Influence of Dispersed TiO2 Nanoparticles via Steric Interaction on the Antifouling Performance of PVDF/TiO2 Composite Membranes
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Membrane Preparation
2.3. Dispersion Analysis of TiO2 Nanoparticles
2.4. Membrane Characterization
2.5. Evaluation of Membrane Antifouling Performance
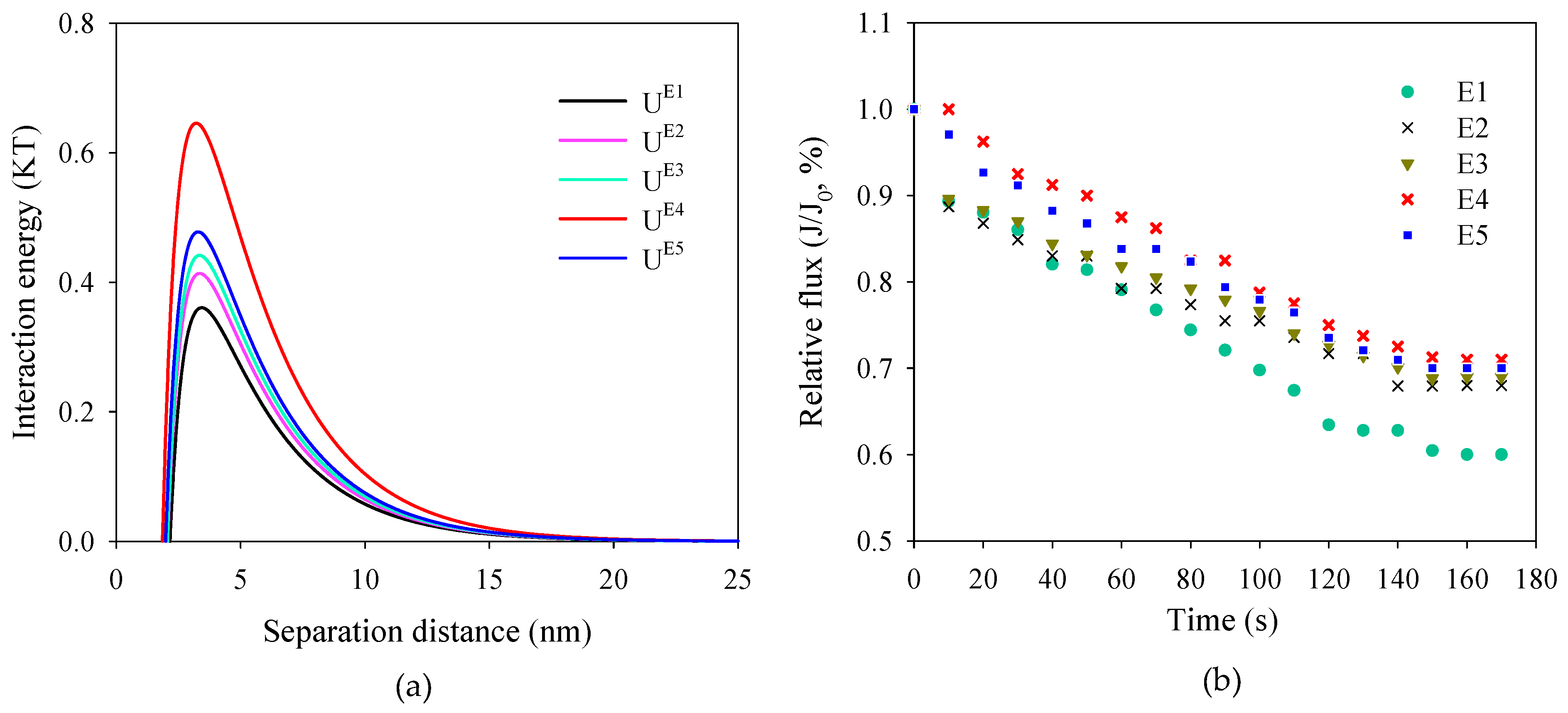
2.5.1. XDLVO Theory Analysis
2.5.2. SMP Filtration
2.5.3. Critical Flux
2.5.4. Fouling Rate
2.5.5. Filtration Process in A/O-MBR
3. Results and Discussion
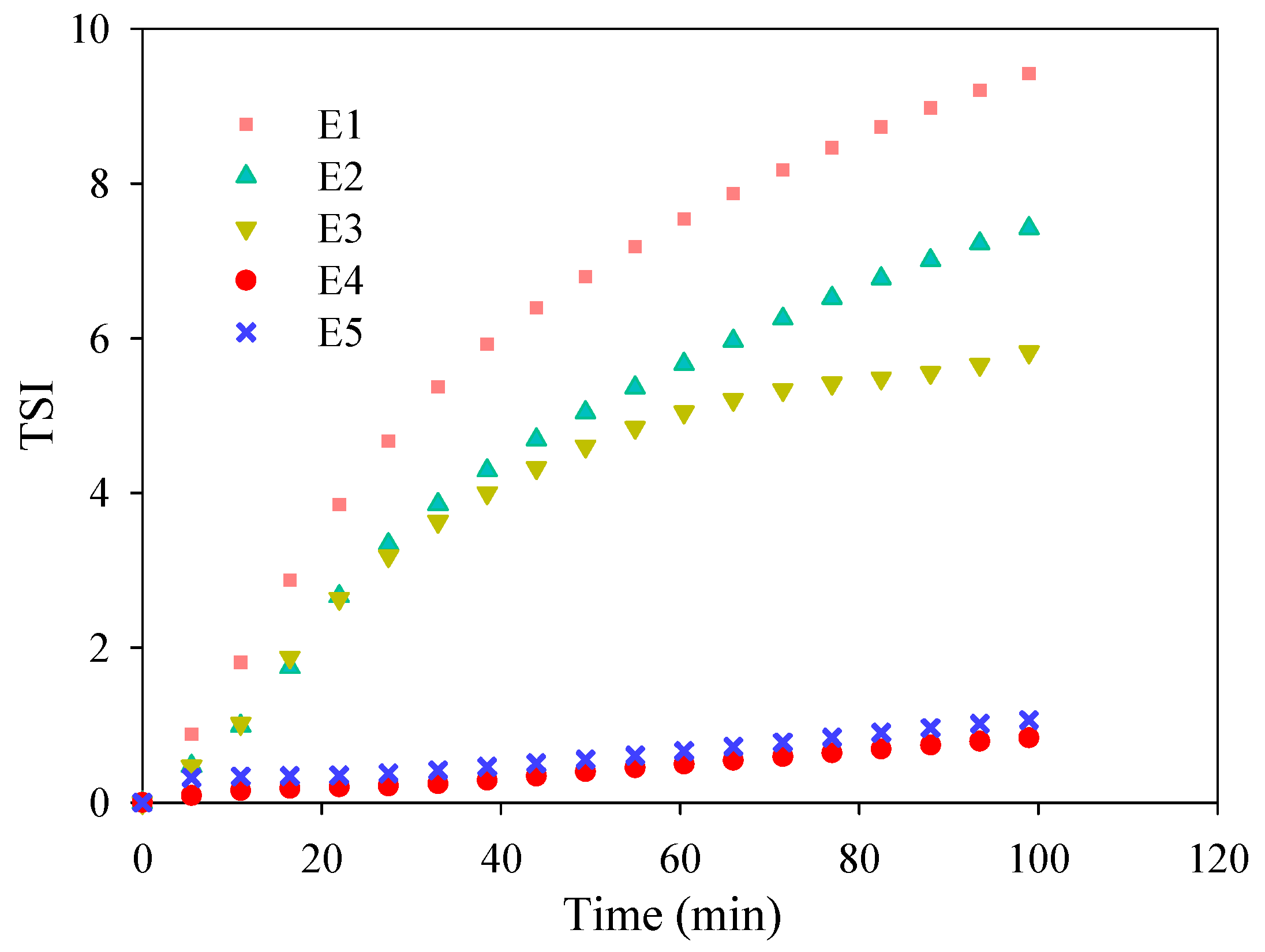
3.1. TiO2 Nanoparticles Dispersion
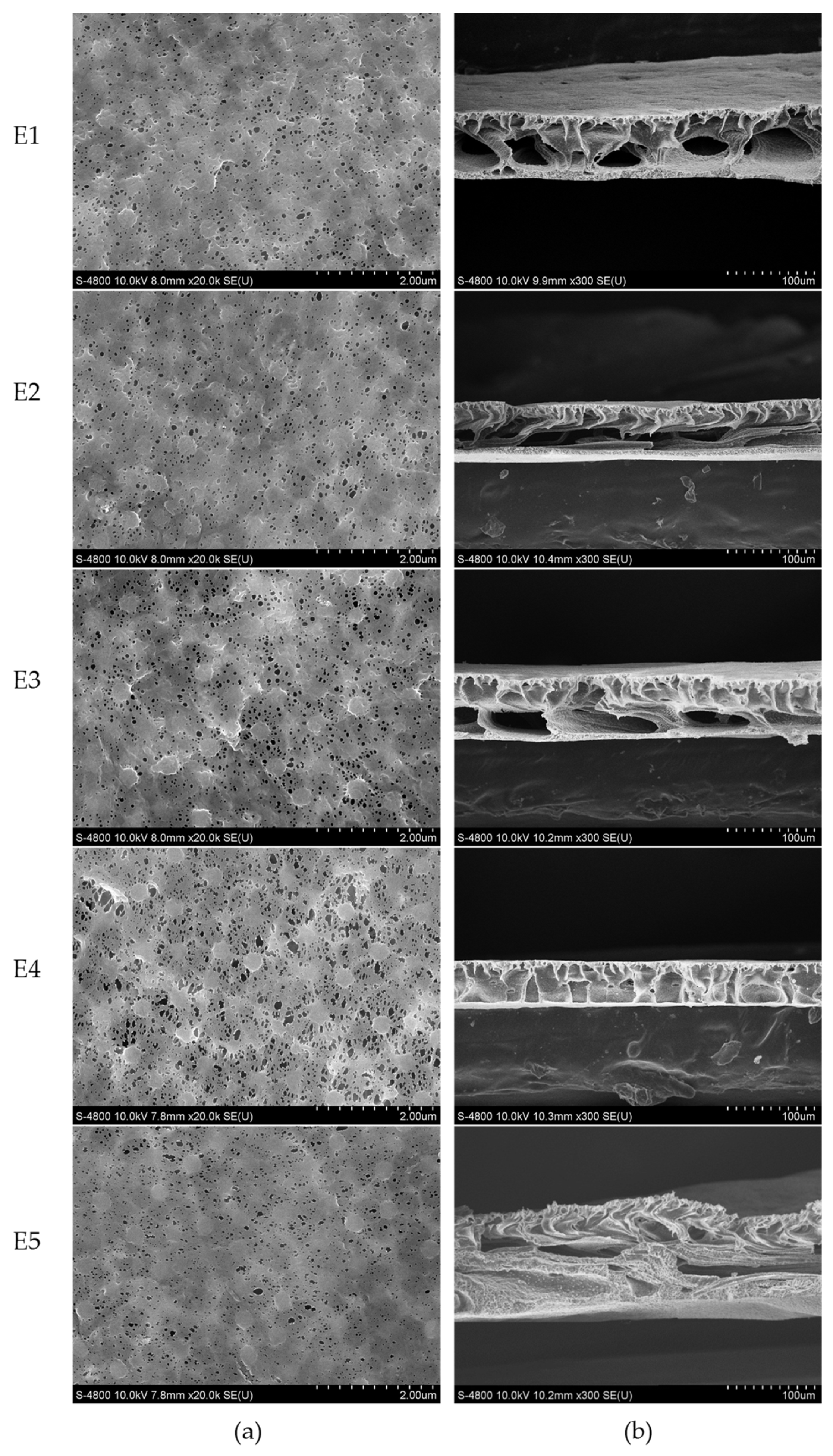
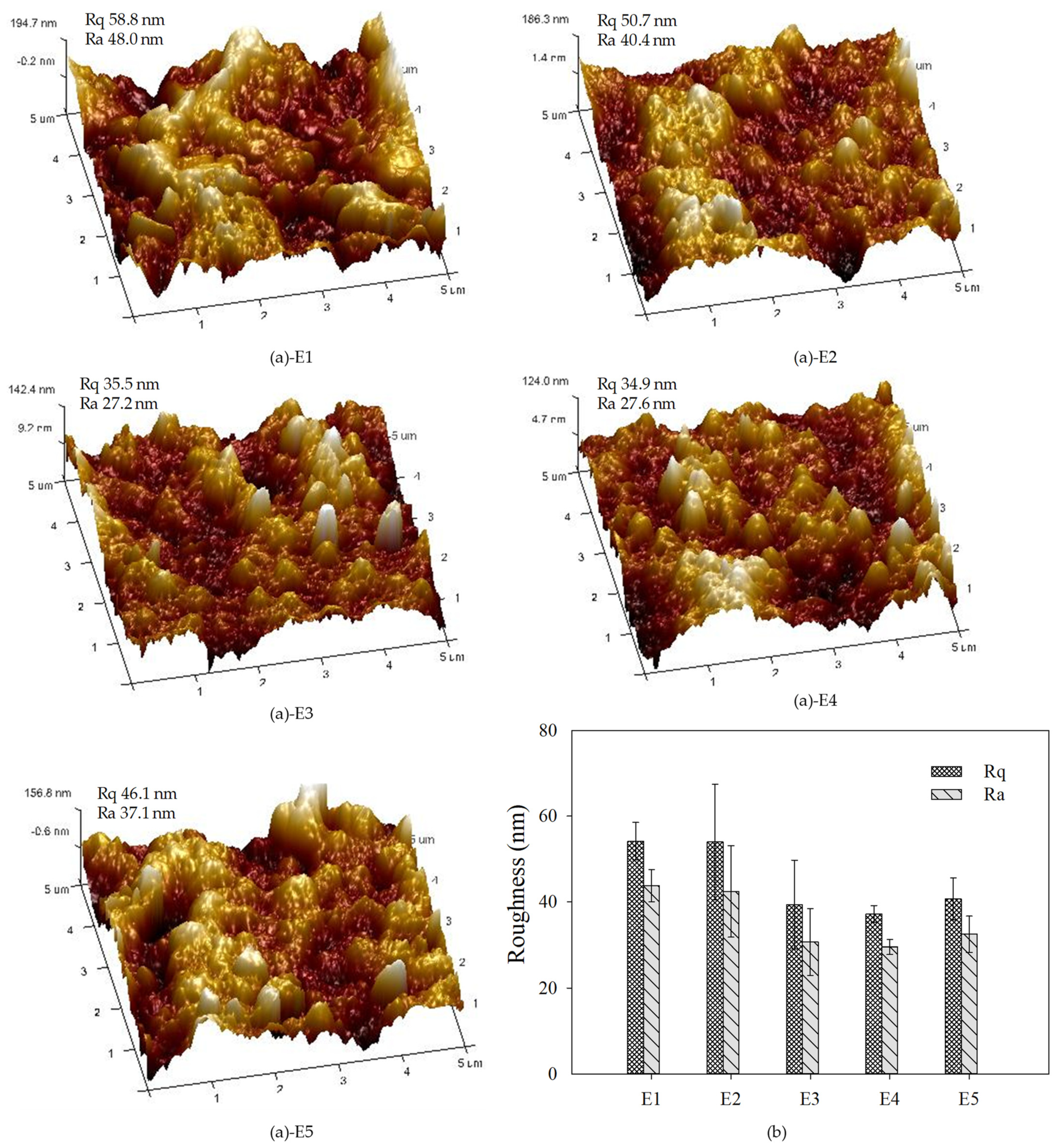
3.2. Membrane Characterizations
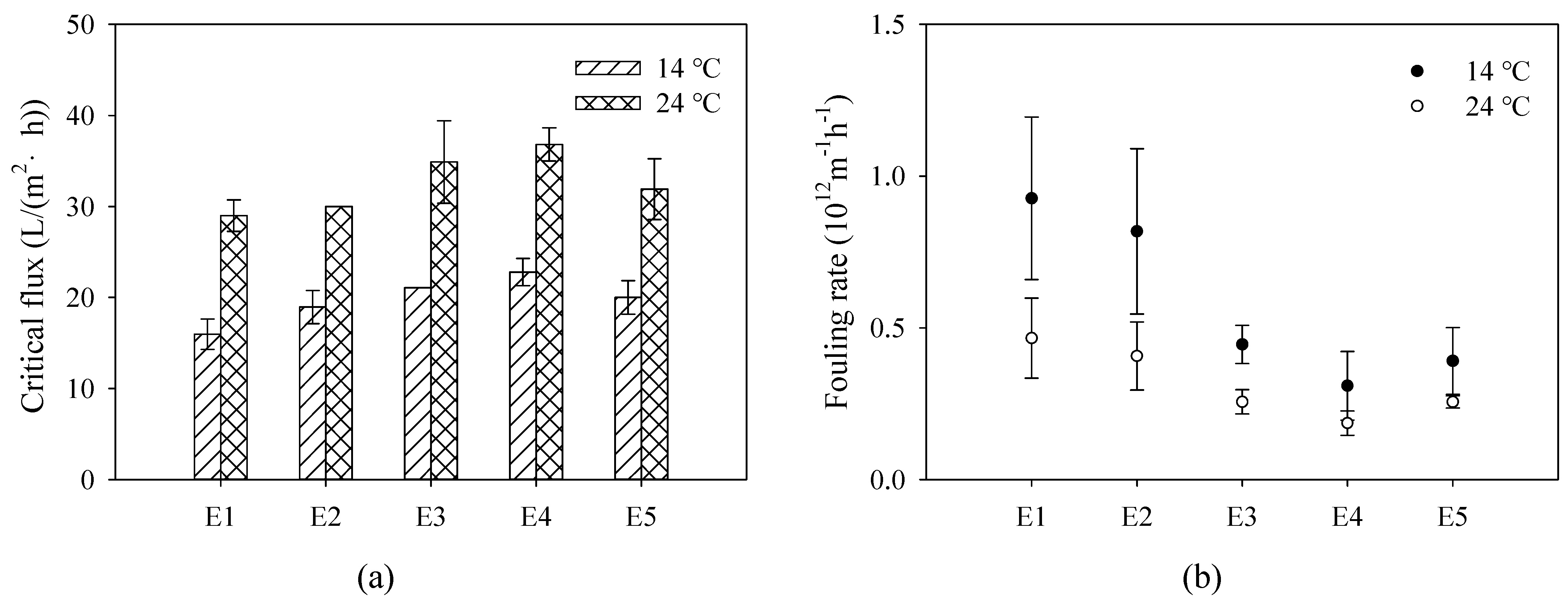
3.3. Assessment of Membrane Antifouling Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xiao, K.; Liang, S.; Wang, X.; Chen, C.; Huang, X. Current state and challenges of full-scale membrane bioreactor applications: A critical review. Bioresour. Technol. 2019, 271, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.; Kim, H.S.; Lee, J.H.; Ham, S.Y.; Park, J.H.; Park, H.D. Development of a new method to evaluate critical flux and system reliability based on particle properties in a membrane bioreactor. Chemosphere 2021, 280, 130763. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Q.; Li, J.; Ngo, H.H.; Guo, W.; Hu, J.; Gao, M.T.; Wang, Q.; Hou, Y. Effect of magnetic powder on membrane fouling mitigation and microbial community/composition in membrane bioreactors (MBRs) for municipal wastewater treatment. Bioresour. Technol. 2018, 249, 377–385. [Google Scholar] [CrossRef]
- Goh, P.S.; Ng, B.C.; Lau, W.J.; Ismail, A.F. Inorganic Nanomaterials in Polymeric Ultrafiltration Membranes for Water Treatment. Sep. Purif. Rev. 2015, 44, 216–249. [Google Scholar] [CrossRef]
- Qian, Y.; Chi, L.; Zhou, W.; Yu, Z.; Zhang, Z.; Zhang, Z.; Jiang, Z. Fabrication of TiO2-modified polytetrafluoroethylene ultrafiltration membranes via plasma-enhanced surface graft pretreatment. Appl. Surf. Sci. 2016, 360, 749–757. [Google Scholar] [CrossRef]
- Tran, D.T.; Mendret, J.; Mericq, J.P.; Faur, C.; Brosillon, S. Performance of PVDF-TiO2 Membranes during Photo-Filtration in the Presence of Inorganic and Organic Components. Membranes 2022, 12, 245. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.J.; Kim, N.; Lee, Y.T. Preparation and characterization of PVDF/TiO2 organic–inorganic composite membranes for fouling resistance improvement. J. Membr. Sci. 2009, 345, 13–20. [Google Scholar] [CrossRef]
- Pi, J.-K.; Yang, H.-C.; Wan, L.-S.; Wu, J.; Xu, Z.-K. Polypropylene microfiltration membranes modified with TiO2 nanoparticles for surface wettability and antifouling property. J. Membr. Sci. 2016, 500, 8–15. [Google Scholar] [CrossRef]
- Korayem, A.H.; Tourani, N.; Zakertabrizi, M.; Sabziparvar, A.M.; Duan, W.H. A review of dispersion of nanoparticles in cementitious matrices: Nanoparticle geometry perspective. Constr. Build. Mater. 2017, 153, 346–357. [Google Scholar] [CrossRef]
- Li, Y.; Kröger, M.; Liu, W.K. Endocytosis of PEGylated nanoparticles accompanied by structural and free energy changes of the grafted polyethylene glycol. Biomaterials 2014, 35, 8467–8478. [Google Scholar] [CrossRef]
- Zhang, J.; Zheng, M.; Zhou, Y.; Yang, L.; Zhang, Y.; Wu, Z.; Liu, G.; Zheng, J. Preparation of Nano-TiO2-Modified PVDF Membranes with Enhanced Antifouling Behaviors via Phase Inversion: Implications of Nanoparticle Dispersion Status in Casting Solutions. Membranes 2022, 12, 386. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Roso, M.; Boaretti, C.; Lorenzetti, A.; Martucci, A.; Modesti, M. PVDF-TiO2 core-shell fibrous membranes by microwave-hydrothermal method: Preparation, characterization, and photocatalytic activity. J. Environ. Chem. Eng. 2021, 9, 106250. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, Z.; Zhu, C.; Mei, X.; Wu, Z. Assessment of SMP fouling by foulant–membrane interaction energy analysis. J. Membr. Sci. 2013, 446, 154–163. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Q.; Wang, Z.; Zhu, C.; Wu, Z. Modification of poly(vinylidene fluoride)/polyethersulfone blend membrane with polyvinyl alcohol for improving antifouling ability. J. Membr. Sci. 2014, 466, 293–301. [Google Scholar] [CrossRef]
- Wisniewska, M.; Szewczuk-Karpisz, K. Removal possibilities of colloidal chromium (III) oxide from water using polyacrylic acid. Environ. Sci. Pollut. Res. 2013, 20, 3657–3669. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Chen, J.; Zhang, X.; Xiao, J.; An, S.; Luan, Z.; Liu, F.; Zhang, B. Dynamic demulsification of oil-in-water emulsions with electrocoalescence: Diameter distribution of oil droplets. Sep. Purif. Technol. 2021, 254, 117631. [Google Scholar] [CrossRef]
- Koulivand, H.; Shahbazi, A.; Vatanpour, V.; Rahmandoost, M. Novel antifouling and antibacterial polyethersulfone membrane prepared by embedding nitrogen-doped carbon dots for efficient salt and dye rejection. Mater. Sci. Eng. C 2020, 111, 110787. [Google Scholar] [CrossRef] [PubMed]
- Kamari, S.; Shahbazi, A. Biocompatible Fe3O4@SiO2-NH2 nanocomposite as a green nanofiller embedded in PES-nanofiltration membrane matrix for salts, heavy metal ion and dye removal: Long-term operation and reusability tests. Chemosphere 2020, 243, 125282. [Google Scholar] [CrossRef]
- Subhi, N.; Verliefde, A.R.D.; Chen, V.; Le-Clech, P. Assessment of physicochemical interactions in hollow fibre ultrafiltration membrane by contact angle analysis. J. Membr. Sci. 2012, 403–404, 32–40. [Google Scholar] [CrossRef]
- Lay, H.T.; Chew, J.W. Critical flux of colloidal foulant in microfiltration: Effect of organic solvent. J. Membr. Sci. 2020, 616, 118531. [Google Scholar] [CrossRef]
- Hou, D.; Yuan, Z.; Tang, M.; Wang, K.; Wang, J. Effect and mechanism of an anionic surfactant on membrane performance during direct contact membrane distillation. J. Membr. Sci. 2020, 595, 117495. [Google Scholar] [CrossRef]
- Zhang, L.; Jia, H.; Wang, J.; Wen, H.; Li, J. Characterization of fouling and concentration polarization in ion exchange membrane by in-situ electrochemical impedance spectroscopy. J. Membr. Sci. 2020, 594, 117443. [Google Scholar] [CrossRef]
- Cheng, H.; Li, Y.; Kato, H.; Li, Y.Y. Enhancement of sustainable flux by optimizing filtration mode of a high-solid anaerobic membrane bioreactor during long-term continuous treatment of food waste. Water Res. 2020, 168, 115195. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Wang, Z.; Zhou, Z.; Yu, G.; Gu, G. Sludge rheological and physiological characteristics in a pilot-scale submerged membrane bioreactor. Desalination 2007, 212, 152–164. [Google Scholar] [CrossRef]
- Chen, F.; Shi, X.; Chen, X.; Chen, W. An iron (II) phthalocyanine/poly(vinylidene fluoride) composite membrane with antifouling property and catalytic self-cleaning function for high-efficiency oil/water separation. J. Membr. Sci. 2018, 552, 295–304. [Google Scholar] [CrossRef]
- Tran, D.T.; Mericq, J.P.; Mendret, J.; Brosillon, S.; Faur, C. Influence of Preparation Temperature on the Properties and Performance of Composite PVDF-TiO2 Membranes. Membranes 2021, 11, 876. [Google Scholar] [CrossRef]
- Farahani, M.H.D.A.; Vatanpour, V. A comprehensive study on the performance and antifouling enhancement of the PVDF mixed matrix membranes by embedding different nanoparticulates: Clay, functionalized carbon nanotube, SiO2 and TiO2. Sep. Purif. Technol. 2018, 197, 372–381. [Google Scholar] [CrossRef]
- Hong, J.; He, Y. Effects of nano sized zinc oxide on the performance of PVDF microfiltration membranes. Desalination 2012, 302, 71–79. [Google Scholar] [CrossRef]
- Haponska, M.; Trojanowska, A.; Nogalska, A.; Jastrzab, R.; Gumi, T.; Tylkowski, B. PVDF Membrane Morphology-Influence of Polymer Molecular Weight and Preparation Temperature. Polymers 2017, 9, 718. [Google Scholar] [CrossRef]
- Bi, R.; Zhang, Q.; Zhang, R.; Su, Y.; Jiang, Z. Thin film nanocomposite membranes incorporated with graphene quantum dots for high flux and antifouling property. J. Membr. Sci. 2018, 553, 17–24. [Google Scholar] [CrossRef]
- Chen, L.; Tian, Y.; Cao, C.Q.; Zhang, J.; Li, Z.N. Interaction energy evaluation of soluble microbial products (SMP) on different membrane surfaces: Role of the reconstructed membrane topology. Water Res. 2012, 46, 2693–2704. [Google Scholar] [CrossRef] [PubMed]
- Yuliwati, E.; Ismail, A.F.; Othman, M.H.D.; Shirazi, M.M.A. Critical Flux and Fouling Analysis of PVDF-Mixed Matrix Membranes for Reclamation of Refinery-Produced Wastewater: Effect of Mixed Liquor Suspended Solids Concentration and Aeration. Membranes 2022, 12, 161. [Google Scholar] [CrossRef] [PubMed]




| Membrane No. | Thickness (mm) | Average Pore Size (μm) | Porosity (%) | Water Permeability (L/(m2·h·kPa) |
|---|---|---|---|---|
| E1 | 0.24 ± 0.00 | 0.067 ± 0.012 | 43.4 ± 4.1 | 58.5 ± 1.3 |
| E2 | 0.25 ± 0.00 | 0.072 ± 0.009 | 46.6 ± 3.5 | 58.1 ± 2.3 |
| E3 | 0.24 ± 0.00 | 0.079 ± 0.009 | 45.2 ± 0.6 | 48.1 ± 1.8 |
| E4 | 0.23 ± 0.00 | 0.129 ± 0.011 | 45.4 ± 2.2 | 71.5 ± 1.6 |
| E5 | 0.26 ± 0.00 | 0.066 ± 0.011 | 40.1 ± 1.4 | 21.2 ± 2.4 |
| TOC Concentration (mg/L) | Zeta Potential (mV) | Contact Angle (°) | |||
| Water | Formamide | Diiodomethane | |||
| SMP | 11.4 ± 0.2 | −10.2 ± 0.3 | 71.3 ± 4.1 | 51.7 ± 2.4 | 30.5 ± 0.9 |
| Membrane No. | PEG Content (%) | Zeta Potential (mV) | Contact Angle (°) | ||
| Water | Formamide | Diiodomethane | |||
| E1 | 0% | −16.4 ± 0.7 | 87.2 ± 0.7 | 72.2 ± 2.6 | 62.1 ± 1.1 |
| E2 | 2% | −18.6 ± 1.6 | 84.9 ± 0.3 | 69.5 ± 0.2 | 59.9 ± 0.4 |
| E3 | 4% | −20.0 ± 1.9 | 81.5 ± 0.5 | 60.0 ± 0.4 | 55.5 ± 0.2 |
| E4 | 6% | −30.0 ± 1.6 | 78.5 ± 0.3 | 57.4 ± 0.1 | 55.5 ± 0.5 |
| E5 | 8% | −21.4 ± 0.8 | 80.7 ± 0.5 | 61.2 ± 1.0 | 56.5 ± 0.6 |
| Surface Tension Parameters for Each Membrane (mJ/m2) | ||||||
| Membrane No. | γLW | γ+ | γ− | γAB | γTOT | |
| E1 | 27.39 ± 0.61 | 0.07 ± 0.09 | 6.28 ± 0.64 | 1.05 ± 0.82 | 28.45 ± 1.37 | |
| E2 | 28.64 ± 0.26 | 0.08 ± 0.01 | 6.91 ± 0.20 | 1.45 ± 0.07 | 30.10 ± 0.19 | |
| E3 | 31.19 ± 0.14 | 0.67 ± 0.04 | 5.28 ± 0.22 | 3.76 ± 0.12 | 34.94 ± 0.24 | |
| E4 | 31.18 ± 0.27 | 0.88 ± 0.06 | 6.45 ± 0.33 | 4.77 ± 0.07 | 35.95 ± 0.19 | |
| E5 | 30.58 ± 0.32 | 0.55 ± 0.08 | 6.33 ± 0.21 | 3.71 ± 0.24 | 34.30 ± 0.49 | |
| The Free Energy of Cohesion of Membranes (mJ/m2) | The Free Energy of Adhesion of Membranes (mJ/m2) | |||||
| Membrane No. | ΔG121LW | Δ121GAB | ΔG121SWS | ΔG123LW | ΔG123AB | ΔG123SWS |
| E1 | −0.64 ± 0.13 | −49.18 ± 0.65 | −49.82 ± 0.75 | −2.22 ± 0.23 | −42.14 ± 0.67 | −44.36 ± 0.86 |
| E2 | −0.93 ± 0.07 | −46.24 ± 0.57 | −47.17 ± 0.52 | −2.69 ± 0.09 | −40.69 ± 0.32 | −43.38 ± 0.25 |
| E3 | −1.68 ± 0.05 | −46.58 ± 0.81 | −48.26 ± 0.76 | −3.60 ± 0.05 | −42.09 ± 0.47 | −45.69 ± 0.42 |
| E4 | −1.68 ± 0.09 | −41.27 ± 0.82 | −42.95 ± 0.82 | −3.60 ± 0.09 | −39.27 ± 0.56 | −42.87 ± 0.55 |
| E5 | −1.48 ± 0.10 | −43.69 ± 0.37 | −45.18 ± 0.29 | −3.39 ± 0.11 | −40.20 ± 0.26 | −43.59 ± 0.23 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Jian, Z.; Jiang, M.; Peng, B.; Zhang, Y.; Wu, Z.; Zheng, J. Influence of Dispersed TiO2 Nanoparticles via Steric Interaction on the Antifouling Performance of PVDF/TiO2 Composite Membranes. Membranes 2022, 12, 1118. https://doi.org/10.3390/membranes12111118
Zhang J, Jian Z, Jiang M, Peng B, Zhang Y, Wu Z, Zheng J. Influence of Dispersed TiO2 Nanoparticles via Steric Interaction on the Antifouling Performance of PVDF/TiO2 Composite Membranes. Membranes. 2022; 12(11):1118. https://doi.org/10.3390/membranes12111118
Chicago/Turabian StyleZhang, Jie, Zicong Jian, Minmin Jiang, Bo Peng, Yuanyuan Zhang, Zhichao Wu, and Junjian Zheng. 2022. "Influence of Dispersed TiO2 Nanoparticles via Steric Interaction on the Antifouling Performance of PVDF/TiO2 Composite Membranes" Membranes 12, no. 11: 1118. https://doi.org/10.3390/membranes12111118
APA StyleZhang, J., Jian, Z., Jiang, M., Peng, B., Zhang, Y., Wu, Z., & Zheng, J. (2022). Influence of Dispersed TiO2 Nanoparticles via Steric Interaction on the Antifouling Performance of PVDF/TiO2 Composite Membranes. Membranes, 12(11), 1118. https://doi.org/10.3390/membranes12111118






