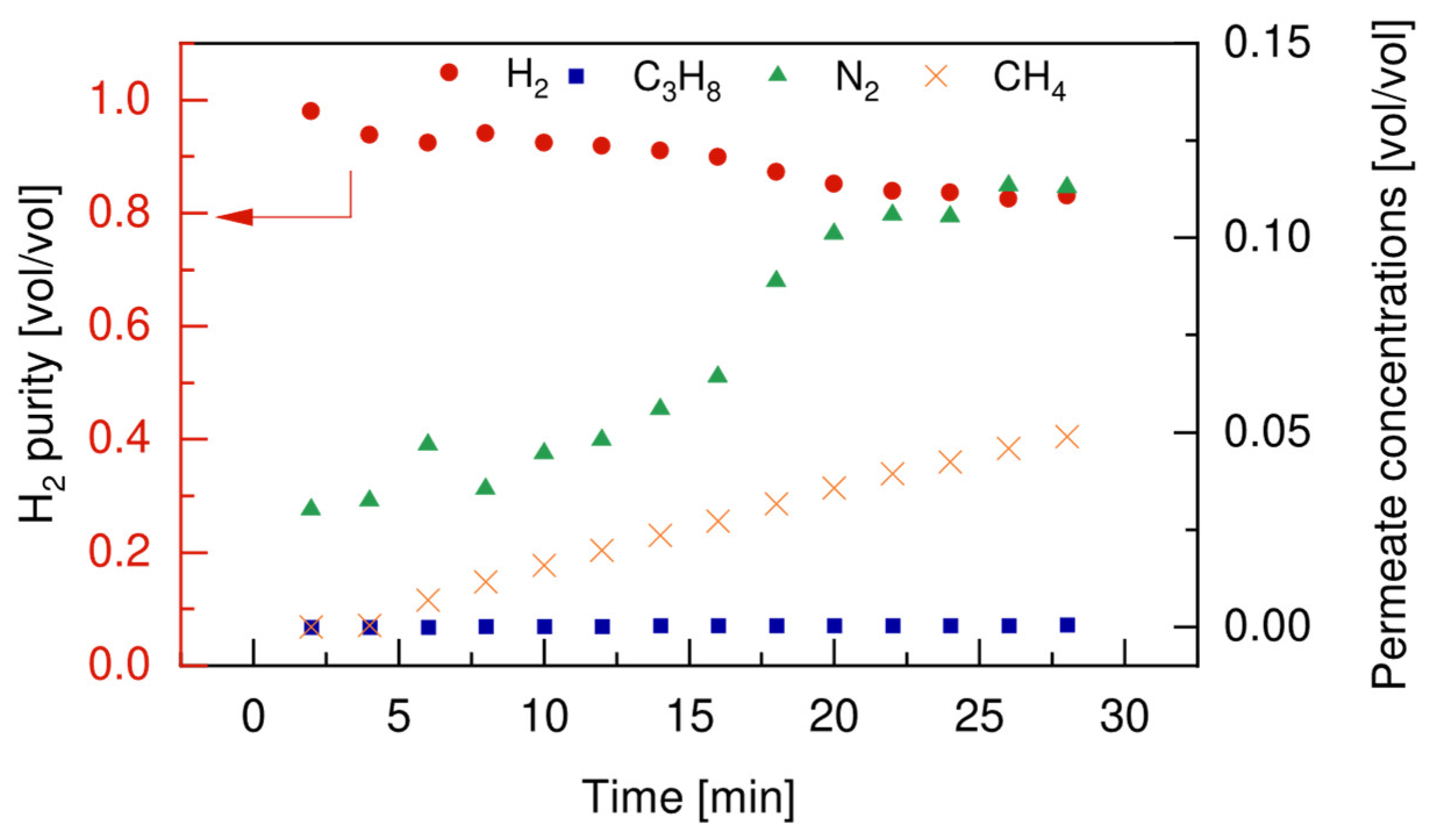
4.4.1. Membrane Stability Tests
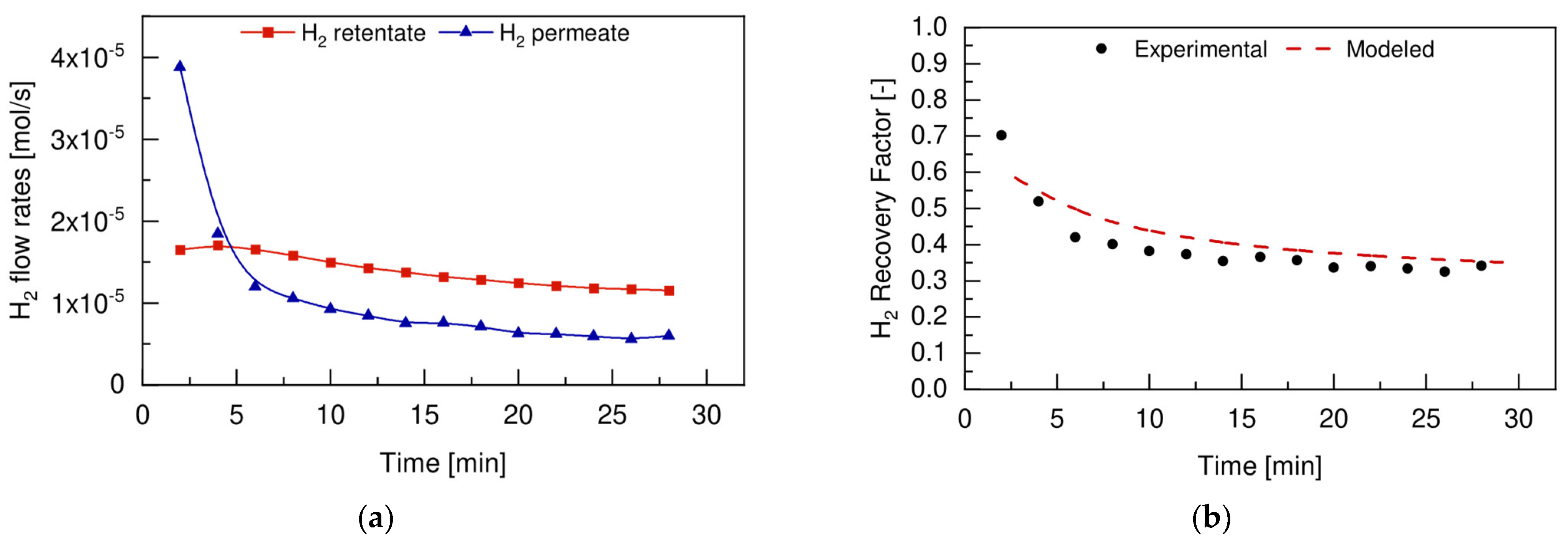
4.4.2. Reaction Tests
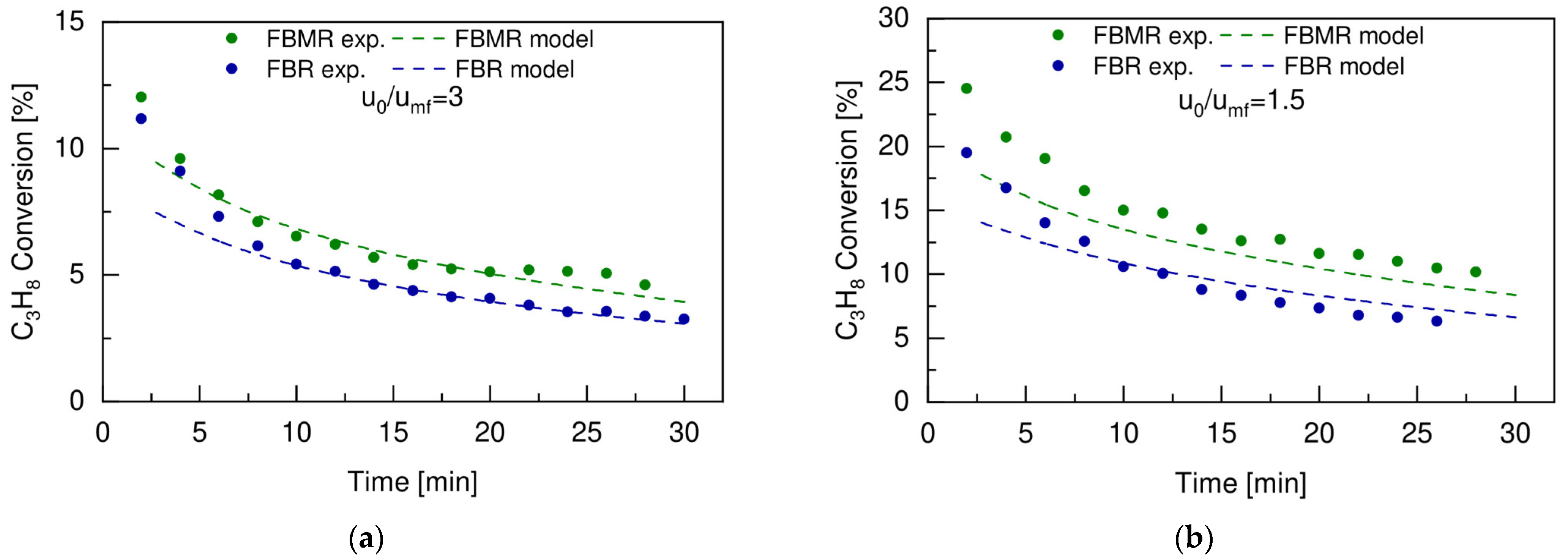
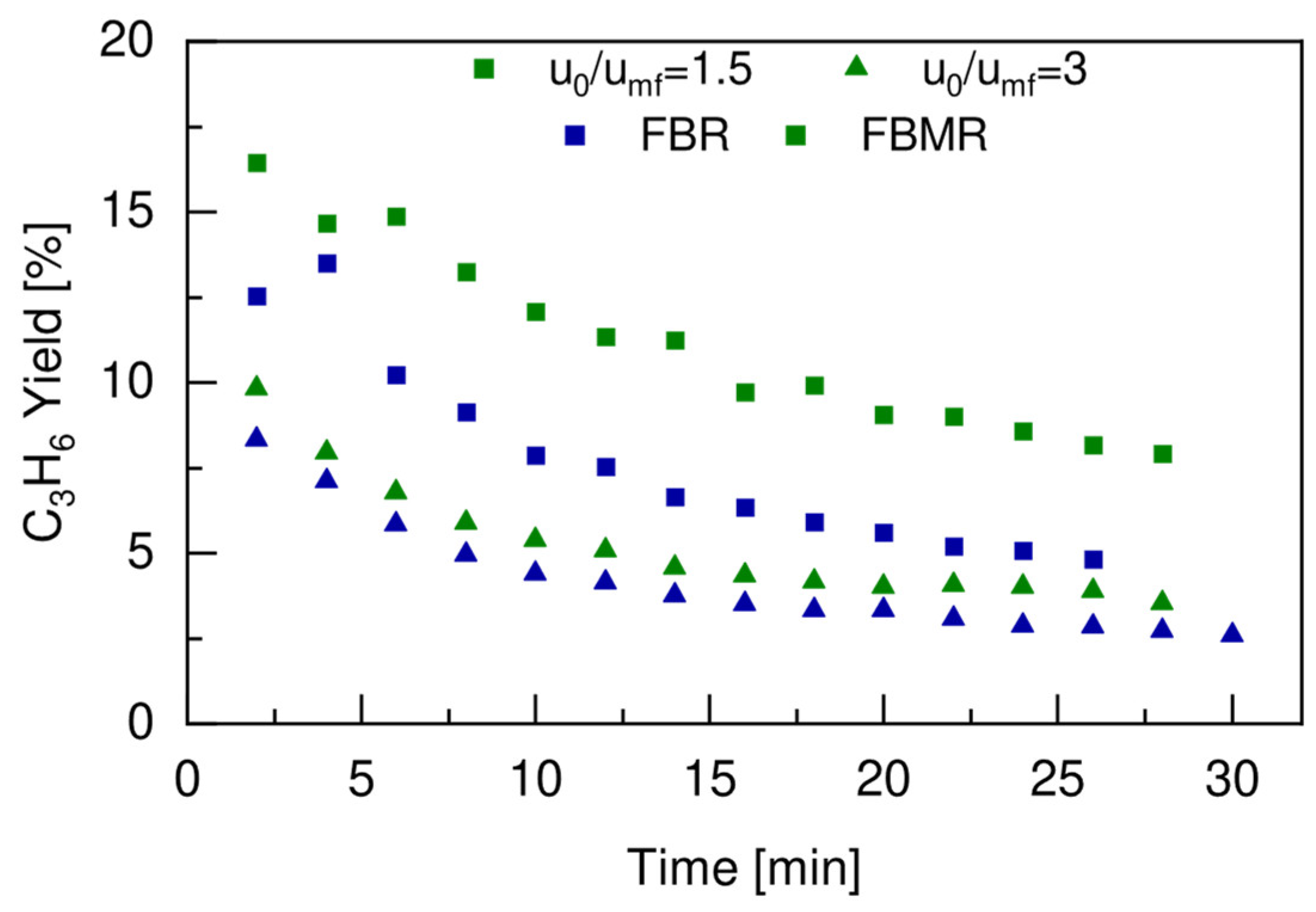
4.5. FBR vs. FBMR and Model Validation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Parameter | Equation | Ref. |
|---|---|---|
| Archimedes number | [35] | |
| Minimum fluidization velocity | ||
| Bed void fraction at minimum fluidization | [36] | |
| Bubble dimensions | [18,37] | |
| Bubble fraction | [18] | |
| Wake fraction | [38] | |
| Emulsion fraction | ||
| Bubble velocity | [18] | |
| Gas emulsion velocity | ||
| Solid Emulsion velocity | ||
| Mass transfer coefficient gas phase | ||
| Mass transfer coefficient solid phase |
Appendix A.1. Reaction Rate Laws and Kinetic Parameters
| Kinetic Parameter Expressions | Parameter | Value | Units |
|---|---|---|---|
| 0.5242 34.57 3.46 −85.817 500 | - °C | ||
| 0.00465 137.31 | |||
| 0.000236 154.54 | |||
| 234 38.43 1.45 × 10−6 125.51 1.04 × 10−3 |
| Kinetic Parameter | Value | Units |
|---|---|---|
| 948.92 | ||
| 9.61 | ||
| 399 | ||
| 40.07 |
| Kinetic Parameter | Value | Units |
|---|---|---|
| 9.989 | ||
| 2.221 × 10−14 | ||
| 1.557 | ||
| 0.096 | ||
| 5.1 | ||
| 1.175e3 | ||
| 1.848 × 10−9 | ||
| 1.822 × 105 | J/mol | |
| 1.007 × 106 | ||
| 405 | °C |
Appendix A.2. Physicochemical Properties of the PtSnK-Al2O3 Catalyst
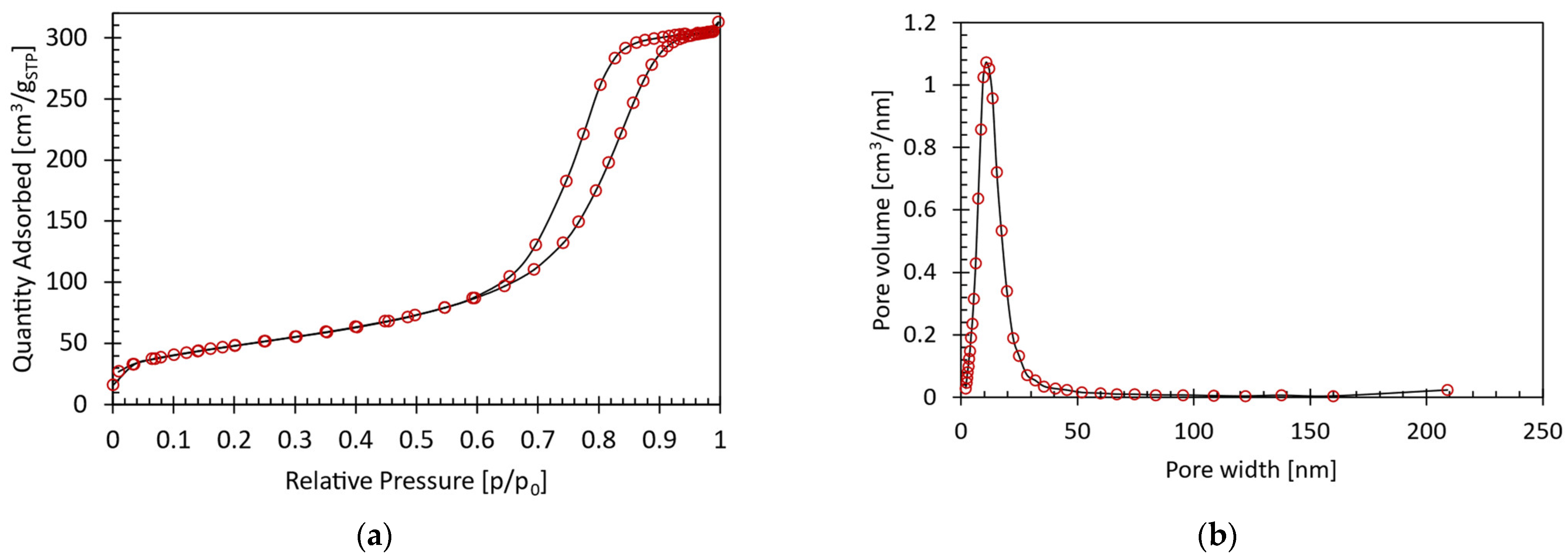


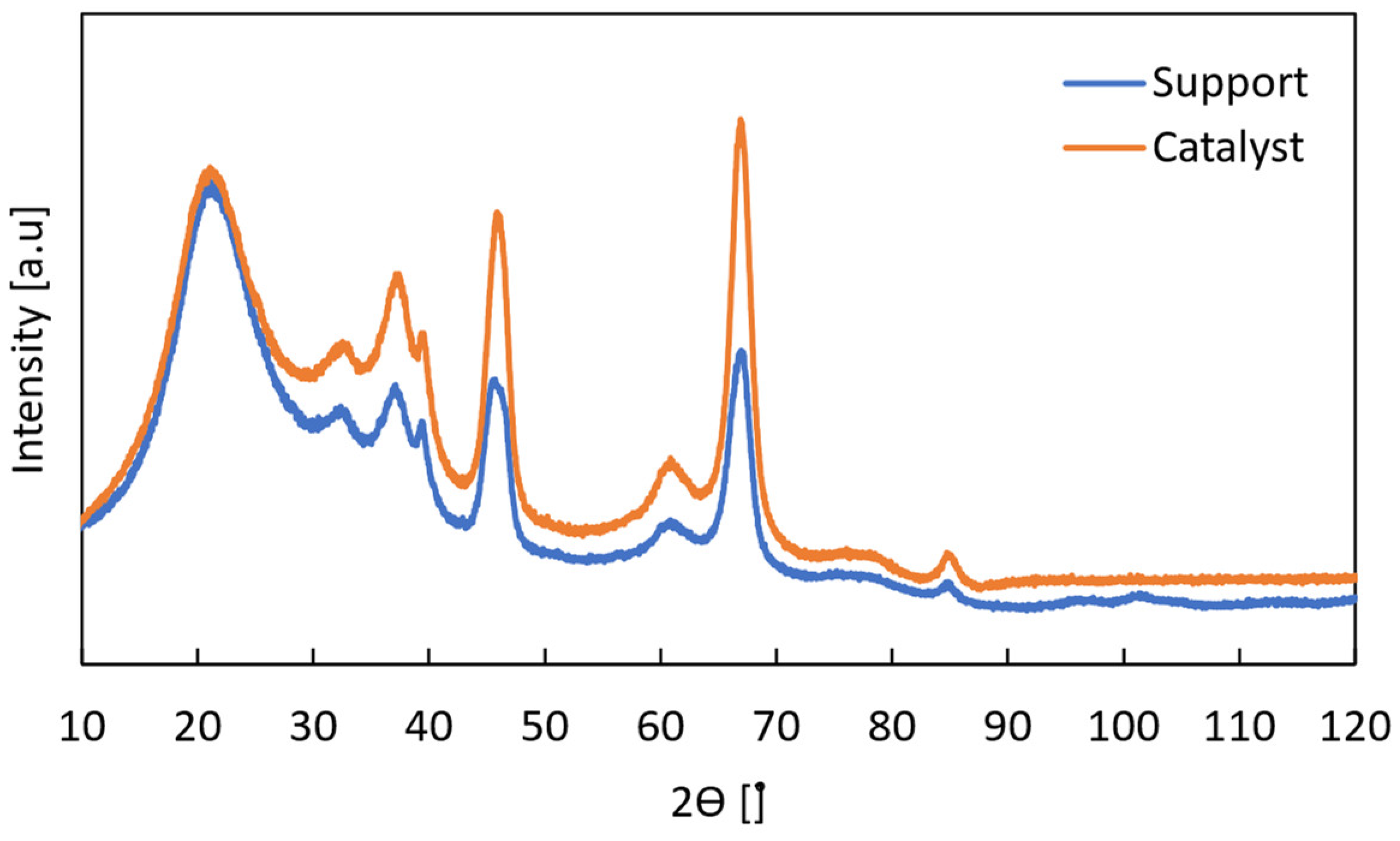
Appendix A.3. Blank Tests
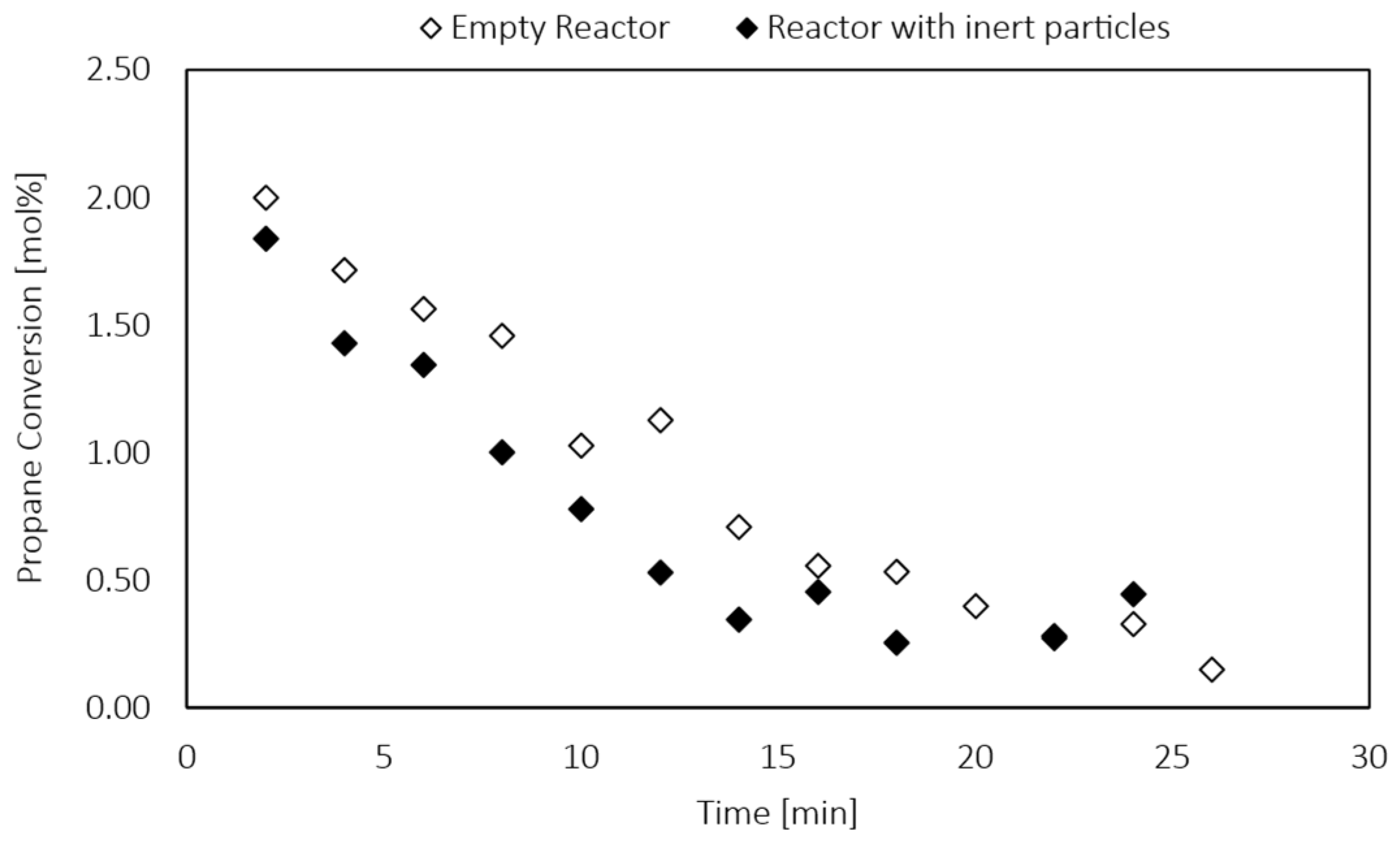
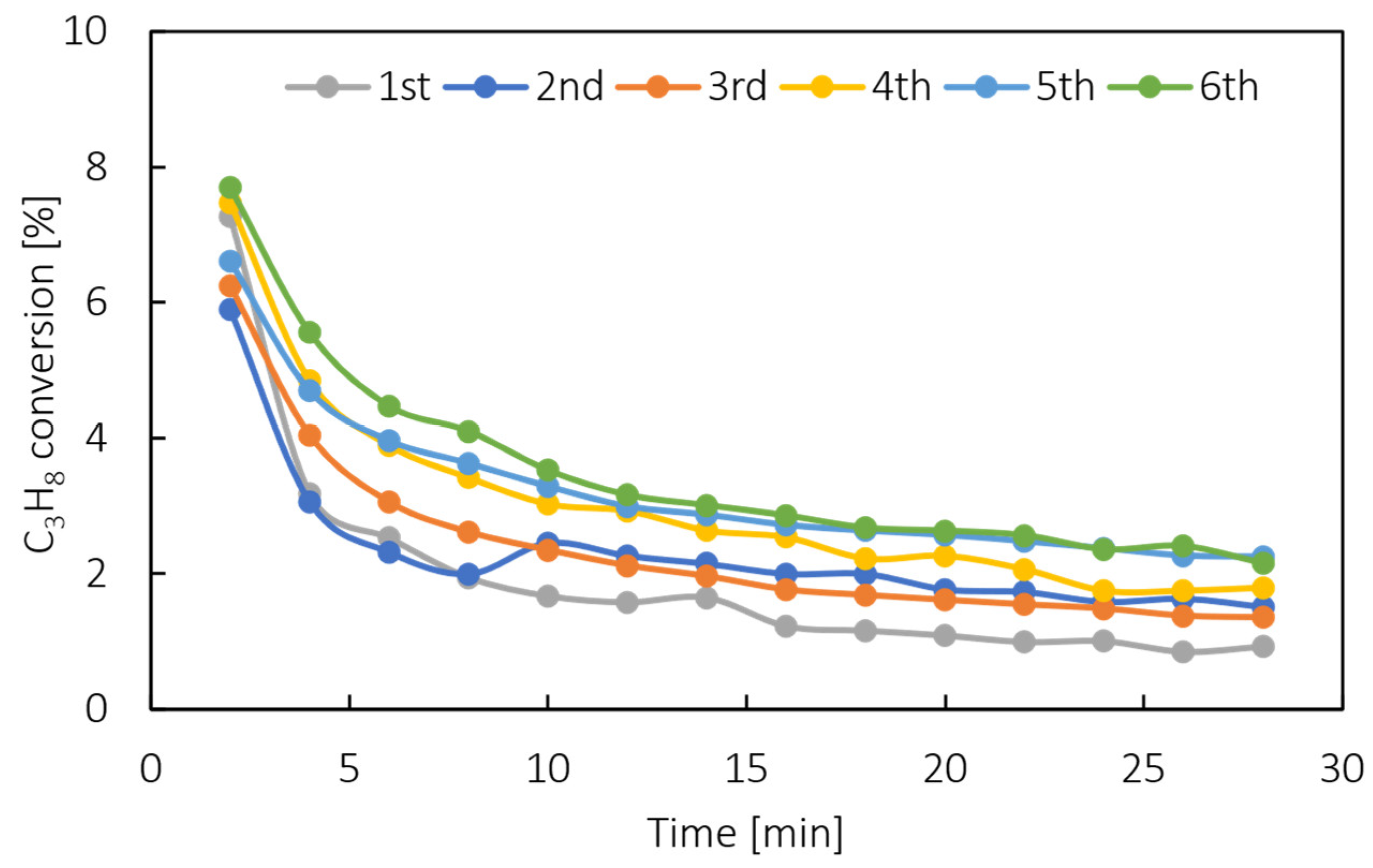
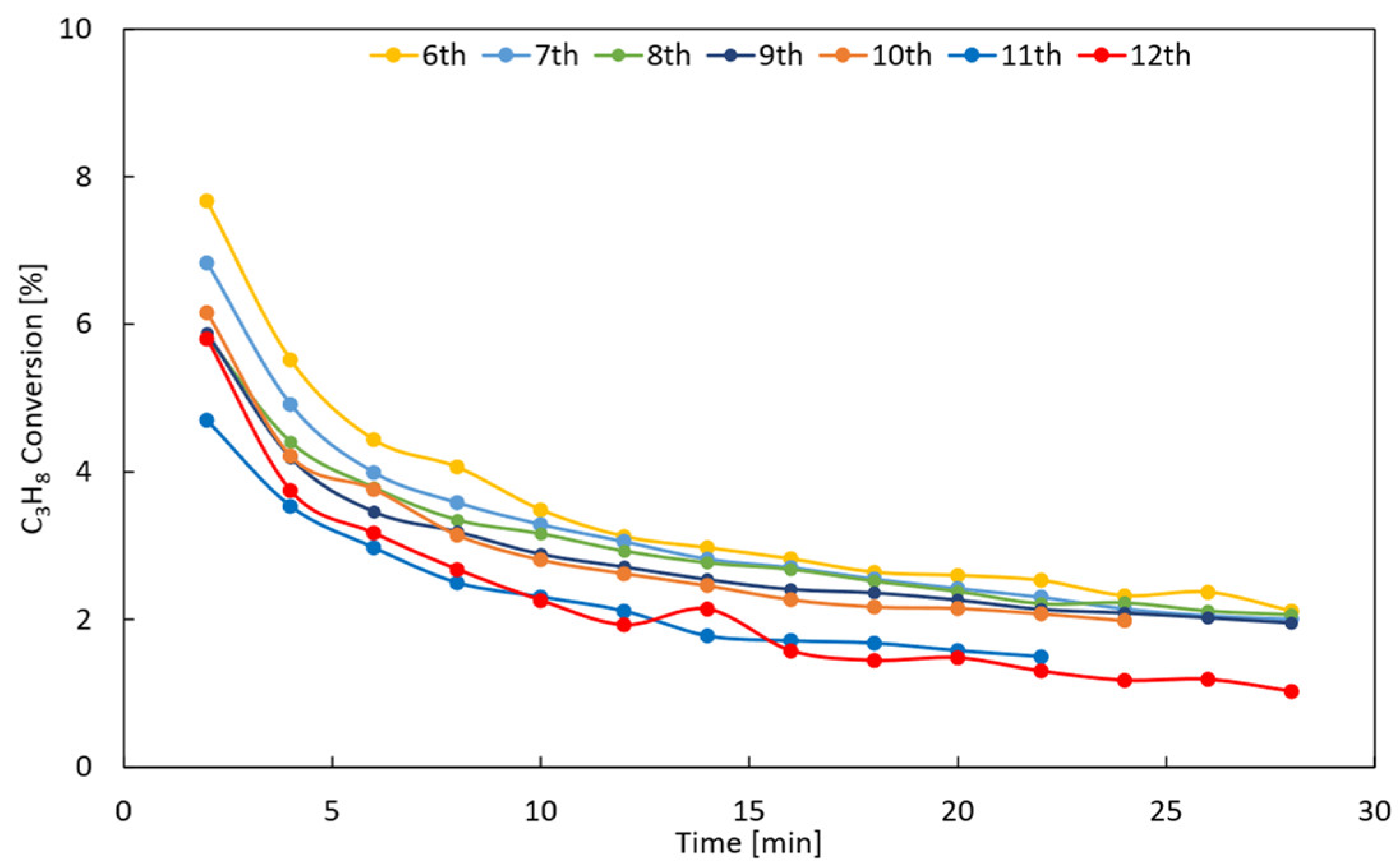
Appendix A.4. Catalyst Stability Tests
| Operating Conditions | |||||
|---|---|---|---|---|---|
| Cycle | Temperature [°C] | Pressure [bar] | Feed Composition | Duration [h] | u0/umf [-] |
| Reduction | 550 | 1 | H2:N2 = 1:2 | 2 | 5 |
| Reaction | 500 | 1 | C3H8:N2 = 2.33 | 0.5 | |
| Oxidation | 500 | 1 | Air:N2 = 1:10 | 1 | |
References
- Global Industry Analysts Global Propylene Industry. Available online: https://www.reportlinker.com/p05799443/Global-Propylene-Glycol-Industry.html?utm_source=GNW (accessed on 1 October 2022).
- Saerens, S.; Sabbe, M.K.; Galvita, V.V.; Redekop, E.A.; Marin, G.B. The Positive Role of Hydrogen on the Dehydrogenation of Propane on Pt (111). ACS Catal. 2017, 7, 7495–7508. [Google Scholar] [CrossRef]
- Nawaz, Z. Light Alkane Dehydrogenation to Light Olefin Technologies: A Comprehensive Review. Rev. Chem. Eng. 2015, 31, 413–436. [Google Scholar] [CrossRef]
- Sattler, J.J.H.B.; Ruiz-Martinez, J.; Santillan-Jimenez, E.; Weckhuysen, B.M. Catalytic Dehydrogenation of Light Alkanes on Metals and Metal Oxides. Chem. Rev. 2014, 114, 10613–10653. [Google Scholar] [CrossRef] [PubMed]
- Martino, M.; Meloni, E.; Festa, G.; Palma, V. Propylene Synthesis: Recent Advances in the Use of Pt-Based Catalysts for Propane Dehydrogenation Reaction. Catalysts 2021, 11, 1070. [Google Scholar] [CrossRef]
- Vora, B.V. Development of Dehydrogenation Catalysts and Processes. Top. Catal. 2012, 55, 1297–1308. [Google Scholar] [CrossRef]
- Lobera, M.P.; Téllez, C.; Herguido, J.; Menéndez, M. Transient Kinetic Modelling of Propane Dehydrogenation over a Pt-Sn-K/Al2O3 Catalyst. Appl. Catal. A Gen. 2008, 349, 156–164. [Google Scholar] [CrossRef]
- Chen, S.; Chang, X.; Sun, G.; Zhang, T.; Xu, Y.; Wang, Y.; Pei, C.; Gong, J. Propane Dehydrogenation: Catalyst Development, New Chemistry, and Emerging Technologies. Chem. Soc. Rev. 2021, 50, 3315–3354. [Google Scholar] [CrossRef]
- Shelepova, E.V.; Vedyagin, A.A. Intensification of the Dehydrogenation Process of Different Hydrocarbons in a Catalytic Membrane Reactor. Chem. Eng. Process. Process Intensif. 2020, 155, 108072. [Google Scholar] [CrossRef]
- Shelepova, E.V.; Vedyagin, A.A.; Mishakov, I.V.; Noskov, A.S. Simulation of Hydrogen and Propylene Coproduction in Catalytic Membrane Reactor. Int. J. Hydrog. Energy 2015, 40, 3592–3598. [Google Scholar] [CrossRef]
- Wang, H.; Wang, B.; Qi, X.; Wang, J.; Yang, R.; Li, D.; Hu, X. Innovative Non–Oxidative Methane Dehydroaromatization via Solar Membrane Reactor. Energy 2021, 216, 119265. [Google Scholar] [CrossRef]
- Shelepova, E.V.; Vedyagin, A.A.; Mishakov, I.V.; Noskov, A.S. Mathematical Modeling of the Propane Dehydrogenation Process in the Catalytic Membrane Reactor. Chem. Eng. J. 2011, 176–177, 151–157. [Google Scholar] [CrossRef]
- Ricca, A.; Montella, F.; Iaquaniello, G.; Palo, E.; Salladini, A.; Palma, V. Membrane Assisted Propane Dehydrogenation: Experimental Investigation and Mathematical Modelling of Catalytic Reactions. Catal. Today 2019, 331, 43–52. [Google Scholar] [CrossRef]
- Didenko, L.P.; Savchenko, V.I.; Sementsova, L.A.; Chizhov, P.E.; Bykov, L.A. Dehydrogenation of Propane in a Combined Membrane Reactor with Hydrogen-Permeable Palladium Module. Pet. Chem. 2013, 53, 27–32. [Google Scholar] [CrossRef]
- He, R.; Wang, Y.; Wang, H.; Lundin, S.T.B.; Wang, B.; Kong, H.; Lu, X.; Wang, J.; Li, W. A Mid/Low-Temperature Solar-Driven Integrated Membrane Reactor for the Dehydrogenation of Propane—A Thermodynamic Assessment. Appl. Therm. Eng. 2021, 193, 116952. [Google Scholar] [CrossRef]
- Iliuta, I.; Tahoces, R.S.; Patience, G. Chemical-Looping Combustion Process: Kinetics and Mathematical Modeling. AIChE J. 2010, 59, 1063–1079. [Google Scholar] [CrossRef]
- Medrano, J.A.; Potdar, I.; Melendez, J.; Spallina, V.; Pacheco-Tanaka, D.A.; van Sint Annaland, M.; Gallucci, F. The Membrane-Assisted Chemical Looping Reforming Concept for Efficient H2 Production with Inherent CO2 Capture: Experimental Demonstration and Model Validation. Appl. Energy 2018, 215, 75–86. [Google Scholar] [CrossRef]
- Levenspiel, O.; Kunii, D. Fluidization Engineering, 2nd ed.; Butterworth-Heinemann: Stoneham, MA, USA, 1991. [Google Scholar]
- Li, Q.; Sui, Z.; Zhou, X.; Chen, D. Kinetics of Propane Dehydrogenation over Pt-Sn/Al2O3 Catalyst. Appl. Catal. A Gen. 2011, 398, 18–26. [Google Scholar] [CrossRef]
- Zangeneh, F.T.; Taeb, A.; Gholivand, K.; Sahebdelfar, S. Kinetic Study of Propane Dehydrogenation and Catalyst Deactivation over Pt-Sn/Al2O3 Catalyst. J. Energy Chem. 2013, 22, 726–732. [Google Scholar] [CrossRef]
- Sahebdelfar, S.; Ravanchi, M.T.; Tahriri Zangeneh, F.; Mehrazma, S.; Rajabi, S. Kinetic Study of Propane Dehydrogenation and Side Reactions over Pt-Sn/Al2O3 Catalyst. Chem. Eng. Res. Des. 2012, 90, 1090–1097. [Google Scholar] [CrossRef]
- Farjoo, A.; Khorasheh, F.; Niknaddaf, S.; Soltani, M. Kinetic Modeling of Side Reactions in Propane Dehydrogenation over Pt-Sn/γ-Al2O3 Catalyst. Sci. Iran. 2011, 18, 458–464. [Google Scholar] [CrossRef]
- Brencio, C.; Gough, R.; de Leeuw den Bouter, A.; Arratibel, A.; Di Felice, L.; Gallucci, F. Kinetic Model for Pd-Based Membranes Coking/Deactivation in Propane Dehydrogenation Processes. Chem. Eng. J. 2023, 452, 139125. [Google Scholar] [CrossRef]
- Brencio, C.; Fontein, F.W.A.; Medrano, J.A.; Felice, L.; Di Arratibel, A.; Gallucci, F. Pd-Based Membranes Performance under Hydrocarbon Exposure for Propane Dehydrogenation Processes: Experimental and Modeling. Int. J. Hydrog. Energy 2021, 47, 11369–11384. [Google Scholar] [CrossRef]
- Arratibel, A.; Pacheco, A.; Laso, I.; Sint, M. Van Development of Pd-Based Double-Skinned Membranes for Hydrogen Production in Fl Uidized Bed Membrane Reactors. J. Memb. Sci. 2018, 550, 536–544. [Google Scholar] [CrossRef]
- Arratibel, A.; Medrano, J.A.; Melendez, J.; Pacheco Tanaka, D.A.; van Sint Annaland, M.; Gallucci, F. Attrition-Resistant Membranes for Fluidized-Bed Membrane Reactors: Double-Skin Membranes. J. Memb. Sci. 2018, 563, 419–426. [Google Scholar] [CrossRef]
- Bariås, O.A.; Holmen, A.; Blekkan, E.A. Propane Dehydrogenation over Supported Pt and Pt–Sn Catalysts: Catalyst Preparation, Characterization, and Activity Measurements. J. Catal. 1996, 158, 1–12. [Google Scholar] [CrossRef]
- Helmi, A.; Fernandez, E.; Melendez, J.; Tanaka, D.A.P.; Gallucci, F.; Van Sint Annaland, M. Fluidized Bed Membrane Reactors for Ultra Pure H2 Production—A Step Forward towards Commercialization. Molecules 2016, 21, 376. [Google Scholar] [CrossRef] [Green Version]
- Fernandez, E.; Helmi, A.; Coenen, K.; Melendez, J.; Viviente, J.L.; Pacheco Tanaka, D.A.; Van Sint Annaland, M.; Gallucci, F. Development of Thin Pd-Ag Supported Membranes for Fluidized Bed Membrane Reactors Including WGS Related Gases. Int. J. Hydrog. Energy 2015, 40, 3506–3519. [Google Scholar] [CrossRef] [Green Version]
- Okazaki, J.; Ikeda, T.; Pacheco Tanaka, D.A.; Llosa Tanco, M.A.; Wakui, Y.; Sato, K.; Mizukami, F.; Suzuki, T.M. Importance of the Support Material in Thin Palladium Composite Membranes for Steady Hydrogen Permeation at Elevated Temperatures. Phys. Chem. Chem. Phys. 2009, 11, 8632–8638. [Google Scholar] [CrossRef]
- Lobera, M.P.; Téllez, C.; Herguido, J.; Menéndez, M. Propane Dehydrogenation over Pt-Sn-K/γ-Al2O3 Catalyst in a Two-Zone Fluidized Bed Reactor. Ind. Eng. Chem. Res. 2008, 47, 9314–9320. [Google Scholar] [CrossRef]
- Nawaz, Z.; Chu, Y.; Yang, W.; Tang, X.; Wang, Y.; Wei, F. Study of Propane Dehydrogenation to Propylene in an Integrated Fluidized Bed Reactor Using Pt-Sn/Al-SAPO-34 Novel Catalyst. Ind. Eng. Chem. Res. 2010, 49, 4614–4619. [Google Scholar] [CrossRef]
- Song, C.; Wang, J.; Wang, S.; Wen, J. Experimental and Theoretical Study of the Impact of Operating Conditions on Catalytic Propane Dehydrogenation in a Fluidized Bed Reactor. Ind. Eng. Chem. Res. 2022, 61, 12434–12447. [Google Scholar] [CrossRef]
- Medrano, J.A.; Julián, I.; Herguido, J.; Menéndez, M. Pd-Ag Membrane Coupled to a Two-Zone Fluidized Bed Reactor (TZFBR) for Propane Dehydrogenation on a Pt-Sn/MgAl2O4 Catalyst. Membranes 2013, 3, 69–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lippens, B.C.; Mulder, J. Prediction of the Minimum Fluidization Velocity. Powder Technol. 1993, 75, 67–78. [Google Scholar] [CrossRef]
- Broadhurst, T.E.; Becker, H.A. Onset of Fluidization and Slugging in Beds of Uniform Particles. AIChE J. 1975, 21, 238–247. [Google Scholar] [CrossRef]
- Mori, S.; Wen, C.Y. Estimation of Bubble Diameter in Gaseous Fluidized Beds. AIChE J. 1975, 21, 109–115. [Google Scholar] [CrossRef]
- Medrano, J.A.; Tasdemir, M.; Gallucci, F.; van Sint Annaland, M. On the Internal Solids Circulation Rates in Freely-Bubbling Gas-Solid Fluidized Beds. Chem. Eng. Sci. 2017, 172, 395–406. [Google Scholar] [CrossRef]
- He, S.; Sun, C.; Bai, Z.; Dai, X.; Wang, B. Dehydrogenation of Long Chain Paraffins over Supported Pt-Sn-K/Al2O3 Catalysts: A Study of the Alumina Support Effect. Appl. Catal. A Gen. 2009, 356, 88–98. [Google Scholar] [CrossRef]


| Parameter | Units | Value |
|---|---|---|
| Pe0 | mol/(m2 s Pa) | 5.73 × 10−10 |
| Ea | kJ/mol | 12.53 |
| δ | μm | 1.75 |
| n | - | 0.75 |
| Material | Dp [μm] | Avg. Skeletal Density [g/cm3] | Apparent Density [g/cm3] | W [g] |
|---|---|---|---|---|
| Al2O3 | 150–250 | 3.300 | 1.691 | 175 |
| 0.05 PtSnK/Al2O3 | 200–250 | 3.317 | 1.204 | 20 |
| Parameter | Value | ||
|---|---|---|---|
| Temperature [°C] | 20 | 400 | 500 |
| umf [m/s] | 0.036 | 0.009 | 0.008 |
| Reactor Configuration | Catalyst | T [°C] and p [bar] | Feed Composition [vol%] | WHSV [h−1] or u0/umf | Conversion [%] | Ref. |
|---|---|---|---|---|---|---|
| PBR | Pt-Sn/γAl2O3 | 580, 1 | 80% C3H8-20% H2 | 5 h−1 | 25% | [22] |
| PBMR | Pt-Sn based | 500, 6 | 80% C3H8-20% H2O | 8 h−1 | <8% | [13] |
| FBR | Pt-Sn-K/γAl2O3 | 500, 1 | 50% C3H8-50% Ar | 4 | <10% | [31] |
| FBR | Pt-Sn/Al-SAPO-34 | 590, 1 | 80% C3H8-20% H2 | 9 h−1 | 30% | [32] |
| FBR | CrOx-based | 550, 1 | 100 C3H8 | 2.49 h−1 | 25% | [33] |
| FBMR | Pt-Sn/MgAl2O4 | 525, 1 | 50% C3H8-50% Ar | 2.5 | 15% | [34] |
| FBR | Pt-Sn-K/γAl2O3 | 500, 2 | 30% C3H8-70% N2 | 1.5 | 19% | This work |
| FBMR | Pt-Sn-K/γAl2O3 | 500, 2 | 30% C3H8-70% N2 | 1.5 | 24.5% | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brencio, C.; Di Felice, L.; Gallucci, F. Fluidized Bed Membrane Reactor for the Direct Dehydrogenation of Propane: Proof of Concept. Membranes 2022, 12, 1211. https://doi.org/10.3390/membranes12121211
Brencio C, Di Felice L, Gallucci F. Fluidized Bed Membrane Reactor for the Direct Dehydrogenation of Propane: Proof of Concept. Membranes. 2022; 12(12):1211. https://doi.org/10.3390/membranes12121211
Chicago/Turabian StyleBrencio, Camilla, Luca Di Felice, and Fausto Gallucci. 2022. "Fluidized Bed Membrane Reactor for the Direct Dehydrogenation of Propane: Proof of Concept" Membranes 12, no. 12: 1211. https://doi.org/10.3390/membranes12121211
APA StyleBrencio, C., Di Felice, L., & Gallucci, F. (2022). Fluidized Bed Membrane Reactor for the Direct Dehydrogenation of Propane: Proof of Concept. Membranes, 12(12), 1211. https://doi.org/10.3390/membranes12121211







