Three- and Multi-Phase Extraction as a Tool for the Implementation of Liquid Membrane Separation Methods in Practice
Abstract
1. Introduction
2. Materials and Methods
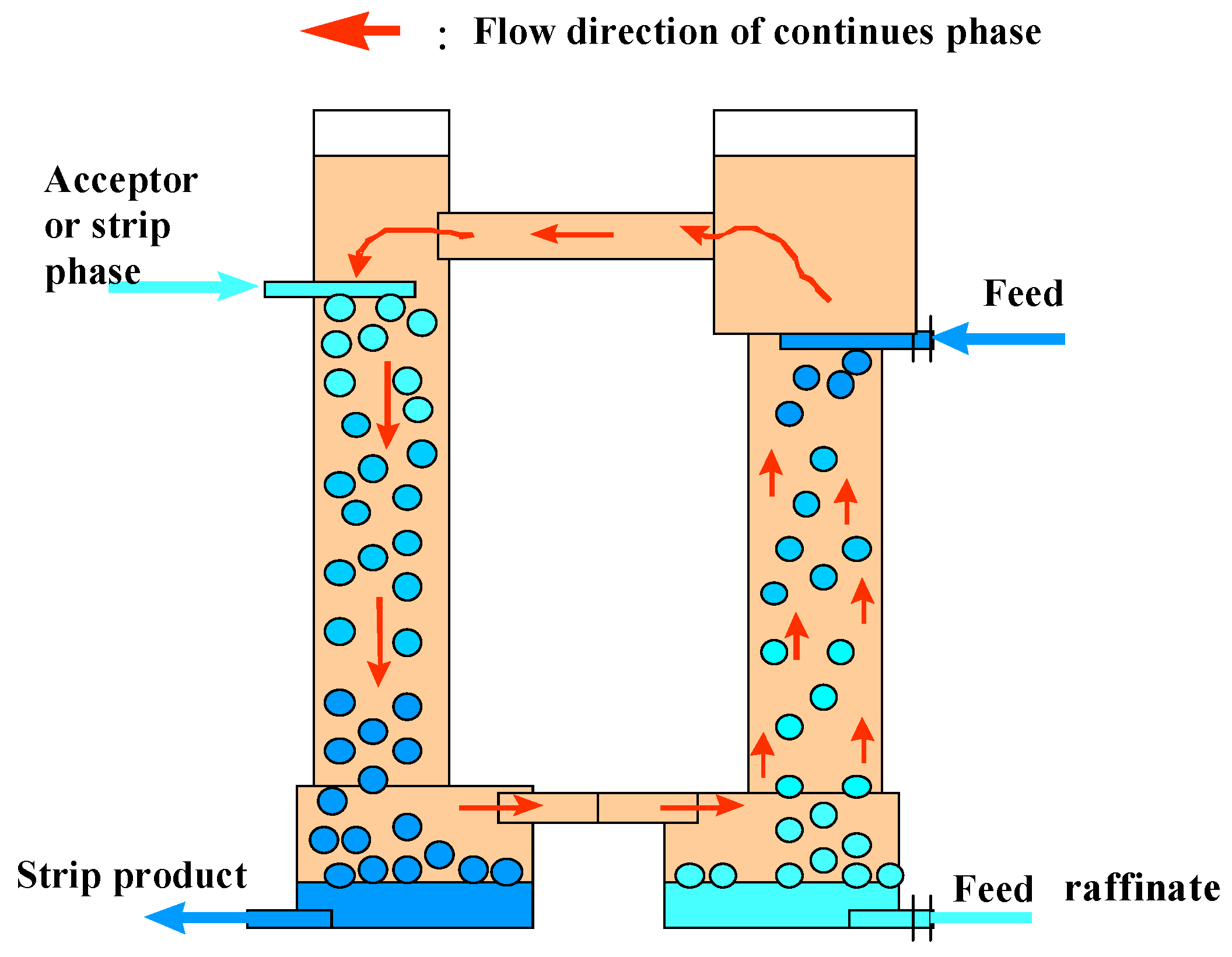
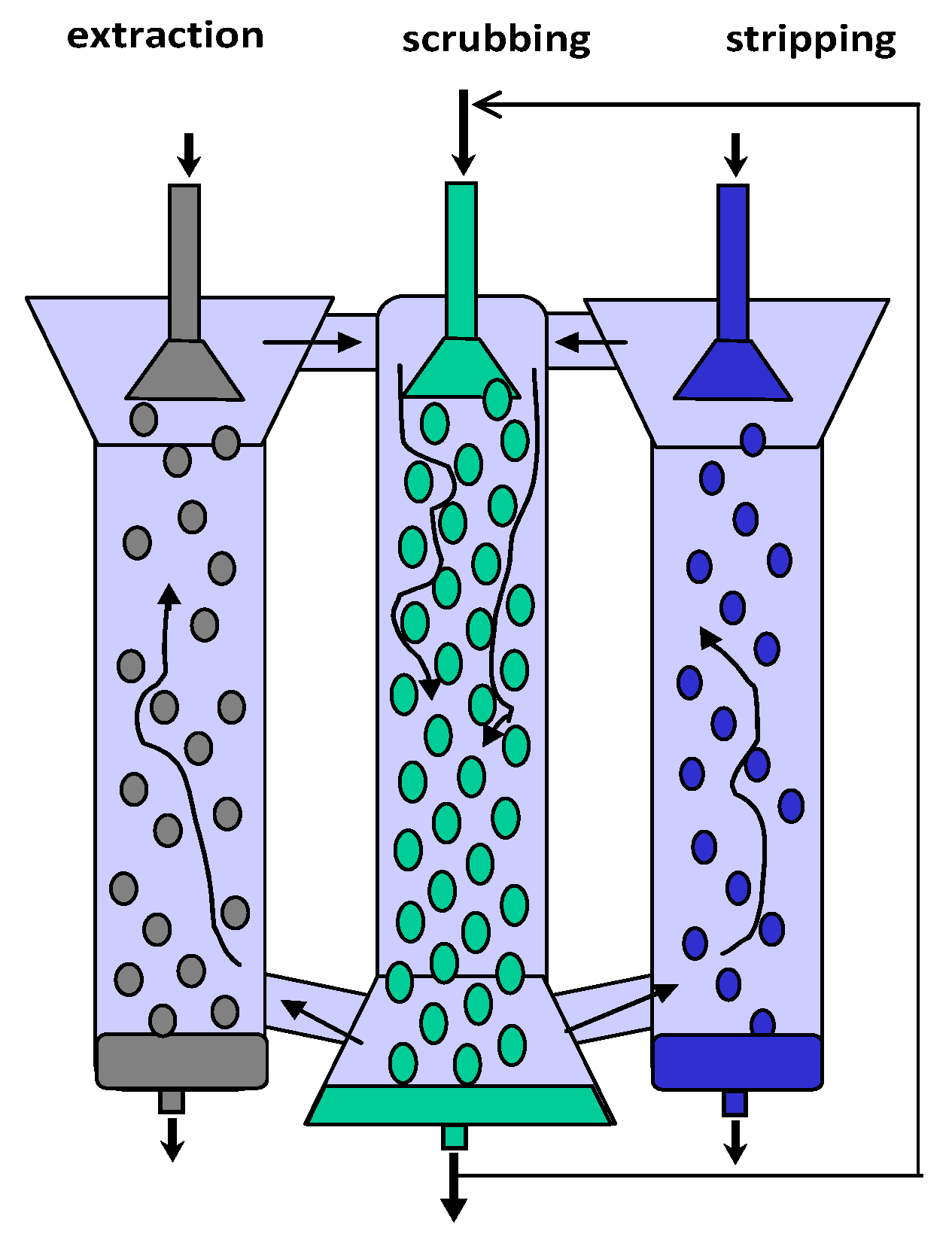
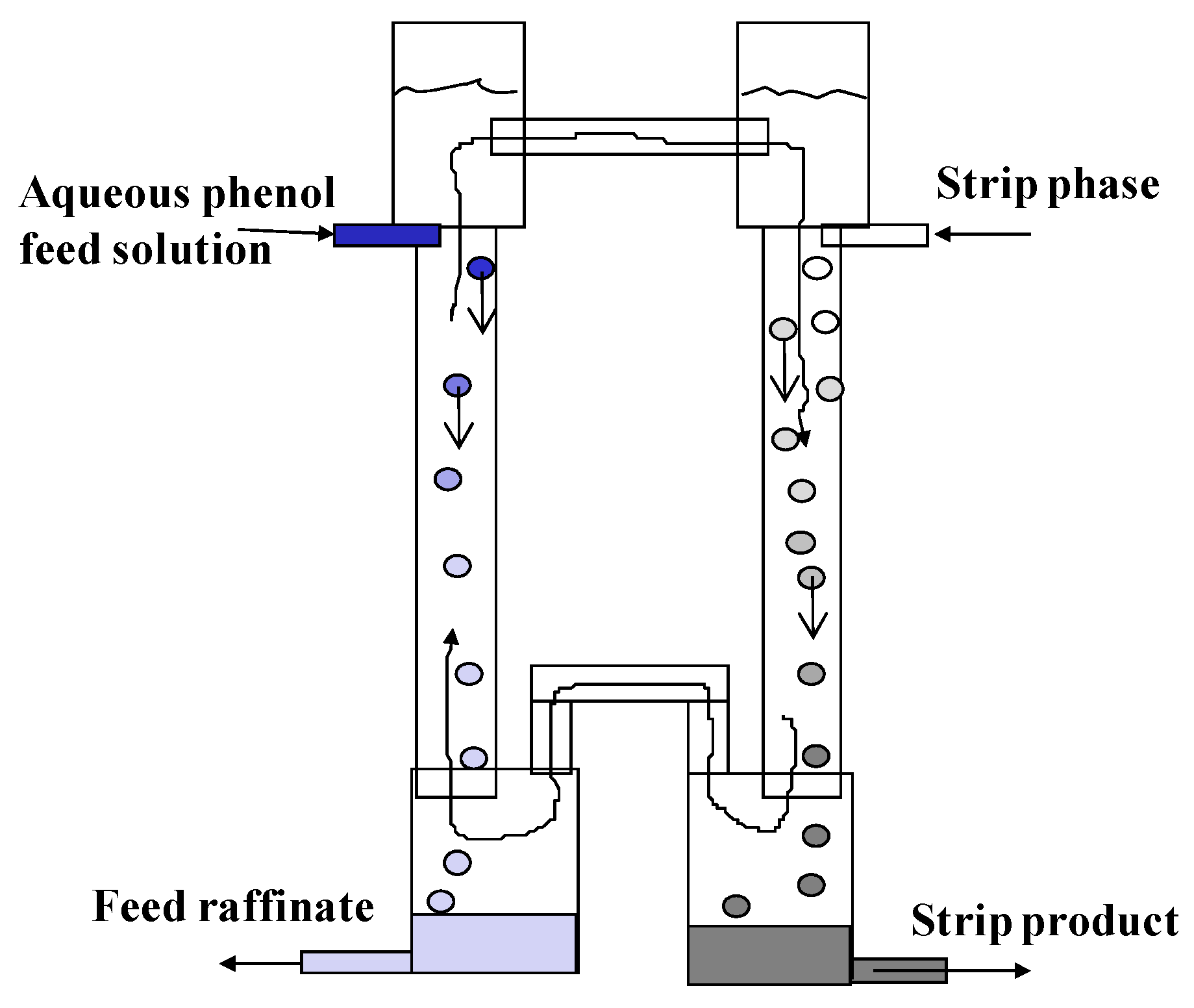
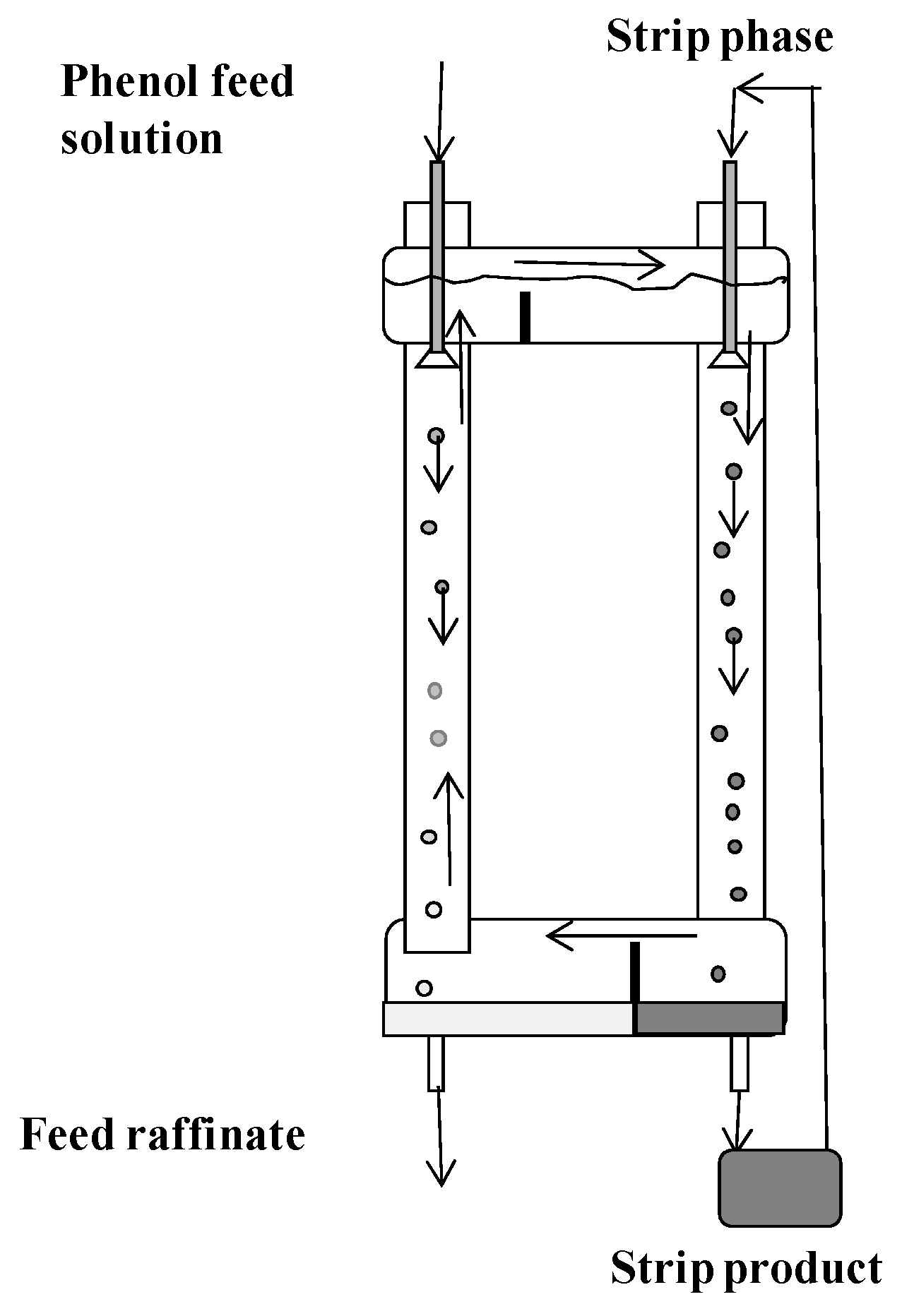
2.1. Apparatuses
2.2. Feed Solution and Extraction System
2.3. Experimental Procedures
3. Results
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Albu, P.C.; Tanczos, S.-K.; Ferencz (Dinu), A.; Pîrtac, A.; Grosu, A.R.; Pascu, D.; Grosu, V.-A.; Bungău, C.; Nechifor, A.C. pH and design on n–alkyl alcohol bulk liquid membranes for improving phenol derivative transport and separation. Membranes 2022, 12, 365. [Google Scholar] [CrossRef] [PubMed]
- Ferencz (Dinu), A.; Grosu, A.R.; Al-Ani, H.N.A.; Nechifor, A.C.; Tanczos, S.-K.; Albu, P.C.; Crăciun, M.E.; Ioan, M.-R.; Grosu, V.-A.; Nechifor, G. Operational limits of the bulk hybrid liquid membranes based on dispersion systems. Membranes 2022, 12, 190. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.-Y.; Zhang, N.; Li, Z.-Y.; Lang, Q.-L.; Yan, B.-H.; Liu, Y.; Zhang, Y. Selective separation of acetic and hexanoic acids across polymer inclusion membrane with ionic liquids as carrier. Int. J. Mol. Sci. 2019, 20, 3915. [Google Scholar] [CrossRef] [PubMed]
- Jean, E.; Villemin, D.; Hlaibi, M.; Lebrun, L. Heavy metal ions extraction using new supported liquid membranes containing ionic liquid as carrier. Sep. Purif. Technol. 2018, 201, 1–9. [Google Scholar] [CrossRef]
- Zante, G.; Boltoeva, M.; Masmoudi, A.; Barillon, R.; Trébouet, D. Supported ionic liquid and polymer inclusion membranes for metal separation. Sep. Purif. Rev. 2022, 51, 100–116. [Google Scholar] [CrossRef]
- Naim, M.M.; Moneer, A.A.; Elewa, M.M.; El-Shafei, A.A. Desalination using modified configuration of supported liquid membrane with enhancement of mass transfer of NaCl. Water Sci. Technol. 2019, 79, 175–187. [Google Scholar] [CrossRef]
- Wang, Z.; Sun, Y.; Tang, N.; Miao, C.; Wang, Y.; Tang, L.; Wang, S.; Yang, X. Simultaneous extraction and recovery of gold(I) from alkaline solutions using an environmentally benign polymer inclusion membrane with ionic liquid as the carrier. Sep. Purif. Technol. 2019, 222, 136–144. [Google Scholar] [CrossRef]
- Li, L.; Ma, G.; Pan, Z.; Zhang, N.; Zhang, Z. Research progress in gas separation using hollow fiber membrane contactors. Membranes 2020, 10, 380. [Google Scholar] [CrossRef]
- Rzelewska-Piekut, M.; Regel-Rosocka, M. Liquid membranes for separation of metal ions from wastewaters. Phys. Sci. Rev. 2021, 20210049. [Google Scholar] [CrossRef]
- Amini, M.; Rahbar-Kelishami, A.; Alipour, M.; Vahidi, O. Supported liquid membrane in metal ion separation: An overview. J. Membr. Sci. Res. 2018, 4, 121–135. [Google Scholar]
- León, G.; Hidalgo, A.M.; Miguel, B.; Guzmán, M.A. Pertraction of Co(II) through novel ultrasound prepared supported liquid membranes containing D2EHPA. Optimization and transport parameters. Membranes 2020, 10, 436. [Google Scholar] [CrossRef] [PubMed]
- Pavón, S.; Blaesing, L.; Jahn, A.; Aubel, I.; Bertau, M. Liquid membranes for efficient recovery of phenolic compounds such as vanillin and catechol. Membranes 2020, 11, 20. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Bai, L.; Li, T.; Deng, L.; Liu, L.; Zeng, S.; Han, J.; Zhang, X. Super selective ammonia separation through multiple-site interaction with ionic liquid-based hybrid membranes. J. Membr. Sci. 2021, 628, 119264. [Google Scholar] [CrossRef]
- León, G.; Gómez, E.; Miguel, B.; Hidalgo, A.M.; Gómez, M.; Murcia, M.D.; Guzmá, M.A. Feasibility of adsorption kinetic models to study carrier-mediated transport of heavy metal ions in emulsion liquid membranes. Membranes 2022, 12, 66. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.L.; Kusumastutib, A.; Derek, C.J.C.; Ooi, B.S. Emulsion liquid membrane for heavy metal removal: An overview on emulsion stabilization and destabilization. Chem. Eng. J. 2011, 171, 870–882. [Google Scholar] [CrossRef]
- León, L.; León, G.; Senent, J.; Pérez-Sirvent, C. Optimization of copper removal from aqueous solutions using emulsion liquid membranes with benzoylacetone as a carrier. Metals 2017, 7, 19. [Google Scholar] [CrossRef]
- Lee, S.C. Continuous extraction of penicillin G by emulsion liquid membranes with optimal surfactant compositions. Chem. Eng. J. 2000, 79, 61–67. [Google Scholar] [CrossRef]
- Ahmad, A.L.; Shafie, Z.M.; Zaulkiflee, N.D.; Pang, W.Y. Preliminary Study of emulsion liquid membrane formulation on acetaminophen removal from the aqueous phase. Membranes 2019, 9, 133. [Google Scholar] [CrossRef]
- Razo-Lazcano, T.; González, M.D.P.; Stambouli, M.; Pareau, D.; Hernández-Perales, L.; Avila-Rodriguez, M. Chlorpheniramine recovery from aqueous solutions by emulsion liquid membranes using soy lecithin as carrier. Colloids Surf. A Physicochem. Eng. Asp. 2017, 536, 68–73. [Google Scholar] [CrossRef]
- Dâas, A.; Hamdaoui, O. Removal of non-steroidal anti-inflammatory drugs ibuprofen and ketoprofen from water by emulsion liquid membrane. Environ. Sci. Pollut. Res. 2014, 21, 2154–2164. [Google Scholar] [CrossRef]
- Kohli, H.P.; Gupta, S.; Chakraborty, M. Extraction of Ethylparaben by emulsion liquid membrane: Statistical analysis of operating parameters. Colloids Surf. A Physicochem. Eng. Asp. 2018, 539, 371–381. [Google Scholar] [CrossRef]
- Seifollahi, Z.; Rahbar-Kelishami, A. Diclofenac extraction from aqueous solution by an emulsion liquid membrane: Parameter study and optimization using the response surface methodology. J. Mol. Liq. 2017, 231, 1–10. [Google Scholar] [CrossRef]
- Seifollahi, Z.; Rahbar-Kelishami, A. Amoxicillin extraction from aqueous solution by emulsion liquid membranes using response surface methodology. Chem. Eng. Technol. 2019, 42, 156–166. [Google Scholar] [CrossRef]
- Mohammed, A.A.; Atiya, M.A.; Hussein, M.A. Removal of antibiotic tetracycline using nano-fluid emulsion liquid membrane: Breakage, extraction and studies. Colloids Surf. A Physicochem. Eng. Asp. 2020, 595, 124680. [Google Scholar] [CrossRef]
- Razo-Lazcano, T.A.; Stambouli, M.; González-Muñoz, M.d.P.; Pareau, D.; Ávila-Rodríguez, M. Emulsion liquid membranes for recovery of ibuprofen from aqueous solutions. J. Chem. Technol. Biotechnol. 2014, 89, 890–898. [Google Scholar] [CrossRef]
- Rosly, M.B.; Othman, N.; Rahman, H.A. Liquid membrane component selection for removal of phenol from simulated aqueous waste solution. Malays. J. Anal. Sci. 2018, 22, 702–714. [Google Scholar]
- Masry, B.; Aly, M.; Daoud, J. Selective permeation of Ag+ ions from pyrosulfite solution through Nano-Emulsion Liquid Membrane (NELM) containing CYANEX 925 as carrier. Colloids Surf. A Physicochem. Eng. Asp. 2020, 610, 125713. [Google Scholar] [CrossRef]
- Al-Ani, F.H.; Alsalhy, Q.F.; Al-Dahhan, M. Enhancing emulsion liquid membrane system (ELM) stability and performance for the extraction of phenol from wastewater using various nanoparticles. Desalination Water Treat. 2021, 210, 180–191. [Google Scholar] [CrossRef]
- Björkegren, S.; Karimi, R.F.; Martinelli, A.; Jayakumar, N.S.; Hashim, M.A. A New emulsion liquid membrane based on a palm oil for the extraction of heavy metals. Membranes 2015, 5, 168–179. [Google Scholar] [CrossRef]
- Kostanyan, A.E. Comparison between various schemes of three-phase extraction: Mass transfer between two liquid phases through an exchange medium. Theor. Found. Chem. Eng. 1999, 33, 584–592. [Google Scholar]
- Kostanian, A.E. Multi-Stage Extraction Process. U.S. Patent No. 6,143,178, 7 November 2000. [Google Scholar]
- Kostanyan, A.E.; Safiulina, A.M.; Tananaev, I.T.; Myasoedov, B.F. Multiphase extraction: Design of single-and multistage separation using liquid pseudomembranes. Doklady Chemistry 2005, 404, 203–205. [Google Scholar] [CrossRef]
- Kostanyan, A.E.; Safiulina, A.M.; Tananaev, I.G. Linear models of three-phase extraction processes. Theor. Found. Chem. Eng. 2007, 41, 755–759. [Google Scholar] [CrossRef]
- Kostanian, A.E. Staged versions of liquid membrane extraction processes. Solvent Extr. Ion Exch. 2013, 31, 297–305. [Google Scholar] [CrossRef]
- Kostanyan, A.E. On the application of liquid-membrane principle in a system of mixing-settling extractors. Theor. Found. Chem. Eng. 2008, 42, 718–723. [Google Scholar] [CrossRef]
- Belova, V.V.; Kostanyan, A.E.; Zakhodyaeva, Y.A.; Kholkin, A.I.; Logutenko, O.A. On the application of bulk-supported liquid membrane techniques in hydrometallurgy. Hydrometallurgy. 2014, 150, 144–152. [Google Scholar] [CrossRef]
- Kostanian, A.E. Multistage Three-Phase Extractor. U.S. Patent No. 6,090,352, 18 July 2000. [Google Scholar]
- Kostanian, A.E. Multiphase Extractor. U.S. Patent No. 6,129,842, 10 October 2000. [Google Scholar]
- Backer, W.; Kostanian, A.E. Multiphase Extractor with a Wash Chamber. U.S. Patent No. 6,454,103, 24 September 2002. [Google Scholar]
- Kostanian, A.E. Multiple Phase Extractor. U.S. Patent No. 6,446,815, 10 September 2002. [Google Scholar]
- Kostanian, A.E. Multi-Phase Extraction Apparatus. U.S. Patent No. 6,521,195, 18 February 2003. [Google Scholar]
- Kostanyan, A.E.; Egorova, N.S.; Voshkin, A.A.; Zonov, S.S. Extraction of uranyl, ytterbium, and lanthanum nitrates in a three-compartment multiphase extractor. Theor. Found. Chem. Eng. 2008, 42, 718–723. [Google Scholar] [CrossRef]
- Rodríguez-Llorente, D.; Cañada-Barcala, A.; Muñoz, C.; Pascual-Muñoz, C.; Navarro, P.; Santiago, R.; Águeda, V.I.; Álvarez-Torrellas, S.; García, J.; Larriba, M. Separation of phenols from aqueous streams using terpenoids and hydrophobic eutectic solvents. Sep. Purif. Technol. 2020, 251, 117379. [Google Scholar] [CrossRef]
- Greminger, D.C.; Burns, G.P.; Lynn, S.; Hanson, D.N.; King, C.J. Solvent extraction of phenols from water. Ind. Eng. Chem. Process Des. Dev. 1982, 21, 51–54. [Google Scholar] [CrossRef][Green Version]

| No | pH | Phenol Concentration, ppm | Distribution Coefficient | |
|---|---|---|---|---|
| Aqueous Phase | Organic Phase | |||
| 1 | 7 | 780 | 43,630 | 55.9 |
| 2 | 7 | 460 | 20,600 | 44.8 |
| 3 | 7 | 81 | 4350 | 53.7 |
| 4 | 7 | 58 | 3070 | 52.9 |
| 5 | 2.1 | 40 | 2020 | 50.5 |
| 6 | 2.1 | 20 | 1030 | 51.5 |
| No | ve, L/h | de, mm | vs, L/h | ds, mm | xf, ppm | xr, ppm | E | xs, ppm |
|---|---|---|---|---|---|---|---|---|
| 1 | 5.3 | 2 | 8.5 | 1 | 1050 | 97 | 0.91 | 14,900 |
| 2 | 3.3 | 2 | 5.3 | 1 | 10,600 | 2280 | 0.78 | - |
| 3 | 3.3 | 3 | 5.3 | 2 | 10,600 | 3280 | 0.69 | 66,900 |
| 4 | 3.3 | 2 | 5.3 | 1 | 1020 | 140 | 0.86 | - |
| 5 | 3.3 | 2 | 5.3 | 1 | 1020 | 150 | 0.85 | - |
| 6 | 3.3 | 3 | 5.3 | 1 | 1020 | 170 | 0.83 | - |
| 7 | 3.3 | 3 | 5.2 | 2 | 1020 | 210 | 0.79 | 20,700 |
| No | ve, L/h | de, mm | vs, L/h | ds, mm | xf, ppm | xr, ppm | E | xs, ppm |
|---|---|---|---|---|---|---|---|---|
| 1 | 3.3 | 2 | 5.3 | 1 | 1030 | 14.5 | 0.99 | - |
| 2 | 5.3 | 2 | 8.5 | 1 | 1030 | 18 | 0.98 | 10,350 |
| 3 | 7.0 | 2 | 11 | 1 | 1030 | 19 | 0.98 | - |
| 4 | 9.0 | 2 | 14 | 1 | 1030 | 35 | 0.97 | 17,800 |
| 5 | 3.3 | 3 | 5.3 | 2 | 1060 | 18 | 0.98 | - |
| 6 | 4.2 | 3 | 7.0 | 2 | 1060 | 13 | 0.99 | - |
| 7 | 5.3 | 3 | 8.5 | 2 | 1060 | 27 | 0.97 | 20,000 |
| 8 | 3.3 | 1 | 5.3 | 2 | 1090 | 20 | 0.98 | - |
| 9 | 5.3 | 1 | 8.5 | 2 | 1090 | 80 | 0.93 | - |
| 10 | 7.0 | 1 | 11 | 2 | 1090 | 180 | 0.84 | 17,700 |
| 11 | 3.3 | 1 | 5.3 | 2 | 10,600 | 380 | 0.96 | - |
| 12 | 5.3 | 1 | 8.5 | 2 | 10,600 | 4500 | 0.58 | 73,300 |
| 13 | 3.3 | 2 | 5.3 | 1 | 10,800 | 470 | 0.96 | - |
| 14 | 3.3 | 3 | 5.3 | 2 | 10,800 | 570 | 0.95 | 53,800 |
| 15 | 3.3 | 3 | 5.3 | 2 | 1060 | 11 | 0.99 | - |
| 16 | 4.2 | 3 | 11 | 2 | 1060 | 8 | 0.99 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kostanyan, A.E.; Belova, V.V.; Voshkin, A.A. Three- and Multi-Phase Extraction as a Tool for the Implementation of Liquid Membrane Separation Methods in Practice. Membranes 2022, 12, 926. https://doi.org/10.3390/membranes12100926
Kostanyan AE, Belova VV, Voshkin AA. Three- and Multi-Phase Extraction as a Tool for the Implementation of Liquid Membrane Separation Methods in Practice. Membranes. 2022; 12(10):926. https://doi.org/10.3390/membranes12100926
Chicago/Turabian StyleKostanyan, Artak E., Vera V. Belova, and Andrey A. Voshkin. 2022. "Three- and Multi-Phase Extraction as a Tool for the Implementation of Liquid Membrane Separation Methods in Practice" Membranes 12, no. 10: 926. https://doi.org/10.3390/membranes12100926
APA StyleKostanyan, A. E., Belova, V. V., & Voshkin, A. A. (2022). Three- and Multi-Phase Extraction as a Tool for the Implementation of Liquid Membrane Separation Methods in Practice. Membranes, 12(10), 926. https://doi.org/10.3390/membranes12100926








