Separation of Bioproducts through the Integration of Cyanobacterial Metabolism and Membrane Filtration: Facilitating Cyanobacteria’s Industrial Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Culture Conditions
2.3. Constructed Plasmids and Strains
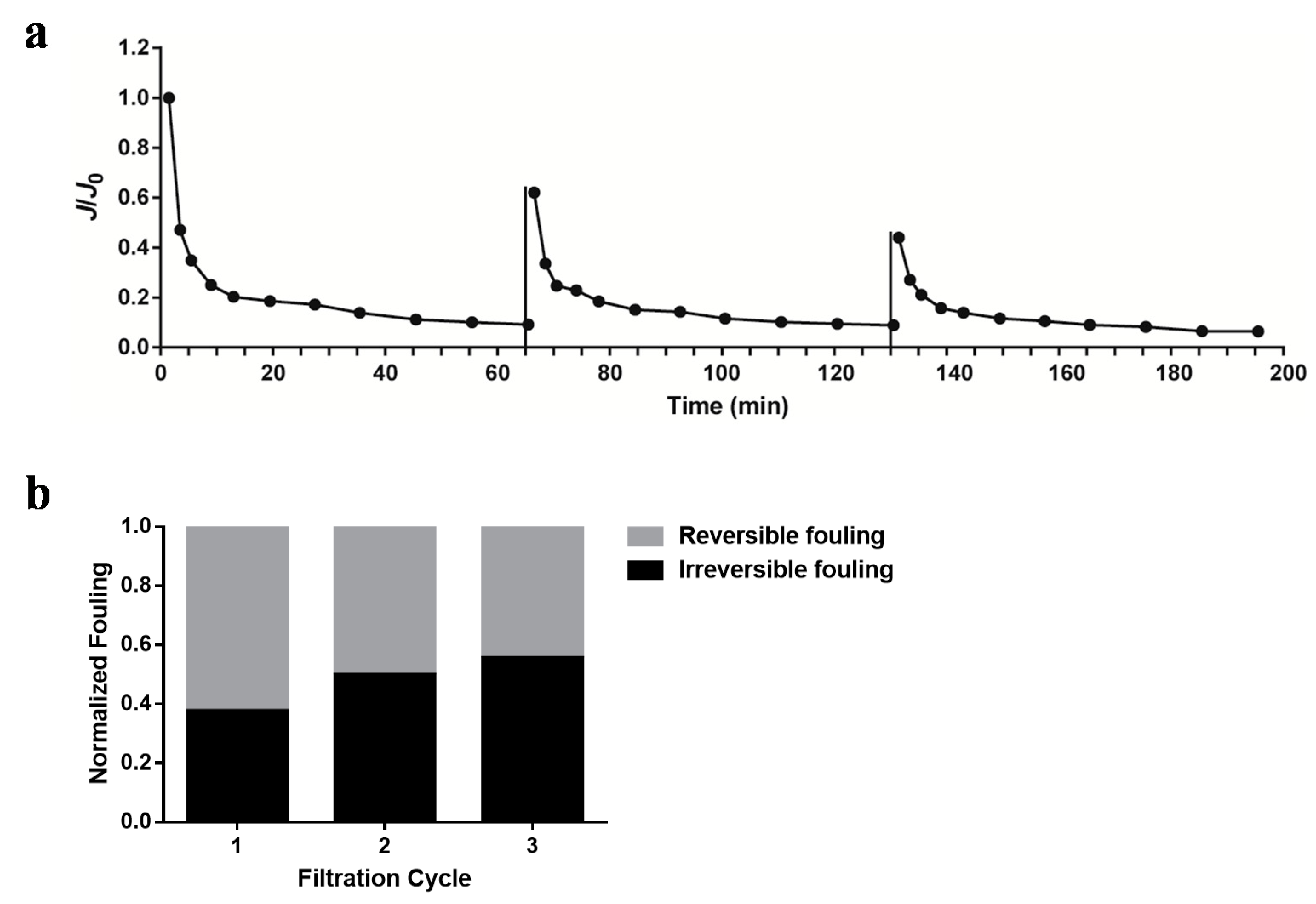
2.4. Membrane Filtration Experiment
2.5. Sucrose Assays
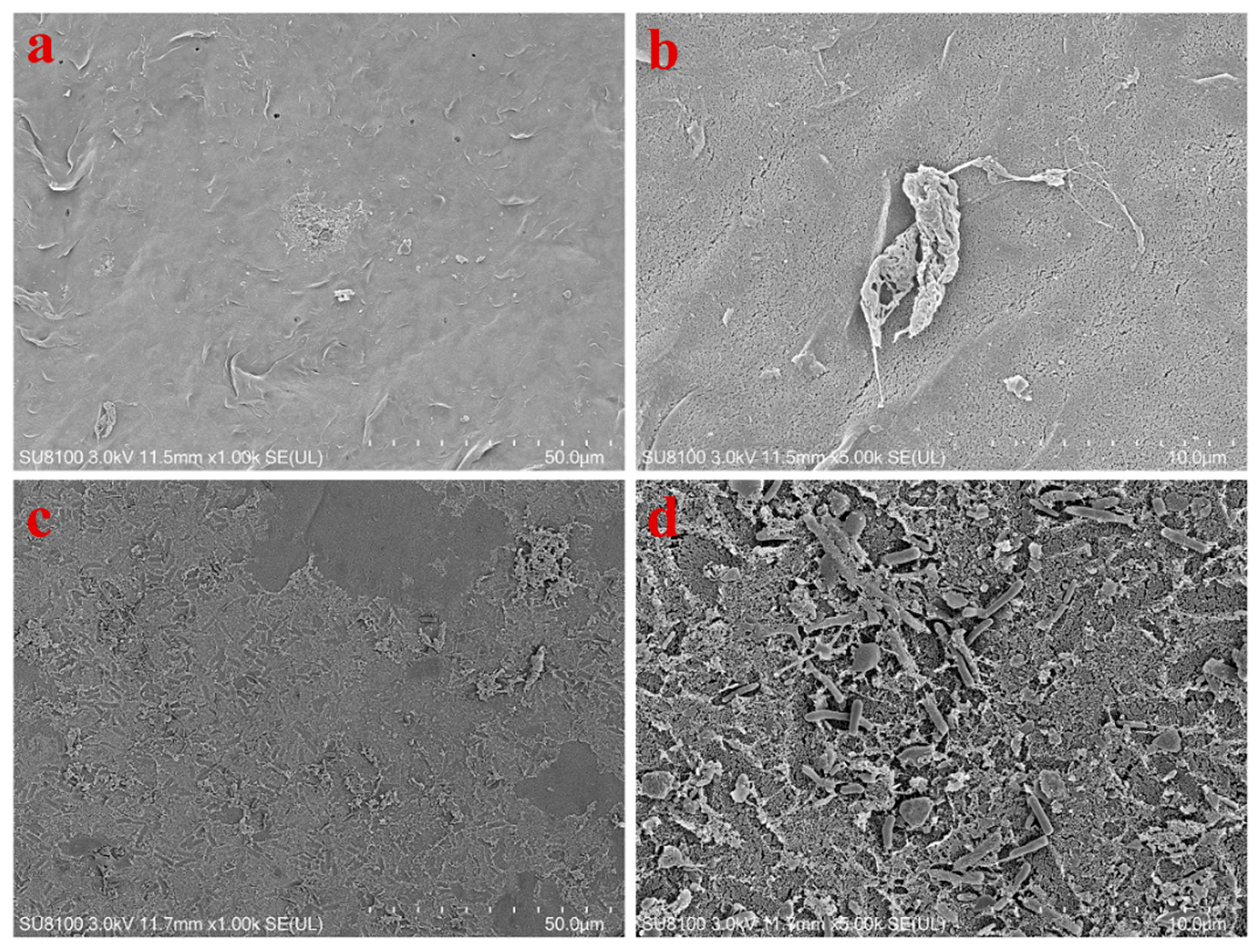
2.6. Fouled Membrane Characterization
3. Results and Discussion
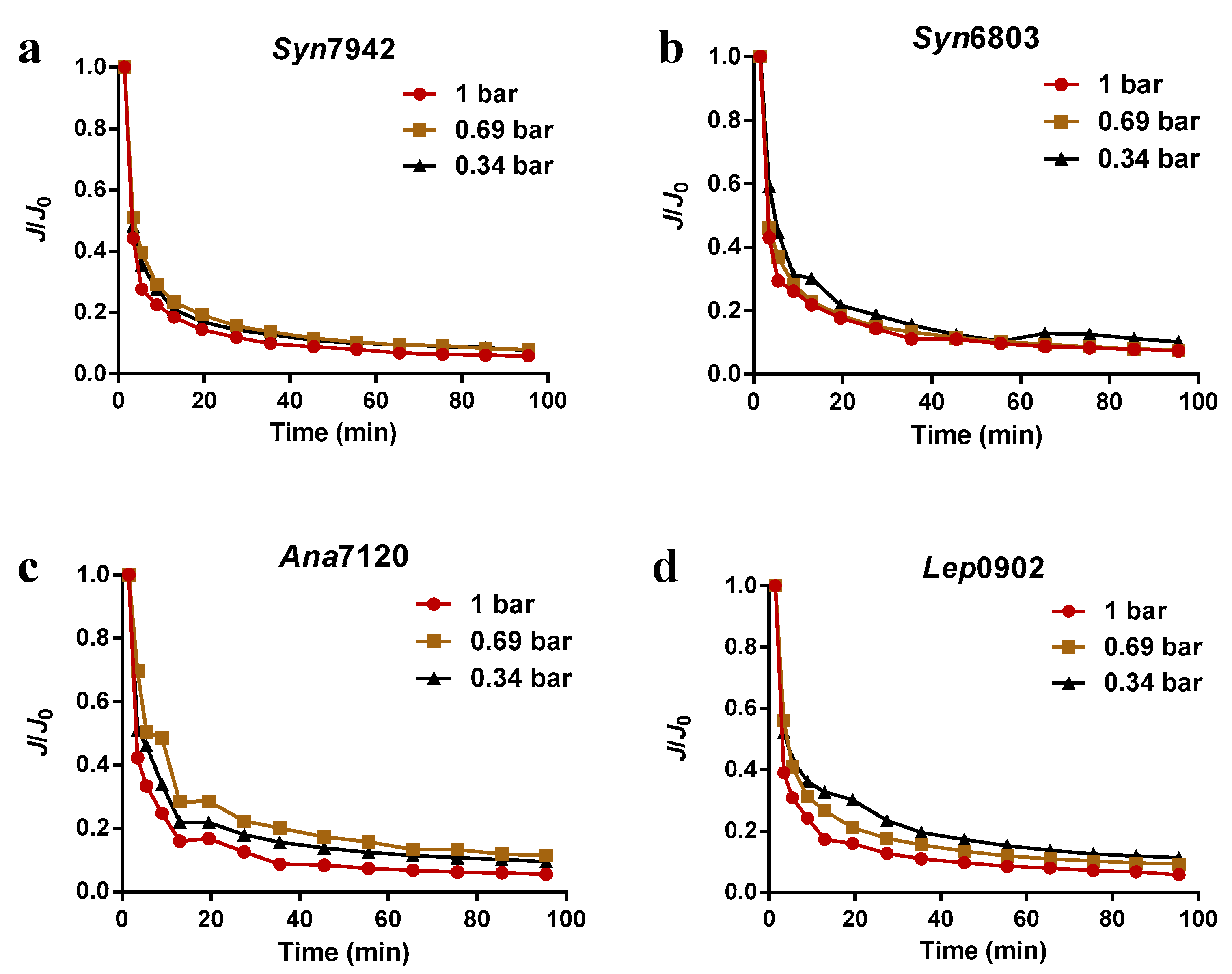
3.1. Membrane Fouling Behavior of Different Kinds of Cyanobacteria
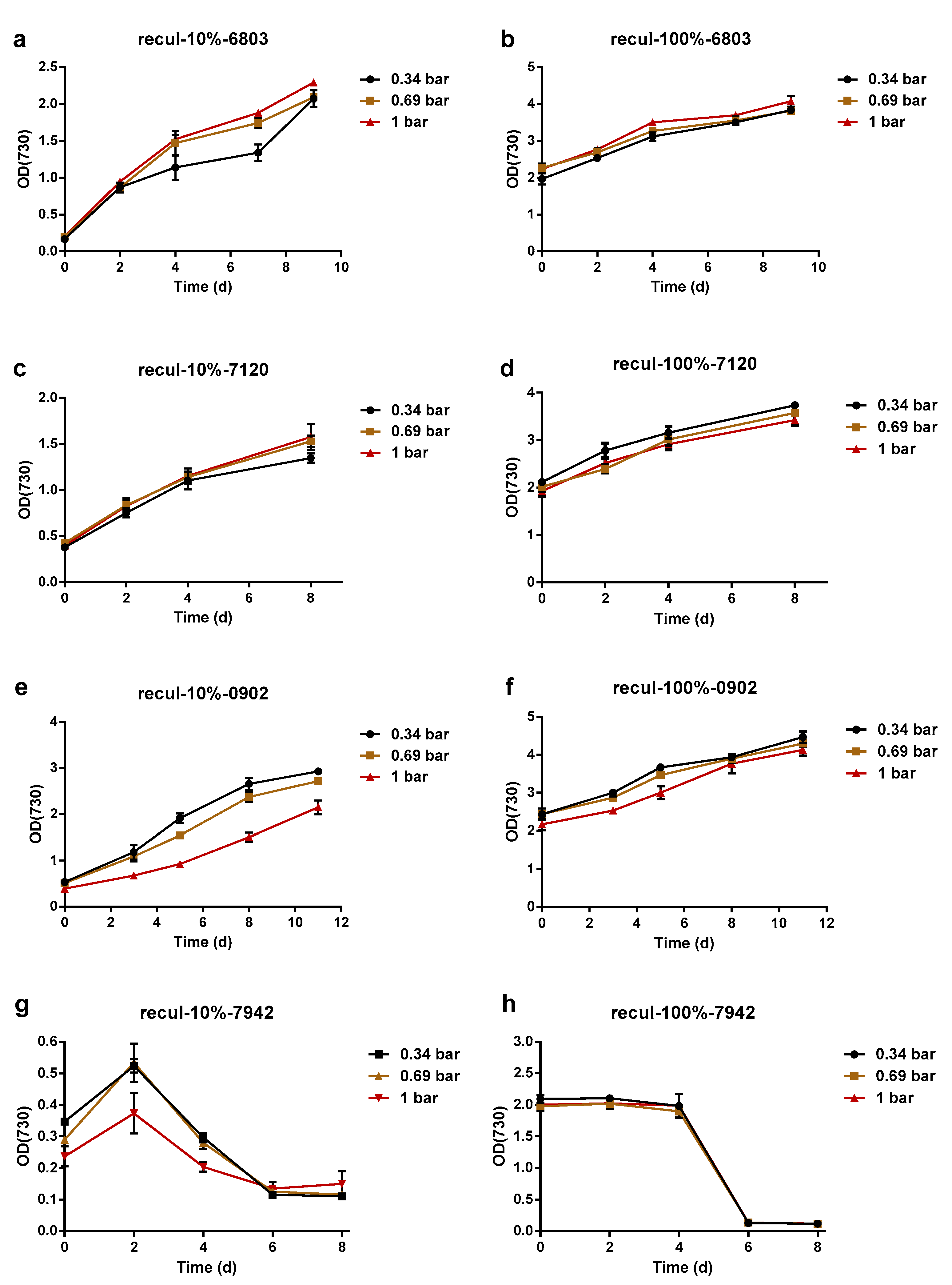
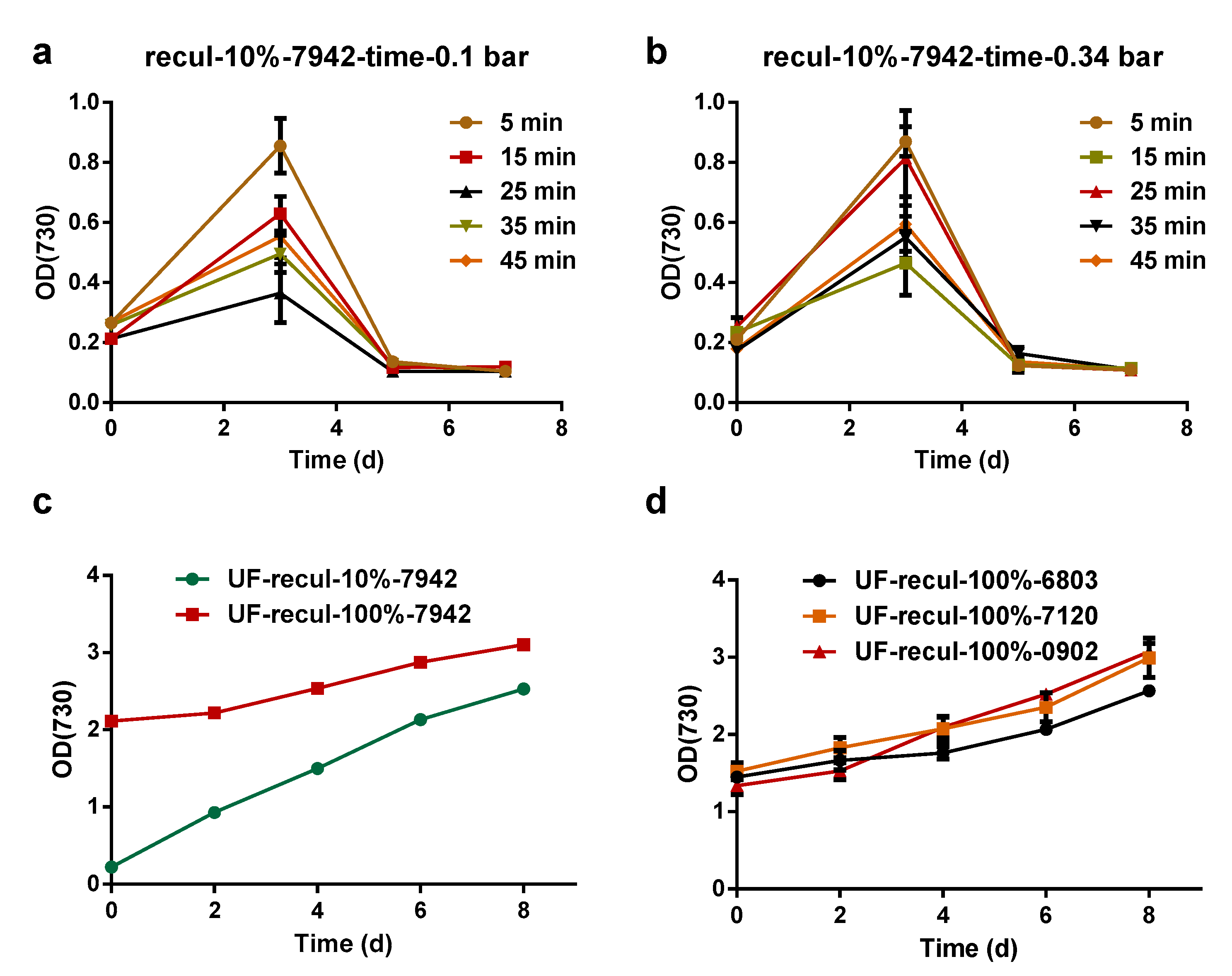
3.2. Cyanobacteria Survival after Pressure-Driven Membrane Filtration
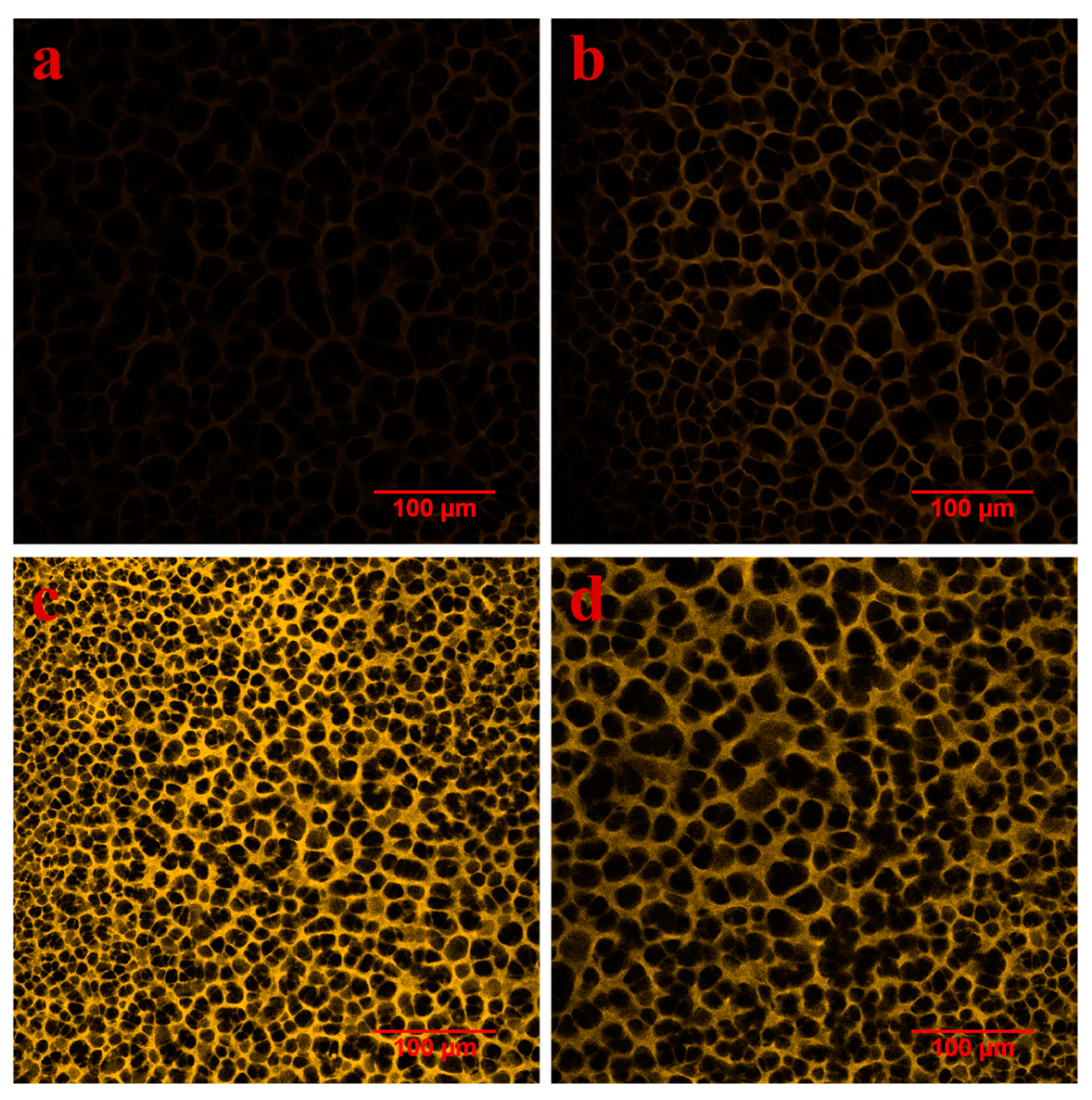
3.3. Characterization of Fouled Membranes
3.4. Construction of cscB+ Syn7942 and the Separation of Produced Sucrose
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, A.; Carroll, A.L.; Atsumi, S. Carbon recycling by cyanobacteria: Improving CO2 fixation through chemical production. FEMS Microbiol. Lett. 2017, 364, fnx165. [Google Scholar] [CrossRef]
- Luan, G.; Lu, X. Tailoring cyanobacterial cell factory for improved industrial properties. Biotechnol. Adv. 2018, 36, 430–442. [Google Scholar] [CrossRef]
- Matson, M.M.; Atsumi, S. Photomixotrophic chemical production in cyanobacteria. Curr. Opin. Biotechnol. 2018, 50, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Xiao, K.; Liang, J.; Wang, X.; Hou, T.; Ren, X.; Yin, P.; Ma, Z.; Zeng, C.; Gao, X. Panoramic insights into semi-artificial photosynthesis: Origin, development, and future perspective. Energy Environ. Sci. 2022, 15, 529–549. [Google Scholar]
- Gudmundsson, S.; Nogales, J. Cyanobacteria as photosynthetic biocatalysts: A systems biology perspective. Mol. Biosyst. 2015, 11, 60–70. [Google Scholar] [CrossRef]
- Gao, X.; Sun, T.; Pei, G.; Chen, L.; Zhang, W. Cyanobacterial chassis engineering for enhancing production of biofuels and chemicals. Appl. Microbiol. Biotechnol. 2016, 100, 3401–3413. [Google Scholar] [CrossRef]
- Lau, N.-S.; Matsui, M.; Abdullah, A.A.-A. Cyanobacteria: Photoautotrophic microbial factories for the sustainable synthesis of industrial products. BioMed Res. Int. 2015, 2015, 754934. [Google Scholar] [CrossRef] [PubMed]
- Abed, R.M.; Dobretsov, S.; Sudesh, K. Applications of cyanobacteria in biotechnology. J. Appl. Microbiol. 2009, 106, 1–12. [Google Scholar] [CrossRef]
- Hirokawa, Y.; Kubo, T.; Soma, Y.; Saruta, F.; Hanai, T. Enhancement of acetyl-CoA flux for photosynthetic chemical production by pyruvate dehydrogenase complex overexpression in Synechococcus elongatus PCC 7942. Metab. Eng. 2020, 57, 23–30. [Google Scholar] [CrossRef]
- Berla, B.M.; Saha, R.; Immethun, C.M.; Maranas, C.D.; Moon, T.S.; Pakrasi, H. Synthetic biology of cyanobacteria: Unique challenges and opportunities. Front. Microbiol. 2013, 4, 246. [Google Scholar] [CrossRef]
- Nozzi, N.E.; Case, A.E.; Carroll, A.L.; Atsumi, S. Systematic approaches to efficiently produce 2,3-butanediol in a marine cyanobacterium. ACS Synth. Biol. 2017, 6, 2136–2144. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.; Wang, W.; Zhang, W.; Chen, L.; Lu, X. Versatility of hydrocarbon production in cyanobacteria. Appl. Microbiol. Biotechnol. 2017, 101, 905–919. [Google Scholar] [CrossRef] [PubMed]
- Johnson, T.J.; Gibbons, J.L.; Gu, L.; Zhou, R.; Gibbons, W.R. Molecular genetic improvements of cyanobacteria to enhance the industrial potential of the microbe: A review. Biotechnol. Prog. 2016, 32, 1357–1371. [Google Scholar] [CrossRef] [PubMed]
- Oliver, J.W.; Atsumi, S. Metabolic design for cyanobacterial chemical synthesis. Photosynth. Res. 2014, 120, 249–261. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Zhao, H.; Li, Z.; Tan, X.; Lu, X. Photosynthetic production of ethanol from carbon dioxide in genetically engineered cyanobacteria. Energy Environ. Sci. 2012, 5, 9857–9865. [Google Scholar] [CrossRef]
- Kanno, M.; Carroll, A.L.; Atsumi, S. Global metabolic rewiring for improved CO2 fixation and chemical production in cyanobacteria. Nat. Commun. 2017, 8, 14724. [Google Scholar] [CrossRef]
- Ungerer, J.; Tao, L.; Davis, M.; Ghirardi, M.; Maness, P.-C.; Yu, J. Sustained photosynthetic conversion of CO2 to ethylene in recombinant cyanobacterium Synechocystis 6803. Energy Environ. Sci. 2012, 5, 8998–9006. [Google Scholar] [CrossRef]
- Wang, X.; Liu, W.; Xin, C.; Zheng, Y.; Cheng, Y.; Sun, S.; Li, R.; Zhu, X.-G.; Dai, S.Y.; Rentzepis, P.M. Enhanced limonene production in cyanobacteria reveals photosynthesis limitations. Proc. Natl. Acad. Sci. USA 2016, 113, 14225–14230. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, T.; Gao, X.; Shi, M.; Wu, L.; Chen, L.; Zhang, W. Biosynthesis of platform chemical 3-hydroxypropionic acid (3-HP) directly from CO2 in cyanobacterium Synechocystis sp. PCC 6803. Metab. Eng. 2016, 34, 60–70. [Google Scholar] [CrossRef]
- Liu, X.; Brune, D.; Vermaas, W.; Curtiss, R. Production and secretion of fatty acids in genetically engineered cyanobacteria. Proc. Natl. Acad. Sci. USA 2010, 107, 13189. [Google Scholar] [CrossRef]
- Hirokawa, Y.; Goto, R.; Umetani, Y.; Hanai, T. Construction of a novel d-lactate producing pathway from dihydroxyacetone phosphate of the Calvin cycle in cyanobacterium, Synechococcus elongatus PCC 7942. J. Biosci. Bioeng. 2017, 124, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Diao, J.; Song, X.; Zhang, L.; Cui, J.; Chen, L.; Zhang, W. Tailoring cyanobacteria as a new platform for highly efficient synthesis of astaxanthin. Metab. Eng. 2020, 61, 275–287. [Google Scholar] [CrossRef] [PubMed]
- Ducat, D.C.; Avelar-Rivas, J.A.; Way, J.C.; Silver, P.A. Rerouting carbon flux to enhance photosynthetic productivity. Appl. Environ. Microbiol. 2012, 78, 2660–2668. [Google Scholar] [CrossRef] [PubMed]
- Farrokh, P.; Sheikhpour, M.; Kasaeian, A.; Asadi, H.; Bavandi, R. Cyanobacteria as an eco-friendly resource for biofuel production: A critical review. Biotechnol. Prog. 2019, 35, e2835. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, L.; Diao, J.; Song, X.; Shi, M.; Zhang, W. Construction and analysis of an artificial consortium based on the fast-growing cyanobacterium Synechococcus elongatus UTEX 2973 to produce the platform chemical 3-hydroxypropionic acid from CO2. Biotechnol. Biofuels 2020, 13, 82. [Google Scholar] [CrossRef]
- Kalil, S.J.; Moraes, C.C.; Sala, L.; Burkert, C.A. Bioproduct extraction from microbial cells by conventional and nonconventional techniques. In Food Bioconversion; Elsevier: Amsterdam, The Netherlands, 2017; pp. 179–206. [Google Scholar]
- Demirbas, A. Use of algae as biofuel sources. Energy Convers. Manag. 2010, 51, 2738–2749. [Google Scholar] [CrossRef]
- Tian, M.; Xu, H.; Yao, L.; Wang, R. A biomimetic antimicrobial surface for membrane fouling control in reverse osmosis for seawater desalination. Desalination 2021, 503, 114954. [Google Scholar] [CrossRef]
- Kong, X.; Ma, J.; Le-Clech, P.; Wang, Z.; Tang, C.Y.; Waite, T.D. Management of concentrate and waste streams for membrane-based algal separation in water treatment: A review. Water Res. 2020, 183, 115969. [Google Scholar] [CrossRef]
- Qu, F.; Liang, H.; Tian, J.; Yu, H.; Chen, Z.; Li, G. Ultrafiltration (UF) membrane fouling caused by cyanobateria: Fouling effects of cells and extracellular organics matter (EOM). Desalination 2012, 293, 30–37. [Google Scholar] [CrossRef]
- Liang, H.; Huang, X.; Wang, H.; Xu, W.; Shi, B. The role of extracellular organic matter on the cyanobacteria ultrafiltration process. J. Environ. Sci. 2021, 110, 12–20. [Google Scholar] [CrossRef]
- Gao, K.; Li, T.; Zhao, Q.; Liu, W.; Liu, J.; Song, Y.; Chu, H.; Dong, B. UF fouling behavior of allelopathy of extracellular organic matter produced by mixed algae co-cultures. Sep. Purif. Technol. 2021, 261, 118297. [Google Scholar] [CrossRef]
- Zhu, T.; Zhou, Z.; Qu, F.; Liu, B.; Van der Bruggen, B. Separation performance of ultrafiltration during the treatment of algae-laden water in the presence of an anionic surfactant. Sep. Purif. Technol. 2022, 281, 119894. [Google Scholar] [CrossRef]
- Hirokawa, Y.; Maki, Y.; Tatsuke, T.; Hanai, T. Cyanobacterial production of 1,3-propanediol directly from carbon dioxide using a synthetic metabolic pathway. Metab. Eng. 2016, 34, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Qu, F.; Liang, H.; Zhou, J.; Nan, J.; Shao, S.; Zhang, J.; Li, G. Ultrafiltration membrane fouling caused by extracellular organic matter (EOM) from Microcystis aeruginosa: Effects of membrane pore size and surface hydrophobicity. J. Membr. Sci. 2014, 449, 58–66. [Google Scholar] [CrossRef]
- Yeganeh-Zare, S.; Farhadi, K.; Amiri, S. Rapid detection of apple juice concentrate adulteration with date concentrate, fructose and glucose syrup using HPLC-RID incorporated with chemometric tools. Food Chem. 2022, 370, 131015. [Google Scholar] [CrossRef]
- Fuszard, M.A.; Ow, S.Y.; Gan, C.S.; Noirel, J.; Ternan, N.G.; McMullan, G.; Biggs, C.A.; Reardon, K.F.; Wright, P.C. The quantitative proteomic response of Synechocystis sp. PCC6803 to phosphate acclimation. Aquat. Biosyst. 2013, 9, 5. [Google Scholar] [CrossRef]
- Li, Y.; Li, D. Competition between toxic Microcystis aeruginosa and nontoxic Microcystis wesenbergii with Anabaena PCC7120. J. Appl. Phycol. 2012, 24, 69–78. [Google Scholar] [CrossRef]
- Hu, Z.Q.; Liu, Y.D.; Li, D.H. Physiological and biochemical analyses of microcystin-RR toxicity to the cyanobacterium Synechococcus elongatus. Environ. Toxicol. Int. J. 2004, 19, 571–577. [Google Scholar] [CrossRef]
- Van De Meene, A.M.; Hohmann-Marriott, M.F.; Vermaas, W.F.; Roberson, R.W. The three-dimensional structure of the cyanobacterium Synechocystis sp. PCC 6803. Arch. Microbiol. 2006, 184, 259–270. [Google Scholar] [CrossRef]
- Yu, J.; Liberton, M.; Cliften, P.F.; Head, R.D.; Jacobs, J.M.; Smith, R.D.; Koppenaal, D.W.; Brand, J.J.; Pakrasi, H.B. Synechococcus elongatus UTEX 2973, a fast growing cyanobacterial chassis for biosynthesis using light and CO2. Sci. Rep. 2015, 5, 8132. [Google Scholar] [CrossRef]
- Hu, B.; Yang, G.; Zhao, W.; Zhang, Y.; Zhao, J. MreB is important for cell shape but not for chromosome segregation of the filamentous cyanobacterium Anabaena sp. PCC 7120. Mol. Microbiol. 2007, 63, 1640–1652. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Taton, A.; Lis, E.; Adin, D.M.; Dong, G.; Cookson, S.; Kay, S.A.; Golden, S.S.; Golden, J.W. Gene Transfer in Leptolyngbya sp. Strain BL0902, a Cyanobacterium Suitable for Production of Biomass and Bioproducts. PLoS ONE 2012, 7, e30901. [Google Scholar]
- Lyons, N.A.; Kolter, R. On the evolution of bacterial multicellularity. Curr. Opin. Microbiol. 2015, 24, 21–28. [Google Scholar] [CrossRef]
- Tong, K.; Bozdag, G.O.; Ratcliff, W.C. Selective drivers of simple multicellularity. Curr. Opin. Microbiol. 2022, 67, 102141. [Google Scholar] [CrossRef] [PubMed]
- Rillema, R.; MacCready, J.S.; Vecchiarelli, A.G. Cyanobacterial growth and morphology are influenced by carboxysome positioning and temperature. Biorxiv 2020. [Google Scholar] [CrossRef]
- Conradi, F.D.; Zhou, R.-Q.; Oeser, S.; Schuergers, N.; Wilde, A.; Mullineaux, C.W. Factors controlling floc formation and structure in the cyanobacterium Synechocystis sp. strain PCC 6803. J. Bacteriol. 2019, 201, e00344-19. [Google Scholar] [CrossRef]
- Springstein, B.L.; Woehle, C.; Weissenbach, J.; Helbig, A.O.; Dagan, T.; Stucken, K. Identification and characterization of novel filament-forming proteins in cyanobacteria. Sci. Rep. 2020, 10, 1894. [Google Scholar] [CrossRef]
- Zhang, X.; Devanadera, M.C.E.; Roddick, F.A.; Fan, L.; Dalida, M.L.P. Impact of algal organic matter released from Microcystis aeruginosa and Chlorella sp. on the fouling of a ceramic microfiltration membrane. Water Res. 2016, 103, 391–400. [Google Scholar] [CrossRef]
- Huang, W.; Chu, H.; Dong, B.; Liu, J. Evaluation of different algogenic organic matters on the fouling of microfiltration membranes. Desalination 2014, 344, 329–338. [Google Scholar] [CrossRef]
- Ladner, D.A.; Vardon, D.R.; Clark, M.M. Effects of shear on microfiltration and ultrafiltration fouling by marine bloom-forming algae. J. Membr. Sci. 2010, 356, 33–43. [Google Scholar] [CrossRef]
- McEwen, J.T.; Machado, I.M.; Connor, M.R.; Atsumi, S. Engineering Synechococcus elongatus PCC 7942 for continuous growth under diurnal conditions. Appl. Environ. Microbiol. 2013, 79, 1668–1675. [Google Scholar] [CrossRef] [PubMed]
- McEwen, J.T.; Kanno, M.; Atsumi, S. 2,3 Butanediol production in an obligate photoautotrophic cyanobacterium in dark conditions via diverse sugar consumption. Metab. Eng. 2016, 36, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Du, W.; Liang, F.; Duan, Y.; Tan, X.; Lu, X. Exploring the photosynthetic production capacity of sucrose by cyanobacteria. Metab. Eng. 2013, 19, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Curatti, L.; Giarrocco, L.; Salerno, G.L. Sucrose synthase and RuBisCo expression is similarly regulated by the nitrogen source in the nitrogen-fixing cyanobacterium Anabaena sp. Planta 2006, 223, 891–900. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hao, F.; Li, X.; Wang, J.; Li, R.; Zou, L.; Wang, K.; Chen, F.; Shi, F.; Yang, H.; Wang, W.; et al. Separation of Bioproducts through the Integration of Cyanobacterial Metabolism and Membrane Filtration: Facilitating Cyanobacteria’s Industrial Application. Membranes 2022, 12, 963. https://doi.org/10.3390/membranes12100963
Hao F, Li X, Wang J, Li R, Zou L, Wang K, Chen F, Shi F, Yang H, Wang W, et al. Separation of Bioproducts through the Integration of Cyanobacterial Metabolism and Membrane Filtration: Facilitating Cyanobacteria’s Industrial Application. Membranes. 2022; 12(10):963. https://doi.org/10.3390/membranes12100963
Chicago/Turabian StyleHao, Fei, Xinyi Li, Jiameng Wang, Ruoyue Li, Liyan Zou, Kai Wang, Fuqing Chen, Feixiong Shi, Hui Yang, Wen Wang, and et al. 2022. "Separation of Bioproducts through the Integration of Cyanobacterial Metabolism and Membrane Filtration: Facilitating Cyanobacteria’s Industrial Application" Membranes 12, no. 10: 963. https://doi.org/10.3390/membranes12100963
APA StyleHao, F., Li, X., Wang, J., Li, R., Zou, L., Wang, K., Chen, F., Shi, F., Yang, H., Wang, W., & Tian, M. (2022). Separation of Bioproducts through the Integration of Cyanobacterial Metabolism and Membrane Filtration: Facilitating Cyanobacteria’s Industrial Application. Membranes, 12(10), 963. https://doi.org/10.3390/membranes12100963






