Applying Chitin Enhanced Diafiltration Process (CEFP) in Removing Cobalt from Synthetic Wastewater
Abstract
1. Introduction
2. Materials and Methods
2.1. Study of Dynamic Metal-Binding Using Chitin in CEFP
2.2. Equilibrium Study of Co Binding Properties of Chitin
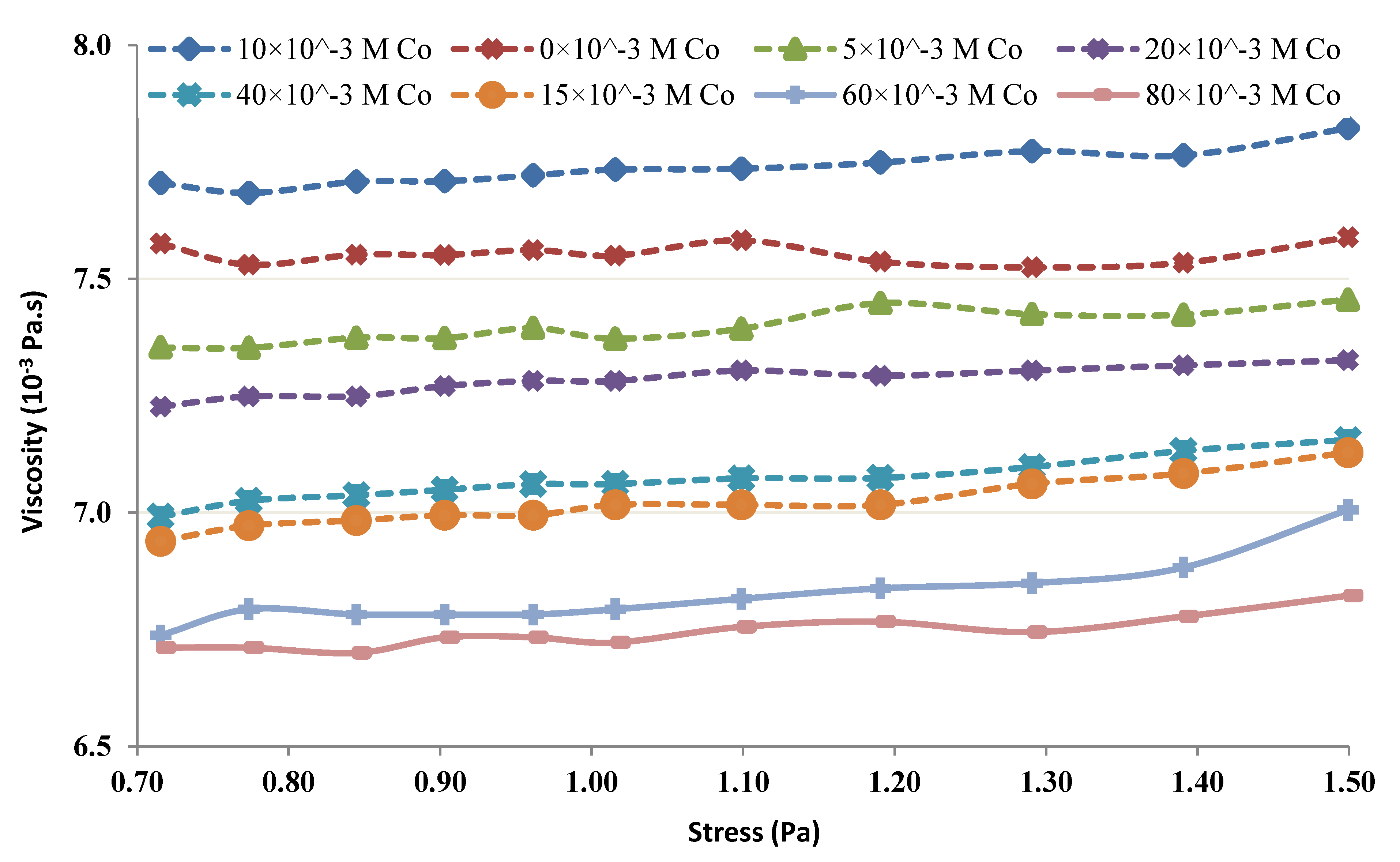
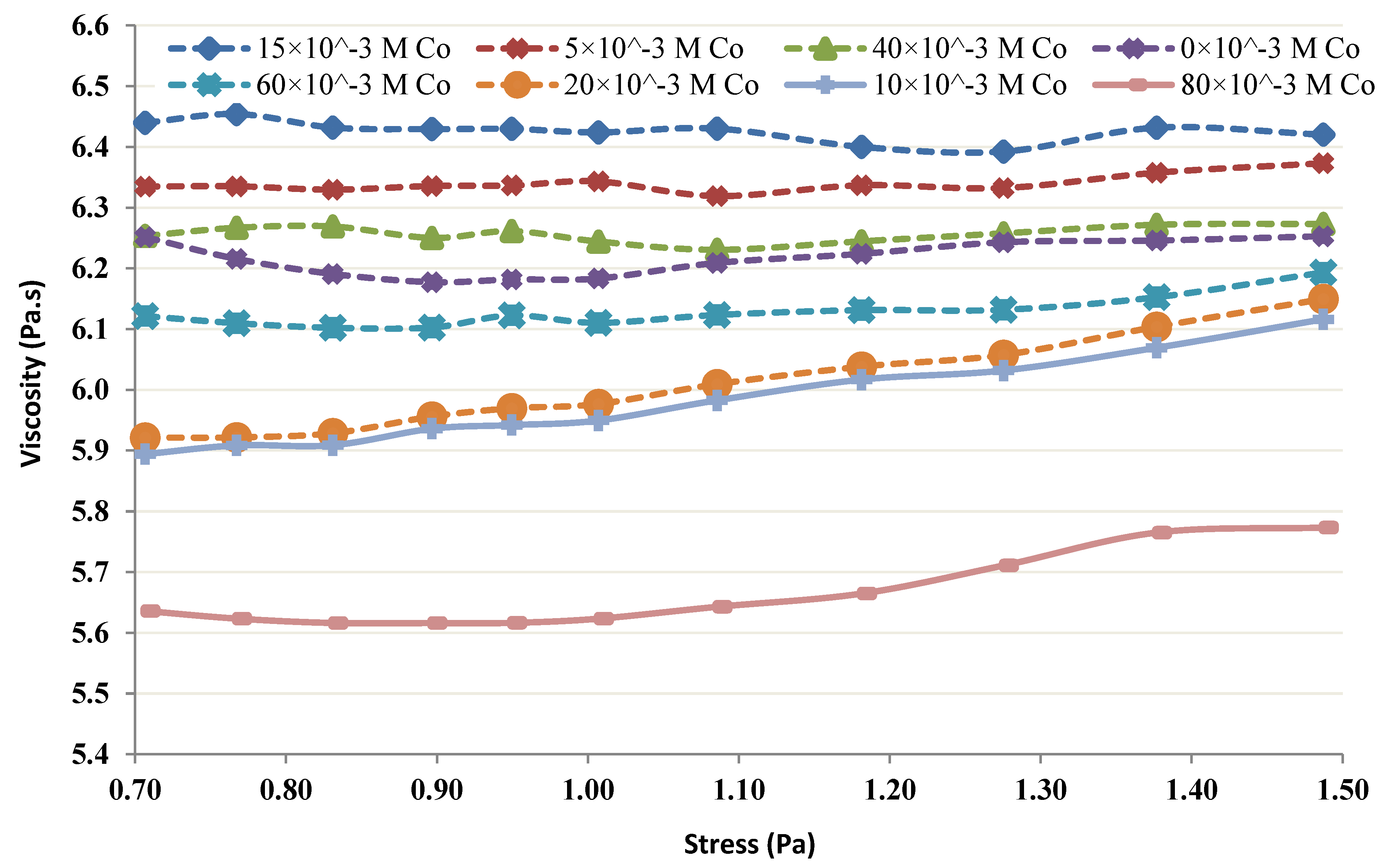
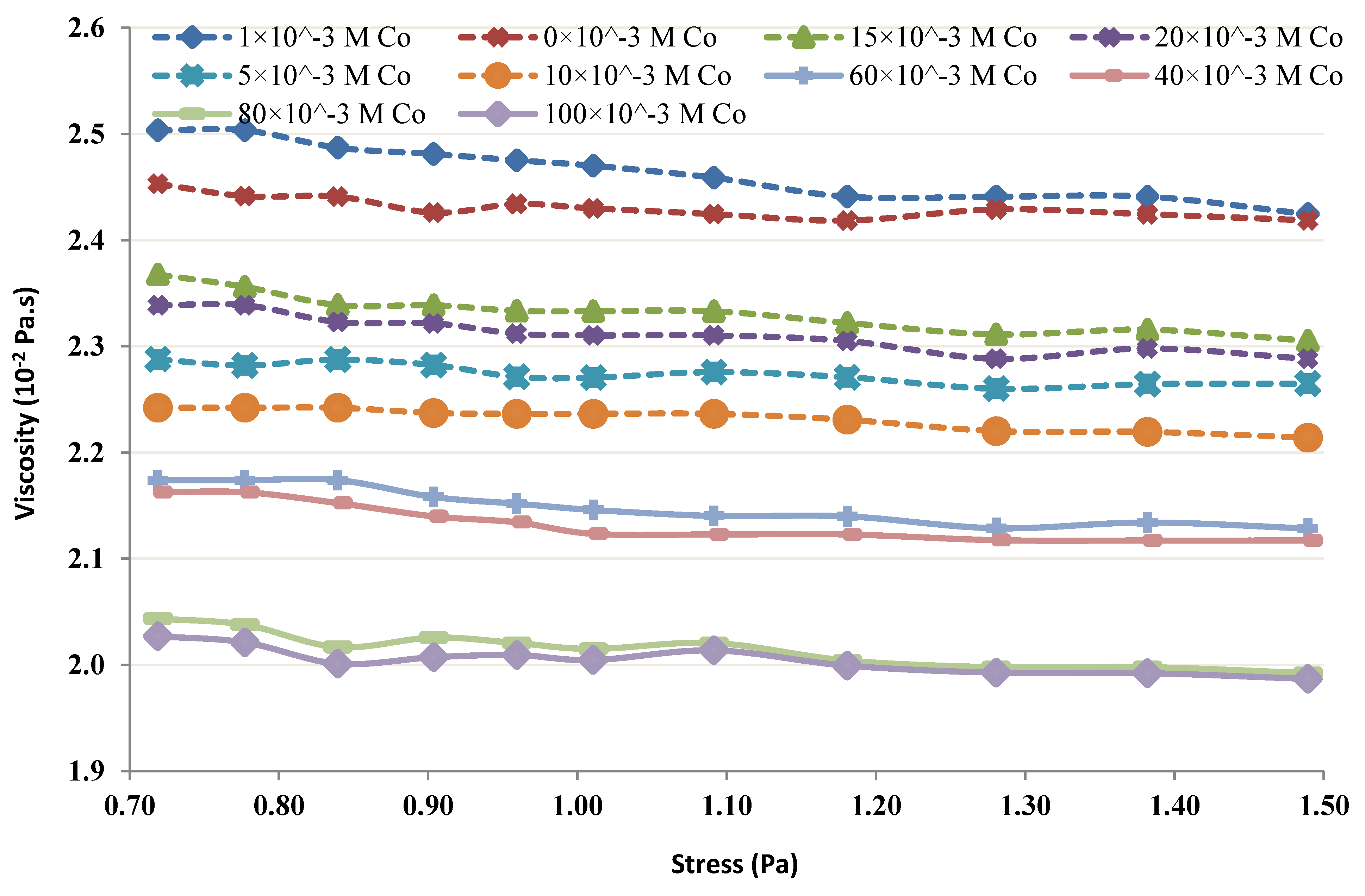
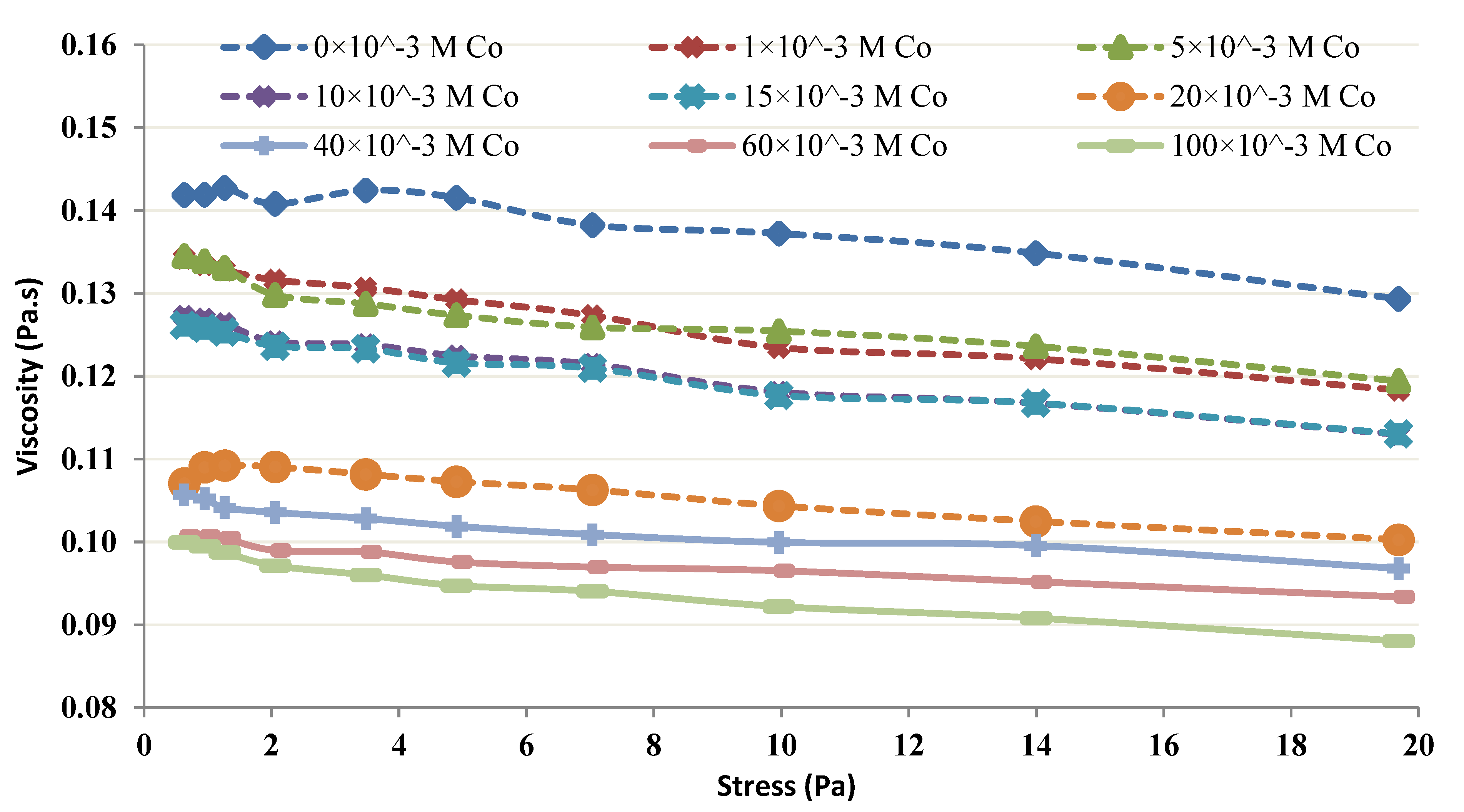
2.3. Rheological Studies of the Chitin-Co Complex
3. Results and Discussion
3.1. Kinetic Study and Equilibrium Isotherm Analysis
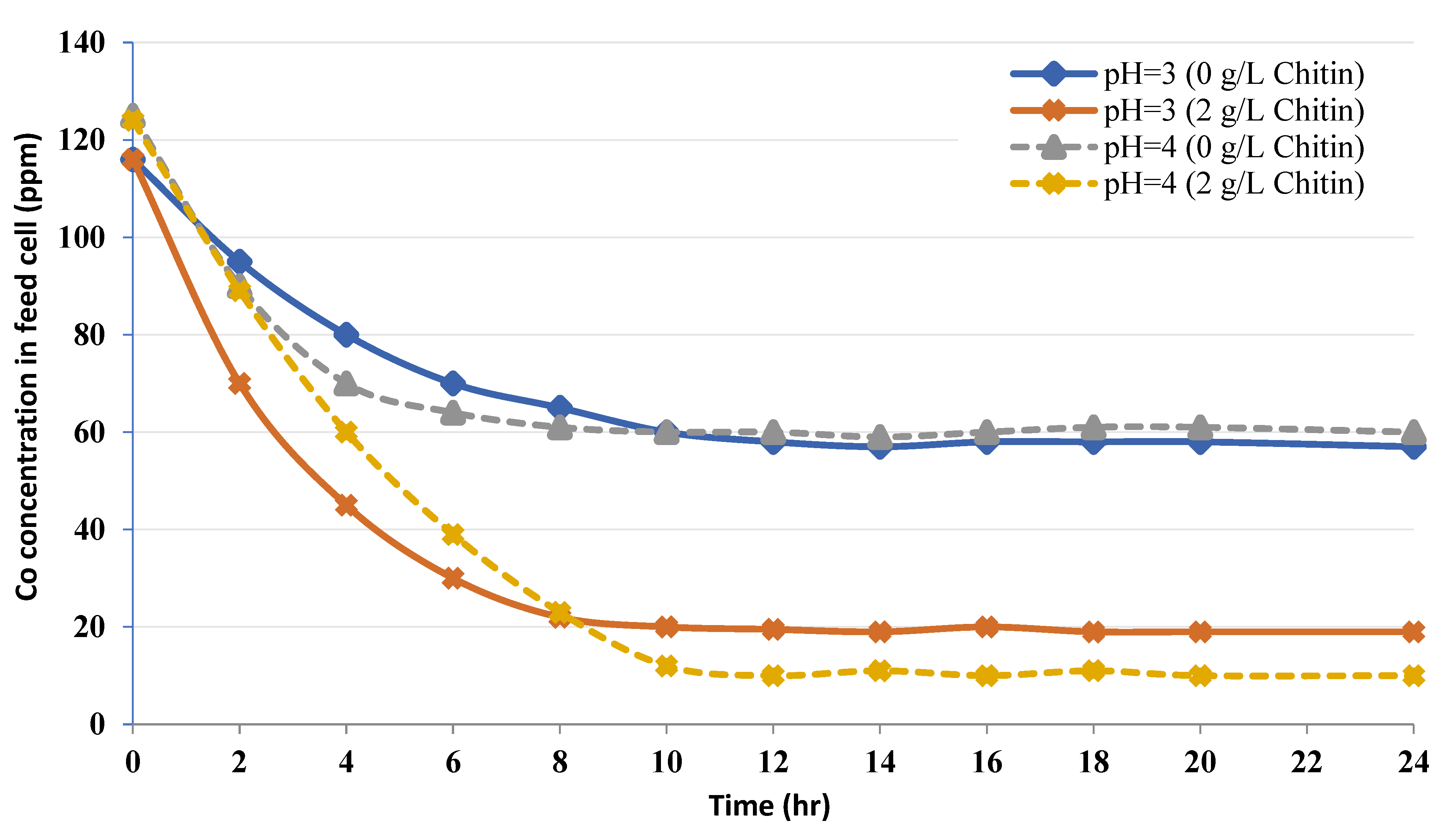
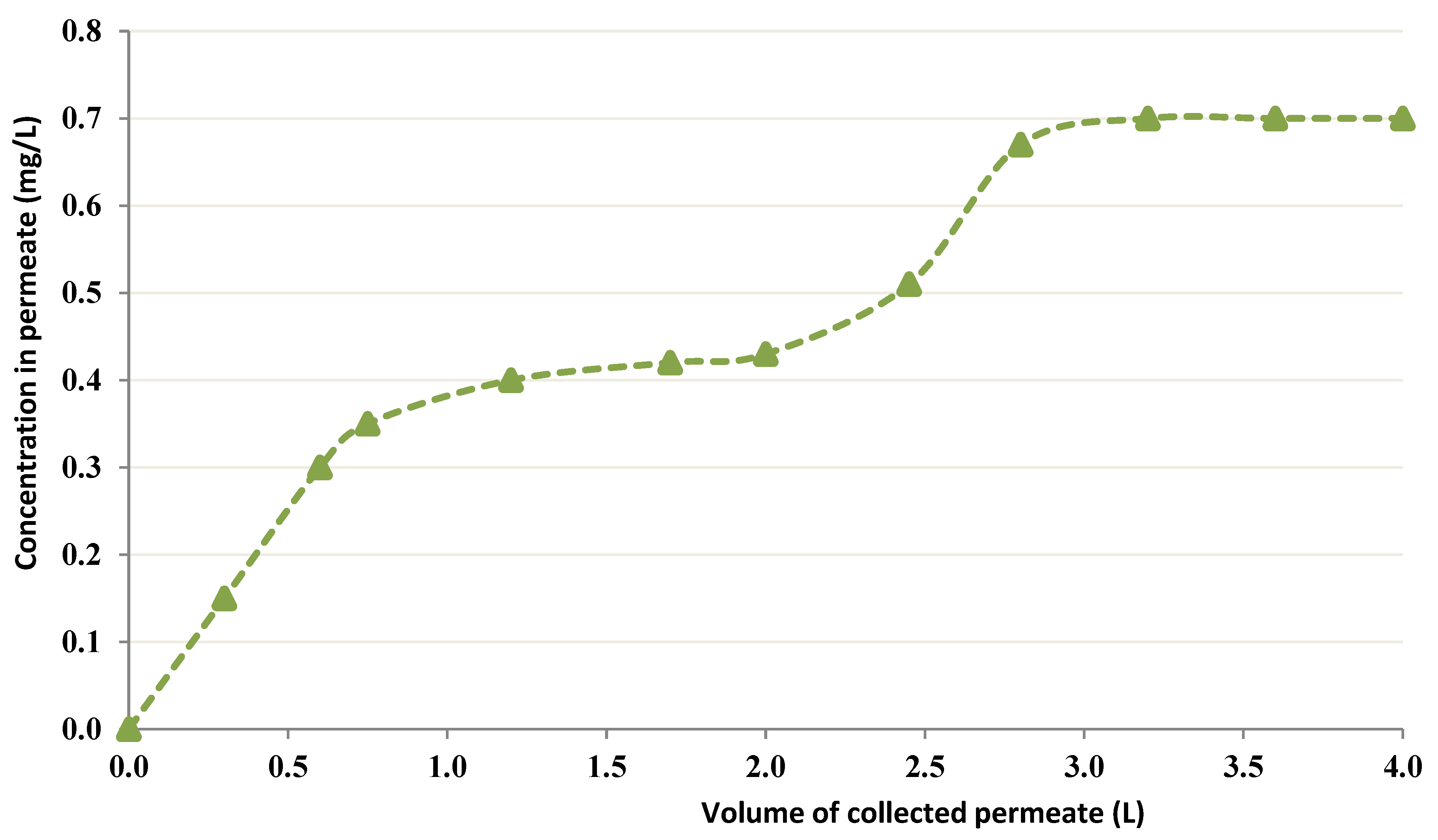
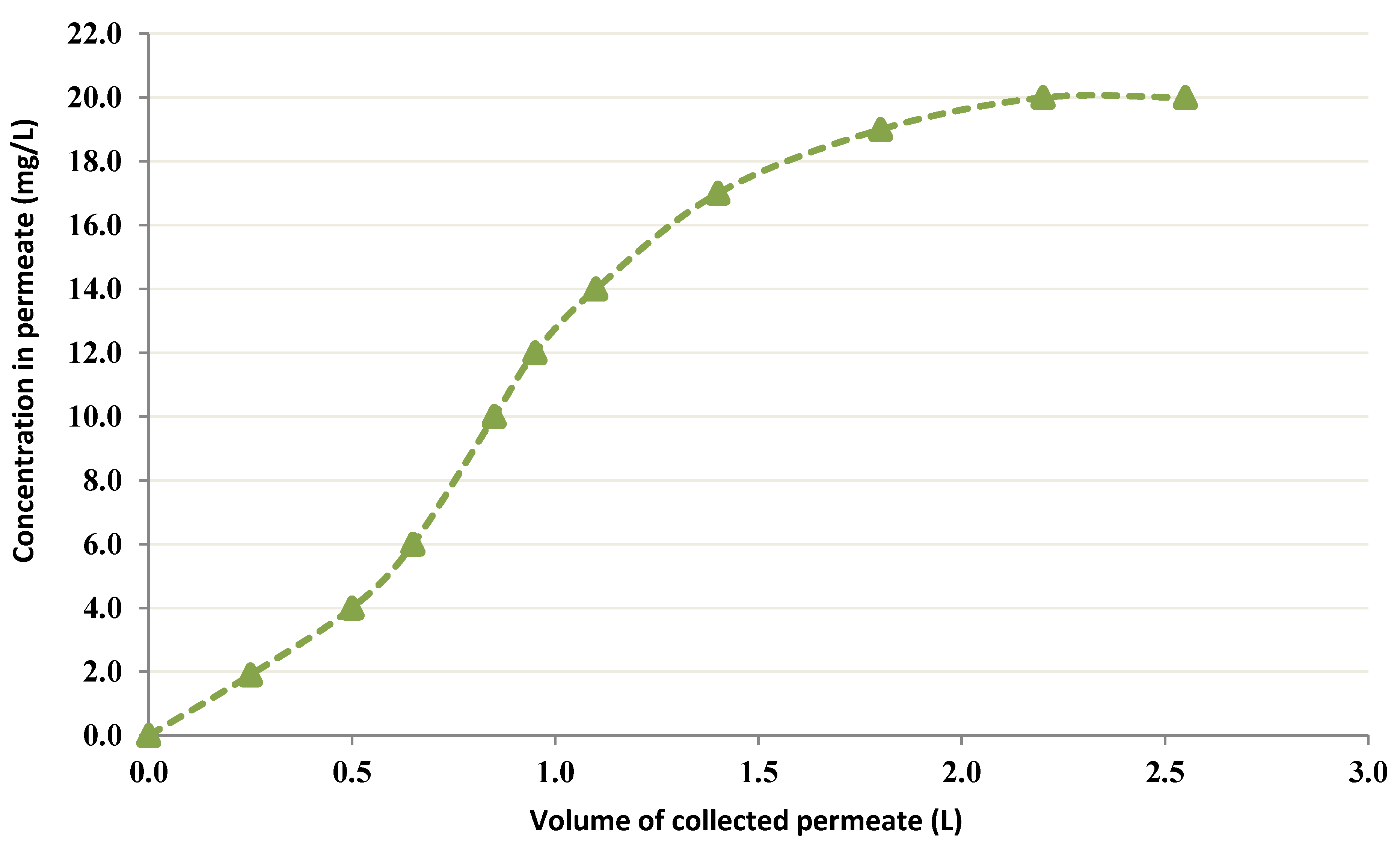
3.2. Dynamic Heavy Metal-Binding Investigations Employing Chitin in CEFP
3.2.1. CEFP with a Feed Cobalt Concentration
3.2.2. CEFP without Chitin
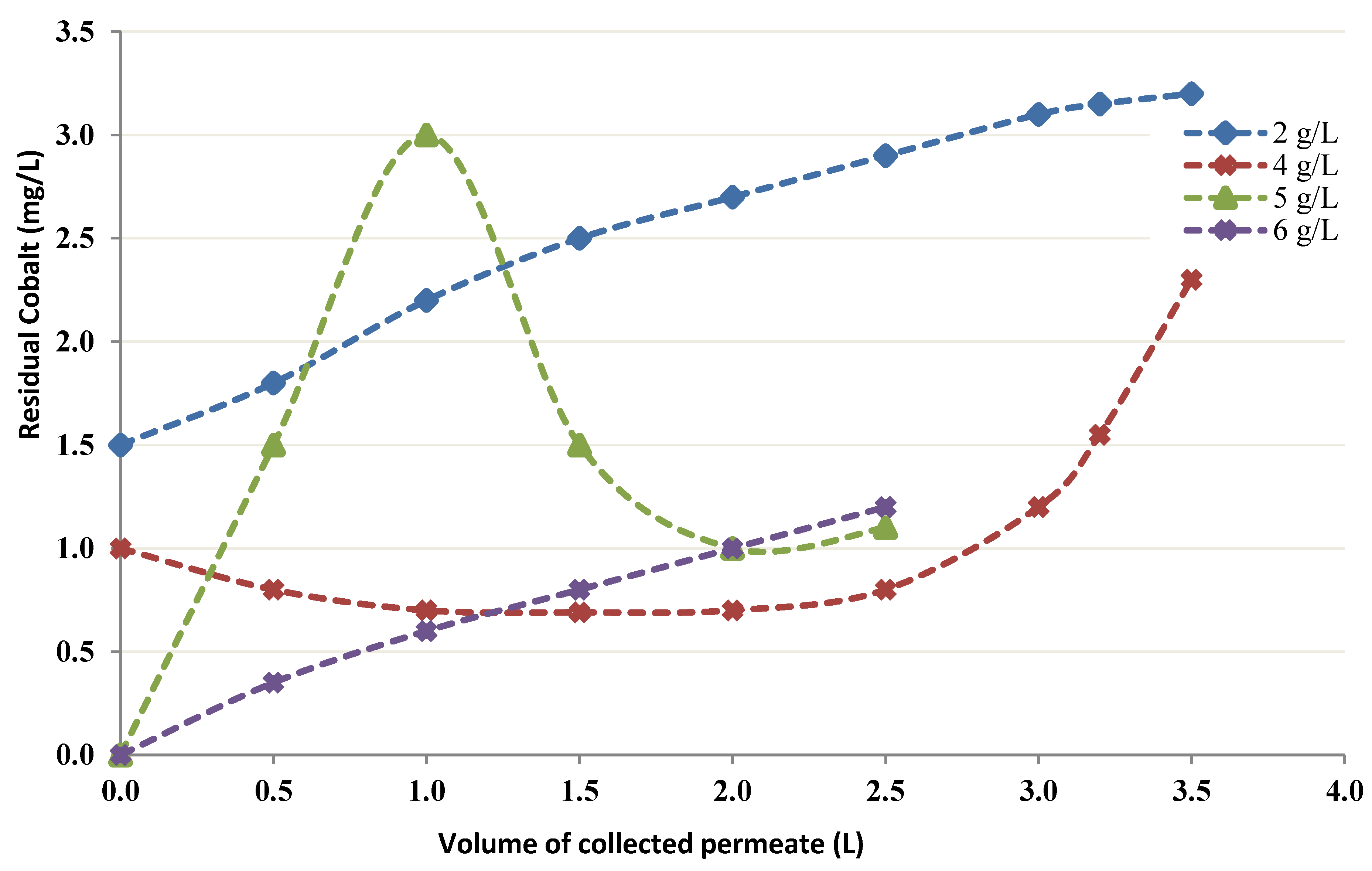
3.2.3. CEFP with Chitin Concentration
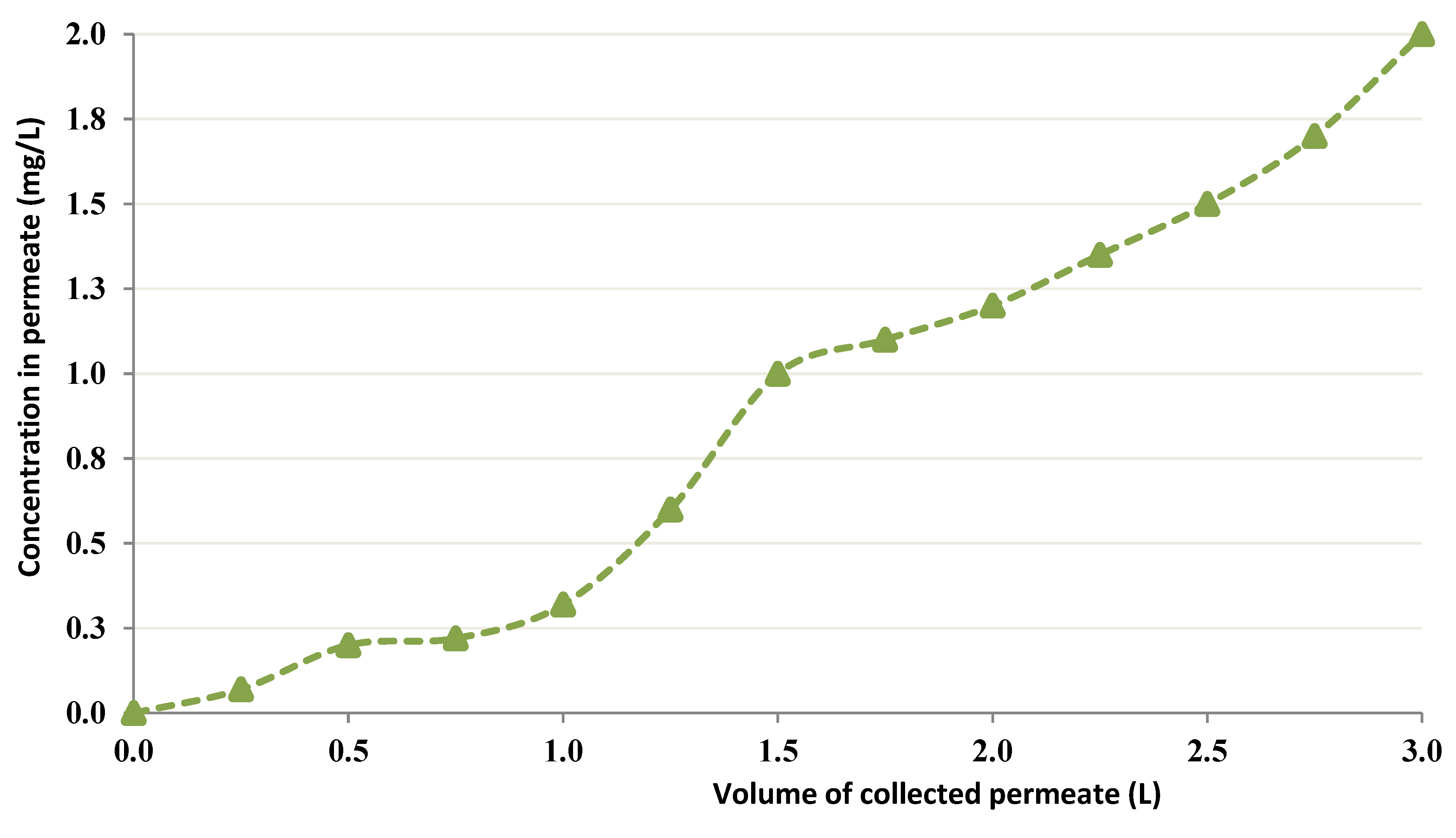
3.2.4. CEFP Employing a 6 g/L Chitin Treating Bigger amount of Co solution
3.2.5. CEFP with 20 g/L Chitin at pH = 2.5
3.2.6. CEFP with Buffer Diafiltration
3.3. Data Modeling of the CEFP
- F: the inlet flow rate (L/h),
- V: the reaction volume (L),
- X: level of the biomass into solution (g/L).
- τ: the residence time in the reactor (h),
- qmax: Polymer’s maximum adsorption capacity (mg/g),
- KS: the dissociation constant (mg/L).
3.4. Rheological Studies of the Chitin-Co Complex
4. Conclusions
- The use of a higher concentration of chitin improved the uptake. It was noted that fewer than 1 mg/L Co into the permeate moved when 6 g/L chitin was employed. Nevertheless, an elevated concentration (20 g/L) conducted a decline in permeate flux. Therefore, it is crucial to select the most favorable level of chitin that does not affect the permeate flux.
- The pH strongly influences the uptake; the optimum uptake amount occurred at pH = 4.
- It seems from the shear thickening behavior that the mutual action of amine groups from multiple chitin molecules is the major cause for the neutralization of lower Co chitin concentrations. This type of behavior is absent at high chitin concentrations.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yildiz, S. Artificial neural network approach for modeling of Ni(II) adsorption from aqueous solution by peanut shell. Ecol. Chem. Eng. S 2018, 25, 581–604. [Google Scholar] [CrossRef]
- Javaid, M.U.; Ali, K.; Qayyum, M.U.; Raza, A.; Gul, M.; Ali, Z. Utilization of fly and coarse ash for removal of heavy metal ions from contaminated water. Acta Sci. Agric. 2021, 5, 18–24. [Google Scholar] [CrossRef]
- Salleh, M.A.M.; Mahmoud, D.K.; Karim, W.A.; Idris, A. Cationic and anionic dye adsorption by agricultural solid wastes: A comprehensive review. Desalination 2011, 280, 1–13. [Google Scholar] [CrossRef]
- Shankar, P.; Gomathi, T.; Vijayalakshmi, K.; Sudha, P.N. Comparative studies on the removal of heavy metals ions onto crosslinked chitosan-g-acrylonitrile copolymer. Int. J. Biol. Macromol. 2014, 67, 180–188. [Google Scholar] [CrossRef]
- Seyed Dorraji, M.S.; Ahadzadeh, I.; Rasoulifard, M.H. Chitosan/polyaniline/MWCNT nanocomposite fibers as an electrode material for electrical double layer capacitors. Int. J. Hydrog. Energy 2014, 39, 9350–9355. [Google Scholar] [CrossRef]
- Bagheri, H.; Roostaie, A.; Baktash, M.Y. A chitosan-polypyrrole magnetic nanocomposite as-sorbent for isolation of naproxen. Anal. Chim. Acta 2014, 816, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Damiri, F.; Andra, S.; Kommineni, N.; Balu, S.K.; Bulusu, R.; Boseila, A.A.; Akamo, D.O.; Ahmad, Z.; Khan, F.S.; Rahman, M.H.; et al. Recent advances in adsorptive nanocomposite membranes for heavy metals ion removal from contaminated water: A comprehensive review. Materials 2022, 15, 5392. [Google Scholar] [CrossRef] [PubMed]
- Othmani, A.; Kadier, A.; Raghuveer, S.; Chinenye, A.I.; Bouzid, M.; Aquatar, M.O.; Khanday, W.A.; Bote, M.E.; Damiri, F.; Gökkuş, Ö.; et al. A comprehensive review on green perspectives of electrocoagulation integrated with advanced processes for effective pollutants removal from water environment. Environ. Res. 2022, 215 Pt 1, 114294. [Google Scholar] [CrossRef]
- Dutta, P.K.; Dutta, J.; Tripathi, V.S. Chitin and chitosan: Chemistry, properties and applications. J. Sci. Ind. Res. 2004, 63, 20–31. [Google Scholar]
- Razieh, S.A.; Mehdi, A.; Rezvan, T.; Meisam, T.M. An efficiency strategy for cobalt recovery from simulated wastewater by biphasic system with polyethylene glycol and ammonium sulfate. Sci. Rep. 2022, 12, 17302. [Google Scholar]
- Guibal, E.; Vincent, T.; Navarro, R. Metal ion biosorption on chitosan for the synthesis of advanced materials. J. Mater. Sci. 2014, 49, 505–518. [Google Scholar] [CrossRef]
- Huang, C.; Chen, S.; Pan, J.R. Optimal condition for modification of chitosan: A biopolymer for coagulation of colloidal particles. Water Res. 2000, 34, 1057–1062. [Google Scholar] [CrossRef]
- Karthik, R.; Meenakshi, S. Removal of Pb(II) and Cd(II) ions from aqueous solution using polyaniline grafted chitosan. Chem. Eng. J. 2015, 263, 168–177. [Google Scholar] [CrossRef]
- Thavamani, S.S.; Rajkumar, R. Removal of Cr(VI), Cu(II), Pb(II) and Ni(II) from Aqueous Solutions by Adsorption on Alumina. Res. J. Chem. Sci. 2013, 3, 44–48. [Google Scholar]
- Zakmout, A.; Said, F.; Velizarov, S.; Crespo, J.G.; Portugal, C.M. Recovery of Cr(III) from tannery effluents by diafiltration using chitosan modified membranes. Water 2021, 13, 2598. [Google Scholar] [CrossRef]
- Rasweefali, M.K.; Sabu, S.; Muhammed Azad, K.S.; Raseel Rahman, M.K.; Sunooj, K.V.; Sasidharan, A.; Anoop, K.K. Influence of deproteinization and demineralization process sequences on the physicochemical and structural characteristics of chitin isolated from Deep-sea mud shrimp (Solenocera hextii). Adv. Biomark. Sci. Technol. 2022, 4, 12–27. [Google Scholar] [CrossRef]
- Zuo, X.J.; Balasubramanian, R. Evaluation of a novel chitosan polymer-based adsorbent for the removal of chromium (III) in aqueous solutions. Carbohydr. Polym. 2013, 92, 2181–2186. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Y.; Hu, X.; Guo, Y. Adsorption of Cr(VI) by modified chitosan from heavy-metal polluted water of Xiangjiang River, China. Trans. Nonferrous Met. Soc. China 2013, 23, 3095–3103. [Google Scholar] [CrossRef]
- Bratskaya, S.; Schwarz, S.; Chervonetsky, D. Comparative study of humic acids flocculation with chitosan hydrochloride and chitosan glutamate. Water Res. 2004, 38, 2955–2961. [Google Scholar] [CrossRef]
- Dantas, T.N.C.; Neto, A.A.D.; Moura, M.C.P.A.; Neto, E.L.B.; Telemaco, E.P. Chromium adsorption by chitosan impregnated with microemulsion. Langmuir 2001, 17, 4256–4260. [Google Scholar] [CrossRef]
- Loulidi, I.; Boukhlifi, F.; Ouchabi, M. Adsorptive removal of chromium (VI) using walnut shell, almond shell, coconut shell and peanut shell. Res. J. Chem. Environ. 2019, 23, 25–32. [Google Scholar]
- Mahmoodi, N.M.; Hayati, B.; Arami, M.; Lan, C. Adsorption of textile dyes on pine cone from colored wastewater: Kinetic, equilibrium and thermodynamic studies. Desalination 2011, 268, 117–125. [Google Scholar] [CrossRef]
- Babazad, Z.; Kaveh, F.; Ebadi, M.; Mehrabian, R.Z.; Juibari, M.H. Efficient removal of lead and arsenic using macromolecule-carbonized rice husks. Heliyon 2021, 7, 120–137. [Google Scholar] [CrossRef]
- Varghese, A.G.; Paul, S.A.; Latha, M.S. Remediation of heavy metals and dyes from wastewater using cellulose-based adsorbents. Environ. Chem. Lett. 2019, 17, 867–877. [Google Scholar] [CrossRef]
- Charazińska, S.; Lochyński, P.; Burszta-Adamiak, E. Removal of heavy metal ions form acidic electrolyte for stainless steel electropolishing via adsorption using Polish peats. J. Water Process Eng. 2021, 42, 613–631. [Google Scholar] [CrossRef]
- Rangel Mendeza, J.R.; Monroy Zeped, R.; Leyva Ramos, E. Chitosan selectivity for removing cadmium (II), copper (II), and lead (II) from aqueous phase: pH and organic matter effect. J. Hazard. Mater. 2009, 162, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Gautam, A.K.; Markandeya, N.; Singh, B.; Shukla, S.P.; Mohan, D. Lead removal efficiency of various natural adsorbents (Moringa oleifera, Prosopis juliflora, peanut shell) from textile wastewater. SN Appl. Sci. 2020, 2, 288. [Google Scholar] [CrossRef]
- Minamisawa, M.; Minamisawa, H.; Yoshida, S.; Takai, N. Adsorption behavior of heavy metals on biomaterials. J. Agric. Food Chem. 2004, 52, 5606–5611. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhu, Y.; Wu, J.; Zheng, J.; Zhao, X.; Lu, B.; Chen, F. Chitosan-coated mesoporous microspheres of calcium silicate hydrate: Environmentally friendly synthesis and application as a highly efficient adsorbent for heavy metal ions. J. Colloid Interface Sci. 2014, 418, 208–215. [Google Scholar] [CrossRef]
- Suganya, S.; Senthil Kumar, P.; Saravanan, A.; Sundar Rajan, P.; Ravikumar, C. Computation of adsorption parameters for the removal of dye from wastewater by microwave assisted sawdust: Theoretical and experimental analysis. Environ. Toxicol. Pharmacol. 2017, 50, 45–57. [Google Scholar]
- Hammari, A.M.; Abubakar, H.; Misau, M.I.; Hamza, U.D. Adsorption equilibrium and kinetic studies of methylene blue dye using groundnut Shell and sorghum husk biosorbent. J. Environ. Bioremediat. Toxicol. 2020, 3, 32–39. [Google Scholar] [CrossRef]

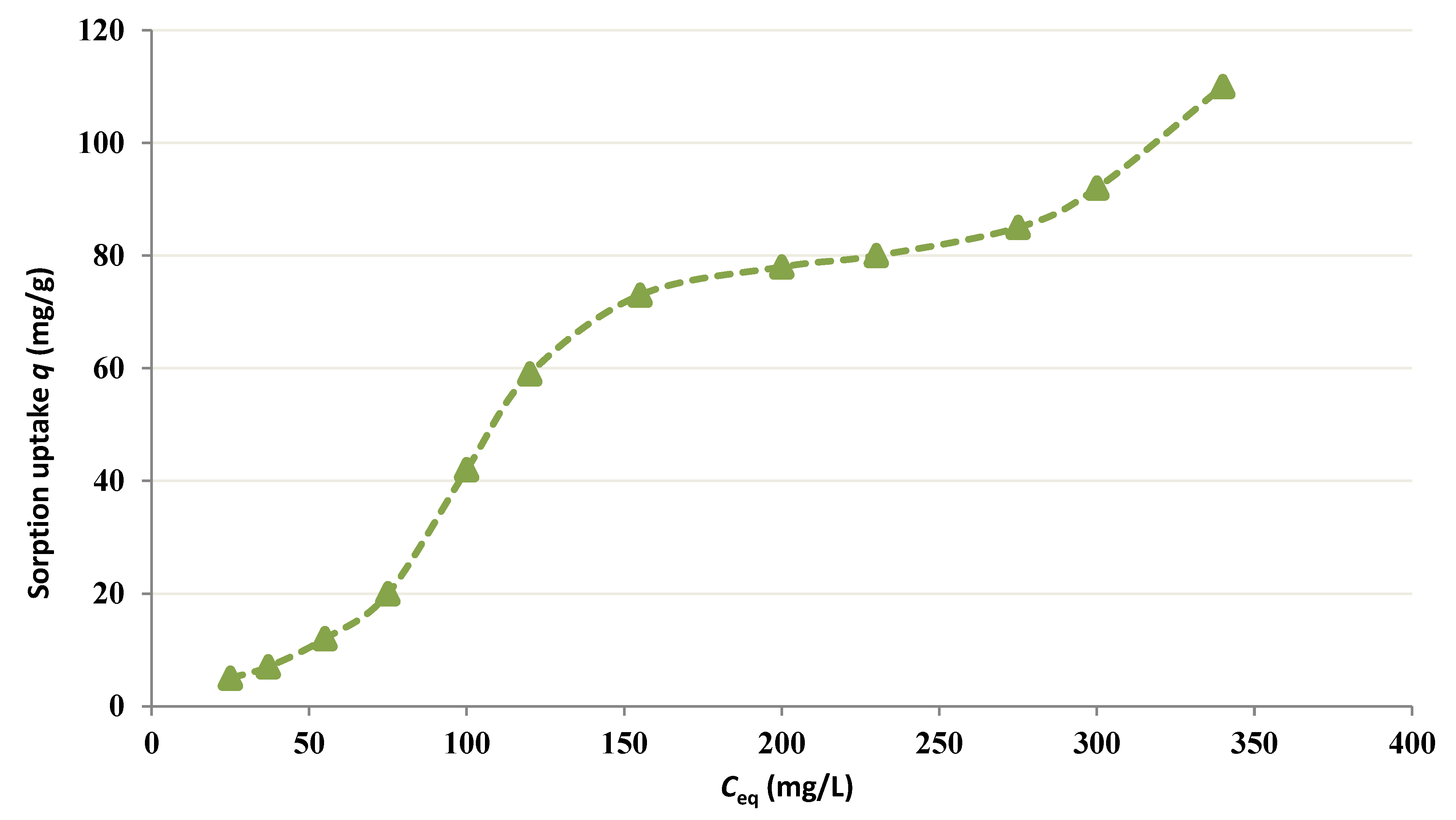



| Initial pH | Uptake (mg/g) | Final pH |
|---|---|---|
| 2 | 29 | 2.0 |
| 3 | 33 | 3.1 |
| 4 | 48 | 4.1 |
| 5 | 5 | 5.2 |
| Concentration of Chitin (g/L) | Volume of Permeate Collected (L) | TMP (psi) | Permeate Flow Rate (mL/min) | pH |
|---|---|---|---|---|
| 2 | 0 | 6.74 | 29 | 4.1 |
| 0.90 | 7.00 | 28 | 4.0 | |
| 1.70 | 7.25 | 28 | 4.1 | |
| 2.55 | 7.25 | 30 | 4.0 | |
| 3.45 | 7.25 | 30 | 4.0 | |
| 4 | 0 | 7.0 | 22 | 4.1 |
| 0.70 | 7.0 | 22 | 4.1 | |
| 1.40 | 7.25 | 22 | 4.1 | |
| 2.10 | 7.25 | 22 | 4.1 | |
| 2.75 | 7.25 | 22 | 4.0 | |
| 3.35 | 7.25 | 22 | 4.0 | |
| 5 | 0 | 6.75 | 20 | 4.0 |
| 0.60 | 6.75 | 20 | 4.1 | |
| 1.10 | 7.00 | 18 | 4.0 | |
| 1.50 | 7.00 | 15 | 4.0 | |
| 1.90 | 7.00 | 15 | 4.0 | |
| 2.25 | 7.00 | 15 | 4.0 | |
| 6 | 0 | 7.5 | 13 | 4.0 |
| 0.40 | 7.75 | 13 | 4.0 | |
| 0.75 | 7.75 | 13 | 4.0 | |
| 1.05 | 7.75 | 13 | 4.1 | |
| 1.40 | 7.5 | 13 | 4.1 | |
| 1.70 | 7.5 | 13 | 4.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elboughdiri, N.; Ghernaout, D.; Gasmi, A.; Khan, M.I.; Ghernaout, B. Applying Chitin Enhanced Diafiltration Process (CEFP) in Removing Cobalt from Synthetic Wastewater. Membranes 2022, 12, 1194. https://doi.org/10.3390/membranes12121194
Elboughdiri N, Ghernaout D, Gasmi A, Khan MI, Ghernaout B. Applying Chitin Enhanced Diafiltration Process (CEFP) in Removing Cobalt from Synthetic Wastewater. Membranes. 2022; 12(12):1194. https://doi.org/10.3390/membranes12121194
Chicago/Turabian StyleElboughdiri, Noureddine, Djamel Ghernaout, Aicha Gasmi, Muhammad Imran Khan, and Badia Ghernaout. 2022. "Applying Chitin Enhanced Diafiltration Process (CEFP) in Removing Cobalt from Synthetic Wastewater" Membranes 12, no. 12: 1194. https://doi.org/10.3390/membranes12121194
APA StyleElboughdiri, N., Ghernaout, D., Gasmi, A., Khan, M. I., & Ghernaout, B. (2022). Applying Chitin Enhanced Diafiltration Process (CEFP) in Removing Cobalt from Synthetic Wastewater. Membranes, 12(12), 1194. https://doi.org/10.3390/membranes12121194










