Electrodialysis Tartrate Stabilization of Wine Materials: Fouling and a New Approach to the Cleaning of Aliphatic Anion-Exchange Membranes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Membranes
2.2. Solutions
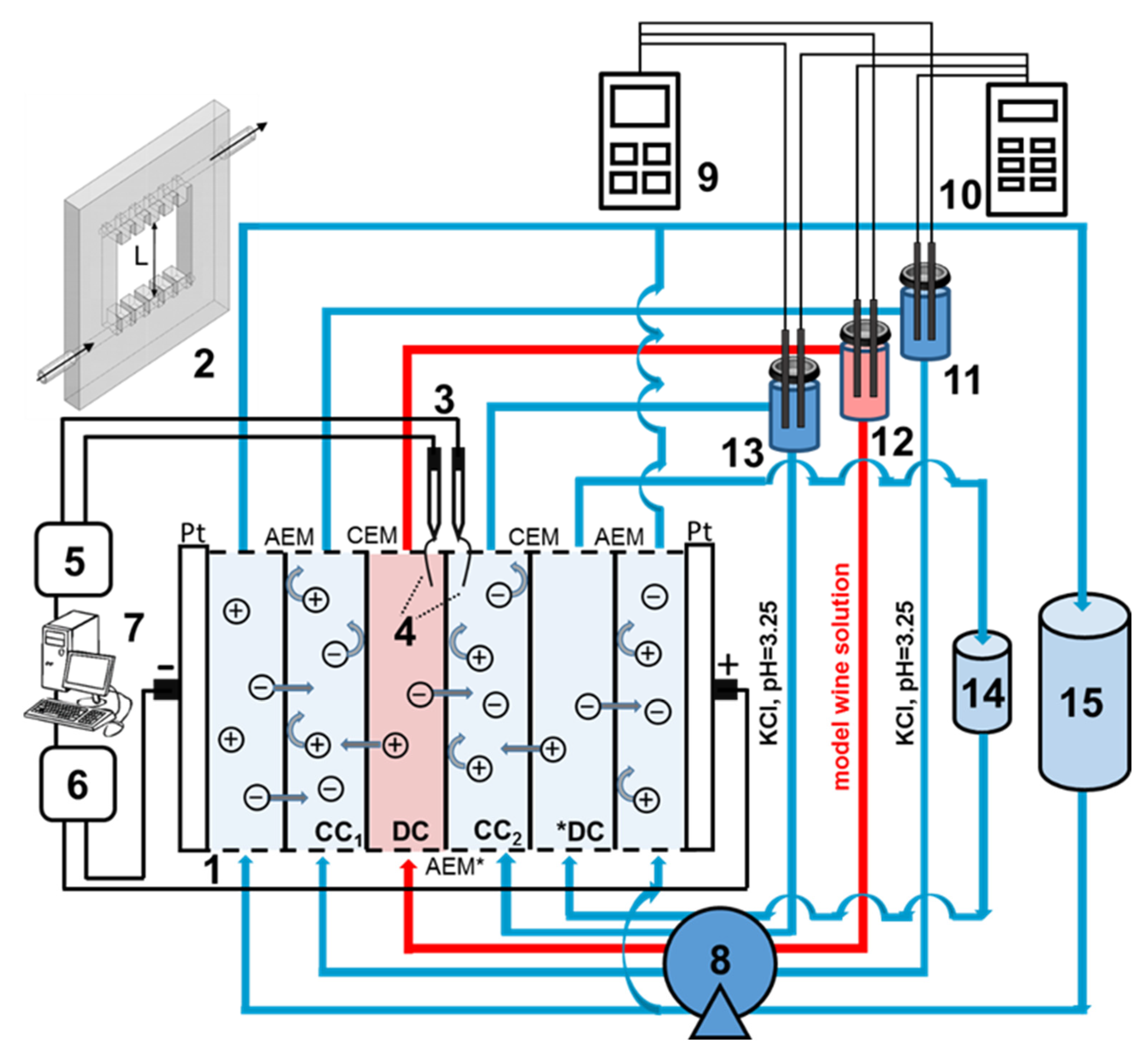
2.3. Electrodialysis Unit and Experimental Technique
2.4. Processing of ED Experimental Data
2.5. Estimation of Biofouling and Antioxidant Activity of Polyphenols
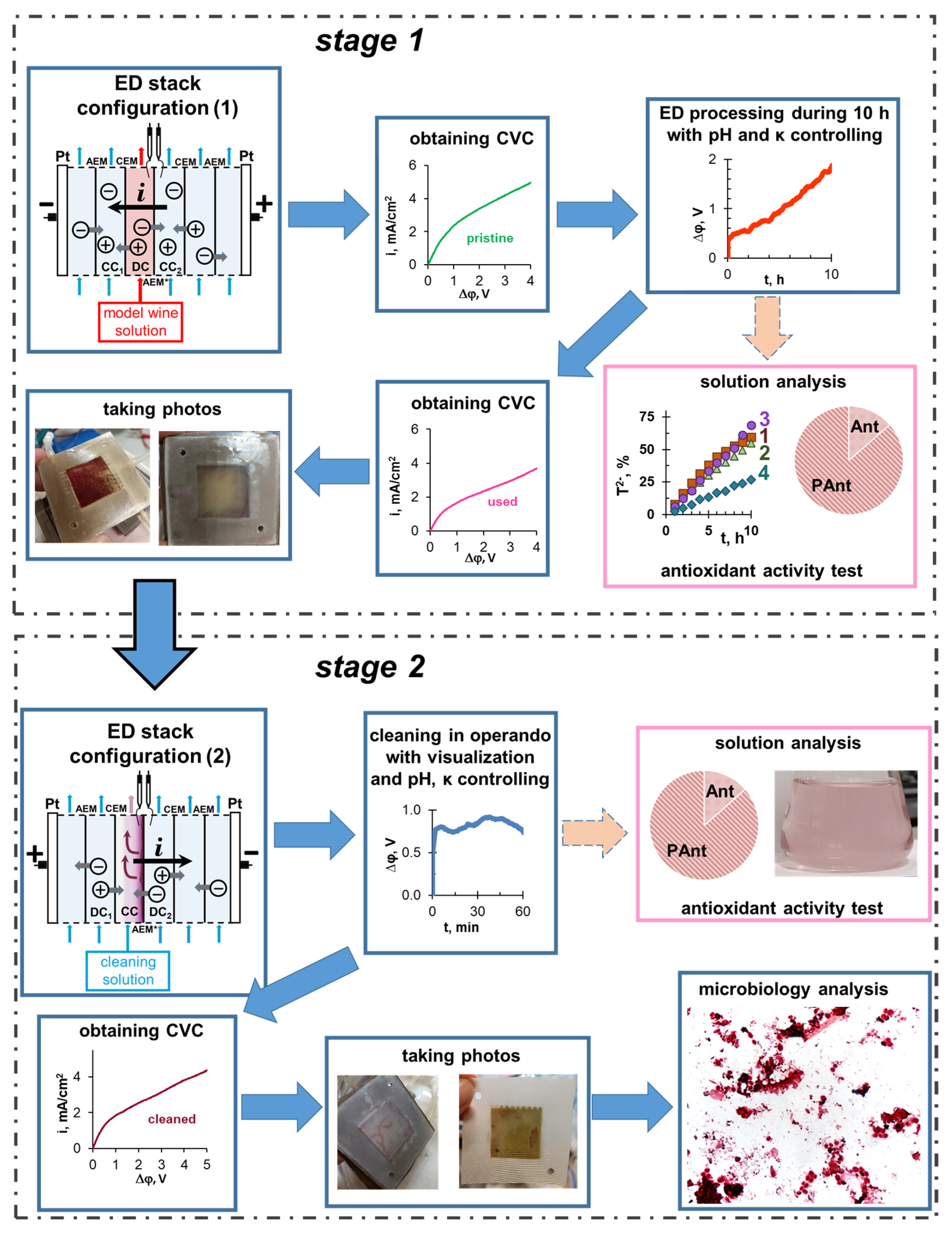
3. Results and Discussion
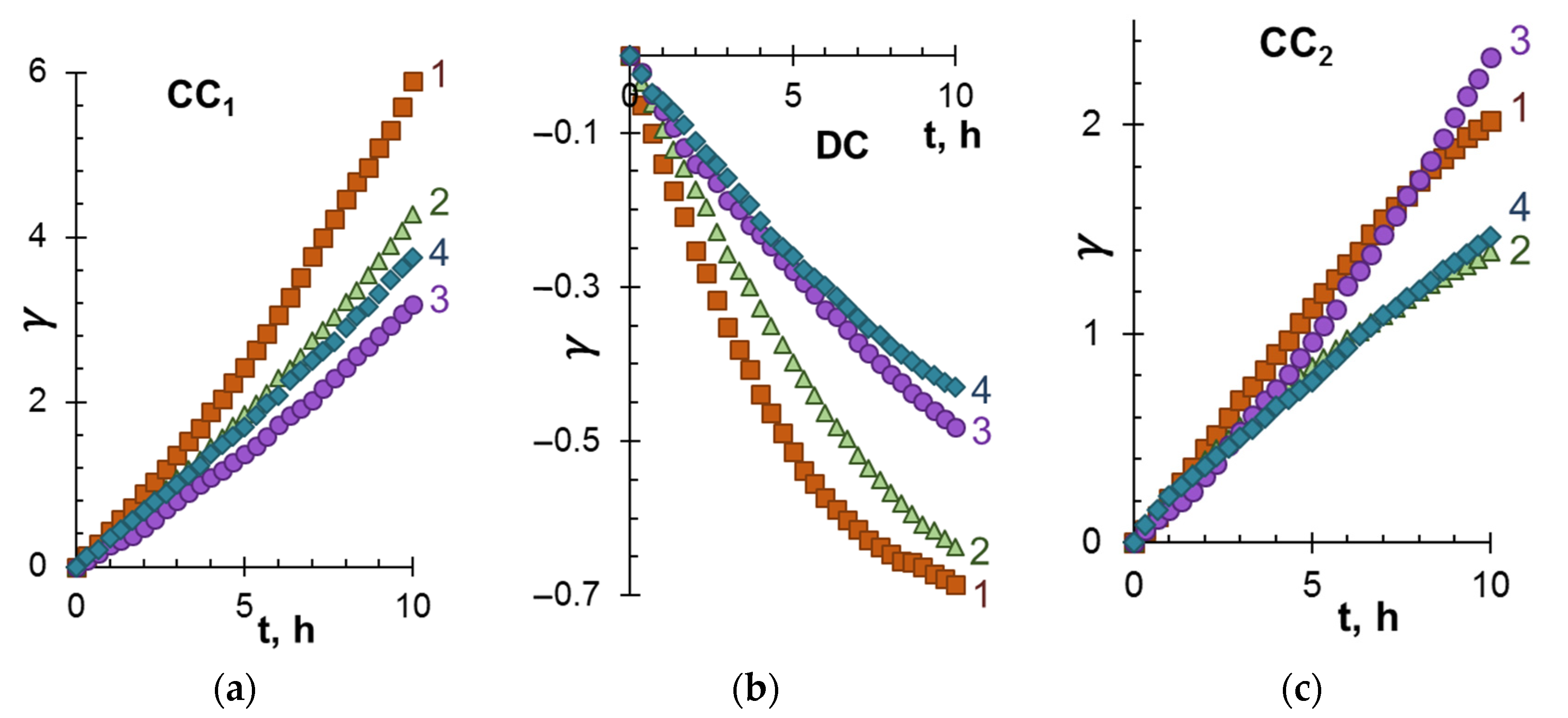
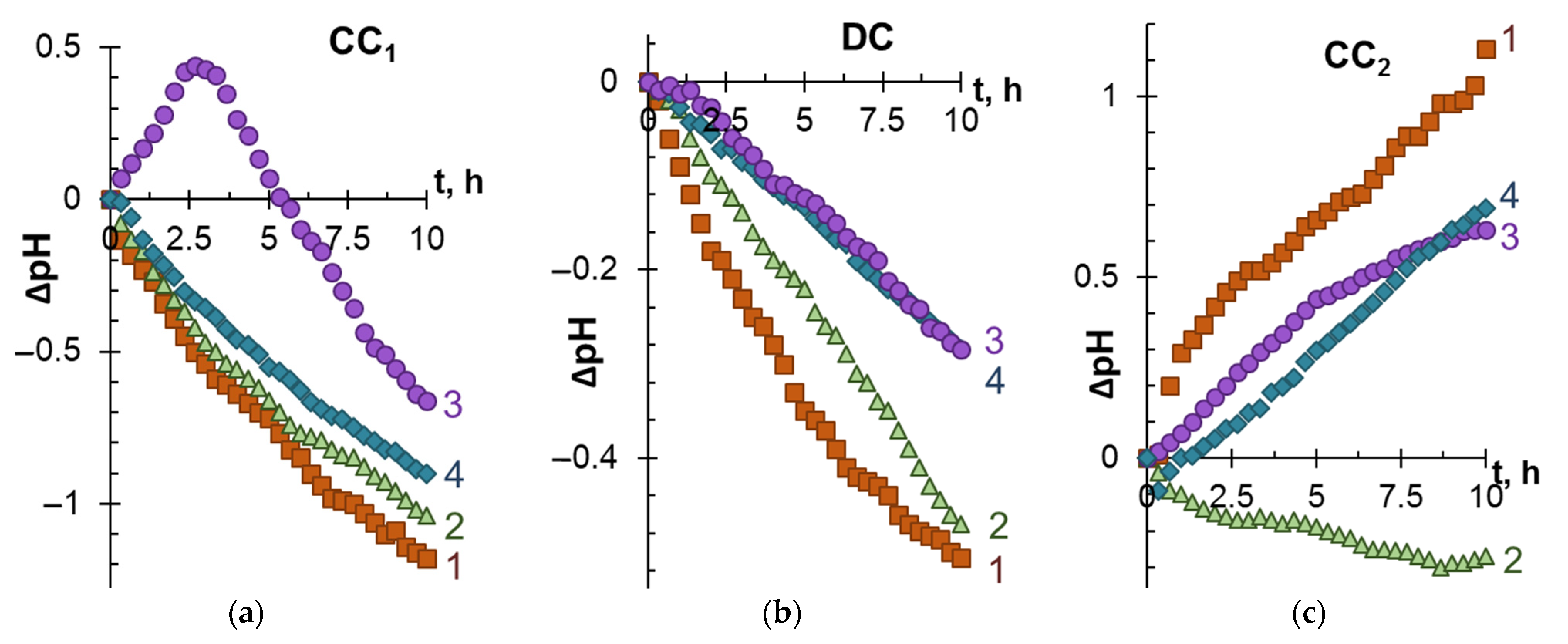
3.1. ED Removal and Concentration of Components of Model Solutions
3.2. The Mechanism of Membrane Fouling by Components of Model Solutions
3.3. Current–Voltage Curves of the CJMA-6 Membrane before and after ED Processing of Model Solutions
3.4. Cleaning of the Anion-Exchange Membrane in Operando
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baenas, N.; Abellán, Á.; Rivera, S.; Moreno, D.A.; Cristina, G.-V.; Domínguez-Perles, R. Foods and Supplements. In Polyphenols: Properties, Recovery, and Applications; Elsevier: Amsterdam, The Netherlands, 2018; pp. 327–362. ISBN 9780128135723. [Google Scholar]
- Vecino, X.; Reig, M.; Gibert, O.; Valderrama, C.; Cortina, J.L. Integration of Monopolar and Bipolar Electrodialysis Processes for Tartaric Acid Recovery from Residues of the Winery Industry. ACS Sustain. Chem. Eng. 2020, 8, 13387–13399. [Google Scholar] [CrossRef]
- Postel, W.; Prasch, E. Studies on the Migration of Coions during Electrodialysis of Wine [Untersuchungen Über Die Coionenwanderung Bei Der Elektrodialyse von Wein]. Zeitschrift für Leb. und Forsch. 1979, 169, 99–105. [Google Scholar] [CrossRef]
- Lopez Leiva, M.H. Use of Electrodialysis in Food Processing. 2. Review of Practical Applications. Leb. Wiss. Technol. Food Sci. Technol. 1988, 21, 177–182. [Google Scholar]
- El Rayess, Y.; Mietton-Peuchot, M. Membrane Technologies in Wine Industry: An Overview. Crit. Rev. Food Sci. Nutr. 2016, 56, 2005–2020. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Pérez, M.P.; Bautista-Ortín, A.B.; Durant, V.; Gómez-Plaza, E. Evaluating Alternatives to Cold Stabilization in Wineries: The Use of Carboxymethyl Cellulose, Potassium Polyaspartate, Electrodialysis and Ion Exchange Resins. Foods 2020, 9, 1275. [Google Scholar] [CrossRef]
- Lasanta, C.; Gomez Benitez, J. Tartrate Stabilization of Wines. Trends Food Sci. Technol. 2012, 28, 52–59. [Google Scholar] [CrossRef]
- Henriques, P.; Brites Alves, A.M.; Rodrigues, M.; Geraldes, V. Controlled Freeze-Thawing Test to Determine the Degree of Deionization Required for Tartaric Stabilization of Wines by Electrodialysis. Food Chem. 2019, 278, 84–91. [Google Scholar] [CrossRef]
- Corti, S.; Paladino, S. Tartaric Stabilization of Wines: Comparison between Electrodyalisis and Cold by Contact. Rev. Fac. Cienc. Agrar. Univ. Nac. Cuyo 2016, 48, 225–238. [Google Scholar]
- Cabrita, M.; Garcia, R.; Catarino, S. Recent Developments in Wine Tartaric Stabilization; Nova Science Publishers: New York, NY, USA, 2016; pp. 49–63. ISBN 978-1-63484-883-1. [Google Scholar]
- Bories, A.; SIRE, Y.; Bouissou, D.; Goulesque, S.; Moutounet, M.; Bonneaud, D.; Lutin, F. Environmental Impacts of Tartaric Stabilisation Processes for Wines Using Electrodialysis and Cold Treatment. South African J. Enol. Vitic. 2011, 32, 174–182. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Jiang, C.; Bazinet, L.; Xu, T. Chapter 10—Electrodialysis-Based Separation Technologies in the Food Industry; Galanakis, C., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 349–381. ISBN 978-0-12-815056-6. [Google Scholar]
- Comuzzo, P.; Battistutta, F. Chapter 2—Acidification and PH Control in Red Wines; Morata, A.B.T.-R.W.T., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 17–34. ISBN 978-0-12-814399-5. [Google Scholar]
- Pismenskaya, N.; Bdiri, M.; Sarapulova, V.; Kozmai, A.; Fouilloux, J.; Baklouti, L.; Larchet, C.; Renard, E.; Dammak, L. A Review on Ion-Exchange Membranes Fouling during Electrodialysis Process in Food Industry, Part 2: Influence on Transport Properties and Electrochemical Characteristics, Cleaning and Its Consequences. Membranes 2021, 11, 811. [Google Scholar] [CrossRef]
- Mikhaylin, S.; Bazinet, L. Fouling on Ion-Exchange Membranes: Classification, Characterization and Strategies of Prevention and Control. Adv. Colloid Interface Sci. 2016, 229, 34–56. [Google Scholar] [CrossRef] [PubMed]
- Ge, S.; Zhang, Z.; Yan, H.; Irfan, M.; Xu, Y.; Li, W.; Wang, H.; Wang, Y. Electrodialytic Desalination of Tobacco Sheet Extract: Membrane Fouling Mechanism and Mitigation Strategies. Membranes 2020, 10, 245. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wang, J.; Nie, Y.; Wang, D.; Xu, H.; Zhang, S. Separation of Soluble Saccharides from the Aqueous Solution Containing Ionic Liquids by Electrodialysis. Sep. Purif. Technol. 2020, 251, 117402. [Google Scholar] [CrossRef]
- Xia, Q.; Qiu, L.; Yu, S.; Yang, H.; Li, L.; Ye, Y.; Gu, Z.; Ren, L.; Liu, G. Effects of Alkaline Cleaning on the Conversion and Transformation of Functional Groups on Ion-Exchange Membranes in Polymer-Flooding Wastewater Treatment: Desalination Performance, Fouling Behavior, and Mechanism. Environ. Sci. Technol. 2019, 53, 14430–14440. [Google Scholar] [CrossRef]
- Ghafari, M.; Mohona, T.M.; Su, L.; Lin, H.; Plata, D.L.; Xiong, B.; Dai, N. Effects of Peracetic Acid on Aromatic Polyamide Nanofiltration Membranes: A Comparative Study with Chlorine. Environ. Sci. Water Res. Technol. 2021, 7, 306–320. [Google Scholar] [CrossRef]
- Šímová, H.; Kysela, V.; Černín, A. Demineralization of Natural Sweet Whey by Electrodialysis at Pilot-Plant Scale. Desalin. Water Treat. Desalin Water Treat 2010, 14, 170–173. [Google Scholar] [CrossRef] [Green Version]
- Jashni, E.; Hosseini, S.M. Polymeric Nano-Enhanced Membranes in Electrodialysis, Electrodialysis Reversal and Capacitive Deionization Technologies. In Advancement in Polymer-Based Membranes for Water Remediation; Nayak, S.K., Dutta, K., Gohil, J., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 569–595. ISBN 978-0-323-88514-0. [Google Scholar]
- Wang, J.; Liu, M.; Feng, Z.; Liu, J.; Liao, S.; Li, X.; Yu, Y. Highly Conductive Anion Exchange Membrane with a Stable Double-Sided Anti-Fouling Structure for Electrodialysis Desalination of Protein Systems. Desalination 2023, 545, 116167. [Google Scholar] [CrossRef]
- Karlin, Y.V.; Kropotov, V.N. Elektrodializnoye Razdeleniye Na+ i Ca2+ v Rezhime Impul’snogo Toka. Ross. Elektrokhim. Z. 1995, 31, 472. [Google Scholar]
- Dufton, G.; Mikhaylin, S.; Gaaloul, S.; Bazinet, L. Systematic Study of the Impact of Pulsed Electric Field Parameters (Pulse/Pause Duration and Frequency) on ED Performances during Acid Whey Treatment. Membranes 2020, 10, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cérémonie, H.; Dudal, Y.; Buret, F. Pulsed Electric Field Induced Redistribution of Fluorescent Compounds from Water-Extractable Soil Organic Matter. Eur. J. Soil Biol. 2008, 44, 10–17. [Google Scholar] [CrossRef]
- Pelletier, S.; Serre, É.; Mikhaylin, S.; Bazinet, L. Optimization of Cranberry Juice Deacidification by Electrodialysis with Bipolar Membrane: Impact of Pulsed Electric Field Conditions. Sep. Purif. Technol. 2017, 186, 106–116. [Google Scholar] [CrossRef]
- Cserhalmi, Z.; Sass-Kiss, Á.; Tóth-Markus, M.; Lechner, N. Study of Pulsed Electric Field Treated Citrus Juices. Innov. Food Sci. Emerg. Technol. 2006, 7, 49–54. [Google Scholar] [CrossRef]
- Nifong, T.P.; Gerhard, G.S. Separation of IgG and IgM from Albumin in Citrated Human Plasma Using Electrodialysis and Metal Ion Affinity Precipitation. ASAIO J. 2002, 48, 645–649. [Google Scholar] [CrossRef]
- Pirot, F.; Faivre, V.; Bourhis, Y.; Aulagner, G.; Falson, F. Faster Phenytoin Removal from Serum by Electrodialysis: A Potential Use in Hemodialysis? J. Memb. Sci. 2002, 207, 265–272. [Google Scholar] [CrossRef]
- Vorobiev, E.; Lebovka, N. Processing of Sugar Beets Assisted by Pulsed Electric Fields. Res. Agric. Eng. 2022, 68, 63–79. [Google Scholar] [CrossRef]
- Zhang, R.; Vorobiev, E.; Zhu, Z.; Grimi, N. Effect of Pulsed Electric Fields Pretreatment on Juice Expression and Quality of Chicory. Innov. Food Sci. Emerg. Technol. 2021, 74, 102842. [Google Scholar] [CrossRef]
- Persico, M.; Mikhaylin, S.; Doyen, A.; Firdaous, L.; Hammami, R.; Bazinet, L. How Peptide Physicochemical and Structural Characteristics Affect Anion-Exchange Membranes Fouling by a Tryptic Whey Protein Hydrolysate. J. Memb. Sci. 2016, 520, 914–923. [Google Scholar] [CrossRef]
- Hansima, M.A.C.K.; Makehelwala, M.; Jinadasa, K.B.S.N.; Wei, Y.; Nanayakkara, K.G.N.; Herath, A.C.; Weerasooriya, R. Fouling of Ion Exchange Membranes Used in the Electrodialysis Reversal Advanced Water Treatment: A Review. Chemosphere 2021, 263, 127951. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Xie, S.; Hu, Z.; Wu, G.; Morrison, L.; Croot, P.; Hu, H.; Zhan, X. Nutrient Recovery from Pig Manure Digestate Using Electrodialysis Reversal: Membrane Fouling and Feasibility of Long-Term Operation. J. Memb. Sci. 2019, 573, 560–569. [Google Scholar] [CrossRef]
- Chon, K.; Jeong, N.; Rho, H.; Nam, J.-Y.; Jwa, E.; Cho, J. Fouling Characteristics of Dissolved Organic Matter in Fresh Water and Seawater Compartments of Reverse Electrodialysis under Natural Water Conditions. Desalination 2020, 496, 114478. [Google Scholar] [CrossRef]
- Hansima, M.A.C.K.; Jayaweera, A.T.; Ketharani, J.; Ritigala, T.; Zheng, L.; Samarajeewa, D.R.; Nanayakkara, K.G.N.; Herath, A.C.; Makehelwala, M.; Jinadasa, K.B.S.N.; et al. Characterization of Humic Substances Isolated from a Tropical Zone and Their Role in Membrane Fouling. J. Environ. Chem. Eng. 2022, 10, 107456. [Google Scholar] [CrossRef]
- Turek, M.; Dydo, P.; Waś, J. Electrodialysis Reversal in High CaSO4 Supersaturation Mode. Desalination 2006, 198, 288–294. [Google Scholar] [CrossRef]
- Shi, L.; Hu, Z.; Simplicio, W.S.; Qiu, S.; Xiao, L.; Harhen, B.; Zhan, X. Antibiotics in Nutrient Recovery from Pig Manure via Electrodialysis Reversal: Sorption and Migration Associated with Membrane Fouling. J. Memb. Sci. 2020, 597, 117633. [Google Scholar] [CrossRef]
- Kammerer, J.; Boschet, J.; Kammerer, D.R.; Carle, R. Enrichment and Fractionation of Major Apple Flavonoids, Phenolic Acids and Dihydrochalcones Using Anion Exchange Resins. LWT Food Sci. Technol. 2011, 44, 1079–1087. [Google Scholar] [CrossRef]
- Ibeas, V.; Correia, A.C.; Jordão, A.M. Wine Tartrate Stabilization by Different Levels of Cation Exchange Resin Treatments: Impact on Chemical Composition, Phenolic Profile and Organoleptic Properties of Red Wines. Food Res. Int. 2015, 69, 364–372. [Google Scholar] [CrossRef]
- Pismenskaya, N.; Sarapulova, V.; Klevtsova, A.; Mikhaylin, S.; Bazinet, L. Adsorption of Anthocyanins by Cation and Anion Exchange Resins with Aromatic and Aliphatic Polymer Matrices. Int. J. Mol. Sci. 2020, 21, 7874. [Google Scholar] [CrossRef] [PubMed]
- Bdiri, M.; Perreault, V.; Mikhaylin, S.; Larchet, C.; Hellal, F.; Bazinet, L.; Dammak, L. Identification of Phenolic Compounds and Their Fouling Mechanisms in Ion-Exchange Membranes Used at an Industrial Scale for Wine Tartaric Stabilization by Electrodialysis. Sep. Purif. Technol. 2020, 233, 115995. [Google Scholar] [CrossRef]
- Perreault, V.; Sarapulova, V.; Tsygurina, K.; Pismenskaya, N.; Bazinet, L. Understanding of Adsorption and Desorption Mechanisms of Anthocyanins and Proanthocyanidins on Heterogeneous and Homogeneous Cation-Exchange Membranes. Membranes 2021, 11, 136. [Google Scholar] [CrossRef] [PubMed]
- Sholokhova, A.Y.; Eliseeva, T.V.; Voronyuk, I. V Sorption of Vanillin by Highly Basic Anion Exchangers under Dynamic Conditions. Russ. J. Phys. Chem. A 2018, 92, 2048–2052. [Google Scholar] [CrossRef]
- Evans, P.J.; Bird, M.R.; Rogers, D.; Wright, C.J. Measurement of Polyphenol–Membrane Interaction Forces during the Ultrafiltration of Black Tea Liquor. Colloids Surfaces A Physicochem. Eng. Asp. 2009, 335, 148–153. [Google Scholar] [CrossRef]
- Ronald, S. Jackson Wine Science: Principles and Applications, 3rd ed.; Academic Press: Cambridge, MA, USA, 2008; ISBN 978-0-12-373646-8. [Google Scholar]
- Ribéreau-Gayon, P.; Glories, Y.; Maujean, A.; Dubourdieu, D. Handbook of Enology, Volume 2: The Chemistry of Wine—Stabilization and Treatments, 2nd ed.; John Wiley & Sons Ltd.: Chichester, UK, 2006; ISBN 978-0-470-01038-9. [Google Scholar]
- Garcia-Vasquez, W.; Ghalloussi, R.; Dammak, L.; Larchet, C.; Nikonenko, V.; Grande, D. Structure and Properties of Heterogeneous and Homogeneous Ion-Exchange Membranes Subjected to Ageing in Sodium Hypochlorite. J. Memb. Sci. 2014, 452, 104–116. [Google Scholar] [CrossRef]
- Sarapulova, V.; Pismenskaya, N.; Butylskii, D.; Titorova, V.; Wang, Y.; Xu, T.; Zhang, Y.; Nikonenko, V. Transport and Electrochemical Characteristics of CJMCED Homogeneous Cation Exchange Membranes in Sodium Chloride, Calcium Chloride, and Sodium Sulfate Solutions. Membranes 2020, 10, 165. [Google Scholar] [CrossRef] [PubMed]
- Sarapulova, V.; Nevakshenova, E.; Nebavskaya, X.; Kozmai, A.; Aleshkina, D.; Pourcelly, G.; Nikonenko, V.; Pismenskaya, N. Characterization of Bulk and Surface Properties of Anion-Exchange Membranes in Initial Stages of Fouling by Red Wine. J. Memb. Sci. 2018, 559, 170–182. [Google Scholar] [CrossRef]
- Bdiri, M.; Dammak, L.; Larchet, C.; Hellal, F.; Porozhnyy, M.; Nevakshenova, E.; Pismenskaya, N.; Nikonenko, V. Characterization and Cleaning of Anion-Exchange Membranes Used in Electrodialysis of Polyphenol-Containing Food Industry Solutions; Comparison with Cation-Exchange Membranes. Sep. Purif. Technol. 2019, 210, 636–650. [Google Scholar] [CrossRef]
- Labbé, D.; Bazinet, L. Effect of Membrane Type on Cation Migration during Green Tea Electromigration and Equivalent Mass Transported Calculation. J. Memb. Sci. 2006, 275, 220–228. [Google Scholar] [CrossRef]
- Kozmai, A.; Sarapulova, V.; Sharafan, M.; Melkonian, K.; Rusinova, T.; Kozmai, Y.; Pismenskaya, N.; Dammak, L.; Nikonenko, V. Electrochemical Impedance Spectroscopy of Anion-Exchange Membrane AMX-Sb Fouled by Red Wine Components. Membranes 2020, 11, 2. [Google Scholar] [CrossRef]
- Yan, H.; Wang, Y.; Xu, T. K6-5: Developing Ion Exchange Membrane for Treating High Salinity Water Using Electrodialysis. In Proceedings of the 5th international conferences on Sustainable Chemical Product and Process Engineering (SCPPE), Tianjin, China, 30 July 2019. [Google Scholar]
- Sarapulova, V.; Pismenskaya, N.; Titorova, V.; Sharafan, M.; Wang, Y.; Xu, T.; Zhang, Y.; Nikonenko, V. Transport Characteristics of CJMAEDTM Homogeneous Anion Exchange Membranes in Sodium Chloride and Sodium Sulfate Solutions. Int. J. Mol. Sci. 2021, 22, 1415. [Google Scholar] [CrossRef]
- He, F.; Liang, N.-N.; Mu, L.; Pan, Q.-H.; Wang, J.; Reeves, M.J.; Duan, C.-Q. Anthocyanins and Their Variation in Red Wines I. Monomeric Anthocyanins and Their Color Expression. Molecules 2012, 17, 1571–1601. [Google Scholar] [CrossRef] [Green Version]
- Lide, D.R. CRC Handbook of Chemistry and Physics; CRC Press: Boca Raton, FL, USA, 2004; Volume 85, ISBN 0849304857. [Google Scholar]
- Giusti, M.; Wrolstad, R.E. Anthocyanins. In Handbook of Food Analytical Chemistry; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2005; pp. 5–69. [Google Scholar]
- Bazinet, L.; Cossec, C.; Gaudreau, H.; Desjardins, Y. Production of a Phenolic Antioxidant Enriched Cranberry Juice by Electrodialysis with Filtration Membrane. J. Agric. Food Chem. 2009, 57, 10245–10251. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J.B.T.-M. [2] Ferric Reducing/Antioxidant Power Assay: Direct Measure of Total Antioxidant Activity of Biological Fluids and Modified Version for Simultaneous Measurement of Total Antioxidant Power and Ascorbic Acid Concentration. In Oxidants and Antioxidants Part A.; Academic Press: Cambridge, MA, USA, 1999; Volume 299, pp. 15–27. ISBN 0076-6879. [Google Scholar]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Rybalkina, O.A.; Sharafan, M.V.; Nikonenko, V.V.; Pismenskaya, N.D. Two Mechanisms of H+/OH− Ion Generation in Anion-Exchange Membrane Systems with Polybasic Acid Salt Solutions. J. Memb. Sci. 2022, 651, 120449. [Google Scholar] [CrossRef]
- Sata, T.; Tsujimoto, M.; Yamaguchi, T.; Matsusaki, K. Change of Anion Exchange Membranes in an Aqueous Sodium Hydroxide Solution at High Temperature. J. Memb. Sci. 1996, 112, 161–170. [Google Scholar] [CrossRef]
- Merle, G.; Wessling, M.; Nijmeijer, K. Anion Exchange Membranes for Alkaline Fuel Cells: A Review. J. Memb. Sci. 2011, 377, 1–35. [Google Scholar] [CrossRef]
- Poulin, J.-F.; Amiot, J.; Bazinet, L. Improved Peptide Fractionation by Electrodialysis with Ultrafiltration Membrane: Influence of Ultrafiltration Membrane Stacking and Electrical Field Strength. J. Memb. Sci. 2007, 299, 83–90. [Google Scholar] [CrossRef]
- Persico, M.; Mikhaylin, S.; Doyen, A.; Firdaous, L.; Hammami, R.; Chevalier, M.; Flahaut, C.; Dhulster, P.; Bazinet, L. Formation of Peptide Layers and Adsorption Mechanisms on a Negatively Charged Cation-Exchange Membrane. J. Colloid Interface Sci. 2017, 508, 488–499. [Google Scholar] [CrossRef]
- Loza, S.; Loza, N.; Kutenko, N.; Smyshlyaev, N. Profiled Ion-Exchange Membranes for Reverse and Conventional Electrodialysis. Membranes 2022, 12, 985. [Google Scholar] [CrossRef]
- Eliseeva, T.; Kharina, A. Desalination of Neutral Amino Acid Solutions in an Electromembrane System. Membranes 2022, 12, 665. [Google Scholar] [CrossRef]
- Kozaderova, O.A.; Kim, K.B.; Gadzhiyeva;, C.S.; Niftaliev, S.I. Electrochemical Characteristics of Thin Heterogeneous Ion Exchange Membranes. J. Memb. Sci. 2020, 604, 118081. [Google Scholar] [CrossRef]
- Dukhin, S.S. Electrokinetic Phenomena of the Second Kind and Their Applications. Adv. Colloid Interface Sci. 1991, 35, 173–196. [Google Scholar] [CrossRef]
- Mishchuk, N.A. Concentration Polarization of Interface and Non-Linear Electrokinetic Phenomena. Adv. Colloid Interface Sci. 2010, 160, 16–39. [Google Scholar] [CrossRef]
- Rubinstein, I.; Zaltzman, B. Equilibrium Electroconvective Instability. Phys. Rev. Lett. 2015, 114, 114502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rybalkina, O.A.; Tsygurina, K.A.; Sarapulova, V.V.; Mareev, S.A.; Nikonenko, V.V.; Pismenskaya, N.D. Evolution of Current–Voltage Characteristics and Surface Morphology of Homogeneous Anion-Exchange Membranes during the Electrodialysis Desalination of Alkali Metal Salt Solutions. Membr. Membr. Technol. 2019, 1, 107–119. [Google Scholar] [CrossRef] [Green Version]
- Hyde, A.M.; Zultanski, S.L.; Waldman, J.H.; Zhong, Y.-L.; Shevlin, M.; Peng, F. General Principles and Strategies for Salting-Out Informed by the Hofmeister Series. Org. Process Res. Dev. 2017, 21, 1355–1370. [Google Scholar] [CrossRef]
- Meng, J.; Shi, L.; Hu, Z.; Hu, Y.; Lens, P.; Wang, S.; Zhan, X. Novel Electro-Ion Substitution Strategy in Electrodialysis for Ammonium Recovery from Digested Sludge Centrate in Coastal Regions. J. Memb. Sci. 2022, 642, 120001. [Google Scholar] [CrossRef]
- Rotta, E.H.; Marder, L.; Pérez-Herranz, V.; Bernardes, A.M. Characterization of an Anion-Exchange Membrane Subjected to Phosphate and Sulfate Separation by Electrodialysis at Overlimiting Current Density Condition. J. Memb. Sci. 2021, 635, 119510. [Google Scholar] [CrossRef]
- Czochanska, Z.; Foo, L.Y.; Newman, R.H.; Porter, L.J. Polymeric Proanthocyanidins. Stereochemistry, Structural Units, and Molecular Weight. J. Chem. Soc. Perkin Trans. 1980, 1, 2278–2286. [Google Scholar] [CrossRef]
- Khoo, H.E.; Azlan, A.; Tang, S.; Lim, S. Anthocyanidins and Anthocyanins: Colored Pigments as Food, Pharmaceutical Ingredients, and the Potential Health Benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef] [Green Version]
- Wach, W. Fructose. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiely: Hoboken, NJ, USA, 2004; ISBN 9783527306732. [Google Scholar]
- Helfferich, F. Ion Exchange; McGraw-Hill: New York, NY, USA, 1962; ISBN 0-486-68784-8. [Google Scholar]
- Kozmai, A.E.; Nikonenko, V.V.; Zyryanova, S.; Pismenskaya, N.D.; Dammak, L. A Simple Model for the Response of an Anion-Exchange Membrane to Variation in Concentration and PH of Bathing Solution. J. Memb. Sci. 2018, 567, 127–138. [Google Scholar] [CrossRef]
- Rybalkina, O.; Tsygurina, K.; Melnikova, E.; Mareev, S.; Moroz, I.; Nikonenko, V.; Pismenskaya, N. Partial Fluxes of Phosphoric Acid Anions through Anion-Exchange Membranes in the Course of NaH2PO4 Solution Electrodialysis. Int. J. Mol. Sci. 2019, 20, 3593. [Google Scholar] [CrossRef]
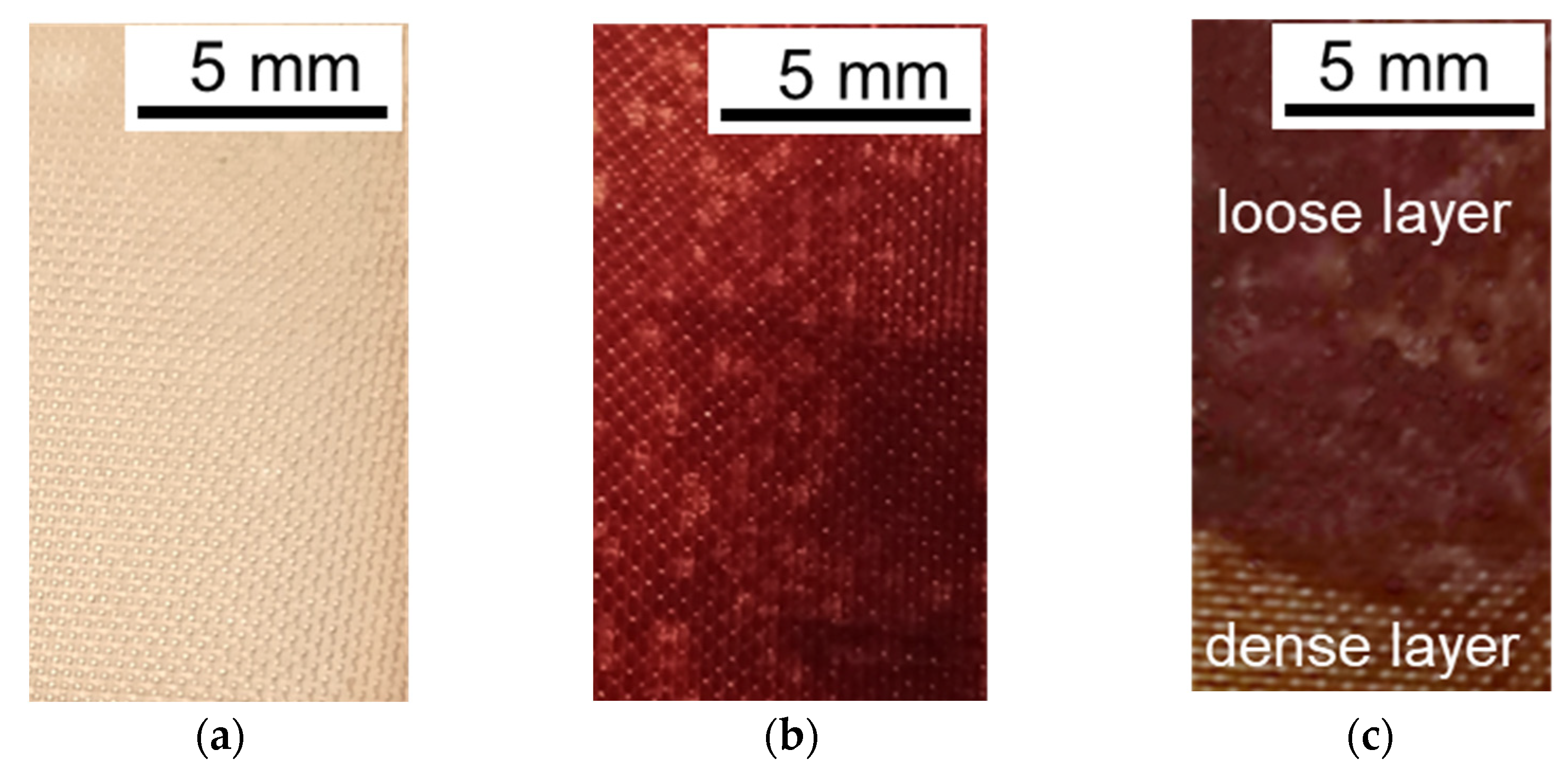
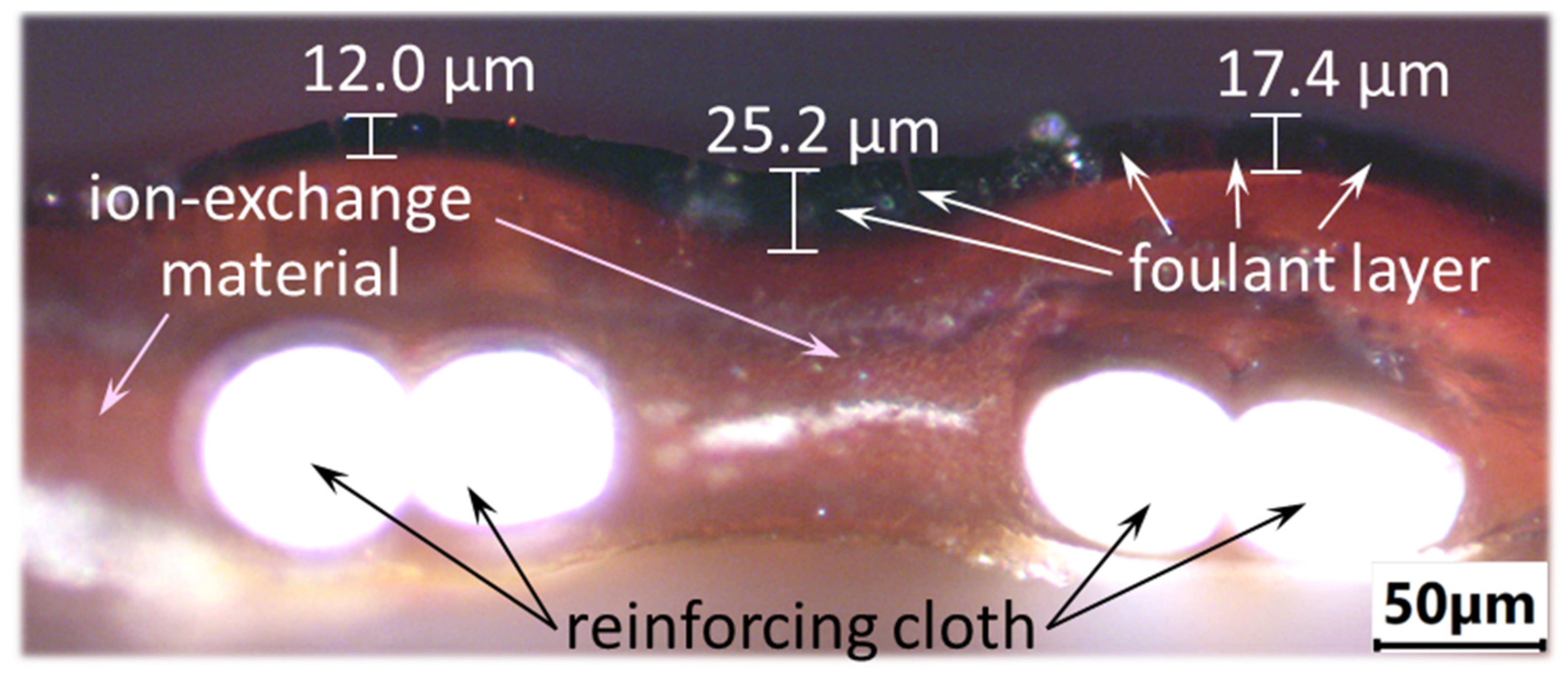
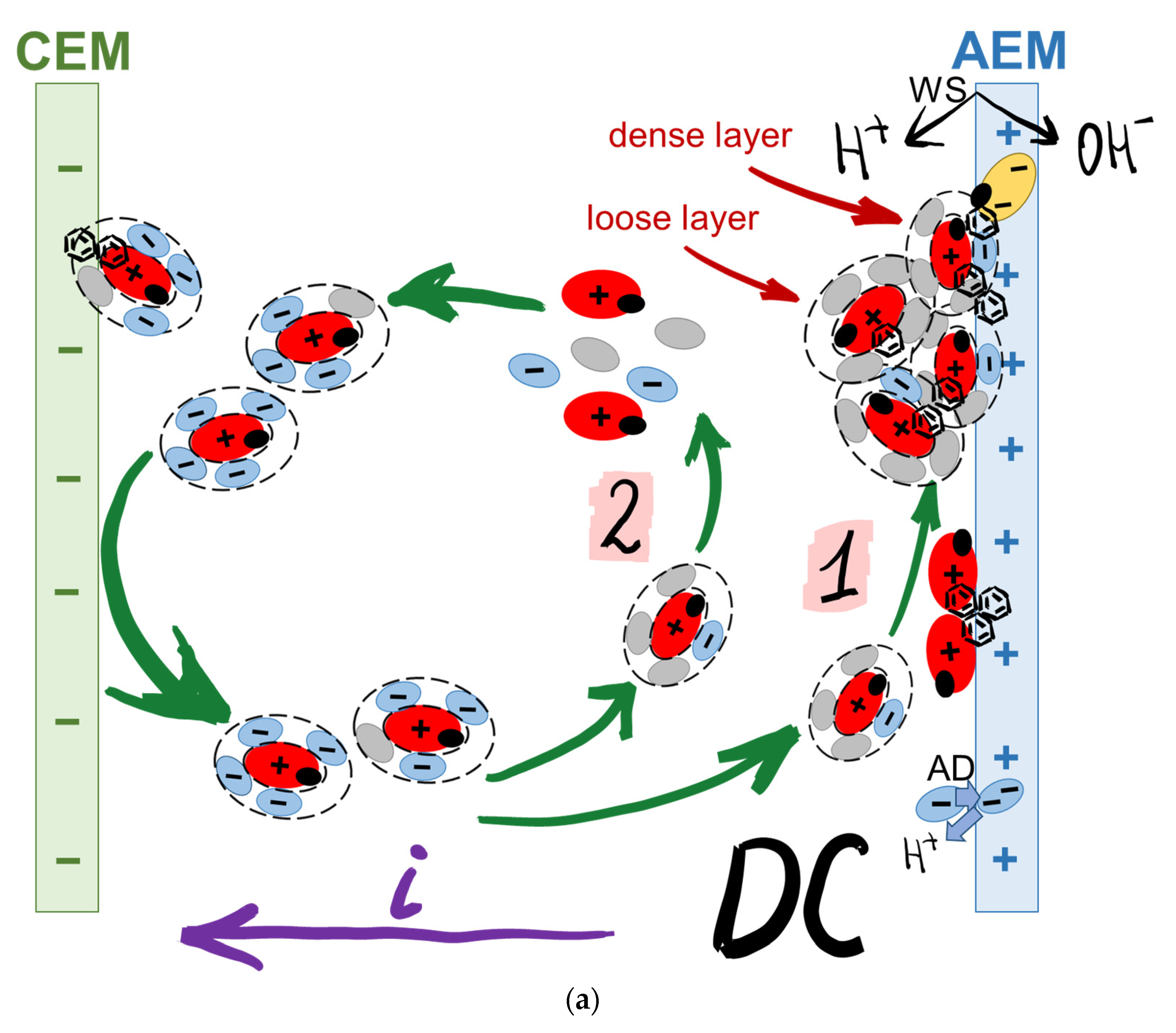
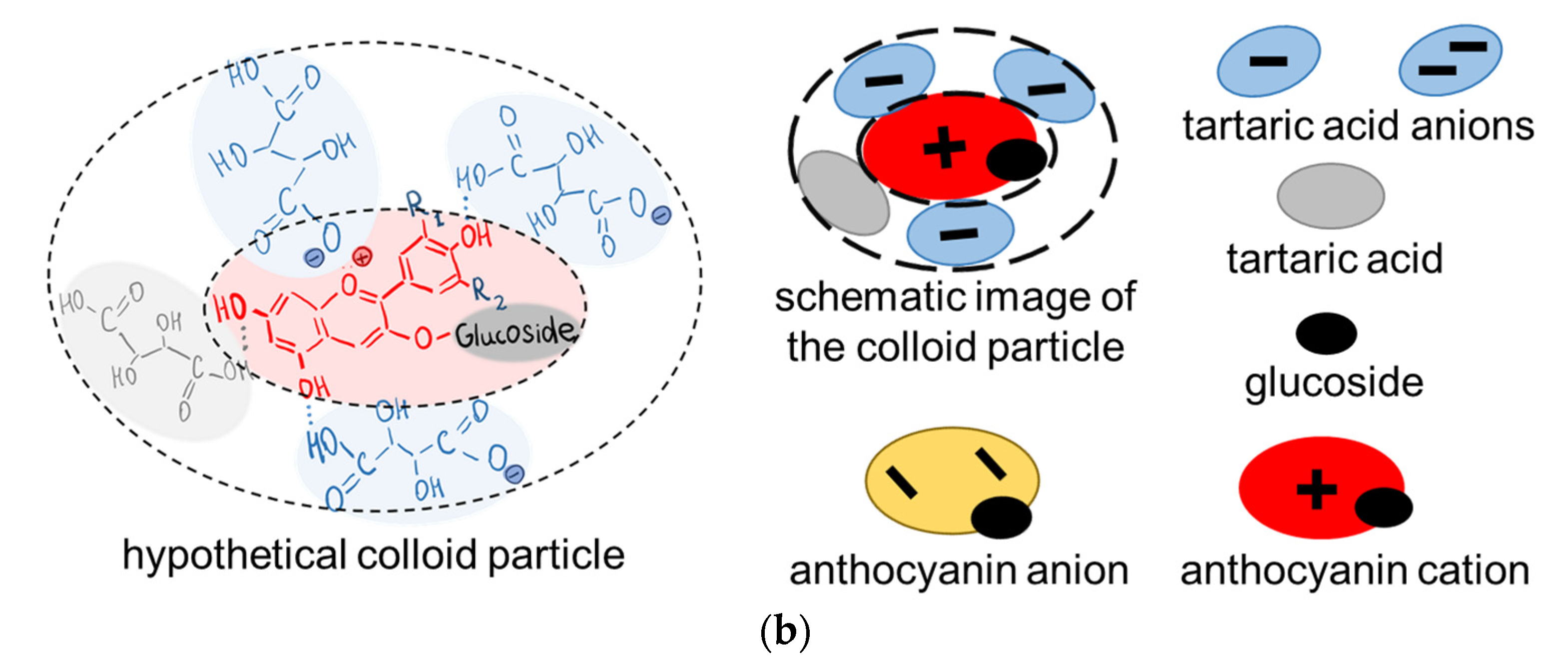

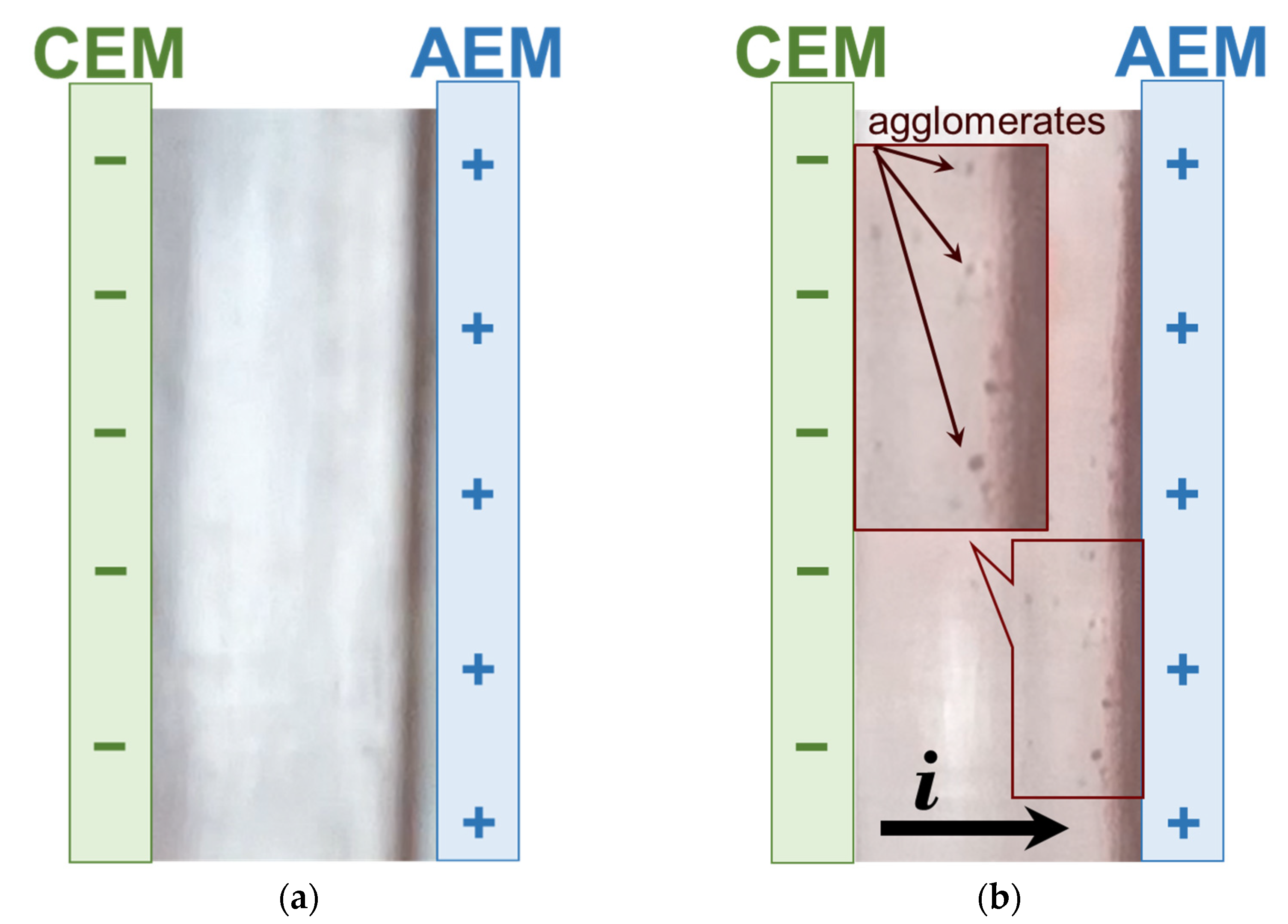
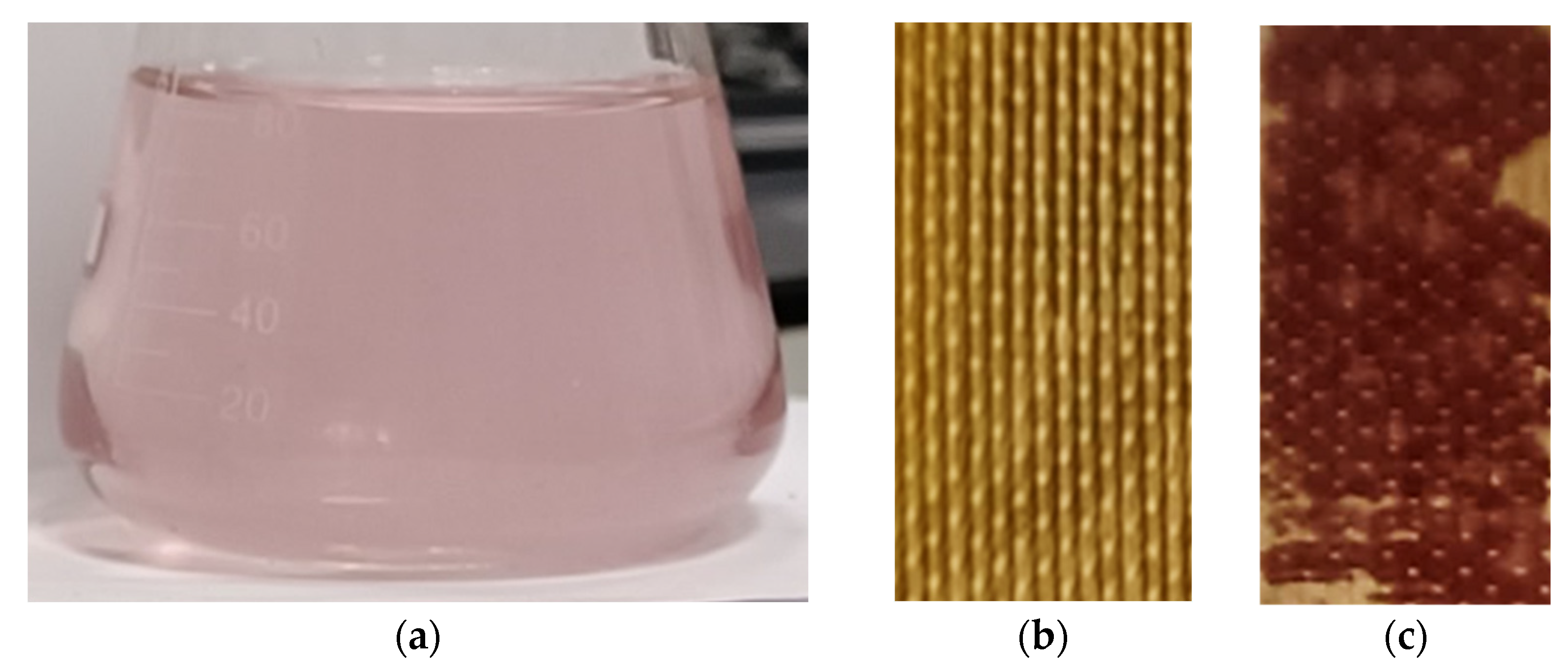
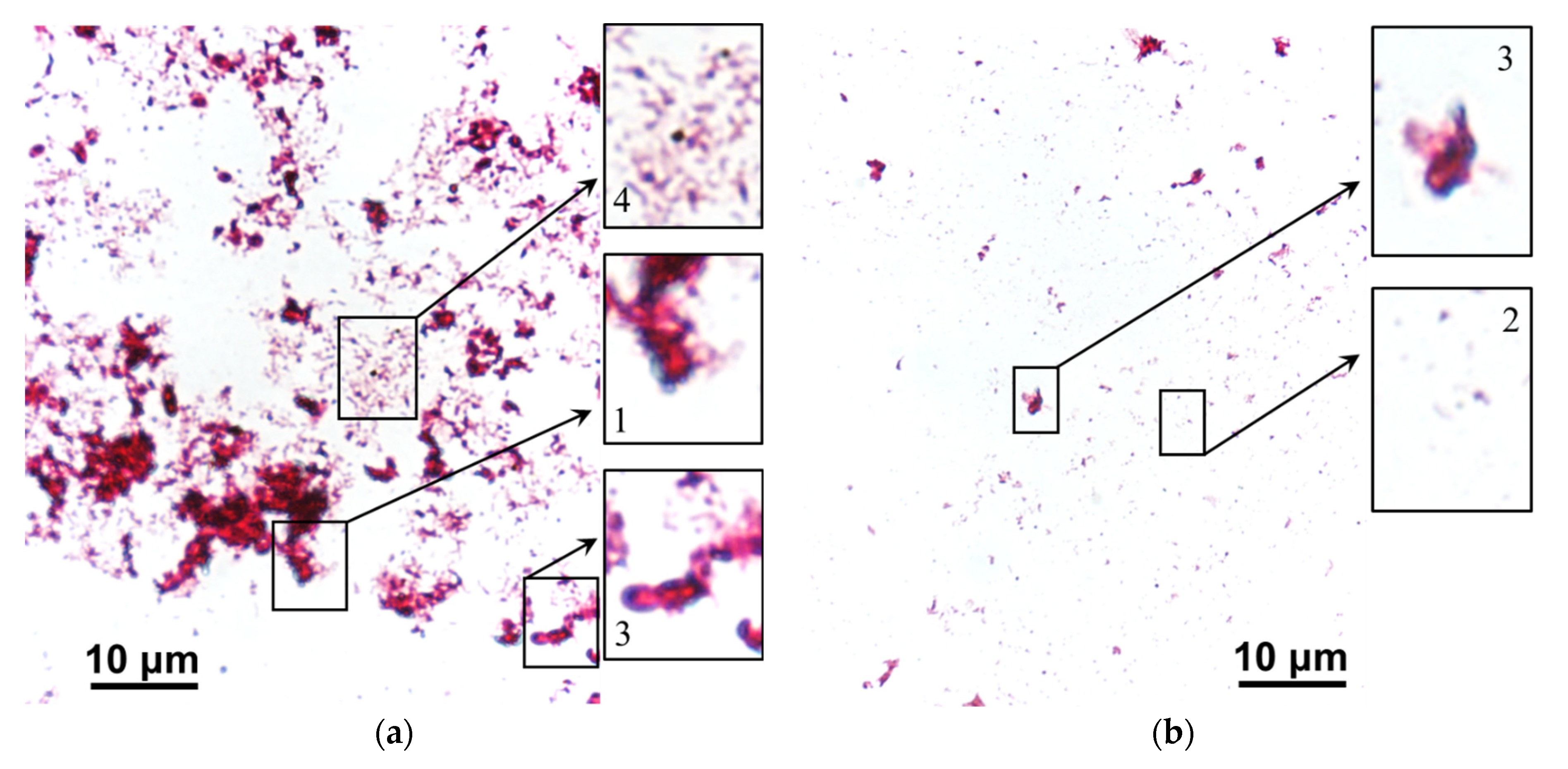

| Designation | Fixed Group | Matrix Material | Membrane Thickness in 0.02 M NaCl Solution, µm | Water Uptake, gH2O/gdry, % | Ion-Exchange Capacity, mmol/gwet |
|---|---|---|---|---|---|
| CJMA-6 | –N+ (CH3)3 | Polyolefin | 120 ± 3 | 18 ± 1 | 0.90 ± 0.05 |
| CJMC-5 | –SO32– | Polyvinylidene fluoride | 154 ± 3 | 32 ± 5 | 0.57 ± 0.07 |
| MA-41 | –N+ (CH3)3, –N+ (CH3)2, –N+ (CH3) | Cross-linked polystyrene with divenylbenzene | 450 ± 50 | 33 ± 3 | 1.22 ± 0.06 |
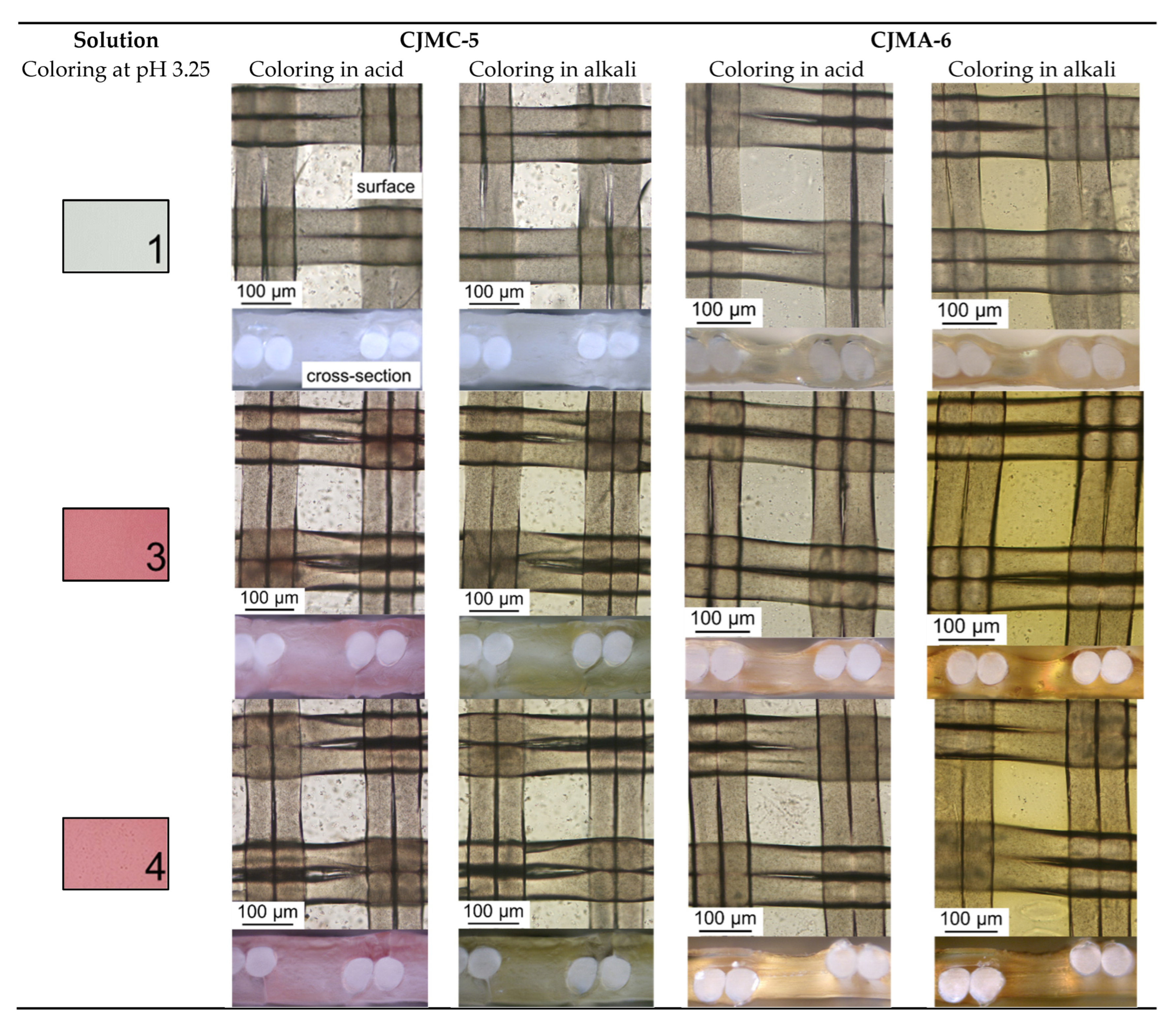
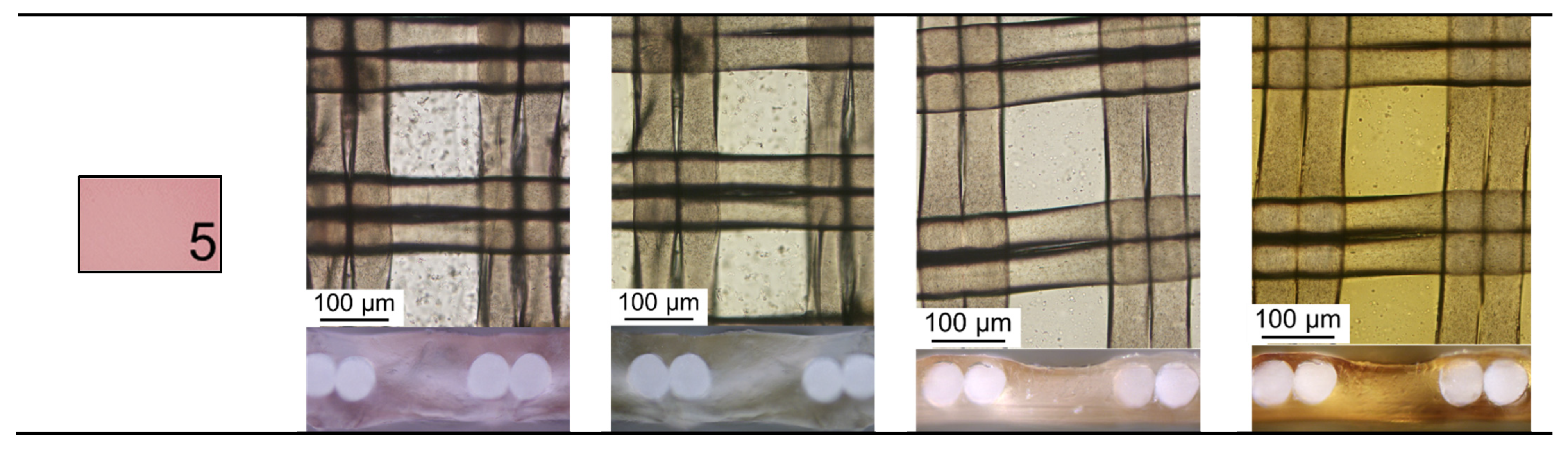
| Solution Designation | KCl, M | 1 KxH(2−x)T, M | C2H5OH, % | Polyphenols, mg/L | Fructose, g/L | pH | κ, mS/cm | |
|---|---|---|---|---|---|---|---|---|
| Ant | PACs | |||||||
| 1 | 0.005 | 0.013 | - | - | - | - | 3.25 | 2.040 |
| 2 | 10 | - | - | - | 1.493 | |||
| 3 | 20 | 130 | - | 2.790 | ||||
| 4 | 1.0 | 2.080 | ||||||
| 5 | - | - | 10 | 20 | 130 | - | 0.935 | |
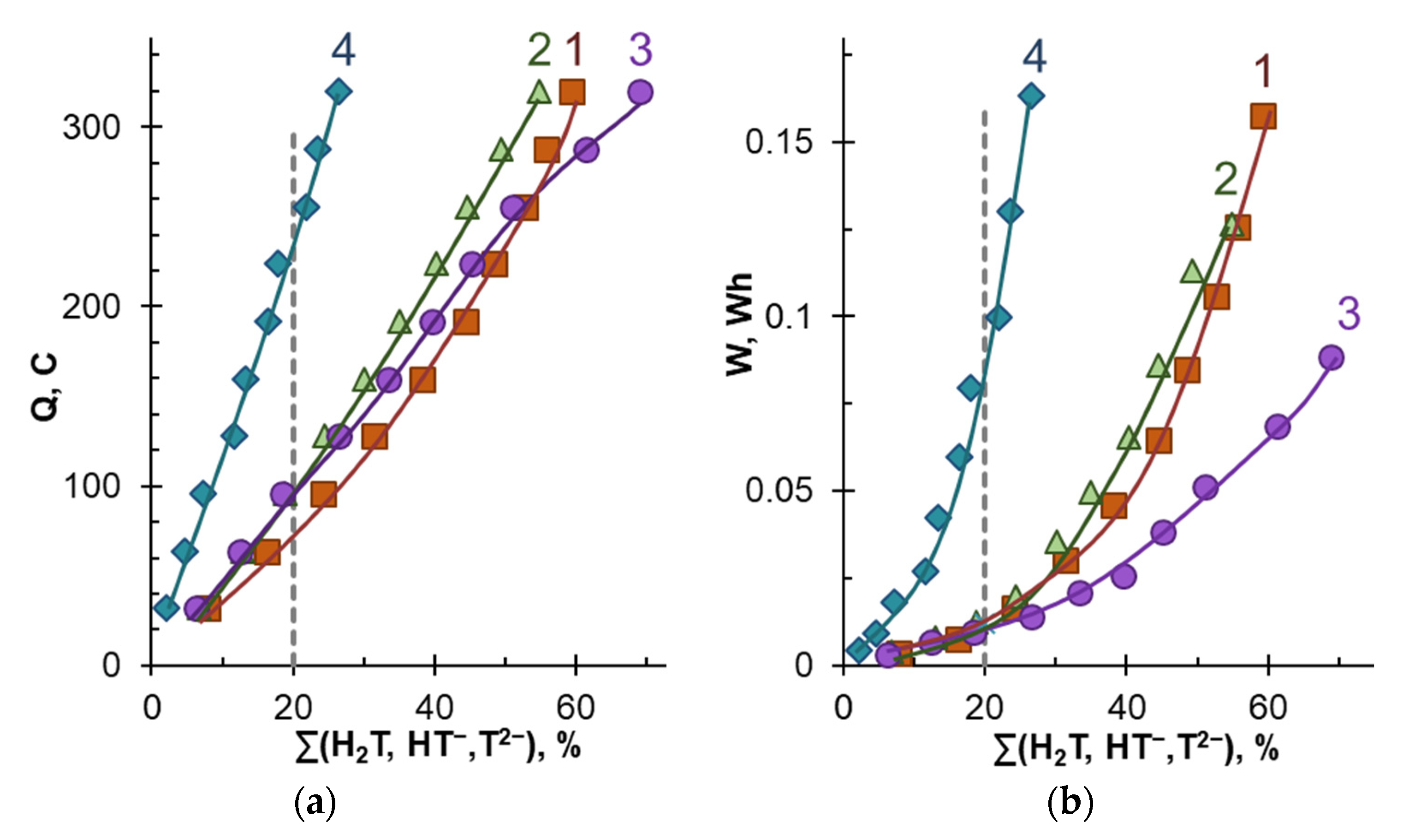
| Solution Designation | The Degree of Tartrates Removal, % | Electrodialysis Duration, s | The Number of Changes Transported, C | Energy Consumption 103, W h |
|---|---|---|---|---|
| 1 | 20 ± 2 | 9000 | 79 ± 5 | 13 ± 1 |
| 2 | 11,580 | 94 ± 5 | 13 ± 1 | |
| 3 | 11,520 | 99 ± 5 | 12 ± 1 | |
| 4 | 27,120 | 235 ± 5 | 85 ± 1 |
| Solution Designation | * Rp, Ω cm2 | Ru/Rp | ilim p exp, mA/cm2 | ilim uexp/ilim pexp | “Length” of the Plateau p, V | “Length” of the Plateau u, V |
|---|---|---|---|---|---|---|
| 1 | 2.78 | 1.00 | 2.01 | 1.08 ± 0.2 | 1.28 | 1.15 |
| 2 | 2.80 | 1.06 | 1.68 | 1.11 ± 0.2 | 1.25 | 1.44 |
| 3 | 2.20 | 1.08 | 4.00 | 0.81 ± 0.2 | >4.22 | >4.22 |
| 4 | 2.84 | 1.03 | 2.37 | 0.59 ± 0.2 | >5.11 | 2.93 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsygurina, K.; Pasechnaya, E.; Chuprynina, D.; Melkonyan, K.; Rusinova, T.; Nikonenko, V.; Pismenskaya, N. Electrodialysis Tartrate Stabilization of Wine Materials: Fouling and a New Approach to the Cleaning of Aliphatic Anion-Exchange Membranes. Membranes 2022, 12, 1187. https://doi.org/10.3390/membranes12121187
Tsygurina K, Pasechnaya E, Chuprynina D, Melkonyan K, Rusinova T, Nikonenko V, Pismenskaya N. Electrodialysis Tartrate Stabilization of Wine Materials: Fouling and a New Approach to the Cleaning of Aliphatic Anion-Exchange Membranes. Membranes. 2022; 12(12):1187. https://doi.org/10.3390/membranes12121187
Chicago/Turabian StyleTsygurina, Kseniia, Evgeniia Pasechnaya, Daria Chuprynina, Karina Melkonyan, Tatyana Rusinova, Victor Nikonenko, and Natalia Pismenskaya. 2022. "Electrodialysis Tartrate Stabilization of Wine Materials: Fouling and a New Approach to the Cleaning of Aliphatic Anion-Exchange Membranes" Membranes 12, no. 12: 1187. https://doi.org/10.3390/membranes12121187
APA StyleTsygurina, K., Pasechnaya, E., Chuprynina, D., Melkonyan, K., Rusinova, T., Nikonenko, V., & Pismenskaya, N. (2022). Electrodialysis Tartrate Stabilization of Wine Materials: Fouling and a New Approach to the Cleaning of Aliphatic Anion-Exchange Membranes. Membranes, 12(12), 1187. https://doi.org/10.3390/membranes12121187










