Amphiphilic Chitosan Porous Membranes as Potential Therapeutic Systems with Analgesic Effect for Burn Care
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Porous Membranes Preparation
2.3. Methods of Characterization
2.3.1. Morpho-Structural Characterization of the Membranes
2.3.2. Mechanical Properties
2.3.3. In vitro Hydrolytic Degradation
2.3.4. Swelling Behavior and Kinetic Characteristics
2.3.5. In Vitro Lidocaine Release
2.3.6. Antibacterial Activity
3. Results and Discussions
3.1. Porous Membrane Preparation
3.2. Structural Characterization of the Membranes
3.3. Membranes Morphological Evaluation
3.4. Pore Size Distribution
3.5. Mechanical Properties
3.6. In Vitro Hydrolytic Degradation
3.7. Swelling Behavior and Kinetic Properties
3.8. In Vitro Release of Lidocaine
3.9. Antibacterial
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Walker, H.; Chipp, E. Short and long term mortality following massive burn injury (>50% TBSA) in a UK burns centre. Burns 2022, in press. [Google Scholar] [CrossRef] [PubMed]
- Battle, C.E.; Evans, V.; James, K.; Guy, K.; Whitley, J.; Evans, P.A. Epidemiology of burns and scalds in children presenting to the emergency department of a regional burns unit: A 7-year retrospective study. Burn. Trauma 2016, 4, 19. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Burns. World Health Organization. 2018. Available online: https://www.who.int/news-room/fact-sheets/detail/burns (accessed on 7 September 2022).
- Yakupu, A.; Zhang, J.; Dong, W. The epidemiological characteristic and trends of burns globally. BMC Public Health 2022, 22, 1596. [Google Scholar] [CrossRef] [PubMed]
- Spiwak, R.; Sareen, S.; Logsetty, S. Techniques to Assess Long-Term Outcomes after Burn Injuries. Eur. Burn J. 2022, 3, 328–339. [Google Scholar] [CrossRef]
- World Health Organization. Disability-Adjusted Life Years (DALYs). Available online: https://www.who.int/data/gho/indicator-metadata-registry/imr-details/158 (accessed on 7 September 2022).
- Hong, R.; Perkins, M.; Gabbe, B.J.; Tracy, L.M. Comparing Peak Burn Injury Times and Characteristics in Australia and New Zealand. Int. J. Environ. Res. Public Health 2022, 19, 9578. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.C.; Joory, K.; Moiemen, N.S. History of burns: The past, present and the future. Burns Trauma 2014, 2, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Askay, S.W.; Patterson, D.R. What are the psychiatric sequelae of burn pain? Curr. Pain Headache Rep. 2008, 12, 94–97. [Google Scholar] [CrossRef] [PubMed]
- Mościcka, P.; Cwajda-Białasik, J.; Szewczyk, M.T.; Jawień, A. Healing Process, Pain, and Health-Related Quality of Life in Patients with Venous Leg Ulcers Treated with Fish Collagen Gel: A 12-Week Randomized Single-Center Study. Int. J. Environ. Res. Public Health 2022, 19, 7108. [Google Scholar] [CrossRef]
- Upton, D.; Solowiej, K. Pain and stress as contributors to delayed wound healing. Wound Pract. Res. 2010, 18, 114–122. [Google Scholar]
- Solowiej, K.; Mason, V.; Upton, D. Review of the relationship between stress and wound healing: Part 1. J. Wound Care 2009, 18, 357–366. [Google Scholar] [CrossRef]
- Navarro-Rodriguez, J.M.; Suarez-Serrano, C.; Martin-Valero, R.; Marcen-Roman, Y.; de-la-Casa-Almeida, M. Effectiveness of Topical Anesthetics in Pain Management for Dermal Injuries: A Systematic Review. J. Clin. Med. 2021, 10, 2522. [Google Scholar] [CrossRef] [PubMed]
- Kau, Y.-C.; Liao, C.-C.; Chen, Y.-C.; Liu, S.-J. Sustained Release of Lidocaine from Solvent-Free Biodegradable Poly[(d,l)-Lactide-co-Glycolide] (PLGA): In Vitro and In Vivo Study. Materials 2014, 7, 6660–6676. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, S.; Contino, V.; Kolli, S. Local Anesthetic Systemic Toxicity (LAST)—A Review and Update. Curr. Anesthesiol. Rep. 2020, 10, 218–226. [Google Scholar] [CrossRef]
- Brusini, R.; Iehl, J.; Clerc, E.; Gallet, M.; Bourdon, F.; Faivre, J. Comparative Preclinical Study of Lidocaine and Mepivacaine in Resilient Hyaluronic Acid Fillers. Pharmaceutics 2022, 14, 1553. [Google Scholar] [CrossRef]
- Juhlin, L.; Evers, H.; Broberg, F. A lidocaine-prilocaine cream for superficial skin surgery and painful lesions. Acta Derm. Vener. 1980, 60, 544–546. [Google Scholar] [CrossRef]
- Lee, F.-Y.; Lee, D.; Lee, T.-C.; Chen, J.-K.; Wu, R.-C.; Liu, K.-C.; Liu, S.-J. Fabrication of Multi-Layered Lidocaine and Epinephrine-Eluting PLGA/Collagen Nanofibers: In Vitro and In Vivo Study. Polymers 2017, 9, 416. [Google Scholar] [CrossRef]
- Hutchins, D.; Rockett, M. The use of atypical analgesics by intravenous infusion for acute pain: Evidence base for lidocaine, ketamine and magnesium. Anaesth. Intensive Care Med. 2019, 20, 415–418. [Google Scholar] [CrossRef]
- Kang, J.-H.; Yoo, K.-H.; Park, H.-Y.; Hyun, S.-M.; Han, S.-D.; Kim, D.-W.; Park, C.-W. Preparation and In Vivo Evaluation of a Lidocaine Self-Nanoemulsifying Ointment with Glycerol Monostearate for Local Delivery. Pharmaceutics 2021, 13, 1468. [Google Scholar] [CrossRef]
- Gudin, J.; Nalamachu, S. Utility of lidocaine as a topical analgesic and improvements in patch delivery systems. Pain Manag. 2019, 132, 28–36. [Google Scholar] [CrossRef]
- Rangappa, S.; Rangan, K.K.; Sudarshan, T.S.; Murthy, S.N. Evaluation of lidocaine loaded clay based dermal patch systems. J. Drug. Deliv. Sci. Technol. 2017, 39, 450–454. [Google Scholar] [CrossRef]
- Ali, A.; Ali, A.; Rahman, M.A.; Warsi, M.H.; Yusuf, M.; Alam, P. Development of Nanogel Loaded with Lidocaine for Wound-Healing: Illustration of Improved Drug Deposition and Skin Safety Analysis. Gels 2022, 8, 466. [Google Scholar] [CrossRef] [PubMed]
- Desai, C.; Wood, F.M.; Schug, S.A.; Parsons, R.W.; Fridlender, C.; Sunderland, V.B. Effectiveness of a topical local anaesthetic spray as analgesia for dressing changes: A double-blinded randomised pilot trial comparing an emulsion with an aqueous lidocaine formulation. Burns 2014, 40, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Tyagi, R.; Parmar, V.S.; Sharma, S.K.; Haag, R. Polyether based amphiphiles for delivery of active components. Polymer 2012, 53, 3053–3078. [Google Scholar] [CrossRef]
- Luu, C.H.; Nguyen, G.; Le, T.-T.; Nguyen, T.-M.N.; Giang Phan, V.H.; Murugesan, M.; Mathiyalagan, R.; Jing, L.; Janarthanan, G.; Yang, D.C.; et al. Graphene Oxide-Reinforced Alginate Hydrogel for Controlled Release of Local Anesthetics: Synthesis, Characterization, and Release Studies. Gels 2022, 8, 246. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-H.; Chang, S.-J.; Tzeng, Y.-S.; Shih, Y.-J.; Adrienne, C.; Chen, S.-G.; Chen, T.M.; Dai, N.T.; Cherng, J.-H. Enhanced wound-healing performance of a phyto-polysaccharide-enriched dressing—A preclinical small and large animal study. Int. Wound J. 2017, 14, 1359–1369. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liu, Y.; Cai, K.; Zhang, B.; Tang, S.; Zhang, W.; Liu, W. Antibacterial polysaccharide-based hydrogel dressing containing plant essential oil for burn wound healing, Burn. Trauma 2021, 9, tkab041. [Google Scholar] [CrossRef]
- Kennedy, J.F.; Knill, C.J.; Thorley, M. Natural Polymers for Healing Wounds. In Recent Advances in Environmentally Compatible Polymers; Kennedy, J.F., Phillips, G.O., Williams, P.A., Eds.; Woodhead Publishing Ltd.: Cambridge, UK, 2001; pp. 97–104. [Google Scholar]
- Li, Z.; Yuan, B.; Dong, X.; Duan, L.; Tia., H.; He, C.; Chenb, X. Injectable polysaccharide hybrid hydrogels as scaffolds for burn wound healing. RSC Adv. 2015, 5, 94248–94256. [Google Scholar] [CrossRef]
- Ibrahim, N.A.; Eid, B.M. Chitosan-Based Composite Materials: Fabrication and Characterization. In Handbook of Composites from Renewable Materials; Thakur, V.K., Thakur, M.K., Kessler, M.R., Eds.; Scrivener Publishing LLC: New York, NY, UK, 2017; Volume 3, pp. 103–136. [Google Scholar]
- Ma, J.; Wang, Y.; Lu, R. Mechanism and Application of Chitosan and Its Derivatives in Promoting Permeation in Transdermal Drug Delivery Systems: A Review. Pharmaceuticals 2022, 15, 459. [Google Scholar] [CrossRef]
- Alemdaroğlu, C.; Değim, Z.; Celebi, N.; Zor, F.; Oztürk, S.; Erdoğan, D. An investigation on burn wound healing in rats with chitosan gel formulation containing epidermal growth factor. Burns 2006, 32, 319–327. [Google Scholar] [CrossRef]
- Singh, R.; Shitiz, K.; Singh, A. Chitin and chitosan: Biopolymers for wound management. Int. Wound J. 2017, 14, 1276–1289. [Google Scholar] [CrossRef]
- Jardin, M.A.; Sayed, S. Chitosan as an Advanced Healthcare Material. In Advanced Biomaterials and Biodevices; Tiwari, A., Nordin, A.N., Eds.; Wiley-Scrivener: Hoboken, NJ, USA, 2014; pp. 147–182. [Google Scholar]
- Enache, A.-C.; Cojocaru, C.; Samoila, P.; Bele, A.; Bostanaru, A.-C.; Mares, M.; Harabagiu, V. Evaluation of Physically and/or Chemically Modified Chitosan Hydrogels for Proficient Release of Insoluble Nystatin in Simulated Fluids. Gels 2022, 8, 495. [Google Scholar] [CrossRef] [PubMed]
- Humelnicu, A.-C.; Samoila, P.; Asandulesa, M.; Cojocaru, C.; Bele, A.; Marinoiu, A.T.; Saccà, A.; Harabagiu, V. Chitosan-Sulfated Titania Composite Membranes with Potential Applications in Fuel Cell: Influence of Cross-Linker Nature. Polymers 2020, 12, 1125. [Google Scholar] [CrossRef] [PubMed]
- Dias, J.R.; Sousa, A.; Augusto, A.; Bártolo, P.J.; Granja, P.L. Electrospun Polycaprolactone (PCL) Degradation: An In Vitro and In Vivo Study. Polymers 2022, 14, 3397. [Google Scholar] [CrossRef]
- Matar, G.H.; Andac, M.; Elmas, A. Locust bean gum-polyvinyl alcohol hydrogels: Synthesis, characterization, swelling behaviors, and mathematical models. J. Appl. Polym. Sci. 2021, 51498. [Google Scholar] [CrossRef]
- Peppas, N. Hydrogels in pharmaceutical formulations. Eur. J. Pharm. Biopharm. 2000, 50, 27–46. [Google Scholar] [CrossRef]
- Concha, L.; Pires, A.L.R.; Moraes, A.M.; Mas-Hernández, E.; Berres, S.; Montelongo, J.H. Cost Function Analysis Applied to Different Kinetic Release Models of Arrabidaea chica Verlot Extract from Chitosan/Alginate Membranes. Polymers 2022, 14, 1109. [Google Scholar] [CrossRef] [PubMed]
- Dash, S.; Murthy, P.N.; Nath, L.; Chowdhury, P. Kinetic modeling on drug release from controlled drug delivery systems. Acta Pol. Pharm. 2010, 67, 217–223. [Google Scholar]
- Fu, Y.; Kao, W.J. Drug release kinetics and transport mechanisms of non-degradable and degradable polymeric delivery systems. Expert Opin. Drug Deliv. 2010, 7, 429–444. [Google Scholar] [CrossRef]
- Anitha, A.; Sanoj Rejinold, N.; Bumgardner, J.D.; Nair, S.V.; Jayakumar, R. Approaches for Functional Modification or Cross-linking of Chitosan. In Chitosan-Based Systems for Biopharmaceuticals: Delivery, Targeting and Polymer Therapeutics, 1st ed.; Sarmento, B., das Neves, J., Eds.; John Wiley & Sons, Ltd.: West Sussex, UK, 2012; pp. 107–124. [Google Scholar]
- Mourya, V.K.; Inamdar, N.N. Chitosan-modifications and applications Opportunities galore. React. Funct. Polym. 2008, 68, 1013–1051. [Google Scholar] [CrossRef]
- Bostanudin, M.F. Amphiphilic Alkylated Pectin Hydrogels for Enhanced Topical Delivery of Fusidic Acid: Formulation and In Vitro Investigation. Sci. Pharm. 2022, 90, 13. [Google Scholar] [CrossRef]
- Lillieborg, S.; Aanderud, L. EMLA anaesthetic cream for debridement of burns: A study of plasma concentrations of lidocaine and prilocaine and a review of the literature. Int. J. Burn. Trauma 2017, 7, 88–97. [Google Scholar]
- Lawrie, G.; Keen, I.; Drew, B.; Chandler-Temple, A.; Rintoul, L.; Fredericks, P.; Grøndahl, L. Interactions between Alginate and Chitosan Biopolymers Characterized Using FTIR and XPS. Biomacromolecules 2007, 8, 2533–2541. [Google Scholar] [CrossRef]
- Mauricio-Sánchez, R.A.; Salazar, R.; Luna-Bárcenas, J.G.; Mendoza-Galván, A. FTIR spectroscopy studies on the spontaneous neutralization of chitosan acetate films by moisture conditioning. Vib. Spectrosc. 2018, 94, 1–6. [Google Scholar] [CrossRef]
- Powell, M.F. Lidocaine and Lidocaine Hydrochloride. Anal. Profiles Drug Subst. 1986, 15, 761–779. [Google Scholar] [CrossRef]
- Bashir, S.; Teo, Y.Y.; Ramesh, S.; Ramesh, K. Physico-chemical characterization of pH-sensitive N -Succinyl chitosan- g -poly (acrylamide-co-acrylic acid) hydrogels and in vitro drug release studies. Polym. Degrad. Stab. 2017, 139, 38–54. [Google Scholar] [CrossRef]
- Kuo, C.-K.; Huang, H.-W.; Chen, L.-G.; Chou, Y.-J. Fabrication and characterization of freeze dried strontium-doped bioactive glasses/chitosan composite scaffolds for biomedical engineering. J. Asian Ceram. Soc. 2021, 9, 1173–1182. [Google Scholar] [CrossRef]
- Reys, L.L.; Silva, S.S.; Pirraco, R.P.; Marques, A.P.; Mano, J.F.; Silva, T.H.; Reis, R.L. Influence of freezing temperature and deacetylation degree on the performance of freeze-dried chitosan scaffolds towards cartilage tissue engineering. Eur. Polym. J. 2017, 95, 232–240. [Google Scholar] [CrossRef]
- Huang, W.-C.; Chen, S.-Y.; Liu, D.-M. An amphiphilic silicone-modified polysaccharide molecular hybrid with in situ forming of hierarchical superporous architecture upon swelling. Soft Matter 2012, 8, 10868. [Google Scholar] [CrossRef]
- Wen, Y.; Li, X.; Zhang, S.; Xie, C.; Ma, W.; Liang, L.; He, Z.; Duan, H.; Mou, Y.; Zhao, G. Preparation of a “Branch-Fruit” structure chitosan nanofiber physical hydrogels with high mechanical strength and pH-responsive controlled drug release properties. RSC Adv. 2022, 12, 17208–17216. [Google Scholar] [CrossRef]
- Antón, E.; Calvo, J.I.; Álvarez, J.R.; Hernández, A.; Luque, S. Fitting approach to liquid–liquid displacement porosimetry based on the log-normal pore size distribution. J. Membr. Sci. 2014, 470, 219–228. [Google Scholar] [CrossRef]
- Bambagiotti-Alberti, M.; Bruni, B.; Di Vaira, M.; Giannellini, V.; Guerri, A. 2-(Diethylamino)-N-(2,6-dimethylphenyl) acetamide, a low-temperature redetermination. Acta Cryst. E 2007, 63, o768–o770. [Google Scholar] [CrossRef]
- Jenkins, S.; Jacob, K.I.; Kumar, S. The effect of hydrogen bonding on the physical and mechanical properties of rigid-rod polymers. J. Polym. Sci. B Polym. Phys. 2000, 38, 3053–3061. [Google Scholar] [CrossRef]
- Wang, D.; Liu, W.; Feng, Q.; Dong, C.; Liu, Q.; Duan, L.; Huang, J.; Zhu, W.; Li, Z.; Xiong, J.; et al. Effect of inorganic/organic ratio and chemical coupling on the performance of porous silica/chitosan hybrid scaffolds. Mater. Sci. Eng. C 2017, 70, 969–975. [Google Scholar] [CrossRef] [PubMed]
- Markiewicz-Gospodarek, A.; Kozioł, M.; Tobiasz, M.; Baj, J.; Radzikowska-Büchner, E.; Przekora, A. Burn Wound Healing: Clinical Complications, Medical Care, Treatment, and Dressing Types: The Current State of Knowledge for Clinical Practice. Int. J. Environ. Res. Public Health 2022, 19, 1338. [Google Scholar] [CrossRef]
- Torp, K.D.; Metheny, E.; Simon, L.V. Lidocaine Toxicity; StatPearls Publishing LLC: Treasure Island, FL, USA, 2019. [Google Scholar]
- Alebachew, T.; Yismaw, G.; Derabe, A.; Sisay, Z. Staphylococcus aureus burn wound infection among patients attending yekatit 12 hospital burn unit, addis ababa, ethiopia. Ethiop. J. Health Sci. 2012, 22, 209–213. [Google Scholar]
- Khan, T.M.; Kok, Y.L.; Bukhsh, A.; Lee, L.H.; Chan, K.G.; Goh, B.H. Incidence of methicillin resistant Staphylococcus aureus (MRSA) in burn intensive care unit: A systematic review. Germs 2018, 8, 113–125. [Google Scholar] [CrossRef]
- Wimberley, N.; Willey, S.; Sullivan, N.; Bartlett, J.G. Antibacterial Properties of Lidocaine. Chest 1979, 76, 37–40. [Google Scholar] [CrossRef]
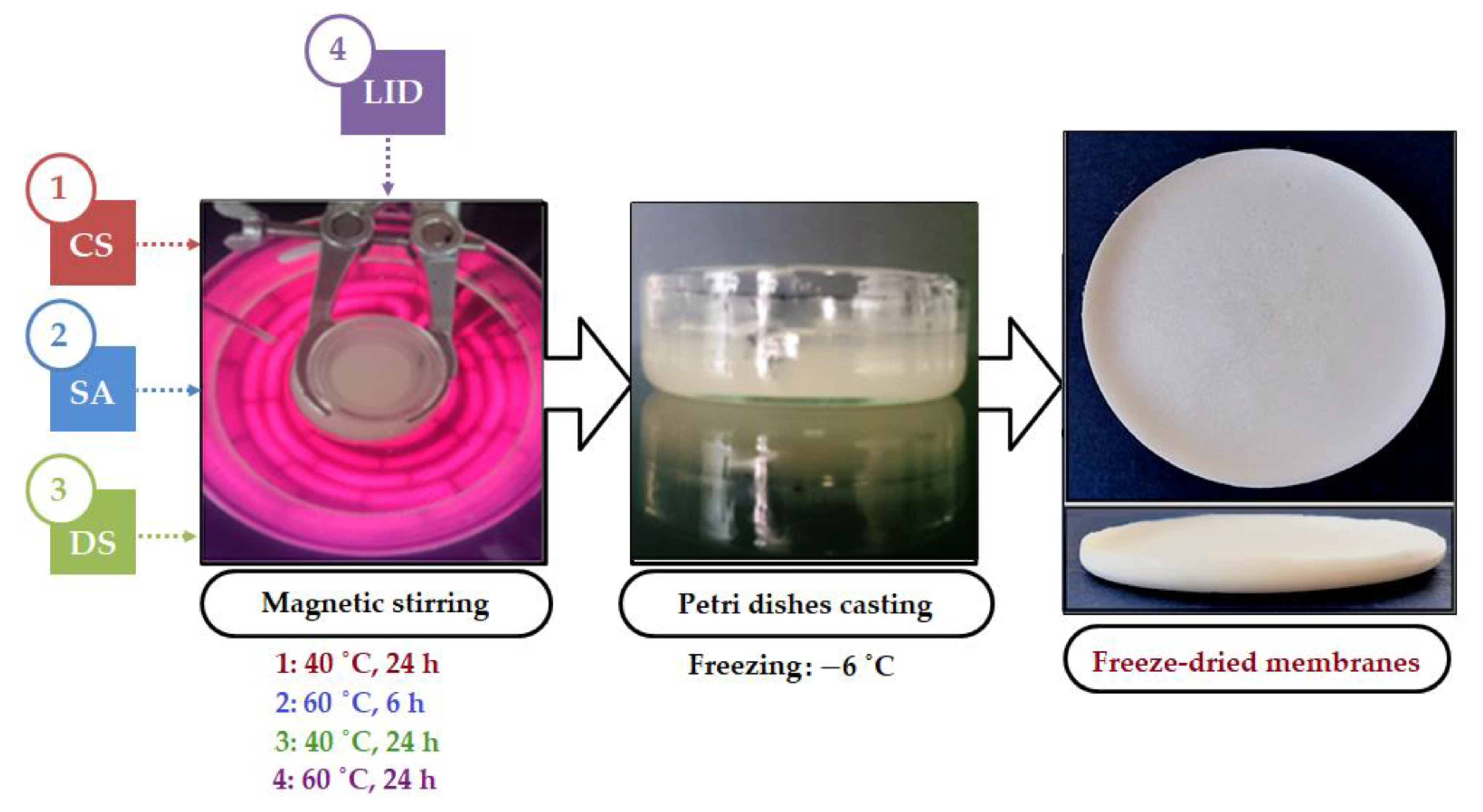



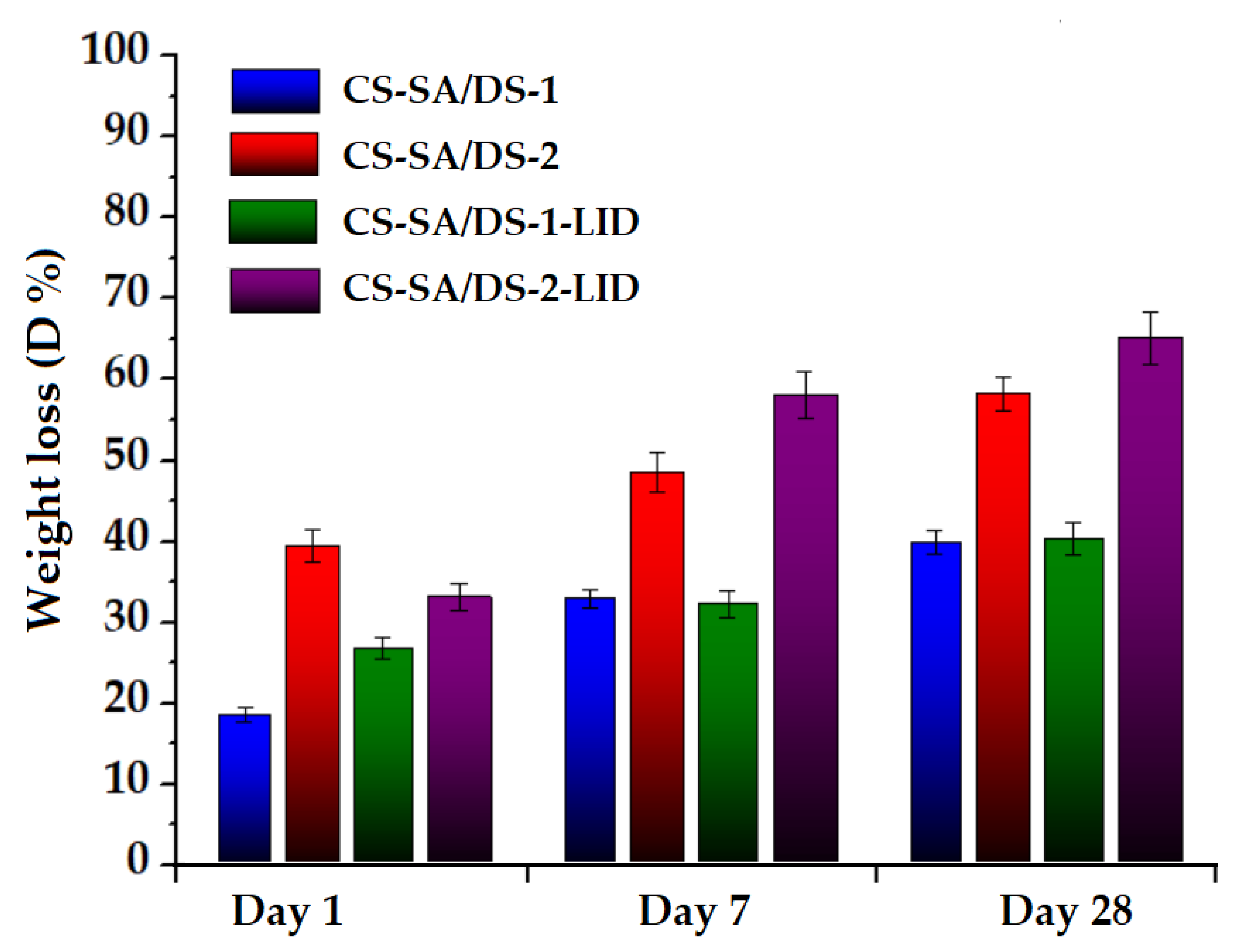

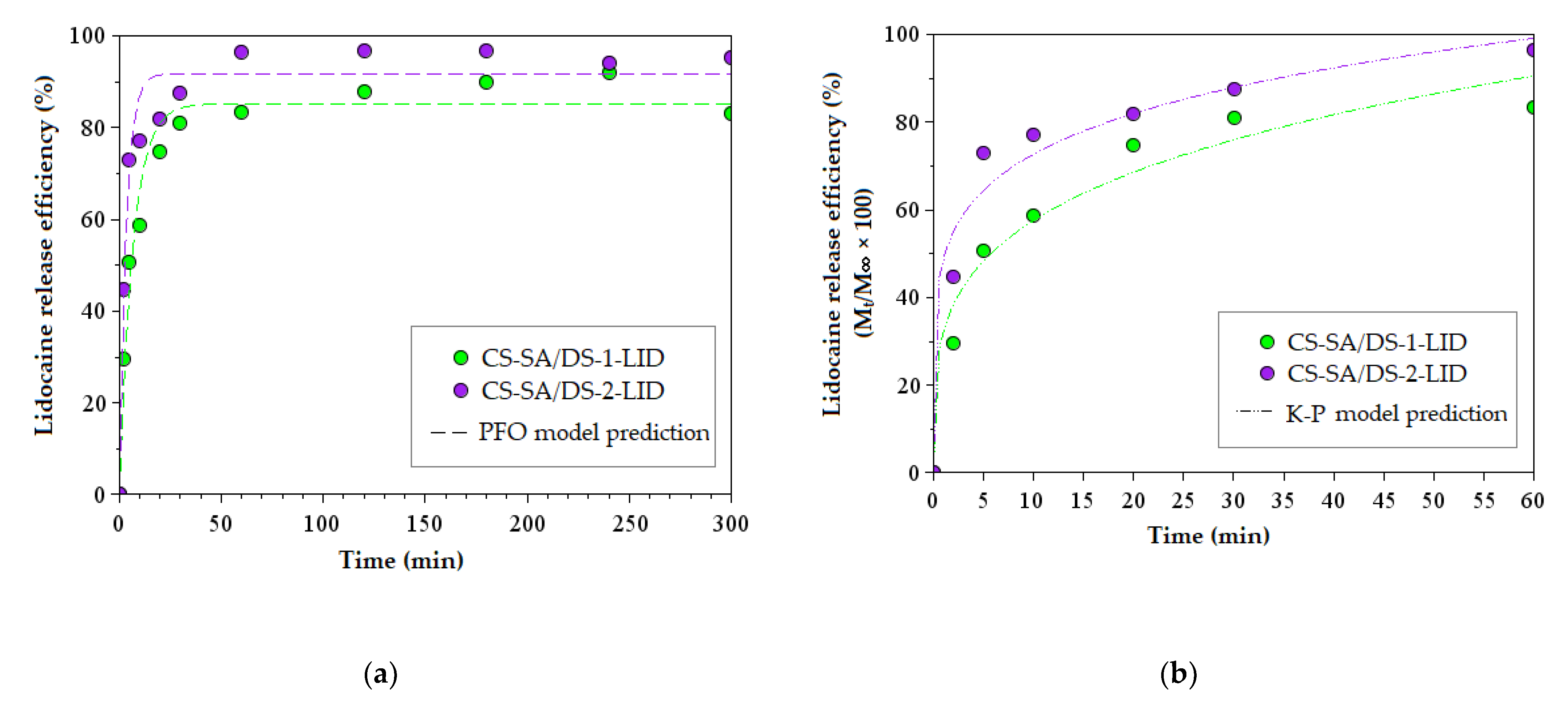

| Hydrogel/Film 1 Code | CS (g) | SA 2 (mg) | DS 2 (mg) | LID (g) |
|---|---|---|---|---|
| CS-A | 0.3 | - | - | |
| CS-SA/DS-1 CS-SA/DS-2 | 0.3 | - | - | |
| CS-SA/DS-1-LID | 0.3 | 15 | 244 | 0.1 |
| CS-SA/DS-2-LID | 0.3 | 75 | 135 | 0.1 |
| Membrane | Maximum Force (N) | Compression Strength (MPa) | Mean Compression Modulus (MPa) |
|---|---|---|---|
| CS-A | 3.05 ± 0.51 | 0.06 ± 0.01 | 0.31 ± 0.05 |
| CS-SA/DS-1 | 1.73 ± 0.29 | 0.03 ± 0.01 | 0.17 ± 0.04 |
| CS-SA/DS-2 | 1.27 ± 0.27 | 0.02 ± 0.01 | 0.13 ± 0.03 |
| CS-SA/DS-1-LID | 4.62 ± 1.60 | 0.09 ± 0.03 | 0.48 ± 0.16 |
| CS-SA/DS-2-LID | 2.86 ± 1.16 | 0.04 ±0.02 | 0.32 ± 0.12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Enache, A.-C.; Samoila, P.; Cojocaru, C.; Bele, A.; Bostanaru, A.-C.; Mares, M.; Harabagiu, V. Amphiphilic Chitosan Porous Membranes as Potential Therapeutic Systems with Analgesic Effect for Burn Care. Membranes 2022, 12, 973. https://doi.org/10.3390/membranes12100973
Enache A-C, Samoila P, Cojocaru C, Bele A, Bostanaru A-C, Mares M, Harabagiu V. Amphiphilic Chitosan Porous Membranes as Potential Therapeutic Systems with Analgesic Effect for Burn Care. Membranes. 2022; 12(10):973. https://doi.org/10.3390/membranes12100973
Chicago/Turabian StyleEnache, Andra-Cristina, Petrisor Samoila, Corneliu Cojocaru, Adrian Bele, Andra-Cristina Bostanaru, Mihai Mares, and Valeria Harabagiu. 2022. "Amphiphilic Chitosan Porous Membranes as Potential Therapeutic Systems with Analgesic Effect for Burn Care" Membranes 12, no. 10: 973. https://doi.org/10.3390/membranes12100973
APA StyleEnache, A.-C., Samoila, P., Cojocaru, C., Bele, A., Bostanaru, A.-C., Mares, M., & Harabagiu, V. (2022). Amphiphilic Chitosan Porous Membranes as Potential Therapeutic Systems with Analgesic Effect for Burn Care. Membranes, 12(10), 973. https://doi.org/10.3390/membranes12100973









