Influence of Membrane Fouling and Reverse Salt Flux on Membrane Impedance of Forward Osmosis Microbial Fuel Cell
Abstract
1. Introduction
2. Materials and Methods
2.1. Ion Diffusion Experiment
2.2. Calculation of Reverse Salt Flux
2.3. Proton Flux Calculation
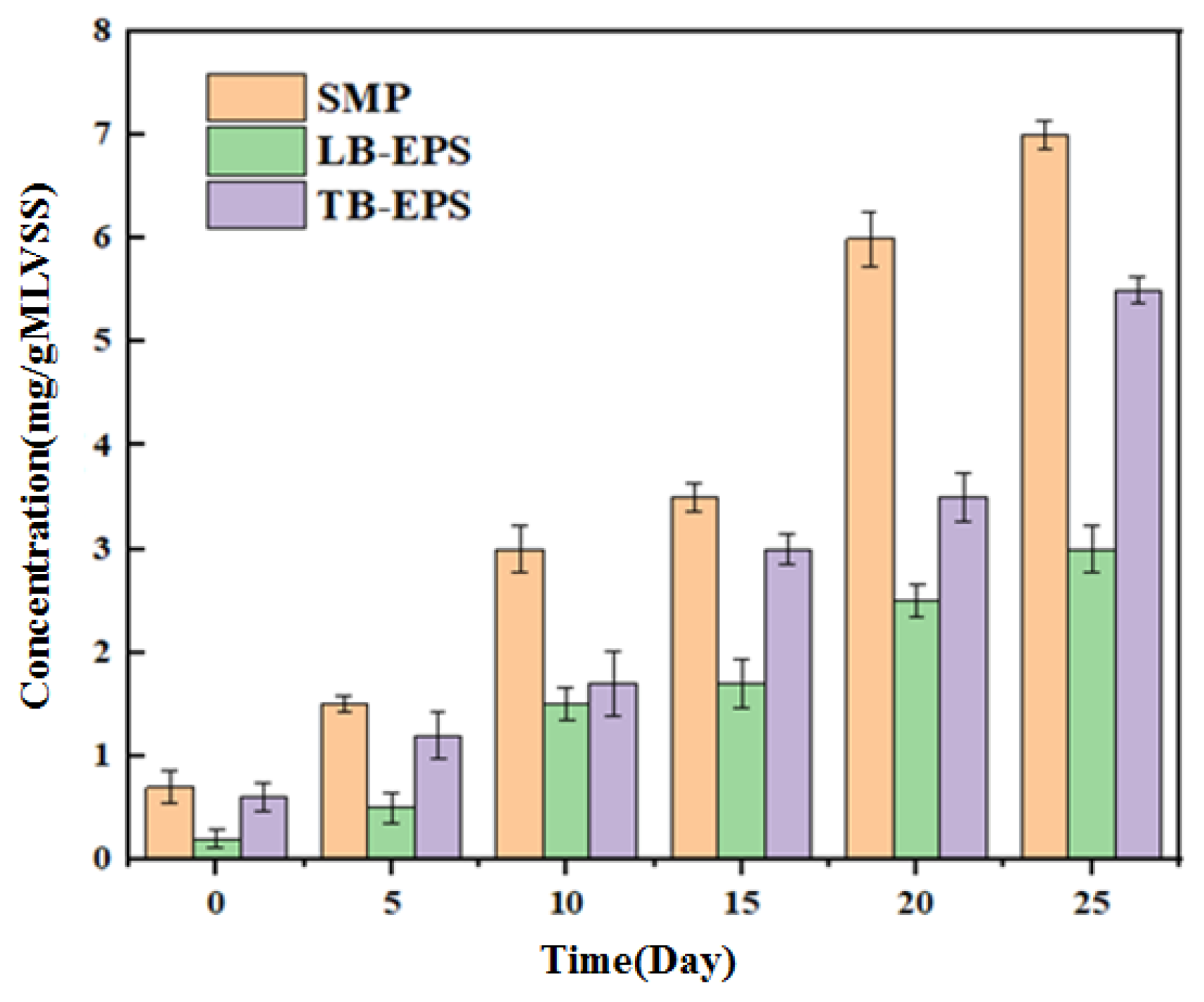
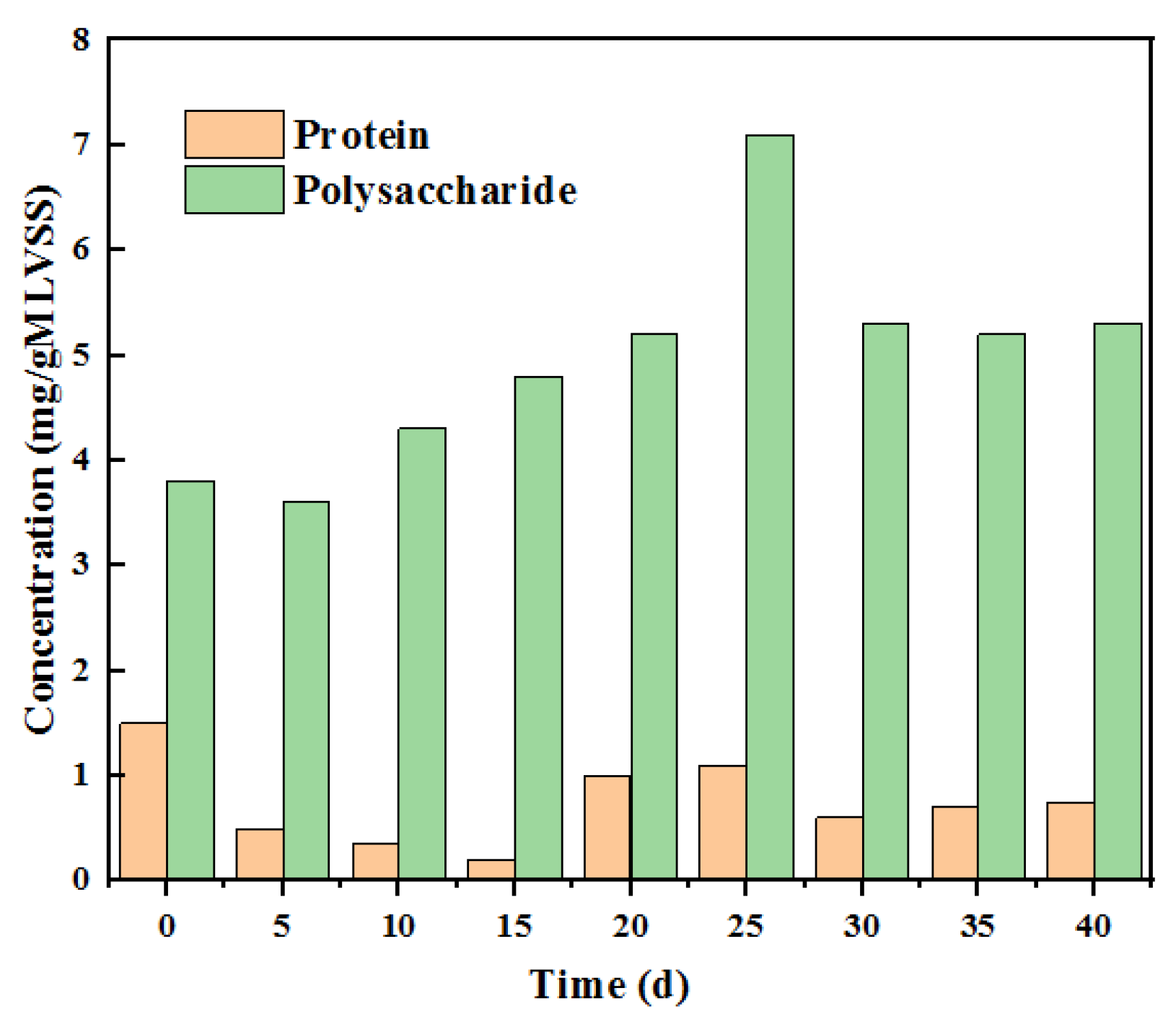
2.4. SMP and EPS Analyses
3. Results and Discussion
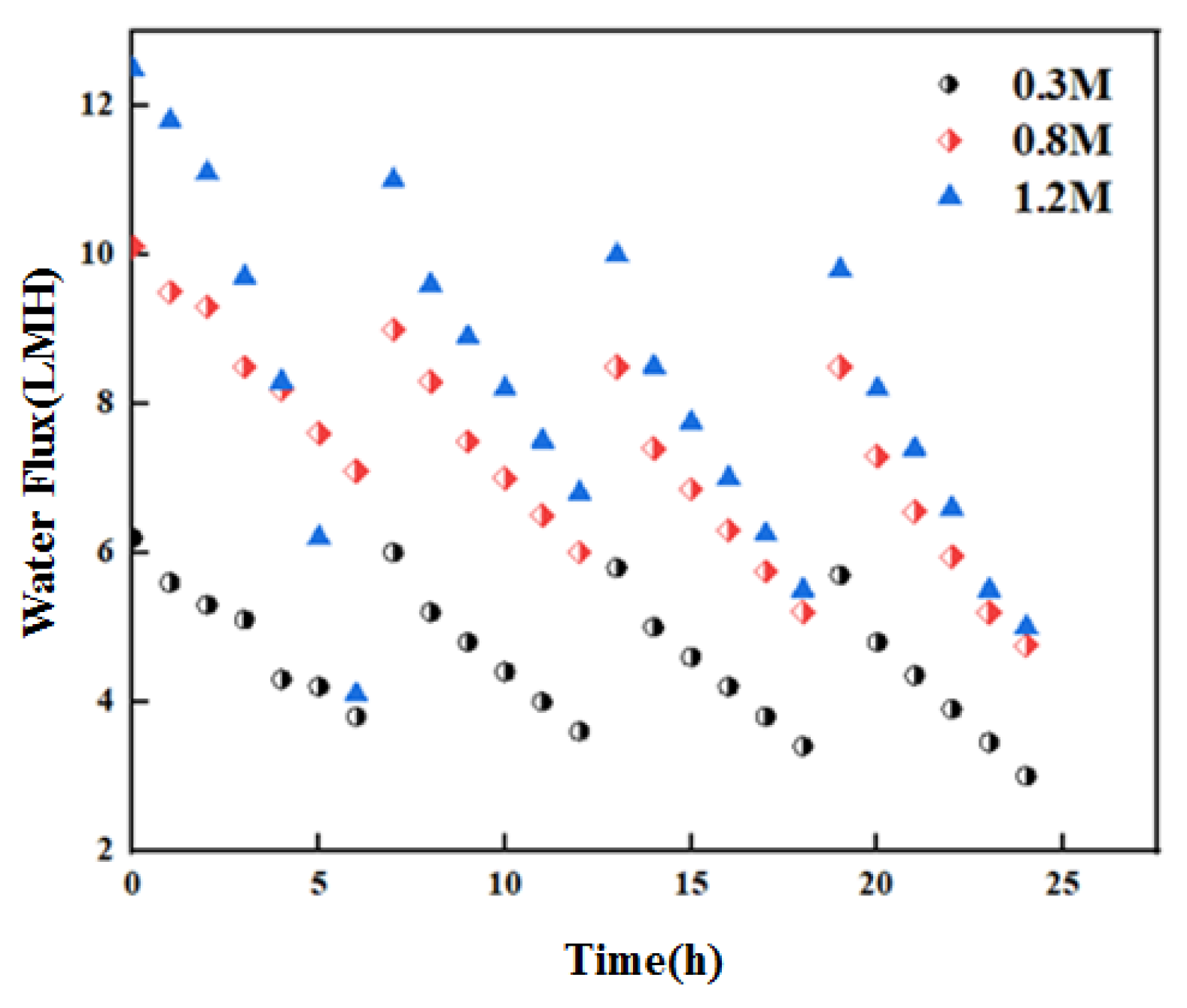
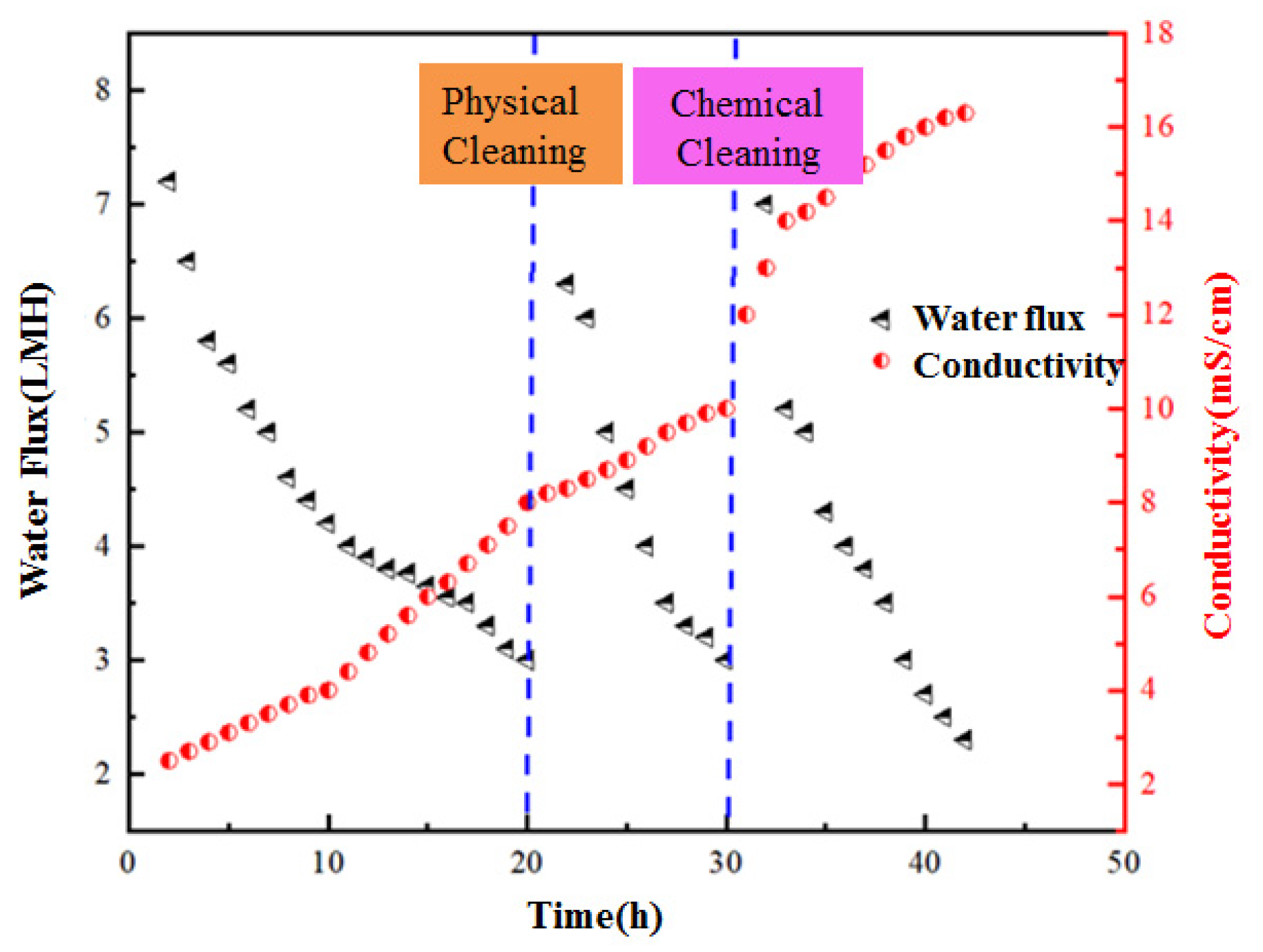
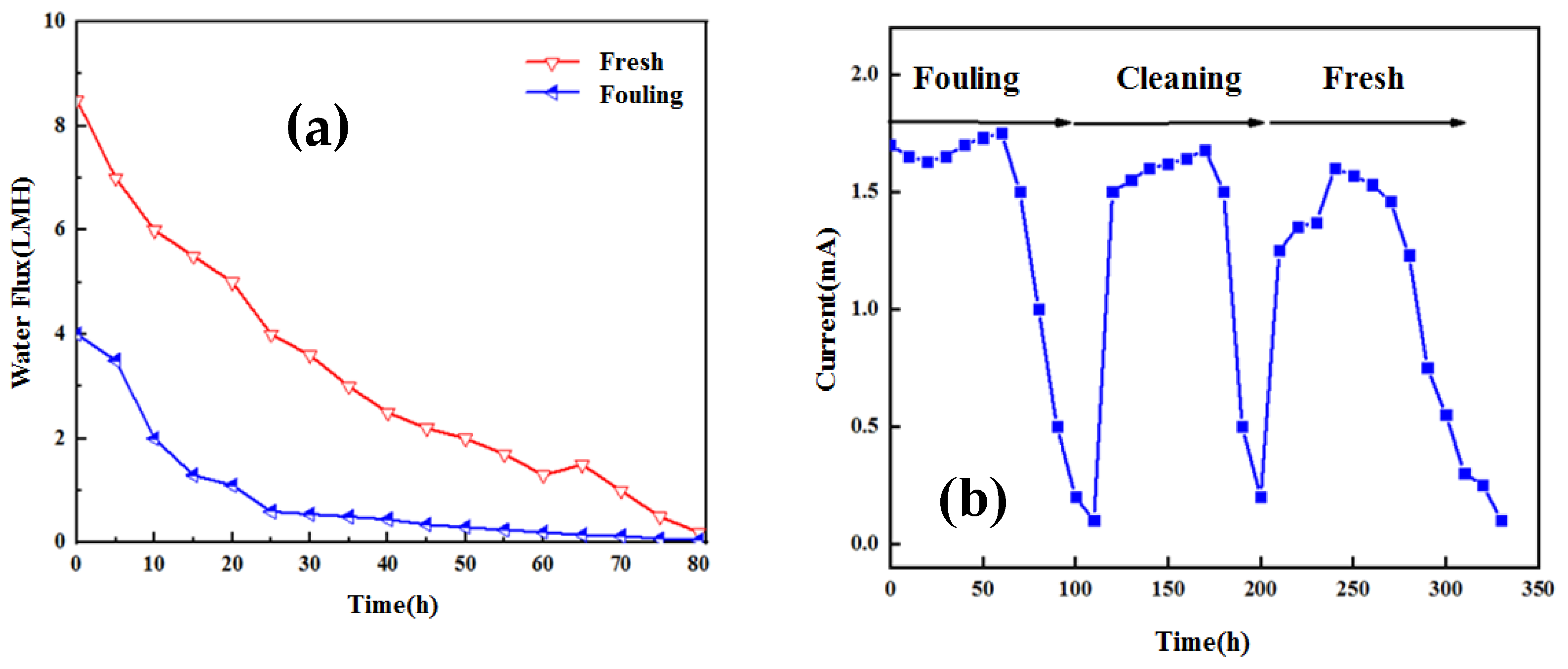
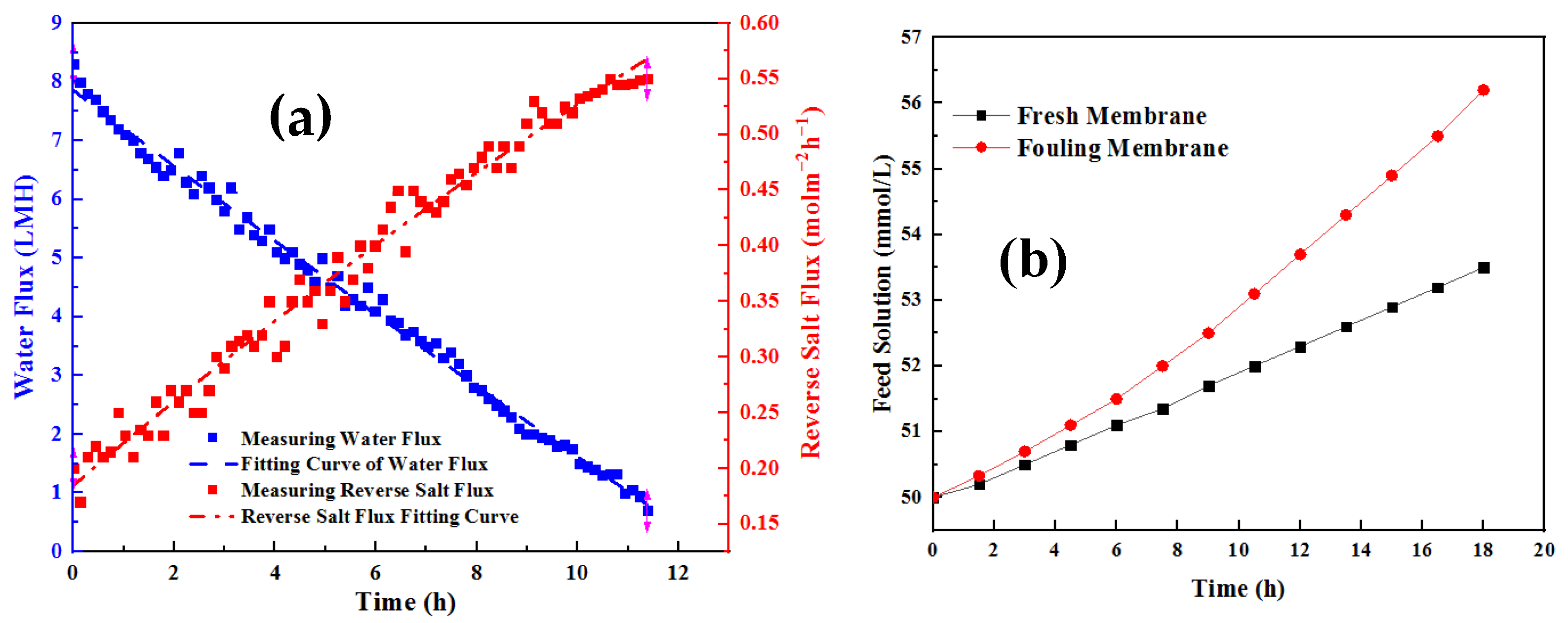
3.1. Water Flux with Long-Term Operation of OsMFC
3.2. Characterization of Anolyte Components
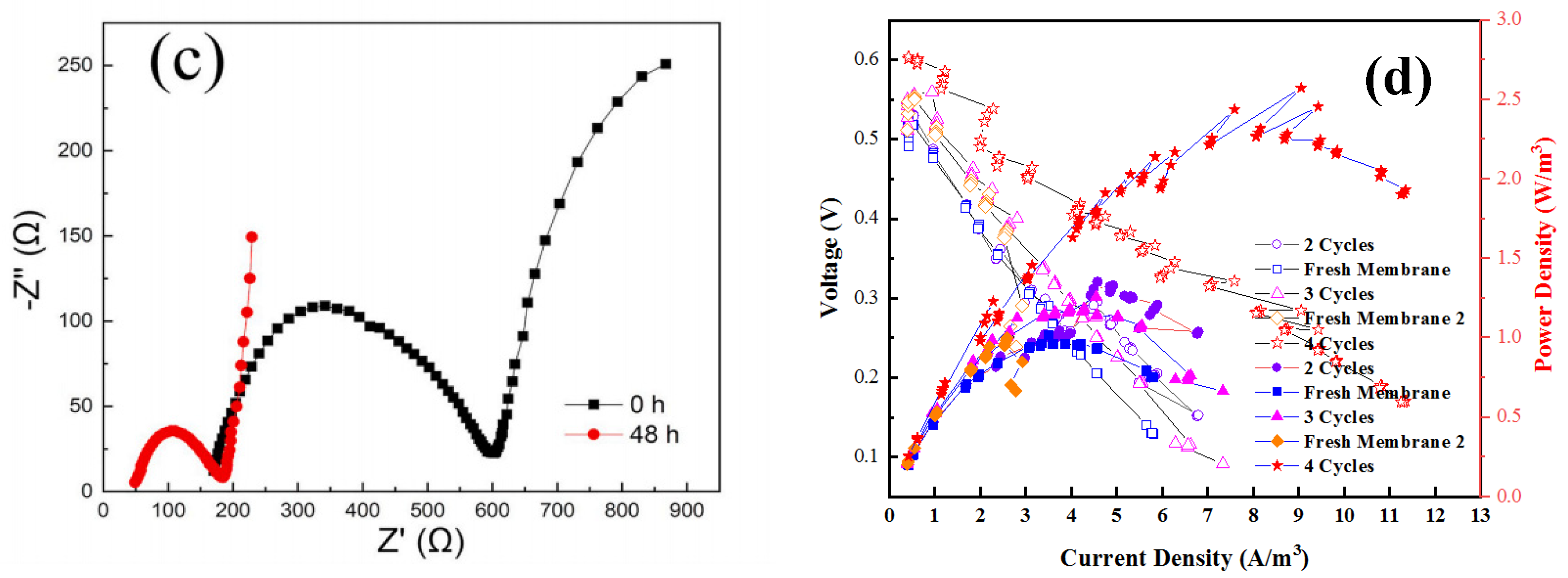
3.3. Effect of Membrane Fouling on Power Generation Performance
3.4. Mass Transfer of Membrane Fouling Model
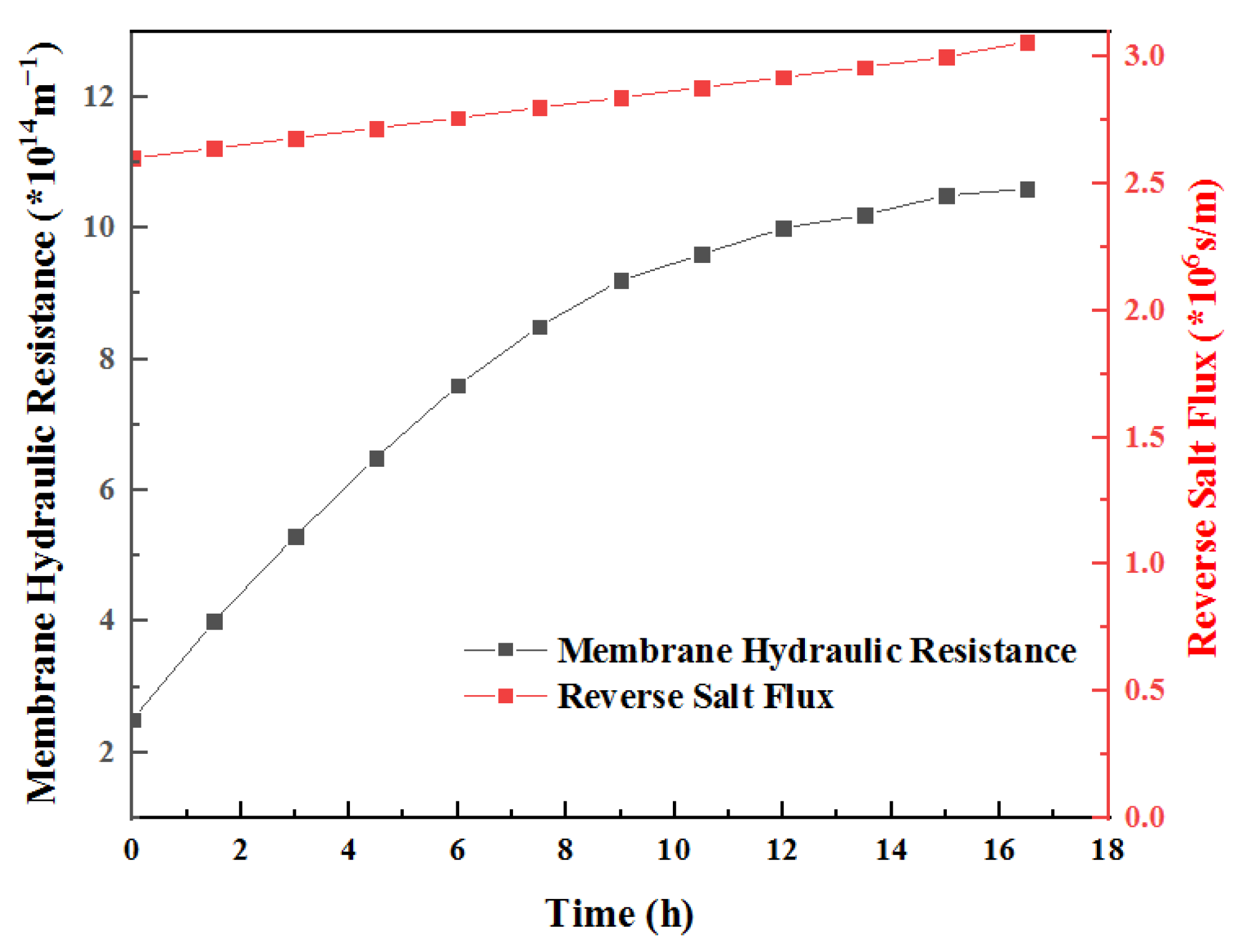
3.5. Calculation of Solute Mass Transfer Resistance
3.6. Hydraulic Resistance Calculation
3.7. Calculation of Solute Concentration on Membrane Surface
3.8. Simulation of Solution Concentration on Both Sides of Membrane under Continuous-Flow OsMFC Operation
3.9. Model Calculation and Analysis
3.10. Concentration Polarization Factor and Effective Concentration Difference
3.11. Hydraulic Resistance and Reverse Salt Resistance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Xiao, F.; Wang, Y.C.; Wu, Z.P.; Chen, G.; Yang, F.; Zhu, S.; Siddharth, K.; Kong, Z.; Lu, A.; Li, J.C.; et al. Recent Advances in Electrocatalysts for Proton Exchange Membrane Fuel Cells and Alkaline Membrane Fuel Cells. Adv. Mater. 2021, 33, 2006292. [Google Scholar] [CrossRef] [PubMed]
- Karibayev, M.; Kalybekkyzy, S.; Wang, Y.; Mentbayeva, A. Molecular Modeling in Anion Exchange Membrane Research: A Brief Review of Recent Applications. Molecules 2022, 27, 3574. [Google Scholar] [CrossRef] [PubMed]
- Mamlouk, M.; Manolova, M. Chapter 6: Alkaline Anionic Exchange Membrane Water Electrolysers. In Electrochemical Methods for Hydrogen Production; Energy and Environment Series; Royal Society of Chemistry: London, UK, 2019; pp. 180–252. [Google Scholar]
- Gu, F.; Dong, H.; Li, Y.; Si, Z.; Yan, F. Highly stable N3-substituted imidazolium-based alkaline anion exchange membranes: Experimental studies and theoretical calculations. Macromolecules 2014, 47, 208–216. [Google Scholar] [CrossRef]
- Zhang, W.; Van Duin, A.C.T. ReaxFF Reactive Molecular Dynamics Simulation of Functionalized Poly(phenylene oxide) Anion Exchange Membrane. J. Phys. Chem. C 2015, 119, 27727–27736. [Google Scholar] [CrossRef]
- Pan, J.; Zhu, H.; Cao, H.; Wang, B.; Zhao, J.; Sun, Z.; Yan, F. Flexible cationic side chains for enhancing the hydroxide ion conductivity of olefinic-type copolymer-based anion exchange membranes: An experimental and theoretical study. J. Memb. Sci. 2021, 620, 118794. [Google Scholar] [CrossRef]
- Wang, J.; Han, Y.; Xu, Z.; Yang, X.; Ramakrishna, S.; Liu, Y. Dissipative Particle Dynamics Simulation: A Review on Investigating Mesoscale Properties of Polymer Systems. Macromol. Mater. Eng. 2021, 306, 2000724. [Google Scholar] [CrossRef]
- Akimov, S.A.; Molotkovsky, R.J.; Kuzmin, P.I.; Galimzyanov, T.R.; Batishchev, O.V. Continuum models of membrane fusion: Evolution of the theory. Int. J. Mol. Sci. 2020, 21, 3875. [Google Scholar] [CrossRef]
- Akimov, S.; Polynkin, M.A.; Jiménez-Munguía, I.; Pavlov, K.V.; Batishchev, O.V. Phosphatidylcholine membrane fusion is pH-dependent. Int. J. Mol. Sci. 2018, 19, 1358. [Google Scholar] [CrossRef]
- Molotkovsky, R.J.; Kuzmin, P.I. Fusion of Peroxisome and Lipid Droplet Membranes: Expansion of a pi-Shaped Structure. Biol. Membr. 2022, 39, 1–13. [Google Scholar]
- Pngin, K.V.; Kondrashov, O.V.; Jiménez-Munguía, I.; Alexandrova, V.V.; Batishchev, O.V.; Galimzyanov, T.R.; Akimov, S.A. Elastic deformations mediate interaction of the raft boundary with membrane inclusions leading to their effective lateral sorting. Sci. Rep. 2020, 10, 4087. [Google Scholar] [CrossRef]
- Zhao, Y.; Song, Y.H.; Duan, L. Study on the Effect of Water Flux in Osmotic Microbial Fuel Cells on Membrane Water Content and Resistance. Water 2022, 14, 848. [Google Scholar] [CrossRef]
- Yao, M.C.; Duan, L.; Song, Y.H.; Hermanowicz, S.W. Degradation mechanism of Ibuprofen via a forward osmosis membrane bioreactor. Bioresour. Technol. 2021, 321, 12444. [Google Scholar] [CrossRef] [PubMed]
- Saadi, K.; Hardisty, S.K.; Tatus-Portnoy, S.S.; Zitoun, D. Influence of loading, metallic surface state and surface protection in precious group metal hydrogen electrocatalyst for H2/Br2 redox-flow batteries. J. Power Source 2022, 536, 231494. [Google Scholar] [CrossRef]
- Lu, Y.; Jia, J.; Miao, H.; Ruan, W.; Wang, X. Performance Improvement and Biofouling Mitigation in Osmotic Microbial Fuel Cells via In Situ Formation of Silver Nanoparticles on Forward Osmosis Membrane. Membranes 2020, 10, 122. [Google Scholar] [CrossRef] [PubMed]
- Trummal, A.; Lipping, L.; Kaljurand, I.; Koppel, I.A.; Leito, I. Acidity of Strong Acids in Water and Dimethyl Sulfoxide. J. Phys. Chem. A 2016, 120, 3663–3669. [Google Scholar] [CrossRef]
- Lin, G.; Chong, P.Y.; Yarlagadda, V.; Nguyen, T.V.; Wycisk, R.J.; Pintauro, P.N.; Bates, M.; Mukerjee, S.; Tucker, M.C.; Weber, A.Z. Advanced Hydrogen-Bromine Flow Batteries with Improved Efficiency, Durability and Cost. J. Electrochem. Soc. 2016, 163, A5049–A5056. [Google Scholar] [CrossRef]
- Zhu, Z.; Luo, X.; Paddison, S.J. DPD simulations of anion exchange membranes functionalized with various cationic groups and associated anions. Solid State Ion. 2019, 340, 115011. [Google Scholar] [CrossRef]
- Lee, M.T. Designing Anion Exchange Membranes with Enhanced Hydroxide Ion Conductivity by Mesoscale Simulations. J. Phys. Chem. C 2020, 124, 4470–4482. [Google Scholar] [CrossRef]
- Lee, M.T. Exploring Side-Chain Designs for Enhanced Ion Conductivity of Anion-Exchange Membranes by Mesoscale Simulations. J. Phys. Chem. C 2019, 123, 10802–10815. [Google Scholar] [CrossRef]
- Marino, M.G.; Kreuer, K.D. Alkaline Stability of Quaternary Ammonium Cations for Alkaline Fuel Cell Membranes and Ionic Liquids. ChemSusChem 2015, 8, 513–523. [Google Scholar] [CrossRef]
- Edson, J.B.; Macomber, C.S.; Pivovar, B.S.; Boncella, J.M. Hydroxide based decomposition pathways of alkyltrimethylammo nium cations. J. Memb. Sci. 2012, 399–400, 49–59. [Google Scholar] [CrossRef]
- Zhao, Y.; Duan, L. Research on Measuring Pure Membrane Electrical Resistance under the Effects of Salinity Gradients and Diffusion Boundary Layer and Double Layer Resistances. Membranes 2022, 12, 816. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Liu, H.; Bae, C.; Tuckerman, M.E.; Hickner, M.A.; Paddison, S.J. Mesoscale Simulations of Quaternary Ammonium Tethered Triblock Copolymers: Effects of the Degree of Functionalization and Styrene Content. J. Phys. Chem. C 2020, 124, 16315–16323. [Google Scholar] [CrossRef]
- Belli, T.J.; Bassin, J.P.; Costa, R.E.; Akaboci, T.R.V.; Battistelli, A.A.; Lobo-Recio, M.A.; Lapolli, F.R. Evaluating the effect of air flow rate on hybrid and conventional membrane bioreactors: Implications on performance, microbial activity and membrane fouling. Sci. Total Environ. 2021, 755, 142563. [Google Scholar] [CrossRef] [PubMed]
- Hou, H.H.; Zhu, M.T.; Duan, L.; Zhao, Y.; Zhang, Z.Z.; Yao, M.C.; Zhou, B.H.; Zhang, H.Y.; Hermanowicz, S.W. Removal performance and biodegradation mechanism of sulfonamides antibiotic contained wastewater by IFAS-MBR bioreactor. J. Mol. Liq. 2022, 367, 120572. [Google Scholar] [CrossRef]
- Wang, D.; Tao, J.; Fan, F.; Xu, R.; Meng, F. A novel pilot-scale IFAS-MBR system with low aeration for municipal wastewater treatment: Linkages between nutrient removal and core functional microbiota. Sci. Total Environ. 2021, 776, 145858. [Google Scholar] [CrossRef]
- Yu, H.; Du, C.; Qu, F.; He, J.; Rong, H. Efficient biostimulants for bacterial quorum quenching to control fouling in MBR. Chemosphere 2022, 286, 131689. [Google Scholar] [CrossRef]
- Hamedi, H.; Ehteshami, M.; Mirbagheri, S.A.; Rasouli, S.A.; Zendehboudi, S. Current Status and Future Prospects of Membrane Bioreactors (MBRs) and Fouling Phenomena: A Systematic Review. Can. J. Chem. Eng. 2018, 97, 32–58. [Google Scholar] [CrossRef]
- Yusuf, Z.; Wahab, N.A.; Sahlan, S. Fouling control strategy for submerged membrane bioreactor filtration processes using aer ation airflow, backwash, and relaxation: A review. Desalination Water Treat. 2016, 57, 17683–17695. [Google Scholar] [CrossRef]
- Guerrini, A.; Romano, G.; Indipendenza, A. Energy Efficiency Drivers in Wastewater Treatment Plants: A Double Bootstrap DEA Analysis. Sustainability 2017, 9, 1126. [Google Scholar] [CrossRef]
- De Sotto, R.; Bae, S. Nutrient removal performance and microbiome of an energy-efficient reciprocation MLE-MBR operated under hypoxic conditions. Water Res. 2020, 182, 115991. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Xiao, S.; Chu, H.; Zhao, F.; Yu, Z.; Zhou, X.; Zhang, Y. Intelligent mitigation of fouling by means of membrane vibration for algae separation: Dynamics model, comprehensive evaluation, and critical vibration frequency. Water Res. 2020, 182, 115972. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.; Smith, S.; Roh, H.K. Alternative energy efficient membrane bioreactor using reciprocating submerged membrane. Water Sci. Technol. 2014, 70, 1998–2003. [Google Scholar] [CrossRef] [PubMed]
- León, G.; Hidalgo, A.M.; Miguel, B.; Guzmán, M.A. Pertraction of Co(II) through novel ultrasound prepared supported liquid membranes containing D2EHPA.Optimization and transport parameters. Membranes 2020, 10, 436. [Google Scholar] [CrossRef]
- Yang, B.; Bai, L.; Li, T.; Deng, L.; Liu, L.; Zeng, S.; Han, J.; Zhang, X. Super selective ammonia separation through multiple-site interaction with ionic liquid-based hybrid membranes. J. Membr. Sci. 2021, 628, 119264. [Google Scholar] [CrossRef]
- Mohammed, A.A.; Atiya, M.A.; Hussein, M.A. Removal of antibiotic tetracycline using nano-fluid emulsion liquid membrane: Breakage, extraction and studies. Colloids Surf. A Physicochem. Eng. Asp. 2020, 595, 124680. [Google Scholar] [CrossRef]
- Al-Ani, F.H.; Alsalhy, Q.F.; Al-Dahhan, M. Enhancing emulsion liquid membrane system(ELM) stability and performance for the extraction of phenol from waste water using various nanoparticles. Desalination Water Treat. 2021, 210, 180–191. [Google Scholar] [CrossRef]


| Parameter | Symbol | Value | Unit | Reference |
|---|---|---|---|---|
| Conditions and Parameters | ||||
| Initial concentration of feed solution | CF, 0 | 0.035 | mol·L−1 | [25] |
| Inlet salt concentration | Cin, F | 0 | mol·L−1 | [25] |
| Volume of feed solution | VF | 3.5 | L | [23] |
| Draw solution concentration | CD, b | 1.2 | mol·L−1 | [25] |
| Effective area of membrane | Am | 3.1 × 10−3 | m2 | [26] |
| Temperature | T | 298.15 | K | [26] |
| Viscosity of pure water | μ | 9.9 × 10−4 | Pa.s | [23] |
| Diffusion coefficient of salt in water | D | 1.35 × 10−9 | m2.s−1 | [23] |
| Membrane property parameters | ||||
| Hydraulic resistance | Rmw | 2.39 × 1014 | m−1 | [22] |
| Salt mass transfer resistance | Rms | 2.85 × 106 | s·m−1 | [22] |
| Structural parameters | S | 425 | μm | [23] |
| Boundary layer thickness | δ | 125 | μm | [25] |
| Boundary layer mass transfer coefficient | Kcecp | 1.3 × 10−5 | m·s−1 | Calculation |
| Mass transfer coefficient of support layer | Kdicp | 3.15 × 10−6 | m/s | Calculation |
| Pollution layer property parameters | ||||
| Salt mass transfer resistance coefficient | Cfs | 1.51 ± 0.07 | 1016 s·m−2 | Correcting |
| Hydraulic resistance coefficient | Cfw | 5.39 ± 0.1 | 1015 m−2 | Correcting |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Duan, L.; Liu, X.; Song, Y. Influence of Membrane Fouling and Reverse Salt Flux on Membrane Impedance of Forward Osmosis Microbial Fuel Cell. Membranes 2022, 12, 1165. https://doi.org/10.3390/membranes12111165
Zhao Y, Duan L, Liu X, Song Y. Influence of Membrane Fouling and Reverse Salt Flux on Membrane Impedance of Forward Osmosis Microbial Fuel Cell. Membranes. 2022; 12(11):1165. https://doi.org/10.3390/membranes12111165
Chicago/Turabian StyleZhao, Yang, Liang Duan, Xiang Liu, and Yonghui Song. 2022. "Influence of Membrane Fouling and Reverse Salt Flux on Membrane Impedance of Forward Osmosis Microbial Fuel Cell" Membranes 12, no. 11: 1165. https://doi.org/10.3390/membranes12111165
APA StyleZhao, Y., Duan, L., Liu, X., & Song, Y. (2022). Influence of Membrane Fouling and Reverse Salt Flux on Membrane Impedance of Forward Osmosis Microbial Fuel Cell. Membranes, 12(11), 1165. https://doi.org/10.3390/membranes12111165








