Polyether Block Amide as Host Matrix for Nanocomposite Membranes Applied to Different Sensitive Fields
Abstract
1. Introduction
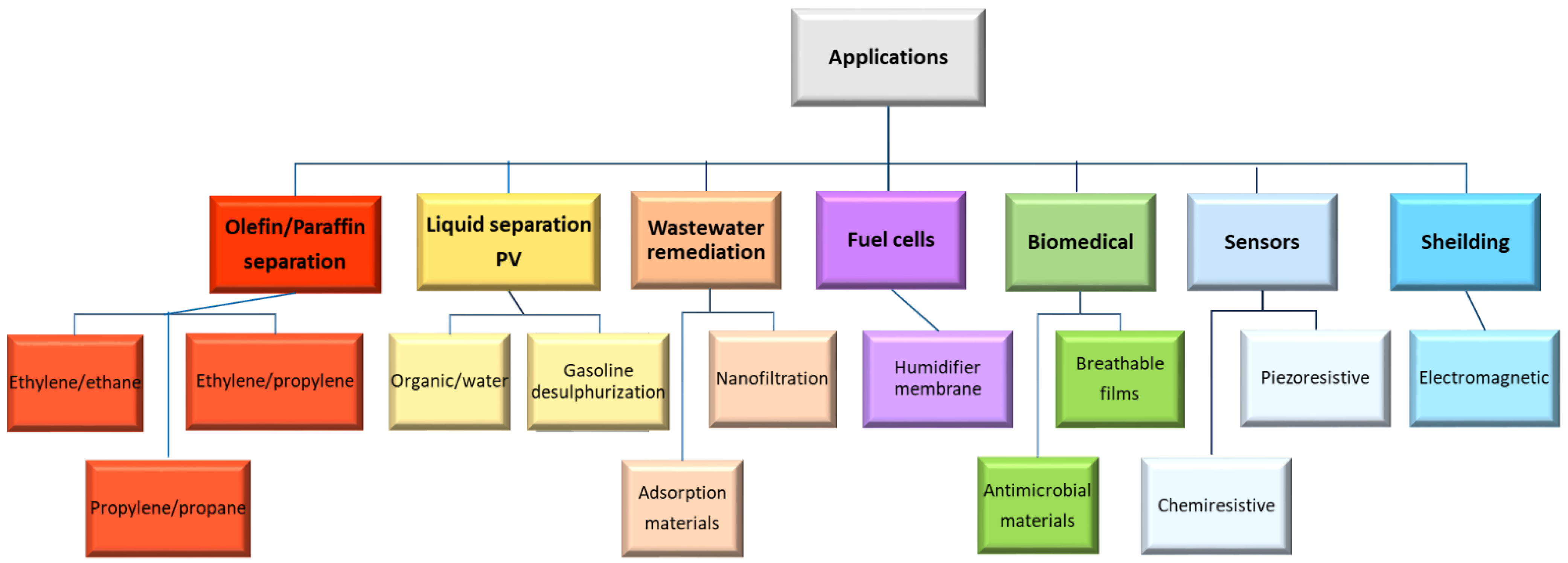
2. Application Domains
3. Filler Categories
4. Preparation and Characterization of the Nanocomposites
- -
- Solution casting,
- -
- Electrospinning,
- -
- Melt electrospinning,
- -
- Hot-melt extrusion,
- -
- Melt compounding,
- -
- Melt blending followed by compression moulding,
- -
- Twin-screw extrusion and compression moulding.
5. Performance of the Nanocomposites in Different Applications
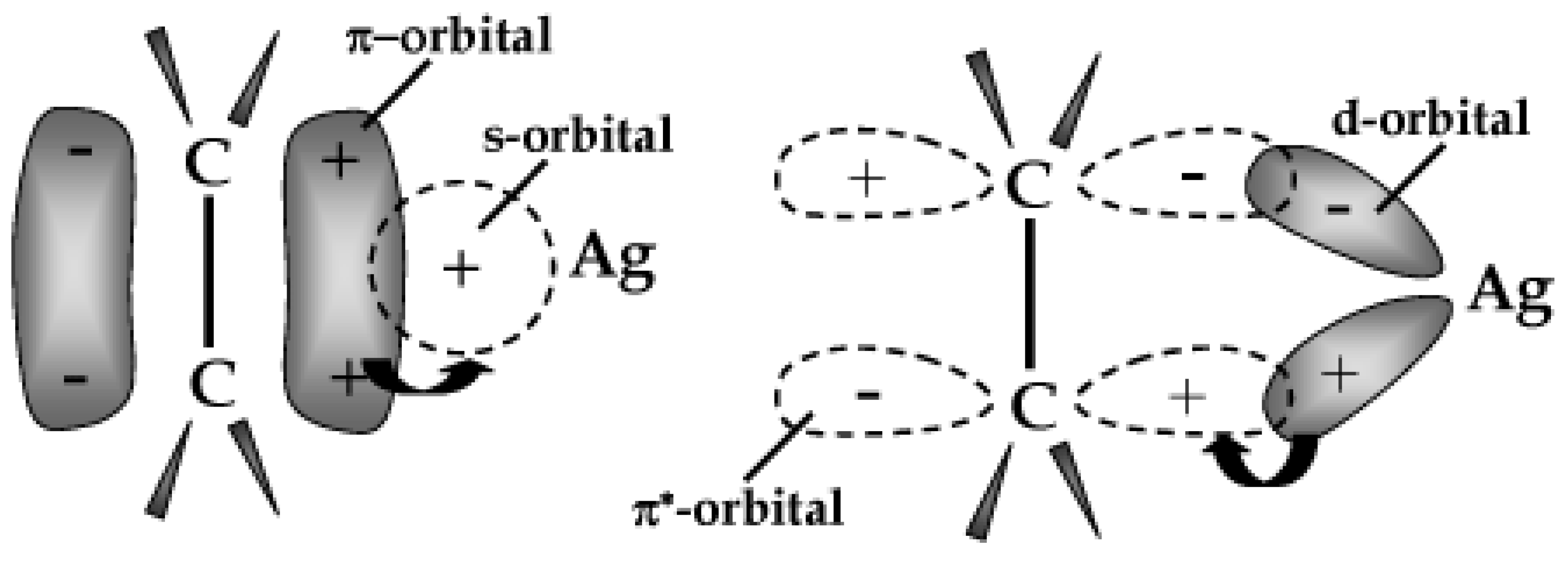
5.1. Olefin/Paraffin Separation
5.2. Pervaporation (PV)
5.2.1. PV MMMs Comprising Non-Porous Fillers
5.2.2. PV MMMs Comprising Porous Fillers
Zeolites
Metal–Organic Frameworks (MOFs)
Other Porous Fillers
5.2.3. Two-Dimensional Nanosheets
5.2.4. Organic Fillers
5.3. Fuel Cells and Batteries
5.4. Membrane Filtration for Water Treatment
6. Other Applications
6.1. Adsorption
6.2. Electromagnetic Shielding/Sensors/Detectors
6.3. Biomedical Applications
6.4. Packaging Applications
6.5. Further Uses of Pebax
7. Conclusions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Clarizia, G.; Bernardo, P. (Eds.) Diverse Applications of Organic-Inorganic Nanocomposites: Emerging Research and Opportunities; Advances in Mechatronics and Mechanical Engineering; IGI Global: Hershey, PA, USA, 2019; ISBN 9781799815303. [Google Scholar]
- Bhat, A.; Budholiya, S.; Aravind Raj, S.; Sultan, M.T.H.; Hui, D.; Md Shah, A.U.; Safri, S.N.A. Review on nanocomposites based on aerospace applications. Nanotechnol. Rev. 2021, 10, 237–253. [Google Scholar]
- Nanocomposites Market Size, Share & COVID-19 Impact Analysis, by Type (Polymer, Metal, Ceramic, Others), by Product (Carbon Nanotubes, Nanoclay, Graphene, Nanofiber, Others), by Application (Packaging, Electrical & Electronics, Automotive, Aerospace & Defense, Others), and Regional Forecast, 2020–2027. Available online: https://www.fortunebusinessinsights.com/nanocomposites-market-104041 (accessed on 20 April 2022).
- Liu, P.; Hou, J.; Zhang, Y.; Li, L.; Lu, X.; Tang, Z. Two-dimensional material membranes for critical separations. Inorg. Chem. Front. 2020, 7, 2560–2581. [Google Scholar] [CrossRef]
- Konyukhova, E.V.; Buzin, A.I.; Godovsky, Y.K. Melting of polyether block amide (Pebax): The effect of stretching. Thermochim. Acta 2002, 391, 271–277. [Google Scholar] [CrossRef]
- Nguyen, Q.T.; Sublet, J.; Langevin, D.; Chappey, C.; Marais, S.; Valleton, J.-M.; Poncin-Epaillard, F. CO2 permeation with Pebax -based membranes for global warming reduction. In Membrane Gas Separation; Yampolskii, Y., Freeman, B., Eds.; Wiley: Chichester, UK, 2010; pp. 255–277. [Google Scholar]
- Yave, W.; Car, A.; Peinemann, K.-V. Nanostructured membrane material designed for carbon dioxide separation. J. Membr. Sci. 2010, 350, 124–129. [Google Scholar] [CrossRef]
- Marcq, J.; Quang, T.N.; Langevin, D.; Brule, B. Abatement of CO2 emissions by means of membranes—Characterization of industrial Pebax™ films. Environ. Prot. Eng. 2005, 31, 13–22. [Google Scholar]
- Kardani, R.; Asghari, M.; Mohammadi, T.; Afsari, M. Effects of nanofillers on the characteristics and performance of PEBA-based mixed matrix membranes. Rev. Chem. Eng. 2018, 34, 797–836. [Google Scholar] [CrossRef]
- Clarizia, G.; Bernardo, P. A review of the recent progress in the development of Nanocomposites based on poly(ether-block-amide) copolymers as membranes for CO2 separation. Polymers 2022, 14, 10. [Google Scholar] [CrossRef]
- Merkel, T.C.; Blanc, R.; Ciobanu, I.; Firat, B.; Suwarlim, A.; Zeid, J. Silver salt facilitated transport membranes for olefin/paraffin separations: Carrier instability and a novel regeneration method. J. Membr. Sci. 2013, 447, 177–189. [Google Scholar] [CrossRef]
- Kim, S.Y.; Cho, Y.; Kang, S.W. Correlation between Functional Group and Formation of Nanoparticles in PEBAX/Ag Salt/Al Salt Complexes for Olefin Separation. Polymers 2020, 12, 667. [Google Scholar] [CrossRef]
- Jeong, S.; Sohn, H.; Kang, S.W. Highly permeable PEBAX-1657 membranes to have long-term stability for facilitated olefin transport. Chem. Eng. J. 2018, 333, 276–279. [Google Scholar] [CrossRef]
- Kim, M.; Kang, S.W. PEBAX-1657/Ag nanoparticles/7,7,8,8-tetracyanoquinodimethane complex for highly permeable composite membranes with long-term stability. Sci. Rep. 2019, 9, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Cho, Y.; Kang, S.W. Preparation and Characterization of PEBAX-5513/AgBF4/BMIMBF4 Membranes for Olefin/Paraffin Separation. Polymers 2020, 12, 1550. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yan, M.; Feng, X.; Wang, X.; Huang, W. Ethylene/propylene separation using mixed matrix membranes of poly (ether block amide)/nano-zeolite (NaY or NaA). Korean J. Chem. Eng. 2021, 38, 576–586. [Google Scholar] [CrossRef]
- Pardo, F.; Gutiérrez-Hernández, S.V.; Zarca, G.; Urtiaga, A. Toward the Recycling of Low-GWP Hydrofluorocarbon/Hydrofluoroolefin Refrigerant Mixtures Using Composite Ionic Liquid–Polymer Membranes. ACS Sustain. Chem. Eng. 2021, 9, 7012–7021. [Google Scholar] [CrossRef]
- Ghanbari, H.; Hojjati, S.M.R.; Azizi, N. Acetone/Water Separation through Polydimethylsiloxane/PEBAX/Titania Nanocomposite Membrane by Pervaporation Method. J. Water Wastewater 2021, 32, 69–81. [Google Scholar]
- Cheshomi, N.; Pakizeh, M.; Namvar-Mahbou, M. Preparation and characterization of TiO2/Pebax/(PSf-PES) thin film nanocomposite membrane for humic acid removal from water. Polym. Adv. Technol. 2018, 29, 1303–1312. [Google Scholar] [CrossRef]
- Matavos-Aramyan, S.; Bagheri, G.; Jazebizadeh, M.H. Pervaporation Separation of Toluene from Aqueous Solutions Using Nano-Based PEBA/NaX Mixed Matrix Membrane. Silicon 2019, 11, 1725–1730. [Google Scholar] [CrossRef]
- Pan, F.; Li, W.; Zhang, Y.; Sun, J.; Wang, M.; Wu, H.; Jiang, Z.; Lin, L.; Wang, B.; Cao, X.; et al. Hollow monocrystalline silicalite-1 hybrid membranes for efficient pervaporative desulfurization. AIChE J. 2019, 65, 196–206. [Google Scholar] [CrossRef]
- Gu, J.; Zhang, X.; Bai, Y.; Yang, L.; Zhang, C.; Sun, Y. ZSM-5 Filled Polyether Block Amide Membranes for Separating EA from Aqueous Solution by Pervaporation. Int. J. Polym. Sci. 2013, 2013. [Google Scholar] [CrossRef]
- Vatani, M.; Raisi, A.; Pazuki, G. Pervaporation separation of ethyl acetate from aqueous solutions using ZSM-5 filled dual-layer poly(ether-block-amide)/polyethersulfone membrane. RSC Adv. 2018, 8, 4713–4725. [Google Scholar] [CrossRef]
- Vatani, M.; Raisi, A.; Pazuki, G. Three-component mixed matrix membrane containing [Hmim][PF6] ionic liquid and ZSM-5 nanoparticles based on poly (ether-block-amide) for the pervaporation process. J. Mol. Liq. 2019, 277, 471–480. [Google Scholar] [CrossRef]
- Liu, S.; Liu, G.; Zhao, X.; Jin, W. Hydrophobic-ZIF-71 filled PEBA mixed matrix membranes for recovery of biobutanol via pervaporation. J. Membr. Sci. 2013, 446, 181–188. [Google Scholar] [CrossRef]
- Liu, S.; Liu, G.; Shen, J.; Jin, W. Fabrication of MOFs/PEBA mixed matrix membranes and their application in bio-butanol production. Sep. Purif. Technol. 2014, 133, 40–47. [Google Scholar] [CrossRef]
- Liu, Q.; Li, Y.; Li, Q.; Liu, G.; Liu, G.; Jin, W. Mixed-matrix hollow fiber composite membranes comprising of PEBA and MOF for pervaporation separation of ethanol/water mixtures. Sep. Purif. Technol. 2019, 214, 2–10. [Google Scholar] [CrossRef]
- Zhang, A.-S.; Li, S.-H.; Ahmad, A.; Mao, H.; Xu, L.-H.; Zhao, Z.-P. Coordinate covalent grafted ILs-modified MIL-101/PEBA membrane for pervaporation: Adsorption simulation and separation characteristics. J. Membr. Sci. 2021, 619, 118807. [Google Scholar] [CrossRef]
- Li, Y.; Yan, D.; Wu, Y. Ionic liquid-modified MCM-41-polymer mixed matrix membrane for butanol pervaporation. R. Soc. Open Sci. 2019, 6, 190291. [Google Scholar] [CrossRef]
- Le, N.L.; Wang, Y.; Chung, T.-S. Pebax/POSS mixed matrix membranes for ethanol recovery from aqueous solutions via pervaporation. J. Membr. Sci. 2011, 379, 174–183. [Google Scholar] [CrossRef]
- Chen, J.H.; Su, Z.B.; Xu, J.P.; Lin, L.J.; Dong, X.F.; Peng, Q.; He, Y.S.; Nie, Y.J. Fabrication of PEBA/Cu2O mixed-matrix membranes and their application in pyridine recovery from aqueous solution. RSC Adv. 2017, 7, 22936–22945. [Google Scholar] [CrossRef]
- Pan, F.; Ding, H.; Li, W.; Song, Y.; Yang, H.; Wu, H.; Jiang, Z.; Wang, B.; Cao, X. Constructing facilitated transport pathway in hybrid membranes by incorporating MoS2 nanosheets. J. Membr. Sci. 2018, 545, 29–37. [Google Scholar] [CrossRef]
- Fang, L.J.; Chen, J.H.; Wang, J.M.; Lin, W.W.; Lin, X.G.; Lin, Q.J.; He, Y.S. Hydrophobic Two-Dimensional MoS2 Nanosheets Embedded in a Polyether Copolymer Block Amide (PEBA) Membrane for Recovering Pyridine from a Dilute Solution. ACS Omega 2021, 6, 2675–2685. [Google Scholar] [CrossRef]
- Najafi, M.; Mousavi, S.M.; Saljoughi, E. Preparation and characterization of poly(Ether block amide)/graphene membrane for recovery of isopropanol from aqueous solution via pervaporation. Polym. Compos. 2018, 39, 2259–2267. [Google Scholar] [CrossRef]
- Yu, S.; Jiang, Z.; Yang, S.; Ding, H.; Zhou, B.; Gu, K.; Yang, D.; Pan, F.; Wang, B.; Wang, S.; et al. Highly swelling resistant membranes for model gasoline desulfurization. J. Membr. Sci. 2016, 514, 440–449. [Google Scholar] [CrossRef]
- Wahab, M.S.A.; Rahman, S.A.; Samah, R.A. Super selective dual nature GO bridging PSF-GO-Pebax thin film nanocomposite membrane for IPA dehydration. Polym.-Plast. Technol. Mater. 2021, 60, 670–679. [Google Scholar]
- Mao, H.; Li, S.-H.; Xu, L.-H.; Wang, S.; Liu, W.-M.; Lv, M.-Y.; Lv, J.; Zhao, Z.-P. Zeolitic imidazolate frameworks in mixed matrix membranes for boosting phenol/water separation: Crystal evolution and preferential orientation. J. Membr. Sci. 2021, 638, 119611. [Google Scholar] [CrossRef]
- Mao, H.; Li, S.-H.; Zhang, A.-S.; Xu, L.-H.; Lu, H.-X.; Lv, J.; Zhao, Z.-P. Furfural separation from aqueous solution by pervaporation membrane mixed with metal organic framework MIL-53(Al) synthesized via high efficiency solvent-controlled microwave. Separ. Purif. Technol. 2021, 272, 118813. [Google Scholar] [CrossRef]
- Liu, L.; Li, Y.; Xu, M.; Wang, C. 2D Co-UMOFNs filled PEBA composite membranes for pervaporation of phenol solution. Separ. Purif. Technol. 2022, 285, 120414. [Google Scholar] [CrossRef]
- Selim, A.; Knozowsk, K.; Ośmiałowskic, B.; Kujawa, J.; Mizsey, P.; Kujawski, W. The fabrication, characterization, and pervaporation performance of poly(ether-block-amide) membranes blended with 4-(trifluoromethyl)-N(pyridine-2-yl)benzamide and 4-(dimethylamino)-N(pyridine-2-yl)benzamide fillers. Separ. Purif. Technol. 2021, 268, 118707. [Google Scholar] [CrossRef]
- Pan, F.; Wang, M.; He, D.; Song, Y.; Li, W.; Hong, W.; Jiang, Z.; Wang, B.; Cao, X. Embedding Ag+ @COFs within Pebax membrane to confer mass transport channels and facilitated transport sites for elevated desulfurization performance. J. Membr. Sci. 2018, 552, 1–12. [Google Scholar] [CrossRef]
- Mousavi, S.R.; Asghari, M.; Mahmoodi, N.M. Chitosan-wrapped multiwalled carbon nanotube as filler within PEBA thin film nanocomposite (TFN) membrane to improve dye removal. Carbohydr. Polym. 2020, 237, 116128. [Google Scholar] [CrossRef]
- Saadati, J.; Pakizeh, M. Separation of oil/water emulsion using a new PSf/pebax/F-MWCNT nanocomposite membrane. J. Taiwan Inst. Chem. Eng. 2017, 71, 265–276. [Google Scholar] [CrossRef]
- Sarwar, Z.; Yousef, S.; Tatariants, M.; Krugly, E.; Čiužas, D.; Danilovas, P.P.; Baltusnikas, A.; Martuzevicius, D. Fibrous PEBA-graphene nanocomposite filaments and membranes fabricated by extrusion and additive manufacturing. Eur. Polym. J. 2019, 121, 109317. [Google Scholar] [CrossRef]
- Nigiz, F.U.; Kibar, M.E. UV-assisted desalination of seawater using titanium dioxide nanotube doped polyether block amide membrane. Water Supply 2020, 20, 2185–2193. [Google Scholar] [CrossRef]
- Li, W.; Chang, Z.; Lin, L.; Xu, X. Effect of montmorillonite on PEBAX® 1074-based mixed matrix membranes to be used in humidifiers in proton exchange membrane fuel cells. e-Polymers 2020, 20, 171–184. [Google Scholar] [CrossRef]
- Wang, F.; Han, S.; Zhang, Y.; Gao, L.; Li, X.; Zhao, L.; Ye, H.; Li, H.; Xin, Q.; Zhang, Y. Constructing rapid water vapor transport channels within mixed matrix membranes based on two-dimensional mesoporous nanosheets. Commun. Chem. 2022, 5, 65. [Google Scholar] [CrossRef]
- Abi, Y.; Li, W.; Chang, Z. PEBAX 3533/PAA/CNC Composite Fiber Membranes as the Humidifier Membrane for Proton Exchange Membrane Fuel Cells. Ind. Eng. Chem. Res. 2022, 61, 1375–1385. [Google Scholar] [CrossRef]
- Zielińska, I.; Polak, D.; Szwast, M. Analysis of the adsorption of selected pharmaceuticals on a composite material PEBAX/GO. J. Water Process Eng. 2021, 44, 102272. [Google Scholar] [CrossRef]
- Sarwar, Z.; Tichonovas, M.; Krugly, E.; Masione, G.; Abromaitis, V.; Martuzevicius, D. Graphene oxide loaded fibrous matrixes of polyether block amide (PEBA) elastomer as an adsorbent for removal of cationic dye from wastewater. J. Environ. Manag. 2021, 298, 113466. [Google Scholar] [CrossRef]
- Zhao, B.; Zhang, X.; Deng, J.; Zhang, C.; Li, Y.; Guo, X.; Zhang, R. Flexible PEBAX/graphene electromagnetic shielding composite films with a negative pressure effect of resistance for pressure sensors applications. RSC Adv. 2020, 10, 1535–1543. [Google Scholar] [CrossRef]
- Wang, G.; Zhao, J.; Ge, C.; Zhao, G.; Park, C.B. Nanocellular poly(ether-block-amide)/MWCNT nanocomposite films fabricated by stretching-assisted microcellular foaming for high-performance EMI shielding applications. J. Mater. Chem. C 2021, 9, 1245–1258. [Google Scholar] [CrossRef]
- Wang, X.-Y.; Ma, L.; Zhao, Q.; Hyun, B.G.; Liu, H.; Yu, L.; Wang, J.; Park, C.B. Flexible Poly(ether-block-amide)/Carbon Nanotube Composites for Electromagnetic Interference Shielding. ACS Appl. Nano Mater. 2022, 5, 7598–7608. [Google Scholar] [CrossRef]
- Jo, Y.K.; Jeong, S.-Y.; Moon, Y.K.; Jo, Y.-M.; Yoon, J.-W.; Lee, J.-H. Exclusive and ultrasensitive detection of formaldehyde at room temperature using a flexible and monolithic chemiresistive sensor. Nat. Commun. 2021, 12, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Wylie, M.P.; Irwin, N.J.; Howard, D.; Heydon, K.; McCoy, C.P. Hot-melt extrusion of photodynamic antimicrobial polymers for prevention of microbial contamination. J. Photochem. Photobiol. B Biol. 2021, 214, 112098. [Google Scholar] [CrossRef] [PubMed]
- Borrego-Sánchez, A.; García-Villén, F.; Miele, D.; Rossi, S.; Sandri, G.; Viseras, C.; Constantino, V.R.L.; Figueiredo, M.P. Polymer/Iron-Based Layered Double Hydroxides as Multifunctional Wound Dressings. Pharmaceutics 2020, 12, 1130. [Google Scholar]
- Geng, W.; Jiang, X.; Liang, S.; Wang, F. Electrospun Functional Poly(ether amide) Composite Nanofibres. TechConnect Briefs 2015, 3, 293–296. [Google Scholar]
- Liang, S.; Zhang, G.; Min, J.; Ding, J.; Jiang, X. Synthesis and Antibacterial Testing of Silver/Poly (Ether Amide) Composite Nanofibers with Ultralow Silver Content. J. Nanomater. 2014, 2014, 684251. [Google Scholar] [CrossRef]
- Clarizia, G.; Bernardo, P.; Carroccio, S.C.; Ussia, M.; Restuccia, C.; Parafati, L.; Calarco, A.; Zampino, D. Heterogenized imidazolium-based ionic liquids in Pebax®Rnew. Thermal, gas transport and antimicrobial properties. Polymers 2020, 12, 1419. [Google Scholar] [CrossRef]
- Sole, B.B.; Lohkna, S.; Chhabra, P.K.; Prakash, V.; Seshadri, G.; Tyagi, A.K. Preparation of mechanically strong poly (ether block amide)/Mercaptoethanol breathable membranes for biomedical applications. J. Polym. Res 2018, 25, 200. [Google Scholar] [CrossRef]
- Sole, B.B.; Seshadri, G.; Tyagi, A.K.; Rattan, S. Effect of Sulphur-chlorine bifunctional diol (SCBD) on antimicrobial, thermal and mechanical behavior of polyether block amide (PEBA) based breathable membranes. J. Polym. Res. 2019, 26, 120. [Google Scholar] [CrossRef]
- Sole, B.B.; Seshadri, G.; Tyagi, A.K.; Rattan, S. Preparation of antibacterial and antifungal breathable polyether block amide/chloropropane diol membranes via solution casting. J. Appl. Polym. Sci. 2018, 135, 46097. [Google Scholar] [CrossRef]
- Préfol, T.; Gain, O.; Sudre, G.; Gouanvé, F.; Espuche, E. Development of breathable pebax®/peg films for optimization of the shelf-life of fresh agri-food products. Membranes 2021, 11, 692. [Google Scholar] [CrossRef]
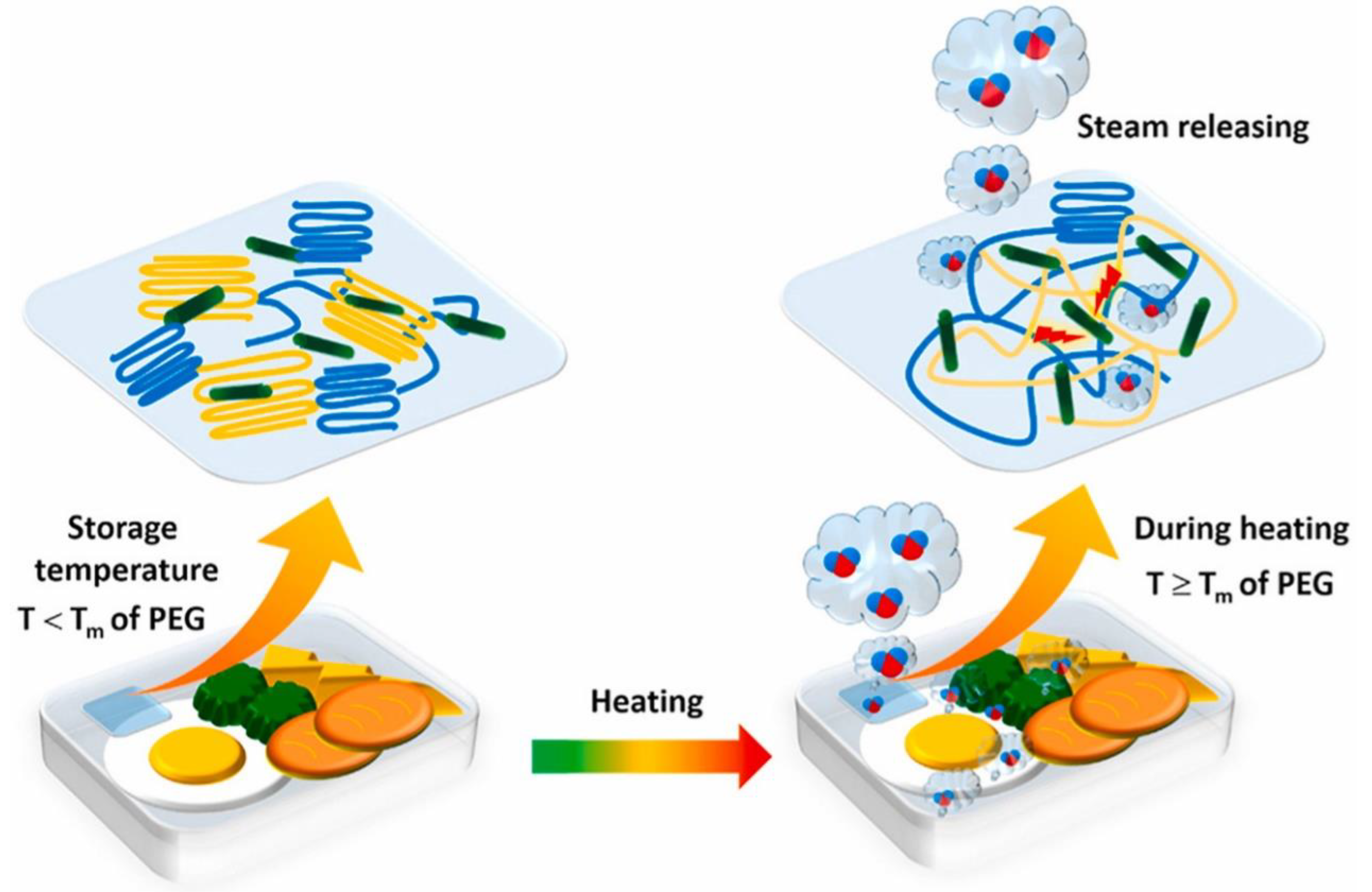
- Thanakkasaranee, S.; Sadeghi, K.; Seo, J. Smart steam release of newly developed temperature-responsive nanocomposite films derived from phase change material. Polymer 2021, 219, 123543. [Google Scholar] [CrossRef]
- Peterson, G.W.; Mundy, L. Incorporation of Metal–Organic Frameworks onto Polypropylene Fibers Using a Phase Inverted Poly(ether-block-amide) Glue. Ind. Eng. Chem. Res. 2022, 61, 13298–13302. [Google Scholar] [CrossRef]
- Eldridge, R.B. Olefin/paraffin separation technology: A review. Ind. Eng. Chem. Res. 1993, 32, 2208–2212. [Google Scholar] [CrossRef]
- Sholl, D.S.; Lively, R.P. Seven chemical separations to change the world. Nature 2016, 532, 435–437. [Google Scholar] [CrossRef] [PubMed]
- Ferraz, H.C.; Duarte, L.T.; Di Luccio, M.; Alves, T.L.M.; Habert, A.C.; Borges, C.P. Recent achievements in facilitated transport membranes for separation processes. Braz. J. Chem. Eng. 2007, 24, 101–118. [Google Scholar] [CrossRef]
- Merkel, T.C.; He, Z.; Morisato, A.; Pinnau, I. Olefin/paraffin solubility in a solid polymer electrolyte membrane. Chem. Commun. 2003, 1596–1597. [Google Scholar] [CrossRef]
- Feng, X.; Huang, R. Liquid Separation by Membrane Pervaporation: A Review. Ind. Eng. Chem. Res. 1997, 36, 1048–1066. [Google Scholar] [CrossRef]
- Kujawski, W. Pervaporation and vapors separation. In Membranes Theory and Practice; Wodzki, R., Ed.; Stowarzyszenie na Rzecz Rozwoju Wydziału Chemii UMK: Toruń, Poland, 2009; Zeszyt III; pp. 110–122. [Google Scholar]
- Liu, F.; Liu, L.; Feng, X. Separation of acetone–butanol–ethanol (ABE) from dilute aqueous solutions by pervaporation. Sep. Purif. Technol. 2005, 42, 273–282. [Google Scholar] [CrossRef]
- Mao, G.; Gao, Y.; Zhou, H.; Jin, W. Tuning of solvent evaporation to prepare PEBA membrane with high separation performance for the pervaporation of phenol aqueous solution. J. Membr. Sci. 2022, 656, 120638. [Google Scholar] [CrossRef]
- Lyons, J.G.; Kennedy, J.E.; Lordan, S.; Geever, L.M.; Higginbotham, C.L. Characterisation of the effects of a titanium micro particle filler on a polyether-block-amide host matrix. J. Mater. Sci. 2010, 45, 3204–3214. [Google Scholar] [CrossRef]
- Lara-Estévez, J.C.I.; Sanchez de Almeida Prado, L.A.; Schulte, K.; Bucio, E. PEBAXTM-Silanized Al2O3 Composite, Synthesis and Characterization. Open J. Polym. Chem. 2012, 2, 63–69. [Google Scholar] [CrossRef]
- Barrer, R.M. Hydrothermal Chemistry of Zeolites; Academic Press: London, UK, 1982. [Google Scholar]
- Rowsell, J.L.C.; Yaghi, O.M. Metal–organic frameworks: A new class of porous materials. Microporous Mesoporous Mater. 2004, 73, 3–14. [Google Scholar] [CrossRef]
- Zhang, Y.; Song, J.; Quispe Mayta, J.; Pan, F.; Gao, X.; Li, M.; Song, Y.; Wang, M.; Cao, X.; Jiang, Z. Enhanced desulfurization performance of hybrid membranes using embedded hierarchical porous SBA-15. Front. Chem. Sci. Eng. 2020, 14, 661–672. [Google Scholar] [CrossRef]
- Kamble, A.R.; Patel, C.M.; Murthy, Z.V.P. A review on the recent advances in mixed matrix membranes for gas separation processes. Renew. Sustain. Energy Rev. 2021, 145, 111062. [Google Scholar] [CrossRef]
- Falla, W.R.; Mulski, M.; Cussler, E.L. Estimating diffusion through flake-filled membranes. J. Membr. Sci. 1996, 119, 129–138. [Google Scholar] [CrossRef]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Althumayri, K.; Harrison, W.J.; Shin, Y.; Gardiner, J.M.; Casiraghi, C.; Budd, P.M.; Bernardo, P.; Clarizia, G.; Jansen, J.C. The influence of graphene and other nanofillers on the gas permeability of the high-free-volume polymer PIM-1. Philos. Trans. R. Soc. A 2016, 374, 20150031. [Google Scholar] [CrossRef] [PubMed]
- Sampranpiboon, P.; Jiraratananon, R.; Uttapap, D.; Feng, X.; Huang, R.Y.M. Pervaporation separation of ethyl butyrate and isopropanol with polyether block amide (PEBA) membranes. J. Membr. Sci. 2000, 173, 53–59. [Google Scholar] [CrossRef]
- Mohammadi, T.; Kikhavandi, T.; Moghbeli, M. Synthesis and characterization of poly(ether-block-amide) membranes for the pervaporation of organic/aqueous mixtures. J. Appl. Polym. Sci. 2008, 107, 1917. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- van der Bruggen, B.; Luis, P. Pervaporation. In Progress in Filtration and Separation; Tarleton, S., Ed.; Academic Press: Oxford, UK, 2015; pp. 101–154. [Google Scholar]
- Raghunath, B.; Hwang, S.T. Effect of boundary layer mass transfer resistance in the pervaporation of dilute organics. J. Membr. Sci. 1992, 65, 147–161. [Google Scholar] [CrossRef]
- Staffell, I.; Scamman, D.; Velazquez Abad, A.; Balcombe, P.; Dodds, P.E.; Ekins, P.; Shah, N.; Ward, K.R. The role of hydrogen and fuel cells in the global energy system. Energy Environ. Sci. 2019, 12, 463–491. [Google Scholar] [CrossRef]
- Potreck, J.; Nijmeijer, K.; Kosinski, T.; Wessling, M. Mixed water vapor/gas transport through the rubbery polymer PEBAX 1074. J. Membr. Sci. 2009, 338, 11–16. [Google Scholar] [CrossRef]
- Park, S.-K.; Cho, E.A.; Oh, I.-H. Characteristics of membrane humidifiers for polymer electrolyte Membrane fuel cells. Korean J. Chem Eng. 2005, 22, 877–881. [Google Scholar] [CrossRef]
- Yang, L.; Luo, D.; Zheng, Y.; Yang, T.; Ma, Q.; Nie, Y.; Dou, H.; Zhang, Y.; Huang, R.; Yu, A.; et al. Heterogeneous Nanodomain Electrolytes for Ultra-Long-Life All-Solid-State Lithium-Metal Batteries. Adv. Funct. Mater. 2022, 32, 2204778. [Google Scholar] [CrossRef]
- Yang, L.; Nie, Y.; Liu, Y.; Zheng, Y.; Luo, D.; Yang, N.; Ma, Q.; Xu, M.; Ma, X.; Yu, A.; et al. The plasticizer-free composite block copolymer electrolytes for ultralong lifespan all-solid-state lithium-metal batteries. Nano Energy 2022, 100, 107499. [Google Scholar] [CrossRef]
- Ajkidkarn, P.; Manuspiya, H. Solution plasma synthesis of bacterial cellulose acetate derived from nata de coco waste incorporated with polyether block amide. Int. J. Biol. Macromol. 2022, 209, 1486–1497. [Google Scholar] [CrossRef]
- Bhat, A.H.; Rehman, W.U.; Khan, I.U.; Khan, I.; Ahmad, S.; Ayoub, M.; Usmani, M.A. 15-Nanocomposite membrane for environmental remediation. In Polymer-Based Nanocomposites for Energy and Environmental Applications; Jawaid, M., Khan, M.M., Eds.; Woodhead Publishing Series in Composites Science and Engineering: Oxford, UK, 2018; pp. 407–440. [Google Scholar]
- Homaeigohar, S.; Elbahri, M. Nanocomposite Electrospun Nanofiber Membranes for Environmental Remediation. Materials 2014, 7, 1017–1045. [Google Scholar] [CrossRef]
- Algieri, C.; Chakraborty, S.; Candamano, S. A Way to Membrane-Based Environmental Remediation for Heavy Metal Removal. Environments 2021, 8, 52. [Google Scholar] [CrossRef]
- Wang, X.; Chen, X.; Yoon, K.; Fang, D.; Hsiao, B.S.; Chu, B. High flux filtration medium based on nanofibrous substrate with hydrophilic nanocomposite coating. Environ. Sci. Technol. 2005, 39, 7684–7691. [Google Scholar] [CrossRef]
- Dzenis, Y. Spinning continuous fibers for nanotechnology. Science 2004, 304, 1917–1919. [Google Scholar] [CrossRef] [PubMed]
- Sarwar, Z.; Krugly, E.; Danilovas, P.P.; Ciuzas, D.; Kauneliene, V.; Martuzevicius, D. Fabrication and characterization of PEBA fibers by melt and solution electrospinning. J. Mater. Res. Technol. 2019, 8, 6074–6085. [Google Scholar] [CrossRef]
- Zastrow, M. 3D printing gets bigger, faster and stronger. Nature 2020, 578, 20–23. [Google Scholar] [CrossRef] [PubMed]
- Ngo, T.D.; Kashani, A.; Imbalzano, G.; Nguyen, K.T.Q.; Hui, D. Additive manufacturing (3D printing): A review of materials, methods, applications and challenges. Compos. Part B Eng. 2018, 143, 172–196. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Tan, W.S.; An, J.; Chua, C.K.; Tang, C.Y.; Fane, A.G.; Chong, T.H. The potential to enhance membrane module design with 3D printing technology. J. Membr. Sci. 2016, 499, 480–490. [Google Scholar] [CrossRef]
- Low, Z.-X.; Chua, Y.T.; Ray, B.M.; Mattia, D.; Metcalfe, I.S.; Patterson, D.A. Perspective on 3D printing of separation membranes and comparison to related unconventional fabrication techniques. J. Membr. Sci. 2017, 523, 596–613. [Google Scholar] [CrossRef]
- Tijing, L.D.; Dizon, J.R.C.; Ibrahim, I.; Nisay, A.R.N.; Shon, H.K.; Advincula, R.C. 3D printing for membrane separation, desalination and water treatment. Appl. Mater. Today 2020, 18, 100486. [Google Scholar] [CrossRef]
- Tsai, H.-Y.; Huang, A.; Soesanto, J.F.; Luo, Y.-L.; Hsu, T.-Y.; Chen, C.-H.; Hwang, K.-J.; Ho, C.-D.; Tung, K.-L. 3D printing design of turbulence promoters in a cross-flow microfiltration system for fine particles removal. J. Membr. Sci. 2019, 573, 647–656. [Google Scholar] [CrossRef]
- Chang, H.; Ho, C.-D.; Chen, Y.-H.; Chen, L.; Hsu, T.-H.; Lim, J.-W.; Chiou, C.-P.; Lin, P.-H. Enhancing the Permeate Flux of Direct Contact Membrane Distillation Modules with Inserting 3D Printing Turbulence Promoters. Membranes 2021, 11, 266. [Google Scholar] [CrossRef]
- Frah, M.A.A.; Pavlushkina, T.; Babinova, A.; Belyaev, V.; Makeev, M.; Osipkov, A.; Konopleva, E.; Zhachkin, V.; Usachev, V. Protection from electromagnetic pollution by using metal based shielding materials. J. Phys. Conf. Ser. 2021, 2056, 012058. [Google Scholar] [CrossRef]
- Liu, C.; Wang, L.; Liu, S.; Tong, L.; Liu, X. Fabrication strategies of polymer-based electromagnetic interference shielding materials. Adv. Ind. Eng. Polym. Res. 2020, 3, 149–159. [Google Scholar] [CrossRef]
- Vadgama, P. Membrane based sensors: A review. J. Membr. Sci. 1990, 50, 141–152. [Google Scholar] [CrossRef]
- Yatsuzuka, K.; Higashiyama, Y.; Asano, K. Fundamental Characteristics of Hydrophilic Polymer (Polyether Block Amide) as a Humidity Sensor. Jpn. J. Appl. Phys. 1993, 32, L461. [Google Scholar] [CrossRef]
- Dong, D.; Ma, J.; Ma, Z.; Chen, Y.; Zhang, H.; Shao, L.; Gao, J.; Wei, L.; Wei, A.; Kang, S. Flexible and lightweight microcellular RGO@Pebax composites with synergistic 3D conductive channels and microcracks for piezoresistive sensors. Compos. Part A Appl. Sci. Manuf. 2019, 123, 222–231. [Google Scholar] [CrossRef]
- Dal Pont, K.; Serghei, A.; Espuche, E. Multifunctional Pd-Based Nanocomposites with Designed Structure from In Situ Growth of Pd Nanoparticles and Polyether Block Amide Copolymer. Polymers 2021, 13, 1477. [Google Scholar] [CrossRef] [PubMed]
- Boyden, B.G.; Nilajkar, A.; O’Neil, C.J. The Effects of Type and Loading of Radiopaque Fillers on the Properties of Polyether Block Amide Compounds. Plast. Eng. 2013, 69, 18–22. [Google Scholar] [CrossRef]
- Halim, K.A.A.; Kennedy, J.E.; Farrell, J.B. Preparation and characterisation of polyether-block-amide/montmorillonite (MMT) nanocomposites for use in angioplasty balloon applications In Proceedings of the 1st International Malaysia Ireland Joint Symposium (IMiEJS 2011), Athlone, Ireland, 9–11 June 2011.
- Murray, K.A.; Kennedy, J.E.; McEvoy, B.; Vrain, O.; Ryan, D.; Cowman, R.; Higginbotham, C.L. Effects of gamma ray and electron beam irradiation on the mechanical, thermal, structural and physicochemical properties of poly (ether-block-amide) thermoplastic elastomers. J. Mech. Behav. Biomed. Mater. 2013, 17, 252–268. [Google Scholar] [CrossRef]
- Murray, K.A.; Kennedy, J.E.; McEvoy, B.; Vrain, O.; Ryan, D.; Cowman, R.; Higginbotham, C.L. Effects of temperature, packaging and electron beam irradiation processing conditions on the property behaviour of Poly (ether-block-amide) blends. Mater. Sci. Eng. C 2014, 39, 380–394. [Google Scholar] [CrossRef]
- Karode, N.; Poudel, A.; Fitzhenry, L.; Matthews, S.; Redington, W.; Walsh, P.; Coffey, A. Crystallisation behaviour of Pebax graphene composite matrix with and without supercritical carbon dioxide assisted polymer processing technique. Cryst. Growth Des. 2018, 18, 3938–3952. [Google Scholar] [CrossRef]
- Sliwa, F.; Bounia, E.L.; Marin, G.; Charrier, F.; Malet, F. A new generation of wood polymer composite with improved thermal stability. Polym. Degrad. Stab. 2012, 97, 496–503. [Google Scholar] [CrossRef]
- Farsi, M.; Tavasoli, A.; Yousefi, H.; Tabar, H.Z. A study on the thermal and mechanical properties of composites made of nanolignocellulose and Pebax® polymer. J. Thermoplast. Compos. Mater. 2019, 32, 1509–1524. [Google Scholar] [CrossRef]
- An, L.; Zhang, N.; Zeng, X.; Zhong, B.; Yu, Y. Quasi-isotropically thermoconductive, antiwear and insulating hierarchically assembled hexagonal boron nitride nanosheet/epoxy composites for efficient microelectronic cooling. J. Colloid Interface Sci. 2022, 608, 1907–1918. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Zhang, G.; Dong, Y.; Zhang, Z.; Xue, S.; Jiang, X. Polyetheramide Templated Synthesis of Monodisperse Mn3O4 Nanoparticles with Controlled Size and Study of the Electrochemical Properties. Nano-Micro Lett. 2014, 6, 38–45. [Google Scholar] [CrossRef]


| Grade | Polyamide (PA) | Polyether (PE) | ||
|---|---|---|---|---|
| Type | Amount (wt%) | Type | Amount (wt%) | |
| 5513 | PA6 | 60 | PTMO | 40 |
| 2080 | PA12 | 50 | PEO | 50 |
| 1074 | PA12 | 45 | PEO | 55 |
| 1657 | PA6 | 40 | PEO | 60 |
| 3533 | PA12 | 30 | PTMO | 70 |
| 2533 | PA12 | 20 | PTMO | 80 |
| Pebax Grade | Filler Type | Loading (wt%) | Separation | Ref. |
|---|---|---|---|---|
| Gas Separation (Olefin/Paraffin) | ||||
| 2533 on a poly(vinylidene fluoride) (PVDF) support | AgBF4 | 20–80 | Ethylene/ethane Propylene/propane | [11] |
| 2533 on a polysulfone (PSf) support | AgBF4/Al(NO3)3 (1/0.1 mol/mol) | 9.7 (AgBF4) | Propylene/propane | [12] |
| 1657 on a PSf support | AgBF4/Al(NO3)3 (1/0.1 mol/mol) | 17 (AgBF4) | Propylene/propane | [13] |
| 1657 on a PSf support | Silver nanoparticle/7,7,8,8-tetracyanoquinodimethane (AgNPs/TCNQ) | 87 | Propylene/propane | [14] |
| 5513 on a PSf support | AgBF4/[BMIM][BF4] | 10 (AgBF4) | Propylene/propane | [15] |
| 2533 | nano-zeolite (NaY or NaA) | 3–10 | Ethylene/propylene | [16] |
| 1657 | Ionic Liquids (ILs) | 20–60 | Recovery of hydrofluorocarbons (HFCs) and hydrofluoroolefins (HFO) from exhausted refrigerant mixtures | [17] |
| Pervaporation | ||||
| 2533 on a PDMS support | TiO2 | up to 1.5 | Recovery of acetone from wastewater | [18] |
| 2533 on a PSf-PES blend support | TiO2 nanoparticles | 0.01–0.05% in the coating solution | Removal of humic acid from water | [19] |
| 2533 | NaX nano-zeolite | 2 | Toluene from aqueous solutions | [20] |
| 2533 | Hollow monocrystalline silicalite-1 (HMS) (200 nm and 350 nm) | 10–50 | Thiophene/n-octane (gasoline desulfurization) | [21] |
| 2533 | ZSM-5 (3–5 micron) | 10–40 | Ethyl acetate from water | [22] |
| 2533 on a polyethersulfone (PES) support | ZSM-5 (35 nm) | 5–15 | Ethyl acetate from water | [23] |
| 2533 on a PES support | ZSM-5 (35 nm) and [Hmim][PF6] | 1% ZSM-5, 2.5% IL | Ethyl acetate from water | [24] |
| 2533 on a PVDF support | Organophilic zeolitic imidazolate frameworks (ZIF-71) | 10–25 | Recovery of biobutanol from acetone–butanol–ethanol (ABE) broth | [25] |
| 2533 on a PVDF support | Zn(BDC)(TED)0.5 | up to 20 | Recovery of biobutanol from acetone–butanol–ethanol (ABE) broth | [26] |
| 2533 on a ceramic support Hollow fibre | MAF-6 | up to 20 | Ethanol from water | [27] |
| 2533 on a PVDF support | ILTf2N@MIL-101 | up to 10 | Ethyl acetate from water | [28] |
| 2533 | Mesoporous molecular sieve MCM-41 modified with [EVIM][Tf2N] (IL1) | 2–10 | Butanol from water | [29] |
| 2533 | Mesoporous molecular sieve MCM-41 modified with [OMPY][Tf2N] (IL2) | 2–10 | Butanol from water | [29] |
| 2533 | Polyhedral oligomeric silsesquioxane (POSS) | 2–10 | Ethanol from water | [30] |
| 2533 | Cu2O nanocrystals | up to 15 | Recovery of Pyridine from water | [31] |
| 2533 on a PSf support | MoS2 Two-dimensional nanosheets | up to 6 | Gasoline desulfurization (Thiophene/n-octane) | [32] |
| 2533 | MoS2 Two-dimensional nanosheets | up to 20 | Recovery of Pyridine from a dilute solution | [33] |
| 2533 | Graphene nanosheets (thickness of 2–18 nm and less than 32 layers) | up to 2 | Recovery of isopropanol from aqueous solution | [34] |
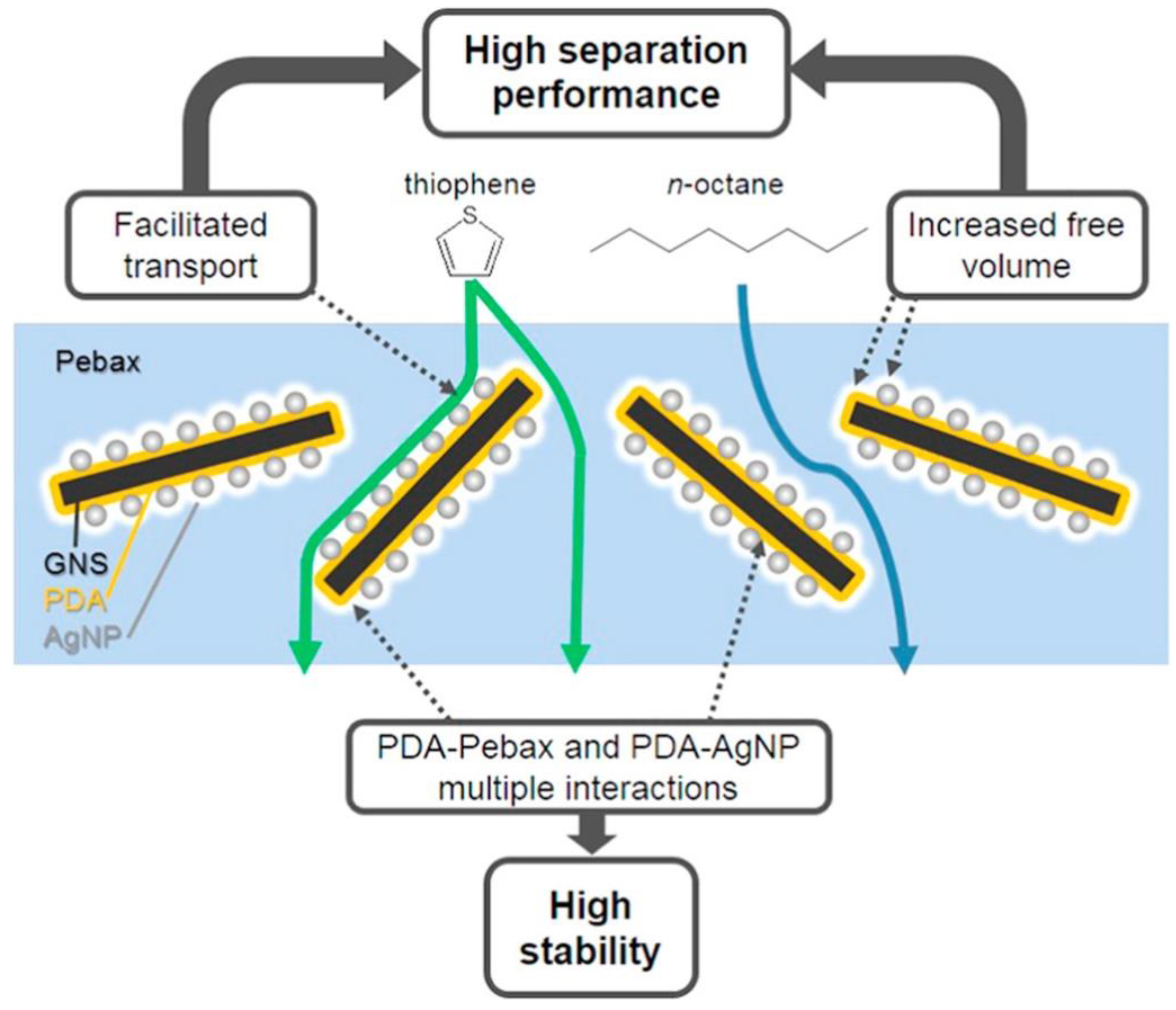
| 2533 on a PSf support | Graphene nanosheets with a dopamine coating and Ag nanoparticle loading (Ag-PDA/GNS) | up to 8 | Gasoline desulfurization (Thiophene/n-octane) | [35] |
| 1657 on a PSf support | Graphene Oxide (GO) | 0.05–0.5 | IPA dehydration | [36] |
| 2533 on a PVDF support | Anisotropic ZIF-L nanosheets (ZLNs) | up to 8 | Phenol from water | [37] |
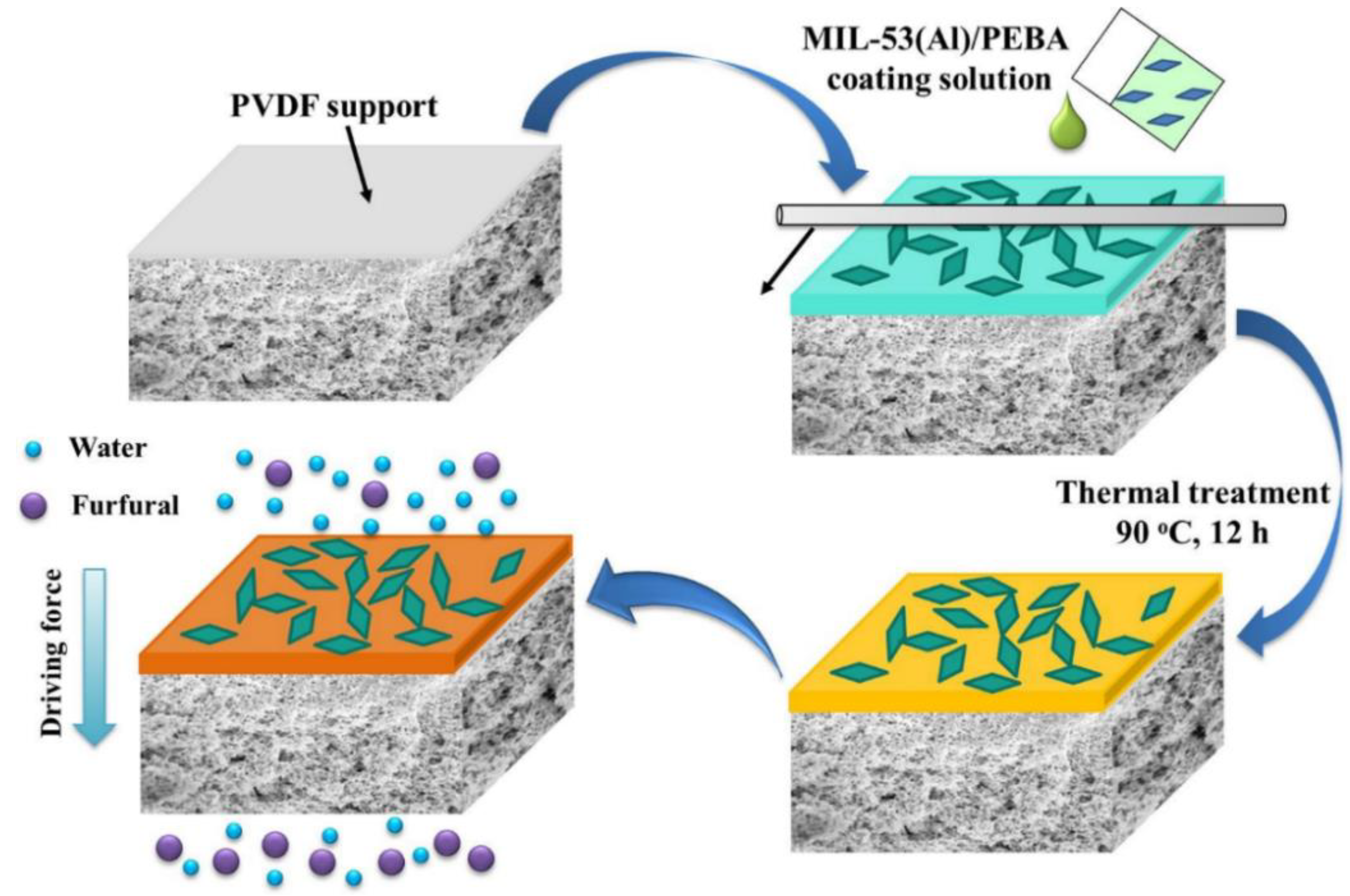
| 2533 on a PVDF support | Metal–organic framework MIL-53(Al) | up to 20 | Furfural from water | [38] |
| 2533 on a PVDF support | Co-UMOFNs | 7.5–40 | Phenol from water | [39] |
| 2533 | 4-(trifluoromethyl)-N-(pyridine-2-yl) benzamide (denoted as F1) | 2.5–10 | Ethanol/water | [40] |
| 2533 | 4-(dimethylamino)-N-(pyridine-2-yl) benzamide (denoted as F2) | 2.5–10 | Ethanol/water | [40] |
| 2533 on a PSf support | Ag-loaded covalent organic frameworks (Ag+@COFs) | 3–15 | Gasoline desulfurization | [41] |
| Water treatment/desalination | ||||
| 1657 on an ultraporous PES support | Chitosan-wrapped multiwalled carbon nanotubes (CWNTs) | up to 2 | Dye removal from water | [42] |
| 2533 on a PSf support | Functionalized multiwall carbon nanotubes (F-MWCNTs) | up to 2 | Nanofiltration of oil/water emulsion | [43] |
| 3533 | Graphene (thickness 10–20 nm, a few micrometers in length) | 0.05–0.4 | Cleanup of oil spills | [44] |
| 1657 | TiO2 nanotubes | 2.5–10 | UV-assisted desalination of seawater | [45] |
| Humidifier membrane in PEMFCs | ||||
| 1074 | APOP-MMT | up to 10.7 | Water vapour/air | [46] |
| 1074 | 2D mesoporous nanosheets of cerium fluoride oxide (F-Ce) and their composite with the IL [Emim][DCA] (IL@F-Ce) | up to 10 | Water vapour/air | [47] |
| 3533 | Cellulose nanocrystals (CNCs) and polyacrylic acid (PAA) | up to 9.1 | Water vapour/air | [48] |
| Pebax Grade | Filler Type | Loading (wt%) | Application | Ref. |
|---|---|---|---|---|
| 2533 | GO | 5 | Removal of pharmaceuticals from wastewater by adsorption | [49] |
| 3533 | GO on PEBA melt electrospun fibre | 0.5–2.0 | Removal of cationic dye from wastewater by adsorption | [50] |
| 2533 | Graphene (surface area of 50–200 m2 g−1, thickness of 1–3 layers) | 1–20 | Electromagnetic shielding | [51] |
| 7233 | MWCNT (outer diameters from 10 to 20 nm, average length of 50 μm) | 5 | Electromagnetic shielding | [52] |
| 4533 2533 2080 | MWCNT (average diameter of 9.5 nm, average length of 1.5 μm) | up to 15 | Electromagnetic shielding | [53] |
| 1657 | ZIF-7 | 2.5–40 | Sensors | [54] |
| 2533 7033 7233 | Photosensitiser, toluidine blue O (TBO) | up to 0.1 | Photodynamic antimicrobial materials | [55] |
| 2533 | Layered double hydroxides (LDH) | up to 13 | Scaffolds as wound dressings | [56] |
| 2533 | LiCl·H2O | 6.7–44.4 | Nanofibers as antibacterial materials | [57] |
| 2533 | AgNO3 | 0.05–0.25‰ | Nanofibers as antibacterial materials | [58] |
| Rnew 25R53 | ILs ([Hdmim][DMSIP], [OOMmim][PF6]) | up to 5 | Antimicrobial films | [59] |
| 1657 | Mercaptoethanol (ME) | 10–40 | Breathable films | [60] |
| 1657 | Sulphur–Chlorine Bifunctional Diol (SCBD) | up to 10 | Breathable films | [61] |
| 1657 | Chloropropane diol (CPD) | 10–40 | Breathable and Antimicrobial films | [62] |
| 1657 | Polyethylene glycol dimethyl ether (PEGDME) and/or hydroxyl-terminated polyethylene glycol (PEGOH) | up to 50 | Breathable films for packaging | [63] |
| 1657 and PEG | Halloysite nanotubes (HNTs) | up to 15 | Films for packaging | [64] |
| 2533 on PP mat | UiO-66-NH2 and HKUST-1 | up to 40 | Materials for personal protective equipment against toxic chemicals | [65] |
| Pebax Grade | Filler Type | Loading (wt%) | Separation | Permeation Flux | Selectivity | Ref. |
|---|---|---|---|---|---|---|
| 2533 on a on a poly(vinylidene fluoride) (PVDF) support | AgBF4 | 67 | Ethylene/ethane | 21 GPU | 210 | [11] |
| 2533 on a PVDF support | AgBF4 | 80 | Ethylene/ethane (mixed gas, 65% C2H4, 35% C2H6) | 79 GPU | 33 | [11] |
| 16 GPU (aged 9 months) | 2.5 | |||||
| 2533 on a PVDF support | AgBF4 using H2O2/HBF4 in the dope | 80 | Ethylene/ethane (mixed gas, 65% C2H4, 35% C2H6) | 49 GPU | 35 | [11] |
| 29 GPU (aged 9 months) | 39 | |||||
| 2533 on a PVDF support | AgBF4 | 40 | Propylene/propane | 10 GPU | 30 | [11] |
| 2533 on a PSf support | AgBF4/Al(NO3)3 (1/0.1 mol/mol) | 9.7 (AgBF4) | Propylene/propane (mixed gas, 50:50) | 10 GPU | 5 | [12] |
| 1657 on a PSf support | AgBF4/Al(NO3)3 (1/0.1 mol/mol) | 17 (AgBF4) | Propylene/propane (mixed gas, 50:50) | 22.5 GPU | 8.8 | [13] |
| 1657 on a PSf support | AgNPs/TCNQ | 87 | Propylene/propane (mixed gas, 50:50) | 10.2 GPU | 12.7 | [14] |
| 5513 on a PSf support | AgBF4/ [Bmim][BF4] | 10 (AgBF4) | Propylene/propane (mixed gas, 50:50) | 12.3 GPU | 3 | [15] |
| 2533 | NaY | 6 | Propylene/ethylene (mixed gas, 80:20) | 262 Barrer | 13.1 | [16] |
| 1657 | - | - | HFC/HFO mixture, R454B (83% R32, 17% R1234yf) | 197 Barrer (R32) | 8.0 | [17,21] |
| 1657 | IL [C2mim][BF4] | 40 | HFC/HFO mixture, R454B (83% R32, 17% R1234yf) | 300 Barrer (R32) | 15 | [17] |
| Pebax Grade | Filler Type | Loading (wt%) | Separation | T (°C) | Feed Conc. | Permeation Flux (g/(m2 h)) | Separation Factor (SF) | Ref. |
|---|---|---|---|---|---|---|---|---|
| 2533 | - | - | Ethanol from water | 23 | 5 wt% ethanol | 118 (thickness 30 micron) | 2.5 | [72] |
| 2533 on a ceramic support hollow fibre | MAF-6 | 7.5 | Ethanol from water | 60 | 5 wt% ethanol | 4446 | 5.6 | [27] |
| 2533 | POSS (AL0136) | 2 | Ethanol from water | Room T | 5 wt% ethanol | 183.5 | 4.6 | [30] |
| 2533 | POSS (SO1440) | 2 | Ethanol from water | Room T | 5 wt% ethanol | 125.8 | 4.1 | [30] |
| 2533 | 4-(trifluoromethyl)-N-(pyridine-2-yl)benzamide (F1) | 2.5 | Ethanol from water | 60 | 5 wt% ethanol | 199 | 4.6 PSI = 916 g/(m2 h) | [40] |
| 2533 | 4-(dimethylamino)-N-(pyridine-2-yl)benzamide (F2) | 10 | Ethanol from water | 60 | 5 wt% ethanol | 173 | 4.6 PSI = 797 g/(m2 h) | [40] |
| 3533 | - | - | IPA from water | 30 | 10 wt% isopropanol | 300 | 3.5 PSI = 750 g/(m2 h) | [83] |
| 1074 | - | - | IPA from water | 50 | 10 wt% isopropanol | 260 | 2.4 PSI = 364 g/(m2 h) | [84] |
| 2533 | Graphene | 1.5 | IPA from water | 50 | 4 wt% isopropanol | 842; Isopropanol: 249 | 10.0 PSI = 7620 g/(m2 h) | [34] |
| 2533 | - | - | Butanol from water | 23 | 5 wt% butanol | 179 (thickness 30 micron) | 5.9 | [72] |
| 2533 | Mesoporous molecular sieve MCM-41 modified with [OMPY][Tf2N] (IL2) | 5 | Butanol from water | 35 | 2.5 wt% butanol | 422 | 25 | [29] |
| 2533 | Mesoporous molecular sieve MCM-41 modified with [EVIM][Tf2N] (IL1) | 5 | Butanol from water | 35 | 2.5 wt% butanol | 420 | 23 | [29] |
| 2533 on a PVDF support | ZIF-71 particles (average size of 1 µm) | 20 | Biobutanol from acetone–butanol–ethanol (ABE) broth | 37 | 0.6 wt% acetone, 1.2 wt% butanol and 0.2 wt% ethanol | Total flux: 520.2 | n-butanol SF: 18.8 PSI = 9780 g/(m2 h) | [25] |
| 2533 on a PVDF support | Zn(BDC)(TED)0.5 | 20 | Biobutanol from acetone–butanol–ethanol (ABE) broth | 40 | 0.6 wt% acetone, 1.2 wt% butanol and 0.2 wt% ethanol | Total flux: 630.2 | n-butanol SF: 17.4 PSI = 10,330 g/(m2 h) | [26] |
| 1074 | - | - | Phenol from water | 80 | 1.5 wt% phenol | 1500 | 78 | [73] |
| 2533 | - | - | Phenol from water | 60 | 1.5 wt% phenol | 2016 | 40 | [73] |
| 2533 | - | - | Phenol from water | 70 | 1.5 wt% phenol | 2133 | 64 | [73] |
| 2533 on a PVDF support | ZIF-L anisotropic nanosheets (ZLNs) (ZLN-0.05) | 4 | Phenol from water | 50 | 1000 ppm phenol | Total flux: 4792; phenol permeance: 4.96 × 105 GPU | 14.4 phenol/water selectivity: 32.5 | [37] |
| 2533 on a PVDF support | - | - | Phenol from water | 70 | 1000 ppm phenol | 1.17 | 27.4 | [39] |
| 2533 on a PVDF support | Co-UMOFNs | 40 | Phenol from water | 70 | 1000 ppm phenol | 420 | 45.5 | [39] |
| 40 | Phenol from water | 80 | 1000 ppm phenol | 603 | 48.0 | [39] | ||
| 1657 on a PSf blend support | Graphene Oxide (GO) | 0.4 for PSf | IPA dehydration | 30 | 20 wt% water | 1190 | 0 wt% IPA in permeate | [36] |
| Pebax Grade | Filler Type | Loading (wt%) | Organic Separated from Water | T (°C) | Organic Conc. in the Feed | Permeation Flux | Separation Factor (SF) | Ref. |
|---|---|---|---|---|---|---|---|---|
| 2533 | MIL-53(Al) | 15 | Furfural | 80 | 1 wt% | 3800 g/(m2 h) | 50.2 | [38] |
| 2533 | Cu2O | 6 | Pyridine | 30 | 1 wt% | 89.3 g/(m2 h) (−13.1%) | 10.2 (+22.8%) | [31] |
| 2533 | Cu2O | 6 | Pyridine | 70 | 1 wt% | 230 g/(m2 h) | 18 PSI = 3910 g/(m2 h) | [31] |
| 2533 | MoS2 | 10 | Pyridine | 30 | 1 wt% | 83.4 g/(m2 h) (−21.5%) | 11.1 (+37.6%) PSI = 855 g/(m2 h) (+16%) | [33] |
| 2533 | MoS2 | 10 | Pyridine | 70 | 1 wt% | 215 g/(m2 h) | 17.1 PSI = 3462 g/(m2 h) | [33] |
| 2533 | NaX | 2 | Toluene | – | 0.01 mg/L | 80 g/(m2 h) | 150 PSI = 11,500 g/(m2 h) Enrichment factor (β) = 920 | [20] |
| 2533 | ZSM-5 (3- micron) | 10 | Ethyl acetate | 50 | 5 wt% | 200 g/(m2 h) | 186 | [22] |
| 2533 on a PES support | ZSM-5 (average size of 35 nm) | 10 | Ethyl acetate | 50 | 5 wt% | laminar flow: 1882 g/(m2 h) | 125 | [23] |
| 2533 on a PES support | ZSM-5 (average size of 35 nm) | 10 | Ethyl acetate | 50 | 5 wt% | turbulent flow: 1985 g/(m2 h) | 134 | [23] |
| 2533 on a PVDf support | ILTf2N@MIL-101 | 7.5 | Ethyl acetate | 30 | 5 wt% | 2853 g/(m2 h) | 108 PSI = 304,900 g/(m2 h) | [28] |
| 2533 on a PVDf support | ILTf2N@MIL-101 | 5 | Ethyl acetate | 30 | 5 wt% | 2354 g/(m2 h) | 208 PSI = 486,220 g/(m2 h) | [28] |
| 2533 on a PSf-PES support | TiO2 nanoparticles | 0.03 wt% TiO2 in the coating solution | Humic acid | - | 20 ppm | 75.32 L/(m2 h) | HA rejection: 98.22% | [28] |
| Filler Type | Loading (wt%) | T (°C) | Feed Conc. (ppm Thiophene) | Permeation Flux (kg/(m2 h)) | Thiophene Enrichment Factor (β) (–) | Ref. |
|---|---|---|---|---|---|---|
| Hollow monocrystalline silicalite-1 (HMS) 200 nm | 20 | 60 | 500 | 20.63 (+82% than Pebax membrane) | 6.11 (+23% than Pebax membrane); PSI: 123 kg/(m2 h) | [21] |
| MoS2 2D nanosheets | 4 | 60 | 1312 | 11.42 (+22.3%) | 9.11 (+65.9%) | [32] |
| Ag+@SNW | 9 | 60 | 1312 | 16.35 (+78.5%) | 6.8 (+30.0%) | [41] |
| PDA/GNS | 6 | 40 | 1300 | 3.94 (+24%) | 7.73 (+10%) | [35] |
| Ag-PDA/GNS | 6 | 40 | 1300 | 4.42 (+40%) | 8.76 (+25%) | [35] |
| Ag-PDA/GNS | 6 | 70 | 1300 | 22.53 | 6.07 | [35] |
| Hierarchical porous SBA-15 | 6 | 60 | 1312 | 22.07 (+30%) | 6.76 (+50%) | [78] |
| Membrane | Filler Amount (wt%) | Vapour Permeability Coefficient, Pv (×104 Barrer) | Air Permeability Coefficient, Pg (Barrer) | Vapour/Air Selectivity (×104) | Ref. |
|---|---|---|---|---|---|
| Nafion 211 | - | 8.21 | 9.05 | 0.93 | [48] |
| Nafion 212 | - | 16.21 | 5.89 | 2.80 | [48] |
| Pebax®3533/PAA + CNC (fibre membrane) | 4.8 (CNCs) | 1.72 | 0.78 | 2.22 (+14%) | [48] |
| Pebax®1074 + MMT | 2.9 | 7.7 | 0.47 | 16.4 | [46] |
| Pebax®1074 + APOP-MMT | 4.8 | 8.4 | 0.49 | 17.3 | [46] |
| Pebax®1074 + IL@F-Ce | 4 | 45.3 | 2.40 (100%) | 18.9 (+83%) | [47] |
| Pebax Grade | Filler Type | Loading (wt%) | Separation | Feed | Permeation Flux (L/(m2 h)) | Rejection (%) | Ref. |
|---|---|---|---|---|---|---|---|
| 1657 on a PES support | Multiwalled carbon nanotubes wrapped with chitosan (CWNTs) | 1 | Dye removal (Malachite green) from water | 30 mg/L | 13.9 | 96.7 | [42] |
| 0.1 | 30 mg/L | 6 | 98.7 | [42] | |||
| 2533 on a PSf support | Functionalized multiwall carbon nanotubes (F-MWCNTs) | 0.5 | Separation of oil/water emulsion by nanofiltration (TMP = 15 bar) | Oil/surfactant (3/1 wt/wt) in water | 230 | 98.6 | [43] |
| 2 | Oil/surfactant (3/1 wt/wt) in water | 174 | 99.3 | [43] | |||
| 1657 | TiO2 nanotubes | 10 | Desalination under UV | Seawater | 8.2 (+100%) | 99.97 (salt) | [45] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Clarizia, G.; Bernardo, P. Polyether Block Amide as Host Matrix for Nanocomposite Membranes Applied to Different Sensitive Fields. Membranes 2022, 12, 1096. https://doi.org/10.3390/membranes12111096
Clarizia G, Bernardo P. Polyether Block Amide as Host Matrix for Nanocomposite Membranes Applied to Different Sensitive Fields. Membranes. 2022; 12(11):1096. https://doi.org/10.3390/membranes12111096
Chicago/Turabian StyleClarizia, Gabriele, and Paola Bernardo. 2022. "Polyether Block Amide as Host Matrix for Nanocomposite Membranes Applied to Different Sensitive Fields" Membranes 12, no. 11: 1096. https://doi.org/10.3390/membranes12111096
APA StyleClarizia, G., & Bernardo, P. (2022). Polyether Block Amide as Host Matrix for Nanocomposite Membranes Applied to Different Sensitive Fields. Membranes, 12(11), 1096. https://doi.org/10.3390/membranes12111096








