Smart Superhydrophobic Filter Paper for Water/Oil Separation and Unidirectional Transportation of Liquid Droplet
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Modification and Characterization of the Filter Paper
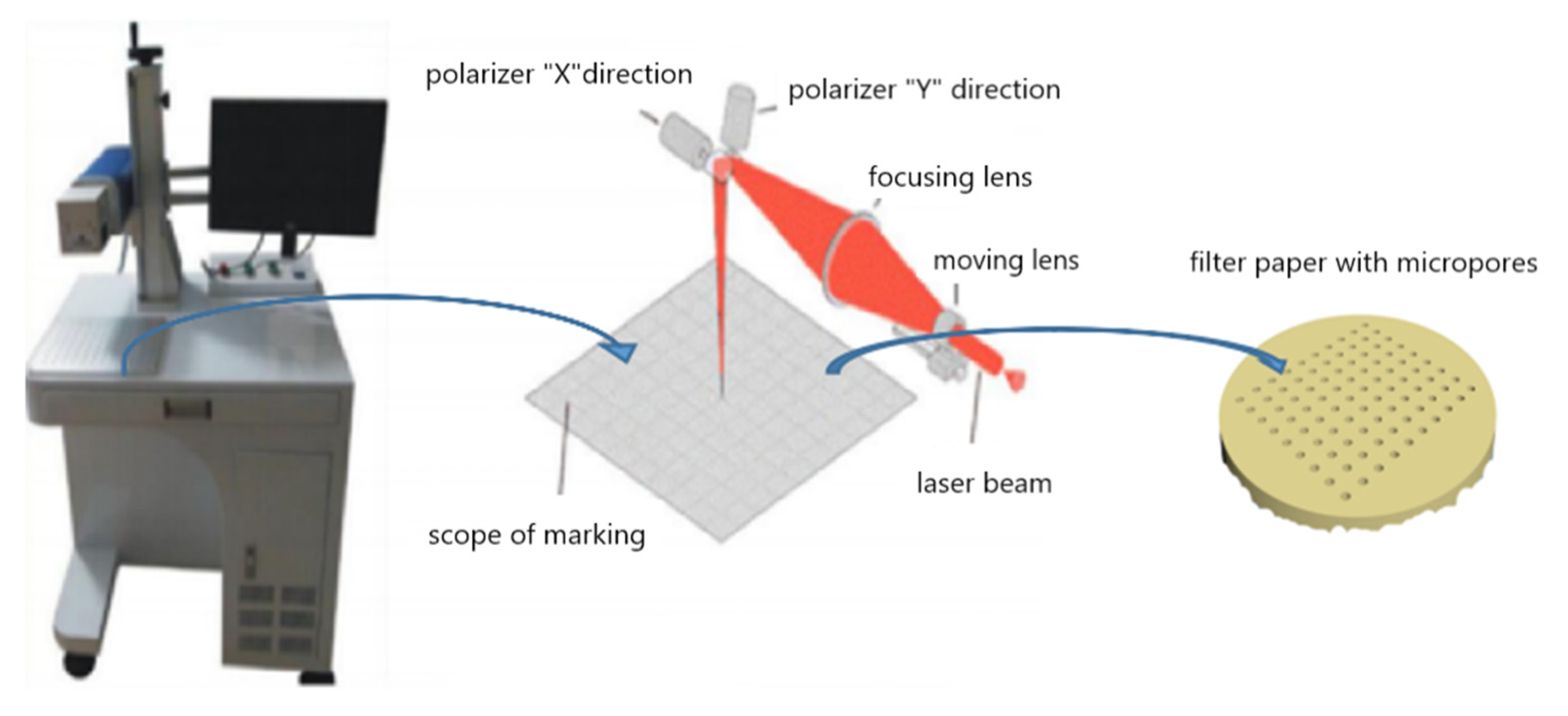
2.3. Boring the Filter Paper by Nano Second Laser
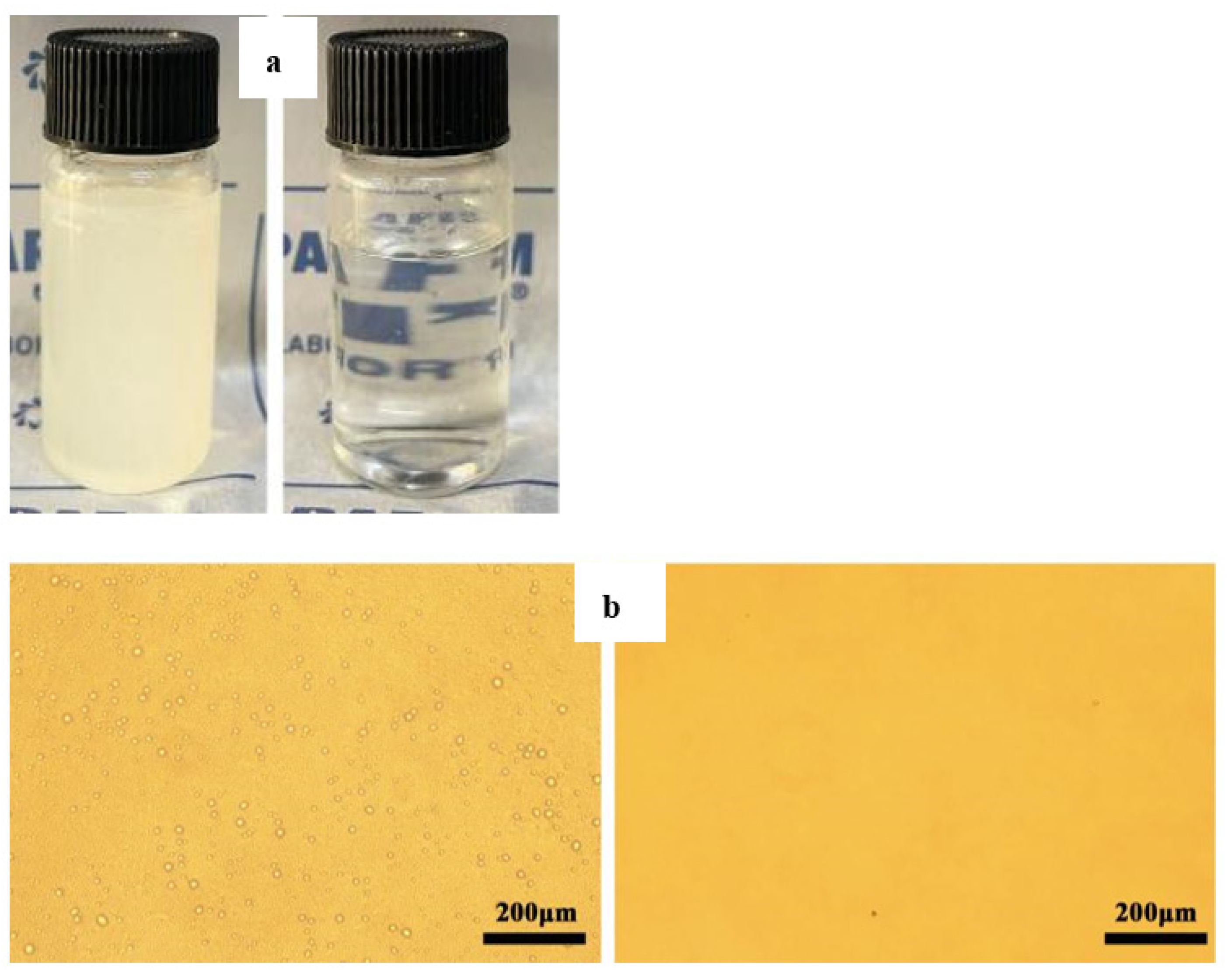
2.4. Preparation of Oil/Water Separation and Surfactant-Stabilized Emulsion Separation
3. Results
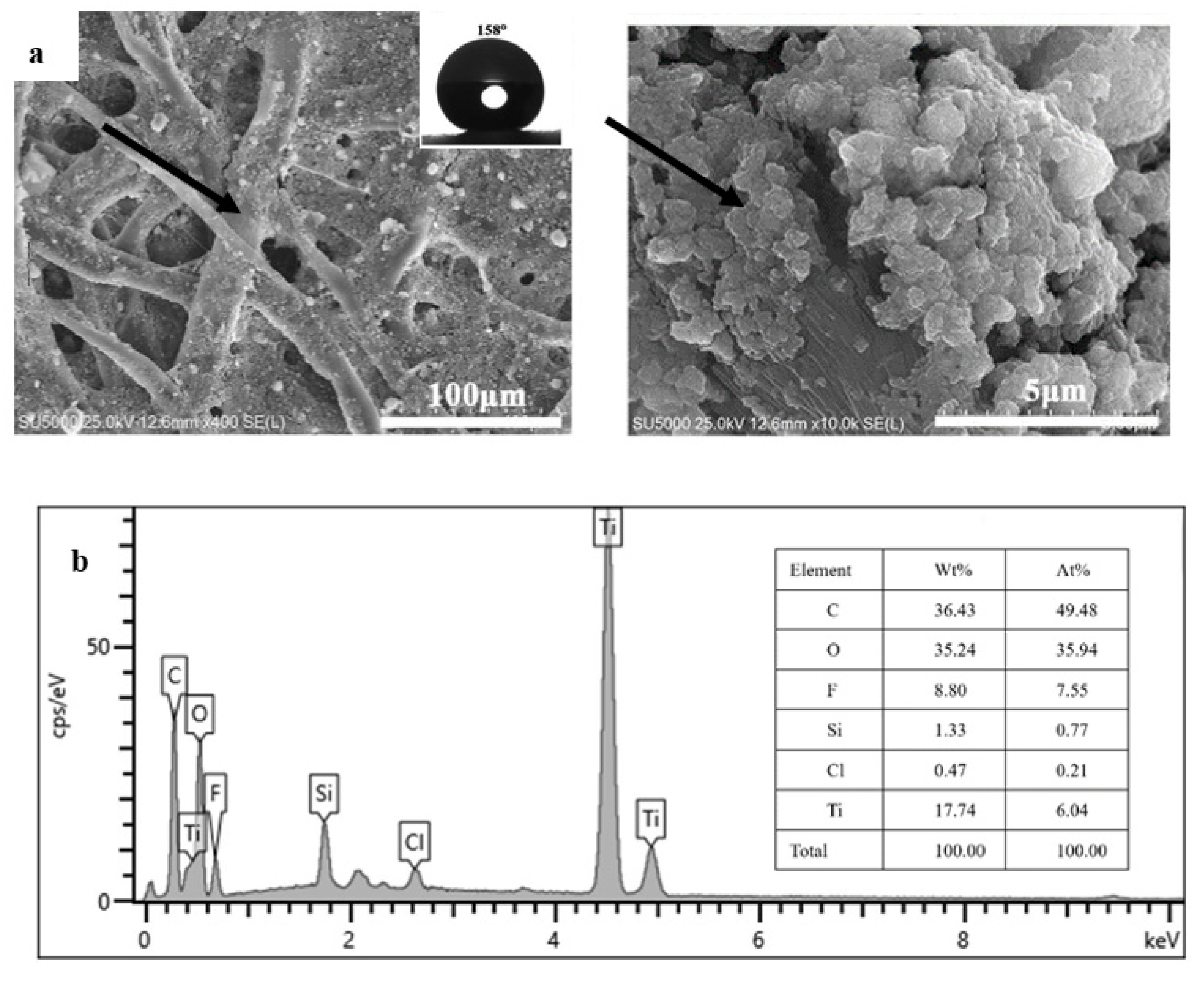
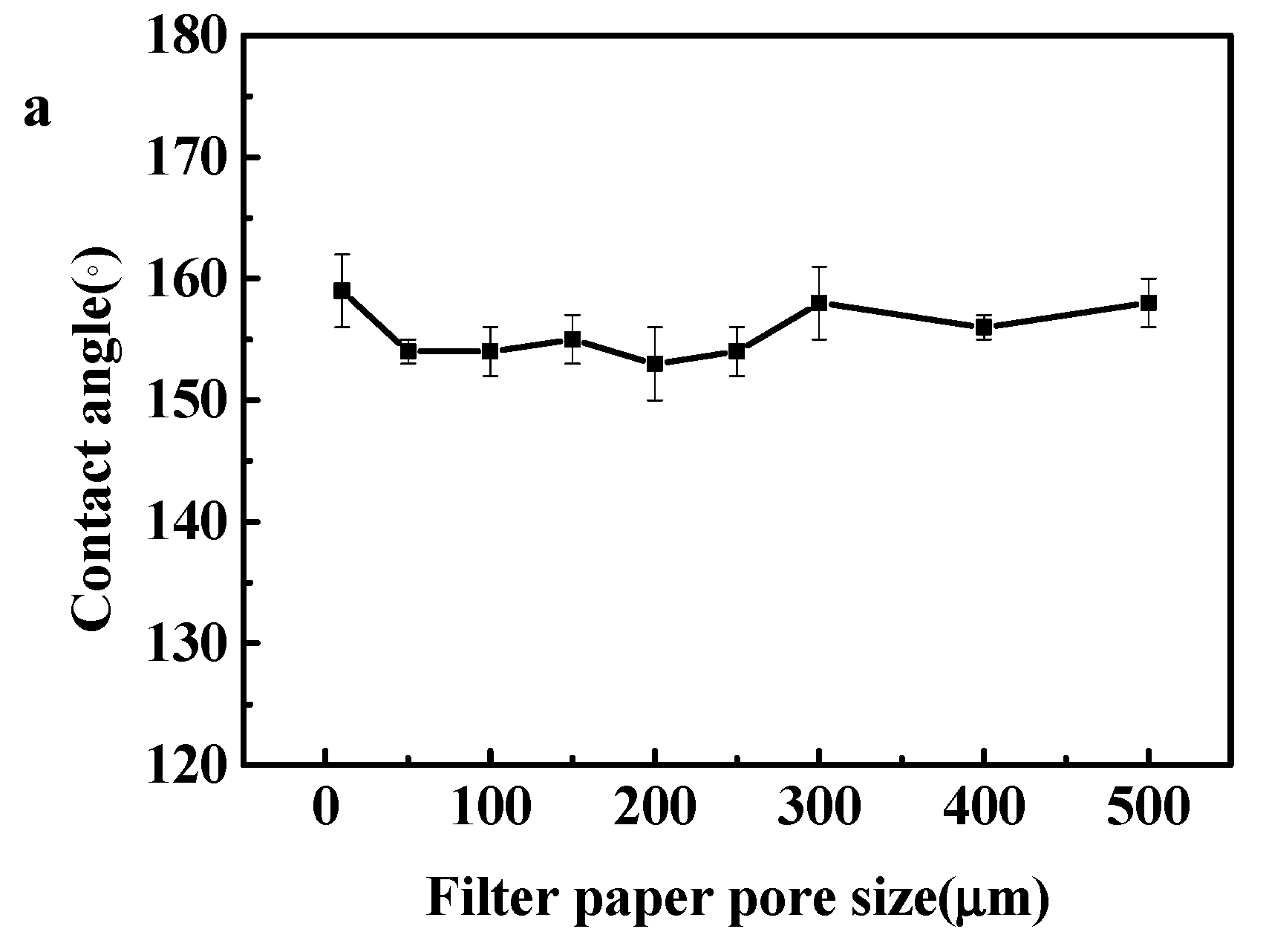
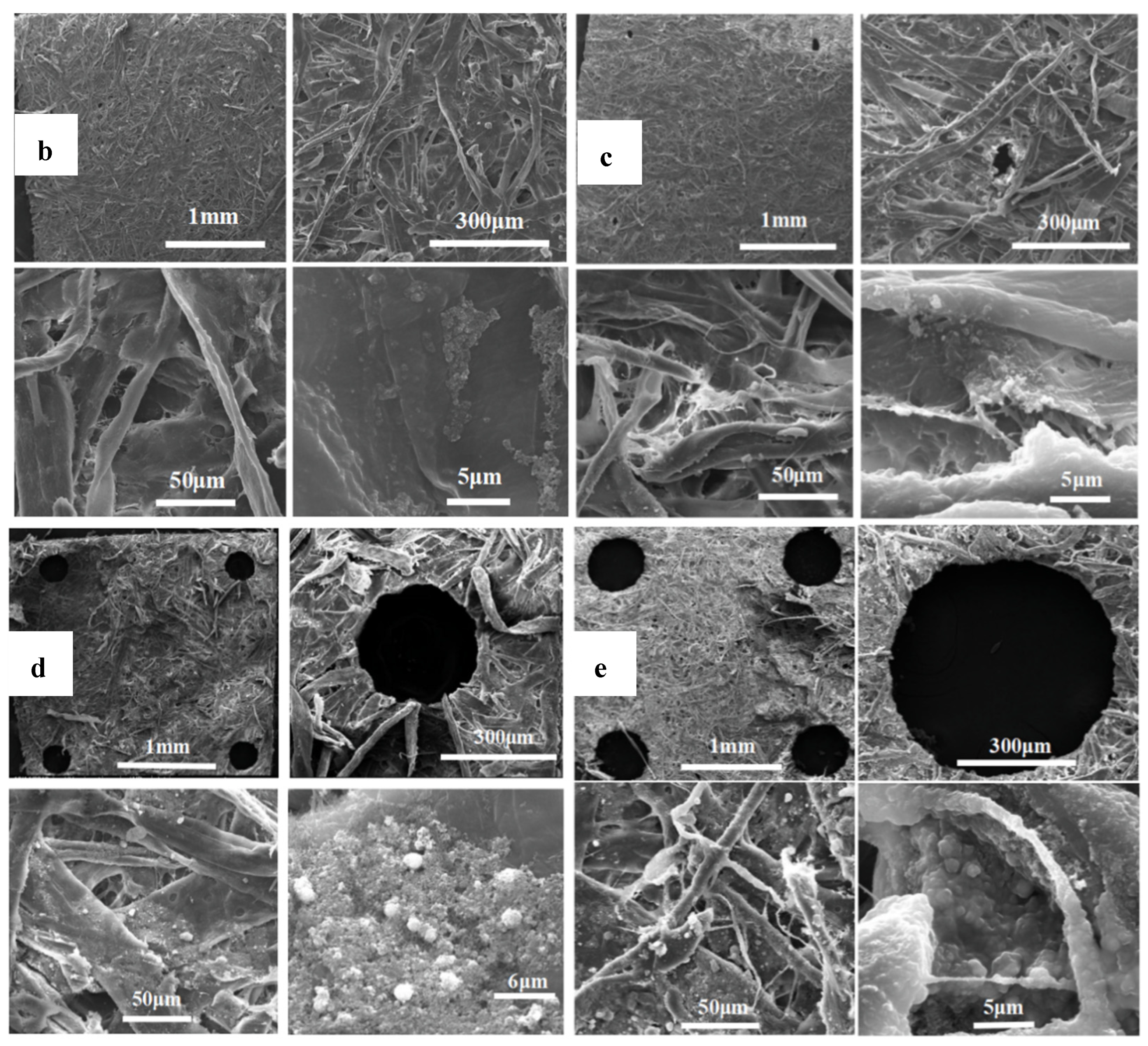
3.1. Fabrication of the Modified Filter Paper
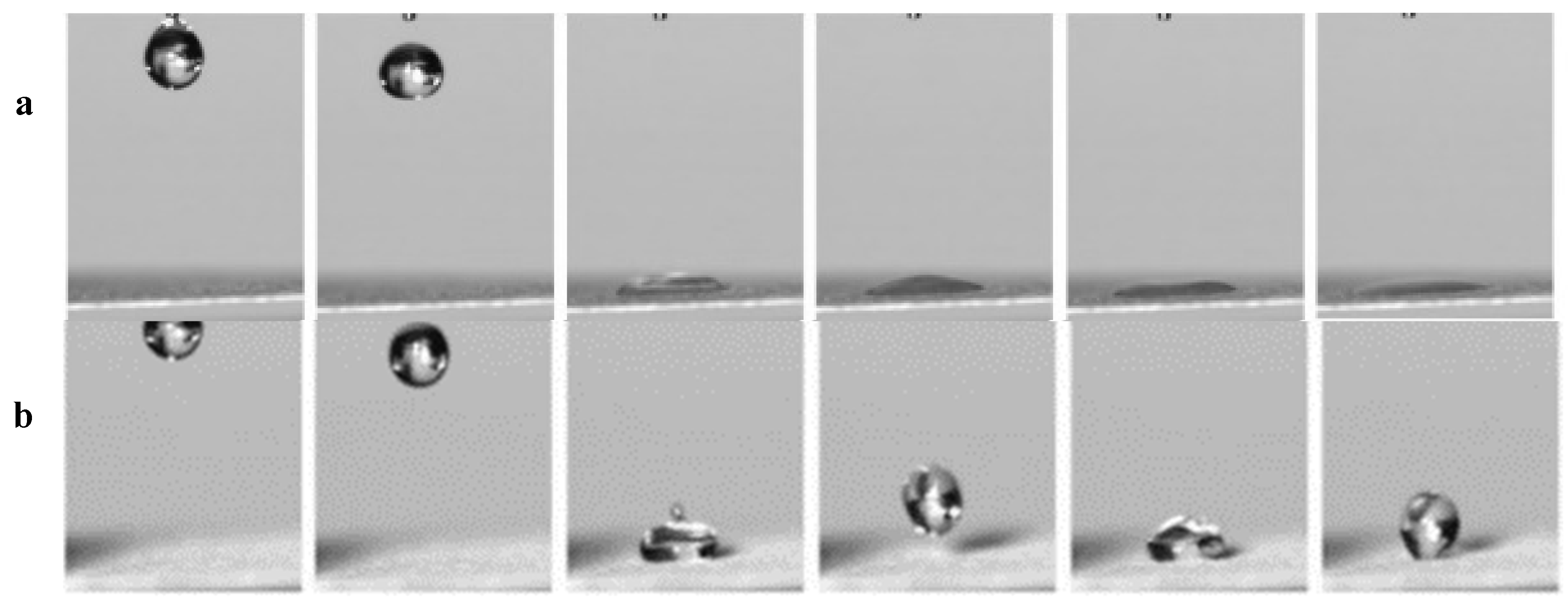
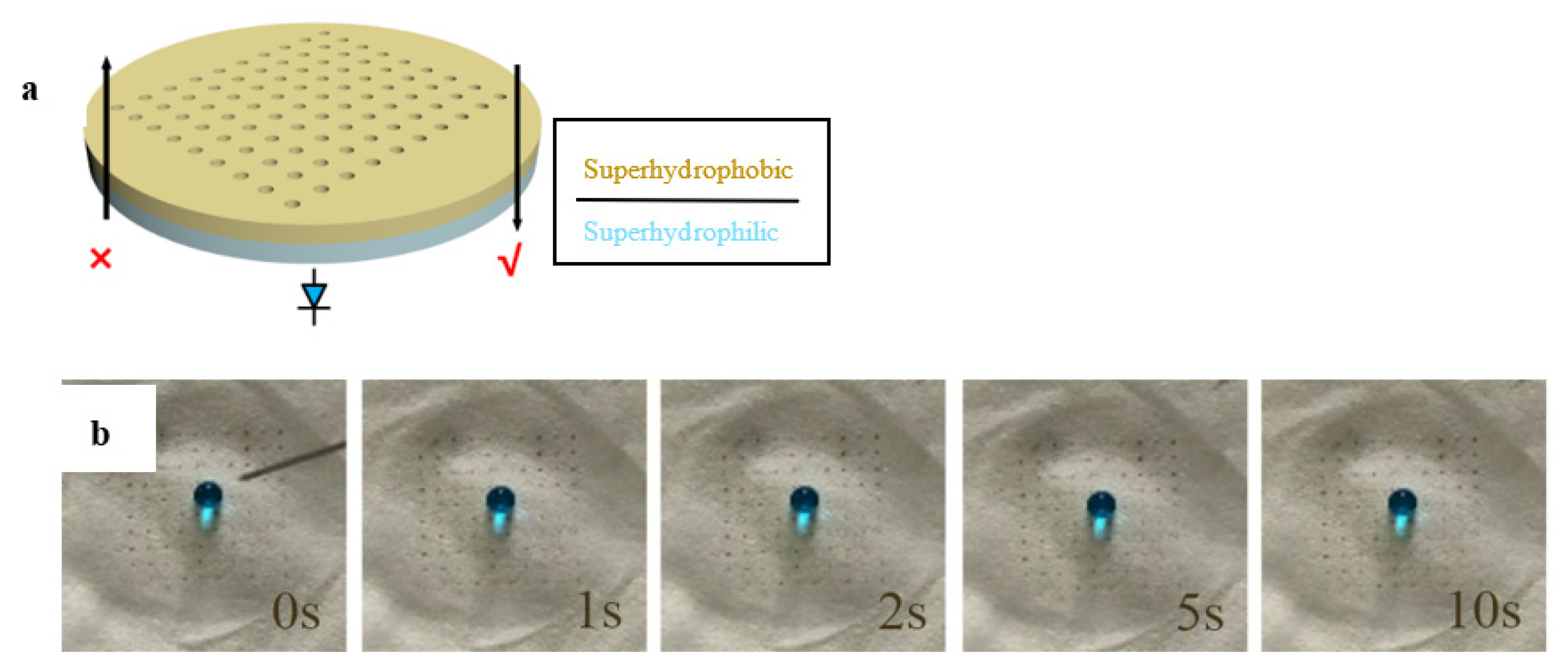
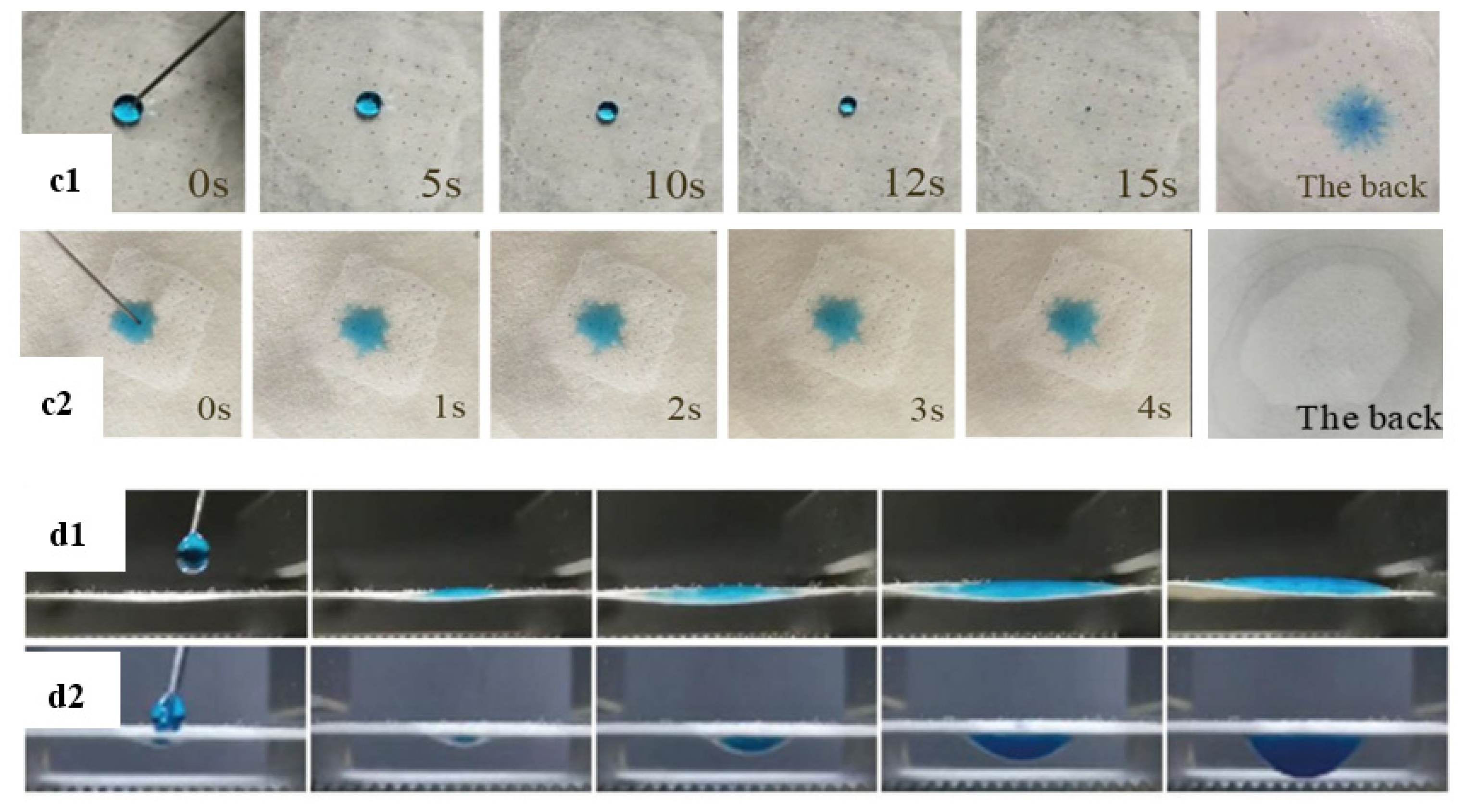
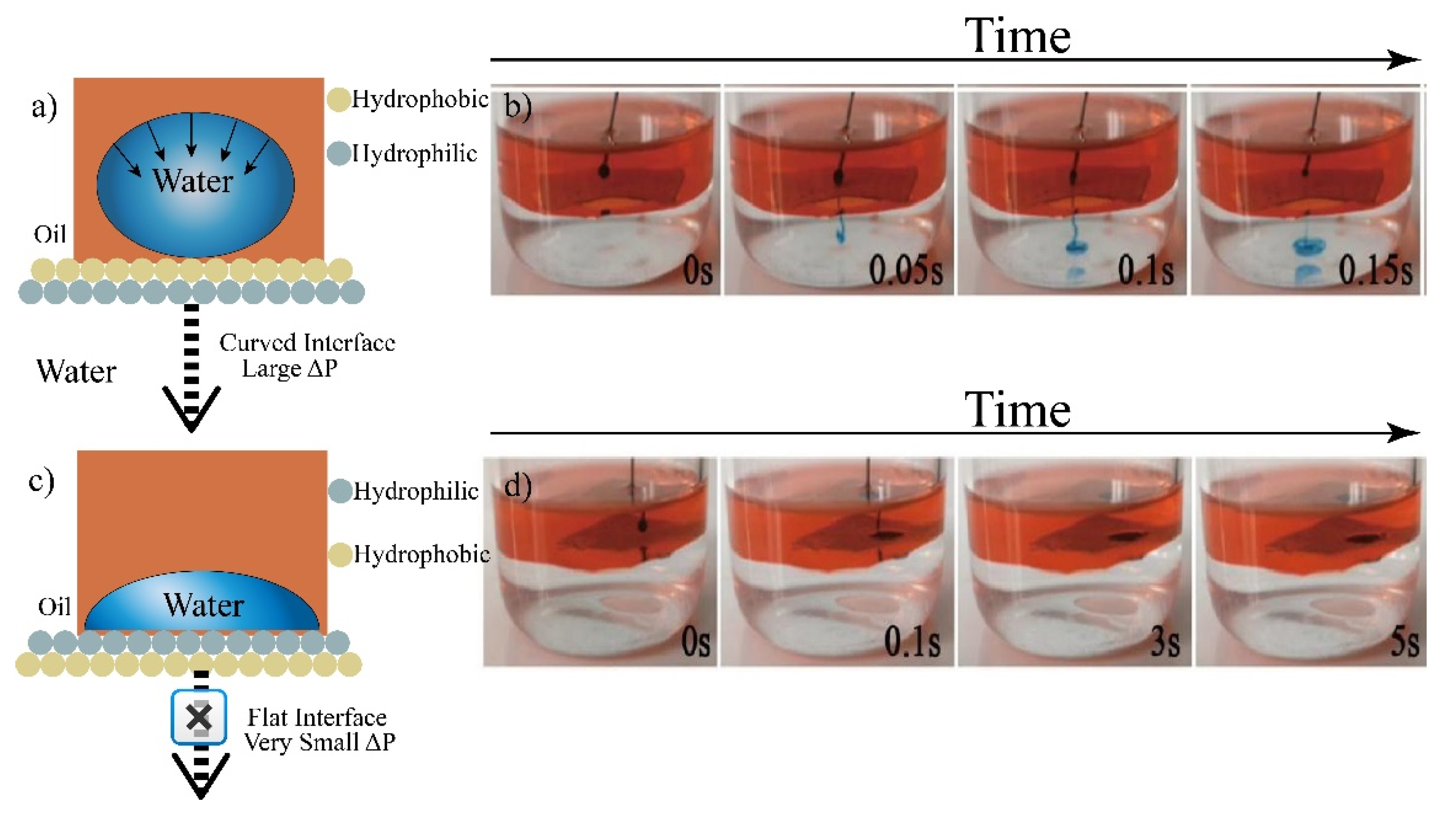
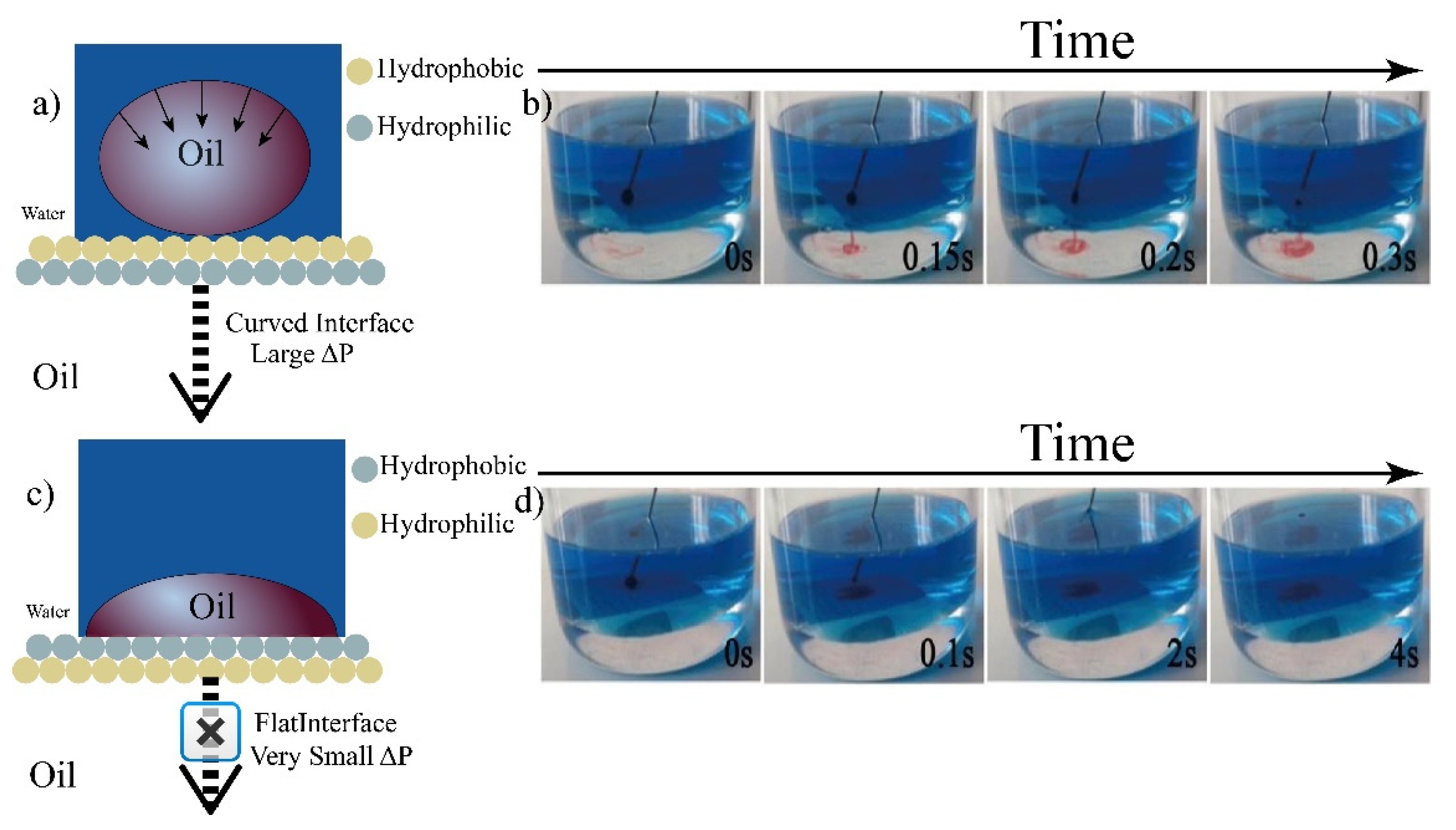
3.2. Constructing Water-Unidirectional Janus Membrane
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, H.; Gao, S.W.; Cai, J.S.; He, C.L.; Mao, J.J.; Zhu, T.X.; Chen, Z.; Huang, J.Y.; Meng, K.; Zhang, K.Q.; et al. Recent progress in fabrication and applications of superhydrophobic coating on cellulose-based substrates. Materials 2016, 9, 124. [Google Scholar] [CrossRef] [Green Version]
- Ullah, A.; Tanudjaja, H.; Ouda, M.; Hasan, S.; Chew, J. Membrane fouling mitigation techniques for oily wastewater: A short review. J. Water Process Eng. 2021, 43, 102293. [Google Scholar] [CrossRef]
- Gomaa, H.; Rao, S. Analysis of flux enhancement at oscillating flat surface membranes. J. Membr. Sci. 2011, 374, 55–66. [Google Scholar] [CrossRef]
- Ullah, A. The influence of interfacial tension on rejection and permeation of the oil droplets through a slit pore membrane. Sep. Purif. Technol. 2021, 266, 118581. [Google Scholar] [CrossRef]
- Hussain, S.; Wan, X.; Fang, Z.; Peng, X. Superhydrophilic and photothermal Fe-TCPP nanofibrous membrane for efficient oil-in-water nanoemulsion separation. Langmuir 2021, 37, 12981–12989. [Google Scholar] [CrossRef] [PubMed]
- Ullah, A.; Ahmad, J.; Khan, H.; Wali Khan, S.; Zamani, F.; Hasan, S.; Starov, V.; Chew, J. Membrane oscillation and slot (pore) blocking in oil-water separation. Chem. Eng. Res. Des. 2019, 142, 111–120. [Google Scholar] [CrossRef]
- Sanjay, R.; Nagarajan, P.; Rabiya, R.; Sabyasachi, G.; Tushar, K.; Suryasarathi, B.; Ramkrishna, S.; Narayan, C. Converting Polymer Trash into Treasure: An approach to prepare MoS2 nanosheets decorated PVDF sponge for oil/water separation and antibacterial applications. Ind. Eng. Chem. Res. 2021, 59, 20141–20154. [Google Scholar] [CrossRef]
- Jiang, Q.; Wang, Y.; Xie, Y.; Zhou, M.; Gu, Q.; Zhong, Z.; Xing, W. Silicon carbide microfiltration membranes for oil-water separation: Pore structure-dependent wettability matters. Water Res. 2022, 216, 118270. [Google Scholar] [CrossRef] [PubMed]
- Ullah, A.; Shahzada, K.; Khan, S.; Starov, V. Purification of produced water using oscillatory membrane filtration. Desalination 2020, 491, 114428. [Google Scholar] [CrossRef]
- Kosak Soz, C.; Trosien, S.; Biesalski, M. Superhydrophobic hybrid paper sheets with Janus-type wettability. ACS Appl. Mater. Interfaces 2018, 10, 37478–37488. [Google Scholar] [CrossRef] [PubMed]
- Song, B.; Xu, Q. Simple yet powerful nanofilters with tunable pore sizes and superhydrophilicity-underwater superoleophobicity for oil spill treatment. Colloids Surf. A Physicochem. Eng. Asp. 2018, 541, 26–35. [Google Scholar] [CrossRef]
- Zhang, S.-M.; Zhang, Y.-P.; Liu, M.-L.; Wang, B.-X.; Liu, P.-F.; Bai, X.; Cui, C.-X.; Qu, L.-B. Smart Janus titanium mesh used as a diode for both liquid droplet and air bubble transport. New J. Chem. 2021, 45, 17862–17870. [Google Scholar] [CrossRef]
- Zhang, Y.P.; Yang, J.H.; Li, L.L.; Cui, C.X.; Li, Y.; Liu, S.Q.; Zhou, X.M.; Qu, L.B. Facile fabrication of superhydrophobic copper- foam and electrospinning polystyrene fiber for combinational oil/water separation. Polymers 2019, 11, 97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Y.; Xie, W.; Zhang, F.; Xing, T.; Jin, J. Superhydrophilic in-situ-cross-linked zwitterionic polyelectrolyte/PVDF-blend membrane for highly efficient oil/water emulsion separation. ACS Appl. Mater. Interfaces 2017, 9, 9603–9613. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Hou, L.A.; Yan, K.; Zhang, L.; Yu, Q.J. Polydopamine nanocluster decorated electrospun nanofibrous membrane for separation of oil/water emulsions. J. Membr. Sci. 2018, 547, 156–162. [Google Scholar] [CrossRef]
- Ge, D.; Yang, L.; Wang, C.; Lee, E.; Zhang, Y.; Yang, S. A multi-functional oil-water separator from a selectively pre-wetted superamphiphobic paper. Chem. Commun. 2015, 51, 6149–6152. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.-B.; Song, Y.; Wang, S.; Meng, J.; Yang, G.; Guo, X.; Feng, L.; Jiang, L. Directly coating hydrogel on filter paper for effective oil-water separation in highly acidic, alkaline, and salty environment. Adv. Funct. Mater. 2015, 25, 5368–5375. [Google Scholar] [CrossRef]
- Cao, C.; Cheng, J. A novel Cu(OH)2 coated filter paper with superhydrophobicity for the efficient separation of water-in-oil emulsions. Mater. Lett. 2018, 217, 5–8. [Google Scholar] [CrossRef]
- Lu, Y.; Sathasivam, S.; Song, J.; Crick, C.R.; Carmalt, C.J.; Parkin, I.P. Robust self-cleaning surfaces that function when exposed to either air or oil. Science 2015, 347, 1132–1135. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wang, Z.; Shao, L. Construction of oil-unidirectional membrane for integrated oil collection with lossless transportation and oil-in-water emulsion purification. J. Membr. Sci. 2018, 549, 67–74. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, X.; Tian, Y. Insights into the wettability transition of nanosecond laser ablated surface under ambient air exposure. J. Colloid Interface Sci. 2019, 533, 268–277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maurer, J.A.; Miller, M.J.; Bartolucci, S.F. Self-cleaning superhydrophobic nanocomposite surfaces generated by laser pulse heating. J. Colloid Interface Sci. 2018, 524, 204–208. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.-F.; Li, P.-P.; Zhang, Y.-P.; Liu, P.-F.; Cui, C.-X.; Wang, J.-C.; Li, X.-J.; Qu, L.-B. Laboratory filter paper from superhydrophobic to quasi-superamphiphobicity: Facile fabrication, simplified patterning and smart application. Cellulose 2019, 26, 3859–3872. [Google Scholar] [CrossRef]
- Wang, B.; Liang, W.; Guo, Z.; Liu, W. Biomimetic super-lyophobic and super-lyophilic materials applied for oil/water separation: A new strategy beyond nature. Chem. Soc. Rev. 2015, 44, 336–361. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.-L.; Zhang, Y.-P.; Cui, C.-X.; Liu, P.-F.; Li, R.-L.; Qu, L.-B. Integrated Janus membrane for smart “dual fluid diode’’ with multifunctional applications. J. Mater. Res. 2021, 36, 1948–1959. [Google Scholar] [CrossRef]
- Tian, X.; Li, J.; Wang, X. Anisotropic liquid penetration arising from a cross-sectional wettability gradient. Soft Matter 2012, 8, 2633–2637. [Google Scholar] [CrossRef]
- Hou, X.; Hu, Y.; Grinthal, A.; Khan, M.; Aizenberg, J. Liquid-based gating mechanism with tunable multiphase selectivity and antifouling behaviour. Nature 2015, 519, 70–73. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.-P.; Wang, N.; Chen, D.-L.; Chen, Y.; Chen, M.-J.; Chen, X.-X. Smart Superhydrophobic Filter Paper for Water/Oil Separation and Unidirectional Transportation of Liquid Droplet. Membranes 2022, 12, 1188. https://doi.org/10.3390/membranes12121188
Zhang Y-P, Wang N, Chen D-L, Chen Y, Chen M-J, Chen X-X. Smart Superhydrophobic Filter Paper for Water/Oil Separation and Unidirectional Transportation of Liquid Droplet. Membranes. 2022; 12(12):1188. https://doi.org/10.3390/membranes12121188
Chicago/Turabian StyleZhang, Yu-Ping, Ning Wang, De-Liang Chen, Yuan Chen, Meng-Jun Chen, and Xin-Xin Chen. 2022. "Smart Superhydrophobic Filter Paper for Water/Oil Separation and Unidirectional Transportation of Liquid Droplet" Membranes 12, no. 12: 1188. https://doi.org/10.3390/membranes12121188
APA StyleZhang, Y.-P., Wang, N., Chen, D.-L., Chen, Y., Chen, M.-J., & Chen, X.-X. (2022). Smart Superhydrophobic Filter Paper for Water/Oil Separation and Unidirectional Transportation of Liquid Droplet. Membranes, 12(12), 1188. https://doi.org/10.3390/membranes12121188




