Spectroscopy Study of Albumin Interaction with Negatively Charged Liposome Membranes: Mutual Structural Effects of the Protein and the Bilayers
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of Liposomes
2.3. Hydrodynamic Diameter and Zeta Potential Measurements
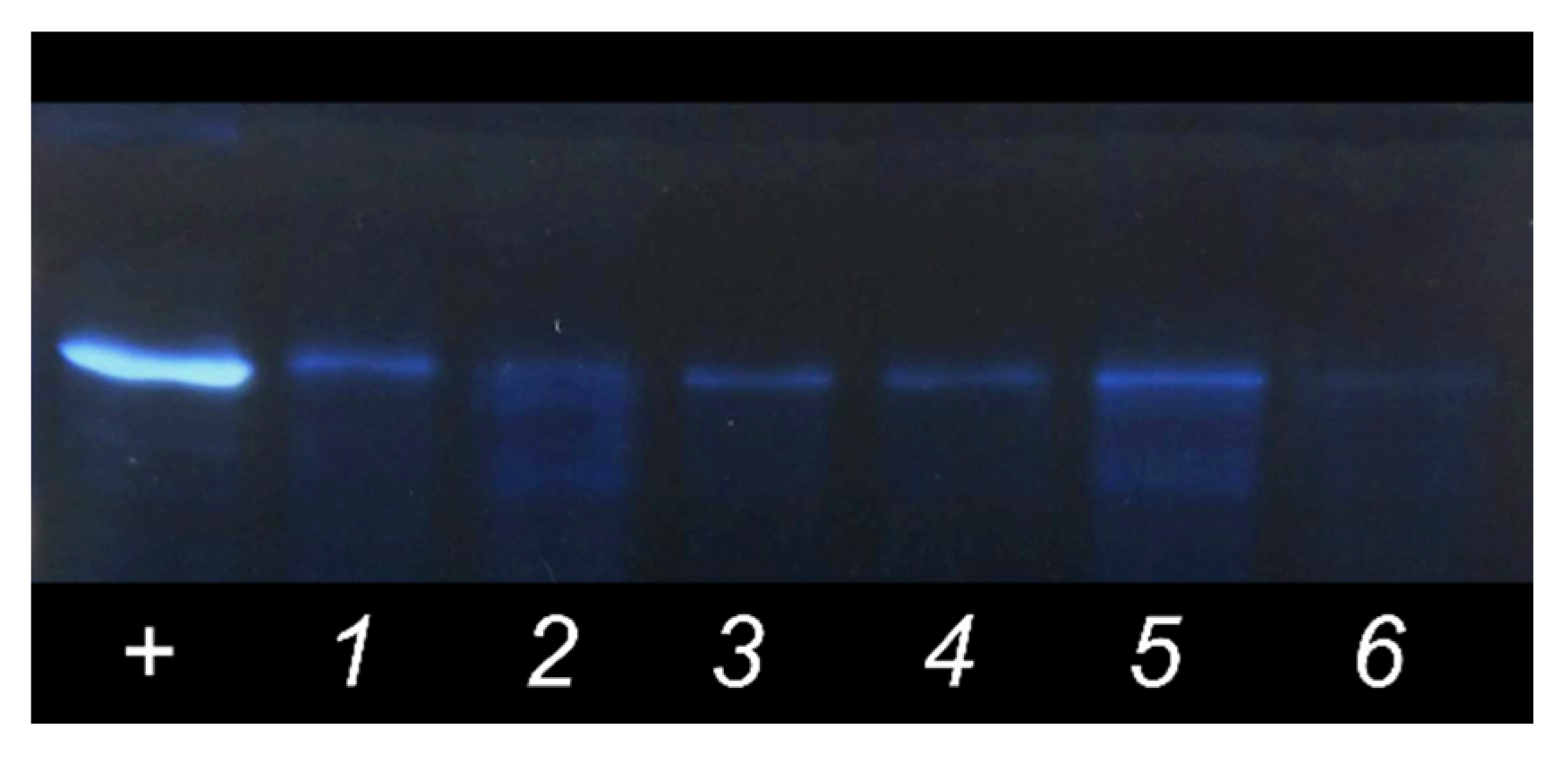
2.4. PAGE with Silver Staining
2.5. Determination of the Protein Binding (PB) Values
2.6. Fluorescence Measurements
2.6.1. ANS Fluorescence
2.6.2. Anisotropy
2.6.3. Calcein Release
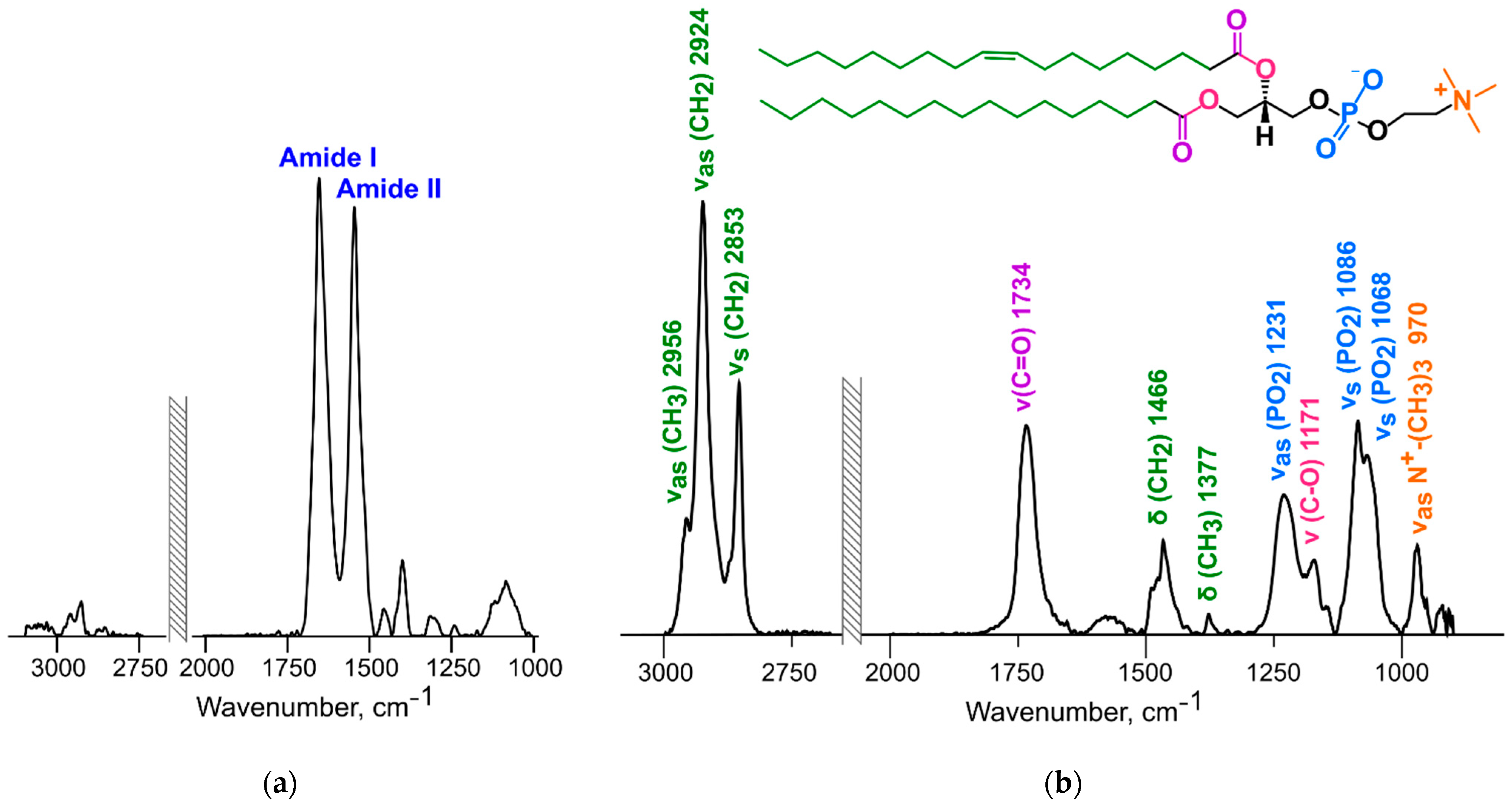
2.7. ATR-FTIR Spectroscopy
2.8. Statistical Analysis of Experimental Data
3. Results and Discussion
3.1. Liposome Preparation and Characteristics
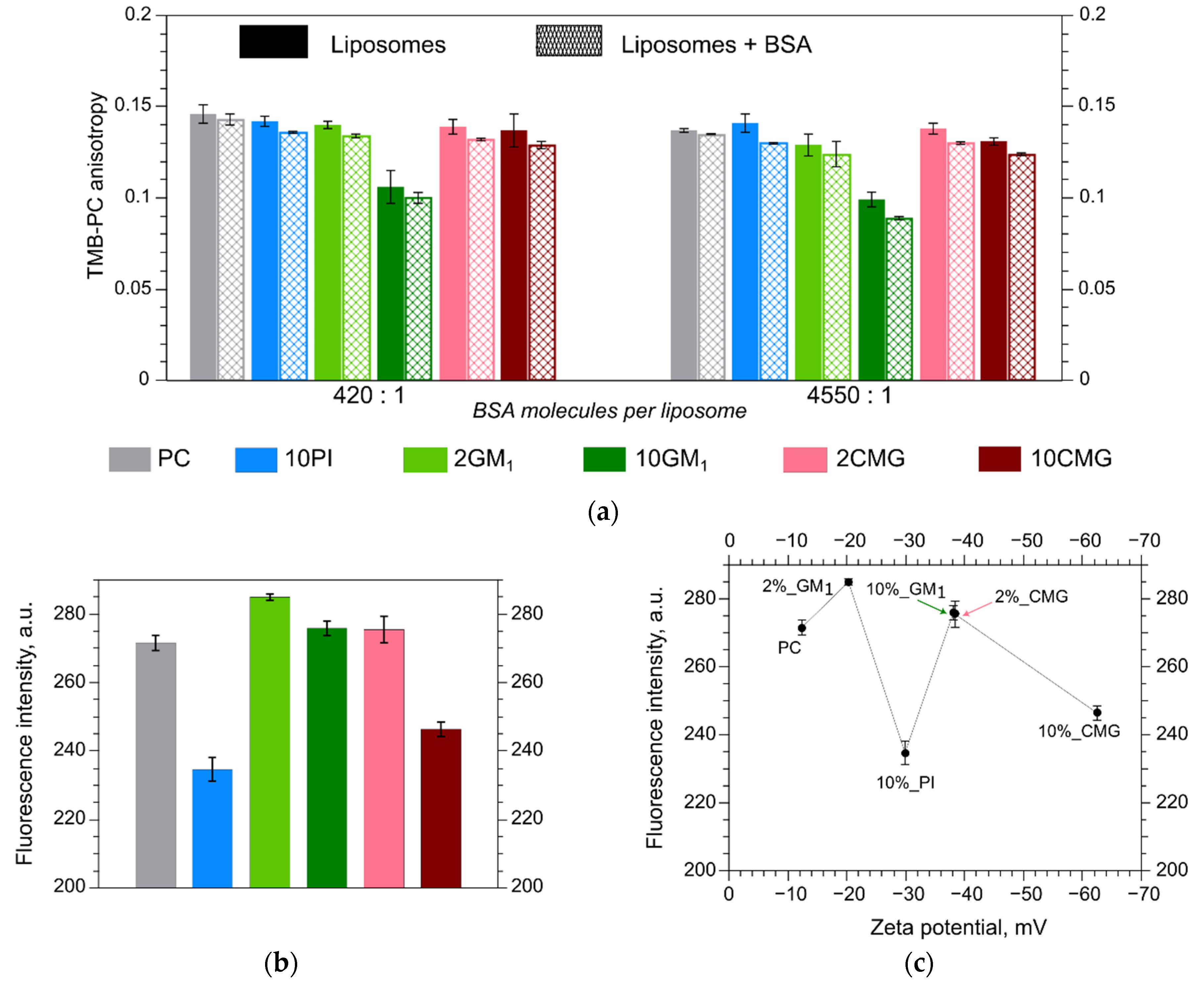
3.2. Albumin Adsorption Evaluation
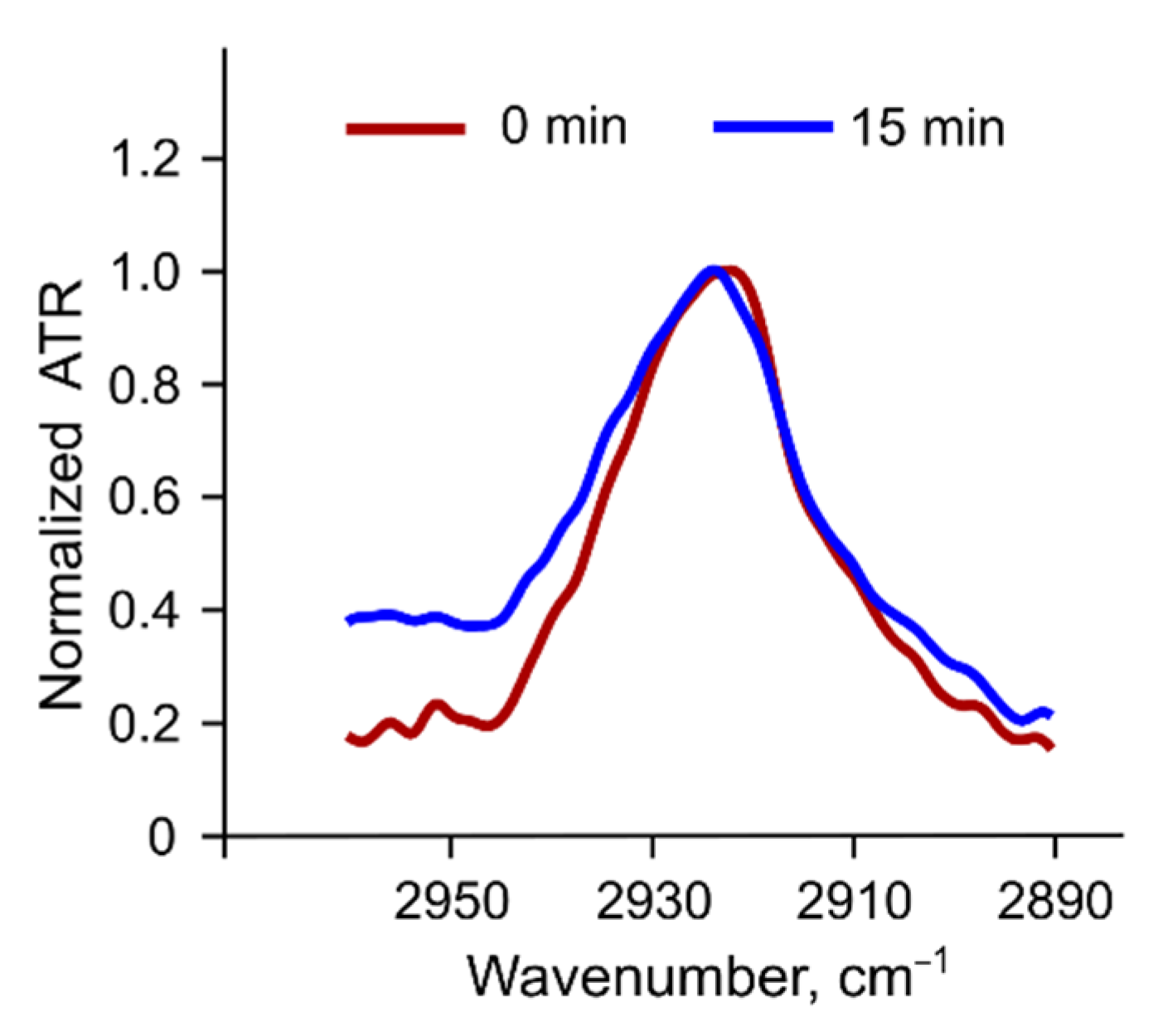
3.3. Fluorescence Anisotropy in Different Bilayers
3.4. Microhydrophobicity of Liposomal Surface Estimated by ANS Fluorescence
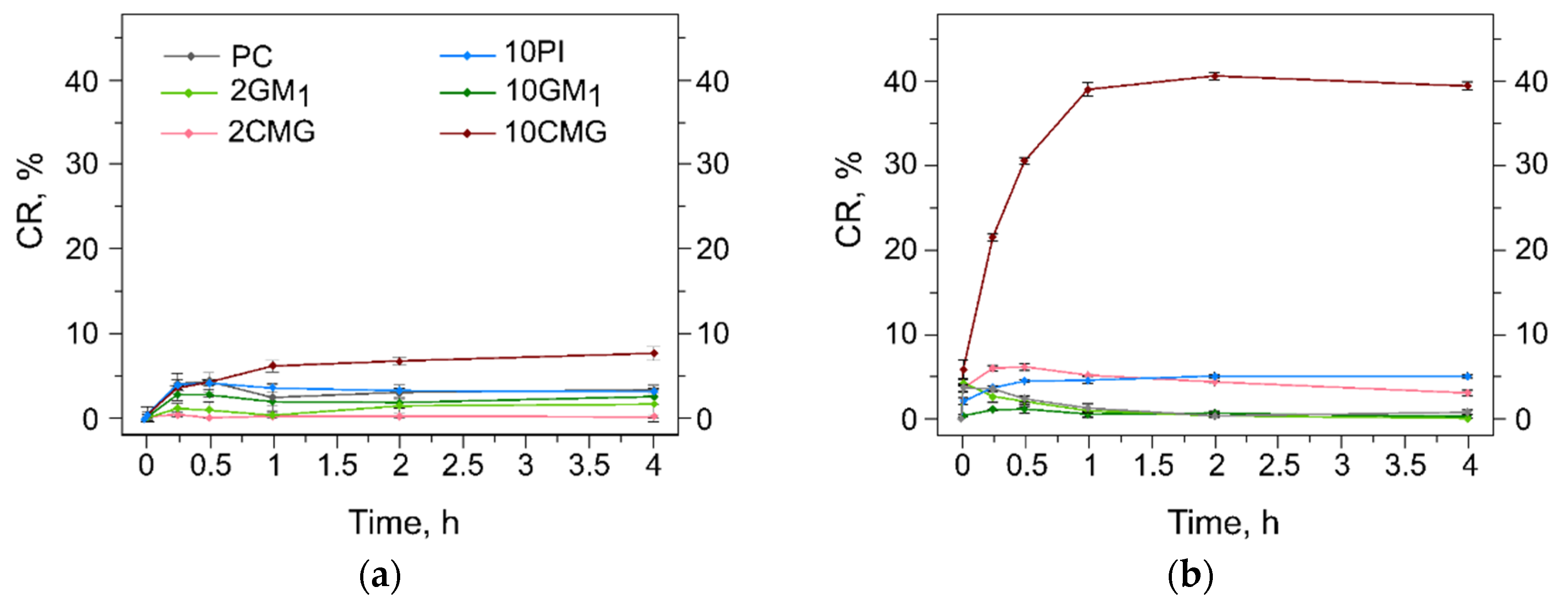
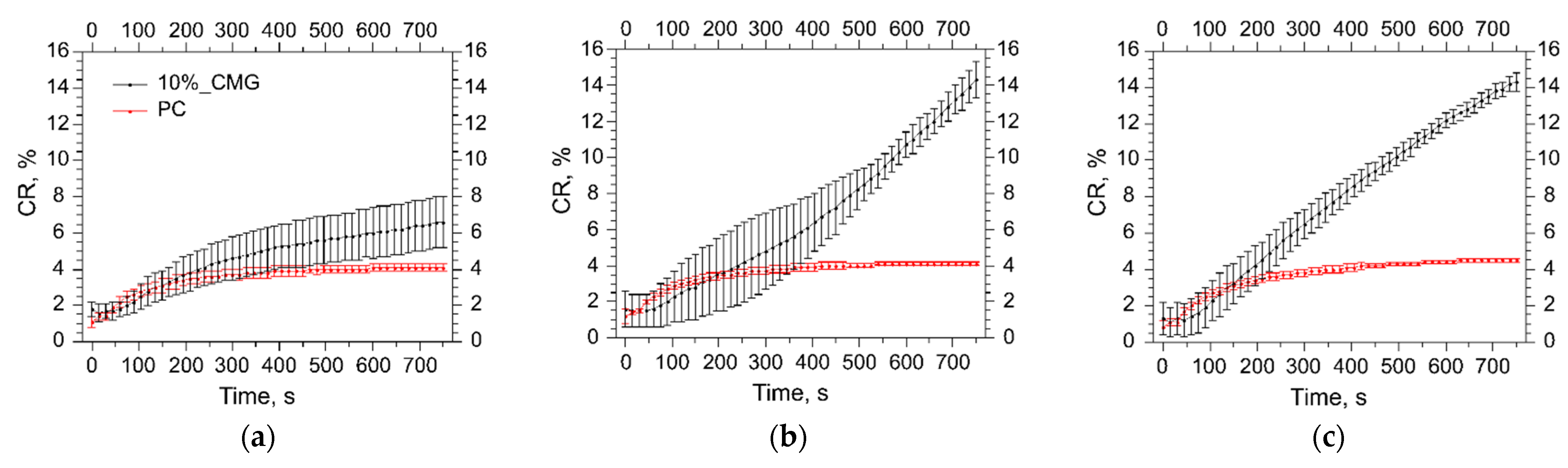
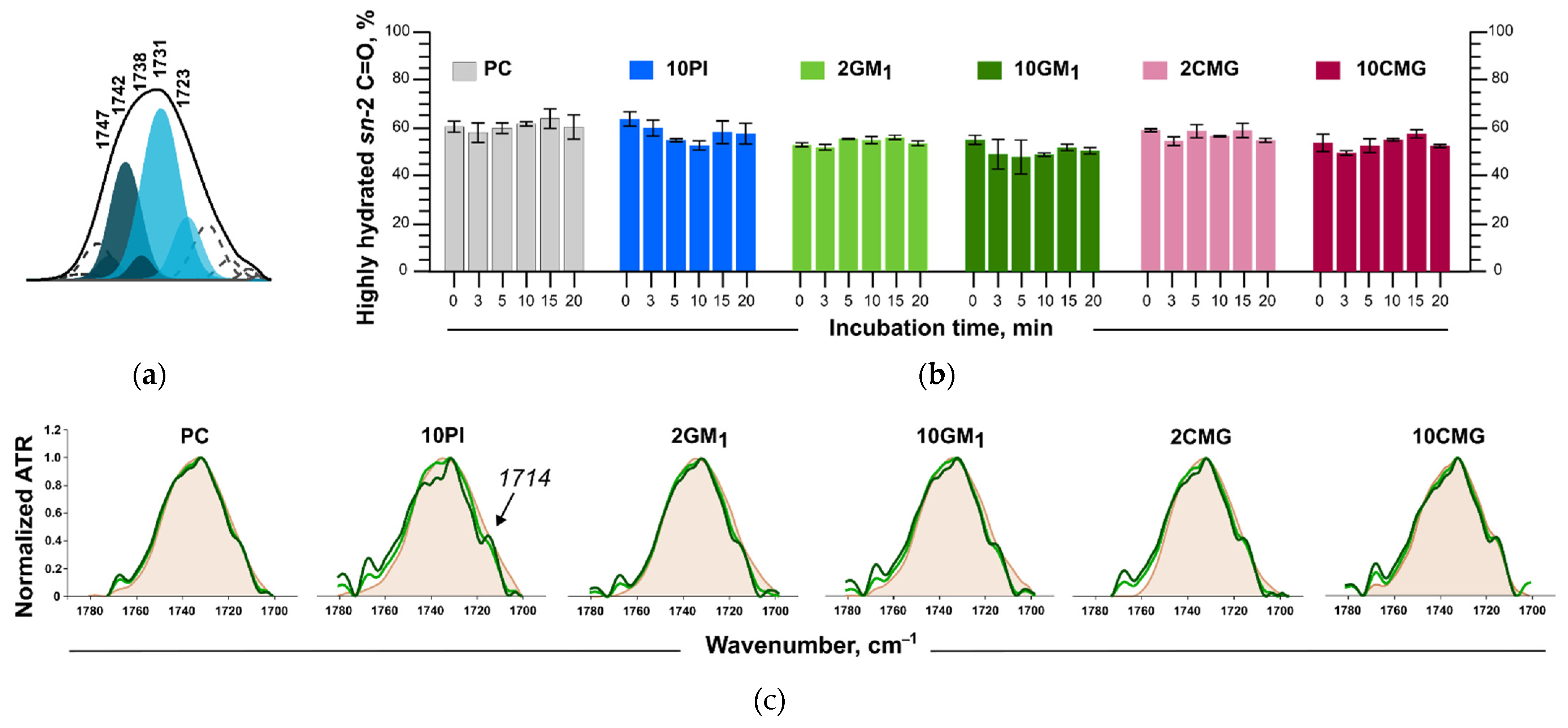
3.5. Assessment of the Liposome Stability during Protein Adsorption
3.6. ATR-FTIR Spectroscopy
3.6.1. Changes in the Structure of the Lipid Bilayer
3.6.2. Changes in the Protein Structure
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Chernecky, C.; Berger, B. Laboratory Tests and Diagnostic Procedures, 6th ed.; Elsevier/Saunders: Philadelphia, PA, USA, 2013; pp. 84–180. ISBN 9781455745029. [Google Scholar]
- Rabbani, G.; Ahn, S.N. Structure, enzymatic activities, glycation and therapeutic potential of human serum albumin: A natural cargo. Int. J. Biol. Macromol. 2019, 123, 979–990. [Google Scholar] [CrossRef] [PubMed]
- van der Vusse, G.J. Albumin as fatty acid transporter. Drug Metab. Pharmakokinet. 2009, 24, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Onishchenko, N.; Tretiakova, D.; Vodovozova, E. Spotlight on the protein corona of liposomes. Acta Biomater. 2021, 134, 57–78. [Google Scholar] [CrossRef] [PubMed]
- Tengvall, P. Protein Interactions with Biomaterials. In Comprehensive Biomaterials; Ducheyne, P., Ed.; Elsevier: Philadelphia, PA, USA, 2011; Volume 4, pp. 63–73. ISBN 9780080552941. [Google Scholar] [CrossRef]
- Schnitzer, J.E.; Sung, A.; Horvat, R.; Bravo, J. Preferential interaction of albumin-binding proteins, gp30 and gp18, with conformationally modified albumins. Presence in many cells and tissues with a possible role in catabolism. J. Biol. Chem. 1992, 267, 24544–24553. [Google Scholar] [CrossRef]
- Fleischer, C.C.; Payne, C.K. Secondary structure of corona proteins determines the cell surface receptors used by nanoparticles. J. Phys. Chem. B 2014, 118, 14017–14026. [Google Scholar] [CrossRef]
- Shang, L.; Wang, Y.; Jiang, J.; Dong, S. pH-dependent protein conformational changes in albumin:gold nanoparticle bioconjugates: A spectroscopic study. Langmuir 2007, 23, 2714–2721. [Google Scholar] [CrossRef]
- Pan, H.; Qin, M.; Meng, W.; Cao, Y.; Wang, W. How do proteins unfold upon adsorption on nanoparticle surfaces? Langmuir 2012, 28, 12779–12787. [Google Scholar] [CrossRef]
- Zuo, G.; Huang, Q.; Wei, G.; Zhou, R.; Fang, H. Plugging into proteins: Poisoning protein function by a hydrophobic nanoparticle. ACS Nano 2010, 4, 7508–7514. [Google Scholar] [CrossRef]
- Bharti, B.; Meissner, J.; Findenegg, G.H. Aggregation of silica nanoparticles directed by adsorption of lysozyme. Langmuir 2011, 27, 9823–9833. [Google Scholar] [CrossRef] [PubMed]
- Cukalevski, R.; Lundqvist, M.; Oslakovic, C.; Dahlbäck, B.; Linse, S.; Cedervall, T. Structural changes in apolipoproteins bound to nanoparticles. Langmuir 2011, 27, 14360–14369. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, K.; Okamoto, Y.; Matsumoto, K.; Otagiri, M.; Chuang, V.T.G. When Albumin Meets Liposomes: A Feasible Drug Carrier for Biomedical Applications. Pharmaceuticals 2021, 14, 296. [Google Scholar] [CrossRef] [PubMed]
- Savay, S.; Szebeni, J.; Baranyi, L.; Alving, C.R. Potentiation of liposome-induced complement activation by surface-bound albumin. Biochim. Biophys. Acta Biomembr. 2002, 1559, 79–86. [Google Scholar] [CrossRef]
- Vuarchey, C.; Kumar, S.; Schwendener, R.A. Albumin coated liposomes: A novel platform for macrophage specific drug delivery. Nanotechnol. Dev. 2011, 1, e2. [Google Scholar] [CrossRef]
- Tretiakova, D.; Onishchenko, N.; Boldyrev, I.; Mikhalyov, I.; Tuzikov, A.; Bovin, N.; Evtushenko, E.; Vodovozova, E. Influence of stabilizing components on the integrity of antitumor liposomes loaded with lipophilic prodrug in the bilayer. Colloids Surf. B Biointerfaces 2018, 166, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Tretiakova, D.S.; Khaidukov, S.V.; Babayants, A.A.; Frolova, I.S.; Shcheglovitova, O.N.; Onishchenko, N.R.; Vodovozova, E.L. Lipophilic Prodrug of Methotrexate in the Membrane of Liposomes Promotes Their Uptake by Human Blood Phagocytes. Acta Nat. 2020, 12, 99–109. [Google Scholar] [CrossRef]
- Boldyrev, I.A.; Zhai, X.; Momsen, M.M.; Brockman, H.L.; Brown, R.E.; Molotkovsky, J.G. New BODIPY lipid probes for fluorescence studies of membranes. J. Lipid Res. 2007, 48, 1518–1532. [Google Scholar] [CrossRef]
- Tretiakova, D.; Le-Deigen, I.; Onishchenko, N.; Kuntsche, J.; Kudryashova, E.; Vodovozova, E. Phosphatidylinositol Stabilizes Fluid-Phase Liposomes Loaded with a Melphalan Lipophilic Prodrug. Pharmaceutics 2021, 13, 473. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, N.; Allen, C.; Doppen, A.M.; Anantha, M.; Cox, K.A.K.; Gallagher, R.C.; Karlsson, G.; Edwards, K.; Kenner, G.; Samuels, L.; et al. Influence of Poly(Ethylene Glycol) Grafting Density and Polymer Length on Liposomes: Relating Plasma Circulation Lifetimes to Protein Binding. Biochim. Biophys. Acta Biomembr. 2007, 1768, 1367–1377. [Google Scholar] [CrossRef]
- Markwell, M.A.; Haas, S.M.; Bieber, L.L.; Tolbert, N.E. A Modification of the Lowry Procedure to Simplify Protein Determination in Membrane and Lipoprotein Samples. Anal. Biochem. 1978, 87, 206–210. [Google Scholar] [CrossRef]
- Yokouchi, Y.; Tsunoda, T.; Imura, T.; Yamauchi, H.; Yokoyama, S.; Sakai, H.; Abe, M. Effect of adsorption of bovine serum albumin on liposomal membrane characteristics. Colloids Surf. B Biointerfaces 2001, 20, 95–103. [Google Scholar] [CrossRef]
- Alekseeva, A.S.; Volynsky, P.E.; Boldyrev, I.A. Estimation of the Phospholipase A2 Selectivity on POPC/POPG Membranes Using the Interaction Map. Biochem. Moscow Suppl. Ser. A 2021, 15, 329–333. [Google Scholar] [CrossRef]
- Cullis, P.R.; Chonn, A.; Semple, S.C. Interactions of liposomes and lipid-based carrier systems with blood proteins: Relation to clearance behaviour in vivo. Adv. Drug Deliv. Rev. 1998, 32, 3–17. [Google Scholar] [CrossRef]
- Ohtsuka, I.; Yokoyama, S. Penetration of bovine serum albumin into dipalmitoylphosphatidylglycerol monolayers: Direct observation by atomic force microscopy. Chem. Pharm. Bull. 2005, 53, 42–47. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wright, A.K.; Thompson, M.R. Hydrodynamic structure of bovine serum albumin determined by transient electric birefringence. Biophys. J. 1975, 15 Pt 1, 137–141. [Google Scholar] [CrossRef]
- Kristensen, K.; Urquhart, A.J.; Thormann, E.; Andresen, T.L. Binding of human serum albumin to PEGylated liposomes: Insights into binding numbers and dynamics by fluorescence correlation spectroscopy. Nanoscale 2016, 8, 19726–19736. [Google Scholar] [CrossRef]
- Fricke, N.; Dimova, R. GM1 Softens POPC Membranes and Induces the Formation of Micron-Sized Domains. Biophys. J. 2016, 111, 1935–1945. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tretiakova, D.S.; Alekseeva, A.S.; Galimzyanov, T.R.; Boldyrev, A.M.; Chernyadyev, A.Y.; Ermakov, Y.A.; Batishchev, O.V.; Vodovozova, E.L.; Boldyrev, I.A. Lateral stress profile and fluorescent lipid probes. FRET pair of probes that introduces minimal distortions into lipid packing. Biochim. Biophys. Acta Biomembr. 2018, 1860, 2337–2347. [Google Scholar] [CrossRef]
- Guliyeva, A.J.; Gasymov, O.K. ANS fluorescence: Potential to discriminate hydrophobic sites of proteins in solid states. Biochem. Biophys. Rep. 2020, 24, 100843. [Google Scholar] [CrossRef] [PubMed]
- Estapé Senti, M.; de Jongh, C.A.; Dijkxhoorn, K.; Verhoef, J.J.F.; Szebeni, J.; Storm, G.; Hack, C.E.; Schiffelers, R.M.; Fens, M.H.; Boross, P. Anti-PEG antibodies compromise the integrity of PEGylated lipid-based nanoparticles via complement. J. Control. Release 2022, 341, 475–486. [Google Scholar] [CrossRef]
- Sabín, J.; Prieto, G.; Ruso, J.M.; Messina, P.V.; Salgado, F.J.; Nogueira, M.; Costas, M.; Sarmiento, F. Interactions between DMPC liposomes and the serum blood proteins HSA and IgG. J. Phys. Chem. B 2009, 113, 1655–1661. [Google Scholar] [CrossRef]
- Mittag, J.J.; Kneidl, B.; Preiβ, T.; Hossann, M.; Winter, G.; Wuttke, S.; Engelke, H.; Rädler, J.O. Impact of plasma protein binding on cargo release by thermosensitive liposomes probed by fluorescence correlation spectroscopy. Eur. J. Pharm. Biopharm. 2017, 119, 215–223. [Google Scholar] [CrossRef]
- Derenne, A.; Claessens, T.; Conus, C.; Goormaghtigh, E. Infrared Spectroscopy of Membrane Lipids. In Encyclopedia of Biophysics; Roberts, G.C.K., Ed.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 1074–1081. [Google Scholar] [CrossRef]
- Tsai, Y.S.; Ma, S.M.; Kamaya, H.; Ueda, I. Fourier transform infrared studies on phospholipid hydration: Phosphate-oriented hydrogen bonding and its attenuation by volatile anesthetics. Mol. Pharmacol. 1987, 31, 623–630. [Google Scholar]
- Wong, P.T.T.; Mantsch, H.H. High-pressure infrared spectroscopic evidence of water binding sites in 1,2-diacyl phospholipids. Chem. Phys. Lipids 1988, 46, 213–224. [Google Scholar] [CrossRef]
- Villalaín, J.; Ortiz, A.; Gómez-Fernández, J.C. Molecular interactions between sphingomyelin and phosphatidylcholine in phospholipid vesicles. Biochim. Biophys. Acta 1988, 941, 55–62. [Google Scholar] [CrossRef]
- Lewis, R.N.; McElhaney, R.N. Studies of mixed-chain diacyl phosphatidylcholines with highly asymmetric acyl chains: A Fourier transform infrared spectroscopic study of interfacial hydration and hydrocarbon chain packing in the mixed interdigitated gel phase. Biophys. J. 1993, 65, 1866–1877. [Google Scholar] [CrossRef]
- Dreier, L.B.; Bonn, M.; Backus, E.H.G. Hydration and Orientation of Carbonyl Groups in Oppositely Charged Lipid Monolayers on Water. J. Phys. Chem. B 2019, 123, 1085–1089. [Google Scholar] [CrossRef]
- Tsai, Y.S.; Ma, S.M.; Nishimura, S.; Ueda, I. Infrared spectra of phospholipid membranes: Interfacial dehydration by volatile anesthetics and phase transition. Biochim. Biophys. Acta 1990, 1022, 245–250. [Google Scholar] [CrossRef]
- Cieślik-Boczula, K.; Koll, A. The effect of 3-pentadecylphenol on DPPC bilayers ATR-IR and 31P NMR studies. Biophys. Chem. 2009, 140, 51–66. [Google Scholar] [CrossRef][Green Version]
- Tretiakova, D.; Kobanenko, M.; Le-Deygen, I.; Boldyrev, I.; Kudryashova, E.; Onishchenko, N.; Vodovozova, E. Spectroscopy Study of Albumin Interaction with Negatively Charged Liposome Membranes: Mutual Structural Effects of the Protein and the Bilayers; Zenodo; Version 1. 2022. Available online: https://doi.org/10.5281/zenodo.7188568 (accessed on 12 October 2022).
- Valentine, M.L.; Cardenas, A.E.; Elber, R.; Baiz, C.R. Physiological Calcium Concentrations Slow Dynamics at the Lipid-Water Interface. Biophys. J. 2018, 115, 1541–1551. [Google Scholar] [CrossRef] [PubMed]
- Roldán-Vargas, S.; Barnadas-Rodríguez, R.; Quesada-Pérez, M.; Estelrich, J.; Callejas-Fernández, J. Surface fractals in liposome aggregation. Phys. Rev. E Stat. Nonlin. Soft Matter Phys. 2009, 79 Pt 1, 011905. [Google Scholar] [CrossRef] [PubMed]
- Mantsch, H.H.; McElhaney, R.N. Phospholipid phase transitions in model and biological membranes as studied by infrared spectroscopy. Chem. Phys. Lipids 1991, 57, 213–226. [Google Scholar] [CrossRef]
- Tatulian, S.A. Attenuated total reflection Fourier transform infrared spectroscopy: A method of choice for studying membrane proteins and lipids. Biochemistry 2003, 42, 11898–11907. [Google Scholar] [CrossRef]
- Tatulian, S.A. Structural characterization of membrane proteins and peptides by FTIR and ATR-FTIR spectroscopy. In Lipid-Protein Interactions: Methods and Protocols, Methods in Molecular Biology; Kleinschmidt, J.H., Ed.; Humana Press: Totowa, NJ, USA, 2013; Volume 974, pp. 177–216. [Google Scholar]
- Kong, J.; Yu, S. Fourier transform infrared spectroscopic analysis of protein secondary structures. Acta Biochim. Biophys. Sin. 2007, 39, 549–559. [Google Scholar] [CrossRef]
- Yang, H.; Yang, S.; Kong, J.; Dong, A.; Yu, S. Obtaining information about protein secondary structures in aqueous solution using Fourier transform IR spectroscopy. Nat. Protoc. 2015, 10, 382–396. [Google Scholar] [CrossRef]
- Usoltsev, D.; Sitnikova, V.; Kajava, A.; Uspenskaya, M. Systematic FTIR spectroscopy study of the secondary structure changes in human serum albumin under various denaturation conditions. Biomolecules 2019, 9, 359. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Schubert, D.; Sawaya, M.R.; Eisenberg, D.; Riek, R. Multidimensional structure-activity relationship of a protein in its aggregated states. Angew. Chem. Int. Ed. Engl. 2010, 49, 3904–3908. [Google Scholar] [CrossRef] [PubMed]
- Holzbaur, I.E.; English, A.M.; Ismail, A.A. FTIR study of the thermal denaturation of horseradish and cytochrome c peroxidases in D2O. Biochemistry 1996, 35, 5488–5494. [Google Scholar] [CrossRef] [PubMed]


| Liposome Sample 1 | Mean Diameter, nm (±SD) | Mean PDI (±SD) | Mean ζ-Potential 2, mV (±SD) | PB, gBSA/mollipids (±SE) | BSA Molecules per Liposome (±SE) |
|---|---|---|---|---|---|
| PC | 121.3 ± 2.4 | 0.084 ± 0.003 | –12.37 ± 0.66 | 4.72 ± 0.60 | 8.31 ± 1.06 |
| 10PI | 104.4 ± 1.8 | 0.068 ± 0.016 | –29.94 ± 1.58 | 5.88 ± 0.71 | 7.84 ± 0.94 |
| 2GM1 | 113.1 ± 0.5 | 0.030 ± 0.012 | –20.28 ± 0.59 | 5.20 ± 1.05 | 7.64 ± 1.54 |
| 10GM1 | 118.5 ± 1.3 | 0.092 ± 0.008 | –38.13 ± 1.02 | 2.48 ± 0.15 | 4.00 ± 0.25 |
| 2CMG | 107.4 ± 0.8 | 0.014± 0.007 | –38.38 ± 1.15 | 3.79 ± 0.47 | 5.57 ± 0.70 |
| 10CMG | 109.6 ± 0.7 | 0.099 ± 0.024 | –62.54 ± 1.46 | 6.61 ± 0.94 | 9.72 ± 1.38 |
| Liposome Sample | Initial Peak Position in Liposomes, cm−1 (±SE) | Peak Position in Liposome–Protein Complex, cm−1 (±SE) | Average Shift, cm−1 |
|---|---|---|---|
| PC | 1230.7 ± 0.3 | 1231.5 ± 0.3 | <1 |
| 10PI | 1230.2 ± 0.1 | 1233.6 ± 0.4 | ~3.4 |
| 2GM1 | 1231.6 ± 0.2 | 1236.0 ± 0.2 | ~4.4 |
| 10GM1 | 1229.9 ± 0.5 | 1234.4 ± 0.6 | ~4.5 |
| 2CMG | 1230.1 ± 0.2 | 1233.8 ± 0.2 | ~3.7 |
| 10CMG | 1228.9 ± 0.5 | 1231.1 ± 0.2 | ~2.2 |
| Secondary Structure Element | Peak Range, cm−1 |
|---|---|
| α-helix | 1650–1660 |
| β-sheet | 1628–1639 |
| β-turn | 1664–1687 |
| random coil | 1640–1649 |
| Intermolecular β-sheet (aggregates) | 1618–1626; 1688–1696 |
| Sample | α-Helix %, (±SE) | p-Value | β-Sheet + Turn %, (±SE) | p-Value | Random Coil %, (±SE) | p-Value |
|---|---|---|---|---|---|---|
| BSA, 0 min | 59.9 ± 1.2 | – | 23.2 ± 2.5 | – | 16.9 ± 2.0 | – |
| BSA, 10 min | 57.6 ± 0.8 | 0.1262 | 25.5 ± 0.7 | 0.2730 | 16.9 ± 0.8 | 0.9814 |
| +PC | 61.0 ± 0.8 | 0.4570 | 20.9 ± 1.2 | 0.4179 | 18.0 ± 1.1 | 0.6174 |
| +10PI | 52.8 ± 1.5 | 0.0058 | 19.1 ± 2.1 | 0.2335 | 28.0 ± 1.2 | 0.0009 |
| +2GM1 | 57.1 ± 0.9 | 0.0872 | 22.9 ± 0.5 | 0.9128 | 20.1 ± 0.8 | 0.1562 |
| +10GM1 | 56.9 ± 0.8 | 0.0664 | 23.5 ± 1.6 | 0.9069 | 19.6 ± 1.2 | 0.2657 |
| +2CMG | 58.3 ± 1.2 | 0.3669 | 24.1 ± 1.7 | 0.7528 | 18.8 ± 0.9 | 0.3923 |
| +10CMG | 55.6 ± 1.1 | 0.0284 | 18.8 ± 1.2 | 0.1242 | 26.2 ± 1.5 | 0.0049 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tretiakova, D.; Kobanenko, M.; Le-Deygen, I.; Boldyrev, I.; Kudryashova, E.; Onishchenko, N.; Vodovozova, E. Spectroscopy Study of Albumin Interaction with Negatively Charged Liposome Membranes: Mutual Structural Effects of the Protein and the Bilayers. Membranes 2022, 12, 1031. https://doi.org/10.3390/membranes12111031
Tretiakova D, Kobanenko M, Le-Deygen I, Boldyrev I, Kudryashova E, Onishchenko N, Vodovozova E. Spectroscopy Study of Albumin Interaction with Negatively Charged Liposome Membranes: Mutual Structural Effects of the Protein and the Bilayers. Membranes. 2022; 12(11):1031. https://doi.org/10.3390/membranes12111031
Chicago/Turabian StyleTretiakova, Daria, Maria Kobanenko, Irina Le-Deygen, Ivan Boldyrev, Elena Kudryashova, Natalia Onishchenko, and Elena Vodovozova. 2022. "Spectroscopy Study of Albumin Interaction with Negatively Charged Liposome Membranes: Mutual Structural Effects of the Protein and the Bilayers" Membranes 12, no. 11: 1031. https://doi.org/10.3390/membranes12111031
APA StyleTretiakova, D., Kobanenko, M., Le-Deygen, I., Boldyrev, I., Kudryashova, E., Onishchenko, N., & Vodovozova, E. (2022). Spectroscopy Study of Albumin Interaction with Negatively Charged Liposome Membranes: Mutual Structural Effects of the Protein and the Bilayers. Membranes, 12(11), 1031. https://doi.org/10.3390/membranes12111031









