Experimental Performance of a Membrane Desorber with a H2O/LiCl Mixture for Absorption Chiller Applications
Abstract
1. Introduction
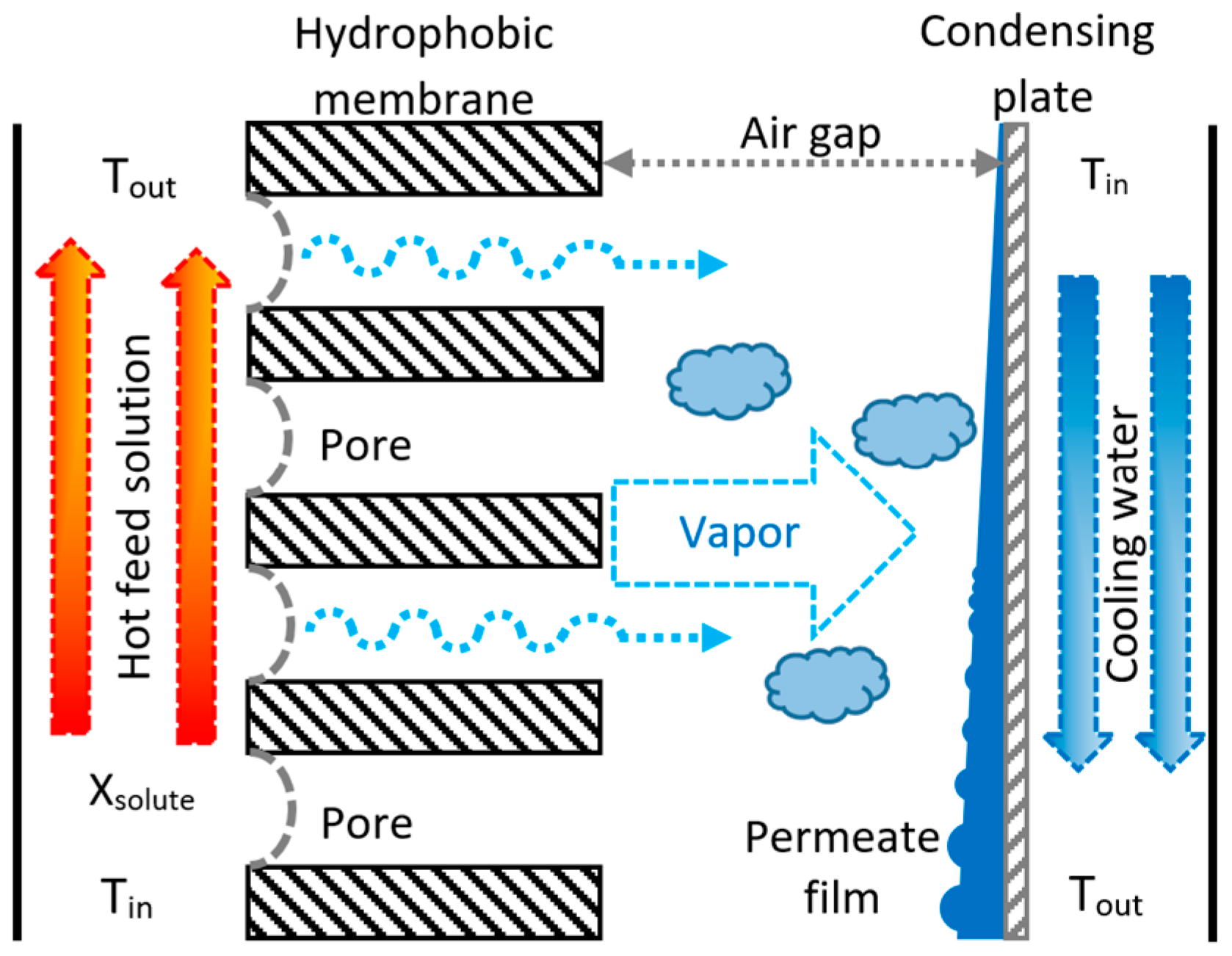
2. Air Gap Membrane Distillation (AGMD) Configuration
3. Methodology
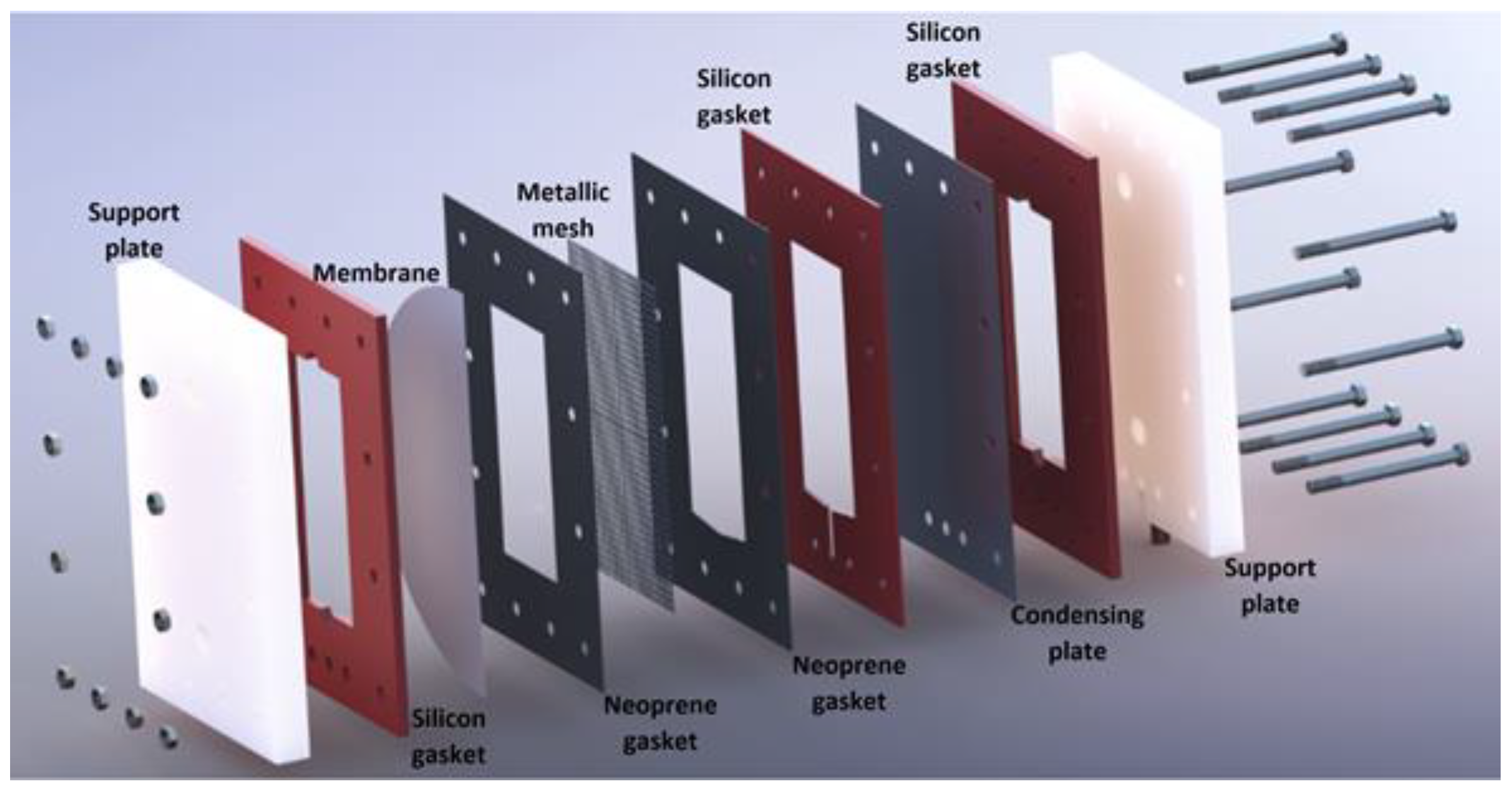
3.1. Experimental Setup
3.2. Heat and Mass Transfer Model
- (1)
- The desorber/condenser unit operates at steady state conditions.
- (2)
- Thermophysical properties are constant.
- (3)
- The heat and mass transfer processes occur in one dimension.
- (4)
- Natural convection is neglected in the air gap region.
- (5)
- Liquid–vapor equilibrium exists at the evaporation and condensation interfaces.
4. Results
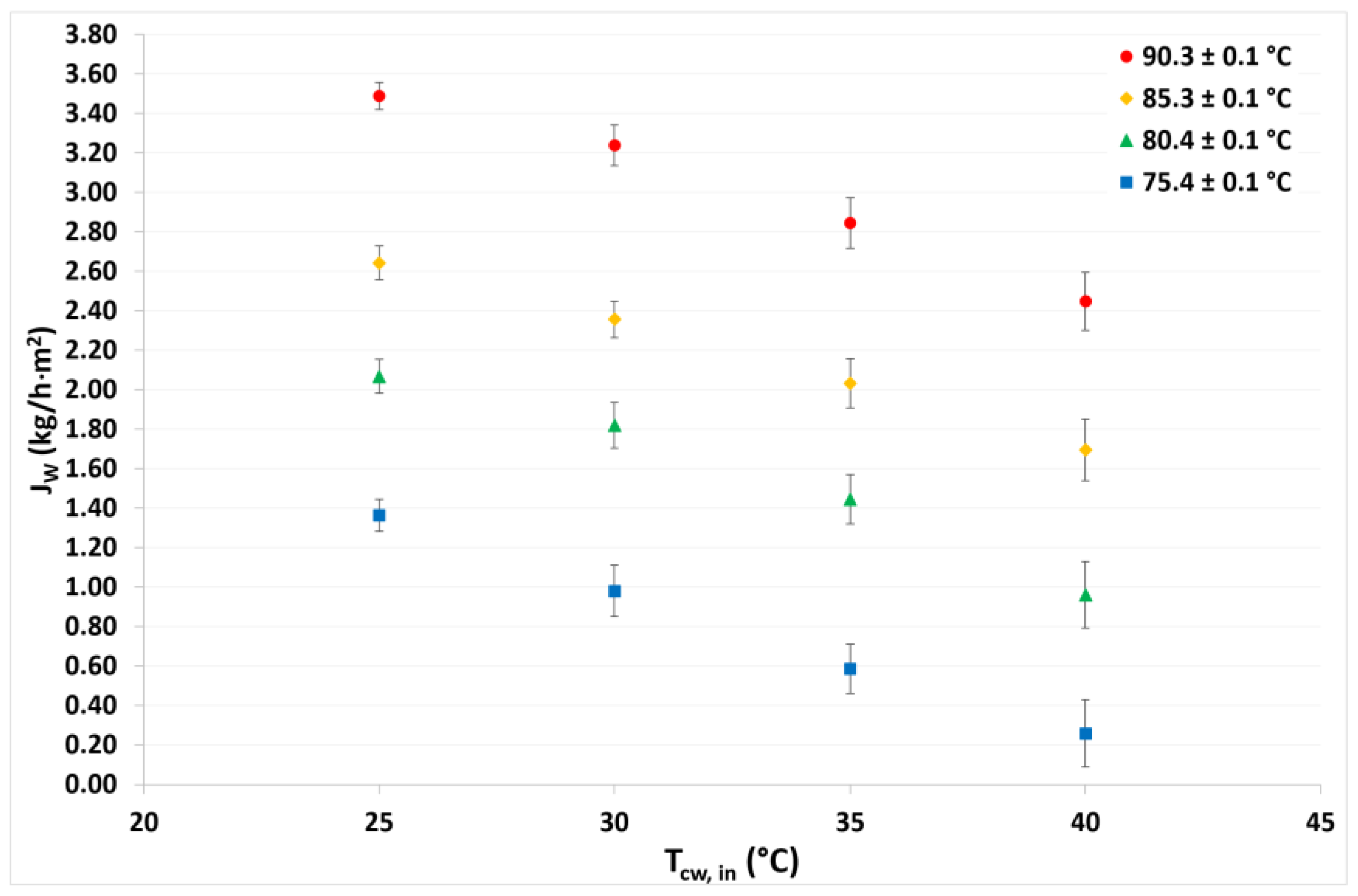
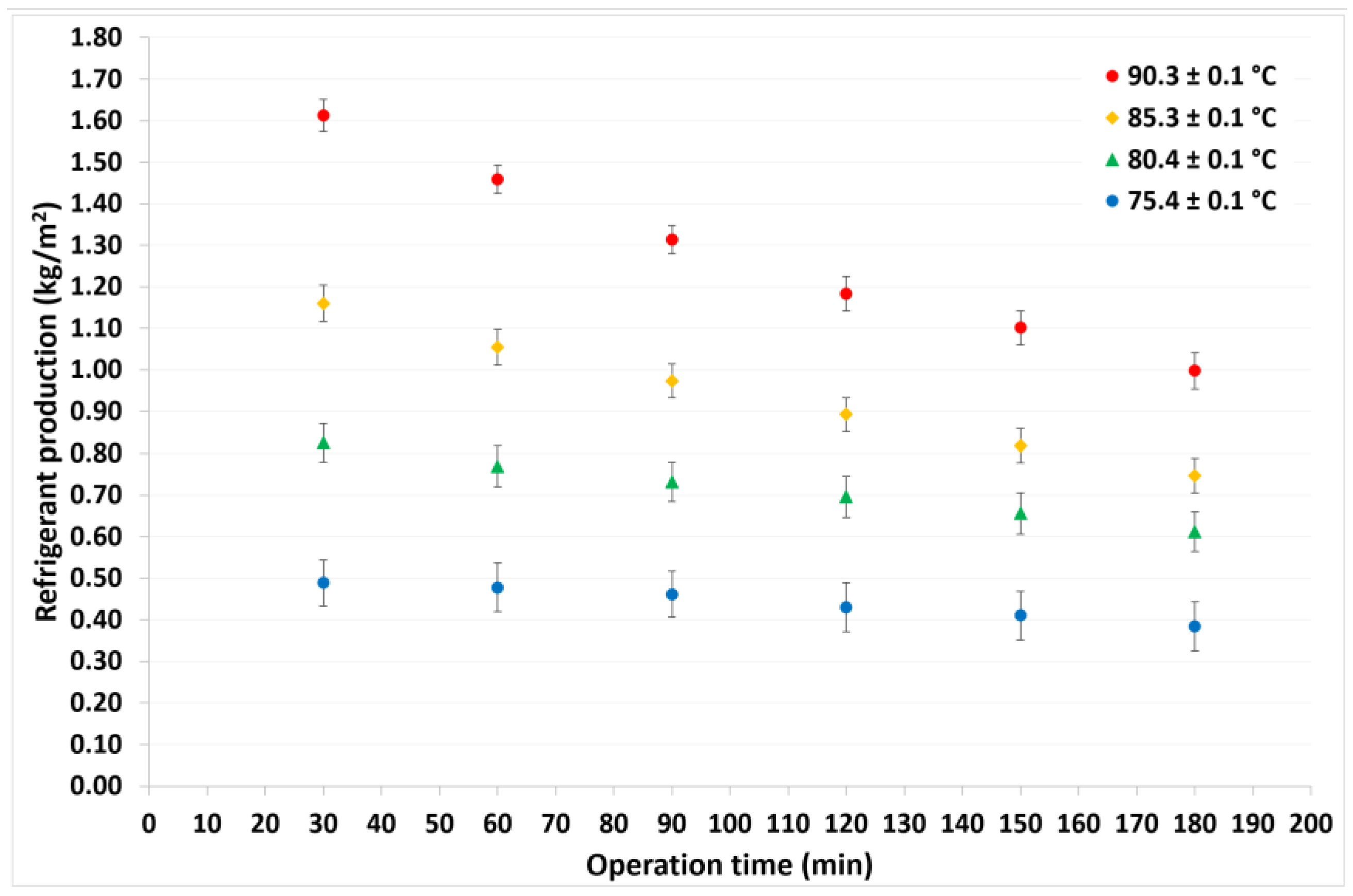
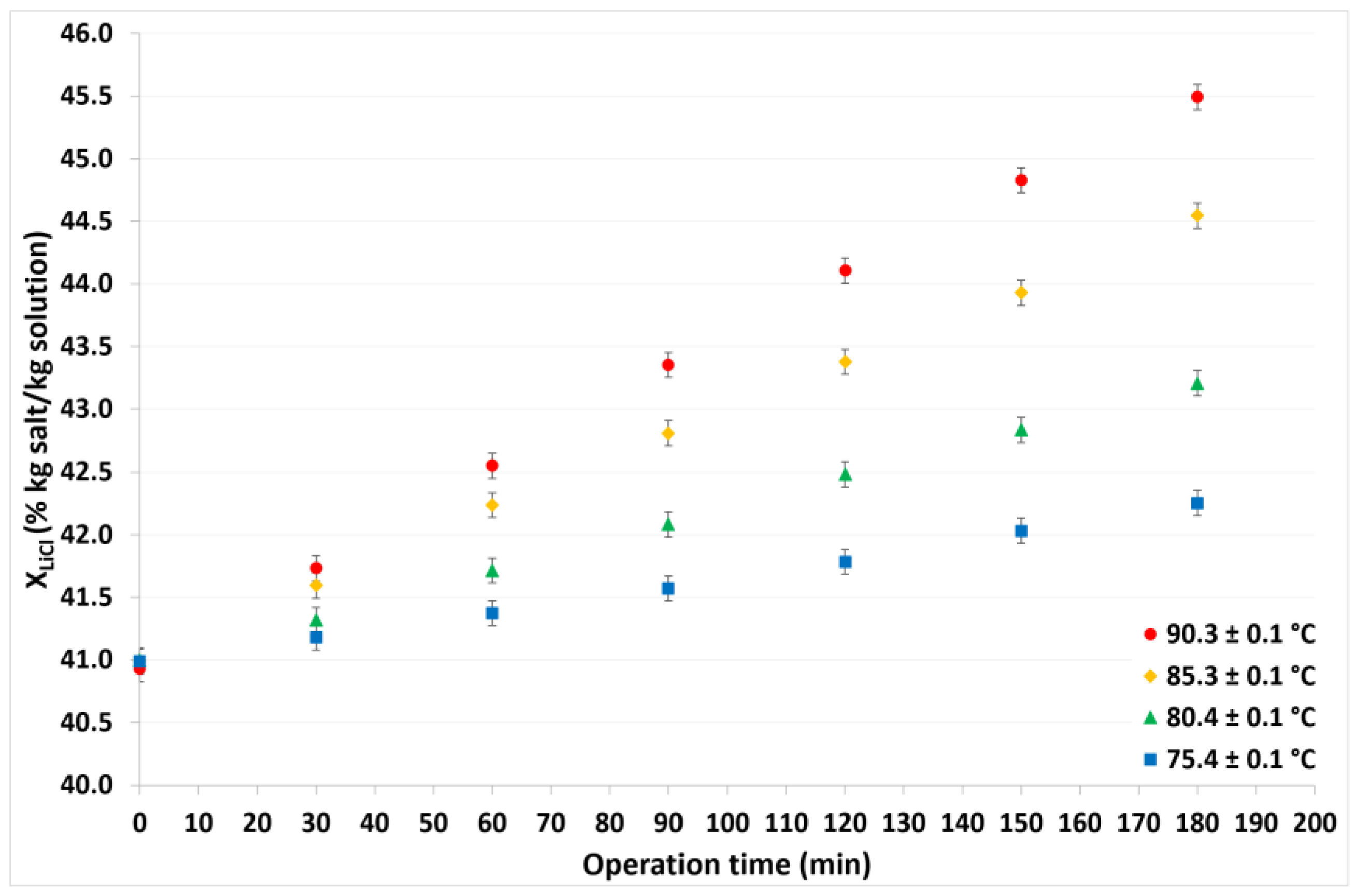
4.1. Continuous Cycle Operation
4.2. Intermittent Operation
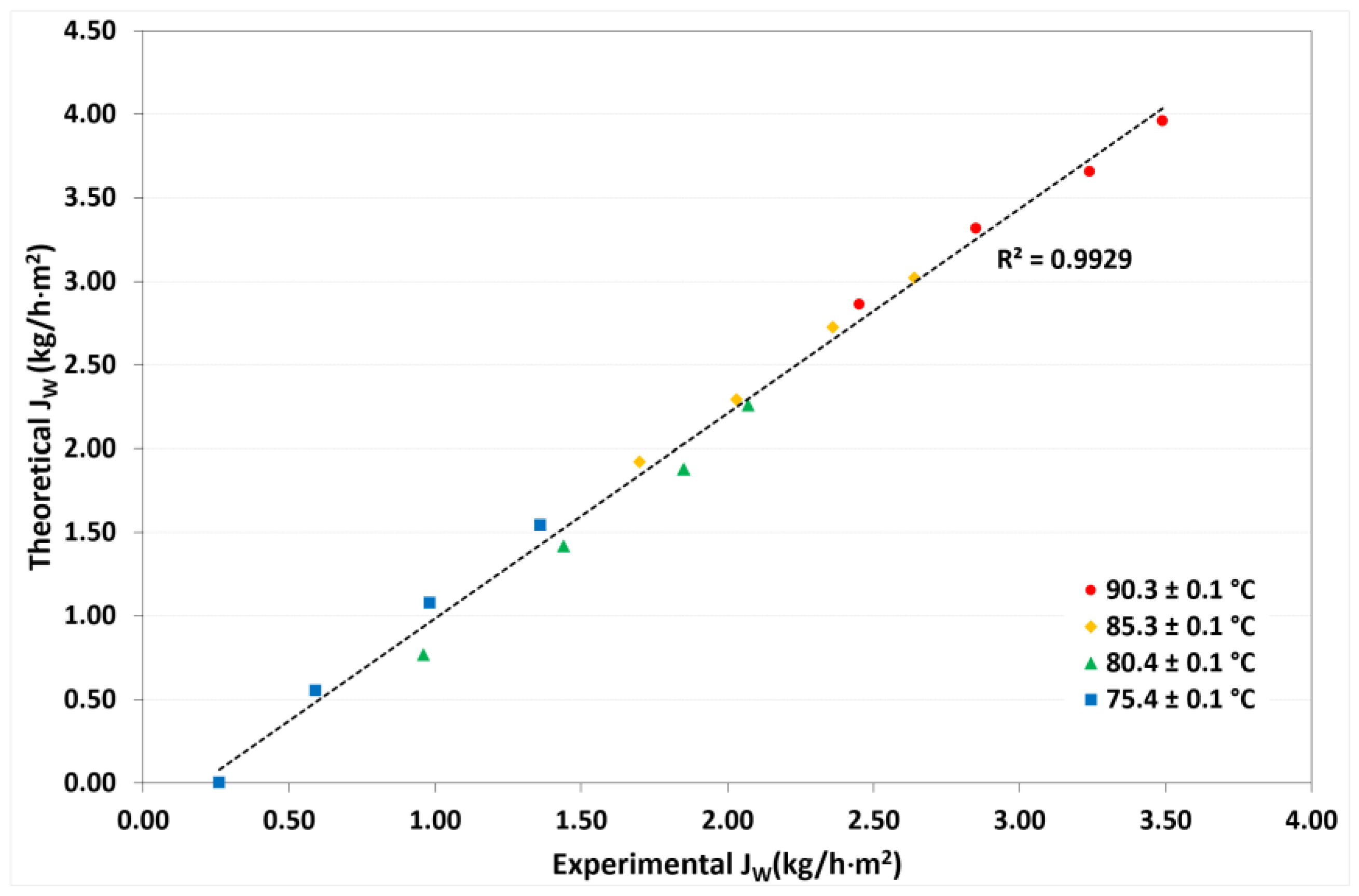
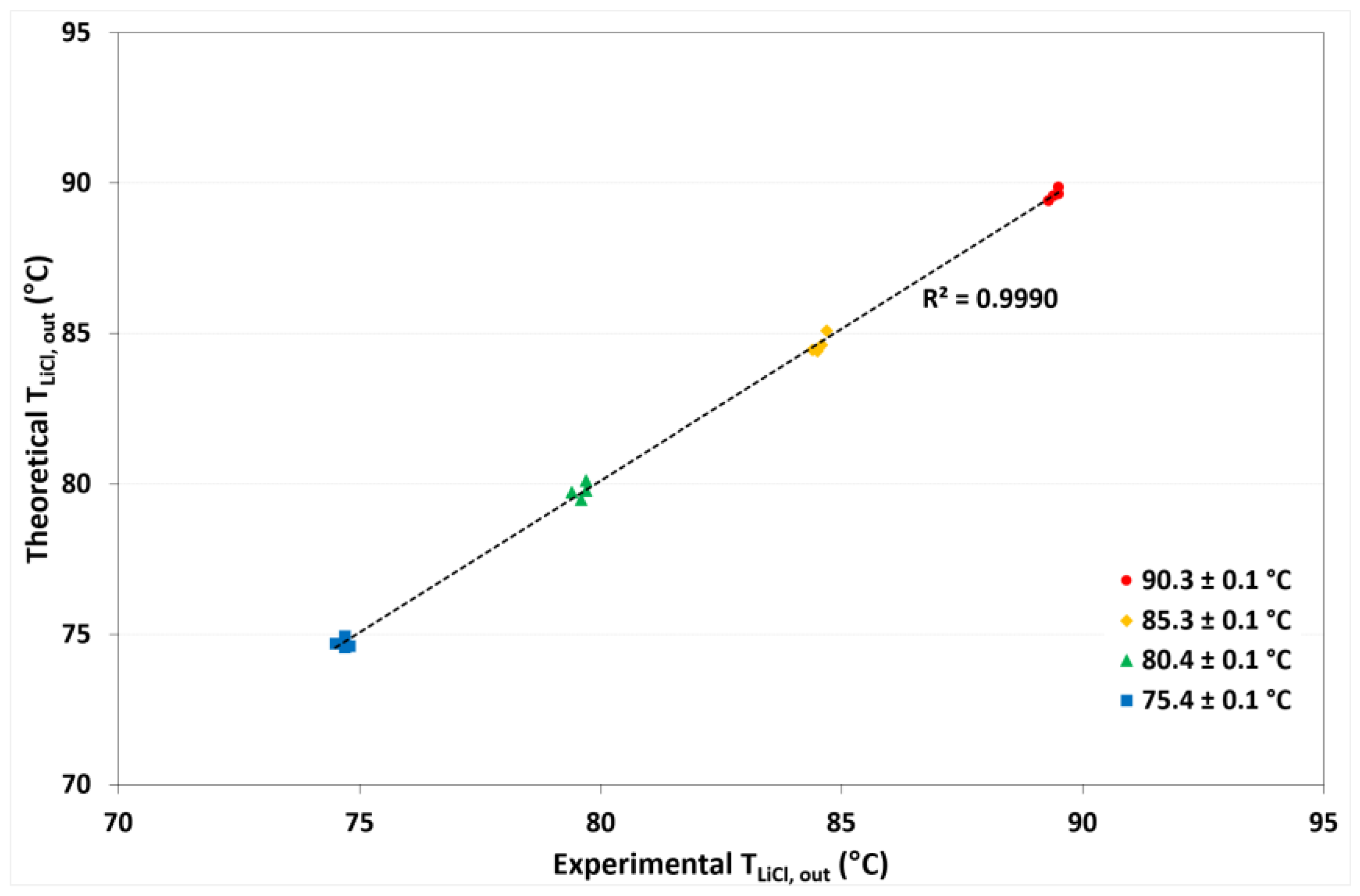
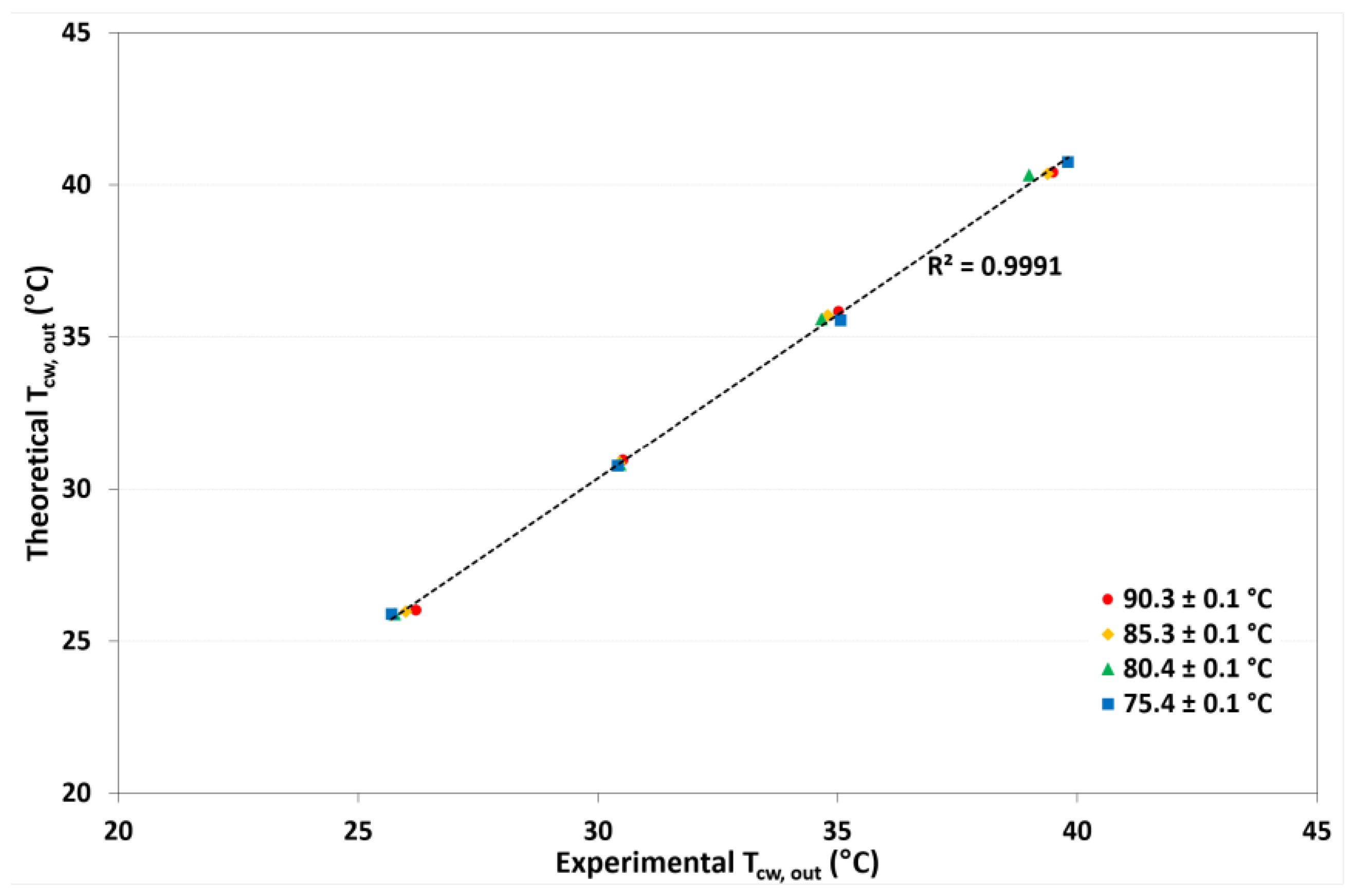
4.3. Model Validation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arshi Banu, P.S.; Sudharsan, N.M. Review of water based vapour absorption cooling systems using thermodynamic analysis. Renew. Sustain. Energy Rev. 2017, 82, 3750–3761. [Google Scholar] [CrossRef]
- Jian, S.; Lin, F.; Zhang, S. A review of working fluids of absorption cycles. Renew. Sustain. Energy Rev. 2012, 16, 1899–1906. [Google Scholar]
- Altamirano, A.; Le Pierrès, N.; Stutz, B. Review of small-capacity single-stage continuous absorption systems operating on binary working fluids for cooling: Theoretical, experimental and commercial cycles. Int. J. Refrig. 2019, 106, 350–373. [Google Scholar] [CrossRef]
- Gommed, K.; Grossman, G.; Ziegler, F. Experimental investigation of a LiCI-water open absorption system for cooling and dehumidification. J. Sol. Energy Eng. 2004, 126, 710–715. [Google Scholar] [CrossRef]
- Kim, K.; Ameel, T.; Wood, B. Performance evaluations of LiCl and LiBr for ab- sorber design applications in the open-cycle absorption refrigeration system. J. Sol. Energy Eng. 1997, 119, 165–173. [Google Scholar] [CrossRef]
- Ahmad, T.; Azhar, M.; Sinha, M.K.; Meraj, M.; Mahbubul, I.M.; Ahmad, A. Energy analysis of lithium bromide-water and lithium chloride-water based single effect vapour absorption refrigeration system: A comparison study. Clean. Eng. Technol. 2022, 7, 100432. [Google Scholar] [CrossRef]
- Saravanan, R.; Maiya, M.P. Thermodynamic comparison of water-based working fluid combinations for a vapour absorption refrigeration system. Appl. Therm. Eng. 1998, 18, 553–568. [Google Scholar] [CrossRef]
- Horuz, I. A comparison between ammonia-water and water-lithium bromide solutions in vapor absorption refrigeration systems. Int. Commun. Heat Mass Transf. 1998, 25, 711–721. [Google Scholar] [CrossRef]
- Barragán Reyes, R.M.; Arellano Gómez, V.M.; García-Gutiérrez, A. Performance modelling of single and double absorption heat transformers. Curr. Appl. Phys. 2010, 10, S244–S248. [Google Scholar] [CrossRef]
- Patel, J.; Pandya, B.; Mudgal, A. Exergy based analysis of LiCl-H2O absorption cooling system. Energy Procedia 2017, 109, 261–269. [Google Scholar] [CrossRef]
- Bhowmick, A.; Kundu, B. Thermo-economic optimization and comparison study of LiBr-H2O and LiCl-H2O working pair in absorption cooling systems based on genetic algorithm. Int. J. Energy Res. 2021, 45, 3938–3954. [Google Scholar]
- Bellos, E.; Tzivanidis, C.; Antonopoulos, K.A. Exergetic and energetic comparison of LiCl-H2O and LiBr-H2O working pairs in a solar absorption cooling system. Energy Convers. Manag. 2016, 123, 453–461. [Google Scholar] [CrossRef]
- Bellos, E.; Tzivanidis, C.; Pavlovic, S.; Stefanovic, V. Thermodynamic investigation of LiCl-H2O working pair in a double effect absorption chiller driven by parabolic trough collectors. Therm. Sci. Eng. Prog. 2017, 3, 75–87. [Google Scholar] [CrossRef]
- Labus, J.M.; Bruno, J.C.; Coronas, A. Review on absorption technology with emphasis on small capacity absorption machines. Therm. Sci. 2013, 17, 739–762. [Google Scholar] [CrossRef]
- Altamirano, A.; Stutz, B.; Le Pierrès, N. Review of small-capacity single-stage continuous absorption systems operating on binary working fluids for cooling: Compact exchanger technologies. Int. J. Refrig. 2020, 114, 118–147. [Google Scholar] [CrossRef]
- Yu, D.; Chung, J.; Moghaddam, S. Parametric study of water vapor absorption into a constrained thin film of lithium bromide solution. Int. J. Heat Mass Transf. 2012, 55, 5687–5695. [Google Scholar] [CrossRef]
- De Vega, M.; Venegas, M.; García-Hernando, N. Modeling and performance analysis of an absorption chiller with a microchannel membrane-based absorber using LiBr-H2O, LiCl-H2O, and LiNO3-NH3. Int. J. Energy Res. 2018, 42, 3544–3558. [Google Scholar] [CrossRef]
- Asfand, F.; Stiriba, Y.; Bourouis, M. Performance evaluation of membrane-based absorbers employing H2O/(LiBr + LiI + LiNO3 + LiCl) and H2O/(LiNO3 + KNO3 + NaNO3) as working pairs in absorption cooling systems. Energy 2016, 115, 781–790. [Google Scholar] [CrossRef]
- Yang, M.; Low, E.; Lim Law, C.; Chen, J.-C.; Show, P.L.; Huang, S.-M. Heat and mass transfer in a counter flow parallel plate membrane-based absorption heat pump (PMAHP). Int. J. Therm. Sci. 2022, 171, 107227. [Google Scholar] [CrossRef]
- Huang, S.-M. Coupled heat and mass transfer in a cross-flow hollow fiber membrane absorption heat pump (HFMAHP). Appl. Therm. Eng. 2017, 111, 1119–1128. [Google Scholar] [CrossRef]
- Huang, S.M. Heat and mass transfer in a quasi-counter flow parallel-plate membrane-based absorption heat pump (QPMAHP). J. Membr. Sci. 2015, 496, 39–47. [Google Scholar] [CrossRef]
- Woods, J.; Pellegrino, J.; Kozubal, E.; Burch, J. Design and experimental characterization of a membrane-based absorption heat pump. J. Membr. Sci. 2011, 378, 85–94. [Google Scholar] [CrossRef]
- Alsaadi, A.S.; Ghaffour, N.; Li, J.-D.; Gray, S.; Francis, L.; Maab, H.; Amy, G.L. Modeling of air gap membrane distillation process: A theoretical and experimental study. J. Membr. Sci. 2013, 445, 53–65. [Google Scholar] [CrossRef]
- Shahu, V.T.; Thombre, S.B. Air gap membrane distillation: A review. J. Renew. Sustain. Energy 2019, 11, 045901. [Google Scholar] [CrossRef]
- Ibarra-Bahena, J.; Rivera, W.; Nanco-Mejía, S.D.; Romero, R.J.; Venegas-Reyes, E.; Dehesa-Carrasco, U. Experimental performance of a membrane desorber operating under simulated warm weather condensation temperatures. Membranes 2021, 11, 447. [Google Scholar] [CrossRef]
- She, X.; Yin, Y.; Xu, M.; Zhang, X. A novel low-grade heat-driven absorption refrigeration system with LiCl–H2O and LiBr–H2O working pairs. Int. J. Refrig. 2015, 58, 219–234. [Google Scholar] [CrossRef]
- Ibarra-Bahena, J.; Dehesa-Carrasco, U.; Romero, R.J.; Rivas-Herrera, B.; Rivera, W. Experimental assessment of a hydrophobic membrane-based desorber/condenser with H2O/LiBr mixture for absorption systems. Exp. Therm. Fluid Sci. 2017, 88, 145–159. [Google Scholar] [CrossRef]
- Izquierdo-Gil, M.A.; Garcia-Payo, M.C.; Fernandez-Pineda, C. Air gap membrane distillation of sucrose aqueous solutions. J. Membr. Sci. 1999, 155, 291–307. [Google Scholar] [CrossRef]
- Dehesa-Carrasco, U.; Pérez-Rábago, C.A.; Arancibia-Bulnes, C.A. Experimental evaluation and modeling of internal temperatures in an air gap membrane distillation unit. Desalination 2013, 326, 47–54. [Google Scholar] [CrossRef]
- Shah, R.K.; London, A.L. Laminar Flow Forced Convection in Ducts: A Source Book for Compact Heat Exchanger Analytical Data. Advances in Heat Transfer; Academic Press: New York, NY, USA, 1978; Volume 1. [Google Scholar]
- Khayet, M. Membranes and theoretical modeling of membrane distillation: A review. Adv. Colloid Interface Sci. 2011, 164, 56–88. [Google Scholar] [CrossRef] [PubMed]
- Bird, R.B.; Stewart, W.E.; Lightfoot, E.N. Transport Phenomena; Wiley: New York, NY, USA, 1976. [Google Scholar]
- Woods, J.; Pellegrino, J.; Kozubal, E.; Slayzak, S.; Burch, J. Modeling of a membrane-based absorption heat pump. J. Membr. Sci. 2009, 337, 113–124. [Google Scholar] [CrossRef]
- Chaudhari, S.K.; Patel, K.R. Thermodynamic Properties of Aqueous Solutions of Lithium Chloride. Phys. Chem. Liquids 2002, 40, 317–325. [Google Scholar] [CrossRef]
- Wimby, J.M.; Berntsson, T.S. Viscosity and density of aqueous solutions of LiBr, LiCl, ZnBr2, CaCl2, and LiNO3. 1. Single salt solutions. J. Chem. Eng. Data 1994, 39, 68–72. [Google Scholar] [CrossRef]
- Zhai, C.; Wu, W. Experimental evaluation on heat/mass transfer and pressure drop of a microchannel membrane-based desorber for compact and efficient H2O/LiBr absorption refrigeration. Int. J. Heat Mass Tranf. 2022, 195, 123198. [Google Scholar] [CrossRef]
- de Vega, M.; Venegas, M.; García-Hernando, N. Viability on the desorption and air condensation of water in a compact membrane-based microchannel desorber-condenser for cooling applications. Energy Convers. Manag. 2022, 267, 115919. [Google Scholar] [CrossRef]
- He, J.; Hirata, R.; Hihara, E.; Dang, C. Desorption characteristic of LiBr–H2O solution in hydrophobic hollow fiber membrane for absorption chiller. Appl. Therm. Eng. 2021, 195, 117164. [Google Scholar] [CrossRef]
- Venegas, M.; García-Hernando, N.; De Vega, M. Experimental evaluation of a membrane-based microchannel desorber operating at low desorption temperatures. Appl. Therm. Eng. 2020, 167, 114781. [Google Scholar] [CrossRef]
- Ibarra-Bahena, J.; Venegas-Reyes, E.; Galindo-Luna, Y.R.; Rivera, W.; Romero, R.J.; Rodríguez-Martínez, A.; Dehesa-Carrasco, U. Feasibility Analysis of a Membrane Desorber Powered by Thermal Solar Energy for Absorption Cooling Systems. Appl. Sci. 2020, 10, 1110. [Google Scholar] [CrossRef]
- Hong, S.J.; Hihara, E.; Dang, C. Analysis of adiabatic heat and mass transfer of microporous hydrophobic hollow fiber membrane-based generator in vapor absorption refrigeration system. J. Membr. Sci. 2018, 564, 415–427. [Google Scholar] [CrossRef]
- Isfahani, R.N.; Fazeli, A.; Bigham, S.; Moghaddam, S. Physics of lithium bromide (LiBr) solution dewatering through vapor venting membranes. Int. J. Multiph. Flow 2014, 58, 27–38. [Google Scholar] [CrossRef]
- Bigham, S.; Isfahani, R.N.; Moghaddam, S. Direct molecular diffusion and micro-mixing for rapid dewatering of LiBr solution. Appl. Therm. Eng. 2014, 64, 371–375. [Google Scholar] [CrossRef]
- Wang, Z.; Gu, Z.; Feng, S.; Li, Y. Application of vacuum membrane distillation to lithium bromide absorption refrigeration system. Int. J. Refrig. 2009, 32, 1587–1596. [Google Scholar] [CrossRef]
- Sudoh, M.; Takuwa, K.; Iizuka, H.; Nagamatsuy, K. Effects of thermal and concentration boundary layers on vapour permeation in membrane distillation of aqueous lithium bromide solution. J. Membr. Sci. 1997, 131, 1–7. [Google Scholar] [CrossRef]
- Ibarra-Bahena, J.; Rivera, W.; Romero, R.J.; Montiel-González, M.; Dehesa-Carrasco, U. Novel intermittent absorption cooling system based on membrane separation process. Appl. Therm. Eng. 2018, 136, 718–729. [Google Scholar] [CrossRef]


| Material | PTFE (Polytetrafluoroethylene) |
|---|---|
| Mean pore diameter (dp) | 0.22 μm |
| Porosity (ε) | 70% |
| Effective area (Amem) | 144 cm2 |
| Thickness (δmem) | 175 μm |
| Variable | Sensor/Instrument | Operation Range | Uncertainty |
|---|---|---|---|
| Temperature | RTD PT100 | −30 to 350 °C | ±0.1 °C |
| Volumetric flow | Volumetric flowmeter | 0 to 7 L/min | ±5.0% f.s. * |
| Mass flow | Coriolis mass flowmeter | 0 to 4.0 × 10−2 kg/s | ±0.1% |
| Distillate water weight | Electronic balance | 0 to 600 g | ±0.01 g |
| Parameter | Value |
|---|---|
| LiCl concentration (% kg salt/kg solution) | 41.05 ± 0.03 |
| Cooling-water volumetric flow (L/min) | 2.0 ± 0.35 |
| H2O/LiCl solution mass flow (kg/s) | 3.50 × 10−2 ± 1.83 × 10−5 |
| H2O/LiCl solution temperature (°C) | 90.3 ± 0.1 |
| 85.3 ± 0.1 | |
| 80.4 ± 0.1 | |
| 75.4 ± 0.1 | |
| Cooling-water temperature (°C) | 40.1 ± 0.1 |
| 35.1 ± 0.1 | |
| 30.1 ± 0.1 | |
| 25.1 ± 0.1 |
| Parameter | Value |
|---|---|
| LiCl concentration (% kg salt/kg solution) | 40.98 ± 0.03 |
| Cooling-water volumetric flow (L/min) | 2.0 ± 0.35 |
| H2O/LiCl solution mass flow (kg/s) | 3.00 × 10−2 ± 4.90 × 10−5 |
| H2O/LiCl solution temperature (°C) | 90.3 ± 0.1 |
| 85.3 ± 0.1 | |
| 80.4 ± 0.1 | |
| 75.4 ± 0.1 | |
| Cooling-water temperature (°C) | 30.1 ± 0.1 |
| Reference | Configuration | dp (μm) | Working Mixture | X (% w/w) * | Tsol (°C) | TCon (°C) | ṁsol (kg/h) | Jw (kg/h·m2) |
|---|---|---|---|---|---|---|---|---|
| [36] | Flat sheet | 1.0 | H2O/LiBr | 50 to 60 | 65 to 85 | 40 | 15 to 50 | 14 to 72 |
| [37] | Flat sheet | 0.45 | H2O/LiBr | 43 | 70 to 90 | 37 to 47 | 2.4 to 3.6 | 7.2 to 14.4 |
| [38] | Hollow fiber | 0.2 to 0.4 | H2O/LiBr | 58 | 90 | 40 | 10 to 30 | 0.41 to 2.2 |
| [25] | Flat sheet | 0.22 | H2O/LiBr | 49.6 | 80 to 95 | 30 to 45 | 90 to 144 | 1.1 to 6.1 |
| [39] | Flat sheet | 0.45 | H2O/LiBr | 45.8 | 58 to 60 | 25.7 | 0.5 to 1.7 | 5.8 to 15.1 |
| [40] | Flat sheet | 0.22 | H2O/LiBr | 49.8 | 75.2 to 95.3 | 14.4 to 25.4 | 90.0 | 1.5 to 5.7 |
| [41] | Hollow fiber | 0.16 | H2O/LiBr | 51 to 58 | 65 to 83 | NA | 173 to 269 | 0.4 to 3.4 |
| [27] | Flat sheet | 0.45 | H2O/LiBr | 45.7 to 58.7 | 74.4 to 95.9 | 15.6 to 20.0 | 58.7 to 90.0 | 0.3 to 9.7 |
| [42] | Flat sheet | 0.45 | H2O/LiBr | 48 to 51 | 50 to 125 | NA | 0.75 to 3.25 | 0.0 to 37.8 |
| [43] | Flat sheet | 1.00 | H2O/LiBr | 48 | 50 to 125 | NA | 2.5 | 0.0 to 34.2 |
| [44] | Hollow fiber | 0.16 | H2O/LiBr | 50 | 65 to 88 | NA | 40 to 120 | 0.3 to 2.0 |
| [45] | Flat sheet | 0.20 | H2O/LiBr | 35 to 55 | 35 to 100 | 15 | NA | 1.8 to 18 |
| This work | Flat sheet | 0.22 | H2O/LiCl | 41 | 75 to 90 | 25 to 40 | 126 | 0.26 to 3.49 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibarra-Bahena, J.; Dehesa-Carrasco, U.; Galindo-Luna, Y.R.; Medina-Caballero, I.L.; Rivera, W. Experimental Performance of a Membrane Desorber with a H2O/LiCl Mixture for Absorption Chiller Applications. Membranes 2022, 12, 1184. https://doi.org/10.3390/membranes12121184
Ibarra-Bahena J, Dehesa-Carrasco U, Galindo-Luna YR, Medina-Caballero IL, Rivera W. Experimental Performance of a Membrane Desorber with a H2O/LiCl Mixture for Absorption Chiller Applications. Membranes. 2022; 12(12):1184. https://doi.org/10.3390/membranes12121184
Chicago/Turabian StyleIbarra-Bahena, Jonathan, Ulises Dehesa-Carrasco, Yuridiana Rocio Galindo-Luna, Iván Leonardo Medina-Caballero, and Wilfrido Rivera. 2022. "Experimental Performance of a Membrane Desorber with a H2O/LiCl Mixture for Absorption Chiller Applications" Membranes 12, no. 12: 1184. https://doi.org/10.3390/membranes12121184
APA StyleIbarra-Bahena, J., Dehesa-Carrasco, U., Galindo-Luna, Y. R., Medina-Caballero, I. L., & Rivera, W. (2022). Experimental Performance of a Membrane Desorber with a H2O/LiCl Mixture for Absorption Chiller Applications. Membranes, 12(12), 1184. https://doi.org/10.3390/membranes12121184








