Impact of Membrane Modification and Surface Immobilization Techniques on the Hemocompatibility of Hemodialysis Membranes: A Critical Review
Abstract
1. Introduction
2. Physical Modifications
2.1. Polishing and Grinding
2.2. Thermal Treatment
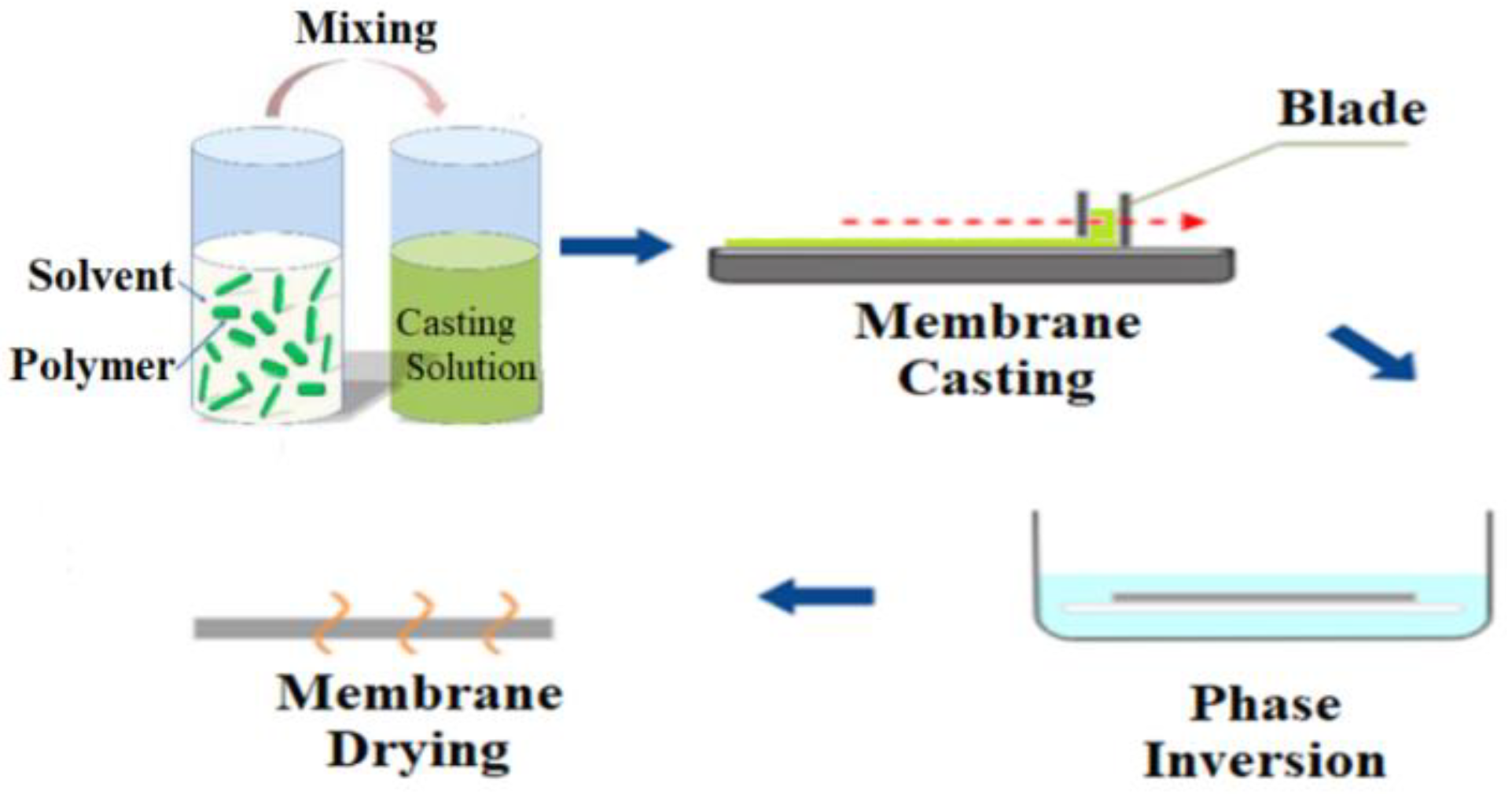
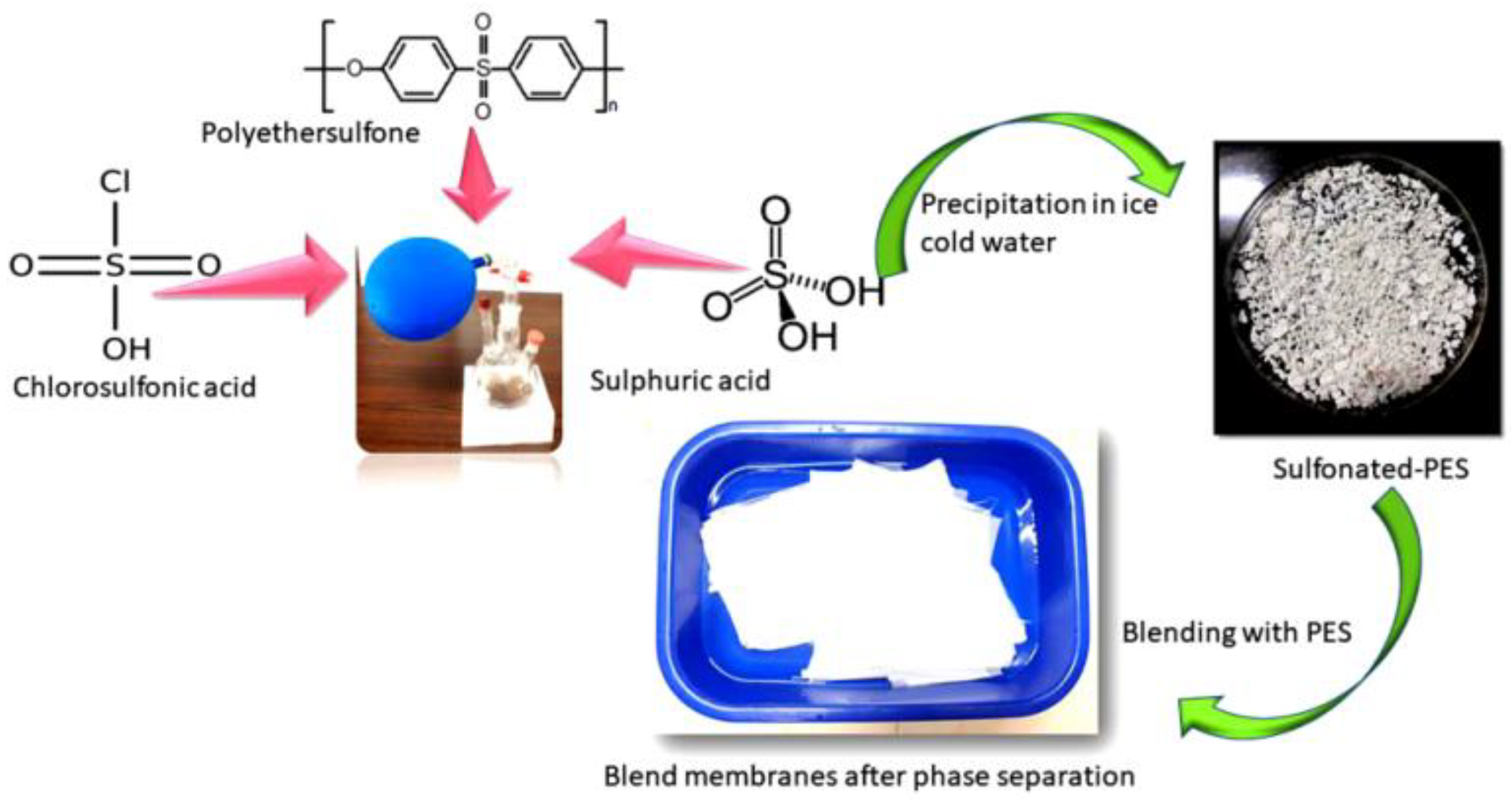
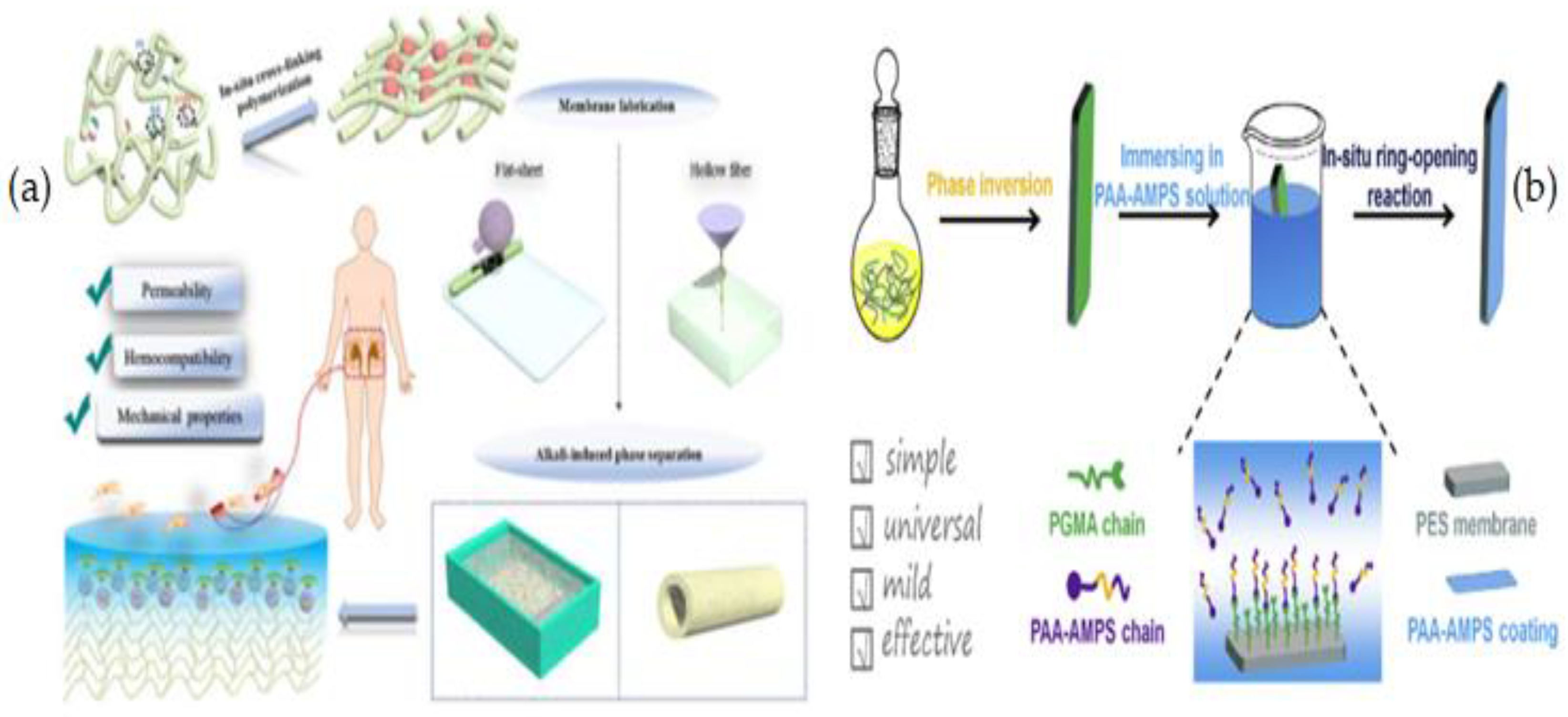
2.3. Blending
2.4. Coating
3. Chemical Modification
3.1. Grafting
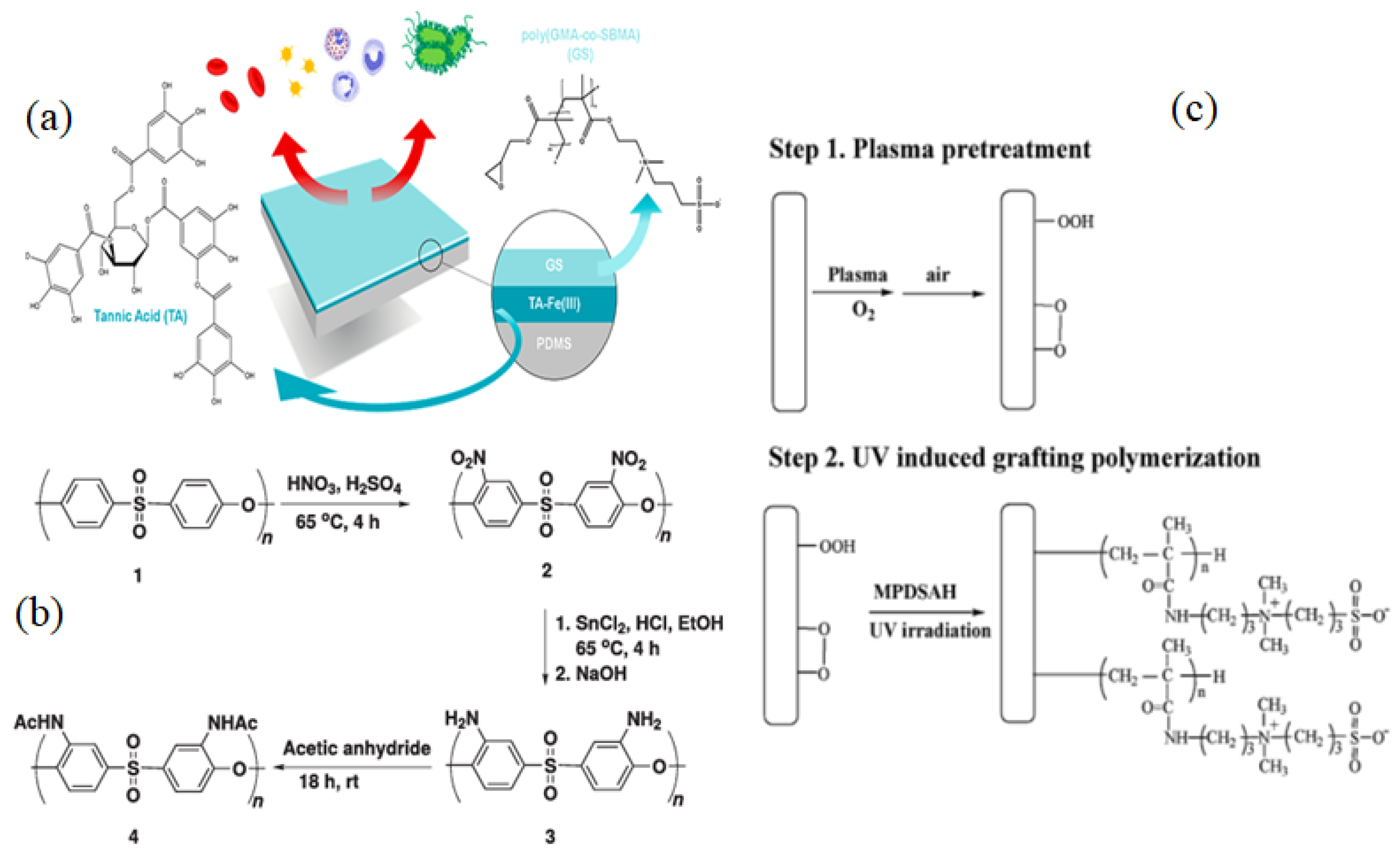
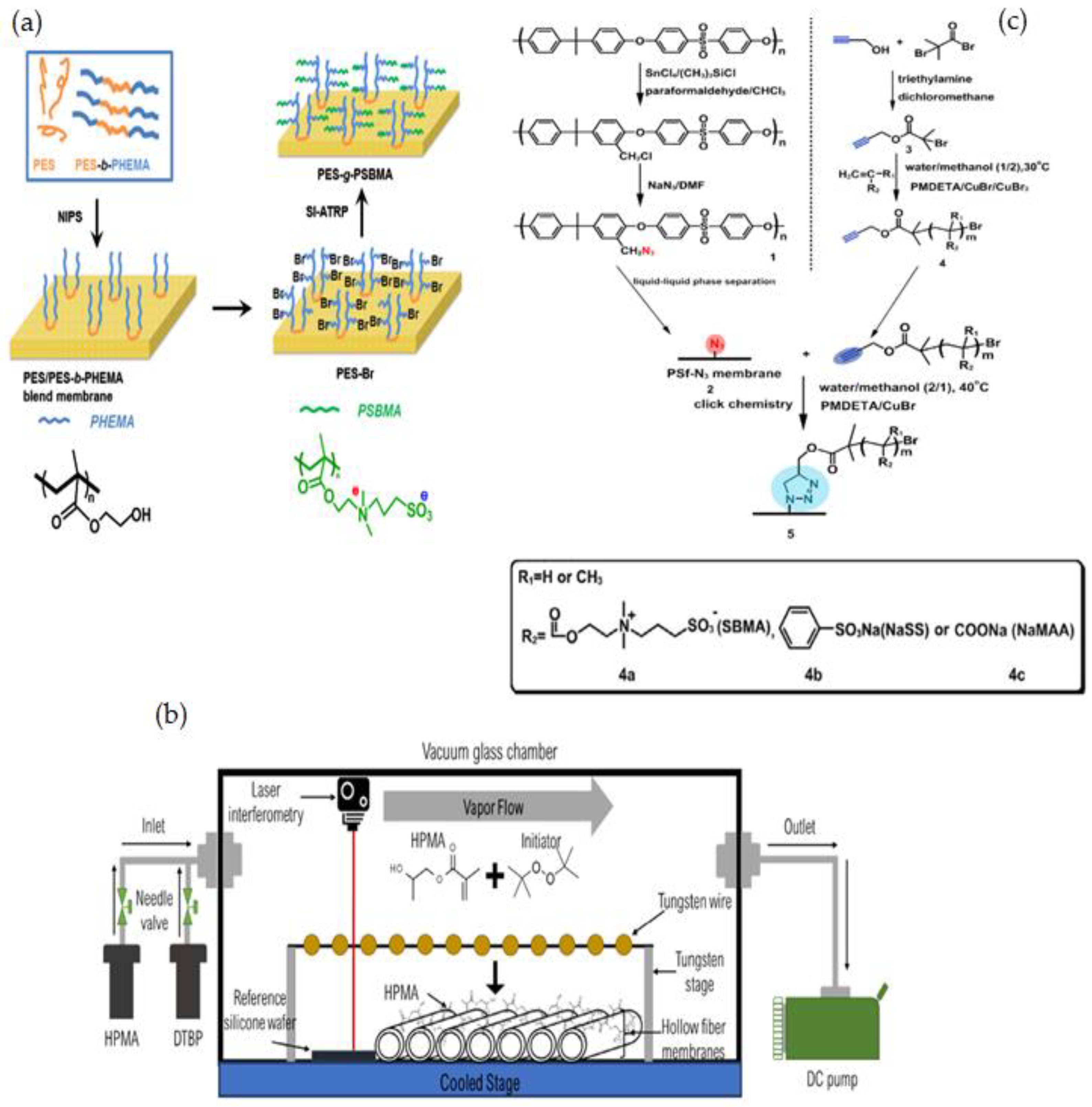
3.1.1. Radical Polymerization, Initiated Vapor Deposition (iCVD) & Click Chemistry
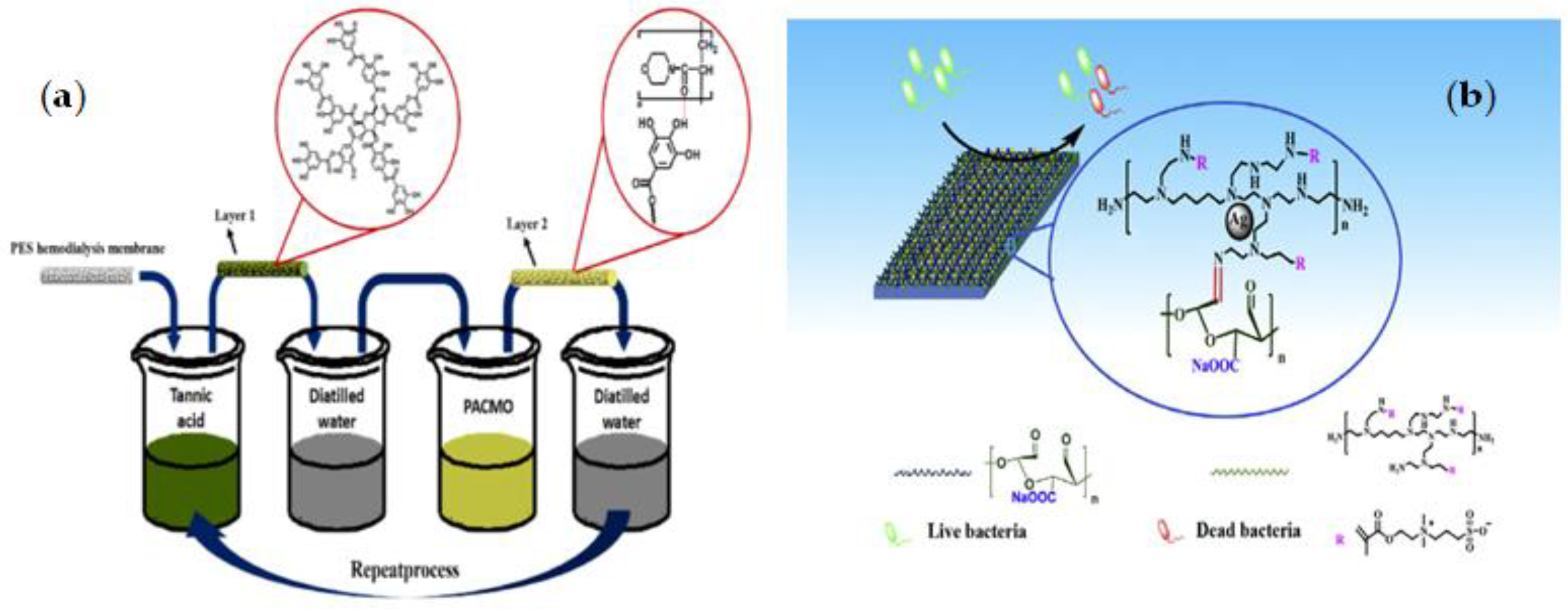
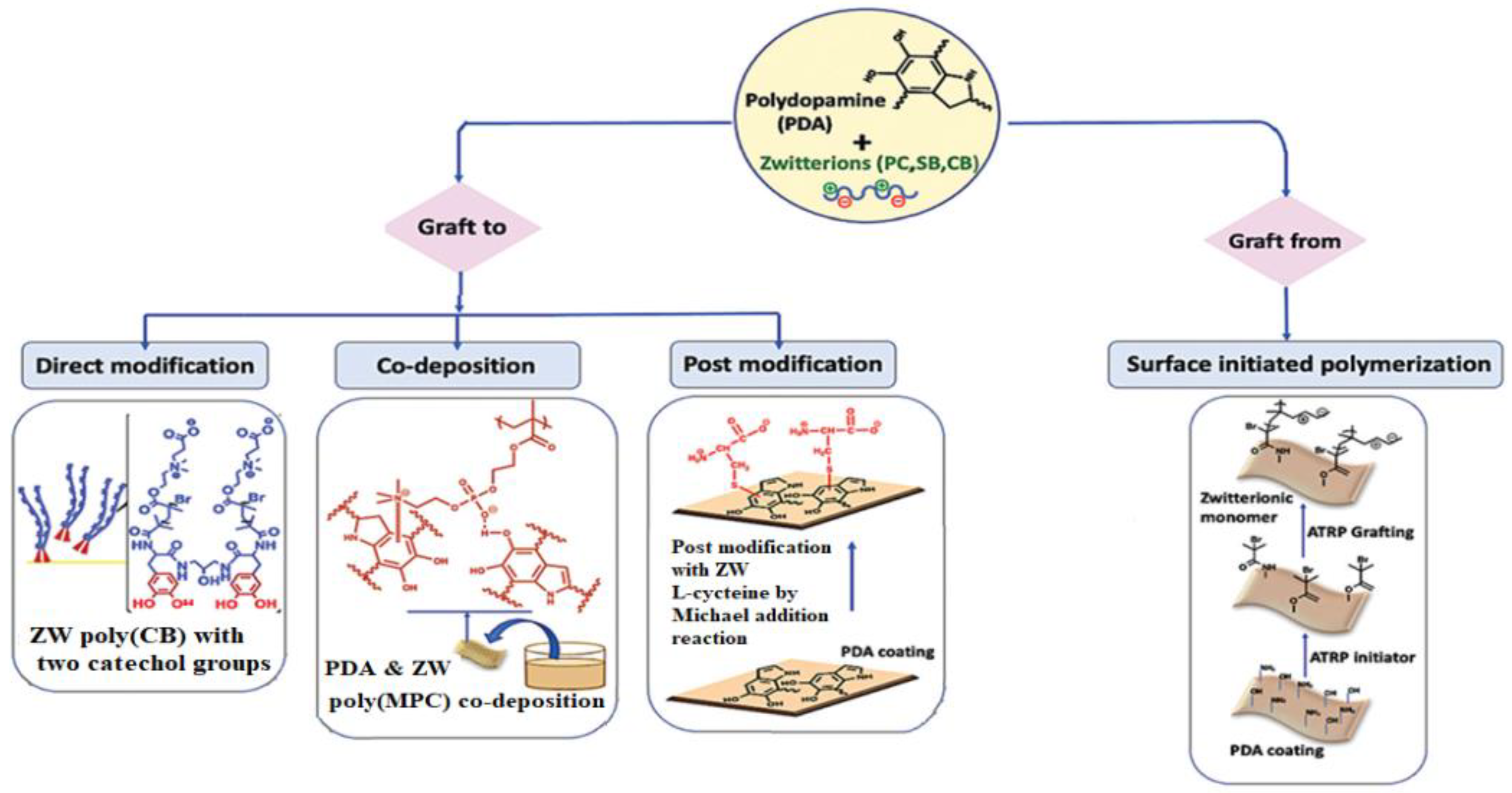
3.1.2. Mussel-Inspired Chemistry

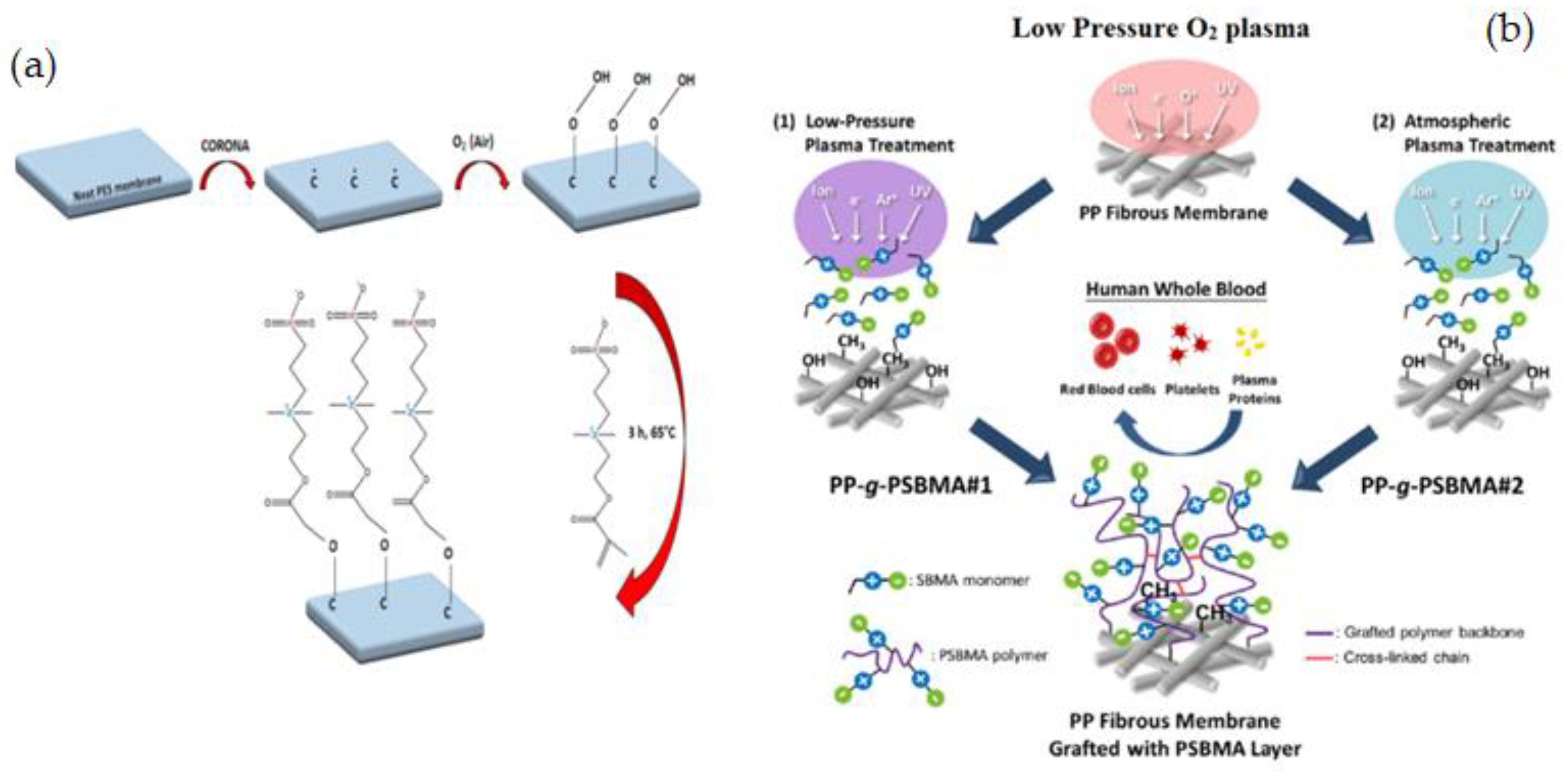
3.1.3. Plasma Technique
3.1.4. Enzymatic Treatment
4. Conclusions and Perspectives
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Brown, M.U.; Triozzi, A.; Emrick, T. Polymer Zwitterions with Phosphonium Cations. J. Am. Chem. Soc. 2021, 143, 6528–6532. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Ji, Y.-L.; Weng, X.-D.; An, Q.-F.; Gao, C.-J. High-flux and fouling-resistant reverse osmosis membrane prepared with incorporating zwitterionic amine monomers via interfacial polymerization. Desalination 2016, 381, 100–110. [Google Scholar] [CrossRef]
- Matin, A.; Shafi, H.; Wang, M.; Khan, Z.; Gleason, K.; Rahman, F. Reverse osmosis membranes surface-modified using an initiated chemical vapor deposition technique show resistance to alginate fouling under cross-flow conditions: Filtration & subsequent characterization. Desalination 2016, 379, 108–117. [Google Scholar]
- Nady, N.; Franssen, M.C.; Zuilhof, H.; Eldin, M.S.M.; Boom, R.; Schroen, K. Modification methods for poly (arylsulfone) membranes: A mini-review focusing on surface modification. Desalination 2011, 275, 1–9. [Google Scholar] [CrossRef]
- Saqib, J.; Aljundi, I.H. Membrane fouling and modification using surface treatment and layer-by-layer assembly of polyelectrolytes: State-of-the-art review. J. Water Process. Eng. 2016, 11, 68–87. [Google Scholar] [CrossRef]
- Song, X.; Ji, H.; Zhao, W.; Sun, S.; Zhao, C. Hemocompatibility enhancement of polyethersulfone membranes: Strategies and challenges. Adv. Membr. 2021, 1, 100013. [Google Scholar] [CrossRef]
- Farahbakhsh, J.; Vatanpour, V.; Khoshnam, M.; Zargar, M. Recent advancements in the application of new monomers and membrane modification techniques for the fabrication of thin film composite membranes: A review. React. Funct. Polym. 2021, 166, 105015. [Google Scholar] [CrossRef]
- Madalosso, H.B.; Machado, R.; Hotza, D.; Marangoni, C. Membrane Surface Modification by Electrospinning, Coating, and Plasma for Membrane Distillation Applications: A State-of-the-Art Review. Adv. Eng. Mater. 2021, 23, 2001456. [Google Scholar] [CrossRef]
- Abdelrasoul, A.; Shoker, A. Induced hemocompatibility of polyethersulfone (PES) hemodialysis membrane using polyvinylpyrrolidone: Investigation on human serum fibrinogen adsorption and inflammatory biomarkers released. Chem. Eng. Res. Des. 2022, 177, 615–624. [Google Scholar] [CrossRef]
- Kochkodan, V.; Johnson, D.J.; Hilal, N. Polymeric membranes: Surface modification for minimizing (bio) colloidal fouling. Adv. Colloid Interface Sci. 2014, 206, 116–140. [Google Scholar] [CrossRef]
- Lange, S.C.; Van Andel, E.; Smulders, M.M.; Zuilhof, H. Efficient and tunable three-dimensional functionalization of fully zwitterionic antifouling surface coatings. Langmuir 2016, 32, 10199–10205. [Google Scholar] [CrossRef]
- Ulbricht, M. Advanced functional polymer membranes. Polymer 2006, 47, 2217–2262. [Google Scholar] [CrossRef]
- Upadhyaya, L.; Qian, X.; Wickramasinghe, S.R. Chemical modification of membrane surface—Overview. Curr. Opin. Chem. Eng. 2018, 20, 13–18. [Google Scholar] [CrossRef]
- Gopal, R.; Zuwei, M.; Kaur, S.; Ramakrishna, S. Surface modification and application of functionalized polymer nanofibers. In Molecular Building Blocks for Nanotechnology; Springer: Berlin/Heidelberg, Germany, 2007; pp. 72–91. [Google Scholar]
- Chan, H.-M.; Erathodiyil, N.; Wu, H.; Lu, H.; Zheng, Y.; Ying, J.Y. Calcium cross-linked zwitterionic hydrogels as antifouling materials. Mater. Today Commun. 2020, 23, 100950. [Google Scholar] [CrossRef]
- Eswari, J.S.; Naik, S. A critical analysis on various technologies and functionalized materials for manufacturing dialysis membranes. Mater. Sci. Technol. 2020, 3, 116–126. [Google Scholar] [CrossRef]
- Lin, X.; Wu, K.; Zhou, Q.; Jain, P.; Boit, M.O.K.; Li, B.; Hung, H.-C.; Creason, S.A.; Himmelfarb, J.; Ratner, B.D. Photoreactive carboxybetaine copolymers impart biocompatibility and inhibit plasticizer leaching on polyvinyl chloride. ACS Appl. Mater. Interfaces 2020, 12, 41026–41037. [Google Scholar] [CrossRef]
- Ter Beek, O.; Pavlenko, D.; Stamatialis, D. Hollow fiber membranes for long-term hemodialysis based on polyethersulfone-SlipSkin™ polymer blends. J. Membr. Sci. 2020, 604, 118068. [Google Scholar] [CrossRef]
- Westphalen, H.; Abdelrasoul, A.; Shoker, A.; Zhu, N. Assessment of hemodialysis clinical practices using polyaryl ether sulfone-polyvinylpyrrolidone (PAES: PVP) clinical membrane: Modeling of in vitro fibrinogen adsorption, in situ synchrotron-based imaging, and clinical inflammatory biomarkers investigations. Sep. Purif. Technol. 2021, 259, 118136. [Google Scholar]
- Chou, Y.-N.; Venault, A.; Wang, Y.-H.; Chinnathambi, A.; Higuchi, A.; Chang, Y. Surface zwitterionization on versatile hydrophobic interfaces via a combined copolymerization/self-assembling process. J. Mater. Chem. B 2018, 6, 4909–4919. [Google Scholar] [CrossRef]
- Dizon, G.V.; Chou, Y.-N.; Yeh, L.-C.; Venault, A.; Huang, J.; Chang, Y. Bio-inert interfaces via biomimetic anchoring of a zwitterionic copolymer on versatile substrates. J. Colloid Interface Sci. 2018, 529, 77–89. [Google Scholar] [CrossRef] [PubMed]
- Erathodiyil, N.; Chan, H.-M.; Wu, H.; Ying, J.Y. Zwitterionic polymers and hydrogels for antibiofouling applications in implantable devices. Mater. Today 2020, 38, 84–98. [Google Scholar] [CrossRef]
- Ishihara, K. Blood-compatible surfaces with phosphorylcholine-based polymers for cardiovascular medical devices. Langmuir 2018, 35, 1778–1787. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Shi, X.; Chen, X.; Chen, W. Preparation and characterization of amphiphilic copolymer PVDF-g-PMABS and its application in improving hydrophilicity and protein fouling resistance of PVDF membrane. Appl. Surf. Sci. 2018, 427, 787–797. [Google Scholar] [CrossRef]
- Bengani-Lutz, P.; Converse, E.; Cebe, P.; Asatekin, A. Self-assembling zwitterionic copolymers as membrane selective layers with excellent fouling resistance: Effect of zwitterion chemistry. ACS Appl. Mater. Interfaces 2017, 9, 20859–20872. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Gao, K.; Zhou, L.; Jiao, Z.; Wu, M.; Cao, J.; You, X.; Cai, Z.; Su, Y.; Jiang, Z. Zwitterionic materials for antifouling membrane surface construction. Acta Biomater. 2016, 40, 142–152. [Google Scholar] [CrossRef]
- Liu, C.; Lee, J.; Small, C.; Ma, J.; Elimelech, M. Comparison of organic fouling resistance of thin-film composite membranes modified by hydrophilic silica nanoparticles and zwitterionic polymer brushes. J. Membr. Sci. 2017, 544, 135–142. [Google Scholar] [CrossRef]
- Sun, H.; Zhang, Y.; Sadam, H.; Ma, J.; Bai, Y.; Shen, X.; Kim, J.-K.; Shao, L. Novel mussel-inspired zwitterionic hydrophilic polymer to boost membrane water-treatment performance. J. Membr. Sci. 2019, 582, 1–8. [Google Scholar]
- Sun, W.; Liu, J.; Chu, H.; Dong, B. Pretreatment and membrane hydrophilic modification to reduce membrane fouling. Membranes 2013, 3, 226–241. [Google Scholar] [CrossRef] [PubMed]
- Hayama, M.; Yamamoto, K.-I.; Kohori, F.; Sakai, K. How polysulfone dialysis membranes containing polyvinylpyrrolidone achieve excellent biocompatibility? J. Membr. Sci. 2004, 234, 41–49. [Google Scholar]
- Wang, H.; Yu, T.; Zhao, C.; Du, Q. Improvement of hydrophilicity and blood compatibility on polyethersulfone membrane by adding polyvinylpyrrolidone. Fibers Polym. 2009, 10, 1–5. [Google Scholar] [CrossRef]
- Zhu, L.; Song, H.; Wang, J.; Xue, L. Polysulfone hemodiafiltration membranes with enhanced anti-fouling and hemocompatibility modified by poly (vinyl pyrrolidone) via in situ cross-linked polymerization. Mater. Sci. Eng. C 2017, 74, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Daneshamouz, S.; Eduok, U.; Abdelrasoul, A.; Shoker, A. Protein-bound uremic toxins (PBUTs) in chronic kidney disease (CKD) patients: Production pathway, challenges and recent advances in renal PBUTs clearance. NanoImpact 2021, 21, 100299. [Google Scholar] [CrossRef]
- Mollahosseini, A.; Abdelrasoul, A.; Shoker, A. Latest advances in zwitterionic structures modified dialysis membranes. Mater. Today Chem. 2020, 15, 100227. [Google Scholar] [CrossRef]
- Mollahosseini, A.; Abdelrasoul, A.; Shoker, A. A critical review of recent advances in hemodialysis membranes hemocompatibility and guidelines for future development. Mater. Chem. Phys. 2020, 248, 122911. [Google Scholar] [CrossRef]
- Saadati, S.; Eduok, U.; Westphalen, H.; Abdelrasoul, A.; Shoker, A.; Choi, P.; Doan, H.; Ein-Mozaffari, F.; Zhu, N. Assessment of Polyethersulfone and Polyacrylonitrile Hemodialysis Clinical Membranes: In situ Synchrotron-based Imaging of Human Serum Proteins Adsorption, Interaction Analyses, Molecular Docking and Clinical Inflammatory Biomarkers Investigations. Mater. Today Commun. 2021, 29, 102928. [Google Scholar] [CrossRef]
- Saadati, S.; Westphalen, H.; Eduok, U.; Abdelrasoul, A.; Shoker, A.; Choi, P.; Doan, H.; Ein-Mozaffari, F.; Zhu, N. Biocompatibility enhancement of hemodialysis membranes using a novel zwitterionic copolymer: Experimental, in situ synchrotron imaging, molecular docking, and clinical inflammatory biomarkers investigations. Mater. Sci. Eng. C 2020, 117, 111301. [Google Scholar] [CrossRef]
- Mollahosseini, A.; Abdelrasoul, A. Novel Insights in Hemodialysis: Most Recent Theories on the Membrane Hemocompatibility Improvement. Adv. Biomed. Eng. 2022, 3, 100034. [Google Scholar] [CrossRef]
- Mollahosseini, A.; Abdelrasoul, A. Introductory chapter: An overview of recent advances in membrane technologies. In Advances in Membrane Technologies; InTechOpen: London, UK, 2020; Volume 1, Chapter 1; pp. 1–14. [Google Scholar]
- Bolton, W.D.; Cull, D.L.; Taylor, S.M.; Carsten, C.G., III.; Snyder, B.A.; Sullivan, T.M.; Youkey, J.R.; Langan, E.M., III.; Gray, B.H. The use of cryopreserved femoral vein grafts for hemodialysis access in patients at high risk for infection: A word of caution. J. Vasc. Surg. Cases 2002, 36, 464–468. [Google Scholar] [CrossRef]
- Mollahosseni, A.; Abdelrasoul, A. Shoker, A. Challenges and advances in hemodialysis membranes. In Advances in Membrane Technologies; InTechOpen: London, UK, 2020; Volume 1, Chapter 7; pp. 151–170. [Google Scholar]
- Li, H.; Han, J.; Liang, G. Phase Transfer of Hydrophobic Nanoparticles Using a Zwitterionic Sulfobetaine Siloxane Generates Highly Biocompatible and Compact Surfaces. ACS Appl. Nano Mater. 2020, 3, 1489–1496. [Google Scholar] [CrossRef]
- Nazari, S.; Abdelrasoul, A. Surface Zwitterionization of HemodialysisMembranesfor Hemocompatibility Enhancement and Protein-mediated anti-adhesion: A Critical Review. Adv. Biomed. Eng. 2022, 3, 100026. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Z.; Qiu, M.; He, C. Heparin-mimicking semi-interpenetrating composite membrane with multiple excellent performances for promising hemodialysis. J. Membr. Sci. 2021, 618, 118740. [Google Scholar] [CrossRef]
- Zhu, L.; Liu, F.; Yu, X.; Xue, L. Poly (lactic acid) hemodialysis membranes with poly (lactic acid)-block-poly (2-hydroxyethyl methacrylate) copolymer as additive: Preparation, characterization, and performance. ACS Appl. Mater. Interfaces 2015, 7, 17748–17755. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.-J.; Liu, F.; Yu, X.-M.; Gao, A.-L.; Xue, L.-X. Surface zwitterionization of hemocompatible poly (lactic acid) membranes for hemodiafiltration. J. Membr. Sci. 2015, 475, 469–479. [Google Scholar] [CrossRef]
- Westphalen, H.; Saadati, S.; Eduok, U.; Abdelrasoul, A.; Shoker, A.; Choi, P.; Doan, H.; Ein-Mozaffari, F. Case studies of clinical hemodialysis membranes: Influences of membrane morphology and biocompatibility on uremic blood-membrane interactions and inflammatory biomarkers. Sci. Rep. 2020, 10, 14808. [Google Scholar] [CrossRef] [PubMed]
- Abdelrasoul, A.; Westphalen, H.; Saadati, S.; Shoker, A. Hemodialysis biocompatibility mathematical models to predict the inflammatory biomarkers released in dialysis patients based on hemodialysis membrane characteristics and clinical practices. Sci. Rep. 2021, 11, 23080. [Google Scholar] [CrossRef]
- Fang, Y.; Xu, Z.K.; Wu, J. Surface modification of membranes. In Encyclopedia of Membrane Science and Technology; Wiley: Hoboken, NJ, USA, 2013; pp. 1–15. [Google Scholar]
- Bui, V.T.; Abdelrasoul, A.; McMartin, D.W. Investigation on the stability and antifouling properties of polyvinylidene fluoride (PVDF)-zwitterion mixed matrix membranes (MMMs) using molecular dynamics simulation (MDS). Comput. Mater. Sci. 2021, 187, 110079. [Google Scholar] [CrossRef]
- Eduok, U.; Abdelrasoul, A.; Shoker, A.; Doan, H. Recent developments, current challenges and future perspectives on cellulosic hemodialysis membranes for highly efficient clearance of uremic toxins. Mater. Today Commun. 2021, 27, 102183. [Google Scholar] [CrossRef]
- Eduok, U.; Camara, H.; Abdelrasoul, A.; Shoker, A. Influence of UV-irradiation intensity and exposure duration on the hemobiocompatibility enhancement of a novel synthesized phosphobetaine zwitterions polyethersulfone clinical hemodialysis membranes. J. Biomed. Mater. Res. B Appl. Biomater. 2022, 110, 573–586. [Google Scholar] [CrossRef]
- Mollahosseini, A.; Argumeedi, S.; Abdelrasoul, A.; Shoker, A. A case study of poly (aryl ether sulfone) hemodialysis membrane interactions with human blood: Molecular dynamics simulation and experimental analyses. Comput. Methods Programs Biomed. 2020, 197, 105742. [Google Scholar] [CrossRef]
- Mollahosseini, A.; Saadati, S.; Abdelrasoul, A. Effects of mussel-inspired co-deposition of 2-hydroxymethyl methacrylate and poly (2-methoxyethyl acrylate) on the hydrophilicity and binding tendency of common hemodialysis membranes: Molecular dynamics simulations and molecular docking studies. J. Comput. Chem. 2022, 43, 57–73. [Google Scholar]
- Mollahosseini, A.; Abdelrasoul, A. Zwitterionization of common hemodialysis membranes: Assessment of different immobilized structure impact on hydrophilicity and biocompatibility of poly aryl ether sulfone (PAES) and cellulose triacetate (CTA) hemodialysis membranes. Struct. Chem. 2022, 1–18. [Google Scholar] [CrossRef]
- Sun, T.; Yao, B.; Warren, A.P.; Barmak, K.; Toney, M.F.; Peale, R.E.; Coffey, K.R. Surface and grain-boundary scattering in nanometric Cu films. Phys. Rev. B 2010, 81, 155454. [Google Scholar] [CrossRef]
- Awan, I.T.; Rivera, V.; Nogueira, I.; Pereira-da-Silva, M.; Li, M.S.; Ferreira, S.; Marega, E., Jr. Growth process and grain boundary defects in Er doped BaTiO3 processed by EB-PVD: A study by XRD, FTIR, SEM and AFM. Appl. Surf. Sci. 2019, 493, 982–993. [Google Scholar]
- Meghnani, R.; Kumar, M.; Pugazhenthi, G.; Dhakshinamoorthy, V. Synthesis of ceramic membrane using inexpensive precursors and evaluation of its biocompatibility for hemofiltration application. Sep. Purif. Technol. 2021, 256, 117814. [Google Scholar]
- Kwong, M.; Abdelrasoul, A.; Doan, H. Controlling polysulfone (PSF) fiber diameter and membrane morphology for an enhanced ultrafiltration performance using heat treatment. Results Mater. 2019, 2, 100021. [Google Scholar]
- Abdelrasoul, A.; Doan, H.; Lohi, A.; Cheng, C.H. Morphology control of polysulfone membranes in filtration processes: A critical review. ChemBioEng. Rev. 2015, 2, 22–43. [Google Scholar]
- Barzin, J.; Feng, C.; Khulbe, K.; Matsuura, T.; Madaeni, S.; Mirzadeh, H. Characterization of polyethersulfone hemodialysis membrane by ultrafiltration and atomic force microscopy. J. Membr. Sci. 2004, 237, 77–85. [Google Scholar]
- Gholami, M.; Nasseri, S.; Feng, C.; Matsuura, T.; Khulbe, K. The effect of heat-treatment on the ultrafiltration performance of polyethersulfone (PES) hollow-fiber membranes. Desalination 2003, 155, 293–301. [Google Scholar]
- Wenten, I.; Aryanti, P.; Khoiruddin, K.; Hakim, A.; Himma, N. Advances in polysulfone-based membranes for hemodialysis. J. Membr. Sci. Res. 2016, 2, 78–89. [Google Scholar]
- Idris, A.; Zain, N.M.; Noordin, M. Synthesis, characterization and performance of asymmetric polyethersulfone (PES) ultrafiltration membranes with polyethylene glycol of different molecular weights as additives. Desalination 2007, 207, 324–339. [Google Scholar]
- Juber, F.A.H.; Jawad, Z.A.; Teoh, G.H.; Ahmad, A.L.; Chin, B.L.F. Development of novel blend poly (Ethylene Glycol)/Poly (Ethersulfone) polymeric membrane using N-Methyl-2-Pyrollidone and dimethylformamide solvents for facilitating CO2/N2 gas separation. Mater. Today Proc. 2021, 46, 1963–1970. [Google Scholar]
- Lu, S.; Zhuang, L.; Lu, J. Homogeneous blend membrane made from poly (ether sulphone) and poly (vinylpyrrolidone) and its application to water electrolysis. J. Membr. Sci. 2007, 300, 205–210. [Google Scholar] [CrossRef]
- Lusiana, R.A.; Sangkota, V.D.A.; Sasongko, N.A.; Gunawan, G.; Wijaya, A.R.; Santosa, S.J.; Siswanta, D.; Mudasir, M.; Abidin, M.N.Z.; Mansur, S. Permeability improvement of polyethersulfone-polietylene glycol (PEG-PES) flat sheet type membranes by tripolyphosphate-crosslinked chitosan (TPP-CS) coating. Int. J. Biol. Macromol. 2020, 152, 633–644. [Google Scholar] [CrossRef]
- Otitoju, T.A.; Ahmad, A.L.; Ooi, B.S. Recent advances in hydrophilic modification and performance of polyethersulfone (PES) membrane via additive blending. RSC Adv. 2018, 8, 22710–22728. [Google Scholar] [CrossRef] [PubMed]
- De Guzman, M.R.; Andra, C.K.A.; Ang, M.B.M.Y.; Dizon, G.V.C.; Caparanga, A.R.; Huang, S.-H.; Lee, K.-R. Increased performance and antifouling of mixed-matrix membranes of cellulose acetate with hydrophilic nanoparticles of polydopamine-sulfobetaine methacrylate for oil-water separation. J. Membr. Sci. 2021, 620, 118881. [Google Scholar] [CrossRef]
- Knowles, B.R.; Wagner, P.; Maclaughlin, S.; Higgins, M.J.; Molino, P.J. Carboxybetaine functionalized nanosilicas as protein resistant surface coatings. Biointerphases 2020, 15, 011001. [Google Scholar] [CrossRef]
- Shahkaramipour, N.; Lai, C.K.; Venna, S.R.; Sun, H.; Cheng, C.; Lin, H. Membrane surface modification using thiol-containing zwitterionic polymers via bioadhesive polydopamine. Ind. Eng. Chem. Res. 2018, 57, 2336–2345. [Google Scholar] [CrossRef]
- Venault, A.; Hsu, C.-H.; Ishihara, K.; Chang, Y. Zwitterionic bi-continuous membranes from a phosphobetaine copolymer/poly (vinylidene fluoride) blend via VIPS for biofouling mitigation. J. Membr. Sci. 2018, 550, 377–388. [Google Scholar] [CrossRef]
- Sinha, M.; Purkait, M. Increase in hydrophilicity of polysulfone membrane using polyethylene glycol methyl ether. J. Membr. Sci. 2013, 437, 7–16. [Google Scholar] [CrossRef]
- Azhar, O.; Jahan, Z.; Sher, F.; Niazi, M.B.K.; Kakar, S.J.; Shahid, M. Cellulose acetate-polyvinyl alcohol blend hemodialysis membranes integrated with dialysis performance and high biocompatibility. Mater. Sci. Eng. C 2021, 126, 112127. [Google Scholar] [CrossRef]
- Yin, J.; Fan, H.; Zhou, J. Cellulose acetate/poly (vinyl alcohol) and cellulose acetate/crosslinked poly (vinyl alcohol) blend membranes: Preparation, characterization, and antifouling properties. Desalin. Water Treat. 2016, 57, 10572–10584. [Google Scholar] [CrossRef]
- Galiano, F.; Briceño, K.; Marino, T.; Molino, A.; Christensen, K.V.; Figoli, A. Advances in biopolymer-based membrane preparation and applications. J. Membr. Sci. 2018, 564, 562–586. [Google Scholar] [CrossRef]
- Su, B.-H.; Fu, P.; Li, Q.; Tao, Y.; Li, Z.; Zao, H.-S.; Zhao, C.-S. Evaluation of polyethersulfone highflux hemodialysis membrane in vitro and in vivo. J. Mater. Sci. Mater. Med. 2008, 19, 745–751. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kim, C.K. Ultrafiltration membranes prepared from blends of polyethersulfone and poly (1-vinylpyrrolidone-co-styrene) copolymers. J. Membr. Sci. 2005, 262, 60–68. [Google Scholar] [CrossRef]
- Song, H.; Ran, F.; Fan, H.; Niu, X.; Kang, L.; Zhao, C. Hemocompatibility and ultrafiltration performance of surface-functionalized polyethersulfone membrane by blending comb-like amphiphilic block copolymer. J. Membr. Sci. 2014, 471, 319–327. [Google Scholar] [CrossRef]
- Sun, M.; Su, Y.; Mu, C.; Jiang, Z. Improved antifouling property of PES ultrafiltration membranes using additive of silica—PVP nanocomposite. Ind. Eng. Chem. Res. 2010, 49, 790–796. [Google Scholar] [CrossRef]
- Athira, V.; Mohanty, S.; Nayak, S. Preparation and characterization of porous polyethersulfone (PES) membranes with improved biocompatibility by blending sulfonated polyethersulfone (SPES) and cellulose acetate (CA)–A comparative study. Mater. Today Commun. 2020, 25, 101544. [Google Scholar]
- Liu, T.-M.; Wu, X.-Z.; Qiu, Y.-R. Enhanced biocompatibility and antibacterial property of polyurethane materials modified with citric acid and chitosan. J. Biomater. Sci. Polym. Ed. 2016, 27, 1211–1231. [Google Scholar] [CrossRef]
- Kiessling, V.; Wan, C.; Tamm, L.K. Domain coupling in asymmetric lipid bilayers. Biochim. Biophys. Acta-Biomembr. 2009, 1788, 64–71. [Google Scholar] [CrossRef]
- Nie, C.; Yang, Y.; Peng, Z.; Cheng, C.; Ma, L.; Zhao, C. Aramid nanofiber as an emerging nanofibrous modifier to enhance ultrafiltration and biological performances of polymeric membranes. J. Membr. Sci. 2017, 528, 251–263. [Google Scholar] [CrossRef]
- Fang, L.-F.; Jeon, S.; Kakihana, Y.; Kakehi, J.-i.; Zhu, B.-K.; Matsuyama, H.; Zhao, S. Improved antifouling properties of polyvinyl chloride blend membranes by novel phosphate based-zwitterionic polymer additive. J. Membr. Sci. 2017, 528, 326–335. [Google Scholar] [CrossRef]
- Choi, S.-Y.; Li, M.-X.; Kang, J.H.; Yang, D.H.; Joung, Y.K. Anti-thrombotic polymer surfaces modified with zwitterionic and fluorinated surface-migrating oligomers. Surf. Interfaces 2021, 25, 101280. [Google Scholar] [CrossRef]
- Ankoliya, D.; Mehta, B.; Raval, H. Advances in surface modification techniques of reverse osmosis membrane over the years. Sep. Sci. Technol. 2019, 54, 293–310. [Google Scholar] [CrossRef]
- Wang, F.; Li, J.; Zhu, H.; Zhang, H.; Tang, H.; Chen, J.; Guo, Y. Physical modification of polytetrafluoroethylene flat membrane by a simple heat setting process and membrane wetting remission in SGMD for desalination. Desalination 2014, 354, 143–152. [Google Scholar] [CrossRef]
- Zhao, C.; Xue, J.; Ran, F.; Sun, S. Modification of polyethersulfone membranes—A review of methods. Prog. Mater. Sci. 2013, 58, 76–150. [Google Scholar] [CrossRef]
- Duarte, L.T.; Pereira, C.C.; Habert, A.C.; Borges, C.P. Polyurethane/polyethersulphone composite hollow fibers produced by simultaneous spinning of two polymer solutions. J. Membr. Sci. 2008, 311, 12–22. [Google Scholar] [CrossRef]
- Hoseinpour, V.; Noori, L.; Mahmoodpour, S.; Shariatinia, Z. A review on surface modification methods of poly (arylsulfone) membranes for biomedical applications. J. Biomater. Sci. Polym. Ed. 2021, 32, 906–965. [Google Scholar] [CrossRef]
- Bengani, P.; Kou, Y.; Asatekin, A. Zwitterionic copolymer self-assembly for fouling resistant, high flux membranes with size-based small molecule selectivity. J. Membr. Sci. 2015, 493, 755–765. [Google Scholar] [CrossRef]
- Zhu, Y.; Yu, X.; Zhang, T.; Wang, X. Constructing zwitterionic coatings on thin-film nanofibrous composite membrane substrate for multifunctionality. Appl. Surf. Sci. 2019, 483, 979–990. [Google Scholar] [CrossRef]
- Fotovvati, B.; Namdari, N.; Dehghanghadikolaei, A. On coating techniques for surface protection: A review. J. Manuf. Mater. Process. 2019, 3, 28. [Google Scholar]
- Chen, Z.; Liao, M.; Zhang, L.; Zhou, J. Molecular simulations on the hydration and underwater oleophobicity of zwitterionic self-assembled monolayers. AIChE J. 2021, 67, e17103. [Google Scholar]
- Cheng, G.; Liao, M.; Zhao, D.; Zhou, J. Molecular understanding on the underwater oleophobicity of self-assembled monolayers: Zwitterionic versus nonionic. Langmuir 2017, 33, 1732–1741. [Google Scholar] [PubMed]
- Lin, J.; Ye, W.; Zeng, H.; Yang, H.; Shen, J.; Darvishmanesh, S.; Luis, P.; Sotto, A.; Van der Bruggen, B. Fractionation of direct dyes and salts in aqueous solution using loose nanofiltration membranes. J. Membr. Sci. 2015, 477, 183–193. [Google Scholar] [CrossRef]
- Xu, C.; Xu, Y.; Zhu, J. Photocatalytic antifouling graphene oxide-mediated hierarchical filtration membranes with potential applications on water purification. ACS Appl. Mater. Interfaces 2014, 6, 16117–16123. [Google Scholar]
- Venault, A.; Trinh, K.M.; Chang, Y. A zwitterionic zP (4VP-r-ODA) copolymer for providing polypropylene membranes with improved hemocompatibility. J. Membr. Sci. 2016, 501, 68–78. [Google Scholar] [CrossRef]
- Venault, A.; Huang, W.-Y.; Hsiao, S.-W.; Chinnathambi, A.; Alharbi, S.A.; Chen, H.; Zheng, J.; Chang, Y. Zwitterionic modifications for enhancing the antifouling properties of poly (vinylidene fluoride) membranes. Langmuir 2016, 32, 4113–4124. [Google Scholar]
- He, C.; Shi, Z.-Q.; Cheng, C.; Lu, H.-Q.; Zhou, M.; Sun, S.-D.; Zhao, C.-S. Graphene oxide and sulfonated polyanion co-doped hydrogel films for dual-layered membranes with superior hemocompatibility and antibacterial activity. Biomater. Sci. 2016, 4, 1431–1440. [Google Scholar]
- Knowles, B.R.; Wagner, P.; Maclaughlin, S.; Higgins, M.J.; Molino, P.J. Silica nanoparticles functionalized with zwitterionic sulfobetaine siloxane for application as a versatile antifouling coating system. ACS Appl. Mater. Interfaces 2017, 9, 18584–18594. [Google Scholar] [CrossRef]
- Chen, S.-H.; Chang, Y.; Ishihara, K. Reduced blood cell adhesion on polypropylene substrates through a simple surface zwitterionization. Langmuir 2017, 33, 611–621. [Google Scholar]
- Jeon, N.J.; Noh, J.H.; Kim, Y.C.; Yang, W.S.; Ryu, S.; Seok, S.I. Solvent engineering for high-performance inorganic–organic hybrid perovskite solar cells. Nat. Mater. 2014, 13, 897–903. [Google Scholar]
- Kelso, M.V.; Mahenderkar, N.K.; Chen, Q.; Tubbesing, J.Z.; Switzer, J.A. Spin coating epitaxial films. Science 2019, 364, 166–169. [Google Scholar]
- Zhang, Y.; D’Ambra, C.A.; Katsumata, R.; Burns, R.L.; Somervell, M.H.; Segalman, R.A.; Hawker, C.J.; Bates, C.M. Rapid and selective deposition of patterned thin films on heterogeneous substrates via spin coating. ACS Appl. Mater. Interfaces 2019, 11, 21177–21183. [Google Scholar]
- Su, P.; Jia, M.; Huang, J.; Li, W.; Tang, C.Y. Multilayer assembly of thin-film nanocomposite membranes with enhanced NaCl and antibiotic rejection. Desalination 2021, 517, 115261. [Google Scholar]
- Zhou, Z.; Zhou, S.; Cheng, X.; Liu, W.; Wu, R.; Wang, J.; Liu, B.; Zhu, J.; Van der Bruggen, B.; Zhang, Y. Ultrathin polyamide membranes enabled by spin-coating assisted interfacial polymerization for high-flux nanofiltration. Sep. Purif. Technol. 2022, 288, 120648. [Google Scholar] [CrossRef]
- Ren, P.-F.; Yang, H.-C.; Liang, H.-Q.; Xu, X.-L.; Wan, L.-S.; Xu, Z.-K. Highly stable, protein-resistant surfaces via the layer-by-layer assembly of poly (sulfobetaine methacrylate) and tannic acid. Langmuir 2015, 31, 5851–5858. [Google Scholar] [CrossRef]
- Chien, H.-W.; Tsai, C.-C.; Tsai, W.-B.; Wang, M.-J.; Kuo, W.-H.; Wei, T.-C.; Huang, S.-T. Surface conjugation of zwitterionic polymers to inhibit cell adhesion and protein adsorption. Colloids Surf. B Biointerfaces 2013, 107, 152–159. [Google Scholar]
- Chen, Q.; He, Y.; Zhao, Y.; Chen, L. Tannic acid and Poly (N-acryloyl morpholine) layer-by-layer built hemodialysis membrane surface for intervening oxidative stress integrated with high biocompatibility and dialysis performance. J. Membr. Sci. 2021, 621, 118896. [Google Scholar] [CrossRef]
- Xie, Y.; Chen, L.; Zhang, X.; Chen, S.; Zhang, M.; Zhao, W.; Sun, S.; Zhao, C. Integrating zwitterionic polymer and Ag nanoparticles on polymeric membrane surface to prepare antifouling and bactericidal surface via Schiff-based layer-by-layer assembly. J. Colloid Interface Sci. 2018, 510, 308–317. [Google Scholar] [CrossRef]
- Capozzi, L.C.; Mehmood, F.M.; Giagnorio, M.; Tiraferri, A.; Cerruti, M.; Sangermano, M. Ultrafiltration membranes functionalized with polydopamine with enhanced contaminant removal by adsorption. Macromol. Mater. Eng. 2017, 302, 1600481. [Google Scholar] [CrossRef]
- Díez, B.; Rosal, R. A critical review of membrane modification techniques for fouling and biofouling control in pressure-driven membrane processes. Nanotechnol. Environ. Eng. 2020, 5, 15. [Google Scholar] [CrossRef]
- Ding, Y.; Floren, M.; Tan, W. Mussel-inspired polydopamine for bio-surface functionalization. Biosurface Biotribology 2016, 2, 121–136. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-Y.; Yang, E.; Lee, M.-Y.; Chae, K.-J.; Kim, C.-M.; Kim, I.S. Polydopamine coating effects on ultrafiltration membrane to enhance power density and mitigate biofouling of ultrafiltration microbial fuel cells (UF-MFCs). Water Res. 2014, 54, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Nie, C.; Ma, L.; Xia, Y.; He, C.; Deng, J.; Wang, L.; Cheng, C.; Sun, S.; Zhao, C. Novel heparin-mimicking polymer brush grafted carbon nanotube/PES composite membranes for safe and efficient blood purification. J. Membr. Sci. 2015, 475, 455–468. [Google Scholar] [CrossRef]
- Wang, L.; Su, B.; Cheng, C.; Ma, L.; Li, S.; Nie, S.; Zhao, C. Layer by layer assembly of sulfonic poly (ether sulfone) as heparin-mimicking coatings: Scalable fabrication of super-hemocompatible and antibacterial membranes. J. Mater. Chem. B 2015, 3, 1391–1404. [Google Scholar] [CrossRef]
- Yang, H.-C.; Luo, J.; Lv, Y.; Shen, P.; Xu, Z.-K. Surface engineering of polymer membranes via mussel-inspired chemistry. J. Membr. Sci. 2015, 483, 42–59. [Google Scholar]
- Zhou, R.; Ren, P.-F.; Yang, H.-C.; Xu, Z.-K. Fabrication of antifouling membrane surface by poly (sulfobetaine methacrylate)/polydopamine co-deposition. J. Membr. Sci. 2014, 466, 18–25. [Google Scholar]
- Yao, L.; He, C.; Chen, S.; Zhao, W.; Xie, Y.; Sun, S.; Nie, S.; Zhao, C. Codeposition of polydopamine and zwitterionic polymer on membrane surface with enhanced stability and antibiofouling property. Langmuir 2018, 35, 1430–1439. [Google Scholar]
- Zhang, Z.; Zhao, Y.; Luo, X.; Feng, S.; Wu, L. Preparation of a heparin-like functionalized tannic acid-coated polyethersulfone ultrafiltration membrane for hemodialysis by a simple surface modification method. Appl. Surf. Sci. 2022, 572, 151440. [Google Scholar]
- Daels, N.; De Vrieze, S.; Sampers, I.; Decostere, B.; Westbroek, P.; Dumoulin, A.; Dejans, P.; De Clerck, K.; Van Hulle, S. Potential of a functionalised nanofibre microfiltration membrane as an antibacterial water filter. Desalination 2011, 275, 285–290. [Google Scholar]
- Hong, S.H.; Hong, S.; Ryou, M.H.; Choi, J.W.; Kang, S.M.; Lee, H. Sprayable ultrafast polydopamine surface modifications. Adv. Mater. Interfaces 2016, 3, 1500857. [Google Scholar]
- Li, X.; Cai, T.; Chen, C.; Chung, T.-S. Negatively charged hyperbranched polyglycerol grafted membranes for osmotic power generation from municipal wastewater. Water Res. 2016, 89, 50–58. [Google Scholar] [CrossRef]
- Ma, W.; Yang, P.; Li, J.; Li, S.; Li, P.; Zhao, Y.; Huang, N. Immobilization of poly (MPC) brushes onto titanium surface by combining dopamine self-polymerization and ATRP: Preparation, characterization and evaluation of hemocompatibility in vitro. Appl. Surf. Sci. 2015, 349, 445–451. [Google Scholar] [CrossRef]
- Xiang, T.; Lu, T.; Xie, Y.; Zhao, W.-F.; Sun, S.-D.; Zhao, C.-S. Zwitterionic polymer functionalization of polysulfone membrane with improved antifouling property and blood compatibility by combination of ATRP and click chemistry. Acta Biomater. 2016, 40, 162–171. [Google Scholar] [PubMed]
- Dizon, G.V.; Clarin, M.T.R.; Venault, A.; Tayo, L.; Chiang, H.-C.; Zheng, J.; Aimar, P.; Chang, Y. A nondestructive surface zwitterionization of polydimethylsiloxane for the improved human blood-inert properties. ACS Appl. Bio Mater. 2018, 2, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Meng, J.; Xu, M.; Yuan, T.; Yang, N.; Sun, T.; Zhang, Y.; Feng, X.; Cheng, B. A simple but efficient zwitterionization method towards cellulose membrane with superior antifouling property and biocompatibility. J. Membr. Sci. 2015, 492, 547–558. [Google Scholar]
- Şimşek, E.N.; Akdağ, A.; Çulfaz-Emecen, P.Z. Modification of poly (ether sulfone) for antimicrobial ultrafiltration membranes. Polymer 2016, 106, 91–99. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, S.; Dai, L.; Chen, X. Novel zwitterionic poly (arylene ether sulfone) s as antifouling membrane material. J. Membr. Sci. 2010, 349, 217–224. [Google Scholar] [CrossRef]
- Lim, S.J.; Shin, I.H. Graft copolymerization of GMA and EDMA on PVDF to hydrophilic surface modification by electron beam irradiation. Nucl. Eng. Technol. 2020, 52, 373–380. [Google Scholar]
- Jhong, J.-F.; Venault, A.; Hou, C.-C.; Chen, S.-H.; Wei, T.-C.; Zheng, J.; Huang, J.; Chang, Y. Surface zwitterionization of expanded poly (tetrafluoroethylene) membranes via atmospheric plasma-induced polymerization for enhanced skin wound healing. ACS Appl. Mater. Interfaces 2013, 5, 6732–6742. [Google Scholar]
- Zhao, J.; Shi, Q.; Luan, S.; Song, L.; Yang, H.; Shi, H.; Jin, J.; Li, X.; Yin, J.; Stagnaro, P. Improved biocompatibility and antifouling property of polypropylene non-woven fabric membrane by surface grafting zwitterionic polymer. J. Membr. Sci. 2011, 369, 5–12. [Google Scholar]
- Hidzir, N.M.; Hill, D.J.; Taran, E.; Martin, D.; Grøndahl, L. Argon plasma treatment-induced grafting of acrylic acid onto expanded poly (tetrafluoroethylene) membranes. Polymer 2013, 54, 6536–6546. [Google Scholar]
- Wavhal, D.S.; Fisher, E.R. Hydrophilic modification of polyethersulfone membranes by low temperature plasma-induced graft polymerization. J. Membr. Sci. 2002, 209, 255–269. [Google Scholar]
- Tang, Y.P.; Cai, T.; Loh, D.; O’Brien, G.S.; Chung, T.S. Construction of antifouling lumen surface on a poly (vinylidene fluoride) hollow fiber membrane via a zwitterionic graft copolymerization strategy. Sep. Purif. Technol. 2017, 176, 294–305. [Google Scholar]
- Kang, G.-D.; Cao, Y.-M. Application and modification of poly (vinylidene fluoride)(PVDF) membranes–a review. J. Membr. Sci. 2014, 463, 145–165. [Google Scholar]
- Liu, F.; Hashim, N.A.; Liu, Y.; Abed, M.M.; Li, K. Progress in the production and modification of PVDF membranes. J. Membr. Sci. 2011, 375, 1–27. [Google Scholar]
- Chou, Y.-N.; Wen, T.-C.; Chang, Y. Zwitterionic surface grafting of epoxylated sulfobetaine copolymers for the development of stealth biomaterial interfaces. Acta Biomater. 2016, 40, 78–91. [Google Scholar]
- Xie, Y.; Wang, R.; Li, S.; Xiang, T.; Zhao, C.-S. A robust way to prepare blood-compatible and anti-fouling polyethersulfone membrane. Colloids Surf. B Biointerfaces 2016, 146, 326–333. [Google Scholar]
- Wang, C.; Xu, Y.; Sun, S.; Zhao, C. Post-functionalization of carboxylic polyethersulfone composite membranes. Compos. Sci. Technol. 2018, 156, 48–60. [Google Scholar]
- Samperi, F.; Battiato, S.; Puglisi, C.; Asarisi, V.; Recca, A.; Cicala, G.; Mendichi, R. Synthesis and characterization of sulfonated copolyethersulfones. J. Polym. Sci. Part A Polym. Chem. 2010, 48, 3010–3023. [Google Scholar]
- Zhao, W.; Mou, Q.; Zhang, X.; Shi, J.; Sun, S.; Zhao, C. Preparation and characterization of sulfonated polyethersulfone membranes by a facile approach. Eur. Polym. J. 2013, 49, 738–751. [Google Scholar]
- Lee, X.J.; Show, P.L.; Katsuda, T.; Chen, W.-H.; Chang, J.-S. Surface grafting techniques on the improvement of membrane bioreactor: State-of-the-art advances. Bioresour. Technol. 2018, 269, 489–502. [Google Scholar] [CrossRef] [PubMed]
- Puro, L.; Mänttäri, M.; Pihlajamäki, A.; Nyström, M. Characterization of modified nanofiltration membranes by octanoic acid permeation and FTIR analysis. Chem. Eng. Res. Des. 2006, 84, 87–96. [Google Scholar] [CrossRef]
- Yu, H.-Y.; He, J.-M.; Liu, L.-Q.; He, X.-C.; Gu, J.-S.; Wei, X.-W. Photoinduced graft polymerization to improve antifouling characteristics of an SMBR. J. Membr. Sci. 2007, 302, 235–242. [Google Scholar] [CrossRef]
- Galiano, F.; Schmidt, S.A.; Ye, X.; Kumar, R.; Mancuso, R.; Curcio, E.; Gabriele, B.; Hoinkis, J.; Figoli, A. UV-LED induced bicontinuous microemulsions polymerisation for surface modification of commercial membranes–Enhancing the antifouling properties. Sep. Purif. Technol. 2018, 194, 149–160. [Google Scholar]
- Hong Anh Ngo, T.; Mori, S.; Thi Tran, D. Photo-induced grafting of poly (ethylene glycol) onto polyamide thin film composite membranes. J. Appl. Polym. Sci. 2017, 134, 45454. [Google Scholar] [CrossRef]
- Saha, P.; Palanisamy, A.R.; Santi, M.; Ganguly, R.; Mondal, S.; Singha, N.K.; Pich, A. Thermoresponsive zwitterionic poly (phosphobetaine) microgels: Effect of macro-RAFT chain length and cross-linker molecular weight on their antifouling properties. Polym. Adv. Technol. 2021, 32, 2710–2726. [Google Scholar] [CrossRef]
- Nadizadeh, Z.; Mahdavi, H. Grafting of zwitterion polymer on polyamide nanofiltration membranes via surface-initiated RAFT polymerization with improved antifouling properties as a new strategy. Sep. Purif. Technol. 2021, 254, 117605. [Google Scholar] [CrossRef]
- Zhao, Y.-F.; Zhang, P.-B.; Sun, J.; Liu, C.-J.; Yi, Z.; Zhu, L.-P.; Xu, Y.-Y. Versatile antifouling polyethersulfone filtration membranes modified via surface grafting of zwitterionic polymers from a reactive amphiphilic copolymer additive. J. Colloid Interface Sci. 2015, 448, 380–388. [Google Scholar]
- Subramaniam, M.; Goh, P.; Sevgili, E.; Karaman, M.; Lau, W.; Ismail, A. Hydroxypropyl methacrylate thin film coating on polyvinylidene fluoride hollow fiber membranes via initiated chemical vapor deposition. Eur. Polym. J. 2020, 122, 109360. [Google Scholar] [CrossRef]
- Zheng, S.; Yang, Q.; Mi, B. Novel antifouling surface with improved hemocompatibility by immobilization of polyzwitterions onto silicon via click chemistry. Appl. Surf. Sci. 2016, 363, 619–626. [Google Scholar] [CrossRef]
- Cui, M.; Shen, M.; Zhou, L.; Luo, Z.; Zhou, H.; Yang, X.; Hu, H. Enhancing antifouling property of PVA membrane by grafting zwitterionic polymer via SI-ATRP method. J. Biomater. Sci. Polym. Ed. 2020, 31, 1852–1868. [Google Scholar] [CrossRef]
- Gleason, K.K. Controlled Release Utilizing Initiated Chemical Vapor Deposited (iCVD) of Polymeric Nanolayers. Front. Bioeng. Biotechnol. 2021, 9, 11. [Google Scholar]
- Guo, Y.-S.; Mi, Y.-F.; Ji, Y.-L.; An, Q.-F.; Gao, C.-J. One-step surface grafting method for preparing zwitterionic nanofiltation membrane via in situ introduction of initiator in interfacial polymerization. ACS Appl. Polym. Mater. 2019, 1, 1022–1033. [Google Scholar] [CrossRef]
- Yu, S.J.; Pak, K.; Kwak, M.J.; Joo, M.; Kim, B.J.; Oh, M.S.; Baek, J.; Park, H.; Choi, G.; Kim, D.H. Initiated chemical vapor deposition: A versatile tool for various device applications. Adv. Eng. Mater. 2018, 20, 1700622. [Google Scholar]
- Zhao, J.; Yang, J.; Li, Y.; Li, D.; Wang, Q.; Zhang, L.; Zhang, Y.; Zhang, S.; Chen, L. Improved permeability and biofouling resistance of microfiltration membranes via quaternary ammonium and zwitterion dual-functionalized diblock copolymers. Eur. Polym. J. 2020, 135, 109883. [Google Scholar]
- Zhang, J.; Yuan, J.; Yuan, Y.; Shen, J.; Lin, S. Chemical modification of cellulose membranes with sulfo ammonium zwitterionic vinyl monomer to improve hemocompatibility. Colloids Surf. B Biointerfaces 2003, 30, 249–257. [Google Scholar]
- Yuan, J.; Huang, X.; Li, P.; Li, L.; Shen, J. Surface-initiated RAFT polymerization of sulfobetaine from cellulose membranes to improve hemocompatibility and antibiofouling property. Polym. Chem. 2013, 4, 5074–5085. [Google Scholar]
- Seidi, F.; Salimi, H.; Shamsabadi, A.A.; Shabanian, M. Synthesis of hybrid materials using graft copolymerization on non-cellulosic polysaccharides via homogenous ATRP. Prog. Polym. Sci. 2018, 76, 1–39. [Google Scholar]
- Liu, P.-S.; Chen, Q.; Wu, S.-S.; Shen, J.; Lin, S.-C. Surface modification of cellulose membranes with zwitterionic polymers for resistance to protein adsorption and platelet adhesion. J. Membr. Sci. 2010, 350, 387–394. [Google Scholar]
- Zhao, Y.-F.; Zhu, L.-P.; Yi, Z.; Zhu, B.-K.; Xu, Y.-Y. Zwitterionic hydrogel thin films as antifouling surface layers of polyethersulfone ultrafiltration membranes anchored via reactive copolymer additive. J. Membr. Sci. 2014, 470, 148–158. [Google Scholar]
- Moad, G.; Rizzardo, E.; Thang, S.H. End-functional polymers, thiocarbonylthio group removal/transformation and reversible addition-fragmentation-chain transfer (RAFT) polymerization. Polym. Int. 2011, 60, 9–25. [Google Scholar] [CrossRef]
- Hill, M.R.; Carmean, R.N.; Sumerlin, B.S. Expanding the scope of RAFT polymerization: Recent advances and new horizons. Macromolecules 2015, 48, 5459–5469. [Google Scholar] [CrossRef]
- Nothling, M.D.; Fu, Q.; Reyhani, A.; Allison-Logan, S.; Jung, K.; Zhu, J.; Kamigaito, M.; Boyer, C.; Qiao, G.G. Progress and perspectives beyond traditional RAFT polymerization. Adv. Sci. 2020, 7, 2001656. [Google Scholar]
- Grande, C.D.; Tria, M.C.; Jiang, G.; Ponnapati, R.; Advincula, R. Surface-grafted polymers from electropolymerized polythiophene RAFT agent. Macromolecules 2011, 44, 966–975. [Google Scholar]
- Vermette, P.; Meagher, L. Interactions of phospholipid-and poly (ethylene glycol)-modified surfaces with biological systems: Relation to physico-chemical properties and mechanisms. Colloids Surf. B Biointerfaces 2003, 28, 153–198. [Google Scholar] [CrossRef]
- Yang, Q.; Adrus, N.; Tomicki, F.; Ulbricht, M. Composites of functional polymeric hydrogels and porous membranes. J. Mater. Chem. 2011, 21, 2783–2811. [Google Scholar]
- Milleret, V.; Hefti, T.; Hall, H.; Vogel, V.; Eberli, D. Influence of the fiber diameter and surface roughness of electrospun vascular grafts on blood activation. Acta Biomater. 2012, 8, 4349–4356. [Google Scholar]
- Bhalani, D.V.; Trivedi, J.S.; Jewrajka, S.K. Selective grafting of morphologically modified poly (vinylidene fluoride) ultrafiltration membrane by poly (acrylic acid) for inducing antifouling property. Appl. Surf. Sci. 2021, 544, 148905. [Google Scholar]
- Yun, D.; Yim, T.; Kwon, O.J.; Kim, T.-H. Click Chemistry-Induced Terminally Crosslinked Poly (ether sulfone) as a Highly Conductive Anion Exchange Membrane Under Humidity Condition. Macromol. Res. 2019, 27, 1050–1059. [Google Scholar] [CrossRef]
- Shen, B.; Pu, H. Poly (ether sulfone) s with pendent imidazolium for anion exchange membranes via click chemistry. Polymer 2020, 207, 122944. [Google Scholar]
- Yang, W.; Chen, J.; Yan, J.; Liu, S.; Yan, Y.; Zhang, Q. Advance of click chemistry in anion exchange membranes for energy application. J. Polym. Sci. 2022, 60, 627–649. [Google Scholar]
- Kim, E.; Koo, H. Biomedical applications of copper-free click chemistry: In vitro, in vivo, and ex vivo. Chem. Sci. 2019, 10, 7835–7851. [Google Scholar] [CrossRef]
- Yu, H.-Y.; Kang, Y.; Liu, Y.; Mi, B. Grafting polyzwitterions onto polyamide by click chemistry and nucleophilic substitution on nitrogen: A novel approach to enhance membrane fouling resistance. J. Membr. Sci. 2014, 449, 50–57. [Google Scholar]
- Li, F.; Bhushan, B.; Pan, Y.; Zhao, X. Bioinspired superoleophobic/superhydrophilic functionalized cotton for efficient separation of immiscible oil-water mixtures and oil-water emulsions. J. Colloid Interface Sci. 2019, 548, 123–130. [Google Scholar] [PubMed]
- Fang, S.; Zhang, Z.; Yang, H.; Wang, G.; Gu, L.; Xia, L.; Zeng, Z.; Zhu, L. Mussel-inspired hydrophilic modification of polypropylene membrane for oil-in-water emulsion separation. Surf. Coat. Technol. 2020, 403, 126375. [Google Scholar] [CrossRef]
- Xi, Z.-Y.; Xu, Y.-Y.; Zhu, L.-P.; Wang, Y.; Zhu, B.-K. A facile method of surface modification for hydrophobic polymer membranes based on the adhesive behavior of poly (DOPA) and poly (dopamine). J. Membr. Sci. 2009, 327, 244–253. [Google Scholar]
- Liu, Z.; Wu, W.; Liu, Y.; Qin, C.; Meng, M.; Jiang, Y.; Qiu, J.; Peng, J. A mussel inspired highly stable graphene oxide membrane for efficient oil-in-water emulsions separation. Sep. Purif. Technol. 2018, 199, 37–46. [Google Scholar]
- Asha, A.B.; Chen, Y.; Narain, R. Bioinspired dopamine and zwitterionic polymers for non-fouling surface engineering. Chem. Soc. Rev. 2021, 50, 11668–11683. [Google Scholar] [PubMed]
- Gao, C.; Li, G.; Xue, H.; Yang, W.; Zhang, F.; Jiang, S. Functionalizable and ultra-low fouling zwitterionic surfaces via adhesive mussel mimetic linkages. Biomaterials 2010, 31, 1486–1492. [Google Scholar]
- Xu, W.; Li, S.; Ye, Z.; Zhang, J.; Deng, L.; Dong, A. Optimization of sulfonated polyethyleneimine zwitterionic coating mediated by polydopamine for poly (vinyl chloride) antifouling. J. Appl. Polym. Sci. 2021, 138, 49636. [Google Scholar]
- Xie, Y.; Tang, C.; Wang, Z.; Xu, Y.; Zhao, W.; Sun, S.; Zhao, C. Co-deposition towards mussel-inspired antifouling and antibacterial membranes by using zwitterionic polymers and silver nanoparticles. J. Mater. Chem. B 2017, 5, 7186–7193. [Google Scholar] [CrossRef] [PubMed]
- Gillich, T.; Benetti, E.M.; Rakhmatullina, E.; Konradi, R.; Li, W.; Zhang, A.; Schlüter, A.D.; Textor, M. Self-assembly of focal point oligo-catechol ethylene glycol dendrons on titanium oxide surfaces: Adsorption kinetics, surface characterization, and nonfouling properties. J. Am. Chem. Soc. 2011, 133, 10940–10950. [Google Scholar] [CrossRef]
- Kuang, J.; Messersmith, P.B. Universal surface-initiated polymerization of antifouling zwitterionic brushes using a mussel-mimetic peptide initiator. Langmuir 2012, 28, 7258–7266. [Google Scholar]
- Kang, S.M.; Hwang, N.S.; Yeom, J.; Park, S.Y.; Messersmith, P.B.; Choi, I.S.; Langer, R.; Anderson, D.G.; Lee, H. One-step multipurpose surface functionalization by adhesive catecholamine. Adv. Funct. Mater. 2012, 22, 2949–2955. [Google Scholar]
- Sin, M.-C.; Sun, Y.-M.; Chang, Y. Zwitterionic-based stainless steel with well-defined polysulfobetaine brushes for general bioadhesive control. ACS Appl. Mater. Interfaces 2014, 6, 861–873. [Google Scholar]
- Shi, J.-L.; Fang, L.-F.; Li, H.; Zhang, H.; Zhu, B.-K.; Zhu, L.-P. Improved thermal and electrochemical performances of PMMA modified PE separator skeleton prepared via dopamine-initiated ATRP for lithium ion batteries. J. Membr. Sci. 2013, 437, 160–168. [Google Scholar]
- Jiang, J.; Zhang, P.; Zhu, L.; Zhu, B.; Xu, Y. Improving antifouling ability and hemocompatibility of poly (vinylidene fluoride) membranes by polydopamine-mediated ATRP. J. Mater. Chem. B 2015, 3, 7698–7706. [Google Scholar] [CrossRef] [PubMed]
- Davenport, D.M.; Lee, J.; Elimelech, M. Efficacy of antifouling modification of ultrafiltration membranes by grafting zwitterionic polymer brushes. Sep. Purif. Technol. 2017, 189, 389–398. [Google Scholar]
- Li, N.; Li, T.; Qiao, X.-Y.; Li, R.; Yao, Y.; Gong, Y.-K. Universal strategy for efficient fabrication of blood compatible surfaces via polydopamine-assisted surface-initiated activators regenerated by electron transfer atom-transfer radical polymerization of zwitterions. ACS Appl. Mater. Interfaces 2020, 12, 12337–12344. [Google Scholar]
- Jiang, J.-H.; Zhu, L.-P.; Li, X.-L.; Xu, Y.-Y.; Zhu, B.-K. Surface modification of PE porous membranes based on the strong adhesion of polydopamine and covalent immobilization of heparin. J. Membr. Sci. 2010, 364, 194–202. [Google Scholar] [CrossRef]
- Cheng, C.; Sun, S.; Zhao, C. Progress in heparin and heparin-like/mimicking polymer-functionalized biomedical membranes. J. Mater. Chem. B 2014, 2, 7649–7672. [Google Scholar] [CrossRef] [PubMed]
- Fabbri, P.; Messori, M. Surface modification of polymers: Chemical, physical, and biological routes. In Modification of Polymer Properties; Elsevier: Amsterdam, The Netherlands, 2017; pp. 109–130. [Google Scholar]
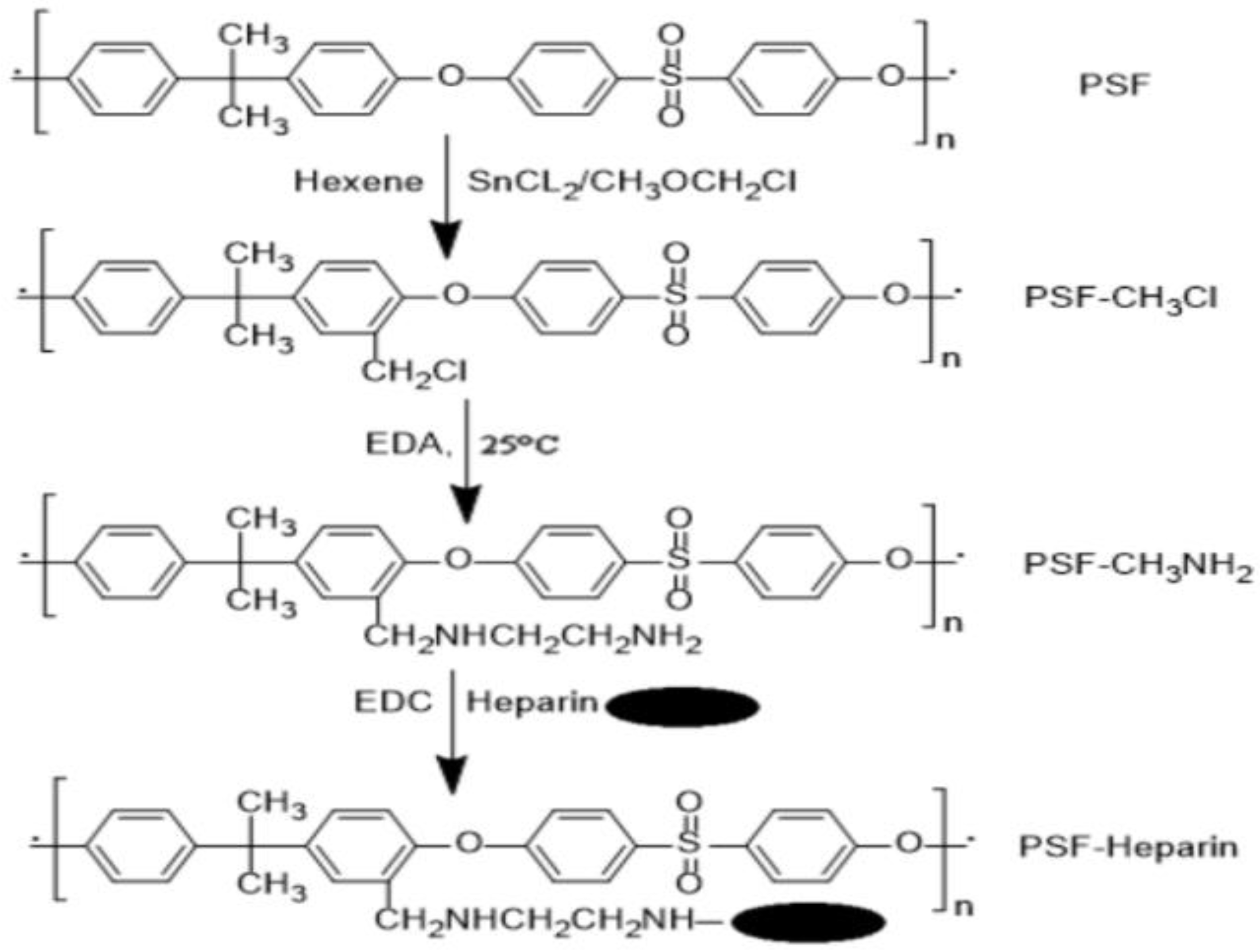
- Huang, X.-J.; Guduru, D.; Xu, Z.-K.; Vienken, J.; Groth, T. Immobilization of heparin on polysulfone surface for selective adsorption of low-density lipoprotein (LDL). Acta Biomater. 2010, 6, 1099–1106. [Google Scholar] [CrossRef]
- Li, J.; Huang, X.J.; Ji, J.; Lan, P.; Vienken, J.; Groth, T.; Xu, Z.K. Covalent heparin modification of a polysulfone flat sheet membrane for selective removal of low-density lipoproteins: A simple and versatile method. Macromol. Biosci. 2011, 11, 1218–1226. [Google Scholar]
- Radu, E.R.; Voicu, S.I. Functionalized Hemodialysis Polysulfone Membranes with Improved Hemocompatibility. Polymers 2022, 14, 1130. [Google Scholar]
- Wang, J.; Peng, C.; Chen, H.; Zhao, W.; Zhao, C. Fabrication of hemocompatible polyethersulfone derivatives by one-step radiation-induced homogeneous polymerization. Mater. Today Commun. 2020, 25, 101548. [Google Scholar]
- Ji, H.; Xu, H.; Jin, L.; Song, X.; He, C.; Liu, X.; Xiong, L.; Zhao, W.; Zhao, C. Surface engineering of low-fouling and hemocompatible polyethersulfone membranes via in-situ ring-opening reaction. J. Membr. Sci. 2019, 581, 373–382. [Google Scholar]
- Chen, S.-H.; Chang, Y.; Lee, K.-R.; Wei, T.-C.; Higuchi, A.; Ho, F.-M.; Tsou, C.-C.; Ho, H.-T.; Lai, J.-Y. Hemocompatible control of sulfobetaine-grafted polypropylene fibrous membranes in human whole blood via plasma-induced surface zwitterionization. Langmuir 2012, 28, 17733–17742. [Google Scholar] [PubMed]
- Zhao, Z.-P.; Li, M.-S.; Li, N.; Wang, M.-X.; Zhang, Y. Controllable modification of polymer membranes by long-distance and dynamic low-temperature plasma flow: AA grafting penetrated through electrospun PP fibrous membranes. J. Membr. Sci. 2013, 440, 9–19. [Google Scholar] [CrossRef]
- Li, Q.; Imbrogno, J.; Belfort, G.; Wang, X.L. Making polymeric membranes antifouling via “grafting from” polymerization of zwitterions. J. Appl. Polym. Sci. 2015, 132, 1–27. [Google Scholar]
- Salimi, P.; Aroujalian, A.; Iranshahi, D. Development of PES-based hydrophilic membranes via corona air plasma for highly effective water purification. J. Environ. Chem. Eng. 2022, 10, 107775. [Google Scholar] [CrossRef]
- Venault, A.; Wei, T.-C.; Shih, H.-L.; Yeh, C.-C.; Chinnathambi, A.; Alharbi, S.A.; Carretier, S.; Aimar, P.; Lai, J.-Y.; Chang, Y. Antifouling pseudo-zwitterionic poly (vinylidene fluoride) membranes with efficient mixed-charge surface grafting via glow dielectric barrier discharge plasma-induced copolymerization. J. Membr. Sci. 2016, 516, 13–25. [Google Scholar] [CrossRef]
- Saxena, N.; Prabhavathy, C.; De, S.; DasGupta, S. Flux enhancement by argon–oxygen plasma treatment of polyethersulfone membranes. Sep. Purif. Technol. 2009, 70, 160–165. [Google Scholar] [CrossRef]
- Chao, A.-C.; Shyu, S.-S.; Lin, Y.-C.; Mi, F.-L. Enzymatic grafting of carboxyl groups on to chitosan––to confer on chitosan the property of a cationic dye adsorbent. Bioresour. Technol. 2004, 91, 157–162. [Google Scholar] [CrossRef]
- Nady, N.; Franssen, M.C.; Boom, R.M.; Mohyeldin, M.S.; Zuilhof, H.; Schroën, C. Modification of PES membranes: An environmentally friendly approach. Presented at the 13th Aachener Membrane Kolloquium, Aachen, Germany, 27–28 October 2010; Available online: https://edepot.wur.nl/154644 (accessed on 20 September 2022).
- Amri, C.; Mudasir, M.; Siswanta, D.; Roto, R. In vitro hemocompatibility of PVA-alginate ester as a candidate for hemodialysis membrane. Int. J. Biol. Macromol. 2016, 82, 48–53. [Google Scholar] [CrossRef] [PubMed]
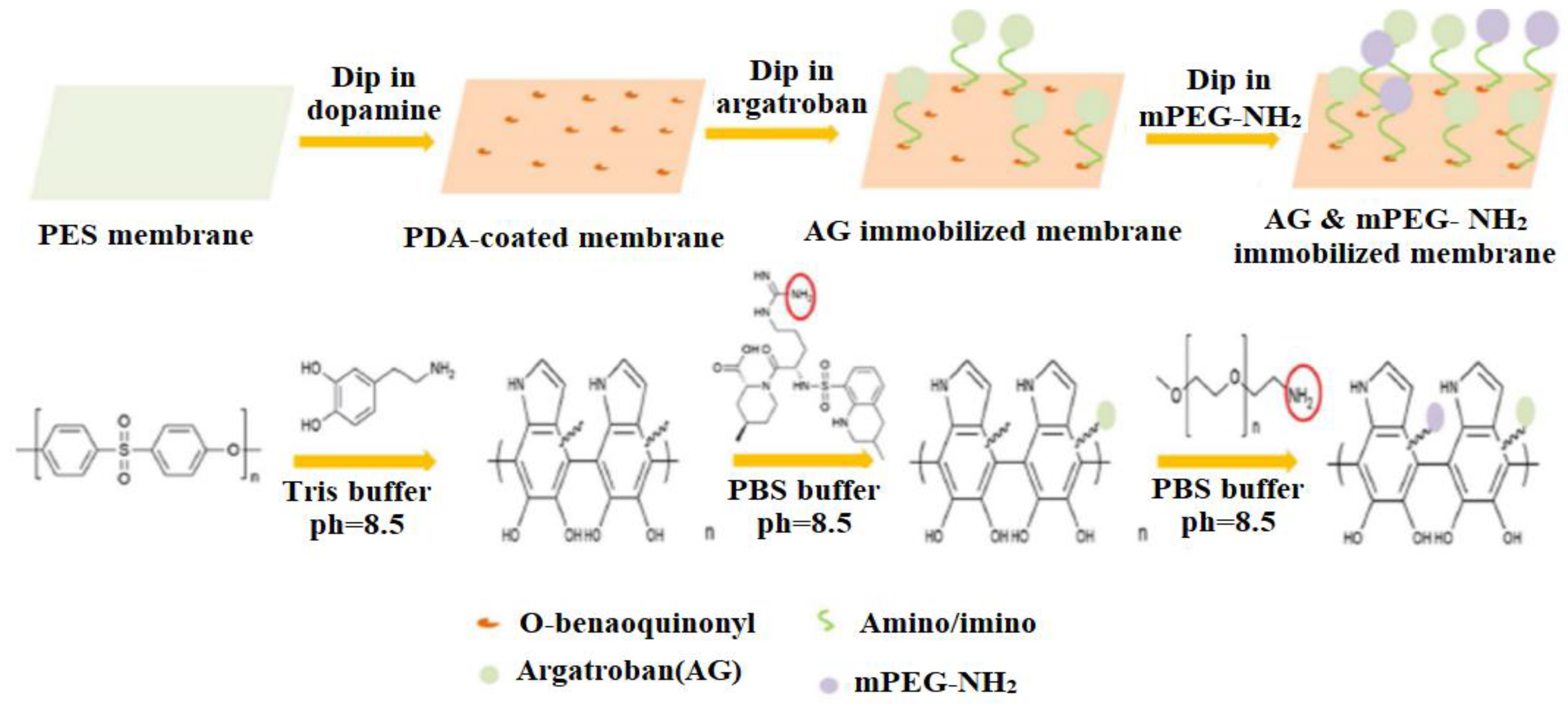
- Dai, Y.; Dai, S.; Xie, X.; Ning, J. Immobilizing argatroban and mPEG-NH2 on a polyethersulfone membrane surface to prepare an effective nonthrombogenic biointerface. J. Biomater. Sci. Polym. Ed. 2019, 30, 608–628. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Qiu, M.; He, C. A zwitterionic polymer/PES membrane for enhanced antifouling performance and promoting hemocompatibility. J. Membr. Sci. 2020, 606, 118119. [Google Scholar] [CrossRef]
- Zhao, Y.-F.; Zhang, P.-B.; Sun, J.; Liu, C.-J.; Zhu, L.-P.; Xu, Y.-Y. Electrolyte-responsive polyethersulfone membranes with zwitterionic polyethersulfone-based copolymers as additive. J. Membr. Sci. 2016, 510, 306–313. [Google Scholar] [CrossRef]
- Huang, Z.; Nazifi, S.; Jafari, P.; Karim, A.; Ghasemi, H. Networked zwitterionic durable antibacterial surfaces. ACS Appl. Bio Mater. 2020, 3, 911–919. [Google Scholar] [CrossRef]
- Wang, T.; Wang, Y.-Q.; Su, Y.-L.; Jiang, Z.-Y. Antifouling ultrafiltration membrane composed of polyethersulfone and sulfobetaine copolymer. J. Membr. Sci. 2006, 280, 343–350. [Google Scholar] [CrossRef]
- Chen, S.; Xie, Y.; Xiao, T.; Zhao, W.; Li, J.; Zhao, C. Tannic acid-inspiration and post-crosslinking of zwitterionic polymer as a universal approach towards antifouling surface. Chem. Eng. J. 2018, 337, 122–132. [Google Scholar]
- Xie, Y.; Li, S.-S.; Jiang, X.; Xiang, T.; Wang, R.; Zhao, C.-S. Zwitterionic glycosyl modified polyethersulfone membranes with enhanced anti-fouling property and blood compatibility. J. Colloid Interface Sci. 2015, 443, 36–44. [Google Scholar] [PubMed]
- Xiang, T.; Luo, C.-D.; Wang, R.; Han, Z.-Y.; Sun, S.-D.; Zhao, C.-S. Ionic-strength-sensitive polyethersulfone membrane with improved anti-fouling property modified by zwitterionic polymer via in situ cross-linked polymerization. J. Membr. Sci. 2015, 476, 234–242. [Google Scholar]
- He, M.; Wang, Q.; Zhao, W.; Zhao, C. A substrate-independent ultrathin hydrogel film as an antifouling and antibacterial layer for a microfiltration membrane anchored via a layer-by-layer thiol–ene click reaction. J. Mater. Chem. B 2018, 6, 3904–3913. [Google Scholar] [PubMed]
- Sun, X.; Hu, W.; Gao, C. Low-fouling polysulfone ultrafiltration membranes with amphiphilic sulfobetaine polyamide as additive. J. Appl. Polym. Sci. 2020, 137, 49039. [Google Scholar]
- Gu, S.; Xia, H.; Du, J.; Yang, L.; Cai, Y.; Zhou, Y.; Huang, J. Surface modification of polysulfones via one-pot ATRP and click chemistry: Zwitterionic graft complex and their hemocompatibility. Fibers Polym. 2016, 17, 161–165. [Google Scholar]
- Xiang, T.; Zhang, L.-S.; Wang, R.; Xia, Y.; Su, B.-H.; Zhao, C.-S. Blood compatibility comparison for polysulfone membranes modified by grafting block and random zwitterionic copolymers via surface-initiated ATRP. J. Colloid Interface Sci. 2014, 432, 47–56. [Google Scholar]
- Yue, W.-W.; Li, H.-J.; Xiang, T.; Qin, H.; Sun, S.-D.; Zhao, C.-S. Grafting of zwitterion from polysulfone membrane via surface-initiated ATRP with enhanced antifouling property and biocompatibility. J. Membr. Sci. 2013, 446, 79–91. [Google Scholar] [CrossRef]




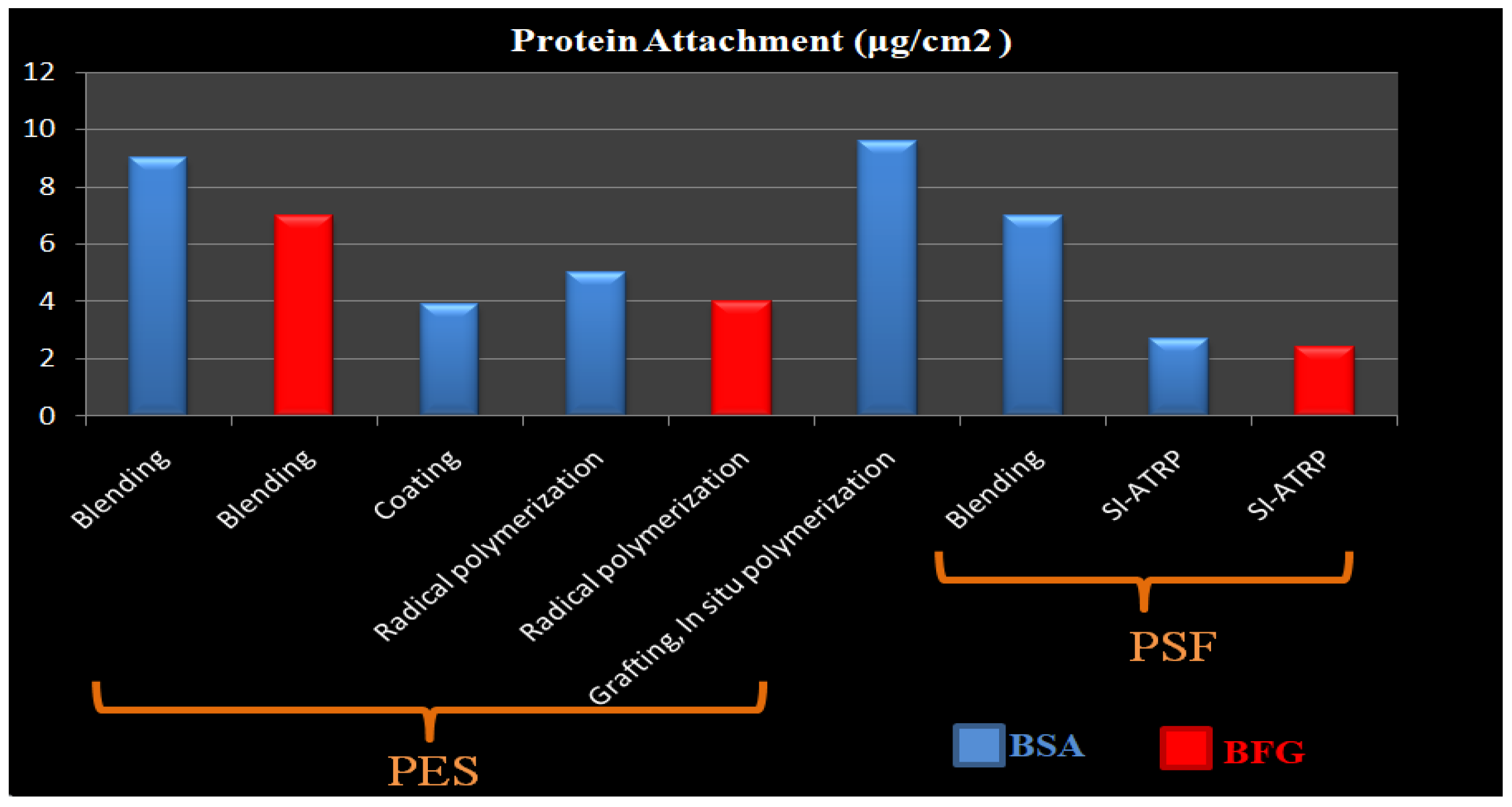
| Name of Additive | Preparation Method of Modification Layer | wt% of Additive | Pure Water Flux (L m−2 h−1) | Contact Angle (θ°) | Antifouling Properties (%) FRR (BSA) | Protein Absorption | Platelet Adhesion | Clotting Time (PT, APTT) | Complement Activation | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| PLA-PHEMA | RAFT | 5, 10, 15, 20 | 236 for 15 wt% PLA-PHEMA content | 71.4° to 60.5° | 54.9 to 86.0% | 28 to 2 μg/cm2 | Suppressed platelet adhesion | Prolonged plasma recalcification times | [45] | |
| PEG-PVA | Phase inversion method, | 1. 1.5, 2, 2.5 | 16.4 to 42.484 | 37.3 to 46° | 82 to 90% | low amount of protein | Suppressed platelet adhesion | Plasma recalcification times: 240 ± 5 s to300 ± 3 s | ~5% ± 0.15 to ~6.5% ± 0.15 thrombus formation | [74] |
| PVP-PES | RAFT | 1.11, 2.97, 4.50, 6.29, 8.12 and 9.47. | - | - | - | - | 96% reduction in platelet adsorption. | APTT increased from 58 to 93 s. | [79] | |
| SPES | Sulfonation | 3 | 49.55 ± 1.52° | 72 ± 5 ng/cm2 | Decreased | 178 ± 2 s | Minor amount of hemostatic fibrin | [81] | ||
| MPC-PPGMA | Non-solvent induced phase separation | 3.1, 5.1, 6 | 80 L m−2 h−1 | 85–97% | BSA rejections are around 20–35%. | Total cell thickness decreased from about 0.43 µm3/μm2 on the pristine PVC membrane to 0.08 µm3/μm2 | [85] | |||
| PES-Aramid nanofiber | Spin-coating method and phase inversion technique | 0.5% CNT | Higher fluxes | 62.5 ± 3.1° | 92.3% | 4.81 μg/cm2 | 3.1 × 107 cells/cm2 | Clearly improved | [84] | |
| PES and PSF Aramid nanofiber/Blending | Spin-coating method and phase inversion technique | 0.5% CNT | Higher fluxes | 64.6 ± 2.0° | 88.2% | 5.03 μg/cm2 | 3.2 × 107 cells/cm2 | Clearly improved | [84] | |
| PVC- SBTFPU (20%) | 20% | similar level to pristine PVC (89.6◦) | 2 μg/cm2 for BSA | 0.84 × 106 platelets/cm 2 | Fibroblasts (NIH3T3 cell line) test, Higher than 90% cell viability | [86] |
| Membrane | Modification | Method | Main Results | Disadvantages | Ref. |
|---|---|---|---|---|---|
| PP | P(4VP-r-ODA) | SAMs | A versatile approach for developing bio-surfaces in vitro, Accurate control of the packing density and environment of an immobilized recognition center, or many centers, on a substrate surface. | The amphiphile concentration, pH, and ionic content all influence the self-assembly process. | [99] |
| PVDF | p(MAO-DMEA) | SAMs | Facile approach, no damage to bulk membrane properties, nanoscale control, Optimizing of coating variables (coating time, solution concentration), and modifier chemical composition (hydrophilic/hydrophobic ratio). | A two-step process, The thermal evaporation process was used as an additional processing stage that is time-consuming | [100] |
| PES | GO-SPHF | Spin-coating | Produce a thin, uniform coating, Controlling the film thickness, Novel fashion of dual-layered composite membranes with integrated advantages of GO and sulfonated polyanions, GO as a multifunctional nano building block linked to a variety of biomaterials, Maintaining membrane mechanical strength | Difficulty with large area samples. two-step spin coating is time-consuming, low efficiency of spin coating material (95–98% of the material is thrown away during the process), | [101] |
| Gold | SB-functionalized SiNPs | Spin-coating | Thickness can be adjusted easily by varying spin speed or viscosity of liquids. The ability of uniform thin film with low-cost production, Does not need the catalyst, Organize and control the chemistries at a materials’ interface, Relatively inexpensive technique, Quickly and easily deposit thin layers. | Depending on many different parameters make it a complex process, Difficulty with large area samples, Multilayer structure difficulty (more than 2 layers), Inability to control deposition accurately (homogeneity, roughness, etc.); Difficulty in making super-thin films (<10 nm). | [102] |
| PP | poly(MPC-random-BMA), poly(SBAArandom-BMA), poly(HEMA-random-BMA), PMPC-block-PBMA, PSBAA-block-PBMA, PHEMA-block-PBMA | Dip-coating | A simple and efficient method, minimal waste systems. | Difficult control of film thickness and surface roughness, Time consuming method | [103] |
| PEI | LBL | A versatile and simple technique for developing multilayer films with desired qualities, Formationofhighlystablemultilayersofzwitterionicpolymers | LBL is an appropriate method to produce multilayers of ZW polymers, under specific circumstances such as low pH or ionic strength. | [109] | |
| PSF, PDMS | PEI/PAA-g-AZ/PEA-p(SBMA) | LBL | Easy & straightforward method, Undetectable nonspecific protein adsorption& completely inhibit platelet adhesion and L929 cells attachment | Limited by the substrate size, type, and shape, Possibility of different types of polymer surfaces | [110] |
| PES | TA-PACMO | LBL | Produce high blood compatibility surfaces, Reducing the danger of cell bursting and enhancing red blood cell retention during dialysis, Preventing oxidative stress and decreased complication risk | The risk of degradation during the performance as hydrogen bonding is not as strong as covalent bonding | [111] |
| PES | PEI-SBMA/OSA-n-Ag | LBL | The Schiff reaction is a strong approach in the biomedical area since it is simple, reversible, pH-sensitive, and biocompatible, as well as having a high stable modified membrane due to Schiff-based connections., Combination of ZW and Ag NPs also leads toantibacterial and antifouling surface | The possibility of association or dissociation of Schiff bases linkages due to different stimuli consisting of pH, vitamin B6 derivatives, amino acids, and enzymes | [112] |
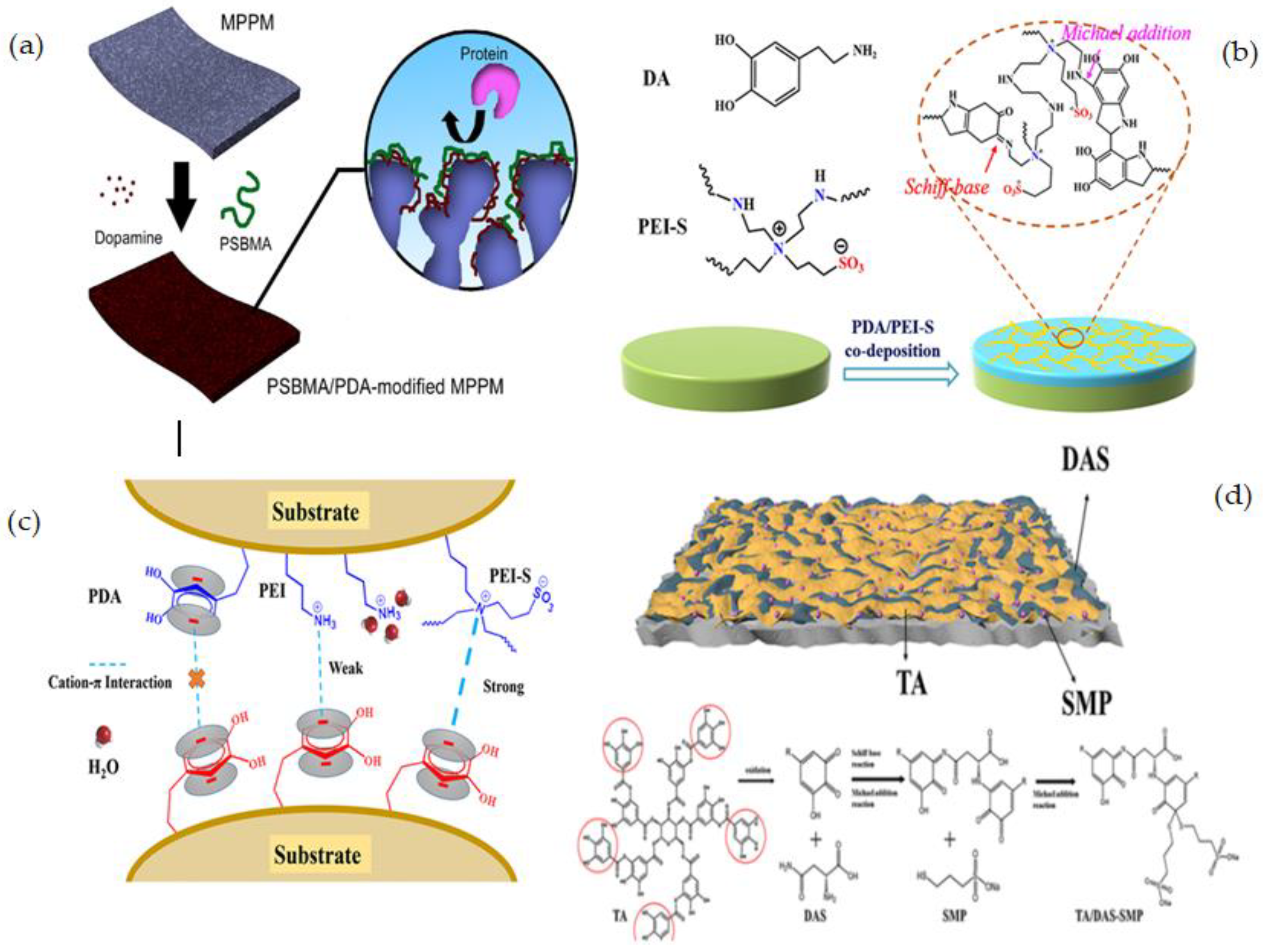
| PP | SBMA/PDA | Co-deposition | A facile and efficient method, One-step process- Short reaction time process | Pore size reduction in a modified membrane, The co-deposition process is influenced by several parameters such as pH, temperature, solution concentration, and deposition time. | [120] |
| PES | M-PDA/PEI-S | Co-deposition | One-step mussel-inspired method, Simple, robust, and material-independent technique, Covalently anchors the PDA to the zwitterionic polymer to improve the coating stability, Producing excellent fouling resistance membrane surface. | Surface morphologies of the M-PDA/PEI and M-PDA/PEI-S noticeably altered compared to M-PDA, Pore size-reduction | [121] |
| PES | TA/DAS-SMP | Co-deposition | No changes to the membrane pores, Production of homogenous surfaces, Good hydrophilic properties, anti-pollution property, hemocompatibility and solute filtration capability, Excellent adhesive qualities, making it an excellent modification surface for materials such as PVDF, PP, PAN, etc. | The risk of degradation during the performance as hydrogen bonding is not as strong as covalent bonding | [122] |
| Types of Surface Modification | Membrane | Monomer | Contact Angle (°) Before/After | Protein Adhesion | Platelet Adhesion | Advantages | Disadvantages | Ref. |
|---|---|---|---|---|---|---|---|---|
| “Grafting-to” method & coating | PDMS | (PGMA-co-SBMA) | 117.93/79.51֩ | 2.89 μg/cm2 For FB (90% Fibrinogen adhesion reduction) | 63.67% Platelet reduction | Non-destructive, simple, innovative method, Precise control of localized grafting, High chemical stability, Maintaining the basic properties of PDMS, such as its mechanical properties and transparency. | Not applicable for large-scale modification, Possibility of changing pore structure as a function of the grafting procedure | [128] |
| Bulk grafting method | PES | NHAc | 76.6/48.1 | - | - | An efficient technique with a stable modification impact due to chemical reactions, Providing membranes with long-term biocidal activity | Old fashioned and unsuccessful method due to the hazardous organic solvents, harsh environment, and time-consuming process | [130] |
| Thermal induced grafting | PES | CB & SB | 10◦ reduced | 66% reduction BSA | - | Facile, economic, and efficient method | Obtaining active sites usually necessitates chemical initiators as well as cleavage agents, Difficult process on a broad scale | [131] |
| Atmospheric plasma-induced surface copolymerization grafting | ePTFE | PSBMA | 120°/22 | Fibrinogen plasma protein was drastically reduced | Excellent resistance to platelet adhesion | Long-term stability because of covalent bonding, Flexible method in tailoring desired surface features with different monomers | Membrane structure and mechanical properties may be affected by plasma impact, and non-uniformity of modified membranes is possible | [133] |
| O2 plasma pre-treatment and UV-irradiatedgrafting | Polypropylene non-woven fabric (NWF) | MPDSAH | 123/17 | 80% reduction for BSA | Excellent resistance to platelet adhesion | The rapid method that produces clean and uniform functional groups on the membrane surface, Accurate control of the packing density, Higher FRR, Excellent stability | Exactly control of different conditions including O2 plasma treatment time, UV irradiation, monomer concentration, etc. | [134] |
| Ozone induced grafting | PVDF | SBMA | - | Excellent BSA reduction | - | Efficient and simple procedure, Tuning the grafting density and chain length, No remarkablephysical and chemical changes on membrane properties | Excessive ozone leads toPVDF degradation and creates large pores | [137] |
| Ozone and UV-induced grafting to method | PS | Poly(GMA-co-SBMA | 100/45 | 90% reduction of FB | 89.3%, reduction of platelet adhesion, 28.6 * 104 (cells per cm2 | Simple, effective, and cost-effective grafting method, Producing stable chemisorption layer on various surfaces, | Control of several variables like the voltage, oxidation time, etc., UV can impact membrane structure and mechanical characteristics. | [140] |
| Radical graft polymerization | PES | P(SSNa-co-SBMA) | 75/55 | 4.93 µg/cm2 BSA and 4.04 µg/cm2 Fibrinogen(BFG) | 2 × 105 cells/cm2 | Convenient and versatile method, Adjusting of component ratio, Commercial and industrial potential for biomedical requirements. | Non-uniformity surface, the Possibility of changing pore structure as a function of the grafting procedure | [141] |
| Modification Method | Flux after Modification | Antifouling Property | Simplicity/Versatility | Chemical Stability | Functionalization | Eco-Friendly Process | Cost Effectiveness | Industrialization Potential |
|---|---|---|---|---|---|---|---|---|
| Blending |  |  |  |  |  |  |  |  |
| Coating |  |  |  |  |  |  |  |  |
| Grafting |  |  |  |  |  |  |  |  |
| Click chemistry |  |  |  |  |  |  |  |  |
| Radical polymerization |  |  |  |  |  |  |  |  |
| Plasma |  |  |  |  |  |  |  |  |
| Ozone |  |  |  |  |  |  |  |  |
| Enzymatic treatment |  |  |  |  |  |  |  |  |

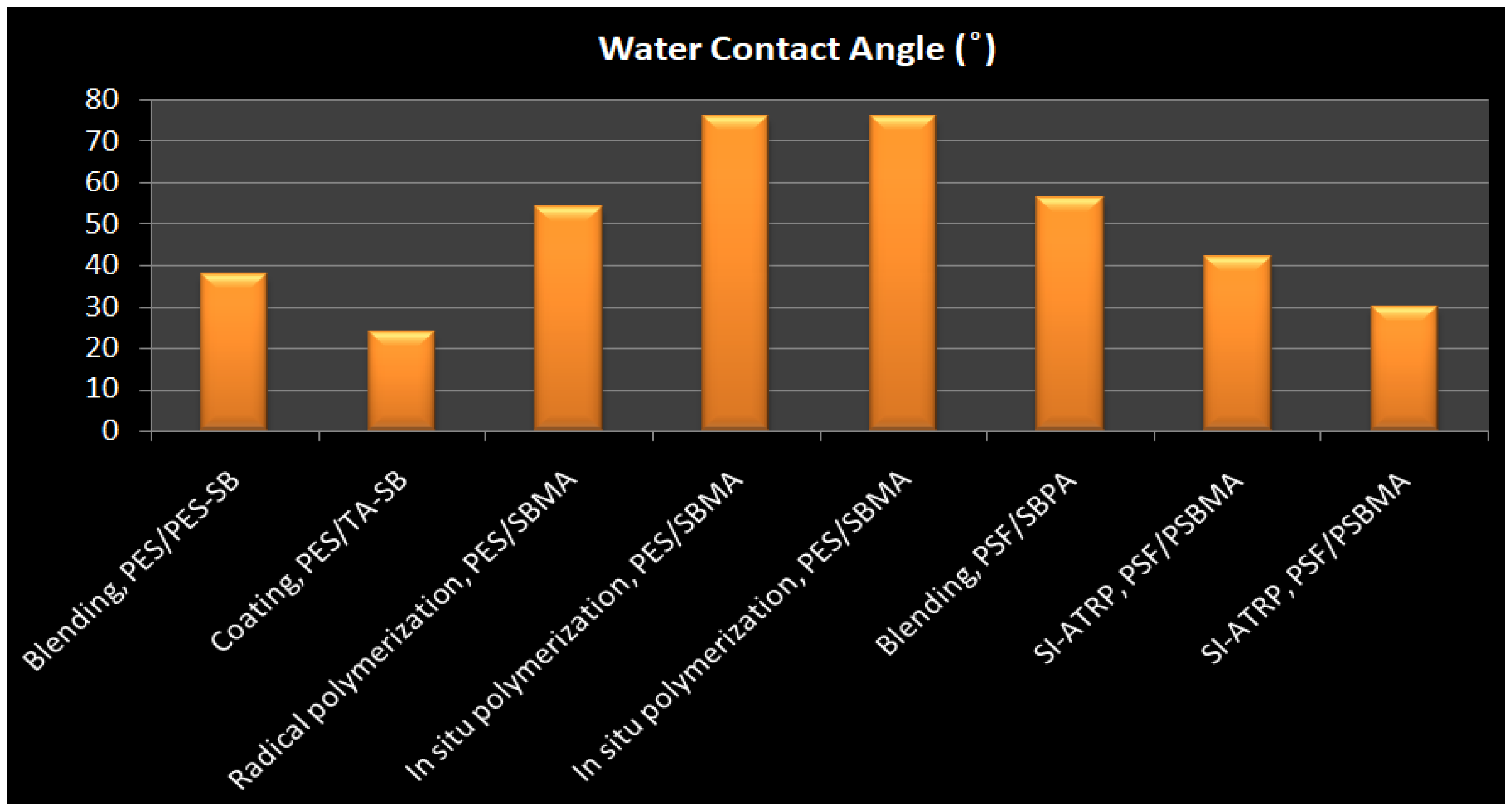
| Membrane-ZW | Immobilization Method ZW | ZW Density(mg/cm2) | Water Contact Angle | Protein Adhesion | Platelet Adhesion | Pure Water Flux (L m−2 h−1) | Antifouling Properties (%) FRR * | Ref. |
|---|---|---|---|---|---|---|---|---|
| PES/PES-SB | Blending | - | 37.8 | 9 μg/cm2 BSA (17% Reduction) & 7 μg/cm2 BFG | Significantly decreased | 243.4 | 93.8%. | [212] |
| PES/PES-SBMA | Blending | - | 59 | 90% BSA reduction | - | 1157 ± 5.6 | 84% | [213] |
| PES/PSBMA | Blending | 78% weight ratio | 25.8 ± 4.6° | 3.88 μm3/μm2 BSA | - | - | - | [214] |
| PES/DMMSA-BMA | Blending | - | 48 | >95% rejection of BSA | - | 82.8% | [215] | |
| PES/TA-SB (M-TA/PEI-S) | Dip-coating | - | 24 | 3.9 μg/cm2 BFG | Very little | [216] | ||
| PES/PGMA-SB | Grafting, In situ cross-linking polymerization | 43 | 0.60 lg/cm2 BSA and 0.37 l g/cm2 BFG | Suppressed platelet adhesion | 43 mL/m2 h mmHg | 100% | [217] | |
| ES-b-PHEM/PSBMA | Grafting, SI-ATRP | High density | 51 | 9 μg/cm2 BSA | Remarkably suppressed and almost no platelets adhered | 39 L/m2 h | 99% | [152] |
| PES/SBMA | Grafting, In situ cross-linking polymerizatio | High density | 76 | 7 μg/cm2 BSA & 10 μg/cm2 BFG | Significantly decreased | 705.21 mL/m2 h mmHg | 99.11% | [218] |
| PES/SBMA | Radical graft polymerization | 0.22 | 54 | 5 μg/cm2 BSA and 4 μg/cm BFG | 2 × 105 cells/cm2 | - | [141] | |
| PES/POEGMS-P(SBMA-co-AA) | LbL thiol-ene “click” chemistry | - | 40 | 4.9 μg/cm2 BSA & 4.6 μg/cm2 BFG, 90% reduction | Nearly no adhered platelet | 61 | - | [219] |
| PSF/SB-PA | Blending | - | 56.2 | 9.6 μg/cm2 BSA, 95% reduction | - | 205 L/m2 h | 85% | [220] |
| PSF/DEPAS | LBL & Click Chemistry | - | 38 | 4 μg/cm2 BSA and 2μg/cm2 BFG, | Significantly decreased | - | - | [221] |
| PSF/PSBMA | Grafting, SI-ATRP | 0.42 mg/cm2 | 42 | 2.7 μg/cm2 BSA& 2.4 μg/cm2 BFG | 0.06 × 107 cell/cm2 | Decreased | Increased | [222] |
| PSF/PSBMA | Grafting | High density | 30 | 98% Reduction | Significantly decreased | 46.72 | 98.1% | [223] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nazari, S.; Abdelrasoul, A. Impact of Membrane Modification and Surface Immobilization Techniques on the Hemocompatibility of Hemodialysis Membranes: A Critical Review. Membranes 2022, 12, 1063. https://doi.org/10.3390/membranes12111063
Nazari S, Abdelrasoul A. Impact of Membrane Modification and Surface Immobilization Techniques on the Hemocompatibility of Hemodialysis Membranes: A Critical Review. Membranes. 2022; 12(11):1063. https://doi.org/10.3390/membranes12111063
Chicago/Turabian StyleNazari, Simin, and Amira Abdelrasoul. 2022. "Impact of Membrane Modification and Surface Immobilization Techniques on the Hemocompatibility of Hemodialysis Membranes: A Critical Review" Membranes 12, no. 11: 1063. https://doi.org/10.3390/membranes12111063
APA StyleNazari, S., & Abdelrasoul, A. (2022). Impact of Membrane Modification and Surface Immobilization Techniques on the Hemocompatibility of Hemodialysis Membranes: A Critical Review. Membranes, 12(11), 1063. https://doi.org/10.3390/membranes12111063






