On-Site Application of Solar-Activated Membrane (Cr–Mn-Doped TiO2@Graphene Oxide) for the Rapid Degradation of Toxic Textile Effluents
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Synthesis of Graphene Oxide (GO) and TiO2 Nanowire (NW)
2.3. Synthesis of Cr–Mn-Doped TiO2/Graphene Oxide Aerogels
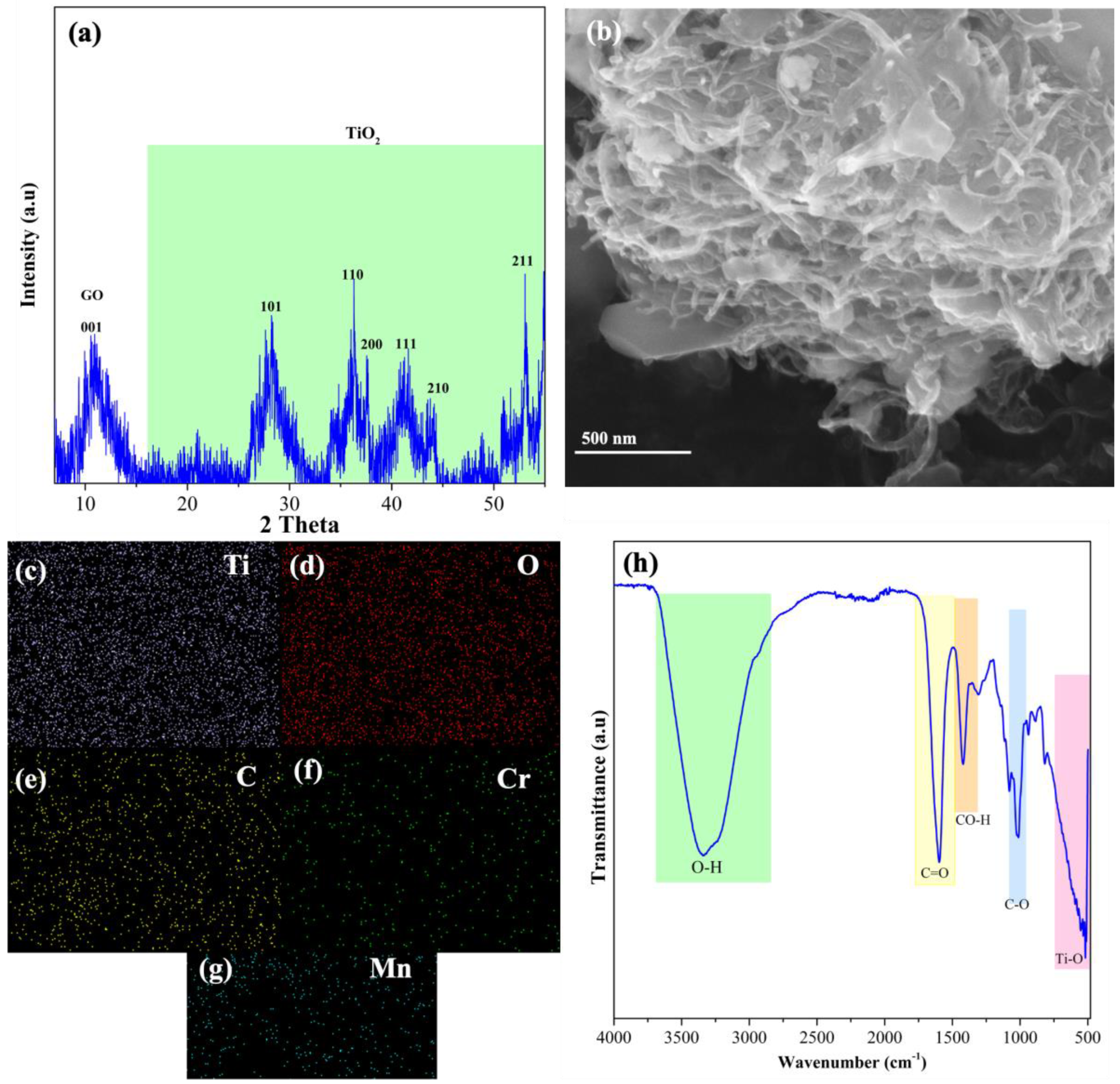
2.4. Characterization of Cr–Mn-Doped TiO2/Graphene Oxide Aerogels
2.5. Statistical Analysis
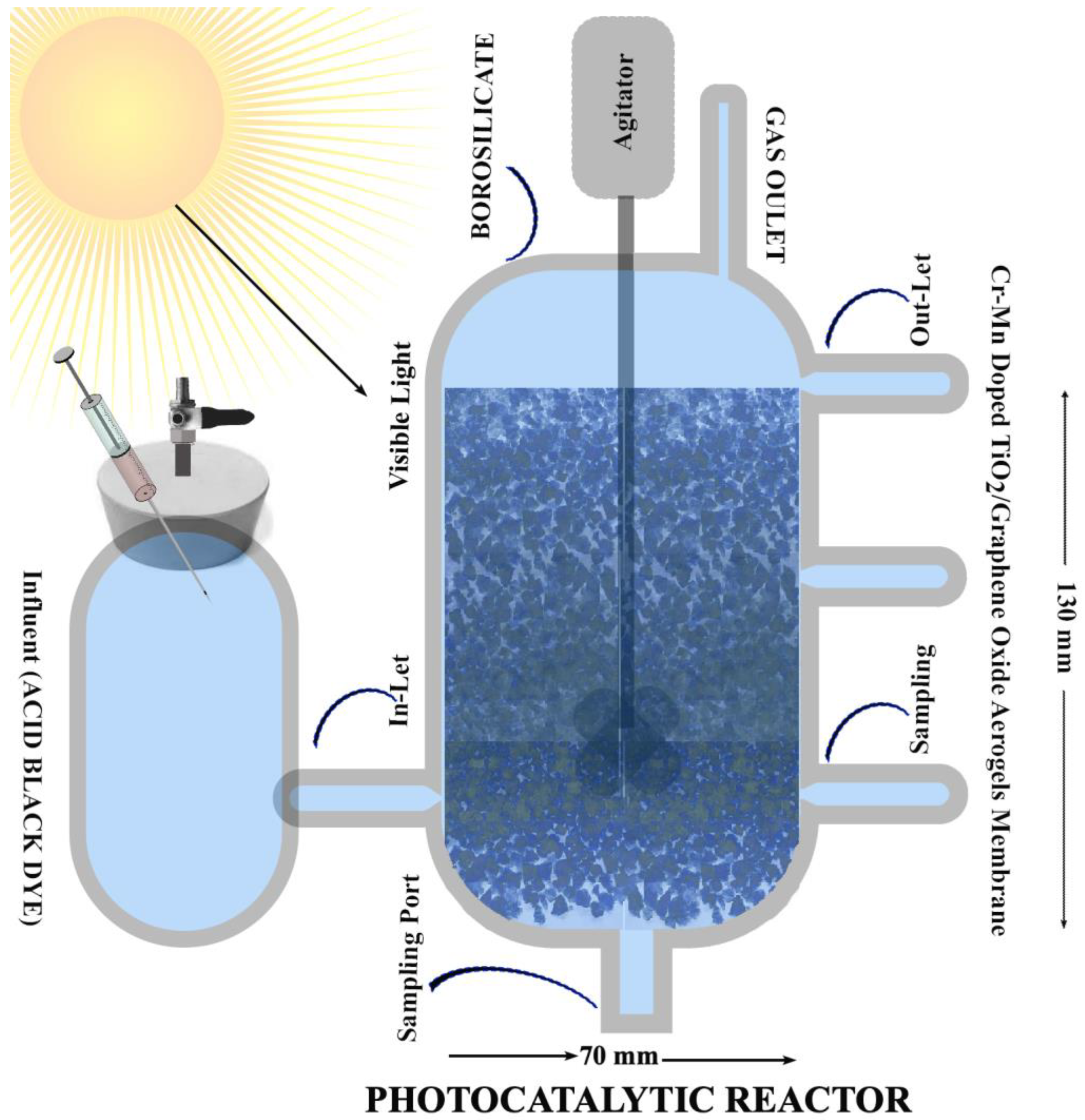
2.6. Photocatalytic Degradation Potential of Acid Black Dye by Cr–Mn-Doped TiO2 NW @GO Aerogels
3. Results and Discussion
3.1. Characterization of Cr–Mn-Doped TiO2 NW @GO Aerogel
3.2. Statistical Evaluation of Photo Catalytic Activity of Cr–Mn-Doped TiO2@GO Aerogel
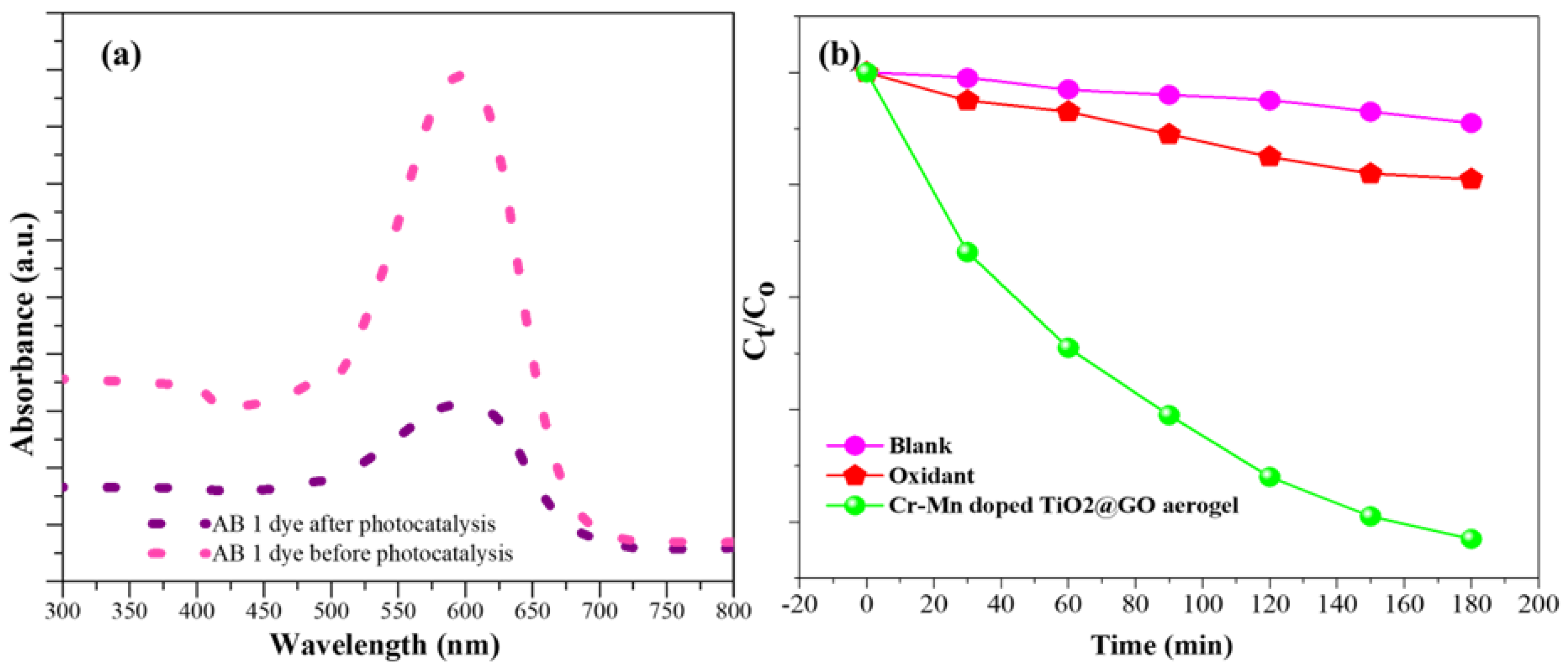
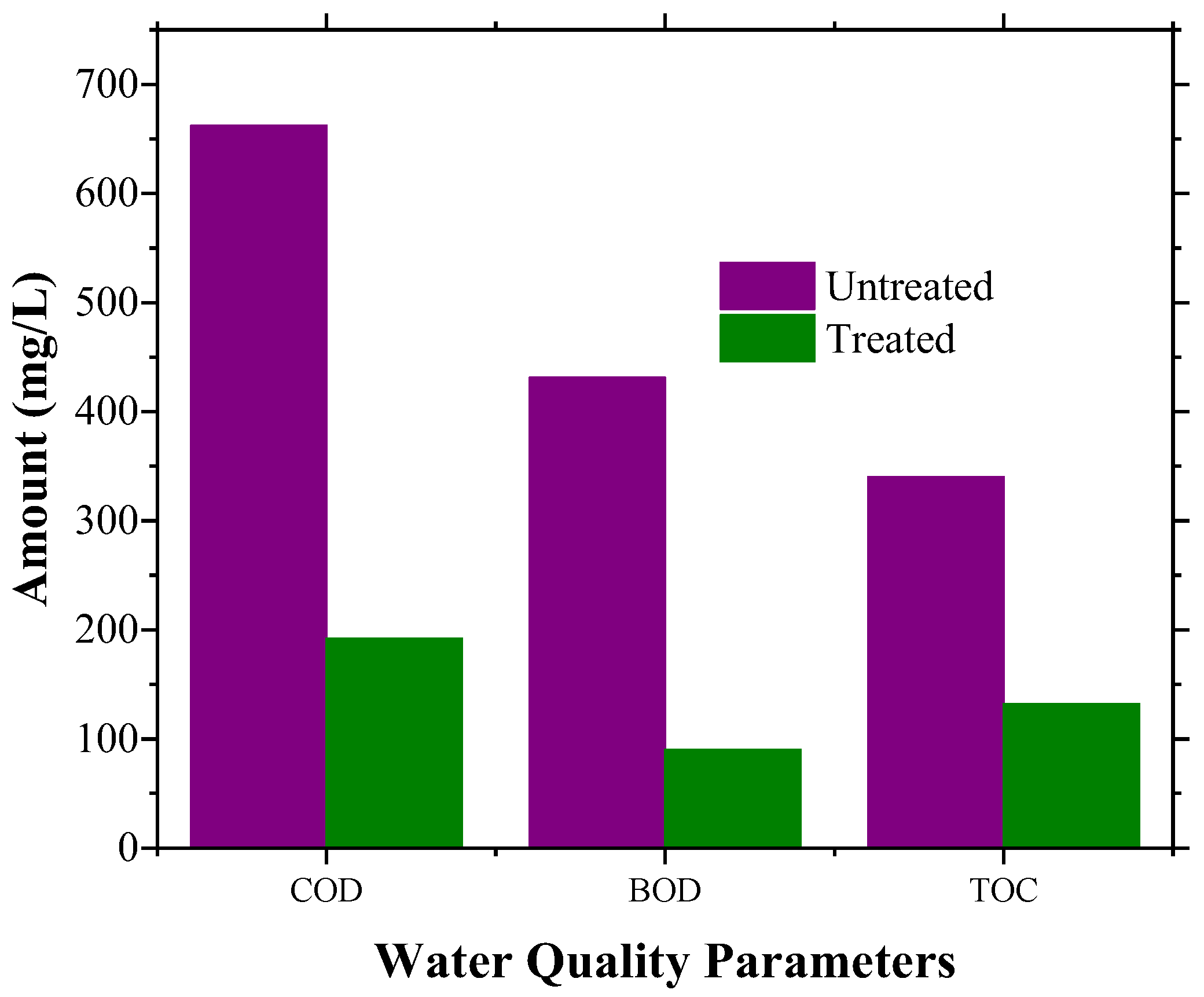
3.3. Evaluation of Extent of AB 1 Dye Degradation by Cr–Mn-Doped TiO2@GO Aerogel
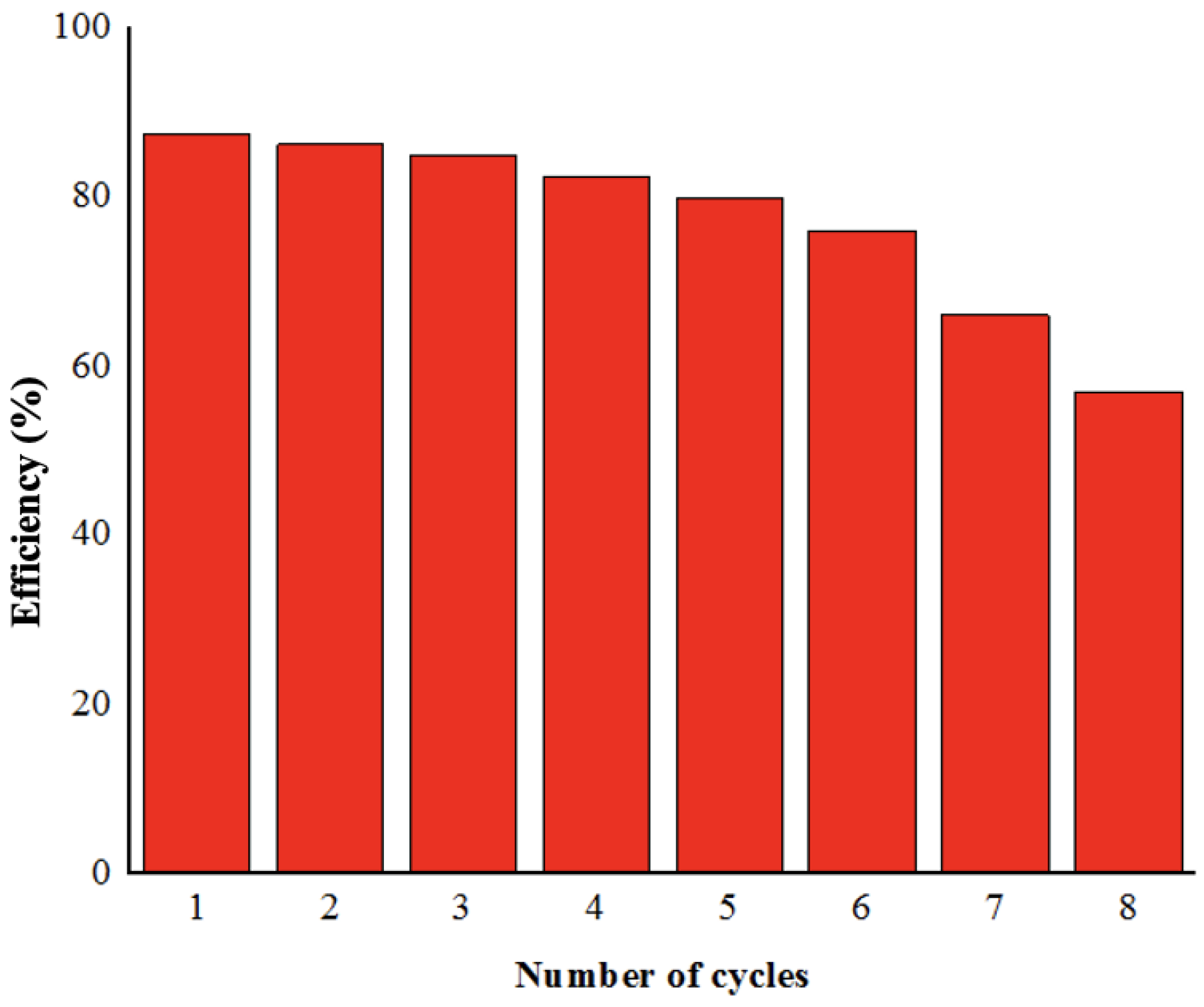
3.4. Reusability of Cr–Mn-Doped TiO2@GO Aerogel Photocatalytic Membrane
3.5. Mechanism of Degradation of AB 1 Dye by Cr–Mn-Doped TiO2@GO Aerogel
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aslam, M.M.; Kuo, H.-W.; Den, W.; Usman, M.; Sultan, M.; Ashraf, H. Functionalized Carbon Nanotubes (CNTs) for Water and Wastewater Treatment: Preparation to Application. Sustainability 2021, 13, 5717. [Google Scholar] [CrossRef]
- Usman, M.; Waseem, M.; Mani, N.; Andiego, G. Optimization of Soil Aquifer Treatment by Chemical Oxidation with Hydrogen Peroxide Addition. Pollution 2018, 4, 369–379. [Google Scholar]
- Benettayeb, A.; Ghosh, S.; Usman, M.; Seihoub, F.Z.; Sohoo, I.; Chia, C.H.; Sillanpää, M. Some Well-Known Alginate and Chitosan Modifications Used in Adsorption: A Review. Water 2022, 14, 1353. [Google Scholar] [CrossRef]
- Benettayeb, A.; Usman, M.; Tinashe, C.C.; Adam, T.; Haddou, B. A Critical Review with Emphasis on Recent Pieces of Evidence of Moringa Oleifera Biosorption in Water and Wastewater Treatment. Environ. Sci. Pollut. Res. 2022, 29, 48185–48209. [Google Scholar] [CrossRef]
- Khan, S.U.; Farooqi, I.H.; Usman, M.; Basheer, F. Energy Efficient Rapid Removal of Arsenic in an Electrocoagulation Reactor with Hybrid Fe/Al Electrodes: Process Optimization Using CCD and Kinetic Modeling. Water 2020, 12, 2876. [Google Scholar] [CrossRef]
- Alam, R.; Khan, S.U.; Usman, M.; Asif, M.; Farooqi, I.H. A Critical Review on Treatment of Saline Wastewater with Emphasis on Electrochemical Based Approaches. Process Saf. Environ. Prot. 2022, 158, 625–643. [Google Scholar] [CrossRef]
- Bai, B.; Bai, F.; Li, X.; Nie, Q.; Jia, X.; Wu, H. The Remediation Efficiency of Heavy Metal Pollutants in Water by Industrial Red Mud Particle Waste. Environ. Technol. Innov. 2022, 28, 102944. [Google Scholar] [CrossRef]
- Shen, G.; Pan, L.; Lü, Z.; Wang, C.; Aleem, F.; Zhang, X.; Zou, J.-J. Fe-TiO2 and Fe2O3 Quantum Dots Co-Loaded on MCM-41 for Removing Aqueous Rose Bengal by Combined Adsorption/Photocatalysis. Chinese J. Catal. 2018, 39, 920–928. [Google Scholar] [CrossRef]
- Ahmad, M.; Yousaf, M.; Nasir, A.; Bhatti, I.A.; Mahmood, A.; Fang, X.; Jian, X.; Kalantar-Zadeh, K.; Mahmood, N. Porous Eleocharis@MnPE Layered Hybrid for Synergistic Adsorption and Catalytic Biodegradation of Toxic Azo Dyes from Industrial Wastewater. Environ. Sci. Technol. 2019, 53, 2161–2170. [Google Scholar] [CrossRef]
- Pathania, D.; Thakur, M.; Mishra, A.K. Alginate-Zr (IV) Phosphate Nanocomposite Ion Exchanger: Binary Separation of Heavy Metals, Photocatalysis and Antimicrobial Activity. J. Alloys Compd. 2017, 701, 153–162. [Google Scholar] [CrossRef]
- Almeida, E.J.R.; Corso, C.R. Decolorization and Removal of Toxicity of Textile Azo Dyes Using Fungal Biomass Pelletized. Int. J. Environ. Sci. Technol. 2019, 16, 1319–1328. [Google Scholar] [CrossRef]
- Saeed, M.; Nadeem, R.; Yousaf, M. Removal of Industrial Pollutant (Reactive Orange 122 Dye) Using Environment-Friendly Sorbent Trapa Bispinosa’s Peel and Fruit. Int. J. Environ. Sci. Technol. 2015, 12, 1223–1234. [Google Scholar] [CrossRef]
- Chen, F.; Ma, J.; Zhu, Y.; Li, X.; Yu, H.; Sun, Y. Biodegradation Performance and Anti-Fouling Mechanism of an ICME/Electro-Biocarriers-MBR System in Livestock Wastewater (Antibiotic-Containing) Treatment. J. Hazard. Mater. 2022, 426, 128064. [Google Scholar] [CrossRef]
- Wang, Q.; Wu, S.; Cui, D.; Zhou, H.; Wu, D.; Pan, S.; Xu, F.; Wang, Z. Co-Hydrothermal Carbonization of Organic Solid Wastes to Hydrochar as Potential Fuel: A Review. Sci. Total Environ. 2022, 850, 158034. [Google Scholar] [CrossRef] [PubMed]
- Katheresan, V.; Kansedo, J.; Lau, S.Y. Efficiency of Various Recent Wastewater Dye Removal Methods: A Review. J. Environ. Chem. Eng. 2018, 6, 4676–4697. [Google Scholar] [CrossRef]
- Bal, G.; Thakur, A. Distinct Approaches of Removal of Dyes from Wastewater: A Review. Mater. Today Proc. 2022, 50, 1575–1579. [Google Scholar] [CrossRef]
- Liu, W.; Huang, F.; Liao, Y.; Zhang, J.; Ren, G.; Zhuang, Z.; Zhen, J.; Lin, Z.; Wang, C. Treatment of CrVI-Containing Mg(OH)2 Nanowaste. Angew. Chem. 2008, 120, 5701–5704. [Google Scholar] [CrossRef]
- Shabir, M.; Yasin, M.; Hussain, M.; Shafiq, I.; Akhter, P.; Nizami, A.-S.; Jeon, B.-H.; Park, Y.-K. A Review on Recent Advances in the Treatment of Dye-Polluted Wastewater. J. Ind. Eng. Chem. 2022, 112, 1–19. [Google Scholar] [CrossRef]
- Chang, S.-H.; Wang, K.-S.; Li, H.-C.; Wey, M.-Y.; Chou, J.-D. Enhancement of Rhodamine B Removal by Low-Cost Fly Ash Sorption with Fenton Pre-Oxidation. J. Hazard. Mater. 2009, 172, 1131–1136. [Google Scholar] [CrossRef]
- Liu, W.; Zheng, J.; Ou, X.; Liu, X.; Song, Y.; Tian, C.; Rong, W.; Shi, Z.; Dang, Z.; Lin, Z. Effective Extraction of Cr(VI) from Hazardous Gypsum Sludge via Controlling the Phase Transformation and Chromium Species. Environ. Sci. Technol. 2018, 52, 13336–13342. [Google Scholar] [CrossRef]
- Singh, R.; Rattan, G.; Singh, M.; Manne, R.; Oberoi, S.K.; Kaur, N. Advanced Oxidation Processes for Wastewater Treatment: Perspective Through Nanomaterials. In Environmental Science and Engineering, Proceedings of the International Conference on Chemical, Bio and Environmental Engineering, Jalandhar, India, 20–22 August 2021; Springer: Berlin/Heidelberg, Germany, 2022; pp. 57–68. [Google Scholar]
- Alemi, A.; Hanifehpour, Y.; Woo Joo, S.; Khandar, A.; Morsali, A.; Min, B.-K. Co-Reduction Synthesis of New LnxSb2−xS3 (Ln: Nd3+, Lu3+, Ho3+) Nanomaterials and Investigation of Their Physical Properties. Phys. B Condens. Matter 2011, 406, 2801–2806. [Google Scholar] [CrossRef]
- Wan, Z.; Zhang, T.; Liu, Y.; Liu, P.; Zhang, J.; Fang, L.; Sun, D. Enhancement of Desulfurization by Hydroxyl Ammonium Ionic Liquid Supported on Active Carbon. Environ. Res. 2022, 213, 113637. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Liu, J.; Shangguan, W. A Review on Photocatalysis in Antibiotic Wastewater: Pollutant Degradation and Hydrogen Production. Chinese J. Catal. 2020, 41, 1440–1450. [Google Scholar] [CrossRef]
- Zhang, Y.; Shaad, K.; Vollmer, D.; Ma, C. Treatment of Textile Wastewater Using Advanced Oxidation Processes—A Critical Review. Water 2021, 13, 3515. [Google Scholar] [CrossRef]
- Shahab-ud-Din; Ahmad, M.Z.; Qureshi, K.; Bhatti, I.A.; Zahid, M.; Nisar, J.; Iqbal, M.; Abbas, M. Hydrothermal Synthesis of Molybdenum Trioxide, Characterization and Photocatalytic Activity. Mater. Res. Bull. 2018, 100, 120–130. [Google Scholar] [CrossRef]
- Ahmad, M.; Yousaf, M.; Cai, W.; Zhao, Z.-P. Formulation of Heterometallic ZIF-8@Cu/Ni/ZnO@CNTs Heterostructure Photocatalyst for Ultra-Deep Desulphurization of Coal and Fuels. Chem. Eng. J. 2023, 453, 139846. [Google Scholar] [CrossRef]
- Yousaf, M.; Ahmad, M.; Zhao, Z.-P. Rapid and Highly Selective Conversion of CO2 to Methanol by Heterometallic Porous ZIF-8. J. CO2 Util. 2022, 64, 102172. [Google Scholar] [CrossRef]
- Liu, P.; Li, S.; Zhang, L.; Yin, X.; Ma, Y. Shearing Bridge Bonds in Carbon Nitride Vesicles with Enhanced Hot Carrier Utilization for Photocatalytic Hydrogen Production. Catal. Sci. Technol. 2022, 12, 4193–4200. [Google Scholar] [CrossRef]
- Feng, C.; Chen, Z.; Li, W.; Zhang, F.; Li, X.; Xu, L.; Sun, M. First-Principle Calculation of the Electronic Structures and Optical Properties of the Metallic and Nonmetallic Elements-Doped ZnO on the Basis of Photocatalysis. Phys. B Condens. Matter 2019, 555, 53–60. [Google Scholar] [CrossRef]
- Miklos, D.B.; Remy, C.; Jekel, M.; Linden, K.G.; Drewes, J.E.; Hübner, U. Evaluation of Advanced Oxidation Processes for Water and Wastewater Treatment—A Critical Review. Water Res. 2018, 139, 118–131. [Google Scholar] [CrossRef]
- Humayun, M.; Raziq, F.; Khan, A.; Luo, W. Modification Strategies of TiO2 for Potential Applications in Photocatalysis: A Critical Review. Green Chem. Lett. Rev. 2018, 11, 86–102. [Google Scholar] [CrossRef]
- Li, G.; Zou, B.; Feng, S.; Shi, H.; Liao, K.; Wang, Y.; Wang, W.; Zhang, G. Synthesis of N-Doped TiO2 with Good Photocatalytic Property. Phys. B Condens. Matter 2020, 588, 412184. [Google Scholar] [CrossRef]
- Serga, V.; Burve, R.; Krumina, A.; Pankratova, V.; Popov, A.I.; Pankratov, V. Study of Phase Composition, Photocatalytic Activity, and Photoluminescence of TiO2 with Eu Additive Produced by the Extraction-Pyrolytic Method. J. Mater. Res. Technol. 2021, 13, 2350–2360. [Google Scholar] [CrossRef]
- Ajmal, A.; Majeed, I.; Malik, R.N.; Idriss, H.; Nadeem, M.A. Principles and Mechanisms of Photocatalytic Dye Degradation on TiO2 Based Photocatalysts: A Comparative Overview. RSC Adv. 2014, 4, 37003–37026. [Google Scholar] [CrossRef]
- Tian, B.; Li, C.; Zhang, J. One-Step Preparation, Characterization and Visible-Light Photocatalytic Activity of Cr-Doped TiO2 with Anatase and Rutile Bicrystalline Phases. Chem. Eng. J. 2012, 191, 402–409. [Google Scholar] [CrossRef]
- Thirupathi, B.; Smirniotis, P.G. Co-Doping a Metal (Cr, Fe, Co, Ni, Cu, Zn, Ce, and Zr) on Mn/TiO2 Catalyst and Its Effect on the Selective Reduction of NO with NH3 at Low-Temperatures. Appl. Catal. B Environ. 2011, 110, 195–206. [Google Scholar] [CrossRef]
- Serga, V.; Burve, R.; Krumina, A.; Romanova, M.; Kotomin, E.A.; Popov, A.I. Extraction–Pyrolytic Method for TiO2 Polymorphs Production. Crystals 2021, 11, 431. [Google Scholar] [CrossRef]
- Tsebriienko, T.; Popov, A.I. Effect of Poly(Titanium Oxide) on the Viscoelastic and Thermophysical Properties of Interpenetrating Polymer Networks. Crystals 2021, 11, 794. [Google Scholar] [CrossRef]
- Khan, I.; Chu, X.; Liu, Y.; Khan, S.; Bai, L.; Jing, L. Synthesis of Ni2+ Cation Modified TS-1 Molecular Sieve Nanosheets as Effective Photocatalysts for Alcohol Oxidation and Pollutant Degradation. Chinese J. Catal. 2020, 41, 1589–1602. [Google Scholar] [CrossRef]
- Kuilla, T.; Bhadra, S.; Yao, D.; Kim, N.H.; Bose, S.; Lee, J.H. Recent Advances in Graphene Based Polymer Composites. Prog. Polym. Sci. 2010, 35, 1350–1375. [Google Scholar] [CrossRef]
- Geim, A.K.; Novoselov, K.S. The Rise of Graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Qi, L.; Ran, J.; Yu, J.; Qiao, S.Z. Ternary NiS/ZnxCd1-XS/Reduced Graphene Oxide Nanocomposites for Enhanced Solar Photocatalytic H2-Production Activity. Adv. Energy Mater. 2014, 4, 1301925. [Google Scholar] [CrossRef]
- Wu, R.; Tan, Y.; Meng, F.; Zhang, Y.; Huang, Y.-X. PVDF/MAF-4 Composite Membrane for High Flux and Scaling-Resistant Membrane Distillation. Desalination 2022, 540, 116013. [Google Scholar] [CrossRef]
- Tan, Z.; Dong, B.; Xing, M.; Sun, X.; Xi, B.; Dai, W.; He, C.; Luo, Y.; Huang, Y. Electric Field Applications Enhance the Electron Transfer Capacity of Dissolved Organic Matter in Sludge Compost. Environ. Technol. 2022, 1–11. [Google Scholar] [CrossRef]
- Ge, D.; Yuan, H.; Xiao, J.; Zhu, N. Insight into the Enhanced Sludge Dewaterability by Tannic Acid Conditioning and PH Regulation. Sci. Total Environ. 2019, 679, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Lu, K.; Hardison, A.K.; Liu, Z.; Xu, X.; Gao, D.; Gong, J.; Gardner, W.S. Membrane Inlet Mass Spectrometry Method (REOX/MIMS) to Measure 15N-Nitrate in Isotope-Enrichment Experiments. Ecol. Indic. 2021, 126, 107639. [Google Scholar] [CrossRef]
- Dong, Y.; Shao, J.; Chen, C.; Li, H.; Wang, R.; Chi, Y.; Lin, X.; Chen, G. Blue Luminescent Graphene Quantum Dots and Graphene Oxide Prepared by Tuning the Carbonization Degree of Citric Acid. Carbon N. Y. 2012, 50, 4738–4743. [Google Scholar] [CrossRef]
- Liu, B.; Chen, H.M.; Liu, C.; Andrews, S.C.; Hahn, C.; Yang, P. Large-Scale Synthesis of Transition-Metal-Doped TiO2 Nanowires with Controllable Overpotential. J. Am. Chem. Soc. 2013, 135, 9995–9998. [Google Scholar] [CrossRef]
- Zhang, H.; Ran, X.; Wu, X.; Zhang, D. Evaluation of Electro-Oxidation of Biologically Treated Landfill Leachate Using Response Surface Methodology. J. Hazard. Mater. 2011, 188, 261–268. [Google Scholar] [CrossRef]
- El-Desoky, M.M.; Morad, I.; Wasfy, M.H.; Mansour, A.F. Synthesis, Structural and Electrical Properties of PVA/TiO2 Nanocomposite Films with Different TiO2 Phases Prepared by Sol–Gel Technique. J. Mater. Sci. Mater. Electron. 2020, 31, 17574–17584. [Google Scholar] [CrossRef]
- Yasin, G.; Arif, M.; Shakeel, M.; Dun, Y.; Zuo, Y.; Khan, W.Q.; Tang, Y.; Khan, A.; Nadeem, M. Exploring the Nickel–Graphene Nanocomposite Coatings for Superior Corrosion Resistance: Manipulating the Effect of Deposition Current Density on Its Morphology, Mechanical Properties, and Erosion-Corrosion Performance. Adv. Eng. Mater. 2018, 20, 1701166. [Google Scholar] [CrossRef]
- Yousaf, M.; Ahmad, M.; Batool, A.; Zhao, Z.-P. Highly-Stable, Bifunctional, Binder-Free & Stand-Alone Photoelectrode (FexNi1-XO@a-CC) for Natural Waters Splitting into Hydrogen. Int. J. Hydrogen Energy 2022, 47, 36032–36045. [Google Scholar] [CrossRef]
- Mohsin, M.; Bhatti, I.A.; Ashar, A.; Khan, M.W.; Farooq, M.U.; Khan, H.; Hussain, M.T.; Loomba, S.; Mohiuddin, M.; Zavabeti, A.; et al. Iron-Doped Zinc Oxide for Photocatalyzed Degradation of Humic Acid from Municipal Wastewater. Appl. Mater. Today 2021, 23, 101047. [Google Scholar] [CrossRef]
- Tan, Z.; Zhu, H.; He, X.; Xi, B.; Tian, Y.; Sun, X.; Zhang, H.; Ouche, Q. Effect of Ventilation Quantity on Electron Transfer Capacity and Spectral Characteristics of Humic Substances during Sludge Composting. Environ. Sci. Pollut. Res. 2022, 29, 70269–70284. [Google Scholar] [CrossRef]
- Mbarek, W.B.; Azabou, M.; Pineda, E.; Fiol, N.; Escoda, L.; Suñol, J.J.; Khitouni, M. Rapid Degradation of Azo-Dye Using Mn–Al Powders Produced by Ball-Milling. RSC Adv. 2017, 7, 12620–12628. [Google Scholar] [CrossRef]
- Yang, J.; Chen, C.; Ji, H.; Ma, W.; Zhao, J. Mechanism of TiO2-Assisted Photocatalytic Degradation of Dyes under Visible Irradiation: Photoelectrocatalytic Study by TiO2-Film Electrodes. J. Phys. Chem. B 2005, 109, 21900–21907. [Google Scholar] [CrossRef]
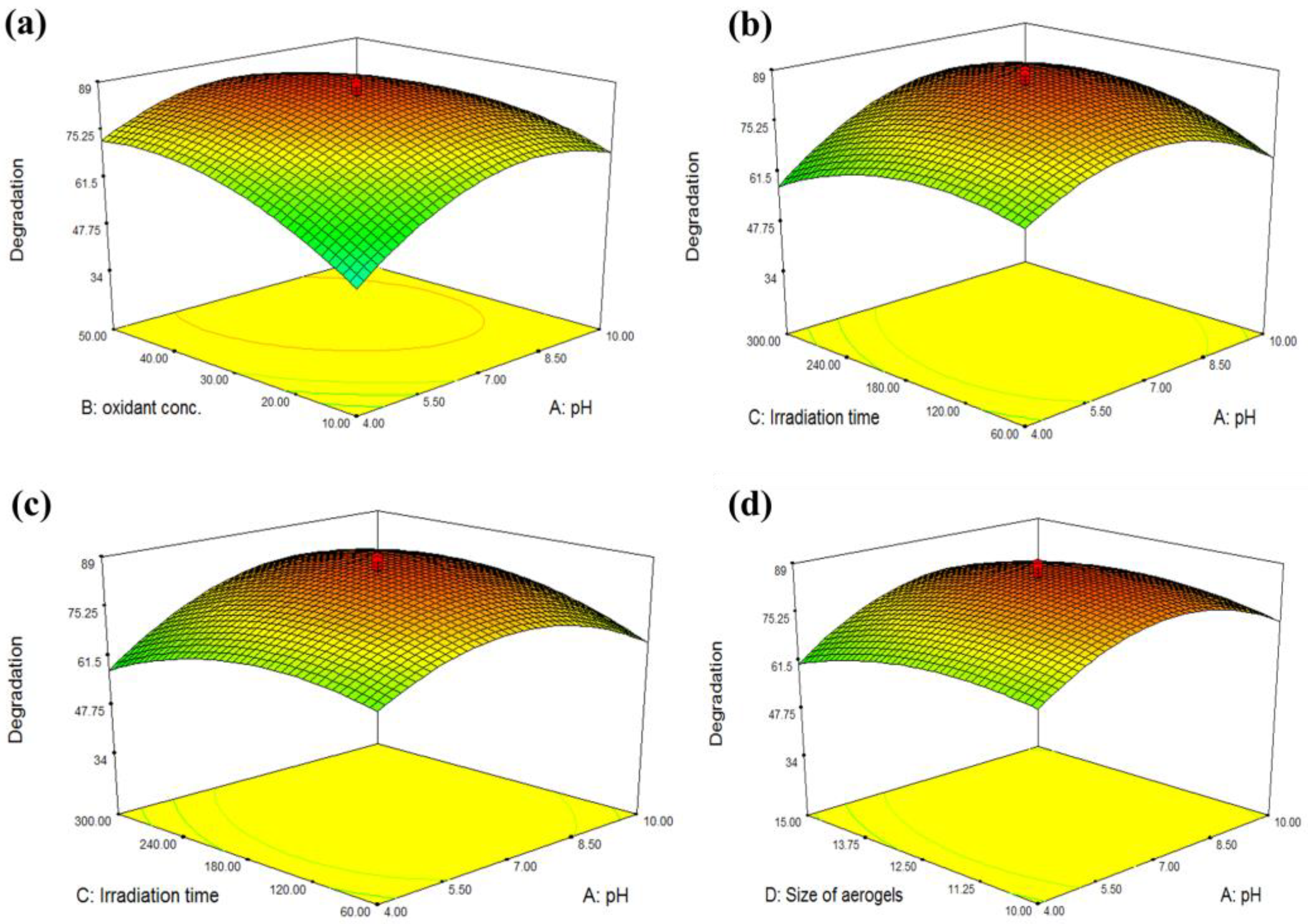

| Factor | Variables | Unit | Low Actual | High Actual |
|---|---|---|---|---|
| A | pH | 4 | 10 | |
| B | Oxidant concentration | mmol | 10 | 50 |
| C | Irradiation Time | min | 60 | 300 |
| D | Size of aerogels | mm | 10 | 15 |
| Sr. No | TiO2 NW | Cr–Mn-Doped TiO2 NW | Cr–Mn-Doped TiO2 NW @GO Aerogel | |
|---|---|---|---|---|
| 1 | SBET (m2/g) | 46.2 | 79.3 | 280.2 |
| 2 | Pore volume (cm3/g) | 0.135 | 0.216 | 0.35 |
| Run | A | B | C | D | Degradation (%) |
|---|---|---|---|---|---|
| 1 | 7 | 70 | 180 | 12.50 | 70 |
| 2 | 4 | 10 | 300 | 15 | 42 |
| 3 | 13 | 30 | 180 | 12.50 | 50 |
| 4 | 7 | 30 | 180 | 7.50 | 76 |
| 5 | 7 | 30 | 180 | 12.50 | 89 |
| 6 | 4 | 50 | 60 | 15 | 59 |
| 7 | 10 | 10 | 300 | 10 | 58 |
| 8 | 7 | 7 | 60 | 12.50 | 55 |
| 9 | 4 | 10 | 60 | 15 | 32 |
| 10 | 10 | 10 | 60 | 10 | 51 |
| 11 | 10 | 10 | 300 | 10 | 62 |
| 12 | 10 | 10 | 60 | 15 | 43 |
| 13 | 10 | 50 | 300 | 15 | 56 |
| 14 | 10 | 50 | 60 | 15 | 58 |
| 15 | 7 | 30 | 160 | 10 | 87 |
| 16 | 10 | 30 | 180 | 10 | 58 |
| 17 | 7 | 30 | 60 | 12.50 | 82 |
| 18 | 7 | 30 | 180 | 7 | 78 |
| 19 | 7 | 30 | 300 | 12.50 | 63 |
| 20 | 7 | 10 | 420 | 15 | 46 |
| 21 | 4 | 50 | 180 | 10 | 50 |
| 22 | 4 | 50 | 60 | 15 | 70 |
| 23 | 7 | 30 | 300 | 12.50 | 87 |
| 24 | 7 | 30 | 300 | 12.50 | 86 |
| 25 | 10 | 10 | 60 | 10 | 89 |
| 26 | 4 | 50 | 80 | 10 | 49 |
| 27 | 1 | 30 | 300 | 10 | 34 |
| 28 | 4 | 10 | 60 | 15 | 30.2 |
| 29 | 7 | 30 | 60 | 12.50 | 60.4 |
| 30 | 4 | 10 | 180 | 15.23 | 41.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yousaf, M.; Akram, M.; Bhatti, I.A.; Ahmad, M.; Usman, M.; Khan, M.U.; Sarwar, A.; Sultan, M.; Sohoo, I. On-Site Application of Solar-Activated Membrane (Cr–Mn-Doped TiO2@Graphene Oxide) for the Rapid Degradation of Toxic Textile Effluents. Membranes 2022, 12, 1178. https://doi.org/10.3390/membranes12121178
Yousaf M, Akram M, Bhatti IA, Ahmad M, Usman M, Khan MU, Sarwar A, Sultan M, Sohoo I. On-Site Application of Solar-Activated Membrane (Cr–Mn-Doped TiO2@Graphene Oxide) for the Rapid Degradation of Toxic Textile Effluents. Membranes. 2022; 12(12):1178. https://doi.org/10.3390/membranes12121178
Chicago/Turabian StyleYousaf, Maryam, Mariam Akram, Ijaz Ahmad Bhatti, Muhammad Ahmad, Muhammad Usman, Muhammad Usman Khan, Abid Sarwar, Muhammad Sultan, and Ihsanullah Sohoo. 2022. "On-Site Application of Solar-Activated Membrane (Cr–Mn-Doped TiO2@Graphene Oxide) for the Rapid Degradation of Toxic Textile Effluents" Membranes 12, no. 12: 1178. https://doi.org/10.3390/membranes12121178
APA StyleYousaf, M., Akram, M., Bhatti, I. A., Ahmad, M., Usman, M., Khan, M. U., Sarwar, A., Sultan, M., & Sohoo, I. (2022). On-Site Application of Solar-Activated Membrane (Cr–Mn-Doped TiO2@Graphene Oxide) for the Rapid Degradation of Toxic Textile Effluents. Membranes, 12(12), 1178. https://doi.org/10.3390/membranes12121178












