Remediation of Water Using a Nanofabricated Cellulose Membrane Embedded with Silver Nanoparticles
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Green Synthesis of Silver Nanoparticles
2.3. Fabrication of AgNPs and Composite Membrane
2.4. Characterization of Biosynthesized Nanoparticles and Composite Membrane
2.5. Efficiency of Biogenic AgNPs for Adsorption of Insecticides and Herbicides from a Contaminated Water Sample
3. Results
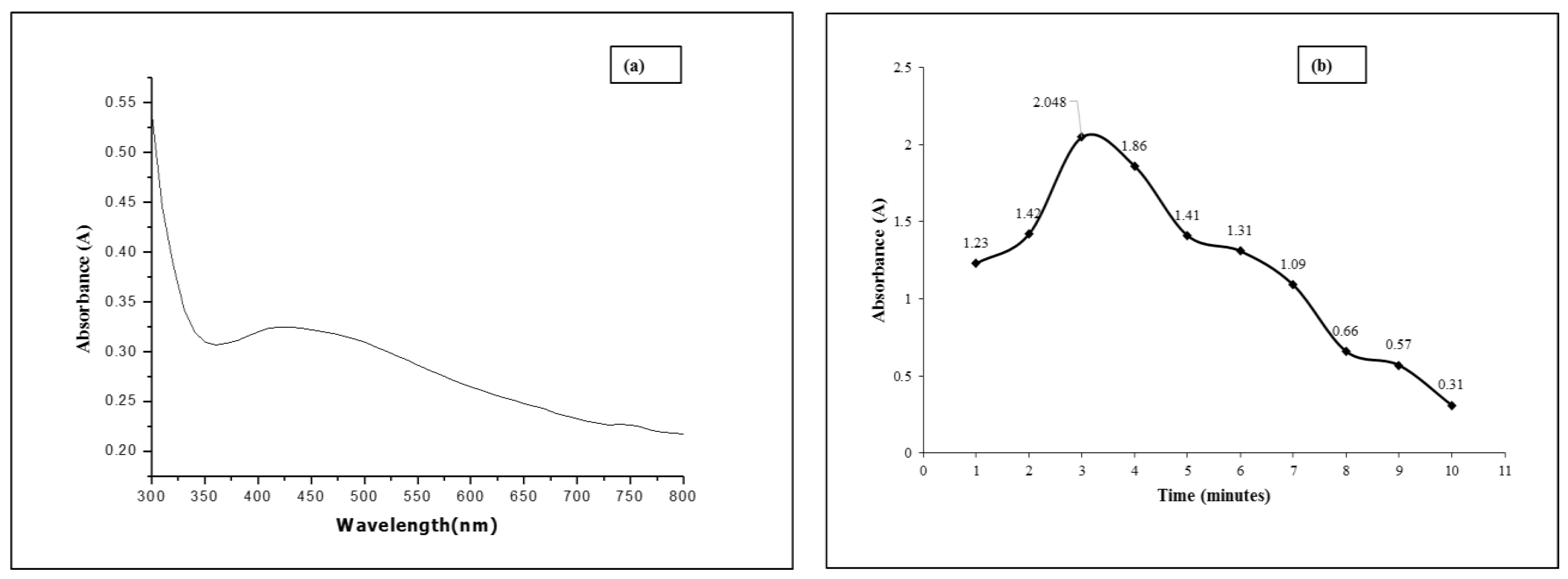
3.1. UV-Visible Analysis of the Synthesized Nanoparticles
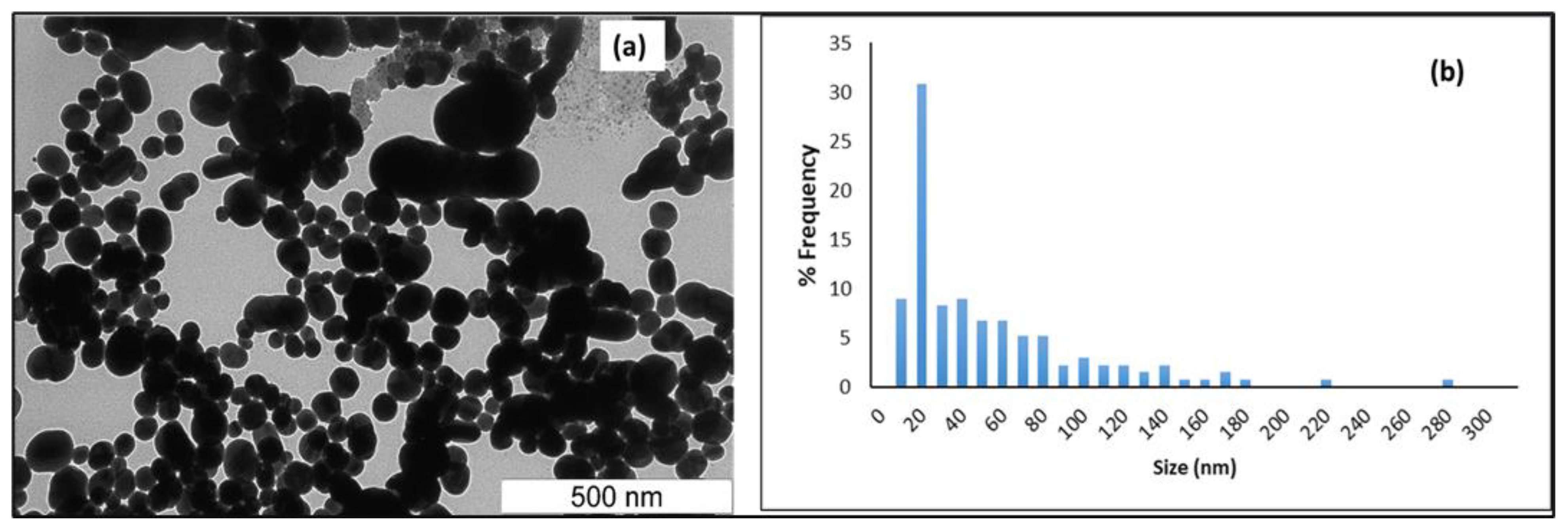
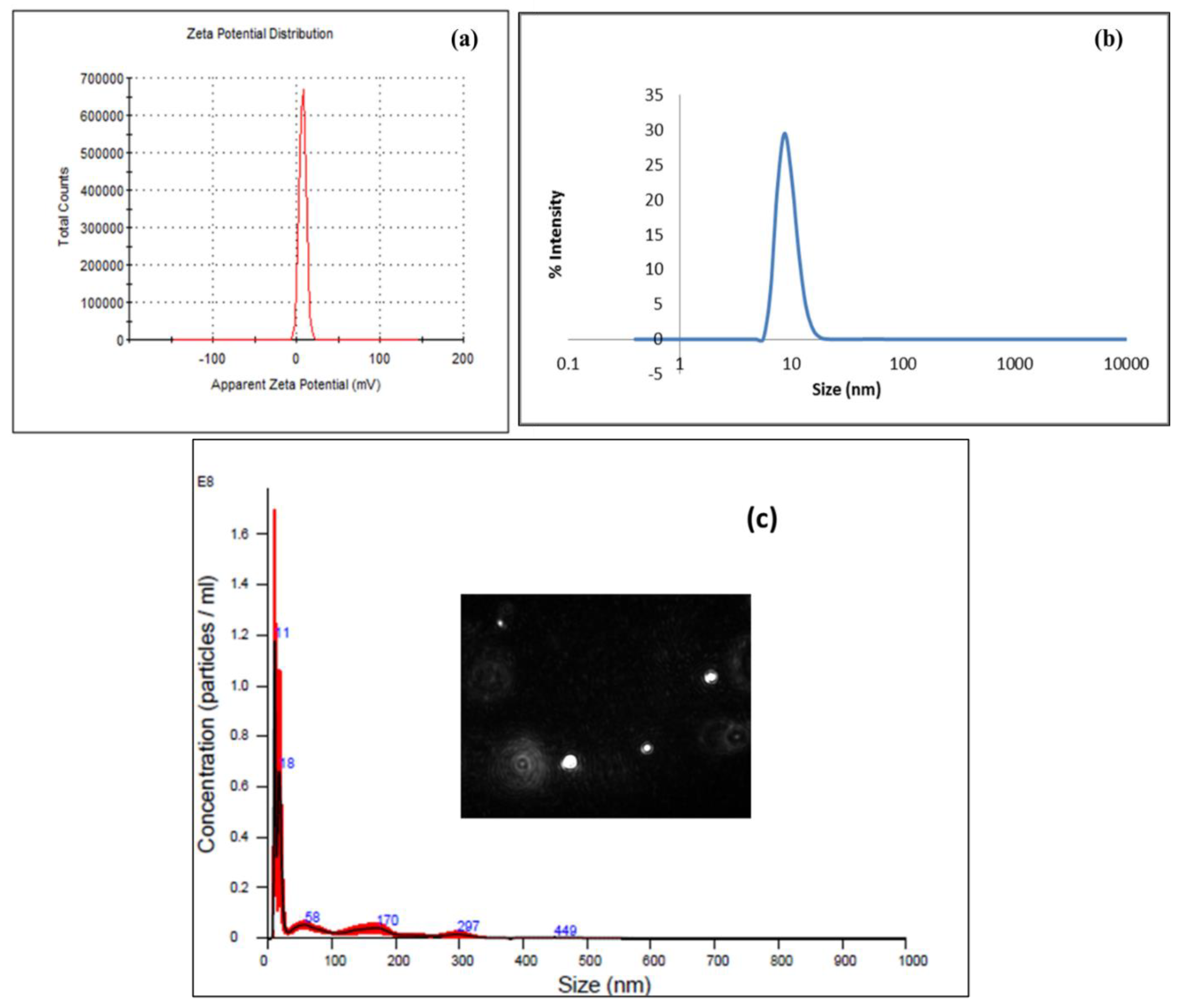
3.2. Transmission Electron Microscopy (TEM) and Dynamic Light Scattering (DLS) Analysis
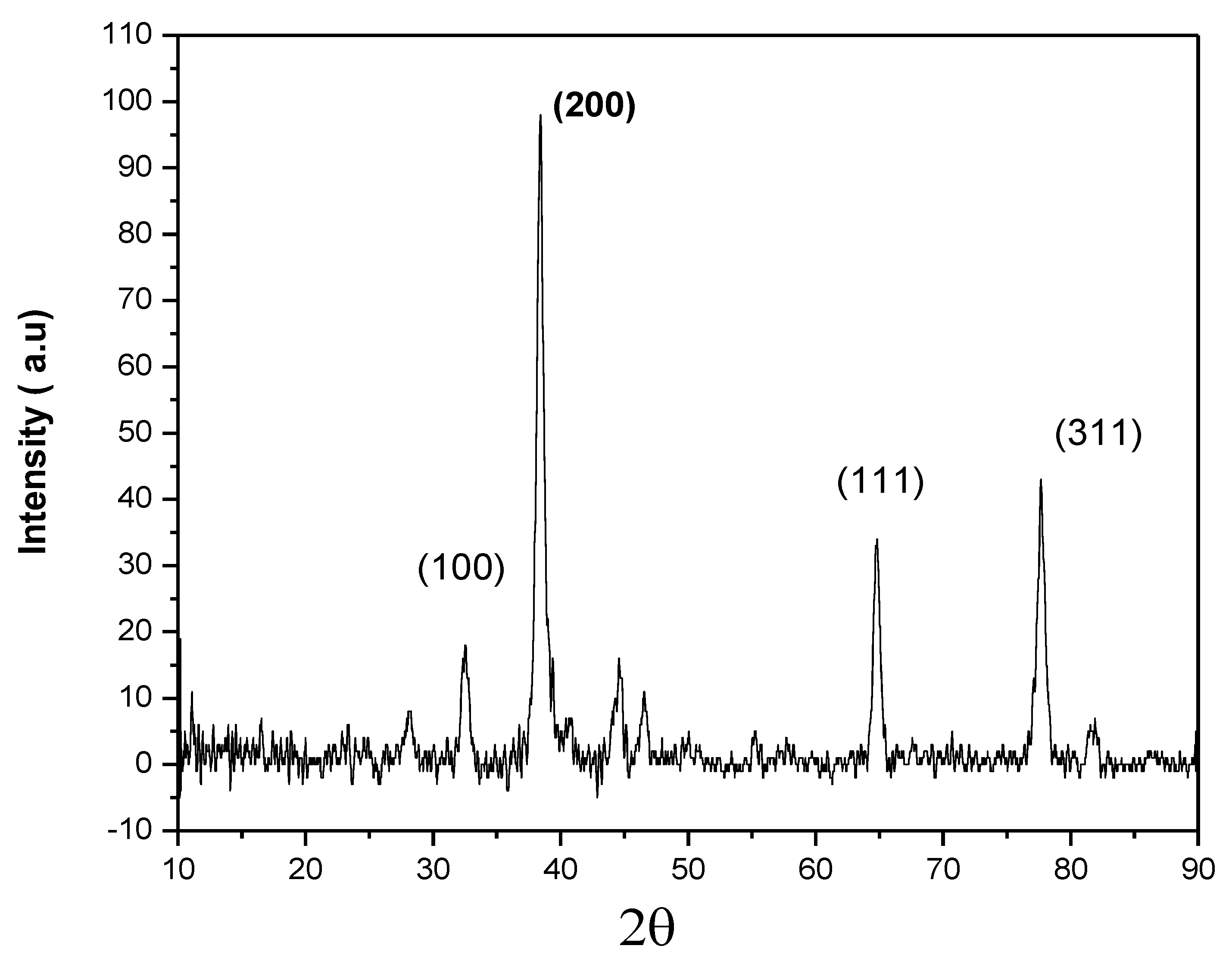
3.3. X-ray Diffraction (XRD) Analysis of AgNPs
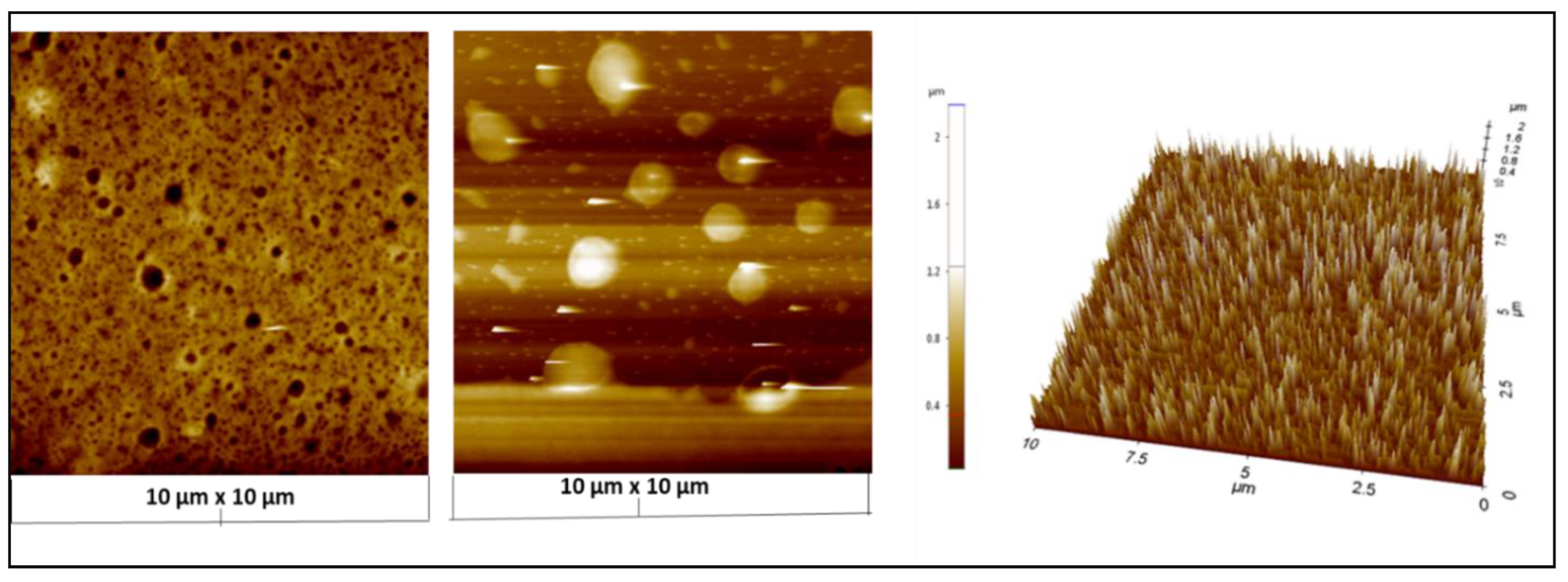
3.4. Atomic Force Microscopy (AFM) Analysis of AgNPs Formation
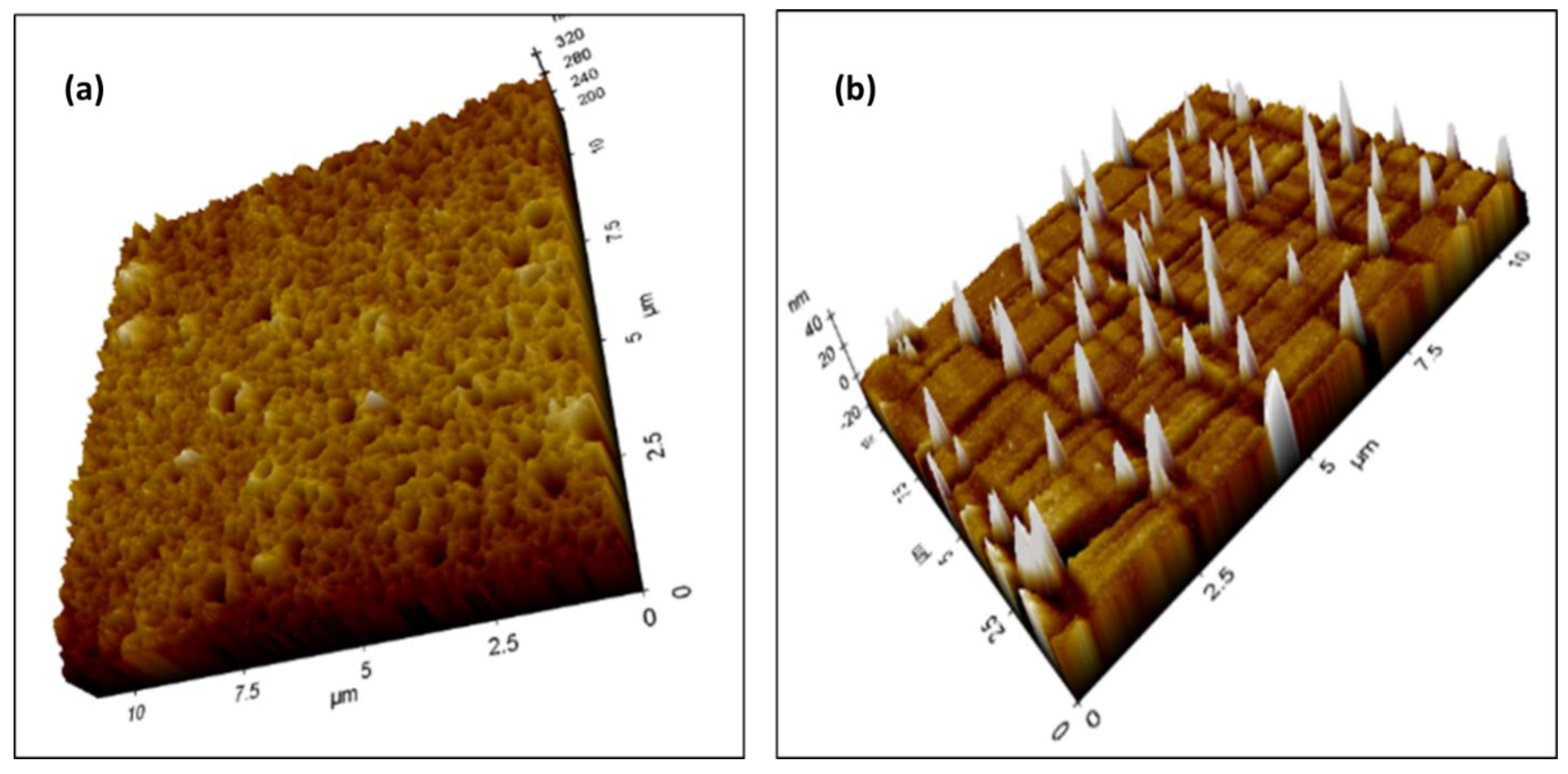
3.5. Incorporation of Ag Nanoparticles into the Cellulose Acetate Membrane
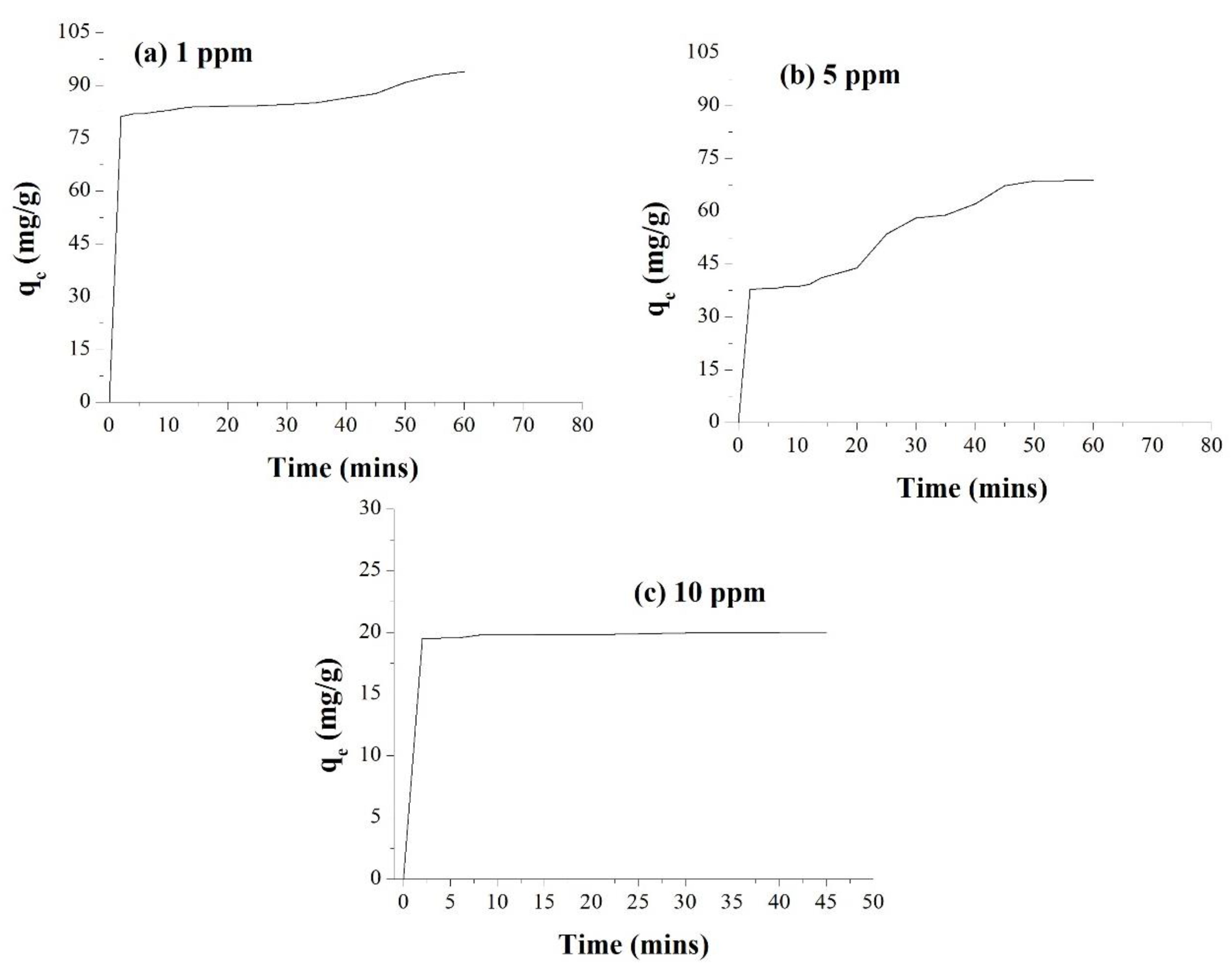
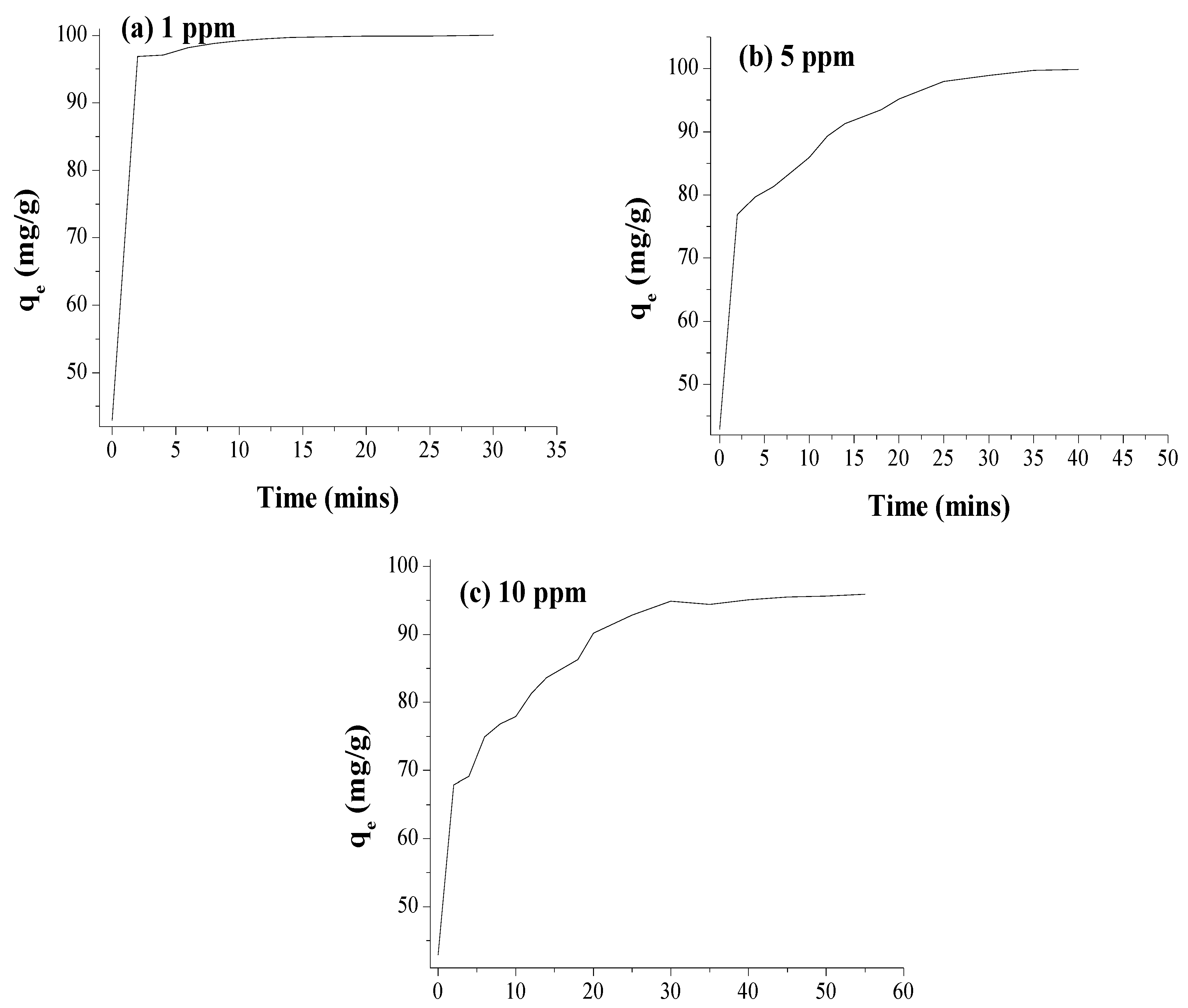
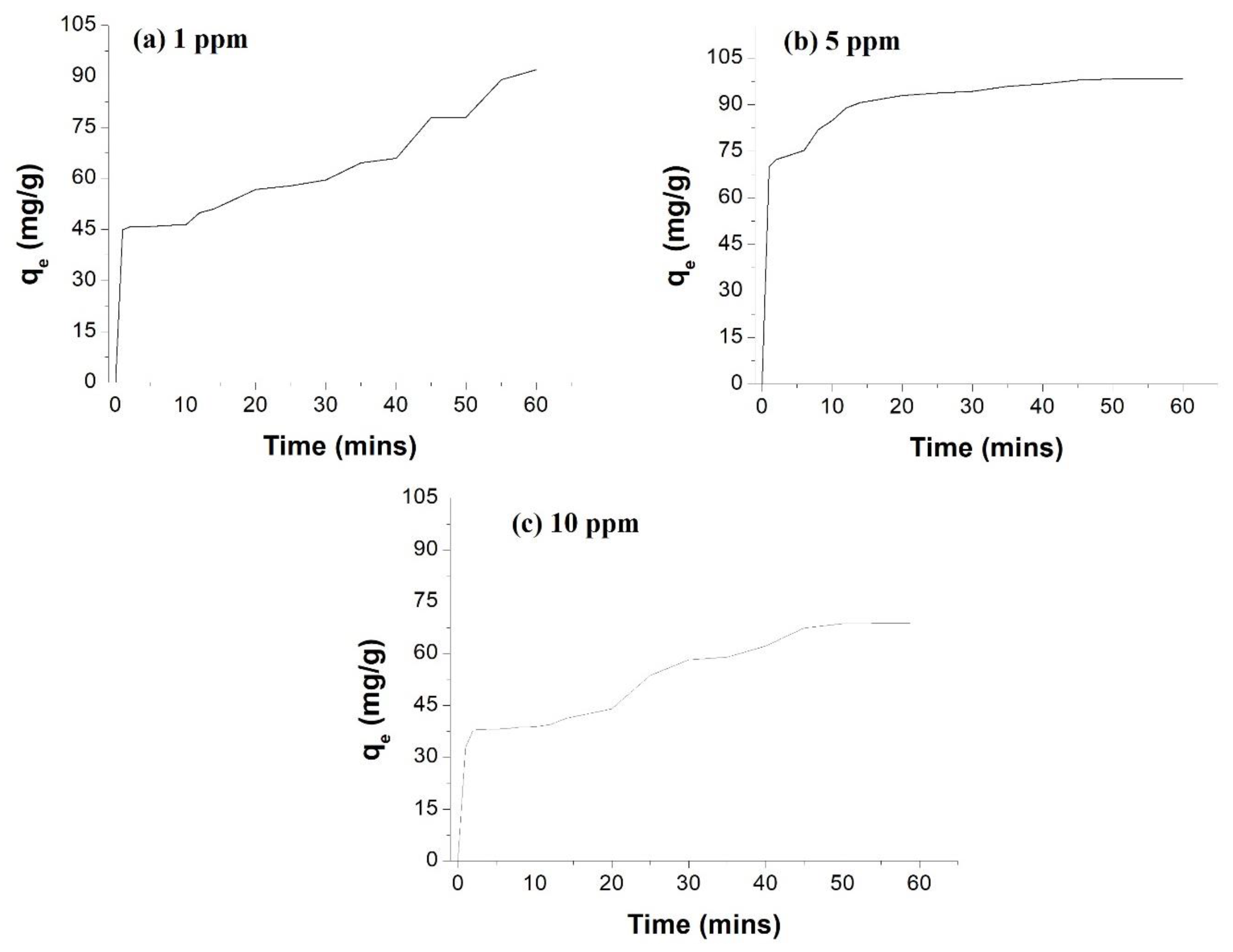
3.6. Removal of Cypermethrin from Water Using AgNPs and Cellulose Acetate Membrane Incorporated with AgNPs
3.7. Removal of CA and PQ by Using AgNPs and Membrane Incorporated with AgNPs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boedeker, W.; Watts, M.; Clausing, P.; Marquez, E. The global distribution of acute unintentional pesticide poisoning: Estimations based on a systematic review. BMC Public Health 2020, 20, 1875. [Google Scholar] [CrossRef] [PubMed]
- de Graaf, L.; Talibov, M.; Boulanger, M.; Bureau, M.; Robelot, E.; Lebailly, P.; Baldi, F.; AGRICAN group. Health of greenspace workers: Morbidity and mortality data from the AGRICAN cohort. Environ. Res. 2022, 212, 113375. [Google Scholar] [CrossRef] [PubMed]
- Scharf, M.E. Neurological Effects of Insecticides and the Insect Nervous System. In Encyclopedia of Entomology; Capinera, J.L., Ed.; Springer: Dordrecht, The Netherlands, 2018. [Google Scholar] [CrossRef]
- Donley, N. The USA lags behind other agricultural nations in banning harmful pesticides. Environ. Health 2019, 18, 44. [Google Scholar] [CrossRef] [PubMed]
- Schwingl, P.J.; Lunn, R.M.; Mehta, S.S. A tiered approach to prioritizing registered pesticides for potential cancer hazard evaluations: Implications for decision making. Environ. Health 2021, 20, 13. [Google Scholar] [CrossRef]
- Neuwirthová, N.; Trojan, M.; Svobodová, M.; Vašíčková, J.; Šimek, Z.; Hofman, J.; Bielská, L. Pesticide residues remaining in soils from the previous growing season (s)-Can they accumulate in non-target organisms and contaminate the food web? Sci. Total Environ. 2019, 646, 1056–1062. [Google Scholar] [CrossRef]
- Cardozo, M.; de Almeida, J.S.; Cavalcante, S.F.D.A.; Salgado, J.R.; Gonçalves, A.S.; França, T.C.C.; Kuca, K.; Bizzo, H.R. Biodegradation of organophosphorus compounds predicted by an enzymatic process using molecular modeling and observed in soil samples through analytical techniques and microbiological analysis: A comparison. Molecules 2020, 25, 58. [Google Scholar] [CrossRef]
- Vivek, C.; Veeraiah, K.; Padmavathi, P.; Rao, D.; Bramhachari, P.V. Acute toxicity and residue analysis of cartap hydrochloride pesticide: Toxicological implications on the fingerlings of freshwater fish Labeo rohita. Biocatal. Agric. Biotechnol. 2016, 7, 193–201. [Google Scholar] [CrossRef]
- Singh, D.P.; Khattar, J.I.S.; Gupta, M.; Kaur, G. Evaluation of the toxicological impact of cartap hydrochloride on some physiological activities of a non-heterocystous cyanobacterium Leptolyngbya foveolarum. Pestic. Biochem. Physiol. 2014, 110, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Ko, D.R.; Chung, S.P.; You, J.S.; Cho, S.; Park, Y.; Chun, B.; Moon, J.; Kim, H.; Kim, Y.H. Effects of paraquat ban on herbicide poisoning-related mortality. Yonsei Med. J. 2017, 58, 859–866. [Google Scholar] [CrossRef]
- Nazir, M.S.; Tahir, Z.; Hassan, S.U.; Ali, Z.; Akhtar, M.N.; Azam, K.; Abdullah, M.A. Remediation of Pesticide in Water. In Sustainable Agriculture Reviews 47; Inamuddin, Ahamed, M.I., Lichtfouse, E., Eds.; Sustainable Agriculture Reviews; Springer: Cham, Switzerland, 2021; Volume 47. [Google Scholar] [CrossRef]
- Bootharaju, M.S.; Pradeep, T. Understanding the Degradation Pathway of the Pesticide, Chlorpyrifos by Noble Metal Nanoparticles. Langmuir 2012, 26, 2671–2679. [Google Scholar] [CrossRef]
- Ratan, Z.A.; Haider, M.F.; Nurunnabi, M.; Shahriar, S.M.; Ahammad, A.J.; Shim, Y.Y.; Cho, J.Y. Green chemistry synthesis of silver nanoparticles and their potential anticancer effects. Cancers 2020, 12, 855. [Google Scholar] [CrossRef]
- Saratale, R.G.; Benelli, G.; Kumar, G.; Kim, D.S.; Saratale, G.D. Bio-fabrication of silver nanoparticles using the leaf extract of ancient herbal medicine, dandelion (Taraxacum officinale), evaluation of their antioxidant, anticancer potential, and antimicrobial activity against phytopathogens. Environ. Sci. Pollut. Res. 2018, 25, 10392–10406. [Google Scholar] [CrossRef] [PubMed]
- Nag, M.; Lahiri, D.; Sarkar, T.; Ghosh, S.; Dey, A.; Edinur, H.A.; Pati, S.; Ray, R.R. Microbial fabrication of nanomaterial and its role in the disintegration of exopolymeric matrices of biofilm. Front. Chem. 2021, 9, 690590. [Google Scholar] [CrossRef] [PubMed]
- Pal, G.; Rai, P.; Pandey, A. Chapter 1-Green synthesis of nanoparticles: A greener approach for a cleaner future. In Green Synthesis; Characterization and Applications of Nanoparticles; Micro and Nano Technologies; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–26. [Google Scholar] [CrossRef]
- Lahiri, D.; Nag, M.; Sheikh, H.I.; Sarkar, T.; Edinur, H.; Siddhartha, P.; Ray, R. Microbiologically synthesized nanoparticles, and their role in silencing the biofilm signaling cascade. Front. Microbiol. 2021, 12, 636588. [Google Scholar] [CrossRef]
- Ravichandran, V.; Vasanthi, S.; Shalini, S.; Shah, S.A.A.; Tripathy, M.; Paliwal, N. Green synthesis, characterization, the antibacterial, the antioxidant and photocatalytic activity of Parkia speciosa leaves extract mediated silver nanoparticles. Results Phys. 2019, 15, 102565. [Google Scholar] [CrossRef]
- Chinnasamy, G.; Chandrasekharan, S.; Koh, T.W.; Bhatnagar, S. Synthesis, characterization, antibacterial and wound healing efficacy of silver nanoparticles from Azadirachta indica. Front. Microbiol. 2021, 12, 611560. [Google Scholar] [CrossRef] [PubMed]
- Jain, N.; Jain, P.; Rajput, D.; Patil, U.K. Green synthesized plant-based silver nanoparticles: Therapeutic perspective for anticancer and antiviral activity. Micro Nano Syst. Lett. 2021, 9, 5. [Google Scholar] [CrossRef]
- Shad, S.; Bashir, N.; Nault, M.F.B.D.; Lynch, I. Incorporation of biogenic zinc nanoparticles into a polymeric membrane. Clean. Eng. Technol. 2021, 5, 100339. [Google Scholar] [CrossRef]
- Factori, I.M.; Amaral, J.M.; Camani, P.H.; Rosa, D.S.; Lima, B.A.; Brocchi, M.; da Silva, E.R.; Souza, J.S. ZnO Nanoparticle/Poly (vinyl alcohol) Nanocomposites via Microwave-Assisted Sol-Gel Synthesis for Structural Materials, UV Shielding, and Antimicrobial Activity. ACS Appl. Nano Mater. 2021, 4, 7371–7383. [Google Scholar] [CrossRef]
- Muzaffar, S.M.; Naeem, S.; Yaseen, S.; Riaz, S.; Kayani, Z.N.; Naseem, S. Microwave-assisted tuning of optical and magnetic properties of zinc oxide nanorods—Efficient antibacterial and photocatalytic agent. J. Sol-Gel Sci. Technol. 2020, 95, 88–100. [Google Scholar] [CrossRef]
- Neto, V.D.O.S.; Freire, P.D.T.C.; Nascimento, R.F.D. Removal of Pesticides and Volatile Organic Pollutants with Nanoparticle. In Nanomaterials Applications for Environmental Matrices; Elsevier Inc.: Amsterdam, The Netherlands, 2019; pp. 403–426. [Google Scholar] [CrossRef]
- Doria, G.; Conde, J.; Veigas, B.; Giestas, L.; Almeida, C.; Assunção, M.; Rosa, J.; Baptista, P.V. Noble metal nanoparticles for bio sensing applications. Sensors 2012, 12, 1657–1687. [Google Scholar] [CrossRef] [PubMed]
- Mendis, P.; de Silva, R.M.; de Silva, K.M.N.; Wijenayaka, L.A.; Jayawardana, K.; Yan, M. Nanosilver Rainbow: A rapid and facile method to tune different colors of nanosilver through the controlled synthesis of stable spherical silver nanoparticles. RSC Adv. 2016, 6, 48792–48799. [Google Scholar] [CrossRef]
- Ayinde, W.B.; Gitari, W.M.; Samie, A. Optimization of microwave-assisted synthesis of silver nanoparticle by Citrus paradisi peel and its application against pathogenic water strain. Green Chem. Lett. Rev. 2019, 12, 225–234. [Google Scholar] [CrossRef]
- Reidy, B.; Haase, A.; Luch, A.; Dawson, K.A.; Lynch, I. Mechanisms of silver nanoparticle release, transformation, and toxicity: A critical review of current knowledge and recommendations for future studies and applications. Materials 2013, 6, 2295–2350. [Google Scholar] [CrossRef] [PubMed]
- Kaegi, R.; Voegelin, A.; Sinnet, B.; Zuleeg, S.; Hagendorfer, H.; Burkhardt, M.; Siegrist, H. Behavior of metallic silver nanoparticles in a pilot wastewater treatment plant. Environ. Sci. Technol. 2011, 45, 3902–3908. [Google Scholar] [CrossRef] [PubMed]
- Hou, L.; Li, K.; Ding, Y.; Li, Y.; Chen, J.; Wu, X.; Li, X. Removal of silver nanoparticles in simulated wastewater treatment processes and its impact on COD and NH4 reduction. Chemosphere 2012, 87, 248–252. [Google Scholar] [CrossRef]
- Li, X.; Lenhart, J.; Walker, H. Dissolution-Accompanied Aggregation Kinetics of Silver Nanoparticles. Langmuir 2010, 26, 16690–16698. [Google Scholar] [CrossRef]
- Gong, A.; Zhu, D.; Mei, Y.-Y.; Xu, X.-H.; Wu, F.-A.; Wang, J. Enhanced biocatalysis mechanism under microwave irradiation in isoquercitrin production revealed by circular dichroism and surface plasmon resonance spectroscopy. Bioresour. Technol. 2016, 205, 48–57. [Google Scholar] [CrossRef]
- Wang, J.; Sui, M.; Ma, Z.; Li, H.; Yuan, B. Antibacterial performance of polymer quaternary ammonium salt–capped silver nanoparticles on Bacillus subtilis in water. RSC Adv. 2019, 9, 25667–25676. [Google Scholar] [CrossRef]
- Beisl, S.; Monteiro, S.; Santos, R.; Figueiredo, A.S.; Sánchez-Loredo, M.G.; Lemos, M.A.; Lemos, F.; Minhalma, M.; de Pinho, M.N. Synthesis and Bactericide Activity of Nanofiltration Composite Membranes–Cellulose Acetate/Silver Nanoparticles and Cellulose Acetate/Silver Ion Exchanged Zeolites. Water Res. 2018, 149, 225–231. [Google Scholar] [CrossRef]
- Raicopol, M.D.; Andronescu, C.; Voicu, S.I.; Vasile, E.; Pandele, A.M. Cellulose acetate/layered double hydroxide adsorptive membranes for efficient removal of pharmaceutical environmental contaminants. Carbohydr. Polym. 2019, 217, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Chen, H.; Wang, R. Preparation of antifouling cellulose acetate membranes with good hydrophilic and oleophobic surface properties. Mater. Lett. 2019, 252, 1–4. [Google Scholar] [CrossRef]
- Deeksha, B.; Sadanand, V.; Hariram, N.; Rajulu, A.V. Preparation and properties of cellulose nanocomposite fabrics with in situ generated silver nanoparticles by bioreduction method. J. Bioresour. Bioprod. 2021, 6, 75–81. [Google Scholar] [CrossRef]
- Andrade, P.F. Improved antibacterial activity of nano-filtration polysulfone membranes modified with silver nanoparticles. Water Res. 2015, 81, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Yu, R.; Jiang, J.; Ding, Z.; Ma, J.; Liang, R. Robust immobilization of anionic silver nanoparticles on cellulose filter paper toward a low-cost point-of-use water disinfection system with improved anti-biofouling properties. RSC Adv. 2021, 11, 4873–4882. [Google Scholar] [CrossRef]
- Zirehpour, A.; Rahimpour, A.; Shamsabadi, A.A.; Gh, M.S.; Soroush, M. Mitigation of thin-film composite membrane biofouling via immobilizing nano-sized biocidal reservoirs in the membrane active layer. Environ. Sci. Technol. 2017, 51, 5511–5522. [Google Scholar] [CrossRef]
- Dong, C.; Wang, Z.; Wu, J.; Wang, Y.; Wang, J.; Wang, S. A green strategy to immobilize silver nanoparticles onto reverse osmosis membrane the for the enhanced anti-biofouling property. Desalination 2017, 401, 32–41. [Google Scholar] [CrossRef]
- Guillen, G.R.; Pan, Y.; Li, M.; Hoek, E.M.V. Preparation, and characterization of membranes formed by nonsolvent induced phase separation: A review. Ind. Eng. Chem. Res. 2011, 50, 3798–3817. [Google Scholar] [CrossRef]
- Sánchez-Márquez, J.A.; Fuentes-Ramírez, R.; Cano-Rodríguez, I.; Gamiño-Arroyo, Z.; Rubio-Rosas, E.; Kenny, J.M.; Rescignano, N. Membrane Made of Cellulose Acetate with Polyacrylic Acid Reinforced with Carbon Nanotubes and Its Applicability for Chromium Removal. Int. J. Polym. Sci. 2015, 2015, 1–12. [Google Scholar] [CrossRef]
- Yadav, N.; Hakkarainen, M. Degradable or not? Cellulose acetate is a model for the complicated interplay between structure, environment, and degradation. Chemosphere 2021, 265, 128731. [Google Scholar] [CrossRef]
- Tahazadeh, S.; Karimi, H.; Mohammadi, T.; Emrooz, H.B.M.; Tofighy, M.A. Fabrication of biodegradable cellulose acetate/MOF-derived porous carbon nanocomposite adsorbent for methylene blue removal from aqueous solutions. J. Solid-State Chem. 2021, 299, 122180. [Google Scholar] [CrossRef]
- Unal, I.; Egri, S. Biosynthesis of silver nanoparticles using the aqueous extract of Rheum ribes, characterization and the evaluation of its toxicity on HUVECs and Artemia salina. Inorg. Nano-Met. Chem. 2022, 23, 1–4. [Google Scholar] [CrossRef]
- Ferdous, Z.; Nemmar, A. Health impact of silver nanoparticles: A review of the biodistribution and toxicity following various routes of exposure. Int. J. Mol. Sci. 2020, 21, 2375. [Google Scholar] [CrossRef]
- Gudikandula, K.; Charya Maringanti, S. Synthesis of silver nanoparticles by chemical and biological methods and their antimicrobial properties. J. Exp. Nanosci. 2016, 11, 714–721. [Google Scholar] [CrossRef]
- Gohil, J.M.; Choudhury, R.R. Introduction to nanostructured and nano-enhanced polymeric membranes: Preparation, function, and application for water purification. In Nanoscale Materials in Water Purification; Elsevier: Amsterdam, The Netherlands, 2019; pp. 25–57. [Google Scholar] [CrossRef]
- Sharma, V.K.; Siskova, K.M.; Zboril, R.; Gardea-Torresdey, J.L. Organic-coated silver nanoparticles in biological and environmental conditions: Fate, stability, and toxicity. Adv. Colloid Interface Sci. 2014, 204, 15–34. [Google Scholar] [CrossRef]
- Trinh, V.T.; Nguyen, T.M.P.; Van, H.T.; Hoang, L.P.; Nguyen, T.V.; Ha, L.T.; Vu, X.H.; Pham, T.T.; Quang, N.V.; Nguyen, X.C. Phosphate adsorption by silver nanoparticles-loaded activated carbon derived from tea residue. Sci. Rep. 2020, 10, 3634. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Bai, R.; Wee, K.H.; Liu, C.; Tang, S.-L. Membrane surfaces immobilized with ionic or reduced silver and their anti-biofouling performances. J. Membr. Sci. 2010, 363, 278–286. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, K.; De Gusseme, B.; Verstraete, W. Biogenic silver nanoparticles (bio-Ag0) decrease biofouling of bio-Ag0/PES nanocomposite membranes. Water Res. 2012, 46, 2077–2087. [Google Scholar] [CrossRef]
- Yorseng, K.; Siengchin, S.; Ashok, B.; Rajulu, A.V. Nanocomposite egg shell powder with in situ generated silver nanoparticles using inherent collagen as reducing agent. J. Bioresour. Bioprod. 2020, 5, 101–107. [Google Scholar] [CrossRef]
- World Health Organization. Silver in Drinking Water: Background Document for Development of WHO Guidelines for Drinking-Water Quality; (No. WHO/HEP/ECH/WSH/2021.7); World Health Organization; Available online: https://www.who.int/westernpacific/publications-detail/WHO-HEP-ECH-WSH-2021.7 (accessed on 1 September 2022).
| Herbicide/ Insecticide | Use | Approval | Toxicity Threshold | Log Kow | LD50 | |
|---|---|---|---|---|---|---|
| Human | Marine life | |||||
| Cypermethrin (CP) | Insecticide for cotton, fruits, and vegetables, Commercial and residential settings | EPA, US for restricted control of insects | Long-term, High | Extra high for fish | >4.5 (bioaccumulation expected) | 57,500 μg/kg (oral, rat) |
| Cartap hydrochloride (CA) | Targets chewing and sucking insects on many crops, including rice. | 1967 Japan, USChewing and sucking pests | Long-term, very low | Long-term, very low | 0.0 | 250–340 mg/kg (oral, rat) |
| Paraquat (PQ) | Herbicide for weeds and grasses in agricultural & non-agricultural areas | EPA, US restricted use of pesticide | Long-term, high | Not available | −4.0 (unlikely to bio accumulate) | 110–150 mg/kg (rats) |
| Adsorption of CP by 0.1 g AgNPs | Adsorption of CP by 0.1 g AgNPs Incorporated into the Cellulose Membrane | Adsorption with the Membrane (without AgNPs) | |||||
|---|---|---|---|---|---|---|---|
| 1 ppm (CP) | 5 ppm (CP) | 10 ppm (CP) | 1 ppm (CP) | 5 ppm (CP) | 10 ppm (CP) | ||
| % Adsorption | 93.9 | 68.8 | 20.02 | 99.8 | 97.9 | 95.1 | 0.6 |
| Adsorption Time (min) | 60 | 60 | 60 | 20 | 30 | 35 | 60 |
| Adsorption by 0.1 g AgNPs | Adsorption of CP by 0.1 g AgNPs Incorporated into the Cellulose Membrane | Adsorption with the Membrane (without AgNPs) | |||||
|---|---|---|---|---|---|---|---|
| 1 ppm (CA) | 5 ppm (CA) | 10 ppm (CA) | 1 ppm (CA) | 5 ppm (CA) | 10 ppm (CA) | ||
| % Adsorption | 100 | 79.1 | 66 | 100 | 91.2 | 89.7 | 0.5 |
| Adsorption Time (min) | 35 | 60 | 60 | 20 | 35 | 45 | 60 |
| Adsorption by 0.1 g AgNPs | Adsorption by 0.1 g AgNPs Incorporated into the Cellulose Membrane | Adsorption with the Membrane (without AgNPs) | |||||
|---|---|---|---|---|---|---|---|
| 1 ppm (PQ) | 5 ppm (PQ) | 10 ppm (PQ) | 1 ppm (PQ) | 5 ppm (PQ) | 10 ppm (PQ) | ||
| % Adsorption | 93.5 | 51.3 | 73.2 | 100 | 96.5 | 92.7 | 0.7 |
| Adsorption Time (min) | 60 | 60 | 60 | 25 | 30 | 30 | 60 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shad, S.; Lynch, I.; Shah, S.W.H.; Bashir, N. Remediation of Water Using a Nanofabricated Cellulose Membrane Embedded with Silver Nanoparticles. Membranes 2022, 12, 1035. https://doi.org/10.3390/membranes12111035
Shad S, Lynch I, Shah SWH, Bashir N. Remediation of Water Using a Nanofabricated Cellulose Membrane Embedded with Silver Nanoparticles. Membranes. 2022; 12(11):1035. https://doi.org/10.3390/membranes12111035
Chicago/Turabian StyleShad, Salma, Iseult Lynch, Syed Waqar Hussain Shah, and Nadia Bashir. 2022. "Remediation of Water Using a Nanofabricated Cellulose Membrane Embedded with Silver Nanoparticles" Membranes 12, no. 11: 1035. https://doi.org/10.3390/membranes12111035
APA StyleShad, S., Lynch, I., Shah, S. W. H., & Bashir, N. (2022). Remediation of Water Using a Nanofabricated Cellulose Membrane Embedded with Silver Nanoparticles. Membranes, 12(11), 1035. https://doi.org/10.3390/membranes12111035








